Abstract
Background: The safety of higher dose vitamin D (vitD) supplementation in women who change from exclusive or full breastfeeding to combination feeding or who continue supplementation after cessation of breastfeeding is unknown.
Objective: Compare vitD supplementation safety of 6,400 to 400 IU/day and 2,400 IU/day using specific laboratory parameters in postpartum women and their infants through 7 months postpartum by feeding type.
Design: In this randomized controlled trial, mothers (exclusively breastfeeding or formula-feeding) were randomized at 4–6 weeks' postpartum to 400, 2,400, or 6,400 IU vitD3 (cholecalciferol)/day for 6 months. Breastfeeding infants in 400 IU group received oral 400 IU vitD3/day; infants in 2,400 and 6,400 IU groups received placebo. Maternal safety parameters (serum vitD, 25-hydroxy-vitamin D [25(OH)D; calcidiol], calcium, phosphorus, intact PTH; urinary calcium/creatinine ratios; and feeding type/changes) were measured monthly; infant parameters were measured at months 1, 4, and 7. Sufficiency was defined as 25(OH)D >50 nmol/L. Feeding type was defined as exclusive/full, combination, or formula-feeding. Data were analyzed using SAS 9.4.
Results: Four hundred nineteen mother-infant pairs were randomized into the three treatment groups and followed: 346 breastfeeding and 73 formula-feeding pairs. A dose of 6400 IU/day safely and significantly increased maternal vitD and 25(OH)D from baseline in all mothers regardless of feeding type (p < 0.0001) and was superior to the 400 and 2,400 IU groups in achieving vitD sufficiency with no other differences in safety parameters by treatment or feeding type. Infants in the 2,400 IU group were more likely vitD-deficient than the other groups; otherwise, there were no infant safety parameter differences.
Conclusions: While 6,400 IU/day was more effective than 400 or 2,400 IU/day in achieving maternal vitD sufficiency in all feeding groups, the groups did not differ on other safety parameters. Similarly, infant safety parameters did not differ by treatment group or feeding status.
Clinical Trial Registration: FDA IND Number: 66,346; ClinicalTrials.gov Number: NCT00412074.
Keywords: vitamin D, cholecalciferol, lactation, infant, postpartum, RCT
Introduction
Maternal vitamin D (vitD) supplementation with 6,400 IU/day alone during full lactation is effective in improving the breastfeeding infant's vitD status.1 Prior reports focused on the effectiveness of supplementation rather than safety.1,2 There remains a concern of the safety of higher dose vitD supplementation in women who change from exclusive or full breastfeeding to combination feeding or who continue supplementation after cessation of breastfeeding.3 To date, a comparison of the safety of sustained high-dose supplementation with vitD in women who are fully breastfeeding with those providing a combination of breast milk and formula (combination feeding) or who are exclusively formula feeding has not been reported; and hence, the focus of this report. It was hypothesized that high-dose vitD supplementation women compared to control postpartum women—lactating and nonlactating—would have improved vitD status with similar safety parameters throughout the study period. The results of this safety aspect of the National Institute of Child Health and Human Development (NICHD)-sponsored vitD supplementation during lactation randomized controlled trial are reported here.
Materials and Methods
Study sites
As previously described, the study was conducted at the Medical University of South Carolina (MUSC; latitude 32.78°N) and the University of Rochester (U of R; latitude 43.15°N).1,2 Approval for this two-site study was granted by: (1) MUSC's Institutional Review Board for Human Subjects HR #16536 and the Clinical and Translational Research Center (CTRC; Protocol #752); and (2) the U of R's Institutional Review Board for Human Subjects (#14460) and the CTRC (Protocol #1129). The study was registered via ClinicalTrials.gov #NCT00412074.
Study design
This was a randomized, double blind, comparative effectiveness, and safety trial of three doses of vitD supplementation (400, 2,400, and 6,400 IU vitD3/day) in breastfeeding mothers and their infants and nonlactating control pairs that met the criteria for an optimized nutrient study.4 The 2,400 IU arm ended due to safety concerns of increased vitD deficiency in the infants in February 2009 (with safety results of the three groups up to that point presented in Supplementary Tables S1, S2, S3, S4A, and B).4 The efficacy results of this study were previously reported.1 As part of the safety concern, a cohort of women who were exclusively formula-feeding were included in the study to assure safety if a woman continued high-dose vitD supplementation and had stopped breastfeeding.
Enrollment and randomizaton of breastfeeding mothers and their infants
Following written informed consent, mothers were randomized using block randomization of 10 to 1 of three vitD supplementation regimens: Group 1: Control, 400 IU (10 μg) vitD3/day (0 IU vitD3 placebo and one prenatal vitamin containing 400 IU vitD3); Group 2: 2,400 IU (60 μg) vitD3/day (2,000 IU vitD3/day and one prenatal vitamin containing 400 IU vitD3); and Group 3: 6,400 IU (160 μg) vitD3/day (6,000 IU vitD3 and one prenatal vitamin containing 400 IU vitD3). The breastfeeding infants were given a liquid suspension (one drop per day) of vitD3 or placebo (Bio-D-Mulsion, Biotics Research Corp, Rosenberg, TX): those infants in Group 1 received 400 IU vitD3 and those in Groups 2 and 3 received placebo (containing 0 IU vitD3).
Enrollment of exclusively formula-feeding mothers and infants
Mothers who chose to exclusively formula-feed were eligible for participation in the study at the Charleston site only. The formula-feeding women were randomized to either treatment group as outlined above. Their infants, who were receiving any commercially available infant formula containing 400 IU vitD/L, were not supplemented as part of the study.
Subjects
Inclusion criteria
Exclusively breastfeeding mothers or formula-feeding mothers and their singleton infants ≥35 weeks of gestation at birth (receiving either exclusive breast milk or formula at the time of study entry5,6) within 4-to-6 weeks'postpartum in good general health were eligible for inclusion in the study. Women who chose to begin combination or exclusively formula-feeding at or after 4 months postpartum were eligible to continue their participation in the study.
Exclusion criteria
Exclusion criteria included any preexisting maternal health problem, twin/multiple pregnancy, or if the infant was <35 weeks of gestation at birth and with a health problem. Infants receiving combination feedings (defined as feedings of <90% breast milk feedings and formula feedings) at the onset of the trial were not eligible to participate.
Definition of feeding type
Feeding type was defined a priori as exclusively or fully breastfeeding (referred to as breastfeeding hereafter), combination feeding (both breast milk/breastfeeding and formula were given to the infant on a daily basis), or exclusive formula-feeding (referred to as formula-feeding hereafter).
Outcome measures
Anthropometric measures
(1) Maternal: at each study visit, maternal weight was obtained; maternal height was measured at visit 1 (V1 at 1 month postpartum) and used to determine monthly BMI, which was calculated as follows: weight (kg)/[height (m)]2. (2) Infant: each infant's weight (kg), length (cm) using a length board, and head circumference (cm) were measured according to standard clinical pediatric practice at V1 and each monthly visit.
Laboratory safety measurements
(1) Maternal and infant total circulating 25-hydroxy-vitamin D (25(OH)D) and vitD (parent compound) were measured using high-performance liquid chromatography (HPLC) and radioimmunoassay techniques, respectively, as previously described.7 Deficiency was defined a priori as total circulating 25(OH)D <50 nmol/L (<20 ng/mL).7 The inter- and intra-assay coefficient of variation was ≤10%. Given the limitation of reagents for the measurement, the total circulating concentration of the parent compound vitD was measured in a subset of sequential mothers and infants using HPLC as previously described.8 The laboratory participated in Vitamin D External Quality Assessment Scheme (DEQAS) throughout the study period.9
(2) Maternal and infant circulating intact parathyroid hormone (iPTH) concentrations were measured by immune-radiometric assay as previously described and expressed as pmol/L (Diasorin Biotechnology Company, Stillwater, MN).10
(3) Measurement of Maternal and infant serum calcium and creatinine (mmol/L) and urinary calcium:creatinine ratios were measured using standard methodology and laboratory normative data at each site. Cross-validation between laboratories was performed for 5% of the samples (interassay variation 5.4%). Maternal values were measured monthly while infant values were reported at V1, visit 4 (V4 at 4 months' postpartum) and visit 7 (V7 at 7 months' postpartum); all values were reported as mmol/L. Maternal urinary calcium/creatinine ratios of 0.8 or higher resulted in notification to the Data and Safety Monitoring Board (DSMB). Infant ratios vary by postnatal age and are variable but decrease with advancing postnatal age.1
Adherence to protocol
Adherence to protocol was assessed through pill count for the mothers and weighing of the vitD bottle for the infant at each visit. The adherence rate was derived from the actual pill count or bottle weight over the expected pill count or weight, respectively, and expressed as a percentage of expected.
Deficiency safety measure and stopping of the 2,400 IU arm
As part of the study's data safety and monitoring plan, any breastfeeding infant with a total circulating 25(OH)D <20 ng/mL at V4 received open-label 400 IU vitD3/day. Because of the safety measure in place at the start of clinical trial, and concern that infants of mothers in the 2,400 IU group would be more likely to have a total circulating 25(OH)D <20 ng/mL, an interim analysis was requested by the DSMB. This concern was based on prior pharmacokinetic studies during lactation.2,11 As previously described,1 the DSMB oversaw this interim analysis and deemed that the 2,400 IU arm of the study should be terminated due to disproportionate number of infants in that arm falling <20 ng/mL threshold for total circulating 25(OH)D adequacy. Results up to the ending of the 2,400 IU arm in February 2009 are reported separately (as Supplementary Tables S1, S2, S3, S4A, and B) from the analyses of the two arms that continued until the completion of the trial.
Statistical analysis
Sample size calculation
The primary study was powered with site included in models to account for variability between sites to assess whether the expected increase in vitD in the experimental group was greater than that of the control group beyond random variability.1 These calculations were conducted with 80% power, two-sided test, and a type I error of 0.05. For the assessment of safety, the approach was a noninferiority design since it was assumed that serum calcium, serum creatinine, urinary calcium: creatinine ratio, and the iPTH would not differ between groups (assumption of equal means). The midlevel value of the normal ranges of these markers was taken as the assumed values of what would be observed in the control group. Our sample size was sufficient to conduct an assessment with 99% power.
Statistical analyses
Statistical analyses were performed using SAS software version 9.4 (SAS, Cary, NC). Descriptive statistics were used to characterize and compare laboratory values between treatment groups at baseline. Analysis of variance was used to test for differences among treatment groups for normally distributed variables (i.e., weight, BMI, dietary calcium, dietary phosphorus, dietary vitD, gestational age, birth weight, and 25(OH)D). The Kruskal–Wallis test was used for analyses of variables that were not normally distributed (i.e., interpregnancy interval, gravidity, parity, and health rating). The percent of women and infants by treatment group with 25(OH)D <50 nmol/L was compared at V1, V4, and V7.1 Continuous variables were summarized using means, standard deviations for normally distributed measures; medians and interquartile ranges for nonparametric measures, as appropirate, and categorical variables were summarized using frequencies and percentages.
Regression methods to model 25(OH)D status (linear and logistic) included variables that showed a significant association with the laboratory values in the bivariate analyses as well as site. Generalized linear mixed-effect models were used to measure the relationship between treatment (400, 2,400, or 6,400 IU), visit (which corresponded to the month postpartum), and treatment by time interaction while adjusting for other covariates, predicting each safety outcome (serum calcium, serum creatinine, iPTH concentration, and urine calcium: creatinine ratio), and accounting for repeated measures per person. The other covariates in the model included baseline maternal 25(OH)D concentration, race/ethnicity (non-Hispanic African American, Hispanic, and white/Caucasian), feeding type (exclusive breastfeeding, combination-feeding or formula-feeding), and BMI. In addition, the respectivate baseline measurement for each outcome was also included in the models. Significance was set a priori as p < 0.05.
Results
Allocation of participants by treatment group
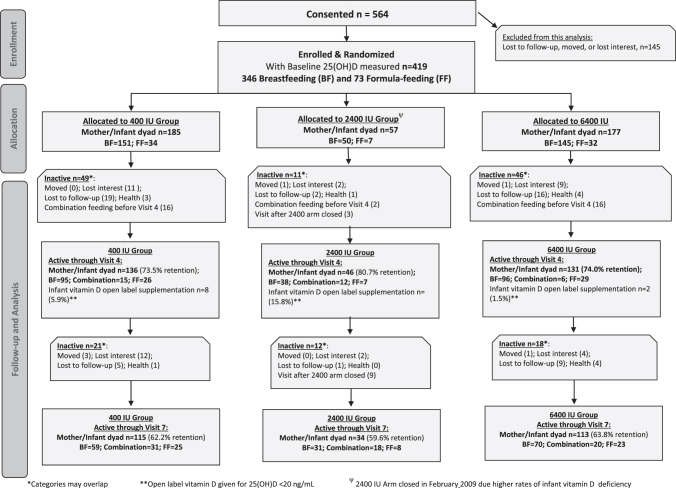
The allocation of participants following enrollment is found in Figure 1 (CONSORT diagram) for the exclusively breastfeeding mother/infant pairs and exclusively formula-feeding mother/infant pairs. Maternal and infant sociodemographic and clinical characteristics for 400 and 6,400 IU groups at study entry are summarized in Table 1. Baseline characteristics did not differ between the groups by treatment. Furthermore, there were no significant differences in baseline characteristics between the treatment groups on any of the measures analyzed before discontinuation of the 2,400 IU group (Supplementary Table S1). The attrition rate for the two remaining arms through completion of the study did not differ (see Supplementary Data).
FIG. 1.
Flow diagram of maternal and infant vitD supplementation study through 7 months postpartum. 25(OH)D, 25-hydroxy-vitamin D; vitD, vitamin D.
Table 1.
Baseline Maternal and Infant Sociodemographic and Clinical Characteristics of 400 and 6,400 IU Groups (n = 362)
| Baseline characteristics at time of enrollmenta | Treatment |
|
|---|---|---|
| 400 IU |
6,400 IU |
|
| n = 185 |
n = 177 |
|
| n (% of treatment) | n (% of treatment) | |
| Site | ||
| MUSC | 116 (62.7) | 102 (57.6) |
| Rochester | 69 (37.3) | 75 (42.4) |
| Feeding at baseline | ||
| Breastfeeding | 151 (81.6) | 145 (81.9) |
| Formula-feeding (at MUSC site only) | 34 (18.4) | 32 (18.1) |
| Maternal race/ethnicity | ||
| African American | 55 (29.7) | 58 (32.8) |
| Hispanic | 62 (33.5) | 58 (32.8) |
| White/Caucasian | 68 (36.8) | 61 (34.4) |
| Maternal education | ||
| Less than High School | 43 (23.4) | 22 (14.7) |
| High School | 38 (20.7) | 48 (27.1) |
| College | 103 (56.0) | 103 (58.2) |
| Maternal employment | ||
| Not full-time employed | 72 (38.9) | 58 (32.8) |
| Full-time employed | 113 (61.1) | 119 (67.2) |
| Maternal insurance status | ||
| Private insurance | 66 (35.7) | 75 (42.4) |
| Medicaid\no insurance | 119 (64.3) | 102 (57.6) |
| Maternal BMI | ||
| BMI ≥30 kg/m2 | 65 (36.5) | 58 (33.9) |
| BMI <30 kg/m2 | 113 (63.5) | 113 (66.1) |
| Season at initial visit | ||
| April–September | 97 (52.4) | 94 (53.4) |
| October–March | 88 (47.6) | 82 (46.6) |
| n | n | |
| Mean ± SD | Mean ± SD | |
| Days postpartum | 184 | 174 |
| 36.8 ± 7.7 | 36.4 ± 7.6 | |
| BMI | 135 | 130 |
| 29.0 ± 6.9 | 27.9 ± 5.2 | |
| Maternal Smart-Probe 400 Site: Forearmb |
184 | 177 |
| 52.3 ± 9.9 | 52.4 ± 9.9 | |
| Maternal dietary calcium (mg/day) | 54 | 50 |
| 967 ± 414 | 979 ± 544 | |
| Maternal dietary phosphorus (mg/day) | 54 | 50 |
| 1,384 ± 560 | 1,377 ± 719 | |
| Maternal dietary vitD (IU/day) | 54 | 50 |
| 172.9 ± 123.2 | 152.8 ± 111.3 | |
| n |
n |
|
|---|---|---|
| Median, 25–75 interquartile range | Median, 25–75 interquartile range | |
| Maternal interpregnancy interval (months) |
133 |
126 |
| 24.0, 12–43 |
24.0, 12–48 |
|
| Maternal gravidity |
184 |
177 |
| 2.0, 1–3 |
2.0, 1–3 |
|
| Maternal parity |
184 |
177 |
| 2.0, 1–3 |
2.0, 1–2 |
|
| Maternal health rating, 0–10c |
184 |
176 |
| 10.0, 8–10 |
10.0, 8–10 |
|
|
Infant characteristics |
n |
n |
|
Mean ± SD |
Mean ± SD |
|
| Infant birth weight (g) |
184 |
177 |
| 3337 ± 519 |
3391 ± 442 |
|
| Infant gestational age at birth (weeks) | 184 |
175 |
| 39.3 ± 1.4 | 39.4 ± 1.0 |
Chi-square analyses were used to test for differences in frequencies between treatment groups; Student's t-test analyses were used to test for differences in means between treatment groups; Mann–Whitney tests were used to test for differences in median between treatment groups.
Total number for each characteristic varies based on available data from the 400 and 6,400 IU arms of the study. The 2,400 IU group was not included in this analysis.
Maternal forearm skin pigmentation score derived using SmartProbe 400 (IMS, Portland, ME), a skin spectrophotometer that derives a score of 0–100 score, with absolute black equal to 0 and absolute white equal to 100, for a given region of the body. Forearm was chosen as the most representative of maximal sun exposure resulting in pigment darkening as a surrogate for sunlight exposure.
Maternal health rating was derived from question asked each mother using a Likert scale of 0–10, with 0 being the worse health and 10 being the best health.
BMI, body mass index; MUSC, Medical University of South Carolina; vitD, vitamin D.
Maternal and infant vitD status and safety parameters by treatment group and feeding type
The effect of treatment on maternal and infant safety laboratory measurements at V1, V4, and V7 for breastfeeding and formula-feeding mothers before discontinuing the 2,400 IU group did not show any differences in safety parameters (Supplementary Table S2). For the two remaining treatment groups—400 and 6,400 IU/day, maternal and infant safety parameters were compared at completion of the study.
In Table 2, a comparison by treatment at the three main timepoints—V1, V4, and V7 is provided for exclusively breastfeeding mothers and infants. There were no statistically significant differences at baseline on any of the vitD and safety parameters. At both V4 and V7, maternal total circulating 25(OH)D was greater in the 6,400 IU group versus the 400 IU group (p < 0.0001). There was a dose effect on the parent compound vitD by treatment with highest concentrations in mothers in the 6,400 IU group versus the 400 IU group (p < 0.0001). Maternal iPTH at V4 and V7 differed statistically between the groups (p = 0.04 and 0.004, respectively), with lowest values in the 6,400 IU group, but all values were within the normal range. Serum calcium and creatinine and urinary calcium:creatinine ratios did not differ at any of the study visits by treatment group with one exception: at V4 only, there was a modest increase in the ratio in the 6,400 IU group (0.3) compared to 400 IU group (0.2), but within the safety parameters of the study. In exclusively breastfeeding infants, 25(OH)D concentration was slightly higher in the 400 IU group compared to the 6,400 IU group at V4 (p = 0.03) only. In the subset of infants whose vitD (parent compound) was measured, there were no statistically significant differences at V4 or V7. When analyzed by treatment group, the infants did not differ on any of the safety measures.
Table 2.
Breastfeeding Maternal and Infant Biochemical Safety Measurements at Visits 1, 4, and 7 by 400 and 6,400 IU groups (n = 296)
| 400 IU (n = 151) | 6,400 IU (n = 145) | p-Value* | |
|---|---|---|---|
| Maternal | |||
| Serum 25(OH)D (nmol/L), mean ± SD | |||
| Visit 1 (n = 296) | 77.2 ± 31.8 | 83.5 ± 34.8 | 0.1 |
| Visit 4 (n = 210) | 78.5 ± 28.7 | 146.5 ± 47.3 | <0.0001 |
| Visit 7 (n = 178) | 73.7 ± 29.5 | 148.4 ± 51.6 | <0.0001 |
| Serum D3 (nmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 175) | 1.6 (1.5–6.4) | 1.7 (1.5–5.6) | 0.9 |
| Visit 4 (n = 125) | 2.0 (1.5–8.4) | 105.5 (66.5–123.2) | <0.0001 |
| Visit 7 (n = 96) | 2.1 (1.5–5.0) | 129.4 (72.0–160.5) | <0.0001 |
| Serum calcium (mmol/L), mean ± SD | |||
| Visit 1 (n = 283) | 2.3 ± 0.1 | 2.3 ± 0.1 | 0.9 |
| Visit 4 (n = 210) | 2.4 ± 0.08 | 2.4 ± 0.08 | 0.7 |
| Visit 7 (n = 175) | 2.3 ± 0.09 | 2.3 ± 0.08 | 0.4 |
| Serum creatinine (mmol/L), mean ± SD | |||
| Visit 1 (n = 295) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.8 |
| Visit 4 (n = 210) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.7 |
| Visit 7 (n = 178) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.3 |
| Urinary calcium: creatinine ratio, mean ± SD | |||
| Visit 1 (n = 294) | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.3 |
| Visit 4 (n = 210) | 0.2 ± 0.2 | 0.3 ± 0.3 | 0.05 |
| Visit 7 (n = 178) | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.1 |
| iPTH (pmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 294) | 3.0 (2.0–4.2) | 2.9 (2.0–4.2) | 0.8 |
| Visit 4 (n = 210) | 2.7 (2.1–3.4) | 2.3 (1.7–3.1) | 0.04 |
| Visit 7 (n = 176) | 3.3 (2.2–4.3) | 2.6 (2.0–3.4) | 0.004 |
| Infant | |||
| 25(OH)D (nmol/L), mean ± SD | |||
| Visit 1 (n = 279) | 40.8 ± 30.9 | 44.6 ± 27.5 | 0.3 |
| Visit 4 (n = 199) | 117.8 ± 52.1 | 104.2 ± 32.3 | 0.03 |
| Visit 7 (n = 152) | 114.8 ± 34.7 | 107.1 ± 35.5 | 0.2 |
| D3 (nmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 71) | 1.5 (1.5–1.5) | 1.5 (1.5–1.4) | 0.9 |
| Visit 4 (n = 51) | 18.8 (2.8–41.5) | 11.6 (2.3–23.4) | 0.2 |
| Visit 7 (n = 43) | 24.6 (3.9–47.7) | 13.3 (3.6–29.8) | 0.2 |
| Serum calcium (mmol/L), mean ± SD | |||
| Visit 1 (n = 212) | 2.6 ± 0.09 | 2.6 ± 0.1 | 0.2 |
| Visit 4 (n = 150) | 2.6 ± 0.09 | 2.6 ± 0.1 | 0.8 |
| Visit 7 (n = 115) | 2.6 ± 0.1 | 2.6 ± 0.08 | 0.4 |
| Serum creatinine (mmol/L), mean ± SD | |||
| Visit 1 (n = 222) | 0.02 ± 0.005 | 0.02 ± 0.006 | 0.6 |
| Visit 4 (n = 160) | 0.02 ± 0.004 | 0.02 ± 0.005 | 0.3 |
| Visit 7 (n = 121) | 0.02 ± 0.004 | 0.02 ± 0.004 | 0.9 |
| Urinary calcium: creatinine ratio, mean ± SD | |||
| Visit 1 (n = 246) | 1.4 ± 1.1 | 1.4 ± 0.9 | 0.5 |
| Visit 4 (n = 193) | 1.4 ± 0.9 | 1.4 ± 0.9 | 0.8 |
| Visit 7 (n = 133) | 1.0 ± 0.7 | 1.1 ± 0.8 | 0.2 |
| iPTH (pmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 168) | 2.4 (1.8–3.5) | 2.2 (1.5–3.1) | 0.2 |
| Visit 4 (n = 131) | 1.8 (1.3–2.5) | 1.9 (1.3–2.5) | 0.6 |
| Visit 7 (n = 105) | 2.0 (1.6–2.6) | 2.0 (1.4–2.7) | 0.8 |
Numbers for each laboratory test vary based on blood sample volume obtained at given visit and number of mothers and infants who were still active in study at time of visit.
For measurement of parent compound vitD, this was measured only in a sequential subset of mothers and infants.
Student's t-test analyses were used to test for differences in mean laboratory values between treatment groups at each visit. Nonparametric analyses were used to test for differences in median laboratory value (D3 and iPTH) between treatment groups at each visit.
25(OH)D, 25-hydroxy-vitamin D; iPTH, intact parathyroid hormone.
For the exclusively formula-feeding mothers (Table 3), there were no statistically significant differences in 25(OH)D concentration at baseline, but by V4, there was a higher maternal 25(OH)D concentration in the 6,400 IU group compared to the 400 IU group that was sustained at V7 (p < 0.006). Similarly, in the sequential subset of the women who were tested, there was a dose effect on the parent compound vitD by treatment with highest concentrations in the mothers assigned to the 6,400 IU group compared to the 400 IU group at V7 (p < 0.003). Maternal iPTH, serum calcium, serum creatinine, and urinary calcium:creatinine ratio did not differ between the groups at baseline or at subsequent visits. Formula-feeding infants did not differ on any of vitD and safety parameters at any time point except for iPTH at V7 with lower levels in 6,400 IU group compared to 400 IU group (p = 0.03); however, their iPTH was not influenced by maternal vitD supplementation.
Table 3.
Maternal and Infant Biochemical Measurements at Visits 1, 4, and 7 by 400 and 6,400 IU Groups in Exclusively Formula-Feeding Mother/Infant Dyads (n = 66)
| 400 IU (n = 34) | 6,400 IU (n = 32) | p-Value* | |
|---|---|---|---|
| Maternal | |||
| 25(OH)D (nmol/L), mean ± SD | |||
| Visit 1 (n = 66) | 68.6 ± 31.1 | 56.6 ± 24.2 | 0.09 |
| Visit 4 (n = 56) | 72.6 ± 37.0 | 96.3 ± 55.5 | 0.07 |
| Visit 7 (n = 50) | 65.2 ± 28.2 | 109.2 ± 71.6 | 0.006 |
| D3 (nmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 42) | 1.5 (1.5–1.5) | 1.5 (1.5–1.5) | 0.8 |
| Visit 4 (n = 28) | 1.5 (1.5–2.7) | 1.5 (1.4–40.9) | 0.4 |
| Visit 7 (n = 25) | 1.5 (1.5–1.5) | 20.5 (1.5–44.3) | 0.003 |
| Serum calcium (mmol/L), mean ± SD | |||
| Visit 1 (n = 66) | 2.3 ± 0.06 | 2.3 ± 0.09 | 0.3 |
| Visit 4 (n = 56) | 2.3 ± 0.08 | 2.3 ± 0.06 | 0.4 |
| Visit 7 (n = 49) | 2.3 ± 0.07 | 2.3 ± 0.09 | 0.7 |
| Serum creatinine (mmol/L), mean ± SD | |||
| Visit 1 (n = 66) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.9 |
| Visit 4 (n = 56) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.9 |
| Visit 7 (n = 49) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 |
| Urinary calcium: creatinine ratio, mean ± SD | |||
| Visit 1 (n = 65) | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.7 |
| Visit 4 (n = 56) | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.7 |
| Visit 7 (n = ) | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.9 |
| iPTH (pmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 66) | 3.4 (2.9–4.4) | 3.8 (3.3–5.3) | 0.2 |
| Visit 4 (n = 56) | 3.5 (3.0–4.3) | 3.4 (2.4–4.0) | 0.3 |
| Visit 7 (n = 49) | 3.3 (2.4–4.3) | 3.2 (2.2–4.1) | 0.8 |
| Infant | |||
| 25(OH)D (nmol/L), mean ± SD | |||
| Visit 1 (n = 65) | 65.4 ± 19.9 | 64.5 ± 17.2 | 0.8 |
| Visit 4 (n = 56) | 93.9 ± 28.8 | 97.6 ± 25.9 | 0.6 |
| Visit 7 (n = 49) | 96.5 ± 27.2 | 93.5 ± 24.6 | 0.8 |
| D3 (nmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 16) | 10.1 (1.5–20.7) | 16.3 (1.5–20.3) | 0.9 |
| Visit 4 (n = 10) | 13.0 (8.7–41.1) | 26.2 (4.9–47.4) | 0.6 |
| Visit 7 (n = 12) | 27.9 (14.4–85.7) | 12.9 (9.2–25.8) | 0.2 |
| Serum calcium (mmol/L), mean ± SD | |||
| Visit 1 (n = 46) | 2.5 ± 0.08 | 2.5 ± 0.05 | 0.04 |
| Visit 4 (n = 39) | 2.6 ± 0.07 | 2.6 ± 0.07 | 0.5 |
| Visit 7 (n = 34) | 2.5 ± 0.1 | 2.5 ± 0.09 | 0.9 |
| Serum creatinine (mmol/L), mean ± SD | |||
| Visit 1 (n = 50) | 0.02 ± 0.008 | 0.02 ± 0.006 | 0.7 |
| Visit 4 (n = 40) | 0.02 ± 0.006 | 0.02 ± 0.006 | 0.7 |
| Visit 7 (n = 35) | 0.02 ± 0.004 | 0.02 ± 0.006 | 0.09 |
| Urinary calcium: creatinine ratio, mean ± SD | |||
| Visit 1 (n = 57) | 0.9 ± 0.6 | 0.9 ± 0.4 | 0.8 |
| Visit 4 (n = 53) | 0.8 ± 0.5 | 0.7 ± 0.5 | 0.5 |
| Visit 7 (n = 46) | 0.9 ± 0.7 | 0.8 ± 0.8 | 0.7 |
| iPTH (pmol/L), median (25–75 IQR) | |||
| Visit 1 (n = 35) | 3.8 (2.5–5.0) | 3.4 (2.1–4.2) | 0.5 |
| Visit 4 (n = 30) | 2.8 (2.0–3.5) | 1.9 (1.6–2.6) | 0.1 |
| Visit 7 (n = 30) | 2.2 (1.7–3.1) | 1.6 (1.3–1.9) | 0.03 |
Numbers for each laboratory test vary based on blood sample volume obtained at given visit and number of mothers and infants who were still active in study at time of visit.
For measurement of parent compound vitD, this was measured only in a sequential subset of mothers and infants.
Student's t-test analyses were used to test for differences in mean laboratory values between treatment groups at each visit. Nonparametric analyses were used to test for differences in median laboratory value (D3 and iPTH) between treatment groups at each visit.
In multiple regression analyses that included baseline 25(OH)D, race/ethnicity, feeding type, visit, and treatment in the model, feeding type was not significant for any maternal safety parameter at any time point. Feeding type was significant for infant serum calcium (with higher mean values for breastfeeding infants) and serum creatinine (with lower mean values for breastfeeding infants) (data not shown).
Infant growth parameters by feeding type and treatment group
Breastfeeding infants by treatment group did not differ at baseline or across subsequent visits on measured growth parameter. Specifically, for the infants who had initially been fully breastfeeding to V4 and then continued on in the study with some breastfeeding, weight, head circumference, and length did not differ by treatment group (Supplementary Table S3).
Rates of deficiency in mothers and infants over time
At study entry, there were no significant differences in the percentages of mothers with 25(OH)D concentrations <50 nmol/L threshold by entrance feeding type (Table 4). By V4, there were significant differences by treatment most dramatically seen in the exclusively breastfeeding group of women: there were consistently more women in the 400 IU group compared to the 6,400 IU group with a 25(OH)D concentration <50 nmol/L. This also was noted with the combination and exclusively formula-feeding mothers.
Table 4.
Percent of Women and Infants by Treatment Group with 25(OH)D <50 nmol/L at Visits 1, 4, and 7 Substratified by Feeding Type
| Feeding type | 25(OH)D <50 nmol/L by treatment, n (%) |
p-Value | |
|---|---|---|---|
| 400 IU |
6,400 IU |
||
| n = 185 | n = 177 | ||
| Mothers | |||
| Visit 1 | |||
| Exclusive breastfeeding | 27 (17.9) | 21 (14.5) | 0.4 |
| Exclusive formula-feeding | 10 (29.4) | 13 (40.6) | 0.3 |
| Visit 4 | |||
| Exclusive breastfeeding | 11 (11.6) | 0 (0.0) | 0.0003 |
| Combination feeding | 7 (46.7) | 0 (0.0) | 0.06 |
| Exclusive formula-feeding | 6 (23.1) | 5 (17.2) | 0.6 |
| Visit 7 | |||
| Exclusive breastfeeding | 10 (17.0) | 0 (0.0) | 0.0003 |
| Combination feeding | 4 (17.4) | 0 (0.0) | 0.1 |
| Exclusive formula-feeding | 8 (32.0) | 5 (21.7) | 0.4 |
| Infants | |||
| Visit 1 | |||
| Exclusive breastfeeding | 100 (69.4) | 83 (61.5) | 0.2 |
| Exclusive formula-feeding | 6 (18.2) | 6 (18.8) | 0.9 |
| Visit 4 | |||
| Exclusive breastfeeding | 8 (8.7) | 3 (3.4) | 0.2 |
| Combination feeding | 0 (0.0) | 0 (0.0) | n/a |
| Exclusive formula-feeding | 1 (3.9) | 0 (0.0) | 0.5 |
| Visit 7 | |||
| Exclusive breastfeeding | 2 (3.6) | 2 (3.3) | 1.0 |
| Combination feeding | 0 (0.0) | 0 (0.0) | n/a |
| Exclusive formula-feeding | 1 (4.0) | 0 (0.0) | 1.0 |
n/a, not applicable.
With regard to infants, there were no differences in the frequency of infants with a 25(OH)D <50 nmol/L threshold. At study entry, the infants who were breastfeeding had more deficiency compared to the formula-fed infants, reflecting the lack of vitD supplementation (88%) in the majority of breastfeeding infants in this cohort during the first month after birth. By V4, the rates of deficiency in the breastfeeding infants had decreased, which was sustained to V7. There were a small number of infants in both groups (5.9% in the 400 IU group and 1.5% in the 6,400 IU group) who required open-label vitD supplementation at the fourth visit due to a total circulating 25(OH)D <50 nmol/L.
Mixed model regression analyses
Mixed models for predicting each maternal safety parameter were initially constructed to compare the 400, 2,400, and 6,400 IU groups before discontinuation of the 2,400 IU arm of the study. There were no treatment by time interaction effects for any of the safety measures for either the mothers or their infants (Supplementary Tables S4A, B). Mixed models were then created for those women who completed the study in the two remaining treatment groups 400 and 6,400 IU.
As shown in Table 5, using a mixed model approach, there were certain associations noted for each maternal safety measure independent of confounders. Time was negatively associated with maternal serum calcium concentration with a slight decline between V4 and V7. Treatment and treatment overtime interaction were not associated with maternal serum calcium concentration.
Table 5.
Mixed Models for Predicting Each Maternal Safety Parameter
| Safety parameter | Beta estimate | Beta standard error | p-Value |
|---|---|---|---|
| 1. Serum calcium | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | −0.00678 | 0.01093 | |
| Treatment | 0.9185 | ||
| Visit 4 | Reference | ||
| Visit 7 | −0.02656 | 0.007972 | |
| Visit | 0.0011 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | 0.01563 | 0.01110 | |
| Treatment by visit interaction | 0.1604 | ||
| 2. Serum creatinine | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | −0.00048 | 0.001224 | |
| Treatment | 0.3464 | ||
| Visit 4 | Reference | ||
| Visit 7 | −0.00144 | 0.000844 | |
| Visit | 0.0009 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | −0.00119 | 0.001171 | |
| Treatment by visit interaction | 0.3095 | ||
| 3. Urinary calcium/creatinine ratio | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | 0.03997 | 0.03242 | |
| Treatment | 0.1736 | ||
| Visit 4 | Reference | ||
| Visit 7 | 0.009965 | 0.02440 | |
| Visit | 0.5428 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | 0.001259 | 0.03403 | |
| Treatment by visit interaction | 0.9705 | ||
| 4. Serum iPTH | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | −0.1752 | 0.2716 | |
| Treatment | 0.3240 | ||
| Visit 4 | Reference | ||
| Visit 7 | 0.4041 | 0.2249 | |
| Visit | 0.0349 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | −0.1315 | 0.3103 | |
| Treatment by visit interaction | 0.6725 | ||
Adjusted for baseline value, baseline 25-hydroxy vitD concentration, 25-hydroxy vitD concentration at visit 4 and visit 7, race, and feeding category.
Maternal serum creatinine was not associated with maternal 25(OH)D concentration. In the model, treatment and treatment overtime interaction were not associated with maternal urinary calcium/creatinine ratios. Similarly, treatment was not associated with maternal iPTH, but treatment over time was associated (p = 0.035).
As shown in Table 6, analysis of infant safety parameters using a mixed model approach for each safety parameter at baseline, 25(OH)D concentration at baseline, 25(OH)D concentration at V4 and 7, race/ethnicity (African American, Hispanic, white/Caucasian), feeding type (exclusively breastfeeding, formula feeding or combination feeding), visit, treatment, and visit by treatment over time were not significant. Urinary calcium/creatinine ratio was positively associated with infant 25(OH)D concentration (p = 0.028). Treatment alone and treatment over time interaction were not associated with infant urinary calcium/creatinine ratios. Infant iPTH was inversely associated with infant 25(OH)D concentration (p < 0.0001) and with treatment (p = 0.0092), but all values were in the normal reported clinical range. Treatment over time interaction was not associated with infant iPTH (p = 0.49).
Table 6.
Mixed Models for Predicting Each Infant Safety Parameter
| Safety parameter | Beta estimate | Beta standard error | p-Value |
|---|---|---|---|
| 1. Serum calcium | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | −0.00651 | 0.01359 | |
| Treatment | 0.8038 | ||
| Visit 4 | Reference | ||
| Visit 7 | −0.02953 | 0.01289 | |
| Visit | 0.0318 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | 0.01856 | 0.01813 | |
| Treatment by visit interaction | 0.3083 | ||
| 2. Serum creatinine | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | −0.00074 | 0.000695 | |
| Treatment | 0.1700 | ||
| Visit 4 | Reference | ||
| Visit 7 | −0.001262 | 0.000664 | |
| Visit | 0.0118 | ||
| Treatment 6,400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | −0.00010 | 0.000921 | |
| Treatment by visit interaction | 0.9121 | ||
| 3. Urinary calcium/creatinine ratio | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | 0.01785 | 0.1038 | |
| Treatment | 0.4641 | ||
| Visit 4 | Reference | ||
| Visit 7 | −0.2565 | 0.08826 | |
| Visit | 0.0017 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | 0.1013 | 0.1257 | |
| Treatment by visit interaction | 0.4218 | ||
| 4. Serum iPTH | |||
| Treatment 400 IU | Reference | ||
| Treatment 6,400 IU | −0.7058 | 0.2366 | |
| Treatment | 0.0124 | ||
| Visit 4 | Reference | ||
| Visit 7 | −0.1839 | 0.2144 | |
| Visit | 0.9402 | ||
| Treatment by visit interaction 400 IU | Reference | ||
| Treatment by visit interaction 6,400 IU | 0.3453 | 0.2723 | |
| Treatment by visit interaction | 0.2122 | ||
Adjusted for baseline value, baseline 25-hydroxy vitD concentration, 25-hydroxy vitD concentration at visit 4 and visit 7, race, and feeding category.
Overall, in both the maternal and infant mixed models, no significant treatment over time interaction effects existed for any maternal and infant safety parameters. Thus, the effect of the two treatments on safety during the intervention period did not differ on the parameters tested.
Discussion
In this vitD supplementation clinical trial of women who were exclusively breastfeeding or formula-feeding at baseline or later transitioned to combination feeding (breastfeeding and formula-feeding) or exclusive formula-feeding, maternal supplementation with 6,400 IU vitD3/day was superior to 400 and 2,400 IU/day in safely achieving maternal vitD sufficiency sustained to 7 months postpartum. As viewed by the DSMB overseeing the study, with the exception of the 2,400 IU group having a greater percentage of infants requiring open-label vitD, there were no instances of adverse events attributable to vitD supplementation.
Our findings are supported by other studies. In their observational study, Vieth Streym et al. explored concentrations of vitD2 and D3 and 25(OH)D in foremilk and hindmilk during the first 9 months of lactation in 107 women,12 with improved vitD status in those supplemented but similar safety profiles in the women regardless of supplementation status. Niramitmahapanya et al.,13 studied 68 lactating women in Thailand who were randomized to placebo versus 1,800 IU vitD3, and showed a strong correlation between the change in maternal 25(OH)D and maternal milk 25(OH)D that were both correlated with the change in infant 25(OH)D with no significant differences in the safety marker urinary calcium/creatinine ratios at 6 weeks by treatment group, which is consistent with our findings. Saadi et al., studied 90 breastfeeding women in the UAE14 with marked deficiency states, who were randomly assigned to 2,000 IU vitD2 daily in oral capsule form (n = 45) or 60,000 IU monthly (avg 2,000 IU/day) in oral tablet form (n = 45) for 3 months, with significantly improved vitD status in some but not all, with no safety issues in any of the women.
Czech-Kowalska et al.15 studied 174 healthy, lactating women and their term infants randomized to receive 1,200 IU/day vitD3 or 400 IU/day for 6 months while breastfeeding. The infants themselves received 400 IU D3/day. At baseline and 3 months, vitD status was similar between the groups, but by 6 months, mean 25(OH)D concentration was higher in the 1,200 IU/day group without safety issues. More recently, Dawodu et al. conducted a longitudinal, 6-month clinical trial of 190 mother-infant exclusively breastfeeding pairs living in Doha, Qatar,16 with a study design similar to this trial: Mothers were randomized to receive either 600 or 6,000 IU vitD3/day starting at ∼4 weeks postpartum. The infants of the mothers in the 600 IU group were randomized to receive 400 IU vitD3/day while the infants in the 6,400 IU group were randomized to receive placebo. The investigators reported a significant improvement in maternal vitD status of the mothers in the 6,000 IU group with no difference in safety parameters between the two groups. The infants receiving 400 IU/day had a slightly higher mean 25(OH)D at the end of the study, but both groups achieved the threshold 25(OH)D concentration of at least 50 nmol/L in the majority of infants. There was a statistically significant and clinically relevant difference in maternal PTH, with a higher mean PTH in the 600 versus 6,000 IU group.
Additional data surrounding the safety and effectiveness of higher dose vitD supplementation during pregnancy and early lactation come from the work of March et al.,17 Wall et al.,18 and Thiele et al.19 and in our earlier pregnancy and lactation studies,1,2,11,20,21 where there were no safety issues ascribed to vitD supplementation. In the study by Wall et al.,18 while 10% of the women participants met their definition of hypercalcemia, there were no statistically significant differences between the groups by treatment and urinary calcium/creatinine ratios also did not differ by treatment.18
This study is unique in that it extends the safety of higher dose vitD to those mothers who provide a combination of breast milk and formula to their infants or who exclusively formula feed. The other strengths of this study are the racially diverse patient population at two distinct geographical locations—Charleston, SC and Rochester, NY, and a sample size that is considerably larger than the prior published studies. Thus, the safety of higher dose vitD supplementation during lactation extends to those women who might choose to provide less breast milk and combination breast- and formula feed and to those women who might choose to stop breastfeeding, but continue higher dose vitD supplementation.
A limitation of the study is that the biological significance of vitD repletion via maternal breast milk versus direct infant supplementation is unknown. Future studies identifying the biological significance of maternal vitD repletion and sufficiency and its effect on the immune signature of maternal breast milk versus direct infant supplementation are underway. Another limitation of the study was that the formula-feeding mothers were studied at only one site, but this site was at a lower latitude with potentially higher risk of hypervitaminosis D with both UV exposure and vitD supplementation.
In conclusion, in this study where safety measures were followed during lactation as well as during the postpartum period in nonlactating women, the sustained safety of 6,400 IU vitD3/day for 6 months was shown. Infants of mothers in the 6,400 IU vitD3 were equivalent in their vitD status to infants receiving supplementation with 400 IU/day without safety issues.
Supplementary Material
Acknowledgments
We want to acknowledge the hundreds of women and infants who participated in this lengthy trial, without whose involvement we would not have been able to conduct this study. We also recognize Renee Oliver Washington, MS for her dedication to all the laboratory details involved in this study and Dr. Jill Newman who assisted us in data analyses and table formatting. We also thank the dedication and hard work of the research and medical staff of the CTSA-sponsored Clinical Research Centers at MUSC and the U of R. Finally, we acknowledge Biotics Research Corp, Rosenberg, Texas, for providing Bio-D-Mulsion vitD drops, and Mead Johnson, Inc. (Mead Johnson, Evansville, IN) for providing vitD–free formula for the breastfeeding infants enrolled in the study (who might require a formula-feed on an emergency basis). Of note, formula-fed infants were on formula with vitamin D (400 IU/L). Neither company was involved in the study design, conduct, and analysis of the data or in the writing of the study results.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Disclosure Statement
The authors have no conflicts of interest relevant to this article to disclose.
Funding Information
Funded, in part, by NIH 5R01HD043921, NIH RR01070, MUSC Department of Pediatrics, and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCAT Grant number UL1 TR000062.
No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the article, and there were no competing interests by any of the authors during the conduct of the study, the analysis of the data and the writing of these study results.
Supplmentary Material
References
- 1. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: A randomized controlled trial. Pediatrics 2015;136:625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wagner CL, Hulsey TC, Fanning D, et al. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: A 6-month follow-up pilot study. Breastfeed Med 2006;1:59–70 [DOI] [PubMed] [Google Scholar]
- 3. Roth DE. Maternal postpartum high-dose vitamin D3 supplementation (6400 IU/day) or conventional infant vitamin D3 supplementation (400 IU/day) lead to similar vitamin D status of healthy exclusively/fully breastfeeding infants by 7 months of age. Evid Based Med 2016;21:75. [DOI] [PubMed] [Google Scholar]
- 4. Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014;72:48–54 [DOI] [PubMed] [Google Scholar]
- 5. Labbok MH, Belsey M, Coffin CF. A call for consistency in defining breast-feeding. Am J Public Health 1997;87:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Labbok MH, Krasovec K. Toward consistency in breastfeeding definitions. Stud Fam Plann 1990;21:226–230 [PubMed] [Google Scholar]
- 7. Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalceferol, cholecaliferol and their major metabolites. J Steroid Biochem 1984:81–86 [DOI] [PubMed] [Google Scholar]
- 8. Hollis BW. Individual quantitation of vitamin D2, vitamin D3, 25(OH)D2 and 25(OH)D3 in human milk. Anal Biochem 1983;131:211–219 [DOI] [PubMed] [Google Scholar]
- 9. Carter GD. Accuracy of 25-hydroxyvitamin D assays: Confronting the issues. Curr Drug Targets 2011;12:19–28 [DOI] [PubMed] [Google Scholar]
- 10. Vieth R, Ladak Y, Walfish P. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinal Metab 2003;88:185–191 [DOI] [PubMed] [Google Scholar]
- 11. Hollis BW, Wagner CL. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr 2004;80(6 Suppl):1752S–1758S [DOI] [PubMed] [Google Scholar]
- 12. Vieth Streym S, Hojskov CS, Moller UK, et al. Vitamin D content in human breast milk: A 9-mo follow-up study. Am J Clin Nutr 2016;103:107–114 [DOI] [PubMed] [Google Scholar]
- 13. Niramitmahapanya S, Kaoiean S, Sangtawesin V, et al. Effect on Vitamin D status of breastfeeding infants after vitamin D3 supplementation during breastfeeding lactation: A double-blind randomizedcontrolled trial. Ann Clin Endocrinol Metab 2017;1:6–14 [Google Scholar]
- 14. Saadi HF, Dawodu A, Afandi B, et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern Child Nutr 2009;5:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Czech-Kowalska J, Latka-Grot J, Bulsiewicz D, et al. Impact of vitamin D supplementation during lactation on vitamin D status and body composition of mother-infant pairs: A MAVID randomized controlled trial. PLoS One 2014;9:e107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dawodu A, Salameh KM, Al-Janahi NS, et al. The effect of high-dose postpartum maternal vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population. A randomized controlled trial. Nutrients 2019;11:1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. March KM, Chen NN, Karakochuk CD, et al. Maternal vitamin D(3) supplementation at 50 mug/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: A randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am J Clin Nutr 2015;102:402–410 [DOI] [PubMed] [Google Scholar]
- 18. Wall CR, Stewart AW, Camargo CA Jr., et al. Vitamin D activity of breast milk in women randomly assigned to vitamin D3 supplementation during pregnancy. Am J Clin Nutr 2016;103:382–388 [DOI] [PubMed] [Google Scholar]
- 19. Thiele DK, Ralph J, El-Masri M, et al. Vitamin D3 supplementation during pregnancy and lactation improves vitamin D status of the mother-infant dyad. J Obstet Gynecol Neonatal Nurs 2017;46:135–147 [DOI] [PubMed] [Google Scholar]
- 20. Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner CL, McNeil RB, Johnson DD, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. J Steroid Biochem Mol Biol 2013;136:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.