Abstract
Background: Hypertensive disorders of pregnancy are a recognized risk factor of a woman's future cardiovascular risk. The potential role of micronutrients in mitigating hypertensive disorders is not fully understood. This study examined maternal postpartum plasma B vitamin profiles by hypertensive disorders of pregnancy in a high-risk multiethnic U.S. population.
Materials and Methods: The analyses included 2584 mothers enrolled within 3 days postpartum at the Boston Medical Center. Hypertensive disorders of pregnancy included gestational hypertension and pre-eclampsia disorders (pre-eclampsia, eclampsia, hemolysis, elevated liver enzymes, and/or low platelets syndrome) as documented in the medical records. Plasma folate, vitamin B12, and homocysteine levels were measured in blood samples collected at enrollment. Kernel density plots and multivariable regressions were used to examine the relationship between hypertensive disorders and postpartum B vitamin profiles.
Results: Of the 2584 mothers, 10% had pre-eclampsia disorders that were associated with significantly lower plasma folate (adjusted beta coefficient (aβ): −0.10; 95% CI: −0.22 to −0.06) and increased homocysteine (aβ: 0.08; 95% CI: 0.04–0.13), but not with vitamin B12 concentrations. These associations remained robust after adjusting for a range of pertinent covariables and were more pronounced in non-Hispanic Black women compared with other groups. However, gestational hypertension was not significantly associated with any postpartum biomarker.
Conclusions: We found that pre-eclampsia disorders, but not gestational hypertension, was associated with lower folate and higher homocysteine levels postpartum, especially among Black mothers. This finding, if further confirmed, may have implications for postpartum care, including attention to maternal micronutrient status to reduce and prevent hypertensive disorders in pregnancy-associated consequences in subsequent pregnancies and lifespan. Registration date: July 25, 2017; Registry website: https://clinicaltrials.gov/ct2/show/NCT03228875.
Keywords: B vitamins, biomarkers, high-risk, homocysteine, hypertension, pre-eclampsia
Introduction
Hypertensive disorders of pregnancy are a leading cause of maternal and infant morbidity and mortality in the United States and currently affects about 8% of pregnancies.1,2 The rates of hypertensive disorders of pregnancy differ by race/ethnicity and is more common among non-Hispanic Black women compared with non-Hispanic White and Hispanic women, in parallel with high rates of chronic hypertension in non-Hispanic Black women.3 Women with hypertensive disorders have a higher risk of recurrence in a subsequent pregnancy.4 Furthermore, the effects of hypertensive disorders extend beyond pregnancy with long-term complications, including chronic hypertension, diabetes mellitus, ischemic heart disease, cerebrovascular disease, kidney disease, thromboembolism, hypothyroidism, and impaired memory.5 Thus, advancing the prevention of hypertensive disorders in pregnancy, and reducing its short- and long-term adverse effects, particularly among high-risk groups, is a key step to reduce maternal mortality and morbidity, a national priority.4
Existing research has explored the association between hypertensive disorders of pregnancy such as pre-eclampsia and genetic, metabolic, environmental, and psychosocial risk factors,1 including maternal micronutrient status and vitamin intake during pregnancy.6 Studies conducted in Iran,7 Peru,8 Poland,9 and India10 demonstrated elevated plasma levels of homocysteine and lower folate and vitamin B12 in women with pre-eclampsia.
There is a need for U.S.-based research exploring the effect of hypertensive disorders in pregnancy on postpartum micronutrient status or whether there are any racial or ethnic differences in these associations. Where possible, hypertensive disorders should also be explored on a spectrum of severity as gestation hypertension is typically considered to be a mild condition, whereas hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome is considered a severe form of pre-eclampsia.11 Given the growing recognition of the impact of hypertensive disorders on long-term maternal cardiometabolic health in addition to pregnancy outcomes, such research is critically needed to inform prevention strategies for pre-eclampsia and appropriate postpartum care for pre-eclamptic women to prevent its recurrence. Optimizing micronutrient status is in general safe, inexpensive, and scalable.
This study explores the postpartum plasma folate, vitamin B12, and homocysteine profiles among mothers on a spectrum of hypertensive disorders during pregnancy in the Boston Birth Cohort (BBC)—a predominantly urban low-income multiethnic U.S. population. We classify women into three groups: (1) no hypertension, (2) gestational hypertension, and (3) pre-eclampsia disorders, including pre-eclampsia, eclampsia, and HELLP. We hypothesize that hypertensive disorders are associated with (i) reduced folate and (ii) higher homocysteine levels. We also explore the role of vitamin B12, which is less studied and posit that for all three biomarkers, the effect of pre-eclampsia disorders is greater than gestational hypertension.
Furthermore, we investigate racial/ethnic differences in the association between hypertensive disorders and postpartum B vitamins and homocysteine levels. Such information is critically needed to inform American College of Obstetricians and Gynecologists (ACOG) guidelines, which currently do not screen women for B vitamin or homocysteine during preconception, pregnancy, nor postpartum, and do not recommend the use of vitamins in the prevention or treatment of hypertensive disorders of pregnancy.12 This research may also have implications for improving maternal postpartum care as well as early prevention of hypertensive disorders in subsequent pregnancies and its associated cardiometabolic consequences throughout the life course.3,13
Materials and Methods
The data are from the ongoing BBC study, which commenced in 1998.14 Eligible mothers for the parent BBC study are those who had a singleton live infant (without major birth defects) at the Boston Medical Center (BMC), a large urban hospital serving a predominantly low-income racial minority inner-city patient population. As of 2016, the BBC study included 8509 mother–infant dyads enrolled postpartum at the BMC. The study protocol was approved by the institutional Review Boards of Boston Medical Center and Johns Hopkins Bloomberg School of Public Health.
This study includes 2584 mothers of the Boston Birth Cohort whose children continued to receive pediatric care at the BMC, and who had measurements of plasma B vitamin biomarkers (folate, vitamin B12, and homocysteine) in blood samples collected at postpartum enrollment. Of note, 2584 mothers had plasma folate and vitamin B12 measurements, whereas 2567 women had homocysteine measurements. The sampling frame is illustrated in Supplement Figure S1. The comparability of the study sample versus the overall sample is presented in Supplementary Table S1.
As described elsewhere,14 hypertensive disorders of pregnancy was defined as the presence of any of the following physician diagnoses as documented in medical records: gestational hypertension, pre-eclampsia, eclampsia, or HELLP syndrome. Furthermore, we created subgroups of hypertensive disorders of pregnancy differentiating gestational hypertension from pre-eclampsia disorders—defined as pre-eclampsia, eclampsia, or HELLP syndrome. Plasma folate and vitamin B12 concentrations were measured by a commercial laboratory via chemiluminescent immunoassay using a MAGLUMI 2000 Analyzer (Snibe Co. Ltd.). Plasma homocysteine was measured using automatic clinical analyzers (Beckman-Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Nanfang Hospital, Guangzhou, China.
The interassay coefficient of variation for all three markers are <6%.15 Maternal B vitamin concentrations were assessed as continuous variables using (i) raw and (ii) log transformed concentrations. Other covariates included sociodemographic factors such as race/ethnicity (non-Hispanic Black [Black, African American, or Haitian], non-Hispanic White, Hispanic, and other), postpartum age (<20, 20–29, and 30+years), nativity (U.S. born vs. non-U.S. born), education (≤elementary, high school, or ≥college), marital status (unmarried vs. married), receipt of public assistance, including WIC (Women Infants and Children), Food Stamps, AFDC (Aid to Families with Dependent Children), Housing assistance or Fuel assistance (yes vs. no), and parity (nulliparous vs. multiparous).
Behavioral risk factors included cigarette smoking (never vs. any), alcohol consumption (never vs. any), and stress (mother's report of life or pregnancy as being very stressful). Biomedical factors included presence of either prepregnancy obesity (BMI ≥30 kg/m2), or diabetes mellitus (gestational or pregestational diabetes), and chronic hypertension (prepregnancy hypertension).
All analyses were conducted using STATA version 15 (StataCorp LP, College Station, TX). Kernel density distribution plots were used to graph the distribution of maternal B vitamins by pre-eclampsia as well as maternal race/ethnic status. Unadjusted and adjusted linear regression models were used to explore the relationship between hypertensive disorders of pregnancy and maternal B vitamin plasma concentrations. Confounders included identified correlates of hypertensive disorders of pregnancy in the literature—maternal race (non-Hispanic Black vs. other groups), age, nativity, education, marital status, receipt of public assistance, parity, cigarette smoking, alcohol consumption, stress, obesity/diabetes, and chronic hypertension.3
Forest plots were conducted on the association of hypertensive disorders status with postpartum folate and homocysteine levels, stratified by key maternal characteristics (age, parity, race/ethnicity [non-Hispanic Black vs. other groups], smoking status and presence of obesity/diabetes).
All p values in the analyses were based on two-sided statistical tests and the Type I error rate was set at 0.05.
Results
Table 1 shows the distribution of maternal characteristics across the spectrum of hypertensive disorders in pregnancy. Women with gestational hypertension and pre-eclampsia disorders were more likely to be older, educated, under more self-reported stress, obese or diabetic, and have chronic hypertension. A full description of the study sample in comparison with all BBC study participants is presented in Supplementary Table S1 highlighting many similarities between this study sample and the entire BBC. Of note, only 3% of women in the sample had gestational hypertension, whereas 10% had pre-eclampsia disorders.
Table 1.
Characteristics of Study Mothers Who Had Measurements of Plasma B Vitamins Stratified by Pre-Eclampsia Status During Pregnancy, A Subsample of the Boston Birth Cohort
| Maternal characteristics | Spectrum of hypertensive disorders of pregnancy |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (N = 2584) |
No hypertension (N = 2246) |
Gestational hypertension (N = 73) |
Pre-eclampsia disordersa(N = 265) |
p value |
|||||
| No. | % | No. | % | No. | % | No. | % | ||
| Race/ethnicity | 0.401 | ||||||||
| Non-Hispanic Blackb | 1772 | 69 | 1522 | 68 | 54 | 74 | 196 | 74 | |
| Hispanic | 504 | 20 | 451 | 20 | 10 | 14 | 43 | 16 | |
| Other | 305 | 12 | 270 | 12 | 9 | 12 | 26 | 10 | |
| Missing | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | |
| Age in years | 0.005 | ||||||||
| <20 | 252 | 10 | 228 | 10 | 6 | 8 | 18 | 7 | |
| 20–29 | 1289 | 50 | 1141 | 51 | 36 | 49 | 112 | 42 | |
| 30+ | 1043 | 40 | 877 | 39 | 31 | 42 | 135 | 51 | |
| Nativity (U.S. born) | 0.856 | ||||||||
| Not born in U.S. | 1531 | 59 | 1336 | 59 | 43 | 59 | 152 | 57 | |
| Born in U.S. | 1005 | 39 | 870 | 39 | 29 | 40 | 106 | 40 | |
| Missing | 48 | 2 | 40 | 2 | 1 | 1 | 7 | 3 | |
| Education | 0.009 | ||||||||
| Less than high school | 727 | 28 | 656 | 29 | 17 | 23 | 54 | 20 | |
| High school/GED | 932 | 36 | 781 | 35 | 29 | 40 | 122 | 46 | |
| Some college and above | 907 | 35 | 792 | 35 | 27 | 37 | 88 | 33 | |
| Missing | 18 | 1 | 17 | 1 | 0 | 0 | 1 | 0 | |
| Marital status | 0.487 | ||||||||
| Married | 902 | 35 | 777 | 35 | 25 | 34 | 100 | 38 | |
| Unmarried | 1656 | 64 | 1448 | 64 | 46 | 63 | 162 | 61 | |
| Missing | 26 | 1 | 21 | 1 | 2 | 3 | 3 | 1 | |
| Receipt of public assistancec | 0.128 | ||||||||
| No | 339 | 13 | 288 | 13 | 16 | 22 | 35 | 13 | |
| Yes | 2231 | 86 | 1944 | 87 | 57 | 78 | 230 | 87 | |
| Missing | 14 | 1 | 14 | 1 | 0 | 0 | 0 | 0 | |
| Parity | 0.763 | ||||||||
| Multiparous | 758 | 29 | 663 | 30 | 18 | 25 | 77 | 29 | |
| Primiparous | 1822 | 71 | 1580 | 70 | 55 | 75 | 187 | 71 | |
| Missing | 4 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | |
| Cigarette smoking | 0.830 | ||||||||
| Never smoked | 2089 | 81 | 1821 | 81 | 58 | 79 | 210 | 79 | |
| Ever smoked | 472 | 18 | 405 | 18 | 15 | 21 | 52 | 20 | |
| Missing | 23 | 1 | 20 | 1 | 0 | 0 | 3 | 1 | |
| Alcohol consumption | 0.702 | ||||||||
| No | 2308 | 89 | 2008 | 89 | 67 | 92 | 233 | 88 | |
| Yes | 199 | 8 | 173 | 8 | 3 | 4 | 23 | 9 | |
| Missing | 77 | 3 | 65 | 3 | 3 | 4 | 9 | 3 | |
| High stressd | 0.001 | ||||||||
| No | 2042 | 79 | 1787 | 80 | 67 | 92 | 188 | 71 | |
| Yes | 529 | 20 | 447 | 20 | 6 | 8 | 76 | 29 | |
| Missing | 13 | 1 | 12 | 1 | 0 | 0 | 1 | 0 | |
| Presence of diabetes mellitus or obesity | <0.001 | ||||||||
| No | 1814 | 70 | 1626 | 72 | 40 | 55 | 148 | 56 | |
| Yes | 769 | 30 | 619 | 28 | 33 | 45 | 117 | 44 | |
| Missing | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Chronic hypertension | <0.001 | ||||||||
| No | 2414 | 93 | 2152 | 96 | 73 | 100 | 189 | 71 | |
| Yes | 170 | 7 | 94 | 4 | 0 | 0 | 76 | 29 | |
Defined as pre-eclampsia, eclampsia, and hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome.
Non-Hispanic Black includes maternal self-report as Black, African American, or Haitian.
Public assistance is defined as receipt of any of the following: WIC (Women Infants and Children), food stamps, AFDC (Aid to Families with Dependent Children), housing assistance, or fuel assistance.
Mother's self-report of life or pregnancy being very stressful. p ≤ 0.5, p ≤ 0.01, p ≤ 0.001.
The prevalence of gestational hypertension was 3.0% among non-Hispanic Blacks, 2.0% among Hispanics, and 3.0% among others. The prevalence of pre-eclampsia disorders was 11.0% among non-Hispanic Blacks, 8.5% among Hispanics, and 8.5% among others. Further breakdown of postpartum B vitamins and homocysteine concentrations by subgroup of hypertensive disorders in pregnancy (gestational hypertension, pre-eclampsia/eclampsia, and HELLP), are presented in Supplementary Table S2.
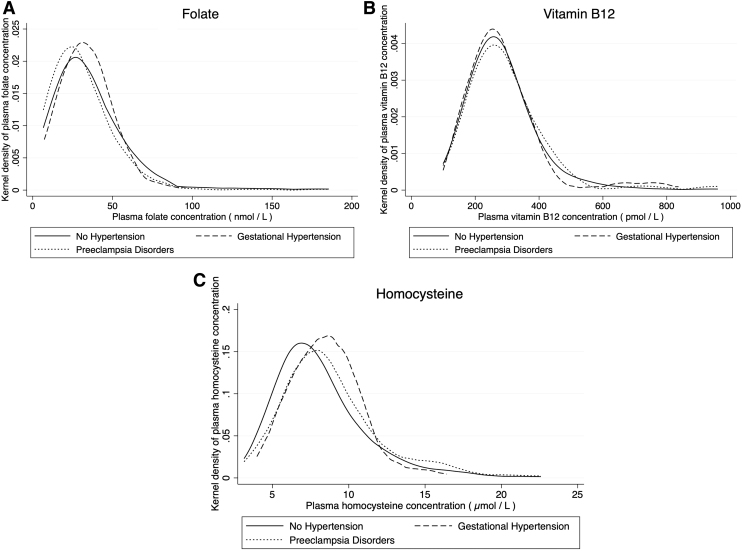
Figure 1 highlights the kernel density of postpartum biomarkers by the hypertensive disorders of pregnancy. Women with gestational hypertension and pre-eclampsia disorders had reduced folate and vitamin B12 levels but higher homocysteine levels.
FIG. 1.
Postpartum plasma folate, vitamin B12, and homocysteine distribution by spectrum of hypertensive disorders in pregnancy: (A) folate, (B) vitamin B12, and (C) homocysteine.
In Table 2, the differences in postpartum maternal B vitamin biomarkers across the spectrum of hypertensive disorders status in overall samples and by race/ethnicity were described. Overall, elevated levels of plasma homocysteine and lower folate and vitamin B12 were seen in women with gestational hypertension and pre-eclampsia disorders. These associations were strongest among non-Hispanic Black women. Table 3 highlights the effect of hypertensive disorders on log transformed maternal B vitamin and homocysteine biomarkers in total sample as well as by race/ethnicity, using multivariable linear regression models. In the overall sample, gestational hypertension did not have a significant association with the postpartum biomarkers.
Table 2.
Mean (SD) and Median (IQR) of Postpartum Plasma Folate, B12, and Homocysteine by Spectrum of Hypertensive Disorders of Pregnancy, Stratified by Race/Ethnicity
| |
No hypertension |
Gestational hypertension |
Pre-eclampsia disordersa |
p valueb | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Folate | |||||||
| Total (N = 2584) | 35.50 (23.83) | 30.33 (24.08) | 33.54 (15.71) | 31.90 (18.39) | 30.37 (20.07) | 26.54 (22.82) | 0.003 |
| Non-Hispanic Black (N = 1775) | 34.61 (22.91) | 29.45 (24.61) | 30.33 (13.32) | 29.97 (15.17) | 29.14 (20.56) | 25.35 (21.61) | 0.003 |
| Hispanic (N = 504) | 35.86 (25.06) | 30.63 (21.11) | 37.97 (8.70) | 39.32 (15.93) | 34.71 (17.60) | 29.59 (20.82) | 0.919 |
| Other (N = 305) | 39.90 (26.23) | 35.07 (24.88) | 47.86 (25.23) | 44.98 (23.73) | 32.51 (19.74) | 31.07 (22.82) | 0.232 |
| Vitamin B12 | |||||||
| Total (N = 2584) | 283.83 (109.51) | 265.39 (109.27) | 288.65 (123.64) | 264.39 (113.14) | 296.97 (132.76) | 269.15 (127.02) | 0.192 |
| Non-Hispanic Black (N = 1775) | 291.75 (112.76) | 272.74 (112.63) | 284.79 (116.50) | 260.80 (117.20) | 304.40 (139.70) | 274.51 (131.09) | 0.312 |
| Hispanic (N = 504) | 269.61 (106.42) | 252.41 (108.59) | 268.03 (84.44) | 235.69 (140.84) | 269.07 (118.32) | 241.49 (105.68) | 0.999 |
| Other (N = 305) | 262.82 (89.25) | 252.29 (91.78) | 334.76 (191.38) | 293.79 (55.66) | 287.12 (92.33) | 254.90 (107.94) | 0.041 |
| Homocysteine | |||||||
| Total (N = 2567) | 8.15 (3.03) | 7.51 (3.33) | 8.50 (2.26) | 8.49 (2.80) | 8.84 (3.28) | 8.13 (3.18) | 0.002 |
| Non-Hispanic Black (N = 1758) | 8.35 (3.14) | 7.68 (3.44) | 8.63 (2.50) | 8.46 (3.43) | 9.00 (3.32) | 8.30 (3.60) | 0.022 |
| Hispanic (N = 504) | 7.77 (2.79) | 7.25 (3.05) | 8.88 (0.98) | 9.15 (0.67) | 8.93 (3.34) | 8.01 (2.92) | 0.021 |
| Other (N = 305) | 7.66 (2.63) | 7.20 (2.85) | 7.31 (1.38) | 7.09 (1.14) | 7.46 (2.61) | 6.95 (3.13) | 0.865 |
Defined as the presence of one of the following: pre-eclampsia, eclampsia, or HELLP syndrome.
Test for significant difference in mean biomarker level by hypertensive disorders of pregnancy status.
IQR, interquartile range; N, number; SD, standard deviation; HELLP, hemolysis, elevated liver enzymes, low platelets.
Table 3.
Crude and Adjusted Mean Difference in Postpartum Plasma Folate, B12, and Homocysteine Among Women with Pre-Eclampsia/Eclampsia/HELLP and Among Women with Gestational Hypertension as Compared with Women Without any of Those Conditions
| Race/ethnicity |
Gestational hypertension |
Pre-eclampsia disordersa |
||||||
|---|---|---|---|---|---|---|---|---|
| Crude linear regression |
Adjustedblinear regression |
Crude linear regression |
Adjustedblinear regression |
|||||
| β (95% CI) | p value | aβ (95% CI) | p value | β (95% CI) | p value | aβ (95% CI) | p value | |
| LnFolate | ||||||||
| Total (N = 2584) | 0.01 (−0.13 to 0.15) | 0.909 | 0.01 (−0.13 to 0.15) | 0.850 | −0.15 (−0.22 to −0.07) | 0.001 | −0.14 (−0.22 to −0.06) | 0.001 |
| Non-Hispanic Black (N = 1775) | −0.06 (−0.22 to 0.11) | 0.486 | −0.06 (−0.22 to 0.11) | 0.488 | −0.17 (−0.26 to −0.08) | 0.001 | −0.17 (−0.26 to −0.07) | 0.001 |
| Hispanic (N = 504) | 0.20 (−0.15 to 0.56) | 0.257 | 0.18 (−0.17 to 0.53) | 0.316 | 0.03 (−0.15 to 0.20) | 0.763 | 0.05 (−0.13 to 0.24) | 0.572 |
| Other (N = 305) | 0.21 (−0.17 to 0.59) | 0.279 | 0.23 (−0.14 to 0.60) | 0.222 | −0.22 (−0.45 to 0.01) | 0.065 | −0.20 (−0.44 to 0.03) | 0.089 |
| Ln Vitamin B12 | ||||||||
| Total (N = 2584) | 0.01 (−0.07 to 0.10) | 0.753 | 0.02 (−0.06 to 0.10) | 0.677 | 0.03 (−0.01 to 0.08) | 0.169 | 0.02 (−0.03 to 0.07) | 0.392 |
| Non-Hispanic Black (N = 1775) | −0.03 (−0.12 to 0.07) | 0.609 | −0.02 (−0.12 to 0.08) | 0.738 | 0.03 (−0.02 to 0.07) | 0.392 | 0.02 (−0.03 to 0.08) | 0.383 |
| Hispanic (N = 504) | 0.01 (−0.20 to 0.23) | 0.899 | 0.01 (−0.21 to 0.23) | 0.947 | 0.02 (−0.13 to 0.09) | 0.725 | −0.03 (−0.15 to 0.08) | 0.562 |
| Other (N = 305) | 0.20 (−0.01 to 0.42) | 0.067 | 0.21 (−0.02 to 0.43) | 0.060 | 0.09 (−0.04 to 0.22) | 0.182 | 0.09 (0.05–0.23) | 0.198 |
| Ln homocysteine | ||||||||
| Total (N = 2567) | 0.07 (−0.01 to 0.15) | 0.096 | 0.06 (−0.01 to 0.14) | 0.111 | 0.08 (0.04– 0.12) | <0.001 | 0.08 (0.04–0.13) | <0.001 |
| Non-Hispanic Black (N = 1758) | 0.05 (−0.04 to 0.15) | 0.259 | 0.06 (−0.03 to 0.16) | 0.192 | 0.08 (0.03– 0.13) | 0.004 | 0.09 (0.03–0.15) | 0.001 |
| Hispanic (N = 504) | 0.18 (−0.02 to 0.39) | 0.081 | 0.17 (−0.04 to 0.38) | 0.104 | 0.13 (0.03– 0.24) | 0.011 | 0.18 (0.07– 0.29) | <0.001 |
| Other (N = 305) | −0.01 (−0.22 to 0.20) | 0.906 | −0.01 (−0.23 to 0.20) | 0.909 | −0.04 (−0.16 to 0.09) | 0.576 | −0.05 (−0.19 to 0.08) | 0.434 |
Defined as the presence of one of the following: pre-eclampsia, eclampsia, or HELLP syndrome.
Adjusted for maternal race, age, nativity, education, marital status, receipt of public assistance, parity, cigarette smoking, alcohol consumption, stress, presence of obesity or diabetes, and chronic hypertension.
aβ, adjusted beta coefficient; β, beta coefficient; Ln, natural log; N, number.
However, the presence of pre-eclampsia disorders was significantly associated with lower plasma folate (adjusted beta coefficient [aβ]: −0.14; 95% CI: −0.22 to −0.06) but higher homocysteine (aβ: 0.08; 95% CI: 0.04–0.13) concentrations postpartum. In contrast, the relationship between pre-eclampsia disorders and vitamin B12 concentration was not significant (aβ: 0.02; 95% CI: −0.03 to 0.07). Racial/ethnic differences were also seen as the relationship between pre-eclampsia disorders and maternal B vitamin/homocysteine biomarkers was strongest in non-Hispanic Black women. Specifically, the presence of pre-eclampsia disorders was significantly associated with lower plasma folate (aβ: −0.17; 95% CI: −0.26 to −0.07) among non-Hispanic Black women only.
Pre-eclampsia disorders were associated with higher homocysteine concentrations postpartum among Hispanic (aβ: 0.18; 95% CI: 0.07–0.29) as well as non-Hispanic Black women (aβ: 0.08; 95% CI: 0.03–0.15).
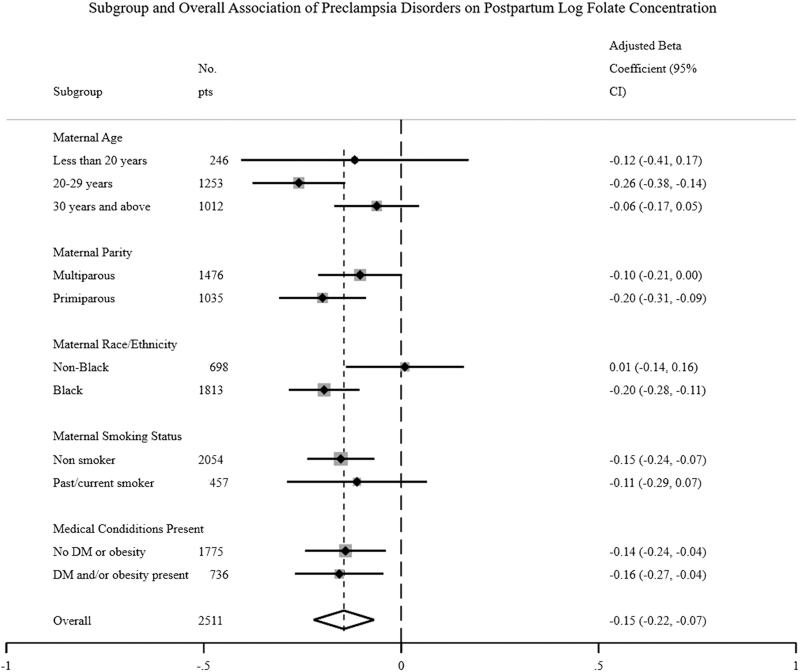
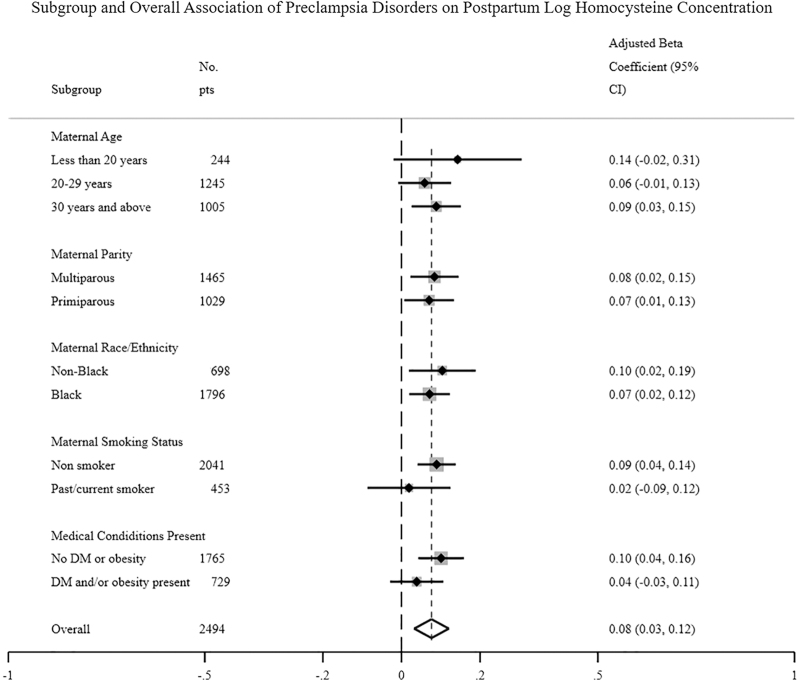
Figures 2 and 3 are forest plots of the association of pre-eclampsia disorders with postpartum plasma folate and homocysteine levels, respectively, stratified by key maternal characteristics—age, parity, race/ethnicity, smoking status, and medical conditions—presence of obesity/diabetes. Subgroups of women with significant associations between pre-eclampsia disorders and postpartum plasma folate levels include women aged 20–29 years, primipara, non-Hispanic Blacks, nonsmokers, and obese/diabetic women. Of note, there was a significant interaction in the relationship between pre-eclampsia disorders and postpartum plasma folate levels among non-Hispanic Blacks versus other groups (confirming our earlier analyses by race/ethnicity). In contrast, all subgroups of women except those <30 years of age as well as current smokers and obese/diabetic women had significant associations between hypertensive disorders and postpartum homocysteine levels.
FIG. 2.
Forest plots on the association of pre-eclampsia status with postpartum folate levels, stratified key maternal characteristics (age, parity, race/ethnicity, smoking status, and medical conditions.
FIG. 3.
Forest plots on the association of pre-eclampsia status with postpartum homocysteine levels, stratified by key maternal characteristics (age, parity, race/ethnicity, smoking status, and medical conditions.
Discussion
In this multiethnic U.S. low-income population, we analyzed postpartum micronutrient biomarkers (folate, B12, and homocysteine) in relation to a spectrum of hypertensive disorders: (1) no hypertension, (2) gestational hypertension, and (3) pre-eclampsia disorders, including pre-eclampsia, eclampsia, and HELLP syndrome. We found that pre-eclampsia disorders were significantly associated with lower plasma folate and higher homocysteine concentrations postpartum, particularly among non-Hispanic Black women. In contrast, gestational hypertension, a mild form of hypertensive disorder during pregnancy, was not associated with these biomarkers.
Although pre-eclampsia disorders were a recognized risk factor of a woman's future cardiovascular risk, little is known about the underlying link and how to break it. Our findings are supportive of previous published data on the role of folate and homocysteine in cardiovascular health.16,17 Folate and homocysteine levels are inversely correlated, and high concentrations of homocysteine are a documented risk factor for vascular disease and higher levels of homocysteine can damage endothelial cells and cause endothelial dysfunction, which is characteristic of pre-eclampsia.
Studies have demonstrated that high homocysteine in early pregnancy is a risk factor for pre-eclampsia.16,17 In addition, elevated levels of homocysteine in women with pre-eclampsia exist from early pregnancy and remain high until postpartum.17 In this study, given the blood samples were collected within a few days after delivery, we speculate that the samples are likely reflective of circulating folate, B12, and homocysteine levels at late pregnancy. Future studies with blood collections at preconception and specific trimesters are needed to determine if the differences might have existed before pregnancy and/or were worsened during the pregnancy.
Our study findings, if further confirmed, have a number of clinical and public health implications. First, this study underscores the importance of optimizing maternal micronutrient status during both prenatal and postnatal periods as they play an important role in cellular growth, replication, and repair; and their deficiencies have been linked with hypertensive disorders.18 Specifically, elevated homocysteine levels are associated with higher risk of cardiovascular diseases in adults, including hypertension, arteriosclerosis, and stroke.19,20
Second, the use of multivitamin supplements particularly in early pregnancy has been advocated to ensure optimal perinatal outcomes and remain a relatively low-cost, safe, and feasible clinical and public health intervention for all women of childbearing age. Our prior study showed that maintaining adequate folate status until the third trimester was associated with lower risk of preterm birth—a complication of hypertensive disorders of pregnancy.14
Folate supplements are an effective treatment to lower homocysteine levels and folate supplementation, or fortification remains a simple and cost-effective intervention, which may prove particularly useful in preventing hypertensive disorders in the populations with low folate and/or high homocysteine status. Even in the U.S. setting with a mandatory folic acid fortification program, this study showed that among high-risk populations such as the BBC, lower folate levels, and elevated homocysteine may be a modifiable cardiovascular risk factor associated with hypertensive disorders in pregnancy.
Third, our data showed a clear linkage of lower postpartum folate levels with pre-eclampsia disorders in pregnancy, a prenatal condition. Several studies have shown the association between folic acid supplementation and pre-eclampsia.15,21 Preconception or prenatal interventions remain key in preventing or ameliorating the risk of pre-eclampsia disorders and its associated adverse effects.22 Given the fact that pre-eclampsia disorders in pregnancy have long-term impacts and tends to recur in subsequent pregnancies, optimal postpartum, and interpregnancy care for women with hypertensive disorders, including correction of low levels of B vitamins and elevated homocysteine, may help to reduce future risk of hypertensive disorders and cardiovascular consequences.
In the United States, women are typically discharged from the hospital on postpartum day 2–4 and ACOG recommend a single blood pressure check between 3 and 10 days postpartum for women with a hypertensive disorder of pregnancy.11 Our findings, if further confirmed, suggest that attention to B vitamin status should be part of the early postpartum visit among women with hypertensive disorders during pregnancy. Furthermore, ACOG and the Society for Maternal Fetal Medicine (SMFM) recommend interpregnancy care for women who have a history of hypertensive disorders of pregnancy, which offers another window of opportunity to follow up B vitamin status among women with hypertensive disorders during pregnancy.
Finally, given the racial/ethnic differences highlighted by this study, it appears that additional attention needs to be given to minority populations, including non-Hispanic Black and Hispanic women.
Other subgroups of interest may potentially include older nulliparous women as well as those with existing cardiometabolic conditions. Attention to these high-risk groups will help reduce long-standing health disparities in maternal perinatal morbidity and mortality and women's health in the United States. Our study results may be also relevant to low-resource settings globally, where there are high rates of hypertensive disorders and a lack of a formal micronutrient fortification program.23 In such settings, there may be an even greater need to improve micronutrient levels to potentially mitigate adverse pregnancy outcomes and associated consequences.24
This study has some limitations. We recognize that a single plasma maternal B vitamins measurement postpartum cannot be used to differentiate between a transitory decrease in dietary intake and chronic deficiency/excess states. However, in populations that have stable sources of micronutrients, such as in the United States where there is mandatory folic acid fortification, plasma concentrations are unlikely to fluctuate dramatically. In addition, the small sample size of women with gestational hypertension did not allow for more rigorous analysis and our observational study design did not permit inferences of causality. Finally, although we have adjusted major known confounders in the analyses, unmeasured or unknown confounding may still be an issue. Owing to these limitations, our findings serve as hypothesis generating rather than as conclusive. Additional prospective studies are needed to confirm our findings.
Conclusion
In summary, our study found that in this predominantly urban low-income multiethnic U.S. population, pre-eclampsia disorders were significantly associated with lower plasma folate and higher homocysteine concentrations postpartum, particularly among non-Hispanic Black women. Our study findings, if further confirmed, may have implications for postpartum and interpregnancy care, including attention to maternal micronutrient status to prevent hypertensive disorders in pregnancy and reduce its associated consequences in subsequent pregnancies and lifespan.
Supplementary Material
Authors' Contributions
B.O., S.A., and X.W. designed research; B.O. performed statistical analyses; G.W. and X.H. performed laboratory assays; B.O. wrote the article; and all other coauthors have participated in data interpretation and presentation, critical review, and revision of the article. X.W. is the PI of the Boston Birth Cohort and had primary responsibility for final content.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605); the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R21HD066471, R01HD086013, R01HD098232, and R01 ES031272); and the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (R40MC27443, UJ2MC31074). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by any funding agencies.
Supplementary Material
References
- 1. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403 [DOI] [PubMed] [Google Scholar]
- 2. Bateman BT, Shaw KM, Kuklina EV, Callaghan WM, Seely EW, Hernández-Díaz S. Hypertension in women of reproductive age in the United States: NHANES 1999–2008. PLoS One 2012;7:e36171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Croke L. Gestational hypertension and preeclampsia: A practice bulletin from ACOG. Am Fam Physician 2019;100:649–650 [PubMed] [Google Scholar]
- 5. Williams D. Long-term complications of preeclampsia. Semin Nephrol 2011;31:111–122 [DOI] [PubMed] [Google Scholar]
- 6. Khaing W, Vallibhakara SA, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: A systematic review and network meta-analysis. Nutrients 2017;9:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahbazian N, Jafari RM, Haghnia S. The evaluation of serum homocysteine, folic acid, and vitamin B12 in patients complicated with preeclampsia. Electron Physician 2016;8:3057–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanchez SE, Zhang C, Rene Malinow M, Ware-Jauregui S, Larrabure G, Williams MA. Plasma Folate, Vitamin B12, and Homocyst(e)ine concentrations in preeclamptic and normotensive Peruvian women. Am J Epidemiol 2001;153:474–480 [DOI] [PubMed] [Google Scholar]
- 9. Sanlikan F, Tufan F, Gocmen A, Kabadayi C, Sengul E. The evaluation of homocysteine level in patients with preeclampsia. Ginekol Pol 2015;86:287–291 [DOI] [PubMed] [Google Scholar]
- 10. Mujawar SA, Patil VW, Daver RG. Study of serum homocysteine, folic Acid and vitamin b(12) in patients with preeclampsia. Indian J Clin Biochem 2011;26:257–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilkerson RG, Ogunbodede AC. Hypertensive Disorders of Pregnancy. Emerg Med Clin North Am 2019;37:301–316 [DOI] [PubMed] [Google Scholar]
- 12. American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy: Executive Summary. Obstet Gynecol 2013;122:1122–1131 [DOI] [PubMed] [Google Scholar]
- 13. Dunlop AL, Kramer MR, Hogue CJ, Menon R, Ramakrishan U. Racial disparities in preterm birth: An overview of the potential role of nutrient deficiencies. Acta Obstet Gynecol Scand 2011;90:1332–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olapeju B, Saifuddin A, Wang G, et al. Maternal postpartum plasma folate status and preterm birth in a high-risk US population. Public Health Nutr 2018:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang G, Hu FB, Mistry KB, et al. Association between maternal prepregnancy body mass index and plasma folate concentrations with child metabolic health. JAMA Pediatr 2016;170:e160845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun F, Qian W, Zhang C, Fan J-X, Huang H-F. Correlation of maternal serum homocysteine in the first trimester with the development of gestational hypertension and preeclampsia. Med Sci Monit 2017;23:5396–5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wadhwani NS, Patil VV, Mehendale SS, Wagh GN, Gupte SA, Joshi SR. Increased homocysteine levels exist in women with preeclampsia from early pregnancy. J Mater Fetal Neonatal Med 2016;29:2719–2725 [DOI] [PubMed] [Google Scholar]
- 18. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 2003;69:1–7 [DOI] [PubMed] [Google Scholar]
- 19. Balakumar P, Singh AP, Ganti SS, Singh M. Hyperhomocysteinemia and cardiovascular disorders: Is there a correlation. Trends Med Res 2007;2:160–166 [Google Scholar]
- 20. Piazzolla G, Candigliota M, Fanelli M, et al. Hyperhomocysteinemia is an independent risk factor of atherosclerosis in patients with metabolic syndrome. Diabetol Metab Syndr 2019;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Serrano NC, Quintero-Lesmes DC, Becerra-Bayona S, et al. Association of pre-eclampsia risk with maternal levels of folate, homocysteine and vitamin B12 in Colombia: A case-control study. PLoS One 2018;13:e0208137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinussen MP, Bracken MB, Triche EW, Jacobsen GW, Risnes KR. Folic acid supplementation in early pregnancy and the risk of preeclampsia, small for gestational age offspring and preterm delivery. Eur J Obstet Gynecol Reprod Biol 2015;195:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3:370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Victora CG, Barros FC, Assunção MC, Restrepo-Méndez MC, Matijasevich A, Martorell R. Scaling up maternal nutrition programs to improve birth outcomes: A review of implementation issues. Food Nutr Bull 2012;33(2_Suppl 1):S6–S26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.