Abstract
Several immunotherapies have demonstrated endogenous insulin preservation in recent-onset type 1 diabetes (T1D). We considered the primary results of rituximab, abatacept, teplizumab, alefacept, high-dose antithymocyte globulin (ATG), low-dose ATG, and low-dose ATG ± granulocyte-colony–stimulating factor trials in an attempt to rank the effectiveness of the agents studied. C-peptide 2-h area under the curve means were modeled using analysis of covariance. The experimental treatment group effect for each study, compared with its internal control, was estimated after adjusting for baseline C-peptide and age. Percentage increase in C-peptide over placebo and the absolute difference within study were calculated to compare and contrast effect size among interventions. Low-dose ATG (55% and 103%) and teplizumab (48% and 63%) ranked highest in C-peptide preservation at 1 and 2 years, respectively. Low-dose ATG and teplizumab show the greatest impact on C-peptide preservation among recent new-onset T1D studies; these should be further explored as core immunotherapies in the T1D prevention setting.
Keywords: Type 1 diabetes, Clinical trial, Intervention, Immunotherapy
Background
Stimulated serum C-peptide levels remain the preferred primary endpoint for most intervention trials in subjects with recent-onset type 1 diabetes (T1D). Although a growing number of interventions have demonstrated capacity to preserve C-peptide, analytical comparisons of efficacy across T1D intervention trials have been limited. Utilizing a model-based adjustment approach, we evaluated, using the least biased methodology possible, C-peptide preservation across the most successful T1D immunotherapy trials. Under the premise that greater effect in recent-onset T1D should increase the probability of efficacy in prevention, we sought to inform selection of immunotherapies for future prevention studies.
Studies chosen for cross-trial comparison included immunotherapy trials from the last 10 years in subjects with recent-onset T1D, demonstrating improved C-peptide compared with placebo. The seven interventions included the Type 1 Diabetes TrialNet-sponsored (1) rituximab, (2) abatacept, (3) low-dose antithymocyte globulin (ATG), and (4) low-dose ATG/granulocyte-colony–stimulating factor (G-CSF) trials, and the Immune Tolerance Network (ITN)-sponsored (5) teplizumab, (6) alefacept, and (7) high-dose ATG trials. All trials included children and adults, were placebo-controlled, blinded, and had 2:1 randomization within 100 days of diagnosis with the exception of the open label teplizumab trial and the 1:1:1 randomized low-dose ATG, ATG/G-CSF, and placebo trial.1–6
The rituximab, alefacept, high-dose ATG, low-dose ATG, and low-dose ATG/G-CSF trials had a primary outcome of 2-h mean area under the curve (AUC) C-peptide as part of a standard mixed meal tolerance test (MMTT) at 1 year. The teplizumab study used a 4-h MMTT AUC C-peptide for the primary analysis (first 2-h of MMTT results included in this comparison), and both the abatacept and teplizumab trials set the primary analysis at 2 years.
Abatacept (CTLA4-Ig), a fusion protein that impairs costimulation needed for full T lymphocyte activation, was given as an intravenous infusion monthly through 2 years.1 Alefacept (LFA-3/IgG1), a fusion protein that impairs CD2-mediated costimulation and specifically targets memory T cells, was given as a weekly intramuscular injection for two 12-week courses.2 Rituximab (anti-CD20), a B cell depleting monoclonal antibody, was provided as intermittent intravenous dosing for 1 month.3 Teplizumab (anti-CD3), a monoclonal antibody targeting effector T cells, was administered through daily intravenous infusion for 14 days with repeat dosing at 1 year (4).
In both the low-dose (2.5 mg/kg) and high-dose (6.5 mg/kg) ATG trials, the proposed mechanism of action was T cell depletion, with poststudy analysis noting relative sparing of regulatory T cells (Tregs) with low-dose ATG. G-CSF was used in combination with low-dose ATG for potential synergy.7,8 Subjects in the low-dose ATG trial received either (1) 2.5 mg/kg intravenous ATG for 2 days, (2) low-dose ATG followed by G-CSF, 6 mg subcutaneously, every 2 weeks for six doses, or (3) placebo.5 The high-dose ATG trial administered 6.5 mg/kg intravenously for 4 days.6 Although a complete review of study drug side effects is beyond the scope of this article, observed adverse events included infusion reactions, cytokine release syndrome, serum sickness, and transient decreases in CD4 T cell counts.
Methods
An analysis of covariance (ANCOVA) model was fitted to 1-year (and separately 2-year) C-peptide AUC means from the 2-h MMTT using publicly available data from all six studies. The C-peptide AUC mean was calculated by first determining the AUC using the trapezoidal rule then dividing by 120 min. The inclusion of study as a covariate assured the treatment effect estimate was relative to their internal control. Each treatment group mean was determined using the fitted fixed-effect model and substituting the mean age and the mean baseline C-peptide of the six-study cohort (i.e., age and baseline C-peptide adjusted) while retaining the coefficient specific to each study. The absolute treatment effect difference is the model-based means of the experimental minus the placebo group. To compare across interventions, the percentage treatment effect, the absolute difference divided by the placebo group mean ( × 100), was calculated for each immunotherapy.
A similarly structured model was applied by Bundy9 and provides details on the valid use of the ANCOVA model when fitted to C-peptide. The transformation ln[Cp +1] to both C-peptide variables satisfied the requirement of normally distributed residuals. We assured that there was no violation of homogeneity of variance among the dichotomous variables of treatment group using the Bartlett's test.10 There was a moderate violation of homogeneity of variance across the continuous variable baseline C-peptide. Although this does not affect the group mean estimates, it could provide inaccurate confidence intervals. For this reason, we used the bootstrap technique11 to estimate the 95% confidence intervals for each group mean and treatment effect by study.
Significance testing of every treatment with every other should be avoided because the control of type I error (large false-positive probability) becomes excessive (21 pair-wise comparisons). If the significance level is controlled for multiple comparisons, then the statistical power would be very low (large false-negative probability). However, we sought to ask a more global question. Namely, how likely was the observed range of the treatment means (maximum treatment effect across studies minus the minimum treatment effect) if the real effect of the seven treatments was the same (i.e., the null hypothesis)?
To explore this question, a permutation test (utilizing repeated random assignment of the experimental treatments to subject values) was computed on the ANCOVA residuals after adjusting for age, baseline C-peptide, and study for year 1 and separately for year 2. To assess the evidence that there was a differential treatment effect by baseline C-peptide, an interaction term between baseline C-peptide and each experimental treatment was tested within the ANCOVA model—none reached even a suggestive level of significance (i.e., <0.15).
Results
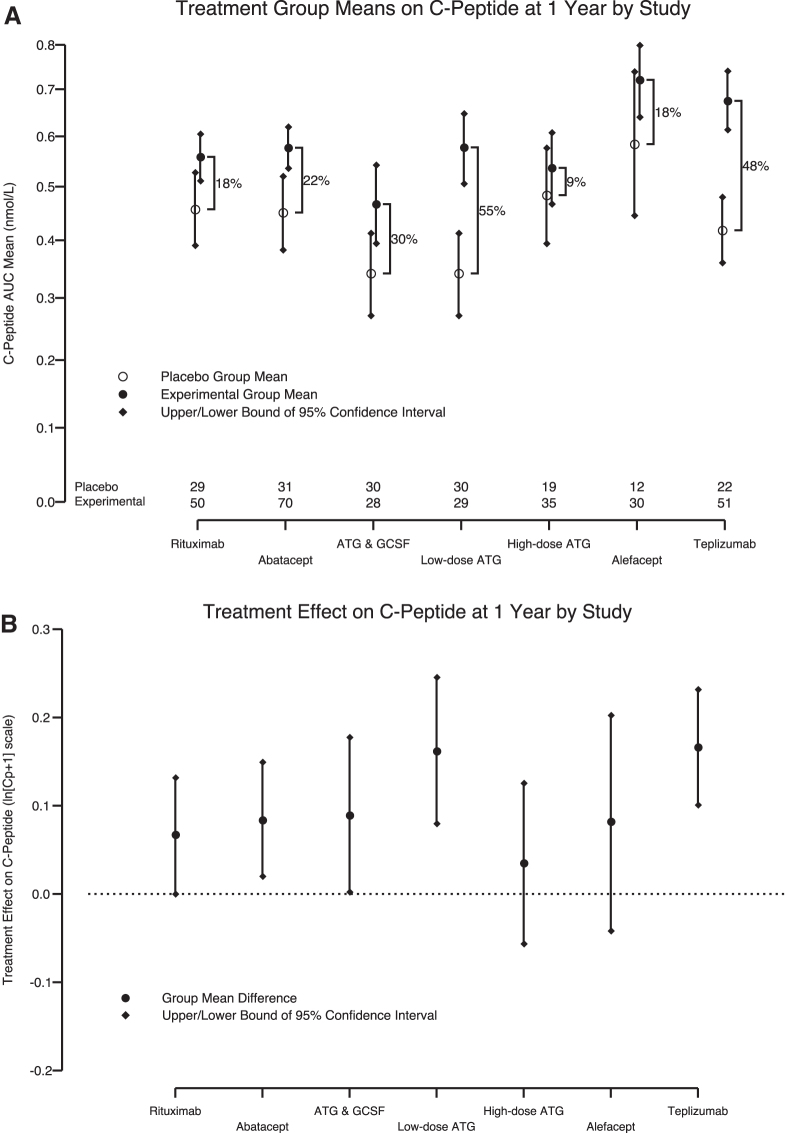
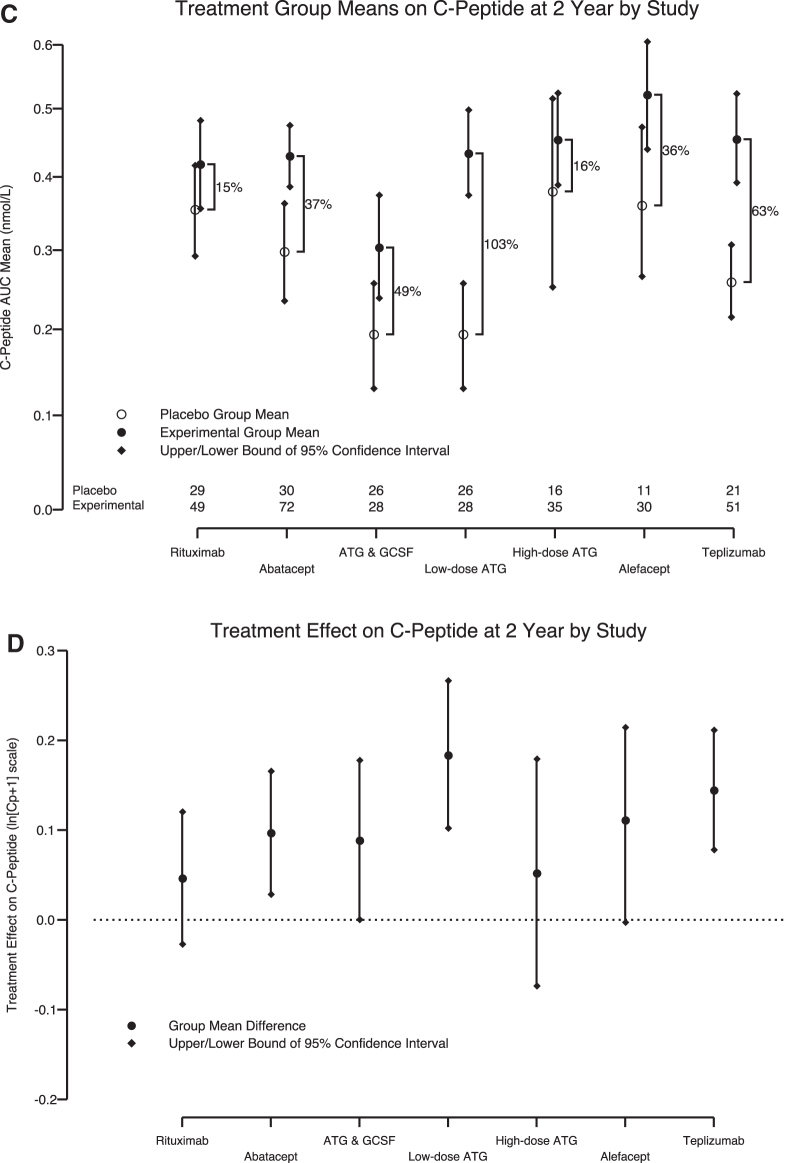
Key features of the seven TrialNet and ITN interventions are provided in Table 1. Treatment effects (percentage change relative to control) for years 1 and 2, respectively, and ordered from lowest to highest for year 1, were high-dose ATG (9% and 16%), rituximab (18% and 15%), alefacept (18% and 36%), abatacept (22% and 37%), low-dose ATG/G-CSF (30% and 49%), teplizumab (48% and 63%), and low-dose ATG (55% and 103%) (Fig. 1A, C).
Table 1.
Key Characteristics of the Interventions in Each Clinical Trial
| n | Age (years) | Regimen | Primary outcome (AUC C-peptide) | P | |
|---|---|---|---|---|---|
| Rituximab | 87 | 8–40 | 375 mg/m2 IV days 1, 8, 15, and 22 | 2 h MMTT at 1 year | 0.03 |
| Abatacept | 112 | 6–36 | 10 mg/kg IV days 1, 14, 28, and then monthly × 2 years | 2 h MMTT at 2 years | 0.0022 |
| ATG—low dose | 60 | 12–45 | ATG 2.5 mg/kg IV over 2 days | 2 h MMTT at 1 year | 0.0003 |
| ATG+G-CSF—low dose | 60 | 12–45 | ATG 2.5 mg/kg IV over 2 days + G-CSF 6 mg SQ × 6 q2 week | 2 h MMTT at 1 year | 0.031 |
| ATG—high dose (START) | 52 | 12–35 | 6.5 mg/kg IV over 4 days | 2 h MMTT at 1 year | 0.591 |
| Alefacept (T1DAL) | 49 | 12–35 | 15 mg IM weekly × 12, repeated 12 weeks later | 2 h MMTT at 1 year | 0.065 |
| Teplizumab (AbATE) | 77 | 8–30 | Median dose ∼11.6 mg IV over 14 days ± repeat at 1 year (n = 40/52) | 4 h MMTT at 2 years | 0.002 |
ATG, antithymocyte globulin; AUC, area under the curve; G-CSF, granulocyte-colony–stimulating factor; MMTT, mixed meal tolerance test.
FIG. 1.
Cross-trial comparison of recent-onset T1D intervention trials. (A) One-year and (C) 2-year percentage increase in C-peptide between placebo and experiment groups comparing C-peptide AUC means (with 95% confidence intervals) from six recent-onset T1D studies adjusted for baseline AUC C-peptide and age with study effect retained. (B) One-year and (D) 2-year absolute C-peptide difference presented as a point estimate with 95% confidence intervals. The low-dose ATG and ATG/G-CSF study arms are presented separately. The number of subjects with available data per study is listed at the bottom of (A) and (C). ATG, antithymocyte globulin; AUC, area under the curve; G-CSF, granulocyte-colony–stimulating factor; T1D, type 1 diabetes.
The treatment effects (absolute difference within each study) on the transformed scale for years 1 and 2, respectively, yielded a slightly different order: high-dose ATG (0.0347 and 0.0517), rituximab (0.0666 and 0.0465), alefacept (0.0817 and 0.112), abatacept (0.0839 and 0.0967), low-dose ATG/G-CSF (0.0889 and 0.0888), low-dose ATG (0.162 and 0.184), and teplizumab (0.166 and 0.144) (Fig. 1B, D). To address whether the treatment effects differ, we calculated a permutation test on the range (maximum minus minimum effect). Evidence remained strongly in favor of the assertion that treatment effects differed (year 1: P = 0.005 and year 2: P = 0.02) among the studies. Furthermore, the evidence suggests that of the agents evaluated, low-dose ATG and teplizumab provided for the greatest retention of C-peptide at 1 and 2 years after initial immunotherapy.
Conclusions
Several immunotherapies have now demonstrated the capacity to slow decline in beta cell function in subjects with recent-onset T1D.12 Short-term and long-term preservation of C-peptide are associated with reduction in severe hypoglycemia and decreased complication risk, respectively.13,14 As such, identifying which therapeutics demonstrate the greatest potential for benefit is of critical importance in designing the most effective and efficient intervention and prevention studies. Practical considerations such as cost, side effect profile, and duration of therapy should also be considered, although they are not the focus of this effort. Herein, we have demonstrated that low-dose ATG and teplizumab provide for the greatest C-peptide preservation among trials in recent-onset T1D.
Appreciating the limitations of this analysis requires further review of the methodologies employed. Randomized trials are designed to remove inherent bias. And although all trials analyzed included contemporaneous intratrial controls, differences in eligibility criteria, demographics of subjects accrued, and baseline C-peptide levels make intertrial comparisons inherently difficult. Differences in the primary outcome between trials may have influenced these results as the teplizumab trial used a 4-h MMTT, whereas all others used a 2-h MMTT, and both teplizumab and abatacept trial primary endpoints were at 2 years versus 1 year. As such, normalization of outcomes across studies (2-h MMTT C-peptide AUC mean), using ANCOVA modeling and adjusting for significant covariates (i.e., subject age and baseline C-peptide), was required to provide the most valid comparison of placebo and treated group differences across studies.
Another limitation of this study is the missing observations for both time periods. Although loss to follow-up was low for all studies examined (median dropout rate 8.5% at 2 years), the analysis could have been biased by dropouts given the relatively small sample sizes of the trials. In addition, the differing C-peptide AUC mean among the six placebo groups presents an inherent challenge for cross-trial comparisons and highlights the importance of retaining the study-specific effects, as was done for this analysis. That said, the randomization process provides for the fairest comparison between groups and eliminates the possibility of selection bias. Although it is impossible to evaluate whether differences in C-peptide decline among the studies had any bearing on the reported treatment effect, we partially addressed this question by quantifying the differential treatment effect as a function of baseline C-peptide by study. There was no evidence of any such relationship.
Beyond the biostatistical limitations of the analysis, we also note that other recent-onset studies that have demonstrated C-peptide preservation were not included due to lack of access to raw data sets. As examples, studies utilizing anti-IL21 monoclonal antibody, liraglutide, and golimumab have demonstrated benefit in recent-onset T1D, but were not included. Future analyses should seek to provide additional comparisons with all agents demonstrating capacity for preservation of C-peptide.
Strengths of this analysis include the novel use of an ANCOVA model, evaluation across multiple clinical trials with a positive C-peptide outcome, and inclusion of critical covariates allowing for comparison of outcomes among a heterogeneous group of subjects. Furthermore, direct hypothesis testing is not advisable as these therapies were not compared in head-to-head trials. Randomized head-to-head comparisons would indeed be the gold standard for efficacy comparisons but have not, in the past, been considered financially or practically feasible in the T1D space. Within the constraints of this cross-trial analysis, the single most accurate way to determine which interventions provided maximal effect was to compare the intratrial placebo-to-treated differences in C-peptide using the ANCOVA model with the included covariates.
In addition to fully appreciating which agents offer the greatest benefits in terms of C-peptide preservation, mechanistic analyses of these interventions are vital to improving our understanding of potential T1D immunotherapeutics. Specifically, differences in C-peptide preservation between trials and between individual participants may be informed by ongoing mechanistic efforts. We must also continue to include assessments of side effect profile, duration of therapy, and cost when trying to guide decision making seeking to identify the “best” therapeutics.
With teplizumab on the verge of becoming the first T1D immunotherapeutic approved by the FDA, we are nearing a major paradigm shift in clinical trial design and standard of care for patients with T1D. Future studies may be forced to ethically and practically bench mark themselves against outcomes seen with teplizumab. As such, next generation trials will need to utilize advanced clinical trial design (e.g., platform trials) with the development of biomarkers to more rapidly inform research teams as to which agents are most effective. A culture change in the T1D space is needed, much like what was seen in pediatric oncology wherein almost every patient with a newly diagnosed malignancy participated in a research study to determine whether the current standard of care plus a new therapeutic could improve outcomes. These efforts in the T1D space will ultimately provide for more meaningful outcomes data.
In conclusion, as additional T1D therapeutics are identified, we believe an obligation exists to determine which therapies should be carried forward in the intervention and, perhaps more importantly, the prevention space. The incidence of T1D continues to rise, but so too does the arsenal of immunomodulatory therapies seemingly capable of combating this complex and heterogeneous disease.
Although all agents included in this analysis demonstrated capacity for C-peptide retention, teplizumab and low-dose ATG provided the greatest effects on C-peptide at 1 and 2 years. Given the recently published teplizumab prevention trial results,15 these data lend further support to the notion that immunotherapies in general, and low-dose ATG and teplizumab specifically, should be the focus of approaches seeking to delay and ultimately prevent T1D.
Acknowledgments
The authors acknowledge the contributions of clinical trial participants and their families in both Type 1 Diabetes TrialNet and the Immune Tolerance Network.
Authors' Contributions
M.J.H. and B.N.B. conceptualized this article. L.M.J. and M.N.G. analyzed the data and wrote the article. B.N.B. and J.P.K. provided statistical analysis. D.A.S., M.A.A., T.M.B., C.E.M., K.C.H., S.E.G., and J.P.K. contributed to data acquisition and interpretation of the data. All authors have provided final approval of the version to be published. M.J.H. is the guarantor of this study and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
M.J.H. is on the scientific advisory board of SAb Biotherapeutics and received grant funding from Sanofi, and The Helmsley Charitable Trust to perform studies of low-dose ATG. MAA holds a patent on ATG plus G-CSF for the treatment of type 1 diabetes. There are no other conflicts of interest to disclose.
Funding Information
We acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided data for this study. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK097835, UC4 DK106993, and JDRF. We thank the Immune Tolerance Network (ITN) studies cited in this analysis. Data for these studies were accessed from the publicly accessible ITN Trial Share database. ITN studies were performed with funding from a National Institutes of Health contract No. N01 AI15416 that established the ITN as an international clinical research consortium headquartered at the Benaroya Research Institute and supported by the National Institute of Allergy and Infectious Diseases and the Juvenile Diabetes Research Foundation. This study was also supported by NIH grants UL1RR024131 UL1RR024139, UL1TR000004, UL1RR024134, UL1TR000003, UL1TR000114, TR000006, and UL1TR000442-06. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
References
- 1. Orban T, Bundy B, Becker DJ, et al. : Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rigby MR, DiMeglio LA, Rendell MS, et al. : Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol 2013;1:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. : Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herold KC, Gitelman SE, Ehlers MR, et al. : Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013;62:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haller MJ, Schatz DA, Skyler JS, et al. : Low-dose anti-thymocyte globulin (ATG) preserves beta-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care 2018;41:1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gitelman SE, Gottlieb PA, Rigby MR, et al. : Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 2013;1:306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker MJ, Xue S, Alexander JJ, et al. : Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haller MJ, Gitelman SE, Gottlieb PA, et al. : Anti-thymocyte globulin/G-CSF treatment preserves beta cell function in patients with established type 1 diabetes. J Clin Invest 2015;125:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bundy BN, Krischer JP; Type 1 Diabetes TrialNet Study Group: A model-based approach to sample size estimation in recent onset type 1 diabetes. Diabetes Metab Res Rev 2016;32:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snedecor GW, Cochran WG. Statistical Methods 8th Edn. Iowa State, UP: Ames, IA, 1989 [Google Scholar]
- 11. Efron B, Tibshirani RJ: An Introduction to the Bootstrap. New York, NY: Chapman & Hall/CRC, 1998 [Google Scholar]
- 12. Jacobsen LM, Newby BN, Perry DJ, et al. : Immune mechanisms and pathways targeted in type 1 diabetes. Curr Diab Rep 2018;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 14. Steffes MW, Sibley S, Jackson M, Thomas W: Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 15. Herold KC, Bundy BN, Long SA, et al. : An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]