Abstract
Background: Though the functional states of other endocrine systems are not defined on the basis of levels of controlling hormones, the assessment of thyroid function is based on levels of the controlling hormone thyrotropin (TSH). We, therefore, addressed the question as to whether levels of thyroid hormones [free thyroxine (fT4), total triiodothyronine (TT3)/free triiodothyronine (fT3)], or TSH levels, within and beyond the reference ranges, provide the better guide to the range of clinical parameters associated with thyroid status.
Methods: A PubMed/MEDLINE search of studies up to October 2019, examining associations of levels of thyroid hormones and TSH, taken simultaneously in the same individuals, with clinical parameters was performed. We analyzed atrial fibrillation, other cardiac parameters, osteoporosis and fracture, cancer, dementia, frailty, mortality, features of the metabolic syndrome, and pregnancy outcomes. Studies were assessed for quality by using a modified Newcastle–Ottawa score. Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. A meta-analysis of the associations was performed to determine the relative likelihood of fT4, TT3/fT3, and TSH levels that are associated with the clinical parameters.
Results: We identified 58 suitable articles and a total of 1880 associations. In general, clinical parameters were associated with thyroid hormone levels significantly more often than with TSH levels—the converse was not true for any of the clinical parameters. In the 1880 considered associations, fT4 levels were significantly associated with clinical parameters in 50% of analyses. The respective frequencies for TT3/fT3 and TSH levels were 53% and 23% (p < 0.0001 for both fT4 and TT3/fT3 vs. TSH). The fT4 and TT3/fT3 levels were comparably associated with clinical parameters (p = 0.71). More sophisticated statistical analyses, however, indicated that the associations with TT3/fT3 were not as robust as the associations with fT4.
Conclusions: Thyroid hormones levels, and in particular fT4 levels, seem to have stronger associations with clinical parameters than do TSH levels. Associations of clinical parameters with TSH levels can be explained by the strong negative population correlation between thyroid hormones and TSH. Clinical and research components of thyroidology currently based on the measurement of the thyroid state by reference to TSH levels warrant reconsideration.
Keywords: thyroid hormones, TSH, clinical parameters, correlation, subclinical thyroid dysfunction
Introduction
Thyroid function testing (1,2) and monitoring (3) are based on the measurement of thyrotropin (TSH) levels. Patients are therefore classified as having euthyroidism (normal TSH and thyroid hormone levels), overt thyroid dysfunction (abnormal TSH and thyroid hormone levels), subclinical thyroid dysfunction (abnormal TSH/normal thyroid hormone levels), and isolated hyper/hypothyroxinemia (normal TSH/abnormal thyroid hormone levels).
This classification of thyroid function is based on the concept of TSH levels being the most sensitive indicator of thyroid function such that subclinical thyroid dysfunction as currently defined is believed to be more significant than isolated hyper/hypothyroxinemia (2), as indicated by the alternative term for the latter, “euthyroid hyper/hypothyroidism” (4).
Subclinical thyroid dysfunction is common and comprises most cases of thyroid dysfunction with a population prevalence of ∼5% (5–9), increasing to 15% in older adults (9). Even though it is generally asymptomatic or associated only with non-specific symptoms, subclinical thyroid dysfunction has been associated with many adverse outcomes across a variety of organ systems (5–9). Therefore, despite the lack of convincing evidence of significant benefit (10,11), treatment for subclinical thyroid dysfunction has been recommended in certain circumstances (6–9).
It has previously been suggested by some authors that the earlier definition of subclinical thyroid dysfunction is overly simple and that its diagnosis should not be based solely on the TSH level being outside of a general population range (12,13). Rather, it is claimed that more accuracy may be achieved by defining a normal reference range for the combination of thyroid hormones and TSH.
However, any model whereby judgment of the thyroid status includes consideration of the TSH level is anomalous, in that the levels of other physiological parameters are not judged by the levels of their controlling hormones. For example, whether or not an individual has hypoglycemia or hypercalcemia is not determined by reference to insulin (14) or parathyroid hormone levels (15), respectively. Adrenocorticotropic hormone (ACTH) levels, though helpful in diagnosing adrenal autonomy, are not considered diagnostic for Cushing's syndrome (16). In general, the level of a controlling hormone is used to determine the cause of a disturbance rather than identifying whether or not there is a disturbance (14–16).
We, therefore, aimed at determining whether or not a systematic review of the literature might indicate the relative merits of thyroid hormone levels and TSH levels, in terms of associations with a broad range of clinical parameters. Because of the strong negative population correlation between free thyroxine (fT4) and TSH (17,18), we expected to find associations between both TSH and fT4 levels and the clinical features of thyroid dysfunction. We further reasoned that if the clinical features were associated better with TSH levels, the current rationale for thyroid function testing and the current consequent clinical and research classifications and practices would be supported, but, if the clinical features were associated better with thyroid hormone levels, these classifications and practices would warrant review. In this latter circumstance, the previously noted associations of clinical features with TSH levels could be attributed to the aforementioned strong negative population correlation between fT4 and TSH.
Methods
Search strategy
Up to October 9, 2019, a systematic search was performed of PubMed/MEDLINE by using the following terms: thyroxine (T4), fT4, total triiodothyronine (TT3), free triiodothyronine (fT3), TSH, and subclinical. No restrictions were placed on language, country, or publication date. The resulting literature was first examined to confirm the previously reported general trends of association between clinical parameters and thyroid status.
On account of the results of this first examination of the literature (see Results section), we studied atrial fibrillation (AF) and other cardiac parameters, bone density and fracture, cancer, death, frailty, dementia and associated pathology, obesity, features of the metabolic syndrome, and pregnancy outcomes. We specifically sought studies that addressed the associations between both TSH and thyroid hormone levels, determined simultaneously in the same individuals, with any of the clinical parameters just mentioned.
Study selection and data extraction
Initially, the titles of the articles were screened for relevance and then the abstracts, with full-text reports of potentially relevant reports were reviewed. Additional relevant articles were searched for in the reference lists of the retrieved full-text studies. If repeated study was made of the same cohort, only the latest was included. The literature search data extraction, identification of additional relevant articles, and critical appraisal were conducted independently by two of the authors (S.P.F. and H.F.), and any discrepancies were resolved by consensus with reference to the criteria described in the next section. Should consensus regarding any article not have been achieved, the default position was that the article would be included. No study that contradicted the results of our work was knowingly excluded.
Studies reporting on associations of levels of fT4, TT3/fT3, and TSH with clinical features related to thyroid dysfunction were included. We included both TT3 and fT3, as there were relatively few studies of fT3. We also included analyses comparing associations with subclinical hypothyroidism and euthyroid hypothyroxinemia, reasoning that this is a comparison of low thyroid function defined on the basis of TSH levels or thyroid hormone levels, respectively. Reports were excluded if the studied population was <100 individuals. Review articles, editorials, meta-analyses, and meeting abstracts were also excluded.
The following information was extracted from each such study: first author, country, number of individuals, sex, age intervals, nature of the study, and the relevant clinical parameter. As there were many subtle different parameters examined, we also grouped the parameters into eight major phenotypes or systems: “cardiac,” bone,” “dementia,” “cancer,” “mortality,” “frailty,” “metabolic,” and “pregnancy.” We recorded any associations with thyroid hormones and/or TSH, in addition to the statistical techniques and degrees of significance of any associations (p-values and/or confidence limits). We also recorded the presence of “incongruent” associations, that is, associations in the opposite direction to that normally expected (e.g., obesity having associations with high thyroid function), or associations of thyroid hormones in the same direction as associations with TSH, as indicators of reverse causation (Supplementary Table S1) (19).
As our study was not directed at a collection of works addressing therapeutic outcomes of an intervention, the use of a quality assessment (the Newcastle–Ottawa scale) was adjusted to suit this setting. Principally, this adjustment consisted of allowing for continuous, as well as binary quantifications, of clinical outcomes and exposure to thyroid hormone levels. Articles were scored according to the representativeness of the subjects, the similarity of the subjects apart from differences in the parameter of interest, the reliability of the classification of thyroid status and parameter status, control for confounding factors, and for prospective studies, the demonstration that outcome was not present at study onset, the adequacy of length and completeness of follow-up. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed (20).
Statistical analysis
To determine whether thyroid hormone levels or TSH levels were associated better with the examined clinical parameters, we analyzed the earlier studies as to the relative frequencies of significant associations of thyroid hormone and TSH levels with the clinical parameters. We then performed further analyses to confirm that these findings did not result from any systematic bias.
We classified each result in a study as showing a significant result or a non-significant result. By a significant result, we mean that a given thyroid test has been shown to be associated with a given condition at a 5% significance level. We treated the result as a binary response variable with the levels of success (significant) and failure (non-significant). We combined TT3 and fT3 as TT3/fT3.
The predictors considered were the type of thyroid test (i.e., TSH, fT4, or TT3/fT3), the clinical system under consideration; the number of subjects in the analysis; and the number of covariates in the model. To account for the repeated analysis within each study, we also incorporated a random intercept term. We considered random intercepts for the study, the cohorts nested within each study, the type of analysis nested within the study, and the complexity of the models nested within the studies.
Pairwise comparisons of the thyroid tests were performed at a 5% overall significance level for those models where a significant effect of the predictor, type of thyroid test, was found. We calculated the Tukey pairwise comparisons between the thyroid tests by using the multcomp package (21). We conducted a McNemar analysis on the contingency tables for each comparative pair of thyroid tests. We tested the null hypothesis that there was no change in the proportion of significant results between the two thyroid tests under consideration. As a final attempt to account for dependency within each study, we performed a simple logistic regression analysis by using only a single randomly chosen analysis from the series of nested models in each study. We performed this for each of the following strata:
smallest number of subjects, simple model;
smallest number of subjects, complex model;
largest number of subjects, simple model; and
largest number of subjects, complex model.
We performed a sensitivity study minimizing the contribution of possible reverse causation, analyzing only the prospective analyses from studies that were free of incongruent associations.
All modeling was performed by using the lme4 (22) and lmerTest (23) packages in R (24), and all codes are available at https://github.com/jonotuke/TSH_2019.
Results
We found, in our first examination of the literature, that though the findings were not unanimous, there was general consistency of the data. In general, consistent with prior work (8), AF (25–31), osteoporosis (32–39), and cancer (40–43) were associated with higher thyroid function that was defined by using TSH and/or thyroid hormone levels, across and beyond the reference range, and steatohepatitis (44–46) and other features of the metabolic syndrome (19,47–66) were associated with lower thyroid function. Both high and low thyroid function, as compared with mid-range thyroid function, were associated with clinical and pathological features of cognitive decline (26,67–75), frailty (76–79), total/cardiovascular mortality (26,80–88), cardiac physiology (89), cardiac disease (apart from AF) (26,31,67,83–85,88,90,91), and pregnancy outcomes (92–99).
There were many series finding these associations in the context of subclinical thyroid dysfunction. Many of these studies (25,50,51,67,83–85,87–89), however, did not address the relative associations of clinical parameters with TSH and thyroid hormone levels, the focus of our study.
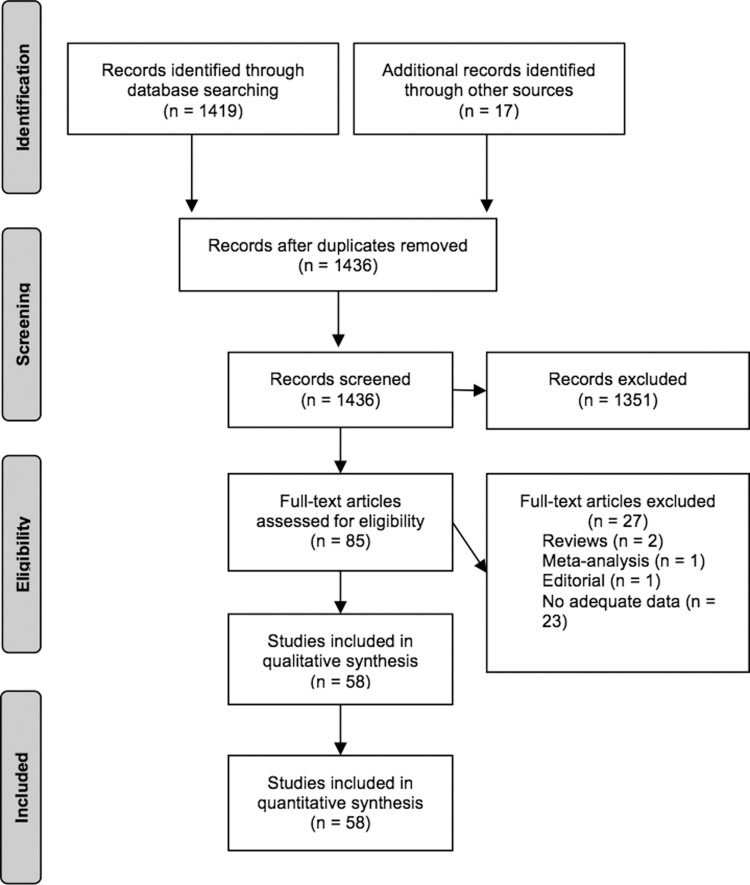
In the end, we identified 58 studies that addressed this question (Fig. 1; Table 1). We found no previous synthesis of the data on the effect of thyroid function, as measured by TSH in comparison to thyroid hormone levels, across a range of organ systems. One meta-analysis restricted to AF (27) was not included in our analysis. Many of the studies addressed multiple parameters summarized by those indicated in Table 1.
FIG. 1.
Description of literature search.
Table 1.
Description and Quality Assessment of Included Studies
| Study | Parameter | Cohort study | Population | N (% female) | Age | NOS |
|---|---|---|---|---|---|---|
| Gammage et al. (28) | AF | Cross-section | U.K. community | 5860 (51) | 72 (65–98) | 9/9 |
| Cappola et al. (26) | Multiple parameters | Prospective | U.S. community | 2843 (56) | 75 ± 5 | 9/9 |
| Heeringa et al. (29) | AF | Cross-section | Netherlands community | 1455 (59) | 68 ± 8 | 9/9 |
| Chaker et al. (30) | AF | Prospective | Netherlands community | 9166 (57) | 65 ± 9.9 | 9/9 |
| Chaker et al. (86) | Sudden cardiac death | Prospective | Netherlands community age ≥45 years | 10,318 (57) | 65 ± 10 | 9/9 |
| Kannan et al. (31) | Heart failure, cardiac outcomes | Cross-section | U.S. heart failure cohort | 1365 (35) | 56.6 ± 14.5 | 9/9 |
| Peixoto de Miranda et al. (91) | Coronary artery disease | Cross-section | Brazil civil servants | 767 (49.3) | 58 | 9/9 |
| Roef et al. (89) | Heart physiology | Cross-section | Belgium community | 2078 (49) | M 46 (41–51) | 9/9 |
| F 45 (40–50) | ||||||
| van de Ven et al. (81) | Mortality | Prospective | Netherlands community | 5816 (53) | 56 ± 18 | 9/9 |
| Inoue et al. (82) | Mortality | Prospective | U.S. community | 5257 (not stated) | 46 ± 17 | 8/9 |
| Yeap et al. (80) | Mortality | Prospective | Australian community men | 3885 (0) | 77 ± 3 | 9/9 |
| Chan et al. (40) | Cancer | Prospective | Australian community | 3649 (56) | 51 ± 15 | 8/9 |
| Tosovic et al. (41) | Cancer | Prospective | Sweden community, women born during 1932–1950 | 17,035 (100) | 56.6 ± 7.1* | 9/9 |
| Khan et al. (42) | Cancer | Prospective | Netherlands community | 10,318 (57) | 61 (57–68) | 8/9 |
| Kuijpens et al. (43) | Breast cancer | Cross-section and prospective | Netherlands community | 2775 (100) | 47–54 | 8/9 |
| van den Beld et al. (78) | Frailty | Prospective + cross-section | Netherlands community men age ≥73 years | 403 (0) | 78 (73–94) | 9/9 |
| Yeap et al. (76) | Frailty | Cross-section | Australia community men | 3943 (0) | 75 ± 4 | 8/9 |
| Bano et al. (77) | Frailty | Prospective | Netherlands community | 9419 (57) | 65 ± 10 | 9/9 |
| n = 9419; age 65 ± 10; female 57% | ||||||
| Gussekloo et al. (79) | Frailty | Prospective | Netherlands community; all age 85 | 558 (66) | 85 | 8/9 |
| Volpato et al. (69) | Dementia | Prospective | U.S. community women age ≥65 years | 464 (100) | 77 ± 0.6 | 8/9 |
| de Jong et al. (71) | Dementia | Cross-section | Netherlands community | 489 (48) | 73 ± 8 | 9/9 |
| Chaker et al. (74) | Dementia | Prospective | Netherlands community | 9446 (43.3) | 64.9 | 9/9 |
| Itterman et al. (75) | Dementia | Cross-section | Germany community | 2557 (55) | 51 ± 10* | 9/9 |
| Yeap et al. (73) | Dementia | Prospective | Australia community men | 3401 (0) | 79.2 ± 3.5* | 8/9 |
| Roef et al. (34) | Osteoporosis | Cross-section | Belgian community, men age 25–45 years | 677 (0) | 34 ± 6 | 9/9 |
| van der Deure et al. (36) | Osteoporosis | Cross-section | Netherlands community age ≥55 years | 1151 (58) | 69 ± 8 | 9/9 |
| Murphy et al. (35) | Osteoporosis | Cross-section | European post-menopausal women | 1278 (100) | 68 ± 7 | 7/9 |
| van Rijn et al. (33) | Osteoporosis | Cross-section | Netherlands post-menopausal women | 1477 (100) | 50 ± 2 | 9/9 |
| Lambrinoudaki et al. (39) | Vertebral fracture | Cross-section | Greece menopause clinic | 335 (100) | 56 ± 7.1* | 9/9 |
| Siru et al. (38) | Hip fracture, bone turnover | Prospective | Australian community men | 338 (0) | 76.7 ± 3.5 | 9/9 |
| Waring et al. (37) | Bone loss, fracture | Prospective | U.S. community men | 1602 (0) | 73.6 ± 5.9* | 9/9 |
| Proces et al. (60) | Obesity | Cross-section | Belgium hospital outpatients | 125 (44) | 57 (13–89) | 7/9 |
| Wolide et al. (61) | Obesity | |||||
| Makepeace et al. (53) | Obesity/Met S | Cross-section | Australian community | 1853 (47) | 49 ± 17 | 9/9 |
| n = 1853; age 49 ± 17; female 47% | ||||||
| Mehran et al. (47) | Obesity/Met S | Prospective | Iran community | 2393 (61) | 38 ± 13 | 9/9 |
| Shon et al. (52) | Obesity/Met S | Cross-section | Korea women medical centre primary health screening | 1572 (100) | 46 ± 11 | 9/9 |
| Roos et al. (48) | Obesity/Met S | Cross-section | Netherlands community | 1581 (46) | 48 ± 12 | 9/9 |
| Jun et al. (55) | Obesity/Met S | Cross-section | Korea medical centre attendees | 6235 (42) | 50 ± 8 | 9/9 |
| Xu et al. (45) | Obesity/Met S | Cross-section | China community | 878 (37) | 72 ± 4 | 9/9 |
| Bano et al. (46) | Obesity/Met S | Prospective | Netherlands community | 9640 (57) | 65 ± 10 | 9/9 |
| Ittermann et al. (44) | Obesity/Met S | Cross-section | Germany community | 3661 (48) | M 51 ± 16 | 9/9 |
| F 48 ± 16 | ||||||
| Knudsen et al. (49) | Obesity/Met S | Cross section | Denmark community | 4082 (“preponderance”) | 18–65 | 9/9 |
| Oh et al. (56) | Obesity/Met S | Cross-section | Korea community | 4275 (50) | 49 | 9/9 |
| Garduño-Garcia et al. (49) | Obesity/Met S | Cross-section | Mexico community | 3033 (51) | 42 ± 10 | 9/9 |
| Temizkan et al. (62) | Met S | Cross-section | Turkey obesity clinic | 1275 (83) | 38 ± 11 | 9/9 |
| Jain et al. (63) | Lipids | Cross-section | U.S. population | 3862 (not stated) | Not stated | 9/9 |
| Boekholdt et al. (64) | Met S | Cross-section | U.K. community | 11,554 (54) | 58 ± 9 | 9/9 |
| Udenze et al. (65) | Met S | Cross-section | Nigeria university staff | 150 (54) | 46.3 ± 8.1 | 8/9 |
| Elgazar et al. (66) | Diabetes | Cross-section | Egypt hospital outpatients | 400 (61) | 54 ± 4.9* | 7/9 |
| Chaker et al. (54) | Diabetes | Prospective | Netherlands community | 8542 (58) | 65 ± 10 | 9/9 |
| Vrijkotte et al. (92) | Foetal growth | Prospective | Netherlands community | 3988 (100) | 31 ± 4.8 | 9/9 |
| Korevar et al. (93) | Premature delivery | Prospective | Netherlands community | 5971 (100) | 29.7 ± 5.0 | 9/9 |
| Medici et al. (94) | Hypertensive pregnancy | Prospective | Netherlands community | 5153 (100) | 29.7 ± 5.1 | 9/9 |
| Cleary-Goldman et al. (95) | Pregnancy outcome | Prospective | U.S. community | 10,990 (100) | 29.6 ± 5.6 | 9/9 |
| Breathnach et al. (96) | Placental abruption | Prospective | Ireland community | 904 (100) | 26 ± 6* | 9/9 |
| Ashoor et al. (97) | Foetal death | Retrospective | England hospital | 3794 (100) | 32.2 (28–36) | 8/9 |
| Knight et al. (98) | Pregnancy-metabolic parameters | Cross-section | England community | 741 (100) | 30.1 ± 5.1 | 9/9 |
| Li et al. (99) | Pregnancy-childhood development | Prospective | China clinic | 1268 (100) | 28 ± 2 | 9/9 |
Age of largest subset of population.
F, female; M, male; Met S, metabolic syndrome; NOS, adapted Newcastle–Ottawa quality assessment scale (the higher number out of 9, the better the study).
We found 22 studies (19,26,30,31,34,35,39,41,44,45,48,55,60–62,65,66,71,75,78,79,91) that examined associations with fT4, TT3/fT3, and TSH and a further 36 studies (26,29,33,36–38,40,42,43,45,47,49,52–54,56,63,64,69,73,74,76,77,80–82,86,90,92–99) that examined associations with only fT4 and TSH levels.
These 58 studies included cross-sectional and prospective cohort studies, diverse populations, and both sexes. They were contemporary and of high quality (Table 1). The study populations comprised strictly euthyroid subjects (26,29,30,34,39,45,48,52–55,62,69,81,82,86,90,91), subjects either euthyroid or with subclinical thyroid dysfunction (19,33,35,36,38,40,42,47,49,60,65,71,73,75–78,80,92,93,95,96,98,99), and subjects euthyroid or with subclinical/overt thyroid dysfunction (28,31,37,41,43,44,46,47,56,61,63,64,66,74,79,94). In some studies, different subsets were examined separately. The 58 articles included in our meta-analysis yielded 1880 results of associations analysis. The supplement catalogues all of these associations in terms of clinical parameters, subgroups, number of participants, statistical methods, statistical significance, and p-values/confidence limits.
The number of subjects for each analysis ranged from 18 to 10,990 with a mean of 3071 (median 2078). The number of results in each study ranged from 3 (60) to 180 (92).
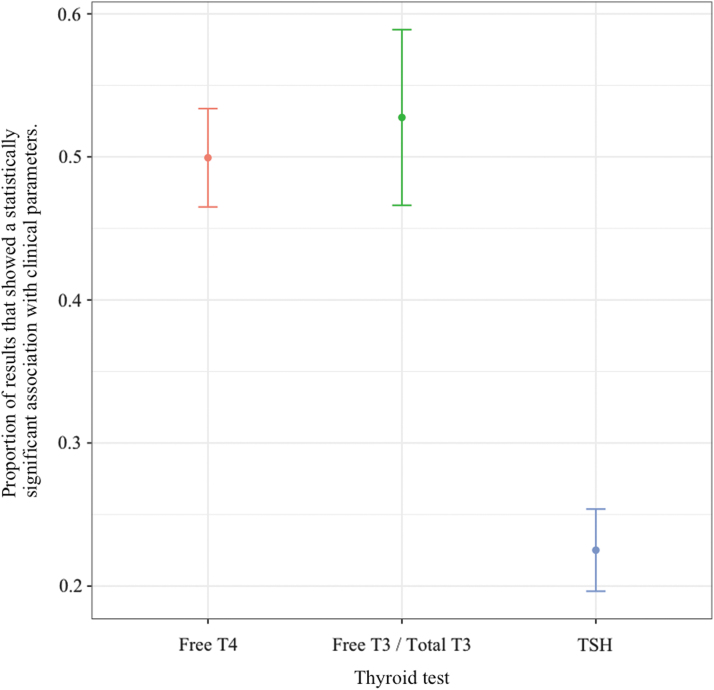
Analysis of all these data confirmed the superiority of associations with thyroid hormone levels (fT4, TT3/fT3) as compared with TSH levels (Fig. 2). fT4 had a significant association with a clinical parameter in 50% of the analyses of the articles. TT3/fT3 had a significant association in 53% of the analyses, whereas TSH had a significant association in only 23%. fT4 levels were associated with clinical parameters and were statistically significantly more often than with TSH levels, (p < 0.0001), as did TT3/fT3, (p < 0.0001). The difference between fT4 and TT3/fT3 levels was not significant (p = 0.71).
FIG. 2.
Overall associations of thyroid hormone and TSH levels with clinical parameters. T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin.
When there was a significant association with fT4, the association with TSH was simultaneously significant 30% of the time; for the converse, the frequency was 66%. For TT3/fT3, the respective figures were similar at 33% and 62%. The McNemar analysis demonstrated these results to be significant; for the comparisons of fT4 versus TSH and TT3/fT3 versus TSH, the null hypothesis was rejected (p < 0.0001). For the comparison of fT4 versus TT3/fT3, we failed to reject the null hypothesis (p < 0.4305).
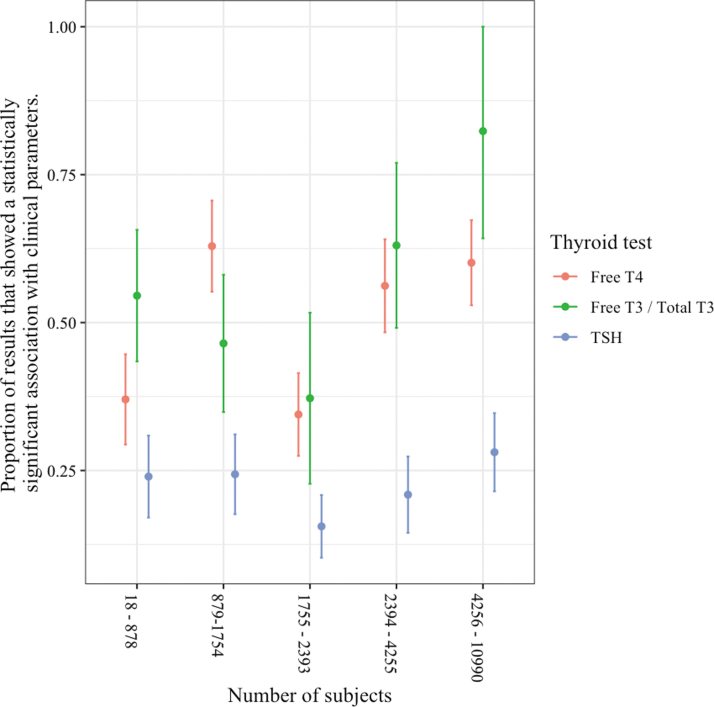
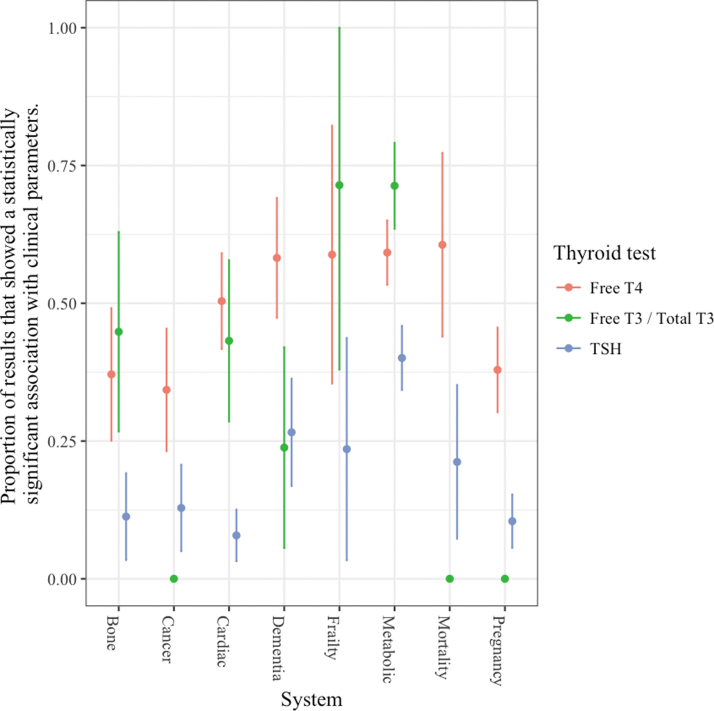
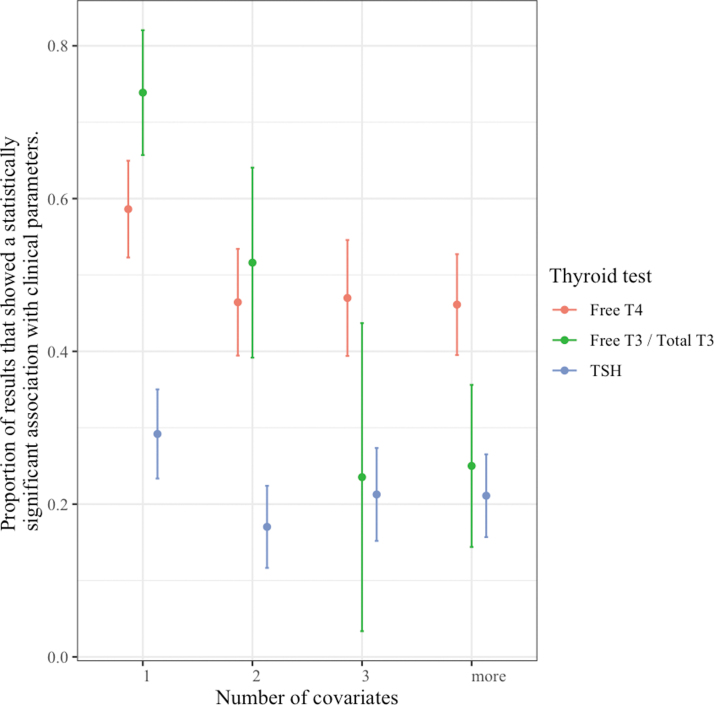
As the number of subjects in the analysis increased, the superior associations with thyroid hormones did not diminish (Fig. 3), and similarly the system did not play a significant role (Fig. 4). In an analysis including the number of covariates in the original result, at higher numbers of covariates, the association of clinical parameters with TT3/fT3 levels was no longer significantly different from the association with TSH levels (Fig. 5).
FIG. 3.
Associations of thyroid hormone and TSH levels with clinical parameters according to sample size.
FIG. 4.
Associations of thyroid hormone and TSH levels with clinical parameters according to clinical system.
FIG. 5.
Associations of thyroid hormone and TSH levels with clinical parameters according to number of covariates.
The basic analysis provided earlier ignores the many sources of dependence between the results reported in each study. To account for this, it was necessary to incorporate a random intercept for study in the model (p = 2.2 × 10−16). There was still then a statistically significant effect of thyroid test in predicting the significance of results (p < 2.2 × 10−16). Post hoc pairwise comparisons show that there was a statistically higher proportion of significant results for fT4 compared with TSH (p < 1 × 10−5), and also a statistically higher proportion of significant results for TT3/fT3 compared with TSH (p < 1 × 10−5). These results confirmed those illustrated in the earlier mentioned confidence interval plots. We found that the additional main effects of system, cohort size, and number of covariates again did not improve the predictive effect of the model, compared with one with just a thyroid test (based on minimizing the Bayesian Information Criterion). We found that a nested random effects structure of cohort within study was a statistically valid addition to the model, but it did not change the observed effects of the thyroid test on what has been cited earlier.
We found, when addressing the issue of dependence of results, a statistically significant effect of thyroid test on the proportion of statistically significant results. Pairwise comparisons revealed that the only significant results in all four models were for fT4 having more significant associations than TSH (Table 2). In this analysis, TT3/fT3 levels did not have more associations with clinical parameters than levels of TSH.
Table 2.
Model Description
| Thyroid test | fT4 vs. TSH | |
|---|---|---|
| Smallest number of subjects, simple model | p = 0.0001891 | p = 0.000191 |
| Smallest number of subjects, complex model | p = 0.000773 | p = 0.000658 |
| Largest number of subjects, simple model | p = 0.01126 | p = 0.00931 |
| Largest number of subjects, complex model | p = 0.0006587 | p = 0.000696 |
fT4, free thyroxine; TSH, thyrotropin.
The results of our sensitivity analysis aimed at minimizing any effect of reverse causation showed no significant change in the proportion of fT4 and TSH levels being associated with clinical parameters, and hence in the statistical conclusions. However, the association of TT3/fT3 levels with clinical parameters was not significantly different than with TSH levels. The proportion of associations with TT3/fT3 was only 13% in this analysis as compared with 53% in the full analysis.
There was no significant change to any of our results regarding the TT3/fT3 combination with consideration of TT3 and fT3 separately.
Only a few of the studies included patients on T4 therapy. In these studies, the proportion of patients on T4 was very low such that separate analyses of these patients were not undertaken. Analyses of cohorts with removal of these patients did not affect the results.
Discussion
We believe this is the first systematic review studying TSH and thyroid hormone associations with various clinical parameters. The results indicate that, contrary to the current paradigm, thyroid hormone levels are associated more strongly with clinical parameters than TSH levels. Any relationship of clinical parameters with TSH levels can be explained by the strong population relationship between thyroid hormone levels and TSH levels, such that TSH levels are merely indirect measures of thyroid hormone levels.
In our sample, we found no indication of, or reference to, any work that suggested that TSH levels consistently indicate thyroid status of any organ or tissue more strongly than thyroid hormone levels.
As our goal was not to estimate the effect size for one treatment, our meta-analysis methodology differed from some other meta-analysis methodologies in that we did not use a weighted technique or pool all original patient data. In addition, it would not have been appropriate to combine all of these factors by using such meta-analysis methodology, as our analysis encompassed multiple studies covering various clinical outcomes, using different methodologies, different assays, and statistical methods.
Theoretically, one could use such other methodology to do a meta-analysis of each clinical parameter, but these individual meta-analyses would still need to be combined by using a method akin to ours (i.e., summing the meta-analyses in some way) to determine whether levels of thyroid hormones or TSH are more likely to be associated in general with clinical parameters. Further, in using such a technique of analysis, the information from many of the studies in our sample would be lost as the parameter/population/statistical method might not be amenable to pooling (27).
The results of the individual patient meta-analysis of AF (27) do, in fact, support our conclusions, showing superior associations with fT4 levels than with TSH levels. Also supportive is a recently published similar meta-analysis of pre-term delivery (100), showing that fT4 levels are associated with the clinical parameter at least as well as TSH levels. One could even argue that the results of these two conventional meta-analyses alone disprove the general hypothesis that TSH levels provide a better guide to thyroid status than fT4 levels.
Potentially, the summation of statistically significant results can be unreliable (20], but we have accounted for the possibilities of bias on account of imbalance in the size of the studies, the nature of the parameters, and the possibility of reverse causation. In all of the studies (except for the few studies comparing individuals with subclinical hypothyroidism with individuals with isolated hypothyroxinemia), each subject was his/her own control, and the study populations of many of the studies were unselected members of a community, so the risk of bias from these considerations was obviated. The convincing degree of superiority of thyroid hormone levels as compared with TSH levels also provides a buffer against the possibility of some unidentified bias influencing our results.
The strictest interpretation of our data would, nevertheless, qualify our conclusions such that they would be valid only to the degree that the chosen parameters truly reflect the thyroid state. Residual confounding, by mechanisms as yet incompletely understood, may have affected the reported associations between thyroid function tests and all of the clinical parameters. This possibility, not considered to be likely in the studies we reviewed, would also compromise the previous literature describing the considered consequences of sub-clinical thyroid dysfunction.
However, even in these circumstances, a slightly weaker conclusion for our study—that is, that there is no evidence supporting the superiority of TSH levels in the assessment of thyroid function—would still stand. Further, there is much evidence to indicate that at least some of the chosen parameters do truly reflect the thyroid state.
In particular, although there are clinical parameters that can affect thyroid function, we do not believe that such reverse causation significantly influence our results. Reverse causation mechanisms have been described for parameters associated with low thyroid function (e.g., obesity (101–103) and dyslipidemia (57,58,104)), but in these circumstances the reverse causation effects would tend to lead to greater associations with TSH levels rather than with fT4 levels.
The sensitivity of TT3/fT3 levels to the sick euthyroid state (105), generated by altered deiodinase activity (106), may also explain some of the associations with TT3/fT3. In particular, mortality and frailty may be associated with low TT3/fT3 levels via reverse causation. As the TSH would also be expected to be low in this situation, one might expect incongruent associations between clinical parameters, and TT3/fT3 (and possibly fT4) and TSH. Our sensitivity study excluded such studies.
We are not aware of any association of a clinical parameter with a high fT4 having been linked to reverse causation. If anything, any component of the sick euthyroid state associated with these conditions, by lowering TSH and fT4 (105), should again favor an association with TSH rather than fT4.
Mendelian randomization studies have provided evidence that the relationship between thyroid function and AF is causal (107,108), whereas there may be reverse causation underlying the relationship between thyroid function and obesity (109). Other indicators supporting a causative relationship between thyroid function and at least some of the parameters we examined include the relationships being seen in otherwise healthy individuals (91–99), the prospective nature of many of our included studies, our sensitivity study, the observed similarity of the relationships to those seen in overt thyroid disease (110–120), basic science evidence (121,122), and positive animal (123) and human (19) intervention studies.
Nevertheless, additional intervention studies could provide further evidence as to the direction of causality in the associations we have studied. Ideally, such intervention studies would be designed to ensure that the intervention, rather than merely normalizing TSH levels, significantly changes the levels of thyroid hormones.
We found TT3/fT3 level associations with fewer parameters than we found for fT4. Although TT3/fT3 levels were associated more strongly than TSH levels, and equally strongly as fT4 levels, with clinical parameters, our sensitivity study showed a fall in the frequency of TT3/fT3 associations, suggesting a component of reverse causation.
Two other analyses, the analysis of associations according to the number of covariates and the sampling analysis, also indicated that the associations of clinical parameters with TT3/fT3 may not be as robust as the associations with fT4 levels. Overall, TT3/fT3 measurement added little to the assessment based on fT4 levels. Future studies may further clarify the relative importance of fT4 and fT3 levels.
fT4 is not the active thyroid hormone at the cellular nuclear level (106). The strong relationships of parameters, especially AF (risk increased up to 9 × across the normal reference range (30)), with levels of fT4 indicate that the active intracellular triiodothyronine generated by thyroid hormone transporters and deiodinases (106) appears to be, at least in the heart, proportional to circulating fT4. Any discrepancy, indicating local regulation of thyroid effect, may be more prominent in more severe pathophysiological circumstances (106), and therefore more relevant in the circumstances of multisystem entities such as frailty, death, and metabolic disturbance.
Our results do not imply that no information can be gleaned from the presence of an abnormal TSH level. In the presence of normal thyroid hormone levels, such TSH levels indicate that the thyroid gland physiology is abnormal. However, for the function of other tissues and organs, the TSH level required to maintain a given level of thyroid hormones appears generally not to be relevant.
It remains possible too that additional analyses might find that TSH levels are providing an additional signal to fT4 levels, in some populations for some conditions. It has been suggested that TSH itself may have physiological effects apart from the stimulation of thyroid hormone levels (36,124), and such effects rather than via the reflection of thyroid status might explain such a TSH signal. Empirically, thus far, the evidence suggests that any of these TSH effects are small.
The association of thyroid hormone and particularly fT4 levels, rather than TSH levels, with clinical features has been noted by many authors, covering many individual parameters (26–28,30,33,35,42,44,46–48,52,53,57,69,73,76,80,81,86). In particular, the meta-analysis regarding AF noted the association with fT4 but not with TSH (27). Authors also previously found evidence of associations of clinical parameters with fT4 in the absence of an association with subclinical thyroid dysfunction as currently diagnosed (33,49,73,80,86). One of these studies also showed associations with TSH (49).
Nevertheless, to date, to the best of our knowledge, this information from the individual studies showing the superiority of thyroid hormone levels in terms of associations with individual clinical parameters has not been synthesized into a formal conclusion regarding the biochemical assessment of thyroid function in general.
It has been suggested that despite TSH being considered a more sensitive indicator of thyroid status, fT4 may be a more sensitive indicator of “cardiac” (28), or “tissue” (47,53) thyroid status. Our study strengthens and generalizes these propositions, indicating that fT4 is the more sensitive indicator of thyroid status because it is the better indicator of tissue and organ effects.
The superior association of clinical parameters with fT4 as compared with TSH levels has more often been attributed to a putative disturbance of set point physiology (42,46,47,69,76,81,86), to a significant difference between pituitary and peripheral sensitivity to fT4 (27,46,48,52), or to statistical/other factors (including reverse causation) (33,36,44,49,58).
The explanations related to set points are denied in the first instance by the evidence that the relatively stable thyroid hormone levels seen in individuals are better explained by a model of “balance points” (or “equilibrium points”) rather than “set points” (125). Notwithstanding this concept, it has been suggested that in older adults there is an alteration of what is termed “set point physiology,” in that TSH may be less suppressed by any given rise in fT4 (42,76). However, in this situation, though the range of TSH may change, any physiological association with greater or lesser TSH levels should remain intact. Further, the greater association of clinical parameters with fT4 rather than TSH levels is apparent across a wide age range (Table 1).
At a population level, TSH levels do, indeed, decrease with rising fT4 levels (17,18), suggesting that in general pituitary sensitivity to thyroid hormones is robust. If for any reason there were a disturbance to pituitary sensitivity in the absence of a corresponding change to peripheral sensitivity, this would in any event provide another reason not to diagnose thyroid function on the basis of TSH levels.
The evidence also suggests that, regardless of the method used, the classification of thyroid function into normal, subclinical disease and overt disease is arbitrary. Thyroid hormones, as previously suggested (9,26), similar to many other biological parameters, exert a continuum of effects across the normal range. There is no clear border between normal and abnormal. There are advantages and disadvantages associated with all levels (9,26,126). Individuals with relatively low levels of fT4, for example, are less likely to develop AF but more likely to develop metabolic syndrome; the converse applies for individuals with higher fT4 levels. At the extremes, the disadvantages clearly outweigh the advantages, and individuals are likely to become symptomatic.
On the other hand, any excursion from the middle of the range has an association with some pathology or other. Some individual pathologies, for example, frailty, mortality, and dementia may increase with deviations either side of the middle of the range. It seems likely that evolutionary mechanisms have arisen to minimize variation from the middle of the reference range of thyroid hormones (127).
The fact that TSH levels reliably identify overt thyroid dysfunction can also be explained by the negative population relationship between TSH and fT4, that is, its extension into the abnormal ranges of fT4 (17,18). This extension is due merely to the fact that the vast majority of all overt thyroid dysfunction is primary rather than secondary (128). This situation differs from other endocrine pathology, for example Cushing's syndrome, where ACTH levels cannot be used as a screening test on account of the likelihood that Cushing's syndrome may be secondary, that is, be due to a disorder of ACTH regulation (129). The fact that TSH levels are thereby very sensitive screening tests for overt thyroid dysfunction (130) does not imply that TSH levels are very specific, that is, that an abnormal TSH level implies thyroid dysfunction. Our work indicates that an abnormal TSH level per se is an imprecise indicator of tissue or organ hyper/hypothyroidism as compared with thyroid hormone levels.
This work addressed diagnosis alone. Extrapolation of our findings appears logical, and there is no apparent a priori reason as to why TSH levels should be preferred over thyroid hormone levels in the context of monitoring thyroid treatments. Randomized trials might, nevertheless, reveal that additional considerations apply in these circumstances. Though there was no suggestion in the studies that we examined of a difference with individuals on thyroid hormone replacement, their numbers were small.
In summary, there is now matching theoretical and empiric evidence from a variety of sources suggesting that the thyroid status of an individual is better defined by thyroid hormone levels than TSH levels. There is evidence of a continuum of thyroid hormone effects along the continuum of thyroid hormone levels, with a possible optimum around the middle of the reference range. Though TSH levels remain good screening tests for overt thyroid dysfunction, it is theoretically and empirically more sound to rely on thyroid hormone and especially fT4 levels to classify the thyroid state.
This work should result in a simplification of the understanding of thyroid physiology and pathophysiology, and bring it more into line with the understanding of the physiology and pathophysiology of other parameters, whereby the status of a parameter is judged by its level rather than the level of any controlling factor. Reconsideration of the TSH-based diagnostic approach to thyroid function appears to be indicated. In turn, this would appear to have implications for clinical guidelines, research methodology, and the rationale of underlying physiological principles.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, Fishman GI, Goldman S, Cooper DS, Mora A, Kudenchuk PJ, Hollenberg AN, McDonald CL, Ladenson PW. 2019. Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention and treatment. Thyroid 29:760–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider C, Feller M, Bauer DC, Collet T-H, da Costa BR, Auer R, Peeters RP, Brown SJ, Bremner AP, O'Leary PC, Feddema P, Leedman PJ, Aujesky D, Walsh JP, Rodondi N. 2018. Initial evaluation of thyroid dysfunction—are simultaneous TSH and fT4 tests necessary? PLoS One 13:e0196631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orgiazzi J. 2016. Dose normal TSH mean euthyroidism in L-T4 treatment? Clin Thyroidol 28:325–328 [Google Scholar]
- 4. Ross DS 2019 Euthyroid hyperthyroxinemia and hypothyroxinemia. Cooper DS (ed) Waltham, MA: UpToDate, Inc. Available at https://www.uptodate.com (accessed November10, 2019)
- 5. Wilson S, Parle JV, Roberts LM, Roalfe AK, Hobbs FDR, Clark P, Sheppard MC, Gammage MD Pattison HM, Franklyn JA. 2006. Prevalence of subclinical thyroid dysfunction and its relation to socioeconomic deprivation in the elderly: a community-based cross-sectional survey. J Clin Endocrinol Metab 91:4809–4816 [DOI] [PubMed] [Google Scholar]
- 6. Biondi B, Cooper DS. 2018. Subclinical hyperthyroidism. N Engl J Med 378:2411–2419 [DOI] [PubMed] [Google Scholar]
- 7. Palacios SS, Pascual-Corrales E, Galofre JC. 2012. Management of subclinical hyperthyroidism. Int J Endocrinol Metab 10:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fatourechi V. 2009. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 84:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor PN, Razvi S, Pearce SH, Dayan CM. 2013. A review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab 98:3562–3571 [DOI] [PubMed] [Google Scholar]
- 10. The TRUST Study Group 2017. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 376:2534–2544 [DOI] [PubMed] [Google Scholar]
- 11. Villar HC, Sacconato H, Valente O, Atallah AN. 2007. Thyroid hormone for subclinical hypothyroidism. Cochrane Database Syst Rev 18:CD003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoermann R, Larisch R, Dietrich JW, Midgley JEM. 2016. Derivation of a multivariate reference range for pituitary thyrotropin and thyroid hormones: diagnostic efficiency compared with conventional single reference method. Eur J Endocrinol 174:735–743 [DOI] [PubMed] [Google Scholar]
- 13. Ross HA, den Hejer M, Hermus Ad RMM, Sweep FCGC. 2009. Composite reference interval for thyroid-stimulating hormone and free thyroxine, comparison with common cutoff values, and reconsideration of subclinical thyroid disease. Clin Chem 55:2019–2025 [DOI] [PubMed] [Google Scholar]
- 14. Cryer PE, Davis SN 2015. Chapter 420: Hypoglycemia. In: Kasper DL, Hauser SL, Jameson JL, Fauci AS, Longo DL, Loscalzo J (eds) Harrison's Principles of Internal Medicine, 19th ed. McGraw Hill, New York, pp. 2430–2435 [Google Scholar]
- 15. Khosla S 2015 Chapter 65: Hypercalcemia and hypocalcemia. In: Kasper DL, Hauser SL, Jameson JL, Fauci AS, Longo DL, Loscalzo J (eds) Harrison's Principles of Internal Medicine, 19th ed. McGraw Hill, New York, pp, 313–314 [Google Scholar]
- 16. Arlt W. 2015. Chapter 406: Disorders of the adrenal cortex. In: Kasper DL, Hauser SL, Jameson JL, Fauci AS, Longo DL, Loscalzo J (eds) Harrison's Principles of Internal Medicine, 19th ed. McGraw Hill, New York, pp. 2316 [Google Scholar]
- 17. Hoermann R, Eckl W, Hoermann C, Larisch R. 2010. Complex relationship between free thyroxine and TSH in the regulation of thyroid function. Eur J Endocrinol 162:1123–1129 [DOI] [PubMed] [Google Scholar]
- 18. Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EU, Walsh JP. 2013. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 98:2936–2943 [DOI] [PubMed] [Google Scholar]
- 19. Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, Jørgensen T. 2005. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 90:4019–4024 [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J 50:346–363 [DOI] [PubMed] [Google Scholar]
- 22. Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effect models using lme4. J Stat Softw 67:1–48 [Google Scholar]
- 23. Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest Package: tests in linear mixed effects models. J Stat Softw 82:1–26 [Google Scholar]
- 24. R Core Team. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. Available at https://www.R-project.org (accessed July15, 2019)
- 25. Selmer C, Olesen JB, Hansen ML, Lindharsen J, Olsen A-MS, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp-Pedersen C, Gislason GH. 2012. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ 345:e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cappola AR, Arnold AM, Wulczn K, Carlson M, Robbins J, Psaty BM. 2015. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab 100:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, Cappola AR, Heckbert SR, Ceresini G, Gussekloo J, den Elzen WPJ, Peeters RP, Luben R, Völzke H, Dörr M, Walsh JP, Bremner A, Iacoviello M, Macfarlane P, Heeringa J, Stott DJ, Westendorp RGJ, Khaw KT, Magnani JW, Aujesky D, Rodondi N; Thyroid Studies Collaboration. 2017. Thyroid Studies Collaboration. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation 136:2100–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gammage MD, Parle JV, Holder RL, Roberts LM, Hobbs FD, Wilson S, Sheppard MC, Franklyn JA. 2007. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med 167:928–934 [DOI] [PubMed] [Google Scholar]
- 29. Heeringa J, Hoogendoorn EH, van der Deure WM, Hofman A, Peeters RP, Hop WC, den Heijer M, Visser TJ, Witterman JC. 2008. High-normal thyroid function and the risk of atrial fibrillation: the Rotterdam study. Arch Int Med 168:2219–2224 [DOI] [PubMed] [Google Scholar]
- 30. Chaker L, Heeringa J, Deghan A, Medici M, Visser WE, Baumgartner C, Hofman A, Rodondi N, Peeters RP, Franco OH. 2015. Normal thyroid function and the risk of atrial fibrillation: the Rotterdam study. J Clin Endocrinol Metab 100:3718–3724 [DOI] [PubMed] [Google Scholar]
- 31. Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, Cappola TP, Cappola AR. 2018. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail 11:e005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan Z, Huang H, Li J, Wang J. 2016. Relationship between subclinical thyroid dysfunction and the risk of fracture: a meta-analysis of prospective cohort studies. Osteoporosis Int 1:115–125 [DOI] [PubMed] [Google Scholar]
- 33. Van Rijn LE, Pop VJ, Williams GR. 2014. Low bone mineral density is related to high physiological levels of free thyroxine in peri-menopausal women. Eur J Endocrinol 170:461–468 [DOI] [PubMed] [Google Scholar]
- 34. Roef G, lapauw B, Goemaere S, Zmierczak H, Fliers T, Kaufman JM, Taes Y. 2011. Thyroid hormone status within the physiological range affects bone mass and density in healthy men at the age of peak bone mass. Eur J Endocrinol 164:1027–1034 [DOI] [PubMed] [Google Scholar]
- 35. Murphy E, Glüer CC, Reid DM, Felsenberg D, Roux C, Eastell R, Williams GR. 2010. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab 95:3173–3181 [DOI] [PubMed] [Google Scholar]
- 36. Van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HA, Peeters RP, Visser TJ. 2008. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam study. Clin Endocrinol (Oxf) 68:175–181 [DOI] [PubMed] [Google Scholar]
- 37. Waring AC, Harrison S, Fink H, Samuels MH, Cawthorn PM, Zmuda JM, Orwoll ES, Bauer D. 2013. A prospective study of thyroid function, bone loss, and fractures in older men: the MrOS study. J Bone Miner Res 28:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siru R, Alfonso H, Chubb SAP, Golledge J, Flicker L, Yeap BB. 2017. Subclinical thyroid dysfunction and circulating thyroid hormones are not associated with bone turnover markers or incident hip fracture in older men. Clin Endocrinol 89:93–99 [DOI] [PubMed] [Google Scholar]
- 39. Lambrinoudaki I, Armeni E, Pliatsika P, Rizos D, Kaparos G, Augoulea A, Alexandrou A, Flokatoula M, Creatsa M, Panoulis C, Triantafyllou N, Papacharalambous X. 2017. Thyroid function and autoimmunity are associated with the risk of vertebral fractures in postmenopausal women. J Bone Miner Metab 35:227–233 [DOI] [PubMed] [Google Scholar]
- 40. Chan YX, Knuiman MW, Divitini ML, Brown SJ, Walsh J, Yeap BB. 2017. Lower TSH and higher free thyroxine predict incidence of prostate but not breast, colorectal or lung cancer. Eur J Endocrinol 177:297–308 [DOI] [PubMed] [Google Scholar]
- 41. Tosovic A, Becker C, Bondeson A-G, Bondeson L, Ericsson U-B, Malm J, Manjer J. 2012. Prospectively measured thyroid hormones and thyroid peroxidase antibodies in relation to breast cancer risk. Int J Cancer 131:226–2133 [DOI] [PubMed] [Google Scholar]
- 42. Khan SR, Chaker L, Ruiter R, Aerts JGJV, Hoffman A, Deghan A, Franco OH, Stricker BHC, Peeters RP. 2016. Thyroid function and cancer risk: the Rotterdam study. J Clin Endocrinol Metab 12:5030–5036 [DOI] [PubMed] [Google Scholar]
- 43. Kuijpens JLP, Nykličtek I, Louwman MWJ, Weetman TAP, Pop VJM, Coebergh J-WW. 2005. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid 15:1253–1259 [DOI] [PubMed] [Google Scholar]
- 44. Ittermann T, Haring R, Wallaschofski H, Baumeister S, Nauck M, Dörr M, Lerch M, Meyer zu Schwabedissen HE, Rosskopf D, Völzke H. 2012. Inverse association between serum free thyroxine levels and hepatic steatosis: results from the study of health in Pomerania. Thyroid 22:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu C, Xu L, Yu M, Li Y. 2011. Association between thyroid function and non alcoholic fatty liver disease in euthyroid elderly Chinese. Clin Endocrinol 75:240–246 [DOI] [PubMed] [Google Scholar]
- 46. Bano A, Chaker L, Plompen EPC, Hofman A, Deghan A, Franco OH, Janssen HLA, Murad SW, Peeters RP. 2016. Thyroid function and the risk of non-alcoholic fatty liver disease: the Rotterdam study. J Clin Endocrinol Metab 101:3204–3211 [DOI] [PubMed] [Google Scholar]
- 47. Mehran L, Amouzegar A, Bakhtiyari M, Mansournia MA, Rahimabad PR, Tohidi M, Azizi F. 2017. Variations in serum free thyroxine concentration within the reference range predicts the incidence of metabolic syndrome in non-obese adults: a cohort study. Thyroid 27:886–893 [DOI] [PubMed] [Google Scholar]
- 48. Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. 2007. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab 92:491–496 [DOI] [PubMed] [Google Scholar]
- 49. Garduño-Garcia J, Alvirde-Garcia U, López-Carrasco G, Mendoza M, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM, Gomez-Perez FJ, Aguilar-Salinas CA. 2010. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol 163:73–278 [DOI] [PubMed] [Google Scholar]
- 50. Lin SY, Wang YY, Liu PH, Lai WA, Sheu WH. 2005. Lower serum free thyroxine levels are associated with metabolic syndrome in a Chinese population. Metabolism 54:1524–1528 [DOI] [PubMed] [Google Scholar]
- 51. Waring AC, Rodondi N, Harrison S, Kanava AM, Simonsick EM, Milkovic I, Satterfield S, Newman AB, Bauer DC; for the Health, Aging and Body Composition (Health ABC) Study 2012. Thyroid function and prevalent and incident metabolic syndrome in older adults: the health, aging, and body composition study. Clin Endocrinol (Oxf) 76:911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shon HS, Jung ED, Kim SH, Lee JH. 2008. Free T4 is negatively correlated with body mass index in euthyroid women. Korean J Intern Med 23:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Makepeace AE, Bremmer AP, O'Leary P, Leedman PJ, Feddema P, Michelangeli V, Walsh JP. 2008. Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: differences between smokers and non-smokers. Clin Endocrinol (Oxf) 69:648–652 [DOI] [PubMed] [Google Scholar]
- 54. Chaker L, Ligthart S, Korevaar TI, Hofman A, Franco OH, Peeters RP, Deghan A. 2016. Thyroid function and risk of type 2 diabetes: a population cohort study. BMC Med 14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jun JE, Jee JH, Bae JC, Jin S-M, Hur KY, Lee M-K, Kim TH, Kim SW, Kim JH. 2017. Association between changes in thyroid hormones and incident type 2 diabetes: a seven-year longitudinal study. Thyroid 27:29–38 [DOI] [PubMed] [Google Scholar]
- 56. Oh H-S, Kwon H, Ahn J, Song E, Park S, Kim M, Han M, Jeon MJ, Kim WG, Kim WB, Shong YK, Rhee E-J, Kim TY. 2018. Association between thyroid dysfunction and lipid profiles differs according to age and sex: results from the Korean National Health and Nutrition Survey. Thyroid 28:849–856 [DOI] [PubMed] [Google Scholar]
- 57. Kim HH, Bae JC, Park HK, Byun DW, Suh K, Yoo MH, Kim JH, Min Y-K, Kim SW, Chung JH. 2016. Triiodothyronine levels are independently associated with metabolic syndrome in euthyroid middle-aged subjects. Endocrinol Metab (Seoul) 31:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strollo F, Carucci I, More M, Marico G, Strollo G, Masini MA, Gentile S. 2012. Free triiodothyronine and cholesterol levels in euthyroid elderly T2DM patients. Int J Endocrinol 2012:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Svare A, Nilsen TI, Bjøro T, Asvold BO, Langhammer A. 2011. Serum TSH related to measures of body mass: longitudinal data from the HUNT Study, Norway. Clin Endocrinol (Oxf) 74:769–775 [DOI] [PubMed] [Google Scholar]
- 60. Proces S, Delgrange E, Vander Borght T, Donckier J, Donckier JE. 2001. Minor alterations in thyroid-function tests associated with diabetes and obesity in outpatients without known thyroid illness. Acta Clinica Belgica 56:86–90 [DOI] [PubMed] [Google Scholar]
- 61. Wolide AD, Zawdie B, Alemayehu T, Tadesse S. 2017. Association between thyroid hormone parameters and dyslipidemia among type 2 diabetes mellitus parameters: comparative cross-sectional study. Diab Metab Syndr Suppl 1:S257–S262 [DOI] [PubMed] [Google Scholar]
- 62. Temizkan S, Balafoulou B, Ozderya A, Avci M, Aydin K, Karaman S, Sargin M. 2016. Effects of thyrotrophin, thyroid hormones and thyroid antibodies on metabolic parameters in a euthyroid population with obesity. Clin Endocrinol 85:616–623 [DOI] [PubMed] [Google Scholar]
- 63. Jain RB. 2017. Associations between the levels of thyroid hormones and lipid/lipoprotein levels: data from national Health and Nutrition Examination Survey 2007–2012. Environ Toxicol Pharmacol 53:133–144 [DOI] [PubMed] [Google Scholar]
- 64. Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DCG, Luben R, Wareham NJ, Khaw K-T. 2009. Initial thyroid status and cardiovascular risk factors: the Epic-Norfolk prospective population study. Clin Endocrinol 72:404–410 [DOI] [PubMed] [Google Scholar]
- 65. Udenze I, Nnaji I, Oshodi T. 2014. Thyroid function in adults with metabolic syndrome. Pan Afr Med J 18:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Elgazar EH, Esheba NE, Shalaby SA, Mohamed WF. 2019. Thyroid dysfunction prevalence and relation to glycemic control in patients with type 2 diabetes mellitus. Diabetes Metab Syndr 13:2513–2517 [DOI] [PubMed] [Google Scholar]
- 67. Vadiveloo T, Donnan PT, Cochrane L, Leese G. 2011. The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab 96:1344–1351 [DOI] [PubMed] [Google Scholar]
- 68. Choi HJ, Byun MS, Yi D, Sohn BK, Lee JH, Kim YK, Lee DY; KBASE Research Group. 2017. Associations of thyroid hormone levels with in vivo Alzheimer's disease pathologies. Alzheimers Res Ther 9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Volpato S, Guralnik JM, Fried LP, Remalay AT, Cappola AR, Launer LJ. 2002. Serum thyroxine level and cognitive decline in older women. Neurology 58:1055–1061 [DOI] [PubMed] [Google Scholar]
- 70. Choi HY, Choe YM, Byun MS, Sohn BK, Baek H, Yi D, Han JY, Woo JI, Lee DY. 2015. Associations between serum thyroid hormone and cerebral amyloidosis in cognitively diverse elderly. Alzheimers Dementia 11:S648–S649 [Google Scholar]
- 71. de Jong FD, Heijer T, Visser TJ, de Rijke YB, Drexhage HA, Hoffman A, Breteler MMB. 2006. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 91:2569–2573 [DOI] [PubMed] [Google Scholar]
- 72. Tan ZS, Beiser A, Ramachandran RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S. 2009. Thyroid function and the risk of Alzheimer's disease: the Framingham Study. Arch Int Med 168:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yeap BB, Alfonso H, Chubb SA, Puri G, Hankey GJ, Flicker L, Almeida OP. 2012. Higher free thyroxine levels predict increased incidence of dementia in older men: the Health in Men Study. J Clin Endocrinol Metab 97:E2230–E2237 [DOI] [PubMed] [Google Scholar]
- 74. Chaker L, Wolters FJ, Korevaar TI, Hofman A, van der Lugt A, Koudstaal PJ, Franco OH, Deghan A, Vernooij MW, Peeters RP, Ikram MA. 2016. Thyroid function and the risk of dementia: the Rotterdam study. Neurology 87:1688–1695 [DOI] [PubMed] [Google Scholar]
- 75. Ittermann T, Wittfeld K, Nauck M, Bülow R, Hosten N, Völzke H, Grabe HJ. 2018. High thyrotropin is associated with reduced hippocampal volume in a population-based study from Germany. Thyroid 28:1434–1442 [DOI] [PubMed] [Google Scholar]
- 76. Yeap BB, Alfonso H, Chubb SAP, Walsh JP, Hankey GJ, Almeida OP, Flicker L. 2012. Higher free thyroxine levels are associated with frailty in older men: the Health In Men Study. Clin Endocrinol 76:741–748 [DOI] [PubMed] [Google Scholar]
- 77. Bano A, Chaker L, Schoufour J, Ikram MA, Kavousi M, Franco OH, Peeters RP, Mattace-Raso FUS. 2018. High circulating free thyroxine levels may increase the risk of frailty: the Rotterdam study. J Clin Endocrinol Metab 103:328–335 [DOI] [PubMed] [Google Scholar]
- 78. van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SWJ. 2005. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab 90:6403–6409 [DOI] [PubMed] [Google Scholar]
- 79. Gussekloo J, van Exel E, de Graen AJM, Meinders AE, Frölich M, Westendorp RGJ. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 80. Yeap BB, Alfonso H, Hankey GJ, Flicker L, Golledge J, Norman PE, Chubb SAP. 2013. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: the Health In Men Study. Eur J Endocrinol 169:401–408 [DOI] [PubMed] [Google Scholar]
- 81. Van de Ven AC, Netea-Maier RT, de Vegt F, Ross HA, Sweep HA, Sween FC, Kiemeney LA, Smit JW, Hermus AR, den Heijer M. 2014. Associations between thyroid function and mortality: the influence of age. Eur J Endocrinol 171:183–191 [DOI] [PubMed] [Google Scholar]
- 82. Inoue K, Tsujimoto T, Saito J, Sugiyama T. 2016. Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid 26:1457–1465 [DOI] [PubMed] [Google Scholar]
- 83. Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp-Pederson C, Gislason GH. 2014. Subclinical and overt thyroid dysfunction and the risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab 99:2372–2382 [DOI] [PubMed] [Google Scholar]
- 84. Moon S, Kim MJ, Yu JM, Yoo HJ, Park YJ. 2018. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid 28:1101–1110 [DOI] [PubMed] [Google Scholar]
- 85. Razvi S, Weaver JU, Vanderpump MP, Pearce SH. 2010. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab 95:1734–1740 [DOI] [PubMed] [Google Scholar]
- 86. Chaker L, van den Berg ME, Niemeijer MN, Franco OH, Deghan A, Hofman A, Rijnbeek PR, Deckers JW, Eijgelsheim M, Stricker BHC, Peeters RP. 2016. Thyroid function and sudden cardiac death: a prospective study. Circulation 134:713–722 [DOI] [PubMed] [Google Scholar]
- 87. Asvold BO, Bjøro T, Nilsen TI, Gunnell D, Vatten LJ. 2008. Thyrotropin levels and risk of fatal coronary heart disease: the HUNT study. Arch Int Med 168:855–860 [DOI] [PubMed] [Google Scholar]
- 88. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. 2005. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Int Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 89. Roef GL, Taes YE, Kaufman J-M, Van Daele CM, De Buyzere ML, Gillebert TC, Rietzschel ER. 2013. Thyroid hormone levels within reference range are associated with heart rate, cardiac structure, and function in middle aged men and women. Thyroid 23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rodondi N, Bauer DC, Cappola AR, Cornuz J, Robbins J, Fried LP, Ladenson PW, Vittinghoff E, Gottdiener JS, Newman AB. 2008. Subclinical thyroid dysfunction, cardiac function and the risk of heart failure: the Cardiovascular Health Study. J Am Coll Cardiol 52:1152–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Peixoto de Miranda EJF, Bittencourt MS, Staniak HL, Sharovsky R, Pereira AC, Foppa M, Santos IS, Lotufo PA, Benseñor IM. 2018. Thyrotropin and free thyroxine levels and coronary artery disease: cross-sectional analysis of the Brazilian longitudinal Study of Adult Health (ELSA-Brasil). Braz J med Biol Res 51:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vrijkotte TGM, Hrudey E, Twickler MB. 2017. Early maternal thyroid function during gestation is associated with fetal growth, particularly in male newborns. J Clin Endo Metab 102:1059–1066 [DOI] [PubMed] [Google Scholar]
- 93. Korevaar TIM, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SMPF, Hofman A, Ross HA, Hooijkaas H, Tiemeier H, Bongers-Schokking JJ, Jaddoe VW, Visser TJ, Steegers EA, Medici M, Peeters RP. 2013. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab 98:4382–4390 [DOI] [PubMed] [Google Scholar]
- 94. Medici M, de Rijke YB, Peeters RP, Visser W, de Muink Keizer-Schrama SM, Jaddoe VV, Hofman A, Hooijkaas H, Steegers EA, Tiemeiei H, Bongers-Schokking JJ, Visser TJ. 2012. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R study. J Clin Endocrinol Metab 97:646–652 [DOI] [PubMed] [Google Scholar]
- 95. Cleary-Goldman J, Malone FD, Lambert-Messerlain G, Sullivan L, Canick J, Porter TF, Luthy D, Gross S, Bianchi DW, D'Alton ME. 2008. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 112:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Breathnach FM, Donnelly J, Cooley SM, Geary M, Malone FD. 2013. Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Aust N Z J Obstetr Gynaecol 53:553–560 [DOI] [PubMed] [Google Scholar]
- 97. Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. 2010. Maternal thyroid function at 11–13 weeks of gestation and subsequent fetal death. Thyroid 20:989–993 [DOI] [PubMed] [Google Scholar]
- 98. Knight BA, Shields BM, Hattersley AT, Vaidya B. 2016. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol 174:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Y, Shan Z, Teng W, Yu X LI Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, Hua T. 2010. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol 72:825–829 [DOI] [PubMed] [Google Scholar]
- 100. Jackson K, Cooper DS. 2019. Subclinical hypothyroidism and thyroid autoimmunity are associated with preterm delivery in an individual participant meta-analysis. Clin Thyroidol 31:410–416 [Google Scholar]
- 101. Rotondi M, Leporati P, La Manna A, Pirali B, Mondello T, Fonte R, Magri F, Chiovato L. 2009. Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol 160:403–408 [DOI] [PubMed] [Google Scholar]
- 102. Rotondi M, Magri F, Chiovato L. 2011. Thyroid and obesity: not a one-way interaction. J Clin Endocrinol Metab 96:344–346 [DOI] [PubMed] [Google Scholar]
- 103. Matzen LE, Kvetny J, Pedersen KK. 1989. TSH, thyroid hormones and nuclear binding of T3 in mononuclear blood cells from obese and non-obese women. Scand J Clin Lab Invest 49:249–253 [PubMed] [Google Scholar]
- 104. Köhrle J. 1990. Thyrotropin (TSH) action on thyroid hormone deiodination and secretion: one aspect of thyrotropin regulation of thyroid cell biology. Horm Metab Res Suppl 23:18–28 [PubMed] [Google Scholar]
- 105. Ross DS 2019 Thyroid function in nonthyroidal illness. Cooper DS (ed) Waltham, MA: UpToDate, Inc. Available at https://www.uptodate.com (accessed September17, 2018)
- 106. Fliers E, Kalsbeek A, Boelen A. 2014. Beyond the fixed setpoint of the hypothalamus-pituitary-thyroid axis. Eur J Endocrinol 171:R197–R208 [DOI] [PubMed] [Google Scholar]
- 107. Ellervik C, Roselli C, Christophersen IE, Alonso A, Pietzner M, Sitlani CMO, Trompet S, Arking DE, Geelhoed B, Guo X, Kleber ME, Lin HJ, Lin H, MacFarlane P, Selvin E, Shaffer C, Smith AV, Verweij N, Weiss S, Cappola AR, Dörr M, Gudnason V, Heckbert S, Mooijaart S, März W, Psaty BM, Ridker PM, Roden D, Stott DJ, Völzke H, Benjamin EJ, Delgado G, Ellinor P, Homuth G, Köttgen A, Jukema JW, Lubitz SA, Mora S, Rienstra M, Rotter JI, Shoemaker MB, Sotoodehnia N, Taylor KD, van der Harst P, Albert CM, Chasman DI. 2019. Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomisation study. JAMA Cardiol 4:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Larsson SC, Allara E, Mason AM, Michaëlsson K, Burgess S. 2019. Thyroid function and dysfunction in relation to 16 cardiovascular diseases: a Mendelian randomisation study. Circ Genom Precis Med 12:e002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Taylor PN, Richmond R, Davies N, Sayers A, Stevenson K, Woltersdorf W, Taylor A, Groom A, Northstone K, Ring S, Okosieme O, Rees A, Nitsch D, Williams GR, Smith GD, Gregory JW, Timpson NJ, Tobias JH, Dayan CM. 2016. Paradoxical relationship between body mass index and thyroid hormone levels: a study using Mendelian randomization. J Clin Endocrinol Metab 101:730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. DeGroot LJ 2000 Graves' disease and the manifestations of thyrotoxicosis. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (eds) Endotext [Internet]. South Dartmouth, MA: MDText.c0m, Inc. Available at https://www.ncbi.n1m.nih.gov/books/NBK285567 (accessed July11, 2015)
- 111. Stabouli S, Papakatsika S Kotsis V. 2010. Hypothyroidism and hypertension. Expert Rev Cardiovasc Ther 8:1559–1565 [DOI] [PubMed] [Google Scholar]
- 112. Ryödi E, Salmi J, Jaatnen P, Huhtala H, Saaristo R, Välimäki M, Auvinen A, Metso S. 2013. Cardiovascular morbidity and mortality in surgically treated hyperthyroidism—a nation-wide cohort study with a long-term follow-up. Clin Endocrinol 80:743–750 [DOI] [PubMed] [Google Scholar]
- 113. Gorka J, Taylor-Gjevre RM, Arnason T. 2013. Metabolic and clinical consequences of hyperthyroidism on bone density. Int J Endocrinol 2013:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fukui T, Hasegawa Y, Takenaka H. 2001. Hyperthyroid dementia: clinicoradiological findings and response to treatment. J Neurol Sci 184:81–88 [DOI] [PubMed] [Google Scholar]
- 115. Duntas LH. 2002. Thyroid disease and lipids. Thyroid 12:287–293 [DOI] [PubMed] [Google Scholar]
- 116. Nikkilä EA, Kekki M. 1972. Plasma triglyceride metabolism in thyroid disease. J Clin Invest 51:2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Prisant LM, Gujral JS, Mulloy AL. 2007. Hyperthyroidism: a secondary cause of isolated hypertension. J Cin Hypertens 8:2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Anantarapu S, Vaikkahura S, Sachan A, Phaneendra BV, Suchitra MM, Reddy AP, Epuri S, Mukka A, Vemvakam D. 2015. Effects of thyroid hormone replacement on glycated hemoglobin levels in non-diabetic subjects with overt hypothyroidism. Arch Endocrinol Metab 59:495–500 [DOI] [PubMed] [Google Scholar]
- 119. Andersen SL, Olsen J, Wu CS, Laurberg P. 2014. Spontaneous abortion, stillbirth and hyperthyroidism: a Danish population-based study. Eur Thyroid J 3:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sahay RK, Sri Nagesh V. 2012. Hypothyroidism in pregnancy. Indian J Endocrinol Metab 16:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Calvo RM, Jauniaux E, Gulbis B, Asunción M, Gervy C, Contempré B, Morreale de Escobar G. 2002. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol 87:1768–1777 [DOI] [PubMed] [Google Scholar]
- 122. Moeller LC, Führer D. 2013. Thyroid hormone, thyroid hormone receptors, and cancer: a clinical perspective. Endocr Relat Cancer 20:R19–R29 [DOI] [PubMed] [Google Scholar]
- 123. Theodossiou C, Schwarzenberger P. 2000. Propylthiouracil reduces xenograft tumour growth in athymic nude mouse prostate cancer model. Am J Med Sci 319:96–99 [DOI] [PubMed] [Google Scholar]
- 124. Fernandez-Ruocco J, Gallego M, Rodriguez-de-Yurre A, Zayas-Arrabal J, Echeazarra L, Alquiza A, Fernández-López V, Rodriguez-Robledo JM, Britto O, Schleier Y, Sepulveda M, Oshiyama NF, Vila-Petroff M, Bassani RA, Medel EH, Casis O. 2019. High thyrotropin is critical for cardiac electrical remodelling and arrhythmia vulnerability in hypothyroidism. Thyroid 29:934–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fitzgerald SP, Bean NG, Fitzgerald LN. 2017. Population data indicate that thyroid regulation is consistent with an equilibrium-point model, but not with a set point model. Temperature (Austin) 4:114–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Walsh JP. 2011. Setpoints and susceptibility: do small differences in thyroid function really matter? Clin Endocrinol 75:158–159 [DOI] [PubMed] [Google Scholar]
- 127. Fitzgerald SP, Bean NG. 2018. Thyroid stimulating hormone (TSH) autoregulation reduces variation in the TSH response to thyroid hormones. Temperature (Austin) 5:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. De Leo S, Lee SY, Braverman LE. 2016. Hyperthyroidism. Lancet 388:906–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. 2015. Cushing's syndrome. Lancet 386:913–927 [DOI] [PubMed] [Google Scholar]
- 130. Sheehan MT. 2016. Biochemical testing of the thyroid: TSH is the best and, oftentimes, only test needed—a review for primary care. Clin Med Res 14:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.