Abstract
Purpose
To determine longitudinal alterations in corneal nerve fiber morphology, dendritic cell (DC) density, and retinal nerve fiber layer (RNFL) thickness over 2 years in patients with multiple sclerosis (MS).
Methods
Thirty-one consecutive patients with relapsing-remitting MS (RRMS) underwent assessment of the Kurtzke Expanded Disability Status Scale (EDSS), Multiple Sclerosis Severity Score (MSSS), corneal confocal microscopy to quantify corneal subbasal nerve morphology and DC density, and spectral-domain optical coherence tomography to quantify RNFL thickness at baseline and after 2 years.
Results
There was a significant reduction in corneal nerve fiber area (CNFA) (P = 0.003), nerve fiber width (CNFW) (P = 0.005), and RNFL thickness (P = 0.004) with an increase in EDSS (P = 0.01) over 2 years. The change in corneal nerve fiber density (CNFD) correlated with the change in EDSS (ρ = −0.468; P = 0.008), MSSS (ρ = −0.442; P = 0.01), DC density (ρ = −0.550; P = 0.001), and RNFL (ρ = 0.472; P = 0.007). The change in corneal nerve fiber length (CNFL) correlated with the change in EDSS (ρ = −0.445; P = 0.01) and MSSS (ρ = −0.490; P = 0.005). Furthermore, there was a significant decrease in CNFL (P < 0.001), CNFA (P = 0.02), CNFW (P = 0.04), corneal total branch density (P = 0.01), and RNFL thickness (P = 0.02) and a significant increase in DC density (P = 0.04) in patients with worsening EDSS (n = 15).
Conclusions
Corneal confocal microscopy can be used to detect progressive corneal nerve fiber loss that relates to a progression of disability in patients with RRMS.
Translational Relevance
Corneal confocal microscopy acts as a sensitive imaging biomarker for progressive nerve degeneration in patients with MS.
Keywords: corneal confocal microscopy, dendritic cells, multiple sclerosis, nerve degeneration, retinal nerve fiber layer
Introduction
Multiple sclerosis (MS) is a chronic inflammatory neurodegenerative disease characterized by repeated episodes of inflammation with demyelination and axonal degeneration in the central nervous system. There is also evidence of peripheral neuropathy and small fiber damage in patients with MS.1–3 Axonal degeneration is considered to underlie progressive neurologic disability4 and can be evaluated by assessing brain atrophy or volume loss on magnetic resonance imaging (MRI) and peripapillary retinal nerve fiber layer (RNFL) thinning measured with optical coherence tomography (OCT).5–8 However, the correlation between MRI measures and clinical disability is poor,9,10 and acute optic neuritis (ON) can increase RNFL thickness.11,12 Therefore, there is a need for more precise measures of axonal degeneration in MS.
Corneal confocal microscopy (CCM) is a noninvasive imaging technique that allows quantification of the corneal subbasal nerve plexus and dendritic cells (DCs).13–15 We and others have previously shown that CCM can detect corneal nerve fiber damage in central16–20 and peripheral21–25 neurodegenerative disorders and increased DCs in diabetic neuropathy, chronic inflammatory demyelinating polyneuropathy, Fabry disease, and Behçet disease.14,24–27
Recently, we and others have shown corneal nerve fiber damage in patients with MS.18,19,28 In the current study, we have evaluated longitudinal alterations in corneal nerve fiber morphology, DC density, and peripapillary RNFL thickness in relation to neurologic disability over 2 years in patients with MS.
Methods
Thirty-one patients with relapsing-remitting MS (RRMS) were enrolled in this prospective study at a single tertiary referral university hospital. These patients were part of a larger cohort from a previously published cross-sectional study demonstrating corneal nerve fiber damage and increased DCs in patients with MS.28 The patients were diagnosed with RRMS according to the revised McDonald criteria.29 A complete neurologic examination was performed for each patient at baseline and after 2 years, and the Kurtzke Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Severity Score (MSSS) were used to assess disease severity and physical disability. Exclusion criteria were a history of ON within 6 months prior to study entry, previous ocular trauma, surgery, contact lens use, or any other systemic and ocular diseases that might affect corneal nerves, DCs, or RNFL. The study was approved by the Institutional Research Ethics Committee and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants after explanation of the nature and possible consequences of the study.
All patients underwent a complete ophthalmologic examination. The peripapillary RNFL thickness measurements were obtained using a spectral-domain OCT device (Spectralis OCT; Heidelberg Engineering GmbH, Heidelberg, Germany). The OCT scanning circle (3.4 mm in diameter) was manually positioned at the center of the optic disc, and the average peripapillary RNFL thickness was recorded in micrometers. The baseline peripapillary RNFL scans were set as reference for the follow-up measurements.
Laser-scanning CCM (Rostock Cornea Module/Heidelberg Retina Tomograph lll; Heidelberg Engineering GmbH) was performed on all patients at baseline and after 2 years of follow-up. The full thickness of the central cornea was scanned using the section mode, and two-dimensional digital images were obtained with a lateral digital resolution of 1 μm/pixel, a depth resolution of 2 μm/pixel, and an image size of 400 × 400 μm. The total duration of examination was approximately 2 minutes per eye. Three to five high-quality subbasal nerve plexus images were selected and analyzed from each patient, and the average of the results was considered. Automated CCMetrics software, version 2.0 (University of Manchester, Manchester, UK), was used to quantify the nerve fibers.30 Six corneal nerve measures were quantified: corneal nerve fiber density (CNFD), the total number of major nerves/mm2; nerve branch density (CNBD), the number of branches arising from major nerves/mm2; nerve fiber length (CNFL), the total length of all nerve fibers and branches (mm/mm2); total branch density (CTBD), the total number of branches/mm2; nerve fiber area (CNFA), the total area of nerve fibers (µm2/mm2); and nerve fiber width (CNFW), the average axial diameter of nerve fibers (µm). The number of highly reflective cells with dendriform structures was manually counted in the same images used to quantify the subbasal nerve plexus using the proprietary software within the corneal confocal microscope, and the density was defined as the total number of cells/mm2.
For all patients, data from one eye were used for analyses. Only the right eyes were assessed if the patient had a history of bilateral ON (n = 6) or no ON in either eye (n = 13). The eyes with previous ON were assessed in patients with a history of unilateral ON (n = 12). The same eye was studied for each patient at baseline and follow-up.
Statistical analysis was performed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA) software. Basic descriptive statistics were calculated and reported as the mean (SD) or median (interquartile range [IQR]), as appropriate. Normal distribution of continuous variables was confirmed with the Kolmogorov-Smirnov test. Paired samples t-test for normally distributed data and Wilcoxon signed rank test for nonnormally distributed data were used to compare the parameters obtained at baseline and after 2 years of follow-up. Independent samples t-test for parametric data and Mann-Whitney U-test for nonparametric data were used to compare the parameters between different treatment groups. The associations between the change in disease severity scores during follow-up and CCM parameters and RNFL were measured using the Spearman correlation coefficient. For all evaluations, a two-sided P value of less than 0.05 was considered statistically significant.
Results
The mean ± SD age of the patients with RRMS at the time of enrollment was 35.0 ± 7.7 years, and 20 (64.5%) of them were female. The mean ± SD duration of MS was 9.5 ± 4.2 years. None of the patients developed confounding ocular disease during the follow-up period. Eighteen patients (58%) had a history of ON, while no patient experienced an ON episode during follow-up. Twenty-eight of the 31 patients (90%) were receiving disease-modifying agents for treatment; 13 (42%) patients were on fingolimod, 10 (32%) were on interferon-β, 4 (13%) were on azathioprine, 1 (3%) was on teriflunomide, and 3 (10%) were not receiving any disease-modifying therapy. Nine (29%) patients had relapses (8 patients had one, 1 patient had two relapses) during 2 years of follow-up. The median (IQR) value of the EDSS score was 3.0 (2.0–3.5) at baseline and increased significantly to 3.0 (2.5–4.0) after 2 years of follow-up (P = 0.01) (Table 1). During the follow-up period, 15 (48%) patients showed a worsening in EDSS score, 3 (10%) showed an improvement, and 13 (42%) had no change. None of the patients reported a history of trigeminal neuralgia.
Table 1.
Corneal Confocal Microscopic Parameters, Peripapillary RNFL Thickness, and Disease Severity Scores in Patients With Multiple Sclerosis at Baseline and After 2 Years of Follow-up
| Characteristic | Baseline | Follow-up | P Value |
|---|---|---|---|
| CNFD (No./mm2) | 26.9 ± 7.2 | 25.7 ± 6.6 | 0.38a |
| CNBD (No./mm2) | 38.1 ± 19.9 | 32.1 ± 14.3 | 0.09a |
| CNFL (mm/mm2) | 16.0 ± 3.2 | 15.1 ± 2.8 | 0.05a |
| CTBD (No./mm2) | 56.1 ± 25.6 | 48.3 ± 20.0 | 0.08a |
| CNFA (µm2/mm2) | 7041.9 ± 1589.9 | 6016.1 ± 1706.8 | 0.003 a |
| CNFW (µm) | 22.0 ± 1.4 | 21.2 ±1.0 | 0.005 a |
| DC density (No./mm2) | 26.0 (6.7–37.0) | 21.2 (4.7–75.3) | 0.16b |
| RNFL thickness (µm) | 84.1 ± 13.8 | 82.8 ± 14.2 | 0.004 a |
| EDSS | 3.0 (2.0–3.5) | 3.0 (2.5–4.0) | 0.01 b |
| MSSS | 4.6 ± 1.8 | 4.3 ± 1.5 | 0.18a |
Data are expressed as mean ± SD for CNFD, CNBD, CNFL, CTBD, CNFA, CNFW, RNFL, and MSSS and median (IQR) for DC density and EDSS. The bold P values represent statistically significant differences.
Paired samples t-test.
Wilcoxon signed rank test.
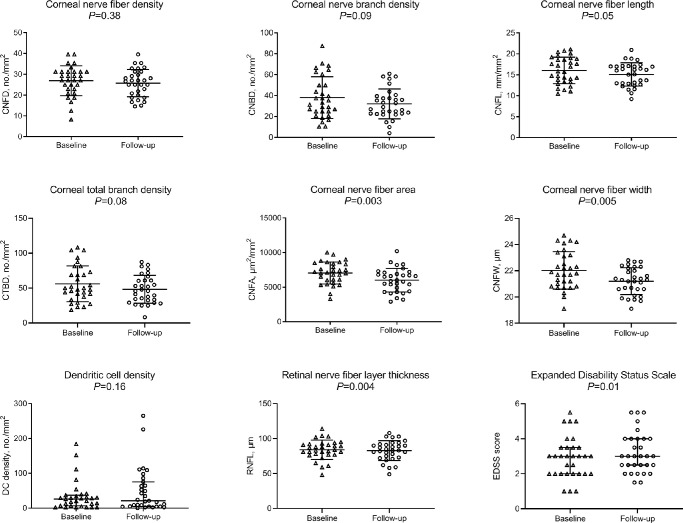
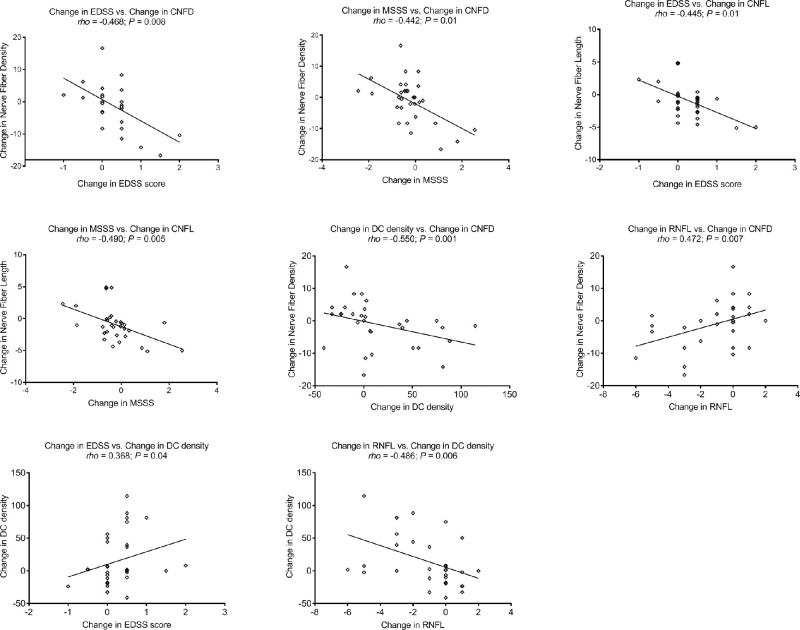
There were significant reductions in CNFA (95% confidence interval [CI], −1685.2 to −366.4; P = 0.003), CNFW (95% CI, −1.4 to −0.3; P = 0.005), and RNFL thickness (95% CI, −2.0 to −0.4; P = 0.004) a trend for reduction in CNFL (95% CI, −1.9 to 0.02; P = 0.05) and CTBD (95% CI, −16.5 to 0.9; P = 0.08) and no significant change in CNFD, CNBD, or DC density over 2 years (Table 1, Fig. 1). The highest percentage of patients with MS showed a decrease in CCM parameters (CNFD [48%], CNBD [65%], CNFL [77%], CTBD [65%], CNFA [74%], and CNFW [71%]), while a proportion showed an increase (CNFD [42%], CNBD [29%], CNFL [23%], CTBD [35%], CNFA [26%], and CNFW [29%]) or no change (CNFD [10%], CNBD [6%]). The change in CNFD during follow-up correlated with the change in EDSS (ρ = −0.468; P = 0.008), MSSS (ρ = −0.442; P = 0.01), DC density (ρ = −0.550; P = 0.001), and RNFL thickness (ρ = 0.472; P = 0.007), and the change in CNFL correlated with the change in EDSS (ρ = −0.445; P = 0.01) and MSSS (ρ = −0.490; P = 0.005). The change in DC density correlated with the change in EDSS (ρ = 0.368; P = 0.04) and RNFL (ρ = −0.486; P = 0.006) during the follow-up period (Fig. 2).
Figure 1.
Corneal confocal microscopic parameters, RNFL thickness, and EDSS score in baseline and follow-up measurements of patients with RRMS, showing a reduction in CNFA, CNFW, and RNFL thickness and an increase in EDSS score, with no change in CNFD, CNBD, CNFL, CTBD, and DC density. Error bars indicate mean (SD) for CNFD, CNBD, CNFL, CTBD, CNFA, CNFW, and RNFL and median (IQR) for DC density and EDSS.
Figure 2.
Scatterplot graphs of significant correlations between corneal confocal microscopic parameters, RNFL thickness, and disease severity scores (EDSS and MSSS) in patients with RRMS.
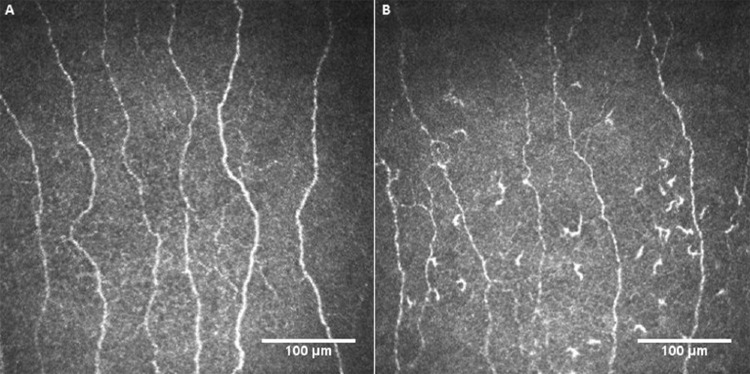
There were significant reductions in CNFL (95% CI, −3.1 to −1.1; P < 0.001), CTBD (95% CI, −29.2 to −4.0; P = 0.01), CNFA (95% CI, −2205.1 to −208.2; P = 0.02), CNFW (95% CI, −1.8 to −0.04; P = 0.04), and RNFL thickness (95% CI, −2.8 to −0.3; P = 0.02) and an increase in DC density (median [IQR], 20.3 [6.7–39.3] vs. 52.7 [4.7–111.0]; P = 0.04) in the subset of patients (48%) with a worsening EDSS score over 2 years, with no significant change in any of the study parameters in patients without progression in EDSS (Table 2). Figure 3 illustrates the CCM images of the central corneal subbasal nerve plexus at baseline and after 2 years of follow-up in a patient with RRMS and worsening EDSS score.
Table 2.
Corneal Confocal Microscopic Parameters, Peripapillary RNFL Thickness, and Disease Severity Scores in Patients With Multiple Sclerosis With and Without a Worsening in EDSS Score Over 2 Years
| Patients with Worsening EDSS (n = 15) | Patients without Worsening EDSS (n = 16) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | Follow-up | P Value | Baseline | Follow-up | P Value |
| CNFD (No./mm2) | 29.6 ± 7.4 | 26.0 ± 7.1 | 0.09a | 24.4 ± 6.2 | 25.5 ± 6.2 | 0.43a |
| CNBD (No./mm2) | 41.1 ± 19.3 | 30.3 ± 14.1 | 0.05a | 35.3 ± 20.7 | 33.8 ± 14.7 | 0.76a |
| CNFL (mm/mm2) | 17.1 ± 2.8 | 15.0 ± 2.4 | <0.001 a | 15.1 ± 3.3 | 15.2 ± 3.2 | 0.86a |
| CTBD (No./mm2) | 61.7 ± 25.4 | 45.1 ± 18.6 | 0.01 a | 50.9 ± 25.6 | 51.4 ± 21.4 | 0.93a |
| CNFA (µm/mm2) | 7360.0 ± 1141.9 | 6153.3 ± 1860.1 | 0.02 a | 6743.7 ± 1908.6 | 5887.5 ± 1600.4 | 0.08a |
| CNFW (µm) | 22.2 ± 1.6 | 21.2 ± 0.9 | 0.04 a | 21.9 ± 1.3 | 21.2 ± 1.7 | 0.07a |
| DC density (No./mm2) | 20.3 (6.7–39.3) | 52.7 (4.7–111.0) | 0.04 b | 28.4 (4.9–35.5) | 14.5 (4.8–45.5) | 0.68b |
| RNFL (µm) | 85.4 ± 12.2 | 83.9 ± 12.4 | 0.02 a | 82.8 ± 15.5 | 81.9 ± 16.0 | 0.09a |
| EDSS | 3.0 (2.0–3.5) | 3.5 (2.5–4.0) | <0.001 b | 3.0 (2.0–3.9) | 2.8 (2.0–3.8) | 0.10b |
| MSSS | 4.4 ± 1.9 | 4.8 ± 1.4 | 0.08a | 4.7 ± 1.8 | 3.9 ± 1.6 | <0.001 a |
Data are expressed as mean ± SD for CNFD, CNBD, CNFL, CTBD, CNFA, CNFW, RNFL and MSSS, and median (interquartile range) for DC density and EDSS. The bold P values represent statistically significant differences.
Paired samples t-test.
Wilcoxon signed rank test.
Figure 3.
Representative corneal confocal microscopic images of the subbasal nerve plexus in a patient with multiple sclerosis at baseline (A) and after 2 years (B) showing reduced nerve fibers and increased dendritic cells. This patient had a relapse and an increase of 1.0 point in EDSS score during follow-up.
The RNFL thickness showed a significant reduction over 2 years in eyes with a history of ON (n = 18; 95% CI, −2.4 to −0.3; P = 0.01) but no change in eyes without ON (n = 13; 95% CI, −2.4 to 0.4; P = 0.13) (Supplementary Table S1). There was no significant difference in the change in EDSS, MSSS, corneal nerve parameters, and peripapillary RNFL thickness, but there was an increase in DC density in patients receiving fingolimod (n = 13) compared with interferon-β (n = 10) (Supplementary Table S2).
Discussion
To our knowledge, this is the first study reporting longitudinal changes in corneal nerve morphology in patients with MS. We show a reduction in corneal nerve fiber area, nerve fiber width, and peripapillary RNFL thickness and significant associations between the reduction in CNFD and CNFL and increasing disease severity (EDSS and MSSS) during 2 years of follow-up in patients with RRMS. The increase in DC density was associated with a reduction in CNFD and RNFL and an increase in EDSS. Furthermore, the reduction in CNFD correlated with a reduction in RNFL thickness. In the subset of patients with worsening EDSS, there were significant reductions in CNFL, CTBD, CNFA, CNFW, and RNFL thickness and an increase in DC density during follow-up.
Several studies have demonstrated a reduction in RNFL in early MS and patients without a history of ON.31–34 We have previously reported a lower RNFL thickness in patients with RRMS with and without a history of ON that was related to EDSS and MSSS.28 Furthermore, a progressive thinning of RNFL has been shown in longitudinal studies of patients with MS,35–37 and a recent longitudinal study of 57 patients with MS reported progressive RNFL thinning over 5 years, regardless of a history of ON.35 Similarly, Graham et al.36 reported a reduction of the temporal RNFL in 45 patients with RRMS without ON over 3 years. In the current study, we confirm a reduction in RNFL thickness over 2 years only in patients with a history of ON. However, episodes of ON can cause a temporary increase in RNFL thickness, which may confound RNFL measurement in longitudinal studies.11
Mikolajczak et al.18 first reported a significant reduction in corneal nerve density and showed that it was related to the severity of MS but not RNFL thickness. We confirmed and extended this finding by demonstrating significantly lower corneal nerve fiber density, corneal nerve branch density, and length.19,28 In this longitudinal study, we now demonstrate the importance of quantifying additional measures of the subbasal nerve plexus and also show that over time, corneal nerve morphology shows dynamic change with concomitant degeneration and regeneration. Although there was no change in the more proximal CNFD, there was a significant reduction in the CNFA and CNFW and a trend for reduction in the more distal CNFL, CNBD, and CTBD. Corneal nerve fiber area is a two-dimensional metric that more fully captures the full spectrum of variation and change in the corneal nerve plexus and may provide a more complete measure of axonal loss and atrophy. Indeed, we have previously shown that CNFA has the greatest utility for assessing longitudinal change, especially in response to therapeutic intervention in patients with diabetic and sarcoid neuropathy.38 Moreover, the reduction in CNFD and CNFL, but not RNFL, correlated with worsening neurologic disability in patients with RRMS. This suggests that CCM may have advantage over OCT to assess the impact of axonal degeneration in relation to clinical disability in patients with MS.
In the current study, although we showed no significant change in DC density over time, an increase in DC density was associated with corneal nerve loss (CNFD reduction) and increasing neurologic disability (worsening EDSS). DCs migrate to the central cornea in immune-mediated and inflammatory conditions,14,15,25,26 and we have previously demonstrated an increase in DC density and decrease in the corneal subbasal nerve plexus in patients with MS,28 chronic inflammatory demyelinating polyneuropathy,25 Fabry disease,24 and Behçet disease.27 A study has also reported that DCs may directly mediate corneal nerve fiber damage in a murine model of diabetic neuropathy.39
Different treatments may alter the trajectory of immune-mediated neurodegeneration and disability in MS.40 Although we found no difference in the change in EDSS, MSSS, corneal nerves, and peripapillary RNFL thickness, there was a greater increase in DC density in patients receiving fingolimod compared with interferon-β.
A limitation of this study is the small sample size and relatively short duration of follow-up. Nevertheless, our study demonstrates a progressive loss of corneal nerves and reduction in peripapillary RNFL thickness over 2 years in patients with RRMS. Furthermore, we show that the reduction in corneal nerves as opposed to RNFL is associated with worsening neurologic disability in RRMS. Further longitudinal studies with a larger sample size and longer follow-up period are required to define the role of CCM as an imaging biomarker of neuronal degeneration and immune activation in patients with MS.
Supplementary Material
Acknowledgments
Disclosure: G. Bitirgen, None; Z. Akpinar, None; A.U. Uca, None; A. Ozkagnici, None; I.N. Petropoulos, None; R.A. Malik, None
References
- 1. Khan A, Kamran S, Ponirakis G, et al.. Peripheral neuropathy in patients with multiple sclerosis. PLoS One. 2018; 13: e0193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warabi Y, Yamazaki M, Shimizu T, Nagao M. Abnormal nerve conduction study findings indicating the existence of peripheral neuropathy in multiple sclerosis and neuromyelitis optica. Biomed Res Int. 2013; 2013: 847670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeLuca GC, Ebers GC, Esiri MM. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain. 2004; 127: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 4. Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 5. Fox NC, Jenkins R, Leary SM, et al.. Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI. Neurology. 2000; 54: 807–812. [DOI] [PubMed] [Google Scholar]
- 6. Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008; 4: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jankowska-Lech I, Wasyluk J, Palasik W, Terelak-Borys B, Grabska-Liberek I. Peripapillary retinal nerve fiber layer thickness measured by optical coherence tomography in different clinical subtypes of multiple sclerosis. Mult Scler Relat Disord. 2019; 27: 260–268. [DOI] [PubMed] [Google Scholar]
- 8. Bross M, Hackett M, Bernitsas E. Approved and emerging disease modifying therapies on neurodegeneration in multiple sclerosis. Int J Mol Sci. 2020; 21: E4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molyneux PD, Barker GJ, Barkhof F, et al.. Clinical-MRI correlations in a European trial of interferon beta-1b in secondary progressive MS. Neurology. 2001; 57: 2191–2197. [DOI] [PubMed] [Google Scholar]
- 10. Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002; 15: 239–245. [DOI] [PubMed] [Google Scholar]
- 11. Zimmermann H, Freing A, Kaufhold F, et al.. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler. 2013; 19: 443–450. [DOI] [PubMed] [Google Scholar]
- 12. Syc SB, Saidha S, Newsome SD, et al.. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012; 135: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tavakoli M, Quattrini C, Abbott C, et al.. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010; 33: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tavakoli M, Boulton AJ, Efron N, Malik RA. Increased Langerhan cell density and corneal nerve damage in diabetic patients: Role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2011; 34: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayer WJ, Mackert MJ, Kranebitter N, et al.. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res. 2012; 37: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 16. Ferrari G, Grisan E, Scarpa F, et al.. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front Aging Neurosci. 2014; 6: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kass-Iliyya L, Javed S, Gosal D, et al.. Small fiber neuropathy in Parkinson's disease: a clinical, pathological and corneal confocal microscopy study. Parkinsonism Relat Disord. 2015; 21: 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikolajczak J, Zimmermann H, Kheirkhah A, et al.. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult Scler. 2017:23: 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petropoulos IN, Kamran S, Li Y, et al.. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest Ophthalmol Vis Sci. 2017; 58: 3677–3681. [DOI] [PubMed] [Google Scholar]
- 20. Kamran S, Khan A, Salam A, et al.. Cornea: a window to white matter changes in stroke; corneal confocal microscopy a surrogate marker for the presence and severity of white matter hyperintensities in ischemic stroke. J Stroke Cerebrovasc Dis. 2020; 29: 104543. [DOI] [PubMed] [Google Scholar]
- 21. Malik RA, Kallinikos P, Abbott CA, et al.. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003; 46: 683–688. [DOI] [PubMed] [Google Scholar]
- 22. Tavakoli M, Marshall A, Pitceathly R, et al.. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010; 223: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. 2014; 31: 431–438. [DOI] [PubMed] [Google Scholar]
- 24. Bitirgen G, Turkmen K, Malik RA, Ozkagnici A, Zengin N. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci Rep. 2018; 8: 12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stettner M, Hinrichs L, Guthoff R, et al.. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. 2015; 3: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajabally YA, Stettner M, Kieseier BC, Hartung HP, Malik RA. CIDP and other inflammatory neuropathies in diabetes—diagnosis and management. Nat Rev Neurol. 2017; 13: 599–611. [DOI] [PubMed] [Google Scholar]
- 27. Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A. In vivo confocal microscopic evaluation of corneal nerve fibers and dendritic cells in patients with Behçet's disease. Front Neurol. 2018; 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 2017; 135: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polman CH, Reingold SC, Banwell B, et al.. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011; 15: 738–747. [DOI] [PubMed] [Google Scholar]
- 31. Maghzi AH, Graves J, Revirajan N, et al.. Retinal axonal loss in very early stages of multiple sclerosis. Eur J Neurol. 2015; 22: 1138–1141. [DOI] [PubMed] [Google Scholar]
- 32. Knier B, Berthele A, Buck D, et al.. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler. 2016; 22: 893–900. [DOI] [PubMed] [Google Scholar]
- 33. Behbehani R, Al-Hassan AA, Al-Khars A, Sriraman D, Alroughani R. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015; 359: 305–308. [DOI] [PubMed] [Google Scholar]
- 34. Albrecht P, Fröhlich R, Hartung HP, Kieseier BC, Methner A. Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol. 2007; 254: 1595–1596. [DOI] [PubMed] [Google Scholar]
- 35. Abalo-Lojo JM, Treus A, Arias M, Gómez-Ulla F, Gonzalez F. Longitudinal study of retinal nerve fiber layer thickness changes in a multiple sclerosis patients cohort: a long term 5 year follow-up. Mult Scler Relat Disord. 2018; 19: 124–128. [DOI] [PubMed] [Google Scholar]
- 36. Graham EC, You Y, Yiannikas C, et al.. Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci. 2016; 57: 2311–2317. [DOI] [PubMed] [Google Scholar]
- 37. Frau J, Fenu G, Signori A, et al.. A cross-sectional and longitudinal study evaluating brain volumes, RNFL, and cognitive functions in MS patients and healthy controls. BMC Neurol. 2018; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brines M, Culver DA, Ferdousi M, et al.. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci Rep. 2018; 8: 4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leppin K, Behrendt AK, Reichard M, et al.. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci. 2014; 55: 3603–3615. [DOI] [PubMed] [Google Scholar]
- 40. von Wyl V, Benkert P, Moser A, et al.. Disability progression in relapse-free multiple sclerosis patients on fingolimod versus interferon-beta/glatiramer acetate [published online May 28, 2020]. Mult Scler. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.