Abstract
Objective: To investigate the association between maternal obesity as measured by prepregnancy body mass index (BMI) and group B streptococcus (GBS) colonization.
Methods: We conducted a secondary analysis from the Consortium on Safe Labor Study (CSL) in the United States cohort study (2002–2008). Pregnant women with deliveries at ≥37 weeks of gestation who attempted labor were included (115,070 assessed deliveries). The association between maternal prepregnancy BMI, categorized as normal weight or below (<25 kg/m2), overweight (25 to <30 kg/m2), class I obesity (30 to <35 kg/m2), class II obesity (35 to <40 kg/m2), and class III obesity (≥40 kg/m2), and GBS colonization was modeled using logistic regression with generalized estimating equations. Models adjusted for maternal age, parity, race, pregestational diabetes, insurance status, study site/region, and year of delivery.
Results: The overall prevalence of GBS colonization was 20.5% (23,625/115,070), which increased with rising maternal BMI, normal weight 19.3% (13,543/70,098), overweight 20.8% (5,353/25,733), class I obesity 23.0% (2,596/11,275), class II obesity 26.1% (1,270/4,850), and class III obesity 27.7% (863/3,114). In multivariable analysis, increasing maternal obesity severity was associated with higher odds of GBS colonization, namely overweight (adjusted odds ratio [AOR]: 1.09, 95% confidence interval [CI]: 1.05–1.13), class I obesity (AOR: 1.20, 95% CI: 1.15–1.26), class II obesity (AOR: 1.42, 95% CI: 1.33–1.51), and class III obesity (AOR: 1.50; 95% CI: 1.38–1.62) compared with normal weight. In secondary analyses, these associations persisted when stratified by maternal race.
Conclusions: In a national U.S. sample, increasing maternal obesity severity as assessed by prepregnancy BMI was associated with a higher likelihood of maternal GBS colonization during pregnancy.
Keywords: group B streptococcus, pregnancy, obesity, GBS, body mass index
Introduction
Maternal Group B streptococcus (GBS) colonization is the leading cause of infectious morbidity and mortality in neonates,1–3 and can also be associated with a range of adverse maternal infectious outcomes, including sepsis, endometritis, urinary tract infection, and chorioamnionitis.4,5 Current guidelines from the American College of Obstetricians and Gynecologists recommend routine intrapartum prophylaxis for women who test positive for GBS between 36 and 37 weeks' gestation.6,7 However, when maternal GBS status is unknown during labor, a risk-based approach is recommended for prophylaxis, which includes factors associated with neonatal GBS infection: a prior infant affected by GBS, anticipated preterm birth <37 weeks, prolonged rupture of membranes, and history of GBS bacteriuria during the current pregnancy.8
There are several epidemiologic risk factors that impact maternal GBS carriage in pregnancy, including Black race, lower socioeconomic status, and vaginal infections.3,9–14 In the United States, routine antepartum screening has been in place since 2002 and as many as 30% of women may test positive for GBS during pregnancy.15,16 Emerging data suggest that maternal obesity may be a risk factor for GBS colonization.17 Maternal obesity has been associated with an increased risk of neonatal early onset GBS disease13 and neonatal death,18 but its association with maternal colonization in pregnancy has been limited to small single institution samples.12,14,19,20 Population-level data across multiple sites offer a larger representative sample to assess for this association in an era of increasing maternal obesity prevalence and severity, and could inform strategies for risk-based prophylaxis for women with unknown GBS status in labor.
We sought to estimate the association between maternal prepregnancy body mass index (BMI) and GBS colonization in pregnancy using the Eunice Kennedy Shriver U.S. National Institute of Child Health and Human Development (NICHD) Consortium on Safe Labor (CSL) study population. Secondarily, we also assessed whether this association varied by maternal self-reported race (i.e., effect modification), given increasing evidence has highlighted racial disparities in GBS colonization.3,10,11
Methods
We conducted a secondary analysis of the CSL cohort, which was a multisite retrospective study of women delivering at ≥23 weeks gestation. This study enrolled women between 2002 and 2008, which coincides with when routine GBS screening and intrapartum prophylaxis was recommended in the United States.2,21 The CSL study included 12 clinical centers with 19 U.S. hospitals. Data from the electronic medical record were abstracted, including demographics, prenatal complications, labor and delivery information, and maternal and neonatal outcomes.22 Multiple strategies were used to assemble a complete dataset, including review of the electronic record, International Classification of Diseases (ICD), Ninth revision (ICD-9) coding, and manual chart review. This analysis was performed using a deidentified dataset and was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
The CSL included 228,438 deliveries at ≥23 weeks gestation, with 9.5% of women (n = 5,053) contributing >1 birth during the study. The current analysis was restricted to deliveries attempting labor and that delivered at term ≥37 weeks gestation because routine GBS screening was recommended to be performed >35 weeks gestation per U.S. guidelines and women who underwent scheduled cesarean delivery may not have been reliably screened for GBS. Consistent with prior analyses from this dataset, a trial of labor was defined as a vaginal delivery or at least two cervical examinations documented in the obstetrical database during the labor admission.23,24 Women contributing multiple deliveries were included, and this was adjusted for in analyses.
Maternal prepregnancy BMI (weight in kilograms divided by the square of height in meters, kg/m2) was categorized as normal weight or below (<25 kg/m2), overweight (25 to <30 kg/m2), class I obesity (30 to <35 kg/m2), class II obesity (35 to <40 kg/m2), and class III obesity (≥40 kg/m2), which is consistent with U.S. Institute of Medicine guidelines.25 The outcome was documented maternal GBS colonization anytime in pregnancy. Information on the gestational age at which GBS status was determined, whether culture data were obtained from urine, vaginal, or rectovaginal samples, and antibiotic sensitivity for women who tested positive was not collected in the CSL dataset.
Descriptive analyses compared maternal characteristics by using chi-square, Fisher's exact, and Wilcoxon rank-sum tests. We utilized generalized estimating equations (GEE) accounting for within-woman correlations (i.e., participants with multiple deliveries during the study period). We calculated adjusted odds ratios (AORs) and 95% confidence intervals (CIs) to assess the association between maternal BMI category and GBS colonization, adjusting for maternal age, parity, race, U.S. region, and year of delivery. Potential confounders were selected using a discrete acyclic graph. We conducted multiple imputation for missing data (age, race, insurance status, and delivery year), consistent with recent analyses from the CSL dataset.24 For each study outcome, 30 imputations of missing covariate values were generated for the same study population and the same covariates as the previously described models assuming that variables were missing at random conditional on those covariates.
In secondary analysis, we included an interaction term for BMI and race to assess effect measure modification by maternal self-reported race (a priori, p < 0.10 was considered statistically significant). The interaction was statistically significant overall (p < 0.001), and thus the multivariable models were also stratified by race. Given the primary analysis was restricted to deliveries in which women attempted labor to minimize potential misclassification of GBS data, we conducted sensitivity analyses by reperforming the primary analysis regardless of trial of labor: (1) restricted to only vaginal deliveries ≥37 weeks gestation at birth (n = 98,090), and then (2) expanded to all deliveries ≥37 weeks (n = 133,328). These additional models adjusted for the same covariates as the primary analysis. All statistical analyses were performed using STATA (STATACORP, version MP 15.1, College Station, TX).
Results
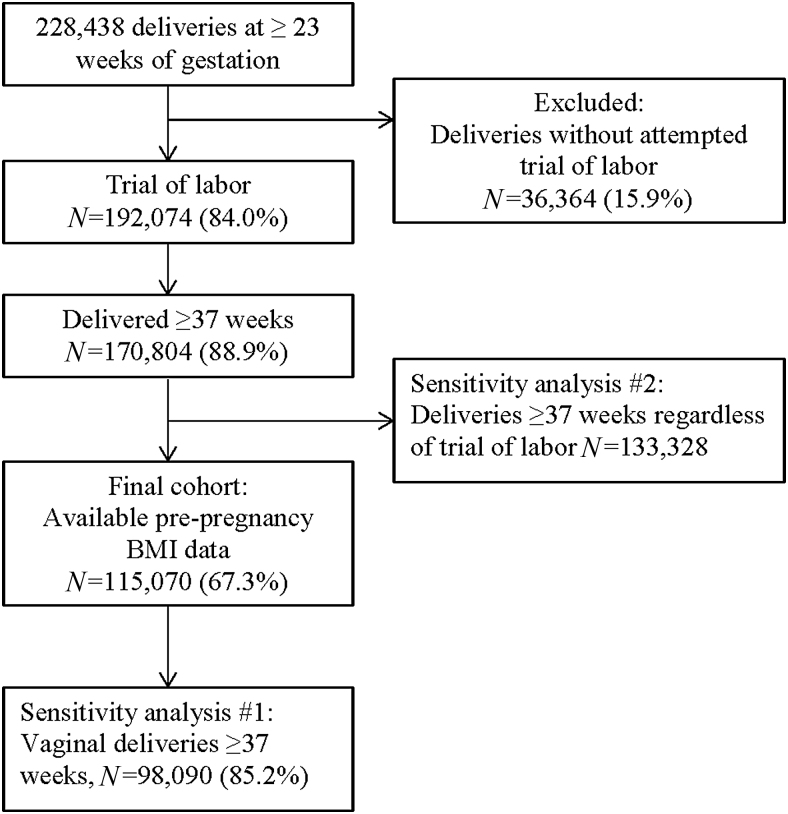
In the original study cohort of 228,438 deliveries at ≥23 weeks of gestation, we excluded 15.9% (n = 36,364) of deliveries without an attempted trial of labor (Fig. 1). We further restricted our cohort to deliveries at ≥37 weeks of gestation with available prepregnancy BMI data. The final study sample for this secondary analysis consisted of 115,070 deliveries (50.3%) at ≥37 weeks of gestation with an attempted trial of labor. A total of 23,625 (20.5%) deliveries were affected by GBS, and the remaining 91,445 (79.5%) did not have documented GBS colonization during pregnancy.
FIG. 1.
Flowchart of women in the CSL cohort included in current analysis. CSL, Consortium on Safe Labor.
The mean maternal age was 27.2 years (standard deviation [SD]: 6.01), 56.7% were parous, and 63.2% had private insurance. By race, 54.8% were White, 19.7% Black, and 19.3% Latina. As described in Table 1, a higher proportion of women who were colonized with GBS were younger (mean age: 27.1 vs. 27.2 years; p = 0.001), had public insurance (36.3% vs. 35.1%; p = 0.003), were unmarried (38.2% vs. 35.8%; p < 0.001), and reported tobacco use (6.6% vs. 6.2%; p = 0.02) when compared to those without GBS colonization. Women who were colonized with GBS were more likely to be of Black race (24.8% vs. 18.3%; p < 0.001). History of preterm birth (5.6% vs. 5.2%; p = 0.03) and pregestational diabetes (1.8% vs. 1.5%; p < 0.001) were more frequent among deliveries with documented GBS colonization. Diagnoses of chronic hypertension, pre-eclampsia, and gestational diabetes did not vary by GBS status, nor did mode of delivery or spontaneous labor on admission.
Table 1.
Characteristics of Deliveries at ≥37 Weeks of Gestation and with a Trial of Labor Overall and by Reported GBS Colonization Status
| Characteristic | Missing n (%) | GBS colonization |
No GBS colonization |
pa |
|---|---|---|---|---|
| n = 23,625 | n = 91,445 | |||
| Sociodemographic characteristics | ||||
| Maternal age, mean (SD), years | 54 (0.0) | 27.1 (6.01) | 27.2 (5.97) | 0.001 |
| Parity ≥1 | — | 13,287 (56.2) | 52,025 (56.8) | 0.07 |
| Race | ||||
| White | 3,733 (3.2) | 12,553 (54.8) | 48,491 (54.8) | <0.001 |
| Black | 5,695 (24.8) | 16,249 (18.3) | ||
| Latina | 3,441 (15.0) | 18,107 (20.4) | ||
| Asian | 805 (3.5) | 3,506 (3.9) | ||
| Other/multi-racial | 410 (1.7) | 2,080 (2.3) | ||
| Delivery year | ||||
| 2002–2003 | 14 (0.0) | 974 (4.1) | 4,190 (4.5) | <0.001 |
| 2004–2005 | 7,747 (32.7) | 30,773 (33.6) | ||
| 2006–2008 | 14,903 (63.0) | 56,469 (61.7) | ||
| Insurance status | ||||
| Private | 17,212 (14.9) | 12,930 (62.4) | 48,994 (63.5) | 0.003 |
| Public | 7,536 (36.3) | 27,115 (35.1) | ||
| Self-pay/other | 252 (1.2) | 1,031 (1.3) | ||
| Marital status, not married | 2,037 (1.7) | 8,876 (38.2) | 32,167 (35.8) | <0.001 |
| Tobacco use | — | 1,568 (6.6) | 5,694 (6.2) | 0.02 |
| Academic delivery hospital | — | 11,049 (46.7) | 41,778 (45.6) | 0.003 |
| Tertiary care hospital | — | 20,706 (87.6) | 79,039 (86.4) | <0.001 |
| U.S. region | ||||
| West | — | 9,881 (41.8) | 41,618 (45.5) | <0.001 |
| Midwest | 5,293 (22.4) | 14,852 (16.2) | ||
| South | 4,313 (18.2) | 21,040 (23.0) | ||
| Northeast | 4,138 (17.5) | 13,935 (15.2) | ||
| Clinical characteristics | ||||
| History of preterm birth | 4,098 (3.5) | 1,294 (5.6) | 4,612 (5.2) | 0.03 |
| Maternal comorbidities | ||||
| Chronic hypertension | — | 592 (2.5) | 2,196 (2.4) | 0.35 |
| Pre-eclampsia | — | 949 (4.0) | 3,492 (3.8) | 0.15 |
| Pregestational diabetes | — | 431 (1.8) | 1,368 (1.5) | <0.001 |
| Gestational diabetes | — | 1,003 (4.2) | 3,850 (4.2) | 0.81 |
| Mode of delivery, overall | ||||
| Cesarean | — | 3,461 (14.6) | 13,519 (14.7) | 0.60 |
| Vaginal | 20,164 (85.3) | 77,926 (85.2) | ||
| Spontaneous labor on L&D admission | — | 13,092 (55.4) | 51,185 (55.9) | 0.12 |
| Gestational age at delivery, mean (SD) | 39.0 (1.12) | 38.9 (1.12) | <0.001 | |
| 37–39 weeks | — | 15,414 (65.2) | 61,295 (67.0) | <0.001 |
| >39 weeks | 8,211 (34.7) | 30,150 (32.9) | ||
p-value comparing with versus without GBS colonization (chi-square for categorical variables and t-test for continuous variables).
GBS, group B streptococcus; SD, standard deviation.
The frequency of maternal GBS colonization increased with rising maternal prepregnancy BMI (Table 2). By BMI exposure category, the frequency of GBS colonization was 19.3% (13,543/70,098) for normal weight or below women, 20.8% (5,353/25,733) for overweight women, 23.0% (2,596/11,275) for class I obesity, 26.1% (1,270/4,850) for class II obesity, and 27.7% (863/3,114) for class III obesity. We noted a similar pattern of an increasing frequency of GBS colonization with rising BMI for White, Black, Latina, and Asian women.
Table 2.
Association Between Maternal Prepregnancy Body Mass Index and Group B Streptococcus Colonization in Pregnancy (n = 115,070)
| Maternal prepregnancy BMI category (kg/m2) | Frequency of GBS colonization (row percentage) |
Unadjusted and adjusted estimatesa,b,c |
||
|---|---|---|---|---|
| Yes n = 23,625 n (%) | No n = 91,445 n (%) | Unadjusted odds ratio, OR (95% CI) | Adjusted odds ratio, AOR (95% CI) | |
| Normal weight or below (<25 kg/m2) | 13,543 (19.3) | 56,555 (80.6) | 1.00 (reference) | 1.00 (reference) |
| Overweight (25–29.9 kg/m2) | 5,353 (20.8) | 20,380 (79.2) | 1.09 (1.05–1.13) | 1.10 (1.06–1.14) |
| Obesity class I (30–34.9 kg/m2) | 2,596 (23.0) | 8,679 (76.9) | 1.24 (1.19–1.31) | 1.25 (1.19–1.32) |
| Obesity class II (35–39.9 kg/m2) | 1,270 (26.1) | 3,580 (73.8) | 1.48 (1.38–1.58) | 1.47 (1.38–1.58) |
| Obesity class III (≥40 kg/m2) | 863 (27.7) | 2,251 (72.2) | 1.60 (1.47–1.73) | 1.59 (1.46–1.72) |
Logistic regression with generalized estimating equations modeled the associations accounting for within-woman correlations (i.e., participants with multiple deliveries during the study period).
Adjusted models included: maternal age, parity, race, pregestational diabetes, insurance status, study site/region, and delivery year.
Data imputation was performed for the following covariates: age, race, insurance status, and delivery year.
BMI, body mass index; CI, confidence interval.
In multivariable analysis, increasing maternal prepregnancy BMI class as a measure of obesity severity was associated with higher odds of colonization with GBS, namely overweight (AOR: 1.09, 95% CI: 1.05–1.13), class I obesity (AOR: 1.20, 95% CI: 1.15–1.26), class II obesity (AOR: 1.42, 95% CI: 1.33–1.51), and class III obesity (AOR: 1.50; 95% CI: 1.38–1.62) compared with normal weight women (Table 2).
We secondarily assessed whether the primary association between increasing maternal BMI and GBS colonization held for different racial groups. In multivariable models stratified by race, this association persisted for White, Black, and Latina women, but not Asian women (data not shown). In sensitivity analyses, we reconfirmed the primary analysis of an association between increasing maternal BMI and GBS colonization both when the analysis was restricted to vaginal deliveries ≥37 weeks, and all deliveries ≥37 weeks regardless of an attempted trial of labor (data not shown).
Discussion
We found that increasing maternal obesity severity as measured by prepregnancy BMI was associated with a higher likelihood of GBS colonization in pregnancy. These results are timely given rising rates of maternal obesity with over one in three pregnant women classified as obese,26 longitudinal data suggesting that maternal GBS colonization is increasing over time,27 and increasing number of countries considering GBS screening in pregnancy.3,11 We also found that the primary association generally held across racial groups, given rates of GBS colonization may be higher among Black women who are more likely to be obese and have a higher risk of intrapartum seroconversion after a negative antepartum screen.3,28
This study demonstrated an association between maternal obesity and GBS colonization in a large multisite cohort. In contrast, three recent studies conducted in single tertiary care medical centers with a total sample size of just over one tenth of the current analysis have assessed the association between BMI and GBS colonization. Unlike this study, which found a significant relationship between obesity severity and GBS colonization across each strata or class of BMI, these earlier studies did not observe a consistent association across BMI strata, which may be attributable to a smaller sample size (n = 7,711, 6,309, and 2,045). Kleweis et al. conducted a retrospective cohort study from 2004 to 2008 among 7,711 mostly Black women and found that obesity was associated with a higher frequency of GBS colonization compared to nonobese women (28.4% vs. 22.2%), with an AOR of 1.35 (95% CI: 1.21–1.50).17 Alvareza et al. showed a weak association between BMI and GBS colonization among 2,045 women who delivered in 2013, which was only significant for class II obesity (1.43; 95% CI: 1.01–2.03).20 They also found that urine versus rectovaginal culture and antibiotic sensitivities did not vary by BMI. The most recent study conducted by Gopal Rao et al. in England from 2014 to 2015 found that among 6,309 screened women, an increased adjusted odds of GBS colonization with class II or III obesity as a single group (1.38; 95% CI: 1.09–1.74), but not class I obesity.10 Earlier smaller observational studies have similarly suggested an association between maternal obesity and GBS colonization during pregnancy.12,14
A biological mechanism for this epidemiological association remains to be elucidated. It is possible that the microbiome is altered in the setting of obesity, which may lead to increased inflammation and risk of infection through changes in vaginal pH or alterations in the host's susceptibility and immune response.29,30 A role for the impact of inflammation on GBS colonization was suggested recently by Ma'ayeh et al. who found that GBS colonization was decreased with vaginal progesterone versus 17α-hydroxyprogesterone caproate,31 which suggested that progestogens may have an anti-inflammatory impact on the vaginal microbiome.32 It is likely that multiple factors interact and influence maternal immune status during pregnancy, including race, obstetric history, anthropometry (including obesity), and consequent maternal GBS colonization.9
This multisite analysis from across the United States has a sample size over 10 times larger than the three most recent single institution studies combined to assess the relationship between maternal obesity and GBS colonization.10,17,20 This allowed for the assessment of a dose-response relationship across individual classes of obesity severity. These results are also more generalizable than recent data from single tertiary care institutions in which specific subpopulations of women may have been overrepresented. Our study population of women who delivered term would be the subset of women for whom additional risk factors, such as obesity, could influence clinical management when GBS status is unknown as they would otherwise not receive prophylaxis. It is possible that obesity should be studied as a putative risk factor in future risk-based models for intrapartum prophylaxis in women at term, including when GBS status is unknown or because of the possibility of a high false negative rate of nearly 10% with antepartum culture.33
There are several study limitations to note. The CSL cohort did not collect data with regards to when maternal samples were collected during pregnancy nor methods used for collection (e.g., rectovaginal swab vs. urine sample) or assay used. Additional information with regards to antibiotic resistance to GBS colonization was not available. We utilized prepregnancy BMI to measure maternal obesity in the current analysis because BMI later in pregnancy was not available. This approach is consistent with prior analyses that have generally used prepregnancy BMI to define the risk of maternal obesity on perinatal outcomes. Future research is needed with regards to whether the association identified in this analysis persists when BMI is ascertained more proximal to delivery (i.e., timing of GBS ascertainment). Additionally, it is possible that women who missed screening were classified as a negative screen rather than an unknown screening result. Early surveillance data of labor and delivery records in 2003–2004, which is shortly after routine screening was implemented, showed that 85% of women had documented antenatal GBS screening, and 98% of screened women had a colonization result available at labor.2,21 In the current analysis, to account for clinical scenarios with a higher probability of missing or unknown GBS data, we limited our analysis to deliveries >37 weeks or to women who attempted labor. The resulting selection bias would have likely inflated the number of women who were identified as GBS negative, which was likely nondifferential with regards to BMI. The prevalence of reported GBS colonization in the current analysis is concordant with national U.S estimates ranging between 20% and 30% based on risk group. Similar to previous studies,10,17 we lacked information on several factors that may impact GBS colonization, including antibiotic use during pregnancy, other vaginal infections, and sexual and health behaviors,34 but it is likely that these possible confounders were nondifferential with regards to maternal obesity.
Conclusion
Increasing severity of maternal obesity was associated with a higher likelihood of maternal GBS colonization in pregnancy in a national sample of U.S women. It is possible that factors driving increasing rates of obesity among pregnant women may also serve to increase rates of GBS colonization, and possibly early onset neonatal sepsis. Understanding the relationship between obesity, and other maternal factors, and GBS could provide new strategies for screening and prevention of the most common cause of neonatal sepsis globally.
Acknowledgments
Institutions involved in the Consortium include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, UT; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville, MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, TX.
Authors' Contributions
K.K.V., C.J.V., and S.D-.K. designed this study. K.K.V., C.J.V., and D.M.S. conducted all analyses. K.K.V., R.A.S., J.M.T., J.S.A.S., and B.L.H. interpreted the results and wrote the article.
Details of Ethics Approval
This secondary cohort analysis was performed using a deidentified dataset under a waiver of informed consent and was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill (IRB study #17-2384; date: September 28, 2017).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The Consortium on Safe Labor was funded by the Intramural Research Program of the NICHD, through Contract No. HHSN267200603425C. The study was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Intramural investigators designed the study and data were collected by clinical site investigators. The corresponding author has full access to the data and final responsibility for preparation and submission of the article for publication.
References
- 1. Boyer K, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665–1669 [DOI] [PubMed] [Google Scholar]
- 2. Schrag S, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: Experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 2013;31:D20–D26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwatra G, Cunnington MC, Merrall E, et al. Prevalence of maternal colonisation with group B streptococcus: A systematic review and meta-analysis. Lancet Infect Dis 2016;16:1076–1084 [DOI] [PubMed] [Google Scholar]
- 4. Bergqvist G, Hurvell B, Malmborg AS, Rylander M, Tunell R. Neonatal infections caused by group B streptococci. Scand J Infect Dis 1971;3:157–162 [DOI] [PubMed] [Google Scholar]
- 5. Pass M, Gray BM, Khare S, Dillon HC Jr. Prospective studies of group B streptococcal infections in infants. J Pediatr 1979;95:437–443 [DOI] [PubMed] [Google Scholar]
- 6. Van Dykem M, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 2009;360:2626–2636 [DOI] [PubMed] [Google Scholar]
- 7. American College of Obstetricians and Gynecologists. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 782. Obstet Gynecol 2019;134:206–210 [DOI] [PubMed] [Google Scholar]
- 8. Lin F, Brenner RA, Johnson YR, et al. The effectiveness of risk-based intrapartum chemoprophylaxis for the prevention of early-onset neonatal group B streptococcal disease. Am J Obstet Gynecol 2001;184:1204–1210 [DOI] [PubMed] [Google Scholar]
- 9. Lao T. Epidemiological factors impact group B streptococcus carriage. BJOG 2019;126:1353. [DOI] [PubMed] [Google Scholar]
- 10. Gopal Rao G, Hiles S, Bassett P, Lamagni T. Differential rates of group B streptococcus (GBS) colonisation in pregnant women in a racially diverse area of London, UK: A cross-sectional study. BJOG 2019;126:1347–1353 [DOI] [PubMed] [Google Scholar]
- 11. Russell N, Seale AC, O'Driscoll M, et al. Maternal colonization with group B streptococcus and serotype distribution worldwide: Systematic review and meta-analyses. Clin Infect Dis 2017;65:S100–S111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stapleton R, Kahn JM, Evans LE, Critchlow CW, Gardella CM. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet Gynecol 2005;106:1246–1252 [DOI] [PubMed] [Google Scholar]
- 13. Håkansson S, Källen K. High maternal body mass index increases the risk of neonatal early onset group B streptococcal disease. Acta Paediatr 2008;97:1386–1389 [DOI] [PubMed] [Google Scholar]
- 14. Ramos E, Gaudier FL, Hearing LR, Del Valle GO, Jenkins S, Briones D. Group B streptococcus colonization in pregnant diabetic women. Obstet Gynecol 1997;89:257–260 [DOI] [PubMed] [Google Scholar]
- 15. Regan J, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol 1991;77:604–610 [PubMed] [Google Scholar]
- 16. Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine 2013;31:D7–D12 [DOI] [PubMed] [Google Scholar]
- 17. Kleweis S, Cahill AG, Odibo AO, Tuuli MG. Maternal obesity and rectovaginal group B streptococcus colonization at term. Infect Dis Obstet Gynecol 2015;2015:586767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nohr E, Vaeth M, Bech BH, Henriksen TB, Cnattingius S, Olsen J. Maternal obesity and neonatal mortality according to subtypes of preterm birth. Obstet Gynecol 2007;110:1083–1090 [DOI] [PubMed] [Google Scholar]
- 19. Shah M, Aziz N, Leva N, Cohan D. Group B streptococcus colonization by HIV status in pregnant women: Prevalence and risk factors. J Womens Health 2011;20:1737–1741 [DOI] [PubMed] [Google Scholar]
- 20. Alvareza M, Subramaniam A, Tang Y, Edwards RK. Obesity as an independent risk factor for group B streptococcal colonization. J Matern Fetal Neonatal Med 2017;30:2876–2879 [DOI] [PubMed] [Google Scholar]
- 21. Van Dyke M, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 2009;360:2626–2636 [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 2010;203:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wenckus D, Gao W, Kominiarek MA, Wilkins I. The effects of labor and delivery on maternal and neonatal outcomes in term twins: A retrospective cohort study. BJOG 2014;121:1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venkatesh K, Glover AV, Vladutiu CJ, Stamilio DM. Association of chorioamnionitis and its duration with adverse maternal outcomes by mode of delivery: A cohort study. BJOG 2018;126:719. [DOI] [PubMed] [Google Scholar]
- 25. Institute of Medicine. Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: National Academies Press, 2009 [PubMed] [Google Scholar]
- 26. Mission J, Marshall NE, Caughey AB. Pregnancy risks associated with obesity. Obstet Gynecol Clin North Am 2015;42:335–353 [DOI] [PubMed] [Google Scholar]
- 27. Gopal Rao G, Nartey G, McAree T, et al. Outcome of a screening programme for the prevention of neonatal invasive early-onset group B streptococcus infection in a UK maternity unit: An observational study. BMJ Open 2017;7:e014634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spiel M, Hacker MR, Haviland MJ, et al. Racial disparities in intrapartum group B streptococcus colonization: A higher incidence of conversion in African American women. J Perinatol 2019;39:433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turnbaugh P, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 206;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 30. Ley R, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature 2006;444:1022–1023 [DOI] [PubMed] [Google Scholar]
- 31. Ma'ayeh M, Rood KM, Walker HC, Oliver EA, Gee SE, Iams JD. Vaginal progesterone is associated with decreased group B streptococcus colonisation at term: A retrospective cohort study. BJOG 2019;126:1141–1147 [DOI] [PubMed] [Google Scholar]
- 32. Kindinger L, Bennett PR, Lee YS, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017;19:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Towers C, Rumney PJ, Asrat T, Preslicka C, Ghamsary MG, Nageotte MP. The accuracy of late third-trimester antenatal screening for group B streptococcus in predicting colonization at delivery. Am J Perinatol 2010;27:785–790 [DOI] [PubMed] [Google Scholar]
- 34. Rocchetti T, Marconi C, Rall VL, Borges VT, Corrente JE, da Silva MG. Group B streptococci colonization in pregnant women: Risk factors and evaluation of the vaginal flora. Arch Gynecol Obstet 2011;283:717–721 [DOI] [PubMed] [Google Scholar]