Abstract
Greater than one-third of adults in the United States have metabolic syndrome (MetS), a cluster of risk factors highly associated with the development of cardiovascular diseases. Premature vascular dysfunction in MetS may lead to accelerated age-related atherogenesis and arterial stiffening, thereby increasing cardiovascular risk. Montmorency tart cherries (Prunus cerasus L.) are rich in bioactive compounds, such as anthocyanins, known to exert cardiovascular protective effects. Previous research suggests that tart cherry juice consumption may improve cardiovascular health. The objective of this study was to evaluate the effects of daily consumption of tart cherry juice on hemodynamics, arterial stiffness, and blood biomarkers of cardiovascular and metabolic health in men and women with MetS. In a randomized, single-blind, placebo-controlled, parallel-arm pilot clinical trial, 19 men and women 20 to 60 years of age with MetS consumed 240 mL of tart cherry juice (Tart Cherry; n = 5 males, 4 females) or an isocaloric placebo-control drink (Control; n = 5 males, 5 females) twice daily for 12 weeks. Arterial stiffness (pulse wave velocity), brachial and aortic blood pressures, wave reflection (augmentation index), and blood biomarkers of cardiovascular and metabolic health were assessed at baseline and 6 and 12 weeks. Oxidized low-density lipoprotein and soluble vascular cell adhesion molecule-1 were significantly lower (P = .047 and P = .036, respectively) in Tart Cherry than Control at 12 weeks, but were not significantly lower than baseline values. There was a trend for total cholesterol to be lower (P = .08) in Tart Cherry than Control at 12 weeks. No significant changes were observed in hemodynamics, arterial stiffness, or other blood biomarkers assessed. These results suggest that daily tart cherry consumption may attenuate processes involved in accelerated atherogenesis without affecting hemodynamics or arterial stiffness parameters in this population. The pilot nature of this study warrants interpreting these findings with caution, and future clinical trials with a larger sample size are needed to confirm these findings.
Keywords: anthocyanins, arterial stiffness, atherosclerosis, blood pressure, cardiovascular disease, polyphenols, tart cherries, vascular function
Introduction
Metabolic syndrome (MetS) is characterized by abdominal obesity, elevated blood pressure, dyslipidemia, and hyperglycemia, as well as a proinflammatory and prothrombotic state.1 It has been estimated that ∼35% of all adults and 50% of adults 60 years of age or older in the United States (U.S.) have MetS.2 MetS is highly associated with the development of chronic diseases including cardiovascular disease (CVD), the leading cause of death in the U.S. and worldwide, and type 2 diabetes mellitus, which further increases CVD risk.1 In fact, premature vascular dysfunction in MetS may lead to atherosclerosis and arterial stiffness, thereby further increasing CVD risk.3–6 These processes are caused, in large part, by oxidative stress and inflammation in the vasculature.7–10
Numerous treatment options for MetS exist, but lifestyle modifications are the recommended initial approach.1 Medications are often prescribed, but can lead to undesirable side effects.11 Thus, effective lifestyle strategies that can improve and/or preserve cardiovascular health in this high-risk population are needed.
Montmorency tart cherries (Prunus cerasus L.) contain polyphenols that have been implicated as cardiovascular protective, in part, due to the antioxidant and anti-inflammatory effects of these compounds and their metabolites in vivo.12,13 Specifically, they are a rich source of anthocyanins, including cyanidin 3-glucosylrutinoside, cyanidin 3-rutinoside, cyanidin sophoroside, and peonidin 3-glucoside, as well as flavonols, including isorhamnetin rutinoside, kaempferol, and quercetin, and flavonols, including catechin, epicatechin, and procyanidins B1 and B2.14,15 There is preclinical evidence that tart cherry consumption improves several parameters associated with MetS and its clinical sequelae, including hyperlipidemia, insulin resistance, inflammation, hepatic steatosis, and abdominal adiposity.16,17
The results of previous clinical studies are promising, but not consistent. For instance, recent clinical research has demonstrated that tart cherry juice consumption lowers blood pressure and blood biomarkers of inflammation and oxidative stress in older adults,18,19 while another study showed improvements in biomarkers of inflammation individuals with overweight and obesity.20 Another clinical trial21 demonstrated that acute consumption of a tart cherry juice concentrate improved systolic blood pressure 2 h postconsumption in men with early hypertension, and improvements were associated with circulating polyphenol metabolites. However, a previous clinical trial22 demonstrated no effect of 6 weeks of tart cherry juice concentrate consumption on arterial stiffness, blood pressure, or blood biomarkers of lipid metabolism or inflammation in healthy men and women, although they did observe an improvement in antioxidant status. Nonetheless, the subjects in that study were relatively young and healthy, which could be a major reason for their observations.
While the effects of tart cherry juice are of interest for the reduction of CVD risk, the effects of chronic consumption of tart cherry juice on cardiovascular health in humans with MetS remain unknown. The objective of this study was to evaluate the effects of daily consumption of tart cherry juice on hemodynamics, arterial stiffness, and blood biomarkers of cardiovascular and metabolic health in men and women with MetS.
Materials and Methods
Participants
Men and women with MetS were recruited from greater Tallahassee, FL area, through campus and community advertisements. Participants were included if they were between the ages of 20 and 40 and had three or more of the MetS diagnostic criteria according to the American Heart Association and the National Heart, Lung, and Blood Institute: elevated waist circumference (≥102 cm [40 in] in men and 88 cm [35 in] in women), elevated triglycerides (TG; ≥150 mg/dL), reduced high-density lipoprotein-cholesterol (HDL-C; ≤40 mg/dL in men and 50 mg/dL in women), elevated blood pressure (systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg), and elevated fasting blood glucose (≥100 mg/dL).1 Exclusion criteria included diagnosed CVD, uncontrolled hypertension (>160/100 mmHg), hormone replacement therapy or insulin use, active cancer, asthma, glaucoma, thyroid, kidney, liver, and pancreatic disease, heavy smoking (>20 cigarettes/day; however, no smokers were enrolled in this trial per chance), and heavy drinking (>7 alcoholic drinks/week for women and >14 alcoholic drinks/week for men).
After an initial prescreening over the telephone, qualified participants were invited to the study site for a screening visit, during which they provided written informed consent, and inclusion and exclusion criteria were confirmed. Anthropometric measurements (height, weight, and waist and hip circumferences) were performed by trained research associates. Measurements of brachial blood pressure were taken in duplicate 5 min apart after 10 min of seated rest using an automatic device (Omron Healthcare, Inc., Bannockburn, IL). A finger stick blood draw was performed to assess blood glucose and lipid profiles (Alere Cholestech LDX® Analyzer). A health history was obtained from participants by a Registered Dietitian Nutritionist for screening purposes.
Recruitment began in April 2014 and continued until October 2016 when the last participant finished the study. In July 2015, due to low enrollment, the inclusion criteria were revised to allow individuals between the ages of 40 and 60 to participate in the study. Final ages included ranged from 20 to 60 years. The Florida State University Institutional Review Board approved the study protocol and all participants provided written informed consent. This trial was registered at clinicaltrials.gov as NCT02154100.
Study design and dietary intervention
We performed a 12-week, randomized, single-blind, placebo-controlled, parallel-arm pilot clinical trial. Using a statistician pregenerated randomization list, the study coordinator randomly assigned qualified participants to one of two treatment groups: (1) 480 mL tart cherry juice (Tart Cherry) or (2) 480 mL calorie-matched placebo-control drink (Control) daily for 12 weeks. Participants were asked to consume 240 mL in the morning and 240 mL in the evening at least 6–8 h apart.
The tart cherry juice consisted of Montmorency tart cherry juice and concentrate (Indian Summer, Inc., Traverse City, MI). The placebo-control drink consisted of water, sugar, malic acid, sodium citrate, natural and artificial flavors, silica, FD&C Red #40, and FD&C Blue #1 (Flavor Dynamics, Inc., South Plainfield, NJ). Nutritional and phenolic compositions of the Tart Cherry Juice and Control Drink are provided in Table 1. To monitor treatment compliance, participants were given daily treatment logs and were asked to record the days and times of treatment consumption, as well as to document any missed treatments and reasons for missing treatment doses. In addition, they were asked to return any unused treatments. Compliance was defined as missing ≤2 doses per week. Participants agreed to maintain their normal diet and physical activity patterns throughout the duration of the study.
Table 1.
Nutritional and Phenolic Composition of the Tart Cherry Juice and Placebo-Control Drinks
| Nutrients and phenolics (per 240 mL) | Tart cherry juicea | Control drinkb |
|---|---|---|
| Calories | 140 (kcal) | 140 (kcal) |
| Total carbohydrate | 34 (g) | 35 (g) |
| Protein | 1 (g) | 0 (g) |
| Sodium | 25 (mg) | 4 (mg) |
| Potassium | 360 (mg) | 1 (mg) |
| Total phenolics (gallic acid equivalents) | 1070 (mg) | — |
| Total anthocyanins (cyanidin-3-glucoside equivalents) | 88 (mg) | — |
| Anthocyanin species | ||
| Cyanidin sophoroside | 1.21 (mg) | — |
| Cyanidin glucosylrutinoside | 0.48 (mg) | — |
| Cyanidin-glucoside | 1.38 (mg) | — |
| Cyanidin xylosylrutinoside | 0.52 (mg) | — |
| Cyanidin rutinoside | 33.34 (mg) | — |
| Peonidin rutinoside | 3.23 (mg) | — |
Nutritional information obtained from Indian Summer, Inc., (Traverse City, MI) and phenolic data provided by Ara Kirakosyan at the University of Michigan. Phenolics were measured using liquid chromatography-mass spectrometry using a TSQ Quantum Ultra AM triple quadrupole mass spectrometer (Thermo-Finnigan, San Jose, CA).
Nutritional information provided by Flavor Dynamics, Inc., (South Plainfield, NJ).
Anthropometric and fat mass assessments
Height without shoes was measured using a wall-mounted stadiometer to the nearest 0.5 cm and weight was assessed using a digital scale (Seca Corporation, Hanover, MD) to the nearest 0.1 kg. Body mass index (BMI) was calculated as kg/m2. Mid-abdominal waist (measured according to the American Heart Association1) and hip circumferences were measured using a Gulick fiber glass measuring tape with a tension handle (Creative Health Products, Inc., Ann Arbor, MI) to the nearest 0.1 cm. Height was measured at baseline, and body weight and waist and hip circumferences were measured at baseline and 6- and 12-week visits. Waist-to-hip ratio was calculated as waist circumference divided by hip circumference. Body composition, including fat mass, android fat, gynoid fat, and android/gynoid ratio, was assessed at baseline and 6- and 12-week visits using dual-energy x-ray absorptiometry (DXA; GE Healthcare Lunar, Madison, WI).
Hemodynamics and arterial stiffness
Cardiovascular measurements were performed at baseline and 6 and 12 weeks after 10 min of supine rest, in a quiet, temperature-controlled room (23°C ± 1°C) after an overnight fast and avoidance of alcohol and caffeine for at least 24 h. Carotid-femoral pulse wave velocity (cfPWV), brachial-ankle pulse wave velocity (baPWV), and femoral-ankle pulse wave velocity (faPWV), all measures of arterial stiffness, were measured using an automatic device (VP-2000; Omron Healthcare, Vernon Hills, IL).
Appropriate-size blood pressure cuffs were wrapped around both arms (brachial artery) and ankles (posterior tibial artery). Electrocardiogram electrodes were placed on the forearms, and a heart sound microphone was placed on the chest. Participants rested for at least 20 min before data collection. Transit time was automatically determined from the time delay between the feet of the pulse waves related to the R-wave of the electrocardiogram. The distance from the carotid to femoral artery was measured with a nonelastic tape measure as a straight line, while the distance from the brachial to tibial arteries, and femoral to tibial arteries, was calculated automatically according to the participant's height. Pulse wave velocity was calculated as distance divided by transit time.23 Two measurements were collected and averaged at each time point. Heart rate was determined from the electrocardiogram.
Aortic blood pressure and augmentation index (AIx) were assessed using pulse wave analysis (SphygmoCor; AtCor Medical Pty Ltd., Australia). Blood pressure waveforms were obtained from the right radial using applanation tonometry with a high-fidelity micromanometer (Millar SPT-301; Millar Instruments, Inc., Houston, TX), and a validated transfer function to generate the corresponding aortic blood pressure and AIx.24 The average of 10 successive waves accepted by the internal quality checks of the software was used for each measurement. Two measurements were collected at each time point and averaged. This technique has been validated and has good repeatability.25–27
Blood collection and biomarker analysis
At baseline and 6- and 12-week visits, fasting venous blood samples were collected in appropriate vacutainers. Serum and plasma were separated through centrifugation using an IEC CL31R multispeed centrifuge (Thermo Electron Corporation, Waltham, MA), aliquoted, and stored at −80°C until analysis.
Plasma samples were analyzed for total cholesterol (TC), HDL-C, low-density lipoprotein-cholesterol (LDL-C), TG, and glucose concentrations, and serum samples were analyzed for total antioxidant status using a AU480 Automated Chemistry Analyzer (Beckman Coulter, Brea, CA) at the University of Colorado-Denver (UC-Denver) Colorado Clinical and Translational Sciences Institute (CCTSI, Denver, CO). Plasma insulin and serum adiponectin and leptin concentrations were measured using radioimmunoassay (Millipore) at the UC-Denver CCTSI (Denver, CO). Serum intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) concentrations were measured using commercially available enzyme-linked immunosorbent assay kits according to the manufacturers' instructions (R&D Systems).
The Homeostatic Model Assessment (HOMA) of insulin resistance (HOMA-IR) and pancreatic β-cell function (HOMA-%B) were calculated using the HOMA2 Calculator v2.2.3 based on fasting insulin and glucose measurements.28
Dietary assessment
A 3-day food record was obtained at baseline and 12-week time points. Analysis of the records was performed using food analysis software (Food Processor version 7.50; ESHA Research, Salem, OR).
Statistical analysis
Statistical analysis was performed using SAS Version 9.3 (SAS Institute, Cary, NC). Baseline values were compared using two-sample t-tests. The main and interaction effects of the treatments and time on outcome variables were evaluated. A split-plot model of repeated measures ANOVA was used for statistical analysis both within and between treatment groups. The mean changes in outcome variables during the intervention periods were compared by analyzing interaction effects of treatment and time, using the SLICE option in a least square means statement. Values are reported as least square means ± SEM. In all statistical comparisons, differences with P < .05 are considered significant, and differences with P < .1 are considered trending toward statistical significance.
Results
Participant characteristics
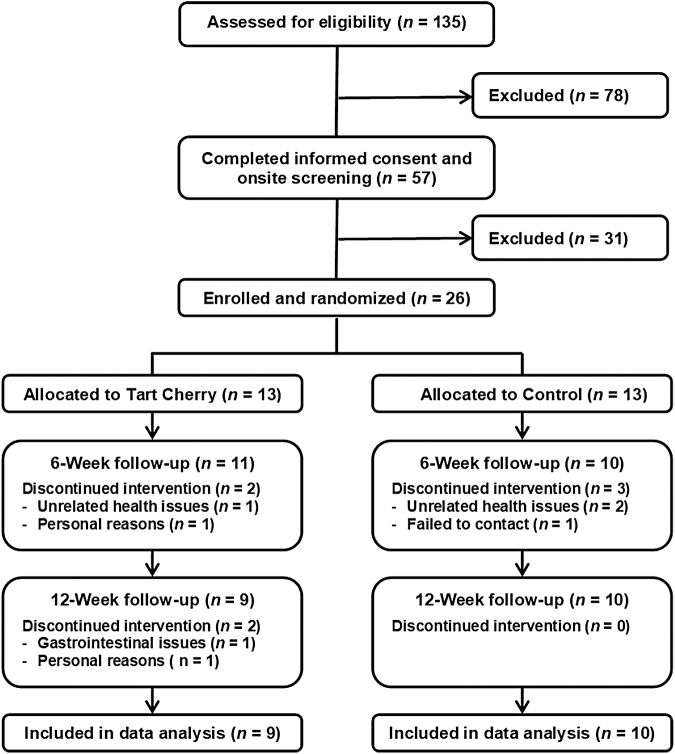
A flowchart of the study enrollment is presented in Figure 1. A total of 26 men and women who met the inclusion and exclusion criteria were randomly assigned to receive either 480 mL of tart cherry juice (n = 13) or 480 mL of a control drink (n = 13) daily for 12 weeks. The overall attrition rate for the 12-week intervention study was ∼27% (31% for the Tart Cherry group and 23% for the Control group). Common reasons for not finishing the study included failure to contact subject, claims of medical and health-related issues such as gastrointestinal complaints, and personal reasons such as lack of time. The 19 participants who completed the study were compliant with their treatments as indicated in their daily dosing diaries.
FIG. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of participant flow throughout the study.
Baseline characteristic data for participants who completed the study are presented in Table 2. Age was significantly (P < .05) different between groups. There were no statistically significant difference between groups for other baseline characteristics including height, weight, BMI, and waist and hip circumferences.
Table 2.
Baseline Characteristics of Study Participants Who Completed the 12-Week Intervention
| Tart Cherry (n = 9) | Control (n = 10) | |
|---|---|---|
| Male:female (n) | 5:4 | 5:5 |
| Age (years) | 29.3 ± 1.1* | 44.2 ± 4.1 |
| Height (cm) | 172.6 ± 2.5 | 171.6 ± 3.3 |
| Weight (kg) | 102.7 ± 6.3 | 98.1 ± 5.9 |
| BMI (kg/m2) | 34.3 ± 1.9 | 33.5 ± 1.8 |
| WC (cm) | 107.4 ± 3.9 | 108.8 ± 3.7 |
| SBP (mmHg) | 124 ± 4 | 128 ± 4 |
| DBP (mmHg) | 77 ± 3 | 78 ± 4 |
| Glucose (mg/dL) | 92.9 ± 15.4 | 117.0 ± 14.7 |
| TG (mg/dL) | 172.6 ± 28.9 | 129.1 ± 27.4 |
| HDL-C (mg/dL) | 44.3 ± 2.1 | 46.6 ± 2.0 |
Data are mean ± SEM.
Significantly different from Control.
BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein-cholesterol; SBP, systolic blood pressure; TG, triglycerides; WC, waist circumference.
Hemodynamics and arterial stiffness
Hemodynamic and arterial stiffness results are presented in Table 3. No significant time or time-by-treatment interaction effects were observed.
Table 3.
Hemodynamics and Arterial Stiffness at Baseline and 6 and 12 Weeks After Daily Tart Cherry Juice and Control Drink Consumption
| Tart Cherry (n = 9) | Control (n = 10) | Time-by-treatment, P-value | |
|---|---|---|---|
| P-SBP (mmHg) | |||
| Baseline | 124 ± 4 | 128 ± 4 | .37 |
| 6 Weeks | 125 ± 4 | 127 ± 2 | .81 |
| 12 Weeks | 122 ± 3 | 124 ± 2 | .67 |
| P-DBP (mmHg) | |||
| Baseline | 77 ± 3 | 78 ± 4 | .86 |
| 6 Weeks | 76 ± 3 | 81 ± 3 | .43 |
| 12 Weeks | 80 ± 5 | 80 ± 3 | .71 |
| P-MAP (mmHg) | |||
| Baseline | 94 ± 4 | 96 ± 4 | .60 |
| 6 Weeks | 93 ± 3 | 98 ± 3 | .32 |
| 12 Weeks | 93 ± 3 | 97 ± 3 | .55 |
| HR (beats/min) | |||
| Baseline | 63 ± 3 | 65 ± 3 | .74 |
| 6 Weeks | 60 ± 2 | 65 ± 2 | .21 |
| 12 Weeks | 60 ± 3 | 68 ± 2 | .05 |
| C-AP (mmHg) | |||
| Baseline | 7 ± 1 | 10 ± 2 | .25 |
| 6 Weeks | 8 ± 1 | 10 ± 1 | .36 |
| 12 Weeks | 8 ± 1 | 8 ± 2 | .91 |
| C-SBP (mmHg) | |||
| Baseline | 113 ± 4 | 118 ± 5 | .39 |
| 6 Weeks | 113 ± 4 | 118 ± 3 | .47 |
| 12 Weeks | 112 ± 3 | 116 ± 4 | .69 |
| C-DBP (mmHg) | |||
| Baseline | 78 ± 3 | 79 ± 4 | .82 |
| 6 Weeks | 77 ± 3 | 82 ± 3 | .34 |
| 12 Weeks | 77 ± 3 | 81 ± 3 | .63 |
| C-MAP (mmHg) | |||
| Baseline | 94 ± 4 | 96 ± 4 | .60 |
| 6 Weeks | 93 ± 3 | 98 ± 3 | .32 |
| 12 Weeks | 93 ± 3 | 97 ± 3 | .55 |
| AIx (%) | |||
| Baseline | 20 ± 2 | 24 ± 4 | .41 |
| 6 Weeks | 21 ± 2 | 27 ± 3 | .19 |
| 12 Weeks | 22 ± 2 | 22 ± 4 | .77 |
| AIx@75 (%) | |||
| Baseline | 15 ± 3 | 19 ± 4 | .40 |
| 6 Weeks | 14 ± 3 | 22 ± 3 | .08 |
| 12 Weeks | 15 ± 2 | 19 ± 4 | .57 |
| cfPWV (cm/s) | |||
| Baseline | 1022 ± 57 | 1102 ± 96 | .41 |
| 6 Weeks | 1044 ± 68 | 1179 ± 77 | .17 |
| 12 Weeks | 991 ± 47 | 1127 ± 65 | .19 |
| baPWV (cm/s) | |||
| Baseline | 1279 ± 41 | 1290 ± 56 | .90 |
| 6 Weeks | 1280 ± 59 | 1321 ± 52 | .63 |
| 12 Weeks | 1244 ± 69 | 1345 ± 64 | .23 |
| faPWV (cm/s) | |||
| Baseline | 997 ± 29 | 933 ± 40 | .50 |
| 6 Weeks | 962 ± 32 | 963 ± 32 | .98 |
| 12 Weeks | 954 ± 39 | 962 ± 36 | .87 |
Data are mean ± SEM.
AIx, augmentation index; AIx@75, augmentation index normalized at heart rate of 75 beats per minute; baPWV, brachial-ankle pulse wave velocity; C-AP, central arterial pressure; C-DBP, central diastolic blood pressure; cfPWV, carotid-femoral pulse wave velocity; C-MAP, central mean arterial pressure; C-SBP, central systolic blood pressure; faPWV, femoral-ankle pulse wave velocity; HR, heart rate; P-DBP, peripheral diastolic blood pressure; P-MAP, peripheral mean arterial pressure; P-SBP, peripheral systolic blood pressure.
Blood biomarkers
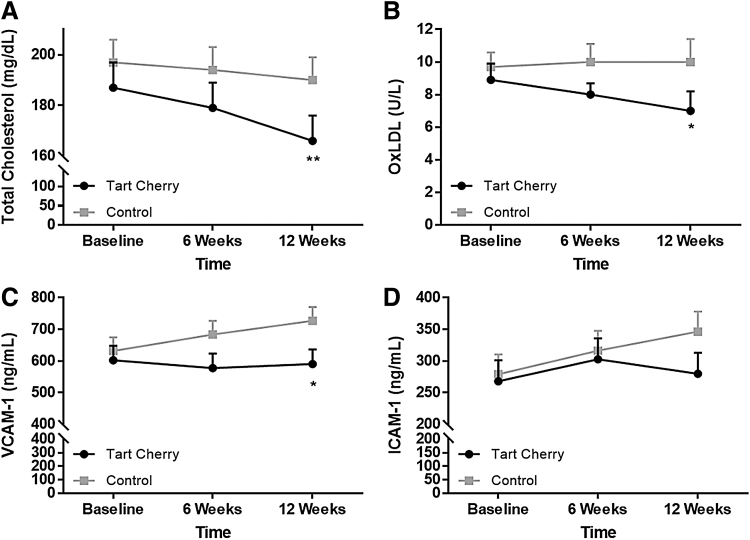
Blood biomarker results are presented in Figure 2 and Table 4. The Tart Cherry group had significantly lower oxidized LDL (7.0 ± 1.0 U/L, time-by-treatment P = .047) and VCAM-1 (591 ± 46 ng/mL, time-by-treatment P = .036) levels than the Control group (10.0 ± 1.4 U/L and 727 ± 43 ng/mL, respectively) at 12 weeks. TC tended (time-by-treatment P = .08) to be lower in Tart Cherry (166 ± 10 mg/dL) than Control (191 ± 9 mg/dL) at 12 weeks. HOMA-%B was significantly higher in Tart Cherry at 6 (149.8 ± 15.5, time-by-treatment P = .002) and 12 (126.6 ± 11.9, time-by-treatment P = .035) weeks than Control (92.1 ± 13.4 and 88.4 ± 13.1, respectively), and when compared to baseline within the Tart Cherry group (100.4 ± 8.9, time P < .05). No significant differences were observed for the remaining parameters.
FIG. 2.
(A) Total cholesterol, (B) oxidized low-density lipoprotein (oxLDL), (C) vascular cell adhesion molecule-1, and (D) intercellular adhesion molecule-1 responses following 6 and 12 weeks of consuming 480 mL tart cherry juice or placebo in adults with metabolic syndrome. Data are presented as mean ± SEM. *P < 0.05, **P < 0.1.
Table 4.
Blood Biomarkers of Cardiovascular and Metabolic Health at Baseline and 6 and 12 Weeks After Daily Tart Cherry Juice and Control Drink Consumption
| Tart cherry (n = 9) | Control (n = 10) | Time-by-treatment, P-value | |
|---|---|---|---|
| TG (mg/dL) | |||
| Baseline | 173 ± 29 | 129 ± 27 | .29 |
| 6 Weeks | 148 ± 29 | 137 ± 27 | .80 |
| 12 Weeks | 162 ± 29 | 128 ± 27 | .40 |
| HDL-C (mg/dL) | |||
| Baseline | 44 ± 2 | 47 ± 2 | .45 |
| 6 Weeks | 41 ± 2 | 45 ± 2 | .21 |
| 12 Weeks | 41 ± 2 | 44 ± 2 | .31 |
| LDL-C (mg/dL) | |||
| Baseline | 104 ± 9 | 121 ± 8 | .16 |
| 6 Weeks | 97 ± 9 | 116 ± 8 | .12 |
| 12 Weeks | 88 ± 9 | 106 ± 8 | .14 |
| TAS (mg/dL) | |||
| Baseline | 1.44 ± 0.06 | 1.49 ± 0.06 | .59 |
| 6 Weeks | 1.43 ± 0.06 | 1.54 ± 0.06 | .25 |
| 12 Weeks | 1.52 ± 0.06 | 1.51 ± 0.06 | .94 |
| Leptin (ng/mL) | |||
| Baseline | 41.2 ± 7.7 | 44.3 ± 6.9 | .76 |
| 6 Weeks | 44.0 ± 7.7 | 44.7 ± 6.9 | .95 |
| 12 Weeks | 42.4 ± 7.7 | 44.5 ± 6.9 | .84 |
| Adiponectin (μg/mL) | |||
| Baseline | 5.2 ± 0.8 | 5.9 ± 0.7 | .52 |
| 6 Weeks | 5.8 ± 0.8 | 5.5 ± 0.7 | .79 |
| 12 Weeks | 5.9 ± 0.8 | 6.0 ± 0.7 | .87 |
| Glucose (mg/dL) | |||
| Baseline | 93 ± 15 | 117 ± 15 | .27 |
| 6 Weeks | 91 ± 15 | 120 ± 15 | .18 |
| 12 Weeks | 91 ± 15 | 121 ± 15 | .17 |
| Insulin (pmol/L) | |||
| Baseline | 74.8 ± 14.8 | 70.1 ± 14.0 | .82 |
| 6 Weeks | 114.2 ± 14.8 | 84.0 ± 14.0 | .15 |
| 12 Weeks | 84.9 ± 14.8 | 72.2 ± 14.0 | .54 |
| HOMA-%B | |||
| Baseline | 100.4 ± 8.9 | 87.8 ± 11.2 | .48 |
| 6 Weeks | 149.8 ± 15.5* | 92.1 ± 13.4 | .002 |
| 12 Weeks | 126.6 ± 11.9* | 88.4 ± 13.1 | .035 |
| HOMA-%S | |||
| Baseline | 102.2 ± 16.0 | 96.6 ± 15.2 | .80 |
| 6 Weeks | 67.1 ± 16.0 | 88.0 ± 15.2 | .35 |
| 12 Weeks | 72.2 ± 16.0 | 94.5 ± 15.2 | .32 |
| HOMA-IR | |||
| Baseline | 1.2 ± 0.3 | 1.4 ± 0.3 | .72 |
| 6 Weeks | 2.1 ± 0.3 | 1.6 ± 0.3 | .21 |
| 12 Weeks | 1.6 ± 0.3 | 1.4 ± 0.3 | .65 |
Data are mean ± SEM.
Statistical significance compared to baseline.
HOMA-%B, homeostatic model assessment of beta cell function; HOMA-%S, homeostatic model assessment of insulin sensitivity; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low-density lipoprotein-cholesterol; TAS, total antioxidant status.
Anthropometrics and fat mass
The mean body weights, BMI, waist and hip circumferences, and body composition assessments at baseline and 6 and 12 weeks are presented in Table 5. At 6 weeks, there was a significant time-by-treatment interaction for waist-to-hip ratio (0.81 ± 0.10 in Tart Cherry vs. 0.95 ± 0.03 in Control, time-by-treatment P = .03). No significant time or time-by-treatment interaction effect for the other parameters or time points was observed.
Table 5.
Anthropometric and Dual-Energy X-ray Absorptiometry Derived Fat Mass Measurements at Baseline and 6 and 12 Weeks After Daily Tart Cherry Juice and Control Drink Consumption
| Tart cherry (n = 9) | Control (n = 10) | Time-by-treatment, P-value | |
|---|---|---|---|
| Weight (kg) | |||
| Baseline | 102.7 ± 6.3 | 98.1 ± 5.9 | .61 |
| 6 Weeks | 104.2 ± 6.3 | 97.6 ± 5.9 | .46 |
| 12 Weeks | 103.9 ± 6.3 | 98.9 ± 5.9 | .57 |
| BMI (kg/m2) | |||
| Baseline | 34.3 ± 1.9 | 33.5 ± 1.8 | .76 |
| 6 Weeks | 34.8 ± 1.9 | 33.3 ± 1.8 | .58 |
| 12 Weeks | 34.7 ± 1.8 | 33.7 ± 1.8 | .72 |
| WC (cm) | |||
| Baseline | 107.4 ± 3.9 | 108.8 ± 3.7 | .80 |
| 6 Weeks | 107.1 ± 3.9 | 109.0 ± 3.7 | .73 |
| 12 Weeks | 109.2 ± 3.9 | 110.6 ± 3.7 | .80 |
| HC (cm) | |||
| Baseline | 121.0 ± 4.5 | 115.0 ± 4.3 | .35 |
| 6 Weeks | 119.2 ± 4.5 | 115.0 ± 4.3 | .50 |
| 12 Weeks | 119.0 ± 4.5 | 115.2 ± 4.3 | .54 |
| WC:HC | |||
| Baseline | 0.89 ± 0.03 | 0.95 ± 0.03 | .38 |
| 6 Weeks | 0.81 ± 0.10 | 0.95 ± 0.03 | .03 |
| 12 Weeks | 0.92 ± 0.02 | 0.96 ± 0.02 | .48 |
| Android fat mass | |||
| Baseline | 4538.6 ± 442.6 | 4416.4 ± 419.8 | .84 |
| 6 Weeks | 4569.8 ± 442.6 | 4354.68 ± 419.8 | .73 |
| 12 Weeks | 4549.5 ± 442.6 | 4265.8 ± 419.8 | .65 |
| Gynoid fat mass | |||
| Baseline | 6882.9 ± 876.7 | 6386.6 ± 831.7 | .69 |
| 6 Weeks | 6887.9 ± 876.7 | 6375.3 ± 831.7 | .68 |
| 12 Weeks | 6863.8 ± 876.7 | 5839.9 ± 831.7 | .41 |
| Total fat mass | |||
| Baseline | 43,305 ± 4385 | 41,806 ± 4159 | .81 |
| 6 Weeks | 42,946 ± 4385 | 41,330 ± 4159 | .79 |
| 12 Weeks | 43,054 ± 4385 | 39,508 ± 4159 | .56 |
Data are mean ± SEM.
HC, hip circumference; WC, waist circumference.
Dietary assessment
Energy and macronutrient intake did not change from baseline to 12 weeks in either the Tart Cherry or Control groups, and there were no significant difference between groups at baseline or 12-week time points (data not shown).
Discussion
The purpose of this study was to investigate the effects of tart cherry juice consumption on hemodynamics, arterial stiffness, and blood biomarkers of cardiovascular and metabolic health in men and women with MetS. We found that consuming two cups of tart cherry juice per day for 12 weeks reduced oxidized LDL and VCAM-1, two cardiometabolic biomarkers involved in atherosclerosis, in men and women with MetS. Other blood biomarkers, hemodynamics, and arterial stiffness parameters were unaltered by the treatments.
Although advancing age is the primary risk factor for developing CVD, MetS can accelerate this process, in large part, due to vascular dysfunction. This includes vascular endothelial dysfunction and arterial stiffness, which are largely driven by oxidative stress and inflammation in the vasculature.7,8 These processes not only impair blood flow and lead to increases in systolic blood pressure but also promote the formation of atherosclerotic plaque within the arterial intima. When activated by stimuli such as high LDL-C levels and reactive oxygen species, circulating immune cells produce proinflammatory cytokines that interact with and provoke inflammation in endothelial cells. Endothelial cell inflammation, in turn, promotes oxidative stress and atherogenesis. Indeed, activated endothelial cells express adhesion molecules, such as VCAM-1 and ICAM-1, which promote the recruitment and adhesion of monocytes to the endothelium and subsequently to the arterial intima where they differentiate into macrophages, take up oxidized LDL, and subsequently form foam cells. Foam cell formation promotes the development of atherosclerotic plaque.9,29
As such, our findings that oxidized LDL and VCAM-1 levels (with a trend for cholesterol levels to be lower in the Tart Cherry group vs. Control) were lower following 12 weeks of tart cherry juice consumption compared to a control drink are promising with respect to reducing the risk of atherosclerosis in this population (Fig. 2). Nonetheless, although there were significant time-by-treatment interactions such that oxidized LDL and VCAM-1 were significantly lower than Control and a trend for cholesterol levels to be lower in the Tart Cherry group than Control, these values were not statistically lower than baseline at 12 weeks and thus should be interpreted with caution.
Limited clinical research has examined the impact of chronic tart cherry juice consumption on parameters implicated in atherosclerosis. Our findings that tart cherry juice consumption significantly reduced oxidized LDL levels are supported by those of Chai et al.19 who observed a borderline significant reduction in oxidized LDL in older adults following 12 weeks of consuming two cups per day of tart cherry juice. They also observed a significant difference between groups for TC (19.11 mg/dL difference), while we observed a trend for a significant difference between groups (25 mg/dL difference, Fig. 2). Chai et al.19 also observed significant reductions in other biomarkers of oxidative stress and inflammation, including C-reactive protein (CRP) and malondialdehyde. Martin et al.20 observed significant improvements in blood biomarkers of inflammation (i.e., erythrocyte sedimentation rate, monocyte chemoattractant protein-1, and tumor necrosis factor-alpha) following 4 weeks of consuming one cup per day of tart cherry juice in adults with overweight and obesity, although they did not observe reductions in CRP. Conversely, a recent study by Lear et al.30 found that consumption of 30 mL tart cherry juice concentrate twice daily for 4 weeks did not alter the proinflammatory biomarkers interleukin-6 or CRP in a middle-aged population. Lipid profiles, oxidized LDL, and adhesion molecules were not assessed in that study. The aforementioned proinflammatory and oxidative stress biomarkers were not assessed in this study; however, we did observe lower levels of the VCAM-1 following tart cherry juice consumption compared to control (Fig. 2). VCAM-1 is an adhesion molecule induced by proinflammatory cytokines and thus is suggestive of reduced inflammation. Although these studies have discrepant study designs, populations, and outcome measures, the findings suggest that daily consumption of tart cherry juice may modulate lipid metabolism, inflammation, and oxidative stress in humans, thereby reducing the risk of atherosclerosis.
We did not observe any improvement in hemodynamic or arterial stiffness parameters in this study (Table 2). With respect to arterial stiffness and vascular function in general, our findings are supported by previous observations that tart cherry juice consumption did not improve measures of arterial stiffness.21,22 Contrary to our findings, others have demonstrated improvements in blood pressure following acute and chronic tart cherry juice consumption in older adults with elevated blood pressure or hypertension.18,21 This discrepancy may be due to not everyone having elevated systolic blood pressure (n = 3 out of 19) or hypertension (n = 9 out of 19) at baseline in our study. Although our study population had MetS, participants in the intervention group were relatively young and MetS characteristics were not required to be the same among all individuals included.
The previous research that observed acute reductions in blood pressure was performed in young and middle-aged adults with elevated blood pressure or stage 1-hypertension.21,31 Although Chai et al.18 reported a significant impact of 12 weeks of tart cherry juice consumption on systolic blood pressure, there was no significant decrease in systolic blood pressure from baseline in the intervention group. Therefore, the significant time-by-treatment interaction may indicate that the change in systolic blood pressure was influenced by the greater value at baseline. Thus, it is possible that tart cherry juice consumption is more effective at improving blood pressure and vascular function in populations with greater impairments at baseline.
It is also possible that the polyphenols in tart cherry juice absorbed in the acute setting, that is, those absorbed in the small intestine vs. colonic metabolites, may be responsible for acute modulation of hemodynamics and vascular function. In fact, Keane et al.21 noted that acute improvements in blood pressure were closely associated with increases in circulating protocatechuic acid and vanillic acid at 1–2 h. Similarly, blueberries are also rich in polyphenols such as anthocyanins and phenolic acids, and acute modulation of vascular endothelial function assessed as flow-mediated dilation has been correlated with circulating phenolic acids absorbed in the small intestine.32–34 Nonetheless, chronic blueberry consumption has been shown to modulate blood pressure, arterial stiffness, and vascular endothelial function.33,35,36 However, those studies used a whole food freeze-dried blueberry powder, whereas the tart cherry interventions were provided in the form of juice, which is devoid of dietary fiber. Whether or not use of a whole food tart cherry intervention would exert differential effects on hemodynamic and vascular function parameters following chronic consumption remains unknown, but is worth exploration. In addition, previous research has not explored the impact of tart cherry juice consumption on vascular endothelial function, with the exception of the study by Keane et al.21 who assessed microvascular reactivity in response to skin perfusion. The acute and chronic effects of tart cherry juice consumption on vascular endothelial function should be investigated in future research.
The mechanisms responsible for the observed improvements in parameters associated with atherogenesis cannot be determined from this study. It is possible that tart cherry polyphenols and their metabolites inhibit vascular oxidative stress and inflammation. Total antioxidant status was not improved by tart cherry juice consumption; however, this and other nonspecific biomarkers may not reflect what is occurring at the cellular level in the vascular endothelium. Importantly, oxidized LDL and VCAM-1 are biomarkers specific to the vascular tissue.37 Future animal studies are needed to directly investigate the impact of tart cherry juice consumption on atherosclerosis, while focusing on determining mechanisms of action. Also, these findings warrant further investigation into the impact of tart cherry juice consumption on factors associated with atherosclerosis in humans. Specifically, larger sample sizes are needed for future clinical studies, and inclusion/exclusion criteria should standardize specific MetS criteria, while ensuring that groups are age matched.
Study limitations
There are several limitations of this study that should be acknowledged. First, the inclusion and exclusion criteria were not designed to standardize which MetS criteria study participants needed to have to qualify for the study (i.e., qualification was based on having any three of the five MetS criteria). Hence, not all individuals met the same criteria. Thus, it is possible that standardization of which three MetS criteria participants were required to have to enroll in the trial may have provided a more homogenous study population. Second, the age requirement for inclusion and exclusion criteria (20–40 years) was revised halfway through the study (to 20–60 years) to increase enrollment. Due to pregenerated randomization, subjects recruited later in the study were primarily in the Control group, many of whom were older in age, thereby leading to a significant difference between groups for age. It is possible this led to selection bias in this study, and thus the findings of this study. Specifically, as age is associated with vascular dysfunction and increased cardiometabolic risk, this could have affected the results of the study. However, the increase in aortic PWV is significant in individuals older than 50 years38 and the mean age of the control group was 44 years. In addition, cardiometabolic parameters were similar in both groups at baseline, despite the age difference, suggesting that arterial aging was not evident in the control group. Nevertheless, the findings of this study are promising, even in the presence of selection bias. Although blood glucose levels were numerically higher in the Control group versus the Tart Cherry group, there were no significant differences between groups at screening or baseline for blood glucose levels. It is possible that the Control group had a higher level of insulin resistance than the Tart Cherry group; however, because fasting blood glucose levels provide a snapshot in time rather than average blood glucose control, and hemoglobin A1c was not assessed in this study, this cannot be confirmed at this time.
Another remaining question, and limitation of this study, is which compounds may have contributed to the effects of tart cherry juice consumption in this study. The tart cherry juice used contained higher amounts of sodium and potassium than the placebo, and both minerals are known to affect cardiovascular health.39 However, the amount of sodium present in the treatment was small (the equivalent to ∼1% of the recommended maximum 2300 mg daily intake of sodium) and thus is not likely to have a major effect. Because hemodynamic and vascular parameters were not affected by the tart cherry juice treatment, the potassium content is also unlikely to have exerted much of an effect on the outcomes assessed in this study. Finally, as this clinical trial was designed as a pilot, the sample size was small and thus, the number of subjects enrolled could be insufficient to detect significant modulation of some of the biomarkers examined. Considering the limitations of this study, our findings should be interpreted with caution. Nonetheless, the study does have several strengths, including the randomized, single-blind, placebo-controlled design, the 12-week duration of treatment, and the population studied. These findings provide enough information to support future clinical trials.
In conclusion, the results of this study suggest that daily consumption of tart cherry juice may attenuate processes involved in atherosclerosis in adults with MetS without having an effect on hemodynamics or arterial stiffness. These findings are important to the field of nutrition as they provide evidence that the addition of a single functional food to the diet may reduce the risk for atherosclerosis in a high-risk population. However, the pilot nature of the study, population, potential selection bias, and strength of the evidence necessitate these findings to be interpreted with caution. Larger, more rigorous randomized controlled trials and mechanistic studies are needed.
Disclaimer
Contents are the authors' sole responsibilities and do not necessarily represent official NIH views.
Authors' Contributions
Conceptualization, S.A.J, B.H.A., and A.F.; Methodology, S.A.J, B.H.A., and A.F.; Data Curation, S.A.J., N.N., N.S.A., and N.L.; Investigation, S.A.J, N.N, S.P., S.J., N.S.A., S.A., G.P., N.S.L., E.A.C., E.M.F., K.S.G., and M.L.E. Formal Analysis, M.E.P.; Writing-Original Draft Preparation, S.A.J.; Writing-Reviewing and Editing, S.A.J., N.N., S.P., S.J.J., N.S.A., S.A., G.P., N.S.L., E.A.C., E.M.F., K.S.G., M.L.E., M.E.P., B.H.A., and A.F.; Visualization, S.A.J.; Supervision, S.A.J., B.H.A., and A.F.; Project Administration, S.A.J., B.H.A., and A.F.; Funding Acquisition, S.A.J., B.H.A., and A.F.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by an unrestricted research grant from the Cherry Research Committee of the Cherry Marketing Institute. The funders had no role in the study design, data collection, data analysis or interpretation, or writing of the article. Blood biochemical analyses performed at the Colorado Clinical and Translational Sciences Institute (University of Colorado-Denver) were supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082-04.
References
- 1. Grundy SM, Cleeman JI, Daniels SR, et al. : Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 2. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ: Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 3. Iglseder B, Cip P, Malaimare L, Ladurner G, Paulweber B: The metabolic syndrome is a stronger risk factor for early carotid atherosclerosis in women than in men. Stroke 2005;36:1212–1217 [DOI] [PubMed] [Google Scholar]
- 4. Tzou WS, Douglas PS, Srinivasan SR, et al. : Increased subclinical atherosclerosis in young adults with metabolic syndrome: the Bogalusa Heart Study. J Am Coll Cardiol 2005;46:457–463 [DOI] [PubMed] [Google Scholar]
- 5. Schillaci G, Pirro M, Vaudo G, et al. : Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension 2005;45:1078–1082 [DOI] [PubMed] [Google Scholar]
- 6. Czernichow S, Bertrais S, Blacher J, et al. : Metabolic syndrome in relation to structure and function of large arteries: A predominant effect of blood pressure. A report from the SU.VI.MAX. Vascular Study. Am J Hypertens 2005;18(9 Pt 1):1154–1160 [DOI] [PubMed] [Google Scholar]
- 7. Grundy SM: Metabolic syndrome update. Trends Cardiovasc Med 2016;26:364–373 [DOI] [PubMed] [Google Scholar]
- 8. LaRocca TJ, Martens CR, Seals DR: Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev 2017;39:106–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hulsmans M, Holvoet P: The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 2010;14:70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson SA, Litwin NS, Seals DR: Age-related vascular dysfunction: What registered dietitian nutritionists need to know. J Acad Nutr Diet 2019;119:1785–1796 [DOI] [PubMed] [Google Scholar]
- 11. Deedwania PC, Volkova N: Current treatment options for the metabolic syndrome. Curr Treat Options Cardiovasc Med 2005;7:61–74 [DOI] [PubMed] [Google Scholar]
- 12. Kelley DS, Adkins Y, Laugero KD: A review of the health benefits of cherries. Nutrients 2018;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A: Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirakosyan A, Seymour E, Llanes DEU, Kaufman PB, Bolling SF: Chemical profile and antioxidant capacities of tart cherry products. Food Chem 2009;115:20–25 [Google Scholar]
- 15. Bonerz D, Würth K, Dietrich H, Will F: Analytical characterization and the impact of ageing on anthocyanin composition and degradation in juices from five sour cherry cultivars. Eur Food Res Technol 2007;224:355–364 [Google Scholar]
- 16. Seymour EM, Singer AA, Kirakosyan A, Urcuyo-Llanes DE, Kaufman PB, Bolling SF: Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food 2008;11:252–259 [DOI] [PubMed] [Google Scholar]
- 17. Seymour EM, Lewis SK, Urcuyo-Llanes DE, et al. : Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food 2009;12:935–942 [DOI] [PubMed] [Google Scholar]
- 18. Chai SC, Davis K, Wright RS, Kuczmarski MF, Zhang Z: Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: a randomized controlled trial. Food Funct 2018;9:3185–3194 [DOI] [PubMed] [Google Scholar]
- 19. Chai SC, Davis K, Zhang Z, Zha L, Kirschner KF: Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients 2019;11:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin KR, Burrell L, Bopp J: Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: a randomized, crossover pilot study. Food Funct 2018;9:5290–5300 [DOI] [PubMed] [Google Scholar]
- 21. Keane KM, George TW, Constantinou CL, Brown MA, Clifford T, Howatson G: Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am J Clin Nutr 2016;103:1531–1539 [DOI] [PubMed] [Google Scholar]
- 22. Lynn A, Mathew S, Moore CT, et al. : Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr 2014;69:122–127 [DOI] [PubMed] [Google Scholar]
- 23. Laurent S, Cockcroft J, Van Bortel L, et al. : Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 24. O'Rourke MF, Pauca AL: Augmentation of the aortic and central arterial pressure waveform. Blood Press Monit 2004;9:179–185 [DOI] [PubMed] [Google Scholar]
- 25. Nichols WW: Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 2005;18(1 Pt 2):3S–10S [DOI] [PubMed] [Google Scholar]
- 26. Wilkinson IB, Hall IR, MacCallum H, et al. : Pulse-wave analysis: clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol 2002;22:147–152 [DOI] [PubMed] [Google Scholar]
- 27. Yamashina A, Tomiyama H, Takeda K, et al. : Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002;25:359–364 [DOI] [PubMed] [Google Scholar]
- 28. Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 29. Gimbrone MA Jr., Garcia-Cardena G: Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 2016;118:620–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lear R, O'Leary M, O'Brien Andersen L, et al. : Tart cherry concentrate does not alter the gut microbiome, glycaemic control or systemic inflammation in a middle-aged population. Nutrients 2019;11:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keane KM, Haskell-Ramsay CF, Veasey RC, Howatson G: Montmorency Tart cherries (Prunus cerasus L.) modulate vascular function acutely, in the absence of improvement in cognitive performance. Br J Nutr 2016;116:1935–1944 [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez-Mateos A, Del Pino-Garcia R, George TW, Vidal-Diez A, Heiss C, Spencer JP: Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol Nutr Food Res 2014;58:1952–1961 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez-Mateos A, Istas G, Boschek L, et al. : Circulating anthocyanin metabolites mediate vascular benefits of blueberries: insights from randomized controlled trials, metabolomics, and nutrigenomics. J Gerontol A Biol Sci Med Sci 2019;74:967–976 [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, et al. : Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr 2013;98:1179–1191 [DOI] [PubMed] [Google Scholar]
- 35. Johnson SA, Figueroa A, Navaei N, et al. : Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: a randomized, double-blind, placebo-controlled clinical trial. J Acad Nutr Diet 2015;115:369–377 [DOI] [PubMed] [Google Scholar]
- 36. Stull AJ, Cash KC, Champagne CM, et al. : Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015;7:4107–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gradinaru D, Borsa C, Ionescu C, Prada GI: Oxidized LDL and NO synthesis—Biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev 2015;151:101–113 [DOI] [PubMed] [Google Scholar]
- 38. McEniery CM, Yasmin, Hall IR, et al. : Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760 [DOI] [PubMed] [Google Scholar]
- 39. National Academies of Sciences, Engineering, and Medicine: Dietary Reference Intakes for Sodium and Potassium: National Academies Press, Washington, DC, 2019 [PubMed] [Google Scholar]