Abstract
Anthracyclines are an integral part of chemotherapy regimens used to treat a variety of childhood-onset and adult-onset cancers. However, the development of cardiac dysfunction and heart failure often compromises the clinical utility of anthracyclines. The risk of cardiac dysfunction increases with anthracycline dose. This anthracycline-cardiac dysfunction association is modified by several demographic and clinical factors, such as age at anthracycline exposure (<4 years and ≥65 years); female sex; chest radiation; presence of cardiovascular risk factors (diabetes, hypertension); and concurrent use of cyclophosphamide, paclitaxel, and trastuzumab. However, the clinical variables alone yield modest predictive power in detecting cardiac dysfunction. Recently, attention has focused on the molecular basis of anthracycline-related cardiac dysfunction, providing an initial understanding of the mechanism of anthracycline-related cardiomyopathy. This review describes the current state of knowledge with respect to the pathogenesis of anthracycline-related cardiomyopathy and identifies the critical next steps to mitigate this problem.
Key Words: anthracycline chemotherapy, cardiomyopathy, heart failure, genomics, metabolomics, prediction
Abbreviations and Acronyms: AUC, area under the curve; CBR, carbonyl reductase; CI, confidence interval; CM, cardiomyocyte; DNA, deoxyribonucleic acid; GST, glutathione S-transferase; hiPSC-CM, human-induced pluripotent stem cell cardiomyocyte; iPSC-CM, induced pluripotent stem cell cardiomyocyte; OR, odds ratio; RNA, ribonucleic acid; SNP, single nucleotide polymorphism; Top2b, topoisomerase-IIβ
Central Illustration
Highlights
-
•
Anthracycline chemotherapy results in an increased risk of cardiac dysfunction.
-
•
Most recent studies have suggested that there is a genetic basis for anthracycline-related cardiac dysfunction.
-
•
Integration of genetics with the clinical characteristics may be used to enhance the ability to predict the risk for anthracycline-related cardiomyopathy.
Anthracyclines (doxorubicin, daunorubicin, epirubicin, idarubicin) form the backbone of chemotherapy regimens used to treat acute lymphoblastic leukemia, acute myeloid leukemia, Hodgkin lymphoma, Ewing sarcoma, osteosarcoma, neuroblastoma, and breast cancer. Up to 60% of patients with cancer receive anthracycline-based regimens (1, 2, 3, 4, 5, 6). In fact, the World Health Organization lists doxorubicin as an essential cytotoxic medicine (7). The mechanisms by which anthracyclines exert antitumor effects include: 1) inhibition of deoxyribonucleic acid (DNA) synthesis and gene expression; 2) DNA binding, alkylation, and cross-linking; 3) interference with DNA strand separation; and 4) free radical formation and lipid peroxidation.
The clinical utility of anthracyclines is compromised by cardiac dysfunction, which is often identified on imaging studies, that may progress to heart failure. Compared with the general population, childhood cancer survivors are at a 5- to 15-fold higher risk of cardiac dysfunction (4, 5, 6, 7, 8, 9). In adults with cancer, the prevalence of cardiac dysfunction approaches 10% (10). Once diagnosed with heart failure, historical data suggest the 5-year survival may be <50% (11). The risk of cardiac dysfunction increases with anthracycline dose (Figure 1) (10,12, 13, 14). Other risk factors include extremes of age at exposure to anthracyclines (<4 years; ≥65 years); female sex; chest radiation; presence of cardiovascular risk factors (diabetes, hypertension); and concurrent use of cyclophosphamide, paclitaxel, and trastuzumab (15, 16, 17). However, despite an established dose-dependent association, there is interpatient variability in the risk of cardiac dysfunction for any given anthracycline dose; clinical variables alone yield modest predictive power in detecting cardiac dysfunction (18,19). Recently, attention has focused on single nucleotide polymorphisms (SNPs) associated with cardiac dysfunction (18,20, 21, 22, 23, 24, 25, 26, 27, 28). The poor prognosis coupled with the interindividual variability in risk has resulted in an increasing interest in developing prediction tools to identify patients at highest risk of this complication (24,29, 30, 31). Such a risk prediction tool could inform personalized decisions regarding anthracycline-based treatment as well as post-treatment surveillance.
Figure 1.
Dose-Response Relationship Between Cumulative Anthracycline Exposure and Risk of Cardiomyopathy
The dose-response relationship between cumulative anthracycline exposure and risk of cardiomyopathy is shown. A total of 170 survivors with cardiomyopathy (cases) were compared with 317 survivors with no cardiomyopathy (control subjects; matched on cancer diagnosis, year of diagnosis, length of follow-up, and race/ethnicity) using conditional logistic regression techniques. A dose-dependent association was observed between cumulative anthracycline exposure and cardiomyopathy risk (0 mg/m2: reference; 1 to 100 mg/m2: odds ratio [OR]: 1.65; 101 to 150 mg/m2: OR: 3.85; 151 to 200 mg/m2: OR: 3.69; 201 to 250 mg/m2: OR: 7.23; 251 to 300 mg/m2: OR: 23.47; >300 mg/m2: OR: 27.59; ptrend <0.001). Reprinted with permission from Blanco et al. (28).
Pathogenesis of Anthracycline-Related Cardiomyopathy
Myocardial injury begins with single-cell myocytolysis. It progresses to patchy myocardial necrosis, leading to interstitial fibrosis, and finally to multifocal myocardial fibrosis (32). This leads to disruption of myocardial structure and eventually, if unmitigated, presents clinically as overt heart failure. The pathogenesis of anthracycline-related cardiomyopathy is an area of active investigation. The tetracyclic ring structure of anthracyclines with the quinone and hydroquinone moieties on adjacent rings permits electron gains and losses. Anthracyclines bind to DNA through intercalation between specific bases and block DNA and ribonucleic acid (RNA) synthesis, causing DNA strand scission and interference with cell replication. Anthracyclines inhibit topoisomerase II and generate semiquinone and free radicals through an iron-dependent, enzyme-mediated reductive process. Anthracyclines bind to cellular membranes to alter fluidity and ion transport (33). Presence of quinone groups cause anthracyclines to produce free radicals that react with oxygen to produce superoxide anion radicals, causing cardiotoxicity (33). Decreased adenosine triphosphate production, direct damage to mitochondria, mitochondria-dependent apoptosis of cardiomyocytes, and lipid peroxidation of the cardiac myocyte membrane are additional mechanisms by which anthracyclines exert cardiotoxicity (34,35). The anthracycline-induced cardiotoxicity is also driven by TOP2Bmediated DNA damage (36). Doxorubicin targets topoisomerase-IIβ (Top2b) and forms iron (III)-doxorubicin complex (37). Cardiomyocyte-specific deletion of Top2b can reduce defective mitochondrial biogenesis and reactive oxygen species formation (36). In particular, the heart is uniquely vulnerable to anthracyclines owing to the high density of mitochondria in cardiomyocytes, which make up 35% of the total cell volume (38). Other cellular changes in anthracycline-exposed cardiomyocytes include depleted cardiac stem cells, impaired DNA synthesis, impaired cell signaling that triggers cell death, altered gene expression, inhibited calcium release from the sarcoplasmic reticulum, impaired formation of the protein titin in sarcomeres, and impaired mitochondrial creatine kinase activity and function (39, 40, 41, 42).
Genetic variants
As summarized in Table 1, existing evidence supports the hypothesis that genetic variation contributes to chemotherapy-related cardiac dysfunction (43,44). The existing evidence has been used to construct the proposed pathophysiologic mechanism of anthracycline-related cardiomyopathy in the Central Illustration. Whereas some studies utilized childhood cancer (21, 22, 23, 24, 25,27,28,45, 46, 47) or adult-onset cancer populations (26,48, 49, 50, 51, 52, 53, 54, 55), others included patients across the age spectrum (20,30,56,57). Several studies examined homogeneous cancer populations (leukemia, osteosarcoma, breast cancer, lymphoma), whereas others examined a mix of several cancers. Doxorubicin, daunorubicin, and epirubicin were the most common anthracyclines examined. A few studies reported cumulative anthracycline dose in doxorubicin-equivalent doses (58). Objective assessments (based on echocardiographic findings, Clinical Terminology Criteria for Adverse Events criteria) were used to define the phenotype for all studies, except one that used self-reports (56). The quest for causal genes has involved the use of a variety of strategies, including examination of single SNPs to customized arrays and agnostic genome-wide approaches. Both cohort and case-control study designs were used; the median number of cases per study was small: 54 (range 12 to 213). Twelve of the 26 studies (46%) reviewed here, did not attempt a replication of their salient findings. However, some of these studies were either examining a single SNP with targeted activity and established mechanism of cardiotoxicity (28,57) or had extended their findings by demonstrating differential gene expression (47) or by validating in preclinical mouse models (26). Furthermore, some of these studies replicated findings from previous studies (30,48, 49, 50, 51).
Table 1.
A Summary of Studies Examining the Role of Genetic Susceptibility to Anthracycline-Related Cardiomyopathy
| First Author (Year) (Ref. #) | Study Design | Age at Anthracycline Exposure (yrs) | Type of Cancer | Definition of Cardiotoxicity | Results | Replication |
|---|---|---|---|---|---|---|
| Pediatric Cancer Populations | ||||||
| Semsei et al. (2012) (45) | Cohort n = 235 | 5.7 ± 3.8 | ALL | Change in LV FS | ABCC1 rs3743527TT genotype and rs3743527TT–rs246221TC/TT genotype combination (OR = NA) | No replication performed |
| Visscher et al. (2012) (24) | Cohort Discovery: n = 156; 38 cases Replication: 1) n = 188; 40 cases 2) n = 96; 43 cases |
Discovery Cases: 5.5 (0-17) Control subjects: 3.9 (0-16.5) Replication 1) Cases: 6.2 (0.4-17.6) Control subjects: 3.7 (0.05-16.9) Replication 2) Cases: 9.0 (0.5-16.8) Control subjects: 10.6 (2.1-17.7) |
ALL, AML, other leukemia, HL, NHL osteosarcoma, rhabdomyosarcoma, Ewing sarcoma, other sarcoma, Wilms tumor, hepatoblastoma, neuroblastoma, carcinoma | 1. FS ≤ 26% 2. Signs and symptoms indicating need for cardiac compromise intervention based on CTCAEv3 |
SLC28A3 rs7853758 (OR: 0.20; p = 7.1 × 10−6FMO2 rs2020870 OR: 0.09; p = 2.1 × 10−4SPG7 rs2019604 (OR: 0.33; p = 0.003 SLC10A2 rs9514091 OR: 0.41; p = 0.007 SLC28A3 rs4877847 OR: 0.54; p = 0.009 UGT1A6 rs6759892 OR: 2.93; p = 2.2 × 10−5ABCB4 rs1149222 OR: 2.31; p = 0.002 ABCC1 rs4148350 OR: 3.77; p = 0.005 HNMT rs17583889 OR: 2.21; p = 0.009 | Replication in a second cohort of 188 children from across Canada and further replication of the top SNP in a third cohort of 96 patients from the Netherlands |
| Visscher et al. (2013) (25) | Replication: 1) n = 128 2) n = 90 |
Replication 1) Cases: 9.1 (0.5-16.8) Control subjects: 11.2 (1.8-17.7) Replication 2) Cases: 12.6 (0.9-17.0) Control subjects: 4.9 (0.5–16.0) |
ALL, AML, HL, NHL osteosarcoma, rhabdomyosarcoma, Ewing sarcoma, other sarcoma, Wilms tumor, hepatoblastoma, neuroblastoma, carcinoma, germ cell tumor | 1. FS ≤ 26% 2. Signs/symptoms of cardiac compromise indicating need for intervention |
UGT1A6 rs17863783 (p = 1.6 × 10−5) and SLC8a3 rs885004 (p = 3.0 × 10−5) were replicated in this analysis | |
| Visscher et al. (2015) (23) | Cohort Discovery: n = 335; 78 cases Replication: n = 185; 44 cases |
Cases: 7.4 (0.04, 17.6) Control subjects: 4.9 (0.1-17.7) |
Leukemia, lymphoma, sarcoma, and others | 1. FS <26% 2. Echo and/or symptoms of cardiac compromise indicating need for intervention |
Significant associations in SLC22A17 rs4982753 (p = 0.0078) and SLC22A7 rs4149178 (p = 0.003), with replication in the second cohort (p = 0.007 and 0.047, respectively) | Significant associations identified in SLC22A17 and SLC22A7 were replicated in the replication cohort (p = 0.0071 and p = 0.047, respectively) |
| Blanco et al. (2012) (28) | Case control Cases: n = 170 Control subjects: n = 317 |
Cases: 7.3 (0-20.7) Control subjects: 7.6 (0-21.1) |
HL, NHL, bone tumors, soft tissue sarcoma, ALL, AML, other | 1. Signs/symptoms of cardiac compromise based on AHA criteria 2. Echo evidence of LV dysfunction (LVEF ≤40%; FS ≤28%) |
CBR3 V244M homozygous G genotypes (CBR3:GG), exposure to low- to moderate-dose anthracyclines increased cardiomyopathy risk when compared with individuals with CBR3:GA/AA genotypes unexposed to anthracyclines (OR: 5.48; p = 0.003), as well as exposed to low- to moderate-dose anthracyclines (OR: 3.30; p = 0.006) | No replication performed |
| Aminkeng et al. (2015) (22) | Cohort Discovery: n = 280; 32 cases Replication European patients: n = 96; 22 cases Non-European patients: n = 80; 19 cases |
Discovery: cases: 9.0 (2.5-14); controls: 4 (2-7.5) Replication (Dutch population): cases: 7.5 (5-12); controls: 11 (6-14) |
ALL, AML, HL, NHL, osteosarcoma, rhabdomyosarcoma, Ewing sarcoma, hepatoblastoma, neuroblastoma, Wilms tumor | Cases were defined as exhibiting FS ≤24% or signs and symptoms of cardiac compromise indicating need for intervention based on CTCAEv3, whereas control subjects had FS ≥30% and no symptoms of cardiac compromise for at least 5 yrs after treatment | Nonsynonymous variant (rs2229774, p.Ser427Leu) in RARG was associated with anthracycline-related cardiomyopathy (p = 5.90 × 10−8, (OR: 4.7; 95% CI: 2.7–8.3) | Replication in similarly treated cohorts of 96 European and 80 non-European patients |
| Krajinovic et al. (2016) (46) | Cohort Discovery: n = 251 Replication: n = 44 |
Mean age of the Discovery cohort: 6.16 (1-18) Mean age of the Replication cohort: 5.27 (1-17) |
ALL | Reduction in FS and EF | Individuals with the ABCC5 TT-1629 genotype had an average of 8% to 12% reduction of EF and FS (EF: p < 0.0001, FS: p = 0.001). A protective effect of the NOS3 TT894 genotype on EF was seen in high-risk patients (p = 0.02), especially in those who did not receive dexrazoxane (p = 0.002). | Analysis of an additional cohort of 44 ALL patients replicated the ABCC5 association but not the NOS3 association |
| Wang et al. (2016) (21) | Discovery Case-control Cases: n = 112 Control subjects: n = 219 Replication Case only: n = 54 |
Discovery: cases: median 7.5 (0-20); controls 7.9 (0-21) Replication: cases 5.6 (1.1-17.3); controls 7.7 (0-20.6) |
Discovery HL, NHL, Sarcoma, AML, ALL, and others Replication HL, NHL, Sarcoma, AML, ALL, and others |
1. Signs/ symptoms of cardiac compromise based on AHA criteria 2. Absence of symptoms/signs with echo evidence of LV dysfunction (EF ≤ 40% and/or FS ≤ 28%) |
Anthracyclines >300 mg/m2, CELF4 rs1786814 GG genotype conferred a 10.2-fold (p < 0.001) increased risk of cardiomyopathy compared with those who had GA/AA genotypes and anthracycline exposure ≤300 mg/m2 | Gene-environment interaction was successfully replicated in an independent set of patients with anthracycline-related cardiomyopathy |
| Singh et al. (2020) (47) | Case-control Cases: n = 75 Control subjects: n = 92 |
Cases: 7.8 (3.8-11.5) Control subjects: 9.6 (3.3-14.8) |
ALL, AML, HL, NHL, bone tumors, kidney tumor, sarcoma, neuroblastoma | LVEF <40% and/or FS <28% | A significant association was observed between the risk of cardiomyopathy and the GSTM1 null genotype (OR: 2.7; 95% CI: 1.3, 5.9; p = 0.007) | No replication performed |
| Pediatric and Adult Cancer Populations | ||||||
| Armenian et al. (2013) (30) | Case-control Cases: n = 77 Control subjects: n = 178 |
Cases: 49.2 (16-68.8) Control subjects: 51.0 (6.4-72.6) |
Leukemia, myeloma, lymphoma status post-hematopoietic cell transplantation | Sign/symptoms of cardiac compromise indicating need for intervention based on AHA criteria | ABCC2 rs8187710 (OR: 4.3; p < 0.01) RAC2 rs13058338 (OR: 2.48; p < 0.01) HFE rs1799945 (OR: 2.5; p = 0.05) | No replication performed |
| Lipshultz et al. (2013) (57) | Cohort n = 184 | 15.2 (3.1-31.4) | ALL | 1. cTnT > 0.01 ng/ml 2. NT-proBNP > 150 pg/ml (<1 yr) 3. NT-proBNP >100 pg/ml (≥1 yr) |
C282Y HFE carriers associated with myocardial injury | No replication performed |
| Wang et al. (2014) (20) | Discovery Case-control: n = 287; 93 cases Replication Case only: n = 76 |
Discovery Cases: 19.4 (0.4-41.7) Control subjects: 18.5 (3.5-49.2) Replication Cases: 48 (13-68) |
Discovery HL, NHL bone tumors, soft tissue sarcoma, ALL, AML, other Replication HL, NHL, ALL, AML, other |
AHA criteria for cardiac compromise: 1. Symptoms/signs of cardiac compromise 2. Echo evidence of LV dysfunction (LVEF ≤ 40% and/or FS ≤ 28%) |
rs2232228, in HAS3 exerted a modifying effect on anthracycline dose-dependent cardiomyopathy risk (p = 5.3 × 10−7) | Gene-environment interaction successfully replicated in an independent set of 76 patients with anthracycline-related cardiomyopathy |
| Leger et al. (2016) (56) | Nested case cohort Cases: n = 79 Comparison cohort: n = 267 |
Adult and pediatric patients | BMT recipients for acute leukemia/MDS, chronic leukemia, lymphoma, multiple myeloma, solid tumors, nonmalignant disorder | Administrative data sources (National Death Index; state hospital discharge and death registry records) and self-report | Identified association with previously reported genetic associations among early onset cardiomyopathy cases, including rs1786814 (CELF4), rs2232228 (HAS3), and rs17863783 (UGT1A6) | No replication performed |
| Adult-Onset Cancer Populations | ||||||
| Reichwagen et al. (2015) (48) | Case-control n = 520 | 68 (61, 80) | NHL | Grade >0 based on CTCAEv2 | Accumulation of RAC2 subunit genotypes TA/AA among cases was statistically significant on adjustment for sex, age, and doxorubicin dose in a multivariate logistic regression analysis (OR: 2.3; p = 0.03) | No replication performed |
| Wojnowski et al. (2005) (26) | Case-control Cases: n = 87 Control subjects: n = 363 |
Cases: 62.0 ± 10.9 Control subjects: 61.3 ± 11.0 |
NHL | 1. Arrhythmia in the absence of arrhythmia before treatment 2. Myocarditis 3. Acute heart failure 4. LVEF <50% or FS <25% |
ABCC1 rs45511401 (OR: 2.39; 95% CI: 1.2–4.9) ABCC2 rs8187710 (OR: 1.98; 95% CI: 1.01–3.90) ABCC2 rs8187694 (OR: 1.98; 95% CI: 1.0–3.9) CYBA rs4673 (OR: 1.93; 95% CI: 1.2–3.2) RAC2 rs13058338 (OR: 1.68; 95% CI: 1.1–2.7) | No replication performed |
| Vulsteke et al. (2015) (49) | Cohort n = 877; 153 cases | Mean: 50.3 | Breast cancer | Asymptomatic decrease of LVEF >10% and cardiac failure grade 3–5 (CTCAEv4.0) | Heterozygous carriers of the rs246221 T allele in ABCC1 relative to homozygous carriers of the T allele were significantly associated with LVEF decline of >10% (OR: 1.3; 95% CI: 1.1–1.4; p < 0.001, and OR: 1.6; 95% CI: 1.1–2.3; p = 0.02) | No replication performed |
| Hertz et al. (2016) (50) | Cohort n = 166; 19 cases | 50 (24-80) | Breast cancer | Asymptomatic (LVEF <55%) | ABCB1 (3435C>T, p = 0.049) and CBR3 (V244M, p = 0.01) (uncorrected) | No replication performed |
| Rossi et al. (2009) (51) | Cohort n = 658; 106 cases | 66 (56-75) | DLBCL | Grade 2–4 cardiac toxicity | NCF4 rs1883112 was an independent predictor against cardiac toxicity (OR: 0.37; p = 0.02) | No replication performed |
| Ruiz-Pinto et al. (2018) (52) | Cohort Discovery: n = 71; 18 cases Replication: n = 83; 31 cases |
Discovery Cases: 49 (27-73) Control subjects: 59.5 (36-72) Replication Cases: 5.1 (1.4-16.9) Control subjects: 10.4 (1.2-21.1) |
Discovery: breast cancer Replication: childhood cancer |
1. Cardiac failure grade 3–5 using CTCAEv4.0 (grade 3: severe symptoms at rest or with minimal activity or exertion, intervention indicated; grade 4: life-threatening consequences, urgent intervention indicated; grade 5: death) 2. Asymptomatic decrease of LVEF >10% |
ETFB, related to anthracycline-mediated mitochondrial dysfunction | Successfully replicated in an independent cohort of 83 anthracycline-treated pediatric cancer patients |
| Garcia-Pavia et al. 2019) (53) | Case-cohort Cases: n = 213 Comparison: n = 2,498 |
Cohort A: adults with diverse cancer with cardiomyopathy: 48.7 ± 17.1 Cohort B: breast cancer patients with cardiomyopathy: 49.6 ± 10.8 Cohort C: children with AML with cardiomyopathy: 10.8 ± 5.6 |
Cases: adults with diverse cancers (n = 99); breast cancer (n = 73); children with AML (n = 41) Comparison cohort: Cancer Genome Atlas participants (n = 2,053); healthy volunteers (n = 445) |
LVEF to <50 (cohort B) or <53% (cohorts A and C) and ≥10% reduction from baseline by echo or <50% and ≥10% reduction from baseline by radionuclide ventriculography, in the absence of established coronary artery disease, cardiomyopathy, primary valvular disease, or uncontrolled hypertension | Titin-truncating variants | No replication performed |
| Wells et al. (2017) (54) | Cohort Discovery: n = 385 Replication: n = 181 |
Discovery: 52 (40-61) Replication: 53 (45-61) |
Diverse cancers | Maximal change in LVEF from pre-chemotherapy measurement | rs7542939—a susceptibility locus near PRDM2. Variation in genes belonging to pathways related to DNA repair, metabolism, and cardiac remodeling may influence changes in LV function after anthracycline exposure. |
rs7542939 successfully replicated |
| Schneider et al. (2017) (55) | Cohort Discovery: n = 1,055; 68 cases Replication 1) n = 930; 47 cases 2) n = 322; 24 cases |
Adults (details not available) | Breast cancer | Centrally reviewed, cardiologist-adjudicated HF | rs28714259—within the binding site for glucocorticoid receptor protein; important roles in the structural and functional maturation of fetal heart | rs28714259 was successfully validated in replication cohorts |
Values are mean ± SD or median (interquartile range) unless otherwise indicated.
AHA = American Heart Association; ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; BMT = bone marrow transplant; CI = confidence interval; CTCAEv3: Clinical Terminology Criteria for Adverse Events version 3; cTnT, cardiac troponin T; DLBCL = diffuse large B-cell lymphoma; DNA = deoxyribonucleic acid; EF = ejection fraction; FS= fractional shortening; HF = heart failure; HL = Hodgkin lymphoma; LV = left ventricular; LVEF = left ventricular ejection fraction; MDS = myelodysplastic syndrome; NA = not available; NHL = non-Hodgkin lymphoma; NT-proBNP = N-terminal pro–B-type natriuretic peptide; OR = odds ratio; SNP = single nucleotide polymorphism.
Central Illustration.
Proposed Pathogenesis of Anthracycline-Related Cardiomyopathy
The identified genetic associations are placed in the context of a proposed pathogenesis of anthracycline-related cardiomyopathy. The proposed mechanisms include transport of anthracyclines across the cardiomyocyte cell membrane, generation of reactive oxygen species (ROS), deoxyribonucleic damage response and repair, mitochondrial dysfunction, generation of cardiotoxic anthracycline metabolites, and sarcomere disruption. Top2b = topoisomerase-IIβ
Drug biotransformation genes
GSTM1
Glutathione S-transferases (GSTs) facilitate elimination of anthracyclines. GSTs could play a role in oxidative damage-induced cardiomyopathy as free radical scavengers. A previous report indicates an association between the GSTM1 null genotype and iron-overload–related cardiomyopathy in patients with thalassemia (59,60). Singh et al. (47) sought to identify an association between the GSTM1 null genotype and anthracycline-related cardiomyopathy in childhood cancer survivors. They examined the GSTM1 gene deletion in 75 survivors with clinically validated cardiomyopathy and 92 survivors without cardiomyopathy. They observed a significant association between the risk of cardiomyopathy and the GSTM1 null genotype (odds ratio [OR]: 2.7; 95% confidence interval [CI]: 1.3 to 5.9; p = 0.007).
Singh et al. (47) measured GSTM1 expression levels in peripheral blood from 20 cases and 20 control subjects and also examined human-induced pluripotent stem cell cardiomyocytes (hiPSC-CMs) from childhood cancer survivors (3 with cardiomyopathy, 3 without) for GSTM1 gene expression levels. There was a significant down-regulation of GSTM1 expression in the peripheral blood RNA from cases compared with from control subjects (average relative expression: 0.67 ± 0.57 vs. 1.33 ± 1.33, respectively; p = 0.049). Along similar lines, hiPSC-CMs from patients who had cardiomyopathy-revealed reduced GSTM1 expression (p = 0.007).
CBR3
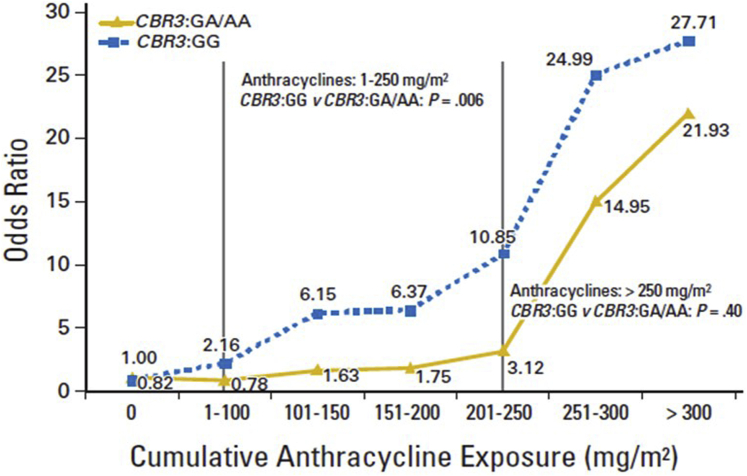
Carbonyl reductases (CBRs) catalyze reduction of anthracyclines to cardiotoxic alcohol metabolites. Polymorphisms in CBR3 influence synthesis of these metabolites. Blanco et al. (28) examined the role of SNPs in CBR3 (CBR3 V244M) in modifying the dose-dependent risk of anthracycline-related cardiomyopathy in childhood cancer survivors. A total of 170 survivors with cardiomyopathy were compared with 317 survivors with no cardiomyopathy. Among individuals with CBR3 V244M homozygous G genotypes (CBR3:GG), exposure to low- to moderate-dose anthracyclines (1 to 250 mg/m2) increased cardiomyopathy risk when compared with individuals with CBR3:GA/AA genotypes unexposed to anthracyclines (OR: 5.48; p = 0.003), as well as in those exposed to low- to moderate-dose anthracyclines (OR: 3.30; p = 0.006). High-dose anthracyclines (>250 mg/m2) were associated with increased cardiomyopathy risk, irrespective of CBR3 genotype status. This study demonstrates increased anthracycline-related cardiomyopathy risk at doses as low as 101 to 150 mg/m2 (Figure 2). An independent study of patients with breast cancer treated with anthracyclines with or without trastuzumab replicated the association between CBR3 and decline in left ventricular ejection fraction (61).
Figure 2.
Dose-Response Relationship Between Cumulative Anthracycline Exposure and Risk of Cardiomyopathy Stratified by Patient CBR3 Genotype Status
The dose-response relationship between cumulative anthracycline exposure and risk of cardiomyopathy stratified by patients’ CBR3 genotype status (CBR3:GG and CBR3:GA/AA) is shown. A total of 170 survivors with cardiomyopathy (cases) were compared with 317 survivors with no cardiomyopathy (control subjects; matched on cancer diagnosis, year of diagnosis, length of follow-up, and race/ethnicity) using conditional logistic regression techniques. Among individuals carrying the variant A allele (CBR1:GA/AA and/or CBR3:GA/AA), exposure to low- to moderate-dose anthracyclines (1 to 250 mg/m2) did not increase the risk of cardiomyopathy. Among individuals with CBR3 V244M homozygous G genotypes (CBR3:GG), exposure to low- to moderate-dose anthracyclines increased cardiomyopathy risk when compared with individuals with CBR3:GA/AA genotypes unexposed to anthracyclines (odds ratio: 5.48; p = 0.003), as well as exposed to low- to moderate-dose anthracyclines (odds ratio: 3.30; p = 0.006). High-dose anthracyclines (>250 mg/m2) were associated with increased cardiomyopathy risk, irrespective of CBR genotype status. Reprinted with permission from Blanco et al. (28).
CBR3 is present in the liver at low basal levels but is highly inducible by the transcription factor Nrf2. Cbr3 messenger RNA and CBR3 protein are highly expressed in the livers of Gclm−/− mice (a mouse model of glutathione deficiency) relative to wild-type mice. Schaupp et al. (62) investigated the ability of CBR3 to metabolize doxorubicin. Incubation of doxorubicin and purified recombinant murine CBR3 were analyzed for doxorubicinol formation using high-performance liquid chromatography, showing that doxorubicin is a substrate of recombinant murine CBR3 (62). Moreover, hepatocytes from Gclm−/− mice produced more doxorubicinol than Gclm+/+ hepatocytes did. In addition, differentiated rat myoblasts (C2C12 cells) cocultured with primary Gclm−/− murine hepatocytes were more sensitive to doxorubicin-induced cytostasis and/or cytotoxicity than incubations with Gclm+/+ hepatocytes were. These results indicate a potentially important role for CBR3 in doxorubicin-induced cardiotoxicity.
SLC28A3, SLC22A17, SLC22A17, UGT1A6
Visscher et al. (24) studied 2,977 SNPs in 220 key drug biotransformation genes in a discovery cohort of 156 anthracycline-treated children from British Columbia, with replication in a second cohort of 188 children and further replication of the top SNP in a third cohort of 96 patients. They identified a significant association of a synonymous coding variant rs7853758 (L461L) within the SLC28A3 gene with anthracycline-related cardiomyopathy (OR: 0.35; p = 1.8 × 10−5 for all cohorts combined). Additional associations (p < 0.01) with risk and protective variants in other genes including SLC28A1 and several adenosine triphosphate-binding cassette transporters (ABCB1, ABCB4, and ABCC1) were present. They then aimed to replicate these findings by testing the association between 23 genetic variants and cardiomyopathy in an independent cohort of 218 patients. They confirmed the association of rs17863783 in UGT1A6 and anthracycline-related cardiomyopathy in the replication cohort (OR: 7.98; p = 0.006). Additional evidence for association of rs7853758 (OR: 0.46; p = 0.058,) and rs885004 (OR: 0.40; p = 0.058) in SLC28A3 was found (combined p = 1.6 × 10−5 and p = 3.0 × 10−5, respectively) (25). They extended the genotyping panel to include over 4,500 SNPs in more than 300 genes relevant in pharmacokinetics and dynamics, including anthracycline transport, metabolism, and toxicity (23). They identified two novel SNPs— rs4982753 (SLC22A17) and rs4149178 (SLC22A7)— in the discovery cohort; these findings were successfully replicated.
Antioxidant mechanisms
HAS3
Co-occurrence of cardiovascular risk factors (diabetes and hypertension) exacerbate the dose-dependent association between anthracyclines and cardiomyopathy (15,63). Institute for Translational Medicine and Therapeutics/Broad/Candidate-gene Association Resource cardiovascular SNP array profiles common SNPs in 2,100 genes considered relevant to de novo cardiovascular disease (20), including cardiovascular risk factors that exacerbate the risk of anthracycline-related cardiomyopathy. Wang et al. (20) investigated host susceptibility to anthracycline-related cardiomyopathy using the Institute for Translational Medicine and Therapeutics/Broad/Candidate-gene Association Resource cardiovascular SNP array. Using a matched case-control design (93 cases; 194 control subjects), they identified a common SNP, rs2232228, in HAS3 that exerted a modifying effect on anthracycline dose-dependent cardiomyopathy risk (p= 5.3 × 10−7) (Figure 3). Among individuals with rs2232228 GG genotype, cardiomyopathy was infrequent and not dose-related. However, in individuals exposed to high-dose (>250 mg/m2) anthracyclines, the rs2232228 AA genotype conferred an 8.9-fold (95% CI: 2.1 to 37.5; p = 0.003) higher cardiomyopathy risk than the GG genotype did. Relative HAS3 messenger RNA levels measured in healthy hearts tended to be lower among individuals with AA compared with those with GA genotypes (p = 0.09). Hyaluronan produced by HAS3 is a ubiquitous component of the extracellular matrix and plays a role in tissue remodeling. In addition, hyaluronan reduces reactive oxygen species–induced cardiac injury. In this setting, cardiomyopathy risk could be due to inadequate remodeling and/or inadequate protection of the heart from reactive oxygen species–mediated injury on high anthracycline exposure.
Figure 3.
Risk of Cardiomyopathy by Anthracycline Dose and HAS3 rs2232228 Genotype Status
The risks of cardiomyopathy by anthracycline dose and HAS3 rs2232228 genotype status (AA, GA, GG) are shown. By using a matched case-control design (93 cases, 194 control subjects), a common single nucleotide polymorphism, rs2232228, in HAS3 was identified. This single nucleotide polymorphism exerted a modifying effect on anthracycline dose-dependent cardiomyopathy risk (p = 5.3 × 10−7). Among individuals with rs2232228 GG genotype, cardiomyopathy was infrequent and not dose related. However, in individuals exposed to high-dose (>250 mg/m2) anthracyclines, the rs2232228 AA genotype conferred an 8.9-fold (95% confidence interval: 2.1- to 37.5-fold; p = 0.003) increased cardiomyopathy risk compared with the GG genotype. Reprinted with permission from Wang et al. (20). OR = odds ratio.
TOP2B-mediated DNA damage
RARG
Aminkeng et al. (22) performed a genome-wide association study in 280 patients of European ancestry (32 cases; 248 control subjects) treated for childhood cancer with independent replication in cohorts of 96 European patients (22 cases; 74 control subjects) and 80 non-European patients (19 cases; 61 control subjects). Cases exhibited fractional shortening of <24% or signs/symptoms of cardiac compromise requiring intervention; controls had no symptoms and fractional shortening ≥30%. A nonsynonymous variant (rs2229774) in RARG was associated with anthracycline-related cardiomyopathy (OR: 4.7; 95% CI: 2.7 to 8.3; p = 5.9 × 10−8). This variant alters RARG function leading to derepression of the key genetic determinant, Top2b. As indicated earlier, Top2b plays a role in the development of cardiomyopathy in a murine model (36), whereas in a rat cardiomyoblast (H9c2) cell line, cardioprotectant dexrazoxane results in decreased Top2b levels (64). RARG is expressed in the heart (Nuclear Receptor Signaling Atlas) and is induced in murine cardiac cells following cardiac injury (65).
Alternative splicing of TNNT2
CELF4
Wang et al. (21) conducted a genome-wide association study in childhood cancer survivors with and without cardiomyopathy (cases and control subjects, respectively), and independently replicated the SNPs that surpassed a pre-specified threshold for statistical significance. They used healthy hearts to seek the mechanistic significance of the validated SNP(s). SNP rs1786814 on CELF4 passed the significance cutoff for gene-environment interaction (pGE = 1.14 × 10−5). Among patients with A allele, cardiomyopathy was infrequent and not dose-related. However, among those exposed to >300 mg/m2 of anthracyclines, the rs1786814 GG genotype conferred a 10.2-fold (95% CI: 3.8 to 27.3; p < 0.001) increased risk of cardiomyopathy compared with those with GA/AA genotypes and anthracycline exposure of ≤300 mg/m2 (Figure 4). cytosine-uridine-guanine repeat binding protein and embryonic lethal abnormal vision-type RNA-binding protein-3–like factor proteins (encoded by CELF4) control developmentally regulated splicing of TNNT2, the gene that encodes for cardiac troponin T, a biomarker of myocardial injury. Coexistence of more than 1 cardiac troponin T variant results in a temporally split myofilament response to calcium, which causes decreased contractility. Analysis of TNNT2 splicing variants in healthy human hearts suggested an association between the rs1786814 GG genotype and coexistence of more than 1 TNNT2 splicing variant (90.5% GG vs. 41.7% GA/AA; p = 0.005). Thus, the modifying effect on the dose-dependent association between anthracyclines and cardiomyopathy possibly occurs through a pathway that involves the expression of abnormally spliced TNNT2 variants.
Figure 4.
Risk of Cardiomyopathy by Anthracycline Dose and CELF4 rs1786814 Genotype Status
The risks of cardiomyopathy by anthracycline dose and CELF4 rs1786814 genotype status (AA, GA, GG) are shown. A genome-wide association study was conducted in childhood cancer survivors with and without cardiomyopathy (cases and control subjects, respectively). No single nucleotide polymorphism was marginally associated with cardiomyopathy. However, single nucleotide polymorphism rs1786814 on CELF4 passed the significance cutoff for gene-environment interaction (pGE = 1.14 × 10−5). Multivariable analyses adjusted for age at cancer diagnosis, sex, anthracycline dose, and chest radiation revealed that, among patients with the A allele, cardiomyopathy was infrequent and not dose related. However, among those exposed to >300 mg/m2 of anthracyclines, the rs1786814 GG genotype conferred a 10.2-fold (95% confidence interval: 3.8- to 27.3-fold; p < 0.001) increased risk of cardiomyopathy compared with those who had GA/AA genotypes and anthracycline exposure of 300 mg/m2 or less. Reprinted with permission from Wang et al. (21). OR = odds ratio.
Metabolomics
Disturbances in cardiac metabolism underlie most cardiovascular diseases. Metabolomics, one of the newer omics technologies, has emerged as a powerful tool for defining changes in both global and cardiac-specific metabolism that occur across a spectrum of cardiovascular disease states (66). Findings from metabolomics studies have contributed to better understanding of the metabolic changes that occur in heart failure and have identified new cardiovascular disease biomarkers. Armenian et al. (67) performed plasma metabolomic analyses (8 pathways; 354 metabolites) in 150 asymptomatic anthracycline-exposed childhood cancer survivors. Median time from cancer diagnosis to study participation was 12.4 years (interquartile range: 2.6 to 37.9 years); median anthracycline dose was 350 mg/m2 (interquartile range: 25 to 642 mg/m2). Thirty-five participants (23%) had cardiac dysfunction defined as left ventricular end-systolic wall stress >2 SD by echocardiogram. Plasma levels of 15 compounds in 3 metabolic pathways (carbohydrate, amino acid, and lipid metabolism) were significantly different between individuals with cardiac dysfunction and those with normal systolic function. After adjusting for multiple comparisons, individuals with cardiac dysfunction had significantly lower plasma carnitine levels (relative ratio: 0.89; p < 0.01) compared with those in individuals with normal systolic function. If confirmed in future studies, findings such as this could suggest a role for carnitine supplementation for primary prevention (before/during anthracycline administration) or secondary prevention in long-term survivors.
iPSC-derived CMs
An innovative approach to study cardiomyopathy is to harness iPSCs and derived differentiated cells as in vitro model systems, shown to serve as powerful model systems for understanding cell type–specific genetic regulation of transcription (68, 69, 70, 71, 72). The response of iPSC-derived CMs has been extensively characterized by Burridge et al. (73,74). iPSC-CMs derived from 4 individuals who developed cardiomyopathy after doxorubicin treatment (DOXTOX group) and 4 who did not (DOX group) showed clear differences in viability (via apoptosis), metabolism, DNA damage, oxidative stress, and mitochondrial function when exposed to doxorubicin. These observations suggest that iPSC-CMs recapitulate in vivo interindividual differences in doxorubicin sensitivity. Knowles et al. (75) used a panel of iPSC-CMs from 45 individuals exposed to 5 different drug concentrations to understand the genetic basis of the interindividual differences in doxorubicin sensitivity. They found several genetic variants that modulate transcriptomic response, including some that act on alternative splicing. The transcriptomic response correlated with cardiac troponin levels in culture. They also showed that the mapped genetic variants were enriched in the top genetic variants in a genome-wide association study of anthracycline-related cardiomyopathy.
Christidi et al. (76) used iPSC-CMs to investigate the functional role of the RARG variant previously shown to be associated with anthracycline-related cardiomyopathy (22). iPSC-CMs from individuals who experienced anthracycline-related cardiomyopathy (cases) showed significantly greater sensitivity to doxorubicin than did iPSC-CMs from doxorubicin-treated individuals who did not develop cardiomyopathy (control subjects) in cell viability and optical mapping experiments. Using CRISPR/Cas9 technology (CRISPR Therapeutics, Cambridge, Massachusetts), isogeneic cell lines differing only at the RARG locus were generated. Genetic correction of RARG-S427L to wild type resulted in reduced doxorubicin-induced double-stranded DNA breaks, reactive oxygen species production, and cell death. Conversely, introduction of RARG-S427L increased susceptibility to doxorubicin. Finally, genetic disruption of RARG resulted in protection from cell death due to doxorubicin treatment. These findings suggest that the presence of RARG-S427L increases sensitivity to anthracycline-related cardiomyopathy.
Holmgren et al. (77) investigated doxorubicin-induced cardiotoxicity in hiPSC-CMs using proteomics. In addition, they combined different sources of omics data (protein, messenger RNA, and microRNA) from the same experimental setup to identify differential expression in data of various origin and types. In this experimental model system, they exposed hiPSC-CMs to doxorubicin for up to 2 days, followed by a washout period of 12 days. Besides an effect on the cell morphology and cardiomyocytes functionality (treated cells exhibited reduced contractility and inhibited cell proliferation), the data showed a strong effect of doxorubicin on all molecular levels investigated. They identified differential expression patterns that showed a linkage between the proteome, transcriptome, and the regulatory microRNA network. Pathway over-representation analysis of the differentially expressed proteins at each time point revealed a clear effect on cardiomyocyte-related signaling pathways, including mitochondrial apoptotic signaling. Effects were also seen on the abundance of proteins that constitute the myofibrils, such as myosin light chains; mutYHomologous genes/proteins; LP18.3, Psb32, and MOLO-1; and troponins; as well as proteins involved in the mitochondrial function, such the cyclooxygenase family, MT-CO2, NADH:ubiquinone oxidoreductase supernumerary subunits family, adenosine triphosphate family, and UQCR10.
Taken together, these studies provide evidence for the use of iPSC-CMs as a suitable platform to identify and characterize the genetic basis and molecular mechanism of anthracycline-related cardiomyopathy. However, there are some limitations that need to be taken into account: these include the immature and indeterminate phenotype of the cardiomyocytes; and the lack of other cardiac cell types, such as endothelial cells and fibroblasts. Nonetheless, by using this model, it should be possible to validate the relevance of genetic variants through SNP arrays.
Prediction of Anthracycline-Related Cardiomyopathy
There have been several attempts at developing risk prediction models for identifying patients at highest risk for anthracycline-related cardiomyopathy. Clinical risk prediction models have yielded modest predictive power for identifying patients at risk for anthracycline-related cardiomyopathy (31,78), resulting in studies that combined genetic variants with clinical and demographic variables to improve the ability to disriminate anthracycline-exposed survivors by their risk of developing cardiomyopathy. Visscher et al. (25) combined multiple variants from drug biotransformation genes together with clinical risk factors into a prediction model and classified patients into 3 risk groups. In the high-risk group, the model was able to predict 75% of the patients who actually developed anthracycline-related cardiomyopathy. Equally as importantly, in the low-risk group, the model was able to predict 96% of the patients who did not to develop cardiomyopathy. They extended the genotyping panel including over 4,500 SNPs in more than 300 genes pre-selected for relevance in pharmacokinetics and dynamics. An improved prediction model constructed using replicated genetic variants as well as clinical factors discriminated significantly better between cases and control subjects than did clinical factors alone in both original (area under the curve [AUC]: 0.77 vs. 0.68; p = 0.003) and replication (AUC: 0.77 vs. 0.69; p = 0.06) cohorts. Armenian et al. (30) included SNPs on genes coding for the NAD(P)H-oxidase subunit RAC2 (rs13058338), HFE (rs1799945), and the doxorubicin efflux transporter ABCC2 (rs8187710) to create a combined (clinical + genetic) heart failure predictive model. The combined model performed better (AUC: 0.79) than the genetic (AUC: 0.67) or the clinical (AUC: 0.69) models alone. However, none of these models were externally validated, presenting a need for robust external validation in independent patient populations. These validated models could provide a framework on which to base future screening strategies and interventions targeted to those at highest risk of developing cardiomyopathy. Equally important is the identification of those at lowest risk, to allow the maximally tolerated dose of anthracyclines as well as to be spared the need for screening for early detection of cardiomyopathy. Thus, there is a possibility for setting an individualized threshold of maximum cumulative anthracycline dose where patients with a favorable risk profile might benefit from an increased cumulative dose with larger antitumor effect. In contrast, those with an unfavorable profile would benefit from alternative therapeutic options and more frequent cardiac monitoring, facilitating early detection of cardiac compromise.
Summary
This review summarizes the current knowledge regarding the etiology and pathogenesis of anthracycline-related cardiomyopathy. Even though a lot of work has been accomplished, there are significant gaps in knowledge. For example, with an exception of few, most studies lack robust patient numbers and functional validation. Most of the studies have not examined gene-environment (gene–anthracycline dose) interactions, or are not powered to do so; thus, genes such as HAS3, CELF4, and CBR3 (all demonstrating strong gene-environment interactions) have been replicated by very few. Most of the studies on the adult-onset cancer patients are restricted to breast cancer of lymphoma survivors; there is a need to expand the portfolio of research to a diverse group of cancer survivors. There is also a need to expand the sample size, to allow the use of an agnostic approach as we integrate genomics, transcriptomics, proteomics, and metabolomics. The ultimate goal is 3-fold: 1) identify those at highest risk such that both primary and secondary measures can be instituted to prevent this complication; 2) use the findings from integrated omics analyses to serve as biomarkers of those at highest risk; and 3) use the leads from these studies to identify therapeutic targets.
Author Disclosures
Dr. Bhatia has received funding from the National Institutes of Health (R35CA220502), Leukemia Lymphoma Society (6563-19), and V Foundation (DT2019-010).
Footnotes
The author attest they are in compliance with human studies committees and animal welfare regulations of the author’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Baech J., Hansen S.M., Lund P.E. Cumulative anthracycline exposure and risk of cardiotoxicity; a Danish nationwide cohort study of 2440 lymphoma patients treated with or without anthracyclines. Br J Haematol. 2018;183:717–726. doi: 10.1111/bjh.15603. [DOI] [PubMed] [Google Scholar]
- 2.Nabhan C., Byrtek M., Rai A. Disease characteristics, treatment patterns, prognosis, outcomes and lymphoma-related mortality in elderly follicular lymphoma in the United States. Br J Haematol. 2015;170:85–95. doi: 10.1111/bjh.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chihara D., Westin J.R., Oki Y. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer. 2016;122:3145–3151. doi: 10.1002/cncr.30173. [DOI] [PubMed] [Google Scholar]
- 4.van Dalen E.C., van der Pal H.J., Kok W.E., Caron H.N., Kremer L.C. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Krischer J.P., Epstein S., Cuthbertson D.D., Goorin A.M., Epstein M.L., Lipshultz S.E. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 6.Smith L.A., Cornelius V.R., Plummer C.J. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
- 8.Oeffinger K.C., Mertens A.C., Sklar C.A. Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 9.Tukenova M., Guibout C., Oberlin O. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz S.E., Lipsitz S.R., Sallan S.E. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 11.Felker G.M., Thompson R.E., Hare J.M. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 12.van der Pal H.J., van Dalen E.C., Hauptmann M. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 13.Yahalom J., Portlock C.S. Long-term cardiac and pulmonary complications of cancer therapy. Hematol Oncol Clin North Am. 2008;22:305–318. doi: 10.1016/j.hoc.2008.01.010. vii. [DOI] [PubMed] [Google Scholar]
- 14.Adams M.J., Lipshultz S.E. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44:600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong G.T., Oeffinger K.C., Chen Y. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotrionte M., Biondi-Zoccai G., Abbate A. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Kremer L.C., van der Pal H.J., Offringa M., van Dalen E.C., Voute P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia S. Role of genetic susceptibility in development of treatment-related adverse outcomes in cancer survivors. Cancer Epidemiol Biomarkers Prev. 2011;20:2048–2067. doi: 10.1158/1055-9965.EPI-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen B.C., McLeod H.L. Pharmacogenomics as a risk mitigation strategy for chemotherapeutic cardiotoxicity. Pharmacogenomics. 2013;14:205–213. doi: 10.2217/pgs.12.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Liu W., Sun C.L. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children's oncology group. J Clin Oncol. 2014;32:647–653. doi: 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Sun C.L., Quinones-Lombrana A. CELF4 variant and anthracycline-related cardiomyopathy: a Children's Oncology Group Genome-Wide Association Study. J Clin Oncol. 2016;34:863–870. doi: 10.1200/JCO.2015.63.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aminkeng F., Bhavsar A.P., Visscher H. Canadian Pharmacogenomics Network for Drug Safety Consortium. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47:1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visscher H., Rassekh S.R., Sandor G.S., CPNDS Consortium Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics. 2015;16:1065–1076. doi: 10.2217/pgs.15.61. [DOI] [PubMed] [Google Scholar]
- 24.Visscher H., Ross C.J., Rassekh S.R. Canadian Pharmacogenomics Network for Drug Safety Consortium. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 25.Visscher H., Ross C.J., Rassekh S.R., CPNDS Consortium Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013;60:1375–1381. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 26.Wojnowski L., Kulle B., Schirmer M. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 27.Blanco J.G., Leisenring W.M., Gonzalez-Covarrubias V.M. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 28.Blanco J.G., Sun C.L., Landier W. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armenian S., Bhatia S. Predicting and preventing anthracycline-related cardiotoxicity. Am Soc Clin Oncol Educ Book. 2018:3–12. doi: 10.1200/EDBK_100015. [DOI] [PubMed] [Google Scholar]
- 30.Armenian S.H., Ding Y., Mills G. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br J Haematol. 2013;163:205–213. doi: 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow E.J., Chen Y., Kremer L.C. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33:394–402. doi: 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cascales A., Pastor-Quirante F., Sanchez-Vega B. Association of anthracycline-related cardiac histological lesions with NADPH oxidase functional polymorphisms. Oncologist. 2013;18:446–453. doi: 10.1634/theoncologist.2012-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano J., Palmeira C.M., Kuehl D.W., Wallace K.B. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta. 1999;1411:201–205. doi: 10.1016/s0005-2728(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 34.Arola O.J., Saraste A., Pulkki K., Kallajoki M., Parvinen M., Voipio-Pulkki L.M. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. [PubMed] [Google Scholar]
- 35.Rebbaa A., Chou P.M., Emran M., Mirkin B.L. Doxorubicin-induced apoptosis in caspase-8-deficient neuroblastoma cells is mediated through direct action on mitochondria. Cancer Chemother Pharmacol. 2001;48:423–428. doi: 10.1007/s002800100375. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S., Liu X., Bawa-Khalfe T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 37.Herman E.H., Zhang J., Hasinoff B.B., Clark J.R., Jr., Ferrans V.J. Comparison of the structural changes induced by doxorubicin and mitoxantrone in the heart, kidney and intestine and characterization of the Fe(III)-mitoxantrone complex. J Mol Cell Cardiol. 1997;29:2415–2430. doi: 10.1006/jmcc.1997.0477. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.D., Kim C.H., Rah B.J., Chung H.I., Shim T.S. Quantitative study on the relation between structural and functional properties of the hearts from three different mammals. Anat Rec. 1994;238:199–206. doi: 10.1002/ar.1092380206. [DOI] [PubMed] [Google Scholar]
- 39.De Angelis A., Piegari E., Cappetta D. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebrecht D., Kokkori A., Ketelsen U.P., Setzer B., Walker U.A. Tissue-specific mtDNA lesions and radical-associated mitochondrial dysfunction in human hearts exposed to doxorubicin. J Pathol. 2005;207:436–444. doi: 10.1002/path.1863. [DOI] [PubMed] [Google Scholar]
- 41.Lebrecht D., Walker U.A. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:108–113. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- 42.Yeh E.T.H., Ewer M.S., Moslehi J. Mechanisms and clinical course of cardiovascular toxicity of cancer treatment I. Oncology. Semin Oncol. 2019;46:397–402. doi: 10.1053/j.seminoncol.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Leong S.L., Chaiyakunapruk N., Lee S.W. Candidate gene association studies of anthracycline-induced cardiotoxicity: a systematic review and meta-analysis. Sci Rep. 2017;7:39. doi: 10.1038/s41598-017-00075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linschoten M., Teske A.J., Cramer M.J., van der Wall E., Asselbergs F.W. Chemotherapy-related cardiac dysfunction: a systematic review of genetic variants modulating individual risk. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.117.001753. [DOI] [PubMed] [Google Scholar]
- 45.Semsei A.F., Erdelyi D.J., Ungvari I. ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol Int. 2012;36:79–86. doi: 10.1042/CBI20110264. [DOI] [PubMed] [Google Scholar]
- 46.Krajinovic M., Elbared J., Drouin S. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16:530–535. doi: 10.1038/tpj.2015.63. [DOI] [PubMed] [Google Scholar]
- 47.Singh P., Wang X., Hageman L. Association of GSTM1 null variant with anthracycline-related cardiomyopathy after childhood cancer—a Children's Oncology Group ALTE03N1 report. Cancer. 2020;126:4051–4058. doi: 10.1002/cncr.32948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichwagen A., Ziepert M., Kreuz M. Association of NADPH oxidase polymorphisms with anthracycline-induced cardiotoxicity in the RICOVER-60 trial of patients with aggressive CD20(+) B-cell lymphoma. Pharmacogenomics. 2015;16:361–372. doi: 10.2217/pgs.14.179. [DOI] [PubMed] [Google Scholar]
- 49.Vulsteke C., Pfeil A.M., Maggen C. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat. 2015;152:67–76. doi: 10.1007/s10549-015-3437-9. [DOI] [PubMed] [Google Scholar]
- 50.Hertz D.L., Caram M.V., Kidwell K.M. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics. 2016;17:231–240. doi: 10.2217/pgs.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi D., Rasi S., Franceschetti S. Analysis of the host pharmacogenetic background for prediction of outcome and toxicity in diffuse large B-cell lymphoma treated with R-CHOP21. Leukemia. 2009;23:1118–1126. doi: 10.1038/leu.2008.398. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Pinto S., Pita G., Martin M. Exome array analysis identifies ETFB as a novel susceptibility gene for anthracycline-induced cardiotoxicity in cancer patients. Breast Cancer Res Treat. 2018;167:249–256. doi: 10.1007/s10549-017-4497-9. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Pavia P., Kim Y., Restrepo-Cordoba M.A. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation. 2019;140:31–41. doi: 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells Q.S., Veatch O.J., Fessel J.P. Genome-wide association and pathway analysis of left ventricular function after anthracycline exposure in adults. Pharmacogenet Genomics. 2017;27:247–254. doi: 10.1097/FPC.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider B.P., Shen F., Gardner L. Genome-wide association study for anthracycline-induced congestive heart failure. Clin Cancer Res. 2017;23:43–51. doi: 10.1158/1078-0432.CCR-16-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leger K.J., Cushing-Haugen K., Hansen J.A. Clinical and genetic determinants of cardiomyopathy risk among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2016;22:1094–1101. doi: 10.1016/j.bbmt.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipshultz S.E., Lipsitz S.R., Kutok J.L. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119:3555–3562. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feijen E.A.M., Leisenring W.M., Stratton K.L. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5:864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mokhtar G.M., Sherif E.M., Habeeb N.M. Glutathione S-transferase gene polymorphism: relation to cardiac iron overload in Egyptian patients with beta thalassemia major. Hematology. 2016;21:46–53. doi: 10.1179/1607845415Y.0000000046. [DOI] [PubMed] [Google Scholar]
- 60.Singh M.M., Kumar R., Tewari S., Agarwal S. Association of GSTT1/GSTM1 and ApoE variants with left ventricular diastolic dysfunction in thalassaemia major patients. Hematology. 2019;24:20–25. doi: 10.1080/10245332.2018.1502397. [DOI] [PubMed] [Google Scholar]
- 61.Serie D.J., Crook J.E., Necela B.M. Genome-wide association study of cardiotoxicity in the NCCTG N9831 (Alliance) adjuvant trastuzumab trial. Pharmacogenet Genomics. 2017;27:378–385. doi: 10.1097/FPC.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaupp C.M., White C.C., Merrill G.F., Kavanagh T.J. Metabolism of doxorubicin to the cardiotoxic metabolite doxorubicinol is increased in a mouse model of chronic glutathione deficiency: a potential role for carbonyl reductase 3. Chem Biol Interact. 2015;234:154–161. doi: 10.1016/j.cbi.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armenian S.H., Sun C.L., Vase T. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyu Y.L., Kerrigan J.E., Lin C.P. Topoisomerase II beta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67:8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 65.Bilbija D., Haugen F., Sagave J. Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Bilsen M., van Nieuwenhoven F.A., van der Vusse G.J. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 67.Armenian S.H., Gelehrter S.K., Vase T. Carnitine and cardiac dysfunction in childhood cancer survivors treated with anthracyclines. Cancer Epidemiol Biomarkers Prev. 2014;23:1109–1114. doi: 10.1158/1055-9965.EPI-13-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas S.M., Kagan C., Pavlovic B.J. Reprogramming LCLs to iPSCs results in recovery of donor-specific gene expression signature. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burrows C.K., Banovich N.E., Pavlovic B.J. Genetic variation, not cell type of origin, underlies the majority of identifiable regulatory differences in iPSCs. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banovich N.E., Li Y.I., Raj A. Impact of regulatory variation across human iPSCs and differentiated cells. Genome Res. 2018;28:122–131. doi: 10.1101/gr.224436.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kilpinen H., Goncalves A., Leha A. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature. 2017;546:370–375. doi: 10.1038/nature22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alasoo K., Rodrigues J., Mukhopadhyay S. Shared genetic effects on chromatin and gene expression indicate a role for enhancer priming in immune response. Nat Genet. 2018;50:424–431. doi: 10.1038/s41588-018-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burridge P.W., Li Y.F., Matsa E. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burridge P.W., Matsa E., Shukla P. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knowles D.A., Burrows C.K., Blischak J.D. Determining the genetic basis of anthracycline-cardiotoxicity by molecular response QTL mapping in induced cardiomyocytes. Elife. 2018;7 doi: 10.7554/eLife.33480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christidi E., Huang H., Shafaattalab S. Variation in RARG increases susceptibility to doxorubicin-induced cardiotoxicity in patient specific induced pluripotent stem cell-derived cardiomyocytes. Sci Rep. 2020;10:10363. doi: 10.1038/s41598-020-65979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmgren G., Sartipy P., Andersson C.X., Lindahl A., Synnergren J. Expression profiling of human pluripotent stem cell-derived cardiomyocytes exposed to doxorubicin-integration and visualization of multi-omics data. Toxicol Sci. 2018;163:182–195. doi: 10.1093/toxsci/kfy012. [DOI] [PubMed] [Google Scholar]
- 78.Armenian S.H., Yang D., Teh J.B. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018;2:1756–1764. doi: 10.1182/bloodadvances.2018019117. [DOI] [PMC free article] [PubMed] [Google Scholar]