Summary
This protocol provides the steps required for a mouse liver orthotopic implantation model. The reliable pre-clinical animal models that have similar characteristics to hepatocellular carcinoma (HCC) are a powerful tool to unveil the mechanisms controlling tumor initiation and progression. Here, we describe a syngeneic orthotopic HCC model that recapitulates the role of a host pro-tumorigenic microenvironment by pre-conditioning mouse livers with a high-fat diet (HFD).
For complete details on the use and execution of this protocol, please refer to Kudo et al. (2020).
Subject Areas: Cell Biology, Cancer, Metabolism, Model Organisms
Graphical Abstract
Highlights
-
•
Liver orthotopic implantation is a useful tool, but consistency is challenging
-
•
Pre-conditioning liver with a high-fat diet improved tumor cell engraftment
-
•
Three injection sites increase the probability of mice harboring tumors
This protocol provides the steps required for a mouse liver orthotopic implantation model. The reliable pre-clinical animal models that have similar characteristics to hepatocellular carcinoma (HCC) are a powerful tool to unveil the mechanisms controlling tumor initiation and progression. Here, we describe a syngeneic orthotopic HCC model that recapitulates the role of a host pro-tumorigenic microenvironment by pre-conditioning mouse livers with a high-fat diet (HFD).
Before You Begin
Note: All mouse experiments must be approved by an Animal Care Committee in your research institution. Personal protective equipment, autoclaved sterile surgical instruments and frequent disinfection of equipment with 70% ethanol are essential for handling mice.
Induction of Fatty Liver by a High-Fat Diet
Timing: ∼8 weeks
-
1.
Acquire High Fat, Fat Calories (60%), Mouse Diet (HFD, Bio-Serv F3282).
-
2.
Mice should be started on HFD at approximately 6–8 weeks-old.
-
3.
Mice will be fed HFD ad libitum for 8 weeks.
CRITICAL: Measuring body weight routinely during the experiment is important. Allocate mice to be equally distributed for each experimental group according to body weight.
CAUTION: Be aware of skin dermatitis. It is important to notice dermatitis as soon as possible and stop HFD if it happens. Autoclaved pieces of absorbant paper can help to prevent skin dermatitis.
-
4.
Eight weeks after starting HFD, proceed to the next step to orthotopically implant HCC cells in the fatty liver. One day before implantation, shave the abdominal region of the mouse.
Preparation for Mouse Surgery
Timing: 30 min
-
5.
All the surgical instruments must be autoclaved and opened on a clean bench.
-
6.
Weigh and prepare the mice for the following procedure.
-
7.
For anesthesia of mice, an isoflurane chamber with nose cone attachment is needed.
-
8.
Prepare a set of autoclaved surgical instruments per mouse as follows: Sharp and blunt scissors; 1 curved fine forceps; 1 Straight blunt forceps: 2 Hartman hemostats.
-
9.
Prepare 1 mL aliquots of Matrigel basement membrane matrix and store at −20°C. Thaw them on ice 2 h before use.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| DPBS | Corning | Cat# 21-031-CV |
| Penicillin-Streptomycin | Corning | Cat# 30-002-CI |
| DMEM | Corning | Cat# 10-017CV |
| Fetal Bovine Serum | Omega Scientific, inc. | Cat# FB-01 |
| L-Glutamine | Corning | Cat# 25-005-CI |
| Trypsin-EDTA (0.25%), phenol red | GIBCO | Cat# 25200056 |
| Matrigel basement membrane matrix | Corning | Cat# 354234 |
| Trypan Blue Solution | Gibco | Cat# 15250061 |
| Experimental Models: Cell Lines | ||
| DihXD3 cells | Moscat lab | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: WT (C57BL/6), (∗) | Moscat lab | N/A |
| Other | ||
| Insulin syringe, 0.3 mL with 31-gauge needle | Becton Dickinson | Cat# 328438 |
| 15- and 50-mL centrifuge tubes | Genesee Scientific | Cat# 28-103 and 28-108 |
| Bright-field microscope | EVOS M5000 | Cat# AMF5000 |
| Caliper | Mitutoyo | Cat# 500-171-30 |
| Scale (capable of measuring mouse weight) | Ohaus | Cat# SP202 |
| Sterile gauze | N/A | N/A |
| Heating pad | N/A | N/A |
| Isoflurane | VETONE | Vet-Rx-MW 502017 |
| Curved fine forceps | FST | Cat# 11159-10 |
| Straight brunt forceps | FST | Cat# 11002-12 |
| Straight scissors | FST | Cat# 14040-10 |
| Hartman Hemostats | FST | Cat# 13002-10 |
| Autoclip applier plus 9-mm autoclips | Kent Scientific | Cat# INS750345; Cat# INS750546 |
| PERMA-HAND SILK 5-0 | VWR | Cat# 95057-064 |
| Germinator 500 | Braintree Scientific, inc. | Cat# GER 5287-120V |
| Surgical microscope | Leica | N/A |
| Wound clip remover | FST | Cat#: 12033-00 |
(∗) Note: This protocol can be used in immunocompromised mice such as NSG mice for liver orthotopic implantation of human HCC cell lines such as SK-HEP-1.
Materials and Equipment
Maintenance Media
| Reagent | Final Concentration | Stock Concentration | Volume (mL) |
|---|---|---|---|
| DMEM | - | - | 440 |
| Glutamine | 200 mM | 2 mM | 5 |
| Penicillin-Streptomycin | 1% | 100% | 5 |
| Fetal Bovine Serum | 10% | 100% | 50 |
Note: Store DMEM at 4°C and all the other components of the media at −20°C.
Step-By-Step Method Details
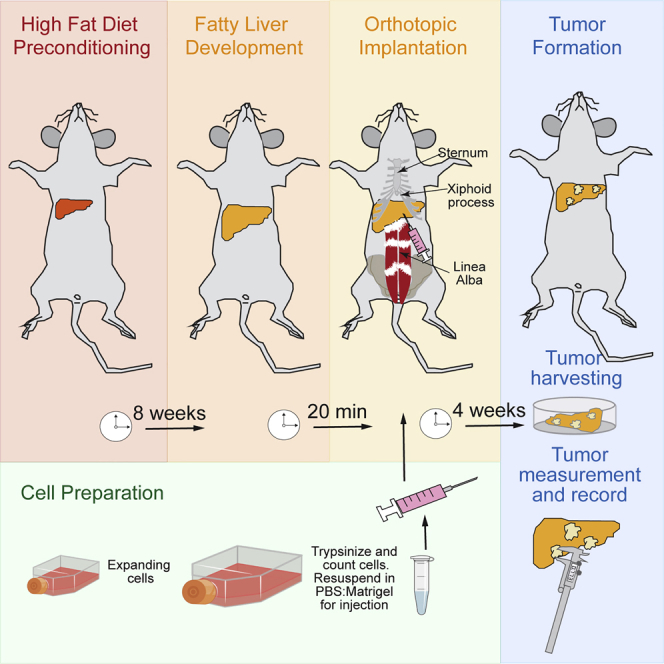
This protocol is aimed at injecting HCC cells orthotopically in preconditioned liver with high-fat diet feeding. Before the injection, the HCC cells are trypsinized, resuspended in serum-free DMEM, and placed in ice until the moment of injection. Following the anesthesia of the mouse, a parallel incision to the linea alba is made in the abdominal wall to expose the liver. Then, HCC cells are mixed 1:1 with Matrigel before injection. The injection is done orthotopically into three different sites: twice in the left-lateral and once in the left-median lobes of the liver. After closing the incision with suture, the skin is closed with wound clips. We show two different methods for closing the peritoneum after the incision by continuous or interrupted suture. Mice are monitored for 2 to 6 weeks after implantation. The main improvement of this protocol is to increase the incidence of tumors thanks to a robust procedure and the injection into three sites in the liver.
Preparation of HCC Cells for Injection
Timing: ∼1 h
Here, the HCC cells are trypsinized and counted before injection.
-
1.
Prewarm DPBS and maintenance media at 37°C and 0.25% trypsin-EDTA at 20°C–22°C before use.
-
2.
Remove the medium from the dish of HCC cells at around 80% confluence.
Note: This protocol uses DihXD3 as HCC cells since they are syngeneic and can be injected and grow in C57BL6 mice, but other HCC cells can also be used.
CRITICAL: Avoid cell passaging for at least 2 days before implantation.
-
3.
Rinse the dish with prewarmed DPBS (6–8 mL per 100 mm dish) and aspirate the DPBS.
-
4.
Add 2 mL of 0.25% trypsin-EDTA to the dish. Place the plates in the 37°C incubator.
-
5.
After 3–5 min, check the cells detaching from the plate by using a bright-field microscope.
CRITICAL: Do not leave the cells in trypsin-EDTA for more than 5 min.
-
6.
When more than 90% of HCC cells are detached, add 6–8 mL per 100 mm dish of maintenance media to neutralize the trypsin in the dish. Gently mix the HCC cell suspension and collect the cells in a 15-mL centrifuge tube.
-
7.
Centrifuge the HCC cell suspension in the 15-mL centrifuge tube for 5 min at 350–400 × g at 20°C–22°C. Wash the cells with 10 mL of PBS. Centrifuge the cells for 5 min at 350–400 × g at 20°C–22°C and resuspend the cells in serum-free DMEM medium.
-
8.
Count the number of HCC cells and adjust the cell concentration to 106 cells per 10 μL in serum-free DMEM (corresponding to the final tumor cell inoculum per injected volume). One injection requires 106 cells in 10 μL. Prepare 10% more volume than the exact volume in the case of the loss during the injection. Keep the cell suspension on wet ice.
Orthotopic Implantation of Syngeneic HCC Cells in Fatty Liver
Timing: ∼20–30 min/mouse
Here, HCC cells are mixed with Matrigel and injected into the liver. HCC cells are mixed 1:1 with Matrigel and kept in ice until the moment of the injection. After anesthetizing the mouse, the liver is exposed, and cells are injected into three sites of the liver: two injections are done into the left-lateral lobe, and the third injection is done into the left-median lobe. Each injection is performed very slowly, and the change of color of the liver is observed during the injection. After injection, the incision and the skin are closed, and mice are set up for recovery.
-
9.
Place the Matrigel solution (10 μL per 1 injection) on wet ice.
-
10.
Place the required materials in the biological safety cabinet to prepare for orthotopic implantation (Figure 1).
-
11.
Anesthetize the mice by using isoflurane inhalation (2%–3% to start the flow of isoflurane).
-
12.
Apply pre-operative analgesia subcutaneously. Either meloxicam SR or buprenorphine can be used, depending on your IACUC protocol.
-
13.
Place the mouse in a supine position on the heating pad to avoid hypothermia under a surgical microscope.
-
14.
Use sterile gauzes with a 10% povidone-iodine solution followed by 70% ethanol to disinfect and clean the shaved abdominal region. Wait until the skin is dry from povidone-iodine and 70% ethanol.
Figure 1.
Surgical Preparation for Orthotopic Implantation
In a disinfected biological safety cabinet, prepare the surgical instruments as follows: (A) Tubes for Matrigel and HCC cell suspension on ice and 0.3-mL 31-gauge needle insulin syringes, (B) gauzes with 10% povidone-iodine solution, (C) gauzes with 70% ethanol, (D) sterilized surgical instruments, (E) autoclip applicator, (F) suture, (G) bead sterilizer, (H) ophthalmic ointment. Missing in the picture but required: an isoflurane chamber and nose cone, and a surgical microscope.
See Methods Video S1.
-
15.
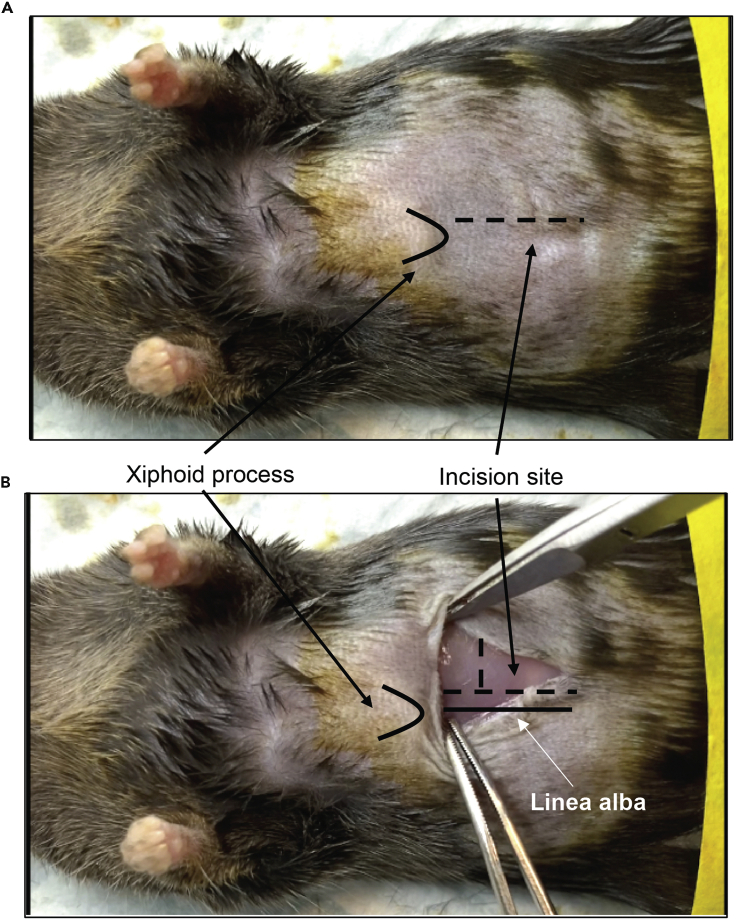
Gently grab the skin with a pair of straight blunt forceps and perform a midline abdominal incision of the skin (starting ∼1 cm under the xiphoid process).
-
16.
Identify the linea alba in the midline of the peritoneum and make a 2.0–2.5 cm incision along the linea alba. To improve the surgical view, perform a lateral incision, perpendicular to the linea alba (see Figure 2).
CRITICAL: Proper incision makes the procedure easier with a good surgical view of the injection site.
Figure 2.
Schematic of Linea Alba and Xiphoid Process in Mouse
(A) Incision in the skin should be parallel to the linea alba. The incision should be 2.0–2.5 cm long.
(B) Incision in the peritoneum should be performed 1 cm below the xiphoid process and along the linea alba. A small lateral incision perpendicular to the linea alba will improve the view.
This video shows how to anesthetize the mouse, apply the analgesia and ophthalmic ointment, and disinfect the abdominal area.
See Methods Video S2.
-
17.
Hold both sides of the peritoneum with two hemostats and flip them to the cranial side to expose the left-medial and/or left-lateral lobe (s) of the liver.
CRITICAL: The investigator should see an enlarged fatty liver.
-
18.
Immediately before orthotopic implantation, 10 μl of HCC cell suspension should be mixed with 10 μL of Matrigel (equal volume to cell suspension). Withdraw the total of 20 μL of Matrigel and the cell suspension with a 0.3-mL insulin syringe attached to a 31-gauge needle. This will ensure a final cell number of 106 cells per 20 μL of the injected volume of the Matrigel/HCC cells suspension.
CRITICAL: Keep the Matrigel-containing solution on ice to prevent gel solidification.
-
19.
Adjust the focus of the surgical microscope on the injection site. Fix the injection site and perform counter-traction by using sterile gauze.
-
20.
Gently insert (approximately 2–3 mm) a 31-gauge needle of 0.3-mL insulin syringe with the Matrigel/HCC cells solution under the liver capsule.
-
21.
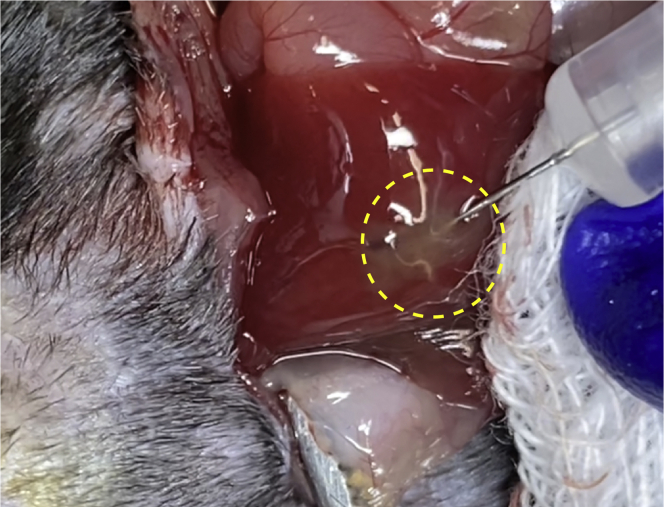
Slowly inject 20 μL of the Matrigel/HCC cells solution (containing 1 × 106 HCC cells) in the subcapsular region of the left-medial and/or left-lateral lobe (s). Successful implantation will change the color of the liver surface to white (see Figure 3).
CRITICAL: Inject the Matrigel/HCC cells solution very slowly as much as possible to avoid leakage and to prevent damage to the liver capsule.
CRITICAL: Maximum 3 injections (2 injections in the left-lateral lobe and 1 injection of the left-median lobe) can be performed.
CRITICAL: This procedure requires surgical skill and practice to obtain reproducible results. In order to get more reproducible results is critical to inject viable cells and this is achieved by a shorter time of the procedure.
-
22.
After injecting the Matrigel/HCC cells solution, keep the needle at least 30 s and slowly retract the needle.
-
23.
Check the liver surface at the needle track for leakage and bleeding.
Figure 3.
Liver Color Change during Cell Injection
During injection, the liver surface will change from dark brown to pale at the injection site.
A correct incision in the abdominal wall results in enough view of the liver to perform the injections of cells.
See Methods Video S3.
-
24.
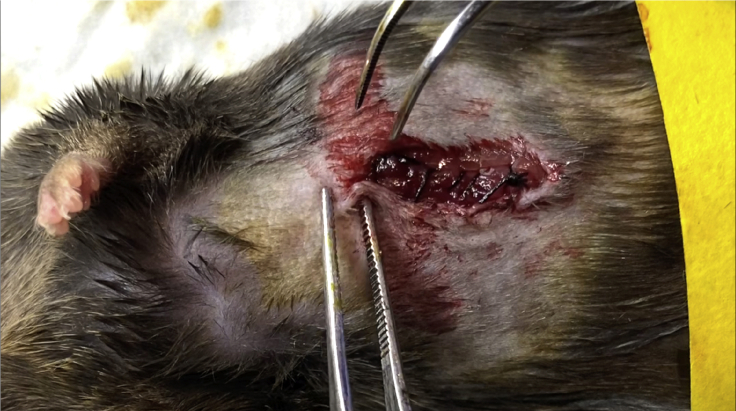
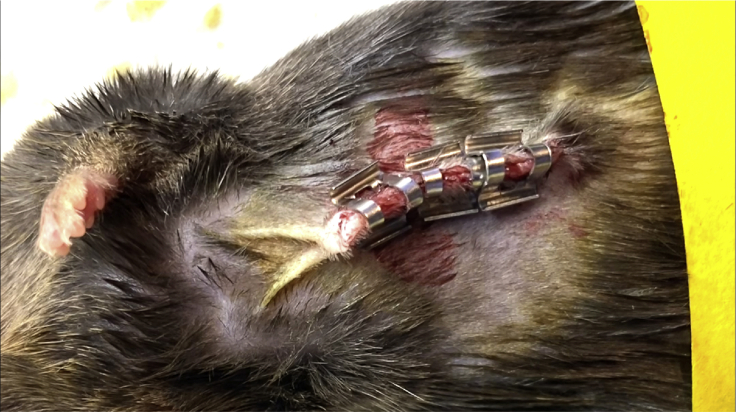
Close the peritoneum of the abdominal wall by a continuous (Figure 4), or interrupted (Figure 5) suture using PERMA-HAND SILK 5-0. Close the skin layer of the abdominal wall by Reflex 9 mm Wound Clips using the autoclip applier (Figure 6).
Figure 4.
Continuous Suture
The continuous suture is one in which a continuous, uninterrupted length of material is used.
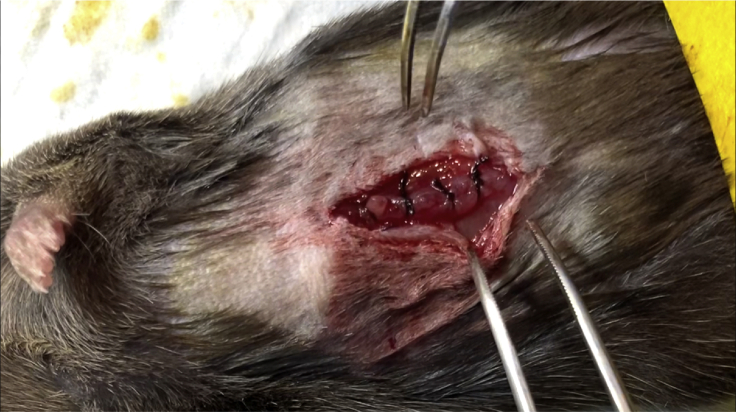
Figure 5.
Interrupted Suture
The interrupted suture is one in which each stitch is made with a separate piece of material.
Figure 6.
Wound Clips
Wound clips can be used for rodent skin closure. They must be applied and removed with a specific applicator.
Three injections are performed in two different liver lobules.
See Methods Video S4 and S5.
-
25.
Leave the mouse on the heating pad and allow the mouse to recover from anesthesia.
-
26.
Apply postoperative analgesia if needed according to the specific animal protocol at your institution.
You can use the continuous suture technique to close the incision.
An alternative to the continuous suture is the interrupted technique.
See Methods Video S6.
Mice need to be monitored after surgery. Mice should be placed on a clean cage and on top of a heat pad.
See Methods Video S7 for a full overview of the surgery procedure.
Monitoring Mouse Condition
Timing: 2–6 weeks
Here, mice are monitored for tumor development.
-
27.
Wound clips must be removed in 10–14 days once the incision has healed.
-
28.
Closely monitor the mice for tumor progression and continue to assess their health until the experiment will be finished. HFD will be continued during the whole experiment.
CRITICAL: If severe weight loss occurs (>20% loss of starting body weight), the mouse should be sacrificed according to your institution’s animal protocol.
-
29.
See the “Quantification and Statistical Analysis” section for more detail into the analysis of the results.
Expected Outcomes
The tumors take 2 to 6 weeks to engraft and grow enough to be analyzed (Figure 7). See Figure 6F-J in our original paper (Kudo et al., 2020). By 6 weeks, if no tumor has formed, it is unlikely that a tumor will form. Our protocol provides an orthotopic model of HCC in which tumor growth is modulated by the fatty liver. Co-implantation with stellate cells also helps to the tumor formation and enables to examine the effect of stellate cells on tumorigenesis and tumor progression. Evaluation of lung metastases can be used to assess the metastatic ability based on both morphology and immunohistochemistry to confirm the expression of typical HCC markers.
Figure 7.
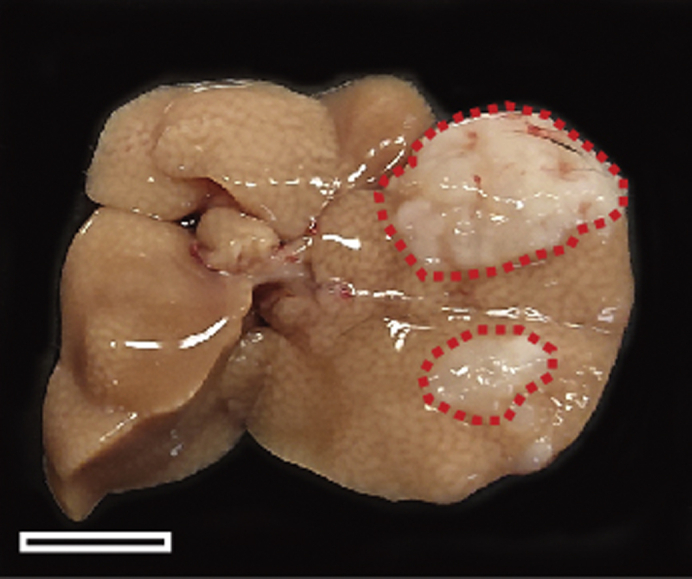
Macroscopic Appearance of an Orthotopic Tumor in the Liver
Successful implantation generates orthotopic liver tumor. Scale bar represents 10 mm.
Quantification and Statistical Analysis
-
1.
We have observed 80%–100% tumor incidence in wild type mice.
-
2.
Create an excel file to record data pre-injection and after tumor harvesting. Data to be recorded should include: sex, date of birth, age, and body weight at the time of liver fatty induction, body weight before surgery, body weight at the endpoint.
-
3.
At the endpoint, the liver weight and diameters (short and long) for each tumor should be recorded. Tumor volume is calculated for each tumor nodule using the following formula: 1/6 × 3.14 × (short diameter)2 × (long diameter).
Limitations
Limitations of liver orthotopic implantation are the long induction time and the difficulty of the surgical procedure. Induction of fatty liver by HFD takes 8 weeks and HCC orthotopic tumor formation requires 2–6 weeks, therefore, a total timeline for an experiment is 2–3 months. Also, the time effort for building surgical expertise is needed. We recommend the use of interrupted suture if there is no previous experience in mouse surgeries. Potential issues for orthotopic tumor implantation are leakage and “accidental” peritoneal metastasis. To avoid these problems, we recommend (1) small-volume Matrigel/HCC cell suspensions (20 μL per injection), (2) mixing Matrigel just before injection to increase the viscosity, and (3) 0.3-mL insulin syringes with 31-gauge needles.
Troubleshooting
Problem 1
Mice show dermatitis as a result of the high-fat diet pre-treatment.
Potential Solution
Place autoclaved pieces of absorbant paper in the cage.
Problem 2
Mice body weight variates widely after HFD.
Potential Solution
Allocate the mice to each group according to their body weight.
Problem 3
Injection makes bubbles in the liver surface.
Potential Solution
Preparing 10 μL aliquots of Matrigel in 1.5 mL Eppendorf tubes helps to resuspend the Matrigel/HCC cells solution without drawing bubbles.
Problem 4
Leakage from the injection site.
Potential Solution
Inject Matrigel/HCC cells suspension as slow as possible and hold for at least 30 s. Reduce the injection volume. Increase the ratio of Matrigel in the Matrigel-HCC cells solution.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jorge Moscat (jom4010@med.cornell.edu).
Materials Availability
DihXD3 cells will be available upon request with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate datasets.
Acknowledgments
This Research was supported by grants by NCI and NIDDK of the National Institutes of Health under awards numbers R01DK108743 and R01CA211794 to J.M., who is the Homer T. Hirst III Professor of Oncology in Pathology. We thank the personnel of the Animal Facility at SBP Medical Discovery Institute for technical assistance.
Author Contributions
Conceptualization, H.K., M.T.D.-M., and J.M.; Investigation, H.K., A.D., T.C.-D., and Y.K.; Writing – Original Draft, H.K. and A.D.; Writing – Review & Editing, H.K., A.D., T.C.-D., Y.K., M.T.D.-M. and J.M.; Funding Acquisition, M.T.D.-M. and J.M.; Supervision, M.T.D.-M. and J.M.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100185.
Contributor Information
Hiroaki Kasashima, Email: m1165063@med.osaka-cu.ac.jp.
Maria T. Diaz-Meco, Email: mtd4001@med.cornell.edu.
Jorge Moscat, Email: jom4010@med.cornell.edu.
References
- Kudo Y., Sugimoto M., Arias E., Kasashima H., Cordes T., Linares J.F., Duran A., Nakanishi Y., Nakanishi N., L'Hermitte A. PKClambda/iota loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression. Cancer Cell. 2020;38:247–262.e11. doi: 10.1016/j.ccell.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows how to anesthetize the mouse, apply the analgesia and ophthalmic ointment, and disinfect the abdominal area.
A correct incision in the abdominal wall results in enough view of the liver to perform the injections of cells.
Three injections are performed in two different liver lobules.
You can use the continuous suture technique to close the incision.
An alternative to the continuous suture is the interrupted technique.
Mice need to be monitored after surgery. Mice should be placed on a clean cage and on top of a heat pad.
Data Availability Statement
This study did not generate datasets.