Abstract
Diabetes management is well suited to use of telehealth, and recent improvements in both diabetes technology and telehealth policy make this an ideal time for diabetes providers to begin integrating telehealth into their practices. This article provides background information, specific recommendations for effective implementation, and a vision for the future landscape of telehealth within diabetes care to guide interested providers and practices on this topic.
Note: This article was written prior to the COVID19 pandemic, and does not include information about recent telehealth policy changes that occurred during or as a result of this public health crisis.
Keywords: Telehealth, Diabetes mellitus, Patient-generated health data, Patient-centered care
Background: Telehealth for Diabetes Finally Comes of Age
Diabetes is an ideal medical condition for telehealth utilization because it relies heavily on patient self-management and use of home medical devices that both generate and capture data. Although diabetes patients have better outcomes with more frequent provider contact,1 subspecialists are both insufficient in number and sparsely geographically distributed,2–4 and frequent office visits are difficult to achieve in practice, even with dedicated care coordinators.5–7 For these reasons, telehealth has been a focus in diabetes care dating back to the early 1990s,8 and a good portion of today's management relies on use of “tele” technologies, including telephone, electronic health record (EHR) portals, e-mail, text messaging, and asynchronous data review.
The last decade has seen a tremendous increase in use of diabetes technology, particularly the use of continuous glucose monitoring (CGM) among people with type 1 diabetes.9 In addition, devices such as CGM systems, insulin pumps, and Bluetooth glucose meters now allow easier upload of data from home to the cloud, and numerous software applications enable clinician review of aggregated diabetes device data.10,11 A number of HIPAA-compliant, Internet-based videoconferencing platforms are also now available for use in clinic-to-clinic or home-to-clinic telehealth care.12–14 These technological advances, combined with improved reimbursement for telehealth encounters,15 have resulted in more opportunities for diabetes providers in clinical practice to utilize telehealth capabilities.
Despite these advances, a minority of diabetes patients are routinely reviewing their own data16 and a minority of providers are utilizing telehealth even in organizations where it has been incentivized.17 Furthermore, although CGM review has been reimbursable for more than a decade18 and video visits are now increasingly reimbursed in the United States,15 subspecialists who do offer telehealth continue to depend primarily on grants, contracts, philanthropy, or institutional investment rather than a fee-for-service billing model.19 An increasing number of start-up companies have stepped into this void by offering mobile apps paired with diabetes health coaching services20,21 and/or basal insulin adjustment advice22–24 for a monthly fee, recognizing that diabetes clinics are not reviewing patient data and providing feedback real time in the way that patients crave.
One philosophical barrier to telehealth adoption by providers may be the desire for unequivocal evidence of benefit. Currently, the body of published literature about diabetes and telehealth is broad, encompassing a wide variety of interventions, patient populations, and outcome measures. There are many published studies that demonstrate benefit, and many that do not.25,26 Just as with in-person diabetes care, the effectiveness and cost-effectiveness of telehealth diabetes care depend on the content, format, and frequency of encounters as well as the populations of patients and providers involved.27,28 However, it is important to understand that telehealth itself is not an intervention but a care modality. Incorporating telehealth into your practice adds another tool for communicating with patients and providing the care they need individually. As an analogy, telephone advice may be less effective than in-person communication for certain scenarios (such as communicating a new diagnosis and complex treatment plan that will elicit many questions and strong emotions) and more effective in others (such as discussing a new exercise plan with a mother of three children who will be distracted by them in office but can keep them busy at home). Providers must use their clinical judgment about when to use each care modality, and this is equally true with the use of telehealth.
Once any philosophical barriers are addressed, practical barriers to the adoption of telehealth by diabetes providers may include uncertainty regarding hardware and software requirements, the process of integrating telehealth into standard workflows in the clinic and EHR, appropriate documentation and reimbursement practices, and determining the optimal frequency and content of telehealth encounters for various patient populations. This article provides guidance on each of these topics (Table 1) and can serve as a framework for clinicians looking to implement or expand telehealth care within their diabetes practices. The recommendations in this article focus primarily on the implementation of patient-to-clinic video encounters, although several subtopics discussed are also applicable to asynchronous review of patient-generated diabetes data or clinic-to-clinic video encounters.
Table 1.
Summary of Top 10 Tips for Diabetes Telehealth
| Category | Tip | Summary |
|---|---|---|
| Technological requirements | 1: Hardware | Invest in a widescreen monitor and quality headphones/microphone. Ensure optimal lighting and room setup. |
| 2: Video software | Numerous options exist for HIPAA-compliant video software. Some can be EHR-integrated. | |
| 3: Diabetes software | Select your preferred software application(s) for reviewing diabetes device data. Consider key features from a provider and patient viewpoint. Discuss privacy and security with your IT staff. | |
| Clinical operations | 4: Scheduling telehealth visits | Template your schedule to allow separate blocks for video visits, or discuss staff intervention if preceding in-person visits run late. |
| 5: Standardizing telehealth visit processes | Develop standardized processes for previsit and postvisit tasks (e.g., data upload, laboratory tests, scheduling follow-up) for telehealth patients. Train staff and patients in these processes. | |
| 6: Reimbursement | Review telehealth reimbursement codes and policies that apply to your practice location (https://www.cchpca.org). Utilize codes for video encounters and review of remotely shared data. | |
| 7: EHR integration | Work with your EHR team to optimize tools for telehealth billing, documentation, and capture of diabetes device data. | |
| Maximizing benefit | 8: Patient expectations | Guide patient expectations about billing, location, timing and frequency of video visits in your practice, as well as appropriate use of telehealth technology and remote data-sharing. |
| 9: Patient-centered care | Use telehealth to promote patient-driven, patient-centered diabetes care with individualized content and timing. | |
| 10: Culture change among providers and institutions | Engage institutional stakeholders early, and develop a formal telehealth onboarding process for providers and staff. |
EHR, electronic health record.
Recommendations: Top 10 Tips for Integrating Telehealth into Your Diabetes Practice
Technological requirements
Tip #1: Hardware
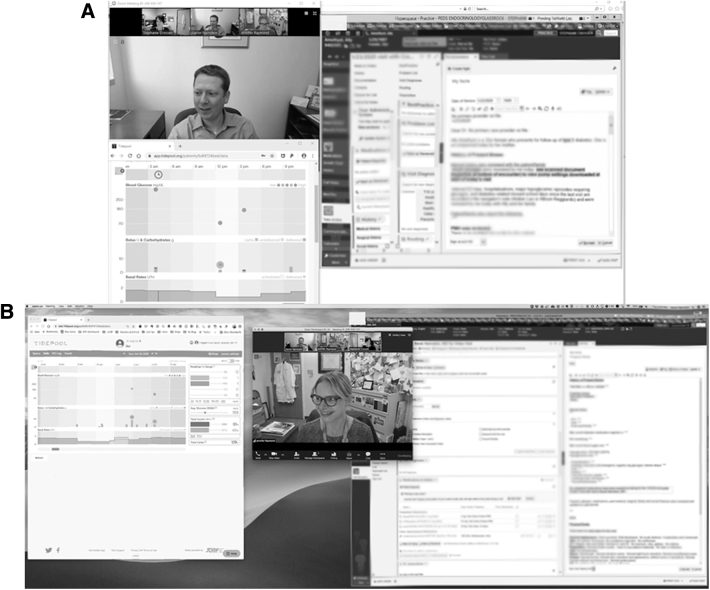
Basic hardware requirements are minimal because video visits can be performed using any mobile device (smartphone or tablet), laptop, or desktop so long as the device has audio and video capabilities, can connect to the Internet, and can download software applications. For the optimal setup, we recommend purchasing an HD video camera that connects to a laptop or desktop via USB. We also recommend purchasing a wide-screen monitor—today's standard for this would be a 38″ LCD monitor—and/or using a two-monitor setup so that several application windows can be open simultaneously. A single wide-screen monitor is preferable as it will better facilitate eye contact with the patient while looking at other application windows on your screen. Use of a single, common-sized monitor such as 24″ leaves insufficient room on screen to view the patient, his/her shared diabetes data, and the EHR simultaneously (Figure 1).
FIG. 1.
Utility of wide-screen monitor for telehealth encounters. Examples of workstation display using 24″ monitor (A) versus 38″ wide-screen monitor (B).
If you are working in a busy space, use noise-canceling headphones with a high-quality built-in microphone to block background noise. If your office is visually distracting or you are connecting from home consider using a backdrop such as a green or blue screen, which can clip on to the back of your chair. Test your camera placement so that you have the best possible eye contact with the patient during the video visit. Finally, test the lighting in your room to ensure that it is soft and even, meaning no harsh bright lights or shadows cutting across your face, and no natural light coming from behind or the side, which could interfere with video quality. In our experience, most patients choose to connect for video visits using smartphones or tablets, but their ability to view shared data would be improved by using a laptop or desktop computer when possible. Depending on their diabetes devices, they will also need to use either a Bluetooth connection or hardware cable to upload and share glucose data via the Internet before telehealth encounters.
Tip #2: Video Software
Many videoconferencing software products now have the requisite security and privacy protections to be HIPAA compliant.12–14 Health care institutions or clinics can purchase access to a given platform and then use it to connect to patients without those patients incurring individual access charges. Patients will typically be required to download the accompanying software application, or to run a temporary application during the encounter. Most video platforms can perform multiparty videoconferencing, which is particularly useful in pediatric practice. For example, an adolescent patient can join the visit from home, his or her parent can join from the workplace, and the physician can join from his or her office. In addition, this capability is key if you hope to initiate shared medical appointments (see Tip #9: Patient-Centered Care) as part of your telehealth program. Certain EHR and videoconferencing vendors allow integration of their applications,13,29 enabling patients to directly launch the video application from the EHR patient portal on their mobile devices. Without this EHR-video platform integration, patients must receive a separate link or invitation to the videoconference session and then independently download and open the videoconference application.
With most video platforms, physicians have the option to connect to visits from off-site, such as from home or a satellite office location, using institutional log-ons to access the necessary software. However, providers should carefully consider the need for additional team members—such as nurses, dietitians, and/or social workers—as well as the ability to ensure privacy and confidentiality before choosing to perform video visits at a location away from the clinic. Patients can also join video visits from any location of their choosing and should be reminded to ensure they are comfortable discussing private health information in the selected environment.
Tip #3: Diabetes Software
An essential step in establishing a diabetes telehealth service is deciding on a method for accessing and reviewing patients' diabetes device data. The array of data-sharing platforms now available for diabetes management can be overwhelming.30–37 These software tools also vary in whether they were developed to meet the needs of patients or providers and whether they are optimized for the care of individual patients or the needs of panel management. In our opinion, helpful features in order of priority are as follows:
Compatibility with the broadest array of devices, including insulin pumps and smart pens, continuous glucose monitors and glucose meters from multiple vendors, as well as the ability to review hybrid closed-loop data for patients utilizing this technology.
Easy upload process for patients at home, ideally allowing passive data-sharing once configured, but at minimum providing a simple active process with minimal additional software or hardware.
Seamless and flexible account administration, enabling access and review by the patient and multiple providers in your office.
Visualization of clinically relevant parameters and key reporting metrics for an individual patient,38 such as frequency of glucose monitoring, glycemic mean and variability, percentage of time in target range, and glucose trends across a modal day.
Technical support from the vendor to instruct patients in how to upload devices from home and/or assist those who encounter difficulty with this process.
Capacity to capture and display patient-generated health data of other types such as food intake, physical activity, and/or menses.
A dashboard or panel view of your diabetes patients, including the ability to sort and filter by various clinical parameters.
Tools to facilitate clinical documentation, such as easily transferring insulin pump settings and CGM analysis into your EHR note.
Fulfilling all these criteria in a single platform is currently a challenge, but you should consider which criteria will be most important for your practice and patient population. The landscape of diabetes data software applications is also changing rapidly, so you should research available options at the time you initiate telehealth, and review applications and their features on a regular basis. Finally, before asking patients to share data with you, you should work with your institutional IT department to ensure your chosen software platform has met your institution's security and privacy standards and that, if required, a business associates' agreement is signed.
Clinical operations
Tip #4: Scheduling Telehealth Visits
Providers offering video visits need to plan their scheduling templates to identify when these visits will be offered. Will you allow any slot on your schedule to become a potential video slot? Will you bunch them together at a separate time from in-person visits? Will you offer after-hours or weekend video visits (which may be in high demand by your patients)? In our experience, video visits tend to be of shorter duration and run more on-time compared with in-person visits. Patients tend to show up on-schedule due to lack of transportation challenges, and no time is needed for check-in, vital sign, and point-of-care HbA1c measurements, or downloading data from patient devices. This improved timeliness is excellent but presents two challenges for the clinician. First, if you typically use check-in time to review the patient's chart, look over downloaded data, and prepare your anticipated recommendations, you will need to find time to do this before the visit or change your practice to do this during your video visit with the patient. Second, if your in-person clinic tends to run late, you risk video patients waiting for long stretches and possibly abandoning the visit before you “arrive.”
In our experience, the best solution to these issues is to schedule video visits in a separate block from in-person visits, either at the start of the day or after lunch, for example. If you choose instead to intersperse video visits with in-person visits, consider asking support staff to notify video patients if you are running behind or arrange for a medical assistant, nurse, or dietitian to begin the video visit while you are finishing the previous encounter. If you choose to do video visits after-hours or off-site (e.g., at home), this may be less possible, but the flexibility offered by video visits works to the benefit of providers as well as patients. Keep in mind that if you involve nonphysician team members in video care, reimbursement codes for this are currently limited [see Tip #6: Reimbursement (Information Applicable to the United States Only)], but may be less so in the future.
Tip #5: Standardizing Telehealth Visit Processes
Video visits omit multiple tasks that are typically performed during diabetes office visits, including downloading data from patients' devices, measuring HbA1c values, obtaining screening tests, and scheduling follow-up. Patients will need to upload and share data from their home devices before each video visit. The timing of when they should do so must be communicated to them, and each practice should consider how to train and support patients who are not familiar with this process. In our experience, the most successful strategy is to train the patient and ensure that the upload process is working seamlessly at an in-person visit before starting video visits. Laboratory work—such as an HbA1c and other recommended screening blood and urine tests—should be ordered ahead of time and patients instructed on when to have these done. Patients will also need to complete training and testing for the video software application to ensure that they have the right application downloaded, that the connection will work on their devices, and that they have sufficient data bandwidth to complete a video visit. Otherwise, technical challenges may lead patients to be late or cancel initial appointments.
If your office has a previsit reminder process for in-person office visits to ensure that patients have their laboratory work done and device data uploaded, you can utilize the same staff members and process to contact patients about upcoming video visits and discuss the necessary previsit tasks. Alternately, your practice can identify one or two staff members who will become the diabetes telehealth navigators and work with patients who are interested in video visits to set up the requisite data-sharing from their diabetes devices and explain the video visit process. Ideally, these individuals will also be available to assist with real-time troubleshooting during video encounters. Clinicians should be clear with patients about whether video visits will be rescheduled in the event that the patient has not uploaded data or obtained requisite laboratory work by a certain time. Finally, you will need to decide how follow-up visits will be scheduled after video encounters since patients will not complete an in-person check-out process. Some options are for the clinician him/herself to discuss follow-up dates during the video visit and send a message to office staff, for a staff member to contact the patient shortly after the visit, or for a staff member to directly join the video visit to assist with scheduling. However you want to handle it, make sure you standardize this process up-front to avoid frustration by all parties.
Tip #6: Reimbursement (Information Applicable to the United States Only)
The information presented in this section is applicable only to clinicians practicing in the United States. If you are practicing outside of the United States, we recommend you contact your professional physician organization for guidance, as this group will likely have the most updated information about telehealth reimbursement policies in your country. In the United States, the last few years have witnessed multiple changes to reimbursement policies for telehealth. Many payers now acknowledge the patient's home as a valid “originating site” for video encounters, and new codes have also been introduced for remote monitoring of physiologic data. In fact, policy is changing so quickly on this topic at the regional and state levels that for the most updated information regarding reimbursement practices in your area, we recommend accessing the Center for Connected Health Policy's website (www.cchpca.org) and from there identifying your regional telehealth resource center.
In general, video visits should be coded using typical current procedural terminology (CPT) codes based on time (e.g., 99214 for an established patient visit lasting 25–39 min) with the addition of the modifier 95 and the point-of-service code 02 to indicate that the visit was conducted via telehealth. Facility fees that are charged for in-person visits do not apply for video visits, but a per-minute transmission fee can be charged using HCPCS T1014. As with in-person visits, additional codes can also be added such as CPT 95251 if CGM review and interpretation were performed. In many states, video visit documentation must include an attestation that the patient was consented verbally to receive video care, and video visits cannot legally be performed if the provider does not hold a medical license in the state where the patient is physically located at the time of encounter.
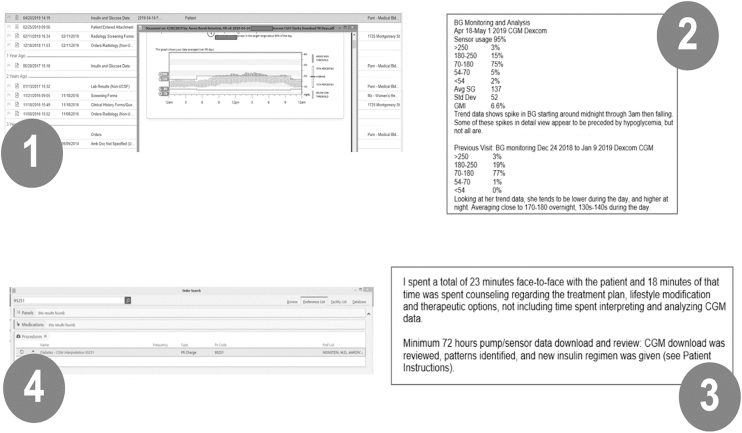
In addition to billing for the real-time clinical encounter, there are now several ways to bill for interpretation of CGM data. One way is to use CPT code 95251 for the review and interpretation of ≥72 h of CGM data (Figure 2). This code can be attached to any real-time office or video visit. Note that you should use a 25 modifier to denote that it was performed on the same day as an E&M visit, and attest to the fact that billed time for the face-to-face visit was not inclusive of time spent interpreting CGM data. The 95251 code can also be attached to a telephone or electronic messaging encounter, but can only be billed by a physician, nurse practitioner, or physician assistant, and will only be reimbursed once every 30 days. A different option for coding your interpretation of diabetes data shared remotely (not to be used simultaneously with 95251) is to use one of CMS's new “remote monitoring” codes—99091 or 99457. These codes are new within the last 2 years and can be used for interpretation of remotely shared glucose data from fingerstick meters or insulin pumps as well as from CGM. For more details about when and how to utilize these remote monitoring codes, refer to published resources.39
FIG. 2.
Steps to use CPT code 95251 for CGM interpretation. (1) Upload CGM documentation to chart—most commonly via scan and PDF upload. (2) Document analysis of CGM data—currently manual but could be automated by diabetes software vendors using automated text generation and copy-paste, or SMART-on-FHIR application. (3) Document service performed—time spent on CGM analysis should not be included in E&M code time. (4) Drop charge for 95251—use 25 modifier if performed on same day as an E&M-coded visit. CGM, continuous glucose monitoring; CPT; FHIR, Fast Healthcare Interoperability Resource; SMART, Substitutable Medical Applications and Reusable Technologies.
Tip #7: EHR Integration
There are several ways in which your EHR can optimally support telehealth activities. Most fundamentally, you must have the correct billing codes built, the ability to designate a separate visit type in providers' schedules, and standardized documentation for video visits. This documentation should include an attestation that patient/guardian consent was obtained for video-based care. EHR note templates can also support the use of billing codes for CGM interpretation by prompting providers to enter information about the device used, the time frame of data reviewed, and your analysis and interpretation of these data, typically with summary measures such as glucose mean and variability, time in range, and data trends. Certain diabetes software platforms are now helping with this process by automating the output of summary glucose statistics, to avoid the time-consuming and mistake-prone process of a clinician manually transcribing numbers.
To utilize billing codes for CGM interpretation, you also need to upload the CGM data to the EHR in some form. This can be done as a scanned PDF in the patient's medical record, and for ease of access should be attached to the applicable encounter and labeled clearly. At one of our institutions, we have built a scanned document type called “Insulin and Glucose Data” so that historical scanned PDFs can be easily identified. At a more advanced level, it has been demonstrated in recent years that CGM data can be directly imported into the EHR for ease of access and visualization by providers, as well as to facilitate improved analytic capabilities.40 This functionality currently requires custom programming, but it is possible that efforts by EHR and/or diabetes software companies will make it a more standard feature in the future. To this end, we advocate that all diabetes software providers design their applications to comply with the SMART (Substitutable Medical Applications and Reusable Technologies) on FHIR (Fast Healthcare Interoperability Resource) application programming interface (API). SMART-on-FHIR was first developed by the Boston Children's Hospital Computational Health Informatics Program as an open specification,41 and in 2020 was adopted by the U.S. Office of the National Coordinator as a mandatory standard for certified health IT.42 The SMART-on-FHIR API standard allows health data to flow between applications, which would enable a clinician to move directly from the patient's EHR into the patient's record in the diabetes application without an additional log-on, pull data from the EHR into the diabetes application and potentially export data and reports back to the EHR as well.43
Maximizing benefit
Tip #8: Patient Expectations
It is important to set expectations with your patients regarding use of telehealth. As mentioned in prior sections, be clear about when video visits will be available (e.g., after-hours vs. Monday–Friday 8am–5pm) and what they will cost. In many scenarios, patients can expect the same copay as for an in-person office visit, but this varies. Also, if their insurance does not cover telehealth, your office may need to institute a self-pay fee schedule. If you are using a CGM interpretation or remote monitoring billing code, keep in mind that your patients may also see required copays or coinsurance for these charges, which may appear new and confusing to them. Patients also need to remember that video applications and device uploads will require data use, so if they have limited data plans on their mobile devices, you should recommend they access a WiFi network. In addition, you must be clear with patients that providers will not be monitoring the data they share real time nor proactively reaching out to address concerning values, and so they will still need to take responsibility for monitoring their own health and contacting the clinical team for assistance when needed.
Many patients may prefer video visits to office visits, so telling them up-front your recommendation about the frequency of remote versus in-person care is also helpful. Research has shown that video visits can be used effectively to replace routine in-person encounters for patients with access barriers44; to provide more frequent, supplemental care to patients who are struggling45; or to facilitate shared medical appointments for cohorts who benefit from mutual support.46 In our experience, they are also useful as quick follow-ups to review the success of a recent change in insulin regimen or behavioral strategy, and as a modality for group education classes. It is important to consider the potential uses of telehealth in your clinical population so that you can guide patients' expectations for the frequency and content of their remote care.
Tip #9: Patient-Centered Care
In many ways, telehealth has the capacity to shift diabetes care delivery to be more patient-driven and patient-centered. Traditional diabetes care has been provider-driven. Frequency, duration, and location of care have been determined by providers' availability. Care decisions have been predicated on HbA1c values and glucose data trends that providers gather and relay to patients in a clinical setting. With telehealth, diabetes care can take place in the home at a frequency customized to the individual, and treatment decisions are based on patient-generated health data that are relayed to the provider. In addition, patients participating in telehealth visits are forced to learn multiple self-management skills, such as uploading diabetes device data and completing necessary laboratory tests off-site, which they may not have acquired during traditional in-person visits. Research has demonstrated an increase in diabetes self-efficacy for those participating in telehealth visits when compared with those participating in standard in-person diabetes visits,47 which could be related to acquiring these skills.
The telehealth model can also support an effective coaching relationship between provider and patient. During video visits, diabetes device data can be reviewed together and used to prompt the patient to ask questions and attempt to interpret his/her own data patterns. Providers in turn can look for teachable moments and opportunities to help the patient problem-solve independently while providing emotional support. The ability to conduct more frequent encounters via telehealth (due to fewer access barriers) also enables visits to focus on one specific change or goal at a time rather than trying to tackle every identifiable issue in one visit, which often overloads the patient and is counterproductive. Unpublished survey data from one of our institutions indicate that video visits help patients feel more heard by their providers and better understand what providers tell them. Finally, there is an additional value in seeing patients in their home environments, including the ability to observe their daily surroundings and to interact with additional caretakers (particularly for pediatric patients) who are not attending office visits.
Tip #10: Culture Change Among Providers and Institutions
When you are working to implement a diabetes telehealth program, keep in mind that learning a new care model can be anxiety-provoking and overwhelming for both providers and institutions. Clinicians and health care systems have been practicing the current in-person medical model for decades. Acknowledging concerns and building supportive practices will increase your likelihood of success. We have found it critical to engage all institutional stakeholders early in the process to allow for successful integration of telehealth practices into routine care. This allows leaders within the institution to provide insight, questions, and feedback during the development phase of your telehealth program. Creating an onboarding process for providers who are new to telehealth is also crucial. This can include practicing telehealth visits before initial appointments so that providers are comfortable using the software for videoconferencing and know how to review and share diabetes data with patients during visits. Scheduling a smaller patient volume during initial telehealth clinics can be helpful to support the learning process, similar to how providers are initially onboarded at a new clinic or institution. In addition, having IT support for providers during their first few telehealth encounters can provide reassurance and increase confidence. Another option is to develop a formal process for telehealth privileges (similar to medical privileges required for all practicing providers) at your institution to facilitate standardized training processes and clarity in expectations for providers participating in telehealth.
Finally, for those at teaching institutions, it is equally important to train the next generation of providers in these new care models. Trainees should be mentored in how to successfully utilize video visits, how to think about panel management, and how to incorporate patient-generated health data into their provision of care. As an example of how this can be done, at one of our institutions (A.N.), we recently began a regularly scheduled educational program called “Diabetes Data Rounds” where we review and discuss a series of recent CGM downloads, akin to how radiologists learn to read X-rays or cardiologists learn to read electrocardiograms.
Conclusion and Future Directions
While barriers remain to the optimal integration of telehealth into clinical care—including persistent billing restrictions and software incompatibilities—recent advances in both technology and policy have now made telehealth a feasible care modality for diabetes management. In this article, we have described a practical set of tips to guide clinicians who are looking to initiate telehealth within their diabetes practices. We hope this article will serve as a launching point for further discussion, and feel that our field would benefit in the near future from the creation of formal telehealth guidelines for diabetes, such as those recently published by the American Psychiatric Association.48
The diabetes community would also benefit from further research evaluating (1) the impact of telehealth as a care modality, and (2) the effectiveness of new types of care that are now possible with use of telehealth. These research categories are distinct, and the interpretation of their findings will be different. For example, research in the first category delivers the same care—such as diabetes nutrition education or discussion of risk-taking behaviors with adolescents—either in-person or via telehealth and then compares outcomes between these two modalities. This type of research will allow us to explore whether telehealth is equivalent, inferior, or superior to an in-person encounter for various types of care. Research in the second category, in contrast, is designed to assess the value of new care models or interventions that are possible with telehealth but cannot be delivered in-person, such as remote patient monitoring. These studies are critical as we enter a highly innovative phase with telehealth and digital health technologies, and their findings should be interpreted as an evaluation of the interventions themselves, rather than of telehealth broadly as a care modality.
In parallel to ongoing research, the integration of telehealth into diabetes practices should be used to support clinical innovation and quality improvement efforts that align closely with the American Diabetes Association standards,49 and to address key issues with provider supply and distribution that have historically limited many patients' access to high-quality care. For example, diabetes care ideally includes clinical diabetes educators, registered dietitians, mental health providers, and health coaches in addition to physicians, but not every diabetes practice has access to all of these provider types. Telehealth could allow remote collaboration between providers to deliver comprehensive interdisciplinary care to people with diabetes. The ability to provide care to patients in their homes also negates the need for lengthy, combined appointments at large multidisciplinary centers. Instead, providers could connect with patients individually at mutually convenient times and correspond with one another before and after these visits to ensure a coordinated approach. Finally, telehealth has the potential to address staffing and space limitations on the provider side by enabling physicians to use their administrative offices and/or homes to conduct patient visits, thereby reducing the demands on typically more expensive shared clinical workspace.
Looking to the future, perhaps the most transformative opportunity for telehealth lies with a population-based, panel management approach to care. In this scenario, telehealth would become the primary modality of care with in-person care reserved for less common circumstances, as proposed in NEJM by Duffy and Lee.50 A provider might start each day with an electronic dashboard populated in real time by patients' most recent laboratory results, CGM data, insulin delivery data, and other patient-reported outcomes captured on a patient's mobile device such as mood or diabetes distress. The provider could then select subcohorts of patients to reach out to via text, phone, video, and/or invite for in-office visits based on their individualized needs. As demonstrated by the Dutch treatment center Diabeter, this approach has the potential to achieve outstanding patient outcomes and deliver value-based care.51
We are currently in an exciting and critical era for diabetes management. New telehealth technologies provide us with the means to extend traditional care in new ways, to improve its patient-centeredness, and to advance the health of populations through novel telehealth-driven care techniques. Appropriate use of these technologies requires sound clinical judgment, thoughtful implementation, and rigorous evaluation to determine what approaches are most effective in specific populations and settings. We can each support this process by sharing the knowledge gained through practical experience in new care modalities, and thus enable the next round of innovators to build on this foundation and move collectively toward the standardization of best practices for telehealth in diabetes management.
Acknowledgments
The authors appreciate the expert input of Linda Branagan, PhD, and Carol Yarbrough, MBA, of the UCSF Telehealth Resource Center.
Author Disclosure Statement
S.C. has no competing financial interests to disclose. J.R. has no competing financial interests to disclose. A.N. has received research support from Cisco Systems, Inc.; has received consulting fees from Nokia Growth Partners and Grand Rounds; serves as advisor to Steady Health (received stock options); has received speaking honoraria from Academy Health and Symposia Medicus; has written for WebMD (receives compensation); and is a medical advisor and cofounder of Tidepool (for which he receives no compensation). The software applications depicted in this article's figures are included as examples only and do not represent the authors' endorsement of those products. The patient information depicted in this article's figures is fictional.
Funding Information
S.C. receives research support from the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001860 and linked award KL2 TR001859.
References
- 1. Nathan DM, Genuth S, Lachin J, et al. : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Lee JM, Davis MM, Menon RK, Freed GL: Geographic distribution of childhood diabetes and obesity relative to the supply of pediatric endocrinologists in the United States. J Pediatr 2008;152:331–336 [DOI] [PubMed] [Google Scholar]
- 3. Vigersky RA, Fish L, Hogan P, et al. : The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab 2014;99:3112–3121 [DOI] [PubMed] [Google Scholar]
- 4. Association of American Medical Colleges. Number of People Per Active Physician By Specialty. 2017. https://www.aamc.org/data-reports/workforce/interactive-data/number-people-active-physician-specialty-2017 (accessed November20, 2019)
- 5. Maldonado MR, D'Amico S, Rodriguez L, et al. : Improved outcomes in indigent patients with ketosis-prone diabetes: effect of a dedicated diabetes treatment unit. Endocr Pract 2003;9:26–32 [DOI] [PubMed] [Google Scholar]
- 6. Laffel LM, Brackett J, Ho J, Anderson BJ: Changing the process of diabetes care improves metabolic outcomes and reduces hospitalizations. Qual Manag Health Care 1998;6:53–62 [DOI] [PubMed] [Google Scholar]
- 7. Holmes-Walker DJ, Llewellyn AC, Farrell K: A transition care programme which improves diabetes control and reduces hospital admission rates in young adults with Type 1 diabetes aged 15–25 years. Diabet Med 2007;24:764–769 [DOI] [PubMed] [Google Scholar]
- 8. Billiard A, Rohmer V, Roques MA, et al. : Telematic transmission of computerized blood glucose profiles for IDDM patients. Diabetes Care 1991;14:130–134 [DOI] [PubMed] [Google Scholar]
- 9. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neinstein A, Wong J, Look H, et al. : A case study in open source innovation: developing the Tidepool Platform for interoperability in type 1 diabetes management. J Am Med Inform Assoc 2016;23:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ekhlaspour L, Tabatabai I, Buckingham B: A review of continuous glucose monitoring data interpretation in the age of automated insulin delivery. J Diabetes Sci Technol 2019;13:645–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zoom: https://zoom.us/plan/healthcare (accessed November1, 2016)
- 13. Vidyo: www.vidyo.com/solutions/healthcare (accessed November1, 2016)
- 14. Cisco: www.cisco.com/c/en/us/solutions/industries/healthcare/care-at-a-distance.html (accessed November1, 2016)
- 15. Center for Connected Health Policy. State Telehealth Laws and Reimbursement Policies: https://www.cchpca.org/telehealth-policy/state-telehealth-laws-and-reimbursement-policies-report (accessed November20, 2019)
- 16. Wong JC, Neinstein AB, Spindler M, Adi S: A minority of patients with type 1 diabetes routinely downloads and retrospectively reviews device data. Diabetes Technol Ther 2015;17:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Modi PK, Kaufman SR, Portney DS, et al. : Telemedicine utilization by providers in accountable care organizations. Mhealth 2019;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Telgener PC, Lowe S: A look at the current reimbursement environment for continuous glucose monitoring (CGM): understanding the fundamentals. J Diabetes Sci Technol 2008;2:681–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olson CA, McSwain SD, Curfman AL, Chuo J: The current pediatric telehealth landscape. Pediatrics 2018;141:e20172334. [DOI] [PubMed] [Google Scholar]
- 20. OneDrop: https://onedrop.today (accessed November20, 2019)
- 21. MySugr: https://mysugr.com/en-us (accessed November20, 2019)
- 22. Sanofi MyStar DoseCoach: https://www.mystarsanofi.com/products/dosecoach (accessed November20, 2019)
- 23. Insulia: https://insulia.com (accessed November20, 2019)
- 24. d-Nav Insulin Guidance Service: https://hygieia.com (accessed November20, 2019)
- 25. Wu C, Wu Z, Yang L, et al. : Evaluation of the clinical outcomes of telehealth for managing diabetes: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2018;97:e12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Guzman KR, Snoswell CL, Taylor ML, et al. : A systematic review of pediatric telediabetes service models. Diabetes Technol Ther 2020. [Epub ahead of print]; DOI: 10.1089/dia.2019.0489 [DOI] [PubMed] [Google Scholar]
- 27. Lee JY, Lee SWH: Telemedicine cost-effectiveness for diabetes management: a systematic review. Diabetes Technol Ther 2018;20:492–500 [DOI] [PubMed] [Google Scholar]
- 28. McDonnell ME: Telemedicine in complex diabetes management. Curr Diab Rep 2018;18:42. [DOI] [PubMed] [Google Scholar]
- 29. Jewett EA, Anderson MR, Gilchrist GS: The pediatric subspecialty workforce: public policy and forces for change. Pediatrics 2005;116:1192–1202 [DOI] [PubMed] [Google Scholar]
- 30. Tidepool: www.tidepool.org (accessed November1, 2016)
- 31. Glooko: https://www.glooko.com (accessed November1, 2016)
- 32. Dexcom Clarity: https://clarity.dexcom.com (accessed November30, 2017)
- 33. Medtronic Carelink: https://carelink.medtronic.com (accessed November30, 2017)
- 34. Tandem TConnect: https://tconnect.tandemdiabetes.com (accessed November30, 2017)
- 35. OneTouch Reveal: https://onetouchreveal.com (accessed November30, 2017)
- 36. Accuchek Connect: https://www.accu-chekconnect.com (accessed November30, 2017)
- 37. Freestyle LibreView: https://www2.libreview.com (accessed November20, 2019)
- 38. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fact Sheet: Finalized CY 2019 Physician Fee Schedule. https://www.cchpca.org/sites/default/files/2018-11/FINAL%20PFS%20CY%202019%20COMBINED_0.pdf (accessed November20, 2019)
- 40. Kumar RB, Goren ND, Stark DE, et al. : Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc 2016;23:532–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. SMART Health kit: https://smarthealthit.org (accessed March9, 2020)
- 42. U.S. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program. 2019:1–1244 [Google Scholar]
- 43. Bloomfield RA Jr., Polo-Wood F, Mandel JC, Mandl KD: Opening the Duke electronic health record to apps: implementing SMART on FHIR. Int J Med Inform 2017;99:1–10 [DOI] [PubMed] [Google Scholar]
- 44. Wood CL, Clements SA, McFann K, et al. : Use of telemedicine to improve adherence to american diabetes association standards in pediatric type 1 diabetes. Diabetes Technol Ther 2016;18:7–14 [DOI] [PubMed] [Google Scholar]
- 45. Crossen SS, Marcin JP, Qi L, et al. : Home visits for children and adolescents with uncontrolled type 1 diabetes. Diabetes Technol Ther 2019;22:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raymond JK: Models of care for adolescents and young adults with type 1 diabetes in transition: shared medical appointments and telemedicine. Pediatr Ann 2017;46:e193–e197 [DOI] [PubMed] [Google Scholar]
- 47. Bakhach M, Reid MW, Pyatak EA, et al. : Home Telemedicine (CoYoT1 Clinic): a novel approach to improve psychosocial outcomes in young adults with diabetes. Diabetes Educ 2019;45:420–430 [DOI] [PubMed] [Google Scholar]
- 48. Shore JH, Yellowlees P, Caudill R, et al. : Best practices in videoconferencing-based telemental health April 2018. Telemed J E Health 2018;24:827–832 [DOI] [PubMed] [Google Scholar]
- 49. American Diabetes Association: 1. Improving care and promoting health in populations: standards of medical care in diabetes-2019. Diabetes Care 2019;42(Suppl 1):S7–S12 [DOI] [PubMed] [Google Scholar]
- 50. Duffy S, Lee TH: In-person health care as option B. N Engl J Med 2018;378:104–106 [DOI] [PubMed] [Google Scholar]
- 51. Diabeter: https://diabeter.nl/en (accessed November20, 2019)