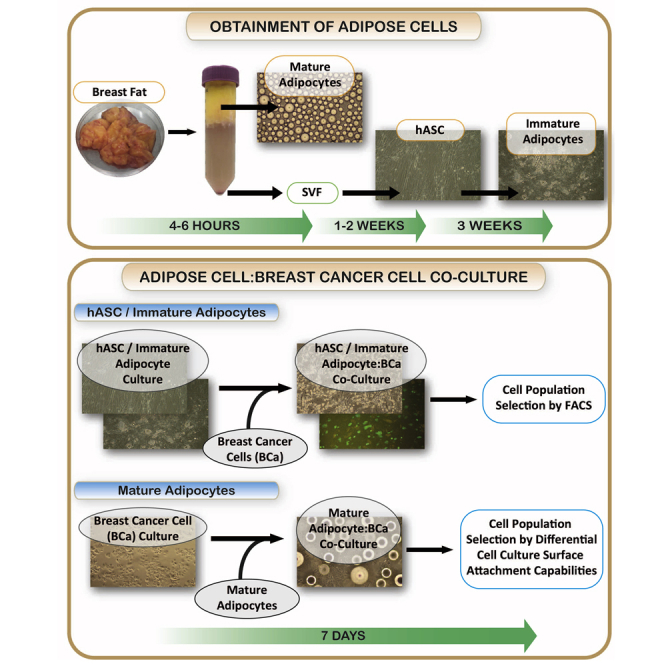
Summary
Primary human breast cancers invade surrounding fat and contact adipocytes, inflammatory infiltrates, and fibrous stroma. This tissue niche influences breast tumor progression. Here, we present a protocol to enable the in vitro study of the complex interactions that occur between breast cancer cells and adipose cells. We describe how to obtain different adipose cell populations, including adipose-derived stem cells, immature adipocytes, and mature adipocytes, from human breast fat tissue and detail the application for co-culture assays with breast cancer cells.
For complete details on the use and execution of this protocol, please refer to Picon-Ruiz et al. (2016) and Qureshi et al. (2020).
Subject Areas: Cell culture, Cell isolation, Cancer, Cell Differentiation
Graphical Abstract
Highlights
-
•
Obtaining different adipose cell populations from human breast fat samples
-
•
Isolation of mature adipocytes, immature adipocytes, and hASC
-
•
Co-culture of breast cancer cells with isolated primary human breast fat populations
Primary human breast cancers invade surrounding fat and contact adipocytes, inflammatory infiltrates, and fibrous stroma. This tissue niche influences breast tumor progression. Here, we present a protocol to enable the in vitro study of the complex interactions that occur between breast cancer cells and adipose cells. We describe how to obtain different adipose cell populations, including adipose-derived stem cells, immature adipocytes, and mature adipocytes, from human breast fat tissue and detail the application for co-culture assays with breast cancer cells.
Before You Begin
Provide Breast Tissue Collection Tubes, Labeled with Date, to the Surgeon
Provide 50 mL tubes with 20 mL DMEM media containing 1% penicillin/streptomycin to the surgeon and store at 4°C until use. For a good yield of mature adipocytes, it is best to request at least a 20 mL sample volume of mammary tissue. Ask operating room personnel to write the time of tissue extraction on the tube and call the lab as soon as the sample is ready for transport.
CRITICAL: Once breast tissue is deposited into the collection tube, store at 4°C. Initiate the cell isolation protocol within 4 h. Cell viability, particularly for mature adipocytes, decreases rapidly overtime so it is not recommended to use any sample more than 24 h after tissue removal from the donor.
CRITICAL: It is important to highlight that the use of human samples in research needs to conform to the principles outlined in the Declaration of Helsinki. Unless the sample is collected as de-identified waste material with no associated clinical information, all human subjects have to provide written informed consent prior to donation of adipose tissue samples and the protocol has to be approved in advance by the appropriate ethical committee.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco's modified Eagle’s medium (DMEM) | VWR | Cat#12-614F |
| Fetal bovine serum (FBS) | Gemini | Cat#100-106 |
| Penicillin/streptomycin | VWR | Cat#45000-652 |
| Collagenase from Clostridium histolyticum Type IA | Sigma-Aldrich | Cat#C9891 |
| Bovine serum albumin (BSA) | Gemini | Cat#700-105P |
| Hank’s balanced salt solution (HBSS) | VWR | Cat#12001-520 |
| Phosphate buffered saline (PBS) | VWR | Cat#12001-676 |
| Improved minimum essential medium (IMEM) | Invitrogen | Cat#10373-017 |
| Critical Commercial Assays | ||
| Human mesenchymal stem cell (hMSC) adipogenic differentiation medium | Lonza | Cat#PT-3004 |
| Experimental Models: Cell Lines | ||
| Human: MCF7 | ATCC | HTB-22 |
| Human: MDA-MB-231 | ATCC | HTB-26 |
| Other | ||
| 50 mL centrifuge tubes | VWR | Cat#89039-658 |
| 20 mL syringe | VWR | Cat#BD302830 |
| 0.20 μm pore syringe filter | VWR | Cat#28144-040 |
| Petri dishes | VWR | Cat#25388-606 |
| Scalpels | VWR | Cat#25863-222 |
| Forceps | VWR | Cat#89259-944 |
| Parafilm | VWR | Cat#52858-076 |
| 200 μm cell strainer | Pluriselect | Cat#43-50200-03 |
| 18G needles | VWR | Cat#89234258 |
| 70 μm cell strainer | VWR | Cat#102095-534 |
| 6-well plates | VWR | Cat#82050-842 |
| 100 mm cell culture dishes | VWR | Cat#82050-916 |
| 150 mm cell culture dishes | VWR | Cat#25383-103 |
| Table top centrifuge for 50 mL tubes | Eppendorf | Cat#5811000620 |
| Vertical tube rotator for 50 mL tubes | VWR | Cat#10136-084 |
| Biological Samples | ||
| Human breast tissue | n/a | n/a |
Materials and Equipment
Alternatives: This protocol uses Human Mesenchymal Stem Cell (hMSC) Adipogenic Differentiation Medium from Lonza for the adipogenic differentiation of human adipose-derived stem cells (hASC) into immature adipocytes. Alternatives to this medium are AdipoMAX Differentiation Medium (Sigma-Aldrich), MesenCult Adipogenic Differentiation Kit (STEMCELL Technologies) or Mesenchymal Stem Cell Adipogenic Differentiation Medium 2 (PromoCell). However, if this reagent is replaced, the protocol for differentiation of immature adipocytes from hASC has to be modified accordingly following the manufacturer′s instructions.
Alternatives: This protocol uses MCF7 and MDA-MB-231 as human breast cancer cell lines. However, this protocol has also been used effectively with other human established breast cancer cell lines including SUM159, SUM1315, T47D, and MDA-MB-361; the human primary breast cancer cell line DT28; and the oncogene-transformed human mammary epithelial cell line HMLER3; and potentially could be applied for use with any breast cancer cell line.
Enzyme Solution
Enzyme Solution for 20 mL Volume of Breast Tissue
| Reagent | Final Concentration | Amount |
|---|---|---|
| Collagenase IA | 1 mg/mL | 20 mg |
| BSA | 1% | 200 mg |
| HBSS | 20 mL | |
| Total | 20 mL |
Note: To avoid loss of enzymatic activity associated with repeated filter sterilization of the collagenase solution, prepare a sterile 100× stock solution by dissolving the vial of lyophilized Collagenase IA in sterile HBSS to a final concentration of 100 mg/mL, and aliquot it in 1 mL/cryotube. The Collagenase 1A stock can be stored at −80°C for up to 6 months until use.
Preparation
Timing: 10 min
-
•
Weigh out appropriate amount of BSA.
-
•
Mix with half of the total HBSS volume and shake vigorously until BSA is dissolved.
-
•
If the solution is foamy, centrifuge for 5 min at 2,000 × g and 22°C–28°C. Recover the supernatant.
-
•
Filter sterilize the solution through a 0.20 μm pore syringe filter.
-
•
Add the appropriate volume of sterile Collagenase 1A 100 mg/mL stock solution to reach a final concentration of 1 mg/mL.
-
•
Add the remaining sterile HBSS to achieve the final volume.
CRITICAL: Prepare fresh 1 mg/mL Collagenase 1A solution immediately before use.
Note: This solution is added to the adipose tissue at 1:1 volume:volume, so it is recommended that it be prepared after the last wash of the tissue so that by then, you will know the exact volume required.
Step-by-Step Method Details
Separation of Mature Adipocytes and Stromal Vascular Fraction (SVF) from Human Mammary Adipose Tissue
Timing: 3–4 h
In this step, breast tissue is digested mechanically and enzymatically to separate mature adipocytes from the stromal vascular fraction (SVF).
-
1.
Carefully decant the adipose tissue into a sterile Petri dish (Figure 1).
Note: It is recommended that you avoid decanting any media in order to reduce the risk of splashes during the performance of the next step.
-
2.
Using a sterile scalpel and forceps, dissociate the tissue mechanically and remove any grossly identified, other undesired tissue types (mainly fibrous tissue highly resistant to the mechanical dissociation) from the adipose sample.
-
3.
Use forceps to sterilely drop adipose tissue pieces into a 50 mL tube.
-
4.
Add PBS to a final volume of 50 mL, close the tube and shake vigorously by hand for 30 s.
-
5.
Centrifuge for 5 min at 400 × g at 22°C–28°C.
-
6.
Decant the top adipose tissue layer into a new 50 mL tube. Try to avoid letting the media flow through into your adipose fraction.
Note: It is recommended that you tilt the tube slowly to an approximately 70 degree angle and use a sterile spoon to help pour the tissue into the new tube.
-
7.
Repeat steps 4–6 until no red pellet can be seen at the bottom of the tube.
Note: These washes ensure removal of all possible red blood cell contaminants from the adipose tissue sample, thus improving adipose cell isolation.
-
8.
Pour the adipose tissue into a new 50 mL tube and add the 1 mg/mL Collagenase 1A enzyme solution in a proportion 1:1 by volume.
-
9.
Seal the tube with Parafilm and place on a vertical tube rotator located inside a 37°C CO2 incubator.
-
10.
Incubate at 37°C for 45 min.
CRITICAL: Incubation time may vary depending on adipose tissue sample characteristics. To achieve the highest yield of viable adipocytes, it is recommended that you check for adequate enzymatic digestion after 30 min and stop incubation when 80%–90% of the tissue is digested. Do not exceed 60 min of incubation.
Note: If Collagenase 1A is supplied by another company, the incubation time may vary.
-
11.
Centrifuge for 5 min at 400 × g at 22°C–28°C.
-
12.After centrifugation the tube should have four layers (Figure 2):
-
a.Clear yellow layer – Lipid released from dead adipocytes.
-
b.Opaque white-yellow layer – mature adipocytes.
-
c.Clear brownish liquid – Enzyme solution.
-
d.Red pellet – Stromal Vascular Fraction (SVF).
-
a.
Note: It is normal to see a portion of undigested adipose tissue between the Adipocyte and Enzyme Solution fractions (Figure 2). The highest yield of viable adipocytes is obtained when the incubation time is enough to disassociate the majority of the adipose tissue without inducing too much damage to the adipocyte population. Adipocyte damage is evidenced by the volume of the clear yellow layer.
-
13.
Discard clear yellow layer using a P1000 pipette.
Note: You might want to cryopreserve it at −80°C for future assays of steroid hormone or lipid content.
-
14.
Carefully decant the opaque layer into a new 50 mL tube, avoiding any flow through of media (go to step 16: Isolation of mature human adipocytes).
Note: If more than one tube was used for the enzymatic digestion of a single breast tissue sample, they should now be combined, allowing a total of 20 mL volume of live adipocytes in each new 50 mL tube.
-
15.
Put the tube containing the SVF, including the enzyme solution, at 4°C during the 1–2 h needed for the isolation of mature human adipocytes (go to step 24: Isolation of hASC).
Figure 1.
Breast Fat Sample before Processing
Figure 2.
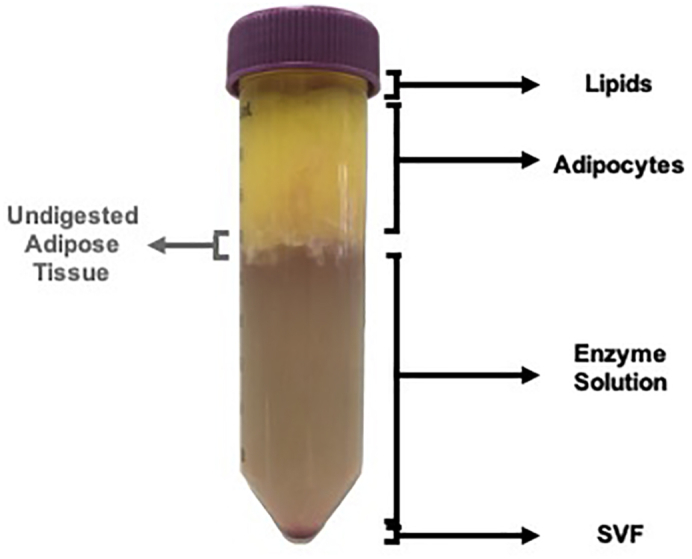
Image of the Four Layers Expected in the Tube after Enzymatic Digestion of Adipose Tissue and Subsequent Centrifugation
Portion of undigested adipose tissue is indicated on the left of the image.
Isolation of Mature Human Adipocytes
Timing: 1–2 h
In this step, mature adipocytes must be filtered and washed for proper isolation.
-
16.
Place a 200 μm cell strainer into a new 50 mL tube (use the same number of tubes as obtained from step 14).
-
17.
Dilute the live adipocytes in PBS to bring the final volume to 40 mL.
-
18.
Gently invert the tube to mix and immediately pipette the suspended adipocytes onto the 200 μm cell strainer.
CRITICAL: Adipocytes are very fragile, so it is recommended that you use a 25 mL pipette and allow the adipocytes to go through the cell strainer by gravity.
-
19.
Repeat step 18 until all of the adipocyte suspension has been filtered.
Note: If the cell strainer becomes blocked, add 3 mL of fresh PBS on the top and resuspend by gently pipetting. Alternately, replace the filter to complete sample filtration.
-
20.
Centrifuge filtered adipocytes for 5 min at 400 × g at 22°C–28°C.
-
21.After centrifugation, the tube should have three layers (Figure 3):
-
a.Clear yellow layer – Lipid released from dead adipocytes.
-
b.Opaque white-yellow layer – mature adipocytes.
-
c.Clear liquid – PBS.
-
a.
CRITICAL: It is normal to see a small pellet in the tube from cell debris. If, however, this pellet is red you should decant the opaque white-yellow layer into a new 50 mL tube as described in step 14, and wash again with PBS (by adding PBS to the 50 mL tube containing the mature adipocytes, to a final volume of 40 mL (as in step 17), and centrifuging for 5 min at 400 × g at 22°C–28°C, as indicated in step 20). This step is included to reduce the risk of hASC contamination during mature adipocyte isolation. After this additional wash, you should observe the three layers detailed in step 21.
-
22.
Discard (or remove and cryopreserve) the clear yellow lipid layer using a P1000 pipette.
-
23.
Carefully place the tip of an 18G needle attached to a 20 mL syringe down into the bottom of the tube and gently aspirate the clear PBS at the bottom of the tube from underneath the floating adipocytes, trying to avoid aspirating any of the floating adipocytes (go to step 36: Breast cancer cell:mature adipocyte co-culture). The remaining middle layer contains your adipocytes.
Note: Adipocytes must be used immediately after isolation.
Figure 3.
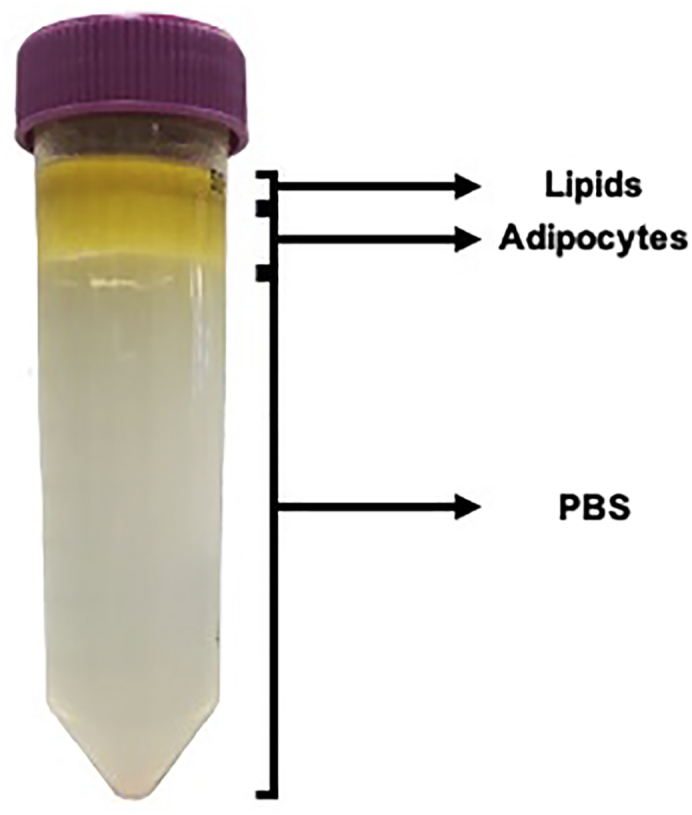
Image of the Three Layers Expected in the Tube after Adipocyte Filtration and Subsequent Centrifugation
Isolation of Human Adipose-Derived Stem Cells (hASCs)
Timing: 1–2 weeks
In this step, the SVF is filtered and washed; and hASC selected by adherent cell culture.
-
24.
Aspirate media from tubes saved previously containing the SVF (step 15).
-
25.
Add 40 mL of PBS and resuspend the pellet.
Note: If more than one tube was used for the enzymatic digestion of a single breast tissue sample, pellets should be collected together in a same tube and then brought to a total volume of 40 mL in PBS (e.g., for four tubes add 10 mL PBS to each tube, resuspend pellets and combine).
-
26.
Place a 70 μm cell strainer into a new 50 mL tube.
-
27.
Filter the cell suspension through the cell strainer.
-
28.
Centrifuge the filtered SVF for 5 min at 400 × g at 22°C–28°C.
-
29.
Discard the supernatant.
CRITICAL: The SVF pellet can easily slip from the tube, so take special care not to lose it in this step.
Pause Point: At this point, SVF can be stored in liquid nitrogen at 106 cells per mL of FBS containing 7% of DMSO, used as freeze media.
-
30.
Resuspend pellet in DMEM media supplemented with 10% FBS and 1% penicillin/streptomycin.
-
31.
For culture, seed cells at a concentration of 300,000 cells/cm2.
CRITICAL: SVF contains different cell populations, so you should only count the big cells to estimate numbers for plating (hASC are at least 2- to 3-fold bigger than contaminating blood cells).
-
32.
Maintain in culture for 2–3 passages, replacing the media every 2 days, to eliminate erythrocytes, white blood cells, and other contaminating SVF cells and obtain a pure hASC primary culture (go to step 46: Breast cancer cell:hASC co-culture).
Note: The identity of your recovered hASC must be phenotypically characterized by flow cytometry and functionally characterized by their ability to differentiate into Osteocytes, Chondrocytes and Adipocytes as in Marchal et al. (2012).
Pause Point: At this point, hASC can be stored in liquid nitrogen at 106 cells/mL of FBS containing 7% of DMSO, used as freeze media.
Differentiation of Immature Adipocytes from hASCs
Timing: 3 weeks
In this step, immature adipocytes are differentiated from isolated hASC using commercially available Adipogenic medium (Lonza #PT-3004).
-
33.
Culture hASC until cells reach 100% confluence.
Note: It is recommended to use P100 dishes for these cultures.
-
34.Induce adipogenesis by three cycles of induction/maintenance as follows.
-
a.3 days of culture with supplemented adipogenesis induction medium.
-
b.1–3 days of culture in supplemented adipogenic maintenance media
-
a.
-
35.
After three complete cycles of induction/maintenance, culture the differentiating adipocytes for 7 more days in adipogenic maintenance medium (go to step 42: Breast cancer cell:immature adipocyte co-culture).
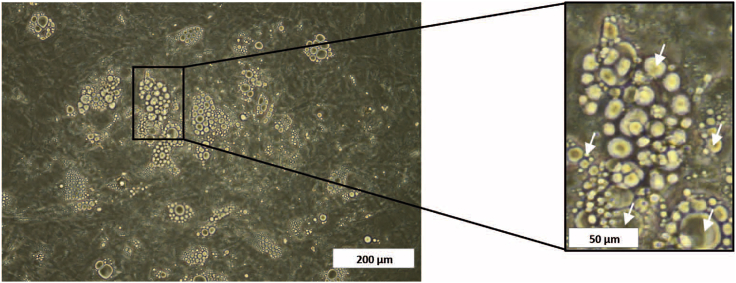
CRITICAL: Adipogenesis is confirmed by visualizing the formation of lipid vacuoles by direct microscopy (Figure 4). Only cultures with a minimum of 70% immature adipocyte confluency should be used for co-culture studies.
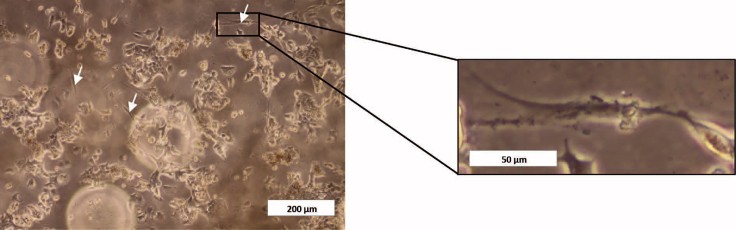
Figure 4.
Optic Microscopy Image of an Immature Adipocyte Culture
White arrows in the amplified section of the picture indicate different size of lipid vacuoles formed. Scale bars, 200 μm (left); 50 μm (right, for the enlarged image).
Note: If another commercially available Adipogenic medium is used for differentiation of immature adipocytes from hASC, this protocol should be replaced by the manufacturer′s protocol.
Breast Cancer Cell:Mature Adipocyte Co-culture
Timing: 7 days
Methodology for breast cancer cell:mature adipocyte co-culture, and subsequent cell population isolation.
-
36.
The day before the planned recovery of mature adipocytes from a fresh human breast fat sample, seed a non-confluent asynchronous culture of the chosen breast cancer cell line onto a 6-well plate, using the appropriate culture media.
Note: For MCF7, seed 50,000 cells/well in IMEM + 5% FBS + 1% penicillin/streptomycin and for MDA-MB-231 lines, seed 50,000 cells/well in DMEM + 10% FBS + 1% penicillin/streptomycin. For other cell lines, estimate the appropriate number of cells to be seeded, taking into consideration that cells will not be passaged during the duration of the co-culture experiment.
-
37.
Resuspend the mature human mammary adipocytes obtained after the isolation protocol (step 23) in the culture media appropriate for the breast cancer cell line that will be used for the co-culture experiment, using 5 mL of media/mL final volume of isolated adipocytes.
-
38.
Remove and discard the culture media from breast cancer cell cultures.
-
39.
Gently invert the tube containing mature adipocytes to mix, and immediately pipette 2 mL of resuspended adipocytes into each well.
Note: For each well to be seeded, step 39 should be repeated in order to use same number of adipocytes in each well, since adipocytes float in the culture media.
-
40.
Add 500 μL of fresh culture media to each well every 2 days.
CRITICAL: Complete change of media is not recommended during these co-culture assays, since the mature adipocytes are suspended in the culture media above the adherent breast cancer cells and removal of the media would lead to adipocyte loss.
-
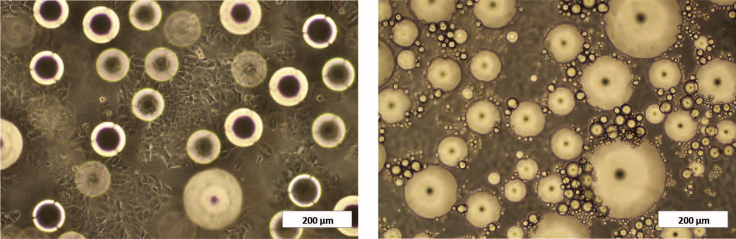
41.At day 7, breast cancer cells and mature adipocytes can be separated easily due to their different attachment to the cell culture surface (Figure 5).
-
a.Mature adipocytes float in the culture and can be collected by recovering the media using a P1000 pipette.Note: Mature adipocytes can be collected for further studies by centrifugating the tube (containing the media and adipocytes recovered from the co-culture) for 5 min at 400 × g at 22°C–28°C, and aspirating the media.
-
b.Breast cancer cells are attached to the cell culture surface and can be isolated by washing off floating adipocytes.
 CRITICAL: Adipocytes can stick to plastic surfaces. It is recommended to perform at least 3–4 washes with PBS to avoid contamination of breast cancer cells with remaining adipocytes in the culture.
CRITICAL: Adipocytes can stick to plastic surfaces. It is recommended to perform at least 3–4 washes with PBS to avoid contamination of breast cancer cells with remaining adipocytes in the culture.
-
a.
Figure 5.
Optic Microscopy Image of a Breast Cancer Cell: Mature Adipocyte Co-culture
Left picture focuses on the MCF7 cells and right picture on mature adipocytes in the co-culture. Scale bar, 200 μm.
Breast Cancer Cell:Immature Adipocyte Co-culture
Timing: 7 days
Methodology for breast cancer cell:immature adipocyte co-culture, and subsequent cell population isolation.
-
42.
Remove the culture media from immature adherent cultures of adipocyte cultures obtained (step 35).
-
43.
Seed breast cancer cells on top of immature adipocytes and add the culture medium appropriate to the breast cancer line.
Note: For MCF7 cells seed 400,000 cells/P100 dish use 7 mL of IMEM + 5% FBS + 1% penicillin/streptomycin and for MDA-MB-231 cells, seed same cell amount but use 7 mL of DMEM + 10% FBS + 1% penicillin/streptomycin.
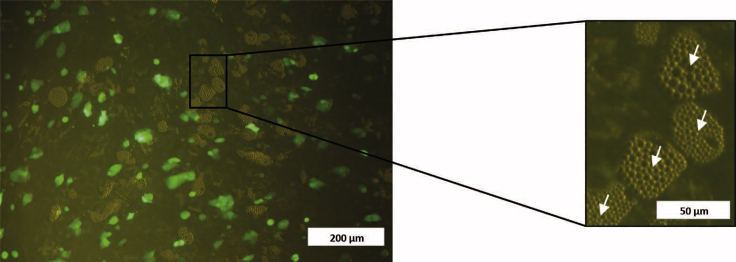
Note: It is highly recommended to use fluorescent-tagged breast cancer cells to facilitate their isolation after co-culture (Figure 6).
Figure 6.
Fluorescent Microscopy Image of a Breast Cancer Cell: Immature Adipocyte Co-culture
In the image can be seen green MCF7 cells and lipid vacuoles of immature adipocytes. White arrows in the amplified section of the picture indicate lipid vacuoles of non-GFP-tagged immature adipocytes. Scale bars, 200 μm (left); 50 μm (right, for the enlarged image).
-
44.
Change culture media every 2 days.
Note: Since both cell populations grow attached, complete change of media is possible during co-culture.
-
45.
At day 7, breast cancer cells and immature adipocytes can be trypsinized, recovered, washed, and separated from each other by FACS, using either anti-cytokeratin as a marker for epithelial cells or fluorescence if the cancer line is GFP-tagged.
Note: We highly recommend the use of fluorescent-tagged breast cancer cells to facilitate their isolation after co-culture. While anti-cytokeratin could serve as a substitute marker, using anti-cytokeratin for FACS will reduce significantly the purity of the populations sorted.
Breast Cancer Cell:hASC Co-culture
Timing: 7 days
Methodology for breast cancer cell:hASC co-culture, and subsequent cell population isolation.
-
46.
Discard culture media from hASC cultures obtained (step 32).
-
47.
Seed breast cancer cells on top of hASC using the culture media appropriate for the breast cancer line.
Note: For MCF7 cells seed 800,000 cells/P150 dish using 18 mL of IMEM + 5% FBS + 1% penicillin/streptomycin and for MDA-MB-231 seed 800,000 cells/P150 dish using 18 mL of DMEM + 10% FBS + 1% penicillin/streptomycin.
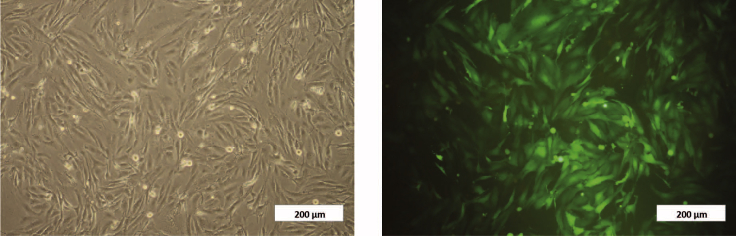
Note: It is highly recommended to use fluorescent-tagged breast cancer cells to facilitate their isolation from hASC after co-culture (Figure 7).
Figure 7.
Optic (Left) and Fluorescent (Right) Microscopy Images of a Breast Cancer Cell: hASC Co-culture
Green cells on the right correspond to MDA-MB-231 cells in the co-culture. Scale bar, 200 μm.
-
48.
Change culture media every 2 days.
Note: Since both cell populations grow as adherent cultures, complete change of media is possible during co-culture.
-
49.
At day 7, breast cancer cells and hASC can be trypsinized, recovered, washed, and separated from each other by FACS, using either anti-cytokeratin as a marker for epithelial cells or fluorescence if the cancer line is GFP-tagged.
Note: We highly recommend the use of fluorescent-tagged breast cancer cells to facilitate their isolation after co-culture. While anti-cytokeratin could serve as a substitute marker, using anti-cytokeratin for FACS will reduce significantly the purity of the populations sorted.
Expected Outcomes
Regarding the anticipated yield of mature human mammary adipose cells using this isolation protocol, approximately 7–10 mL of viable mature adipocytes can be obtained from a 20 mL breast tissue sample. For other breast adipose cell populations, the number of cells obtained for co-culture experiments will depend largely on in vitro expansion of the isolated hASC cultures before use since, unlike adipocytes, these cells can be grown ex vivo.
In regard to breast cancer cell:adipose cell co-culture studies, these experiments are useful to study the effects of breast cancer:adipose cell interaction on inflammation and cancer stem cell enrichment. However, based on our prior work, the effects of co-culture can vary modestly depending on the pre- or post-menopausal state, and the degree of adiposity of the patient donors, the individual breast cancer cell line, and the type and maturity of the adipose cell populations used (Picon-Ruiz et al., 2016; Qureshi et al., 2020).
Generalizability/Application to Other Sources of Human Adipose Tissue
This protocol has been used for adipocyte isolation from human abdominal and breast fat (Picon-Ruiz et al., 2016; Qureshi et al., 2020), and can potentially be adapted for isolation of adipocytes from virtually any source of human fat tissue including pericolic and peritoneal fat.
Limitations
The most important limitation of this protocol is the recovery of mature adipocytes. This adipose cell population is very fragile, has to be used immediately over the course of a week after isolation and cannot be propagated ex vivo.
Another potential limitation is related to the differentiation of immature adipocytes from hASC. Adipogenic differentiation efficiency depends on several factors, and cultures with an immature adipocyte confluency below 70% should be discarded.
Troubleshooting
Problem 1
Low yield of viable mature adipocytes.
Potential Solution
This may be due to different situations:
-
•Inadequate enzymatic digestion (step 10).
-
○If incubation with collagenase solution has been insufficient, adipocyte filtration through 200 μm cell strainer will be difficult. Increase incubation time.
-
○If incubation with collagenase enzyme solution has been excessive, the clear yellow lipid layer will be increased due to adipocyte lysis and the opaque white-yellow layer decreased after centrifugation. Decrease incubation time.
-
○
-
•Inadequate adipocyte filtration (step 19).
-
○If having difficulties with filtering the adipocytes through the various filters in this protocol, add more PBS or replace cell strainer to reduce adipocyte loss.
-
○If, after filtering and centrifugation, the clear yellow layer is increased and opaque white-yellow layer decreased after centrifugation, you may have exerted too much pressure on the cells and caused adipocyte lysis. Be sure that the cell filtration is carried-out by gravity flow.
-
○
Problem 2
Contamination of breast cancer cell:mature adipocyte co-culture by hASC (Figure 8).
Figure 8.
Optic Microscopy Image Showing a Breast Cancer Cell: Mature Adipocyte Co-culture Contaminated by hASC (White Arrows)
Amplified section shows the presence of a single contaminating hASC. Scale bars, 200 μm (left); 50 μm (right, for the enlarged image).
Potential Solution
To avoid contamination of mature adipocyte preps by hASC, it is recommended that you perform an additional wash of mature adipocytes with PBS after (step 21).
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Joyce M. Slingerland (js4915@georgetown.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any unique datasets or code.
Acknowledgments
This work was supported by grants from the Florida Breast Cancer Foundation (J.M.S.), the Breast Cancer Research Foundation (J.M.S.), the NIH (1R01CA210440-01A1; J.M.S.), the Susan G. Komen Foundation (PDF16380958; M.P.-R. and J.M.S.), the European Commission (MSCA-IF-2018 845104; M.P.-R.), the Instituto de Salud Carlos III (PIE16-00045 and DTS19/00145; J.A.M.), and the Spanish Ministry of Science, Innovation and Universities (MICIU) through FEDER (RTI2018-101309-B-C22; J.A.M.).
Author Contributions
M.P.-R. designed the protocol, and J.M.S. and J.A.M. served as mentors to M.P.-R. during the project.
Declaration of Interests
The authors declare no competing interests.
Contributor Information
Manuel Picon-Ruiz, Email: mpicon@ugr.es.
Joyce M. Slingerland, Email: js4915@georgetown.edu.
References
- Picon-Ruiz M., Pan C., Drews-Elger K., Jang K., Besser A.H., Zhao D., Morata-Tarifa C., Kim M., Ince T.A., Azzam D.J. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- Qureshi R., Picon-Ruiz M., Aurrekoetxea-Rodriguez I., Nunes d ePaiva V., D′Amico M., Yoon H., Radhakrishnan R., Morata-Tarifa C., Ince T., Lippman M. The major pre- and post-menopausal estrogens play opposing roles in obesity driven mammary inflammation and breast cancer development. Cell Metab. 2020;31(6):1154–1172.e9. doi: 10.1016/j.cmet.2020.05.008. [DOI] [PubMed] [Google Scholar]
- Marchal J.A., Picón M., Perán M., Bueno C., Jiménez-Navarro M., Carrillo E., Boulaiz H., Rodríguez N., Álvarez P., Menendez P. Purification and long-term expansion of multipotent endothelial-like cells with potential cardiovascular regeneration. Stem Cells Dev. 2012;21(4):562–574. doi: 10.1089/scd.2011.0072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.