Abstract
Purpose: Topical corticosteroids used to treat ocular inflammation are associated with a high risk of clinically significant toxicities. Therefore, corticosteroid-sparing medications to treat ocular inflammation are needed. Noninfectious anterior uveitis (NAU) is a sight-threatening ocular inflammatory condition typically treated with topical corticosteroids. This corticosteroid-controlled comparator trial examines the safety and efficacy of reproxalap, a novel inhibitor of reactive aldehyde species (RASP), for the treatment of ocular inflammation, by using NAU as a model.
Methods: Forty-five patients with mild-to-moderate acute NAU were randomly assigned 1:1:1 to receive reproxalap 0.5% ophthalmic solution (4 times daily for 6 weeks), prednisolone 1% ophthalmic solution (Pred Forte®, 4 times daily taper for 6 weeks), or a combination of reproxalap 0.5% ophthalmic solution (4 times daily for 6 weeks) and prednisolone 1% ophthalmic solution (twice daily taper for 6 weeks).
Results: All treatments improved anterior cell count and grade, and no differences were observed in change from baseline between groups. Reproxalap monotherapy and combination therapy were statistically noninferior to prednisolone. The proportion of patients requiring rescue therapy was comparable across treatment groups. No safety issues were identified for reproxalap-treated patients, whereas treatment with prednisolone resulted in an average increase of intraocular pressure of ∼2 mm Hg.
Conclusions: Reproxalap may be a safe and effective alternative to topical corticosteroids for patients with NAU and other forms of ocular inflammation. These results represent initial clinical evidence of the importance of RASP in ocular inflammation and the applicability of RASP inhibition to immune modulation in ocular disease. Clinical trial (NCT02406209).
Keywords: anti-inflammatory, clinical studies, eye drops, inflammation, reactive aldehyde species, reproxalap, uveitis
Introduction
Topical ocular corticosteroids, a mainstay of treatment for inflammatory diseases of the anterior segment of the eye, are associated with increased risk of cataract formation, increased intraocular pressure (IOP) associated with glaucoma, and ocular surface infections.1,2 Consequently, there is a need for new immune-modulating medications with alternative mechanisms of action that can treat ocular inflammation without the adverse side effects of corticosteroids. Noninfectious anterior uveitis (NAU) is a potentially severe inflammatory condition characterized by the sudden onset of eye pain, blurry vision, hyperemia, photophobia, and vision loss, often resulting in a dramatic negative impact on quality of life.
NAU is caused by a wide variety of underlying pathologies. Experimental models of uveitis demonstrate an association between uveitis and elevated reactive aldehyde species (RASP), such as malondialdehyde and 4-hydroxynonenal.3–9 By covalently binding amino and thiol groups on receptors and kinases, RASP potentiate upstream proinflammatory signaling cascades that involve NF-kB, inflammasomes, scavenger receptor A, and other mediators.16–19 Increased levels of RASP are implicated across diverse ophthalmic inflammatory conditions, including anterior uveitis,10 Behçet disease,11 cataracts,12 pterygium,13 glaucoma,14 and proliferative vitreoretinopathies.15 Findings from these studies suggest that RASP represent a potential therapeutic target for ocular inflammatory diseases.
Reproxalap is a novel small-molecule RASP inhibitor in development to treat NAU, allergic conjunctivitis, and dry eye disease. Results from nonclinical studies indicate that reproxalap and related molecules have the potential to mitigate ocular inflammation related to NAU and other inflammatory eye conditions, such as postsurgical inflammation and diabetic retinopathy.20–22 Results from subsequent clinical trials in patients with dry eye syndrome and allergic conjunctivitis suggest that topical ocular reproxalap may be safe and effective for use in humans.23,24 Herein, we report results from the first randomized, comparator-controlled clinical trial designed to evaluate the potential of reproxalap as an adjunct to or replacement for topical ocular corticosteroid therapy in patients with NAU.
Methods
Study design and treatment
We conducted a randomized, investigator-masked, comparator-controlled, parallel-group, multicenter phase 2 clinical trial (NCT02406209). The trial was conducted in compliance with the Good Clinical Practice Guideline as defined by the International Conference on Harmonization, the Declaration of Helsinki, and all applicable federal and local regulations. The central or local Institutional Review Board for the participating trial sites reviewed and approved the trial protocol and patient information and informed consent forms. All patients provided written informed consent before enrollment.
Eligible participants were randomly assigned 1:1:1 to receive one of the following treatment regimens: reproxalap ophthalmic drops 0.5% 4 times daily for 6 weeks (reproxalap group); prednisolone acetate ophthalmic suspension 1% (Pred Forte®, Allergan, Inc., Dublin, Ireland) starting at 4 times daily, then tapered through week 6 (prednisolone group); or reproxalap ophthalmic drops 0.5% 4 times daily for 6 weeks and prednisolone acetate 1% starting at twice daily, then tapered through week 4 (combination group).
Patients
This trial was exploratory and was not formally powered; however, 15 subjects were deemed by the investigators to represent a sufficient subject number for a pilot trial in NAU. Eligible patients were men or women aged 18–85 years with NAU; an anterior chamber cell grade between 1 and 3 (based on the scale of grade 0 [ ≤ 1 cell], grade 1 [2–10 cells], grade 2 [11–20 cells], and grade 3 [21–50 cells])25 in at least 1 eye; baseline IOP <25 mm Hg; and visual acuity better than or equal to 20/200. Patients experiencing a bilateral episode of NAU were eligible for study in 1 eye, and the nonstudy eye was assigned to receive standard-of-care treatment. Patients were excluded from the trial if more than 1 topical medication to control IOP was required. Other exclusion criteria included any ocular or medical condition or laboratory finding that, in the judgment of the investigator, made the patient unsuitable for the trial; history of malignancy within the past 5 years (except successfully treated squamous or basal cell carcinoma of the skin or in situ cervical cancer); active intermediate or posterior uveitis; history of fibrinoid reaction; previous anterior uveitis episode in the study eye ≤4 weeks before screening; use of an oral corticosteroid ≤14 days before screening; intravitreal or sub-Tenon ocular corticosteroid treatment in the study eye ≤6 months before screening; and current use of nonsteroidal anti-inflammatory agents or immunosuppressive agents, unless the dose had been stable for the past 6 weeks and no change in dosing was anticipated for the duration of the trial.
Rescue therapy was available to patients who did not demonstrate an improvement in anterior chamber cell grade by week 1 or who demonstrated ≥1 unit increase in anterior chamber cell grade any time after week 1. Choice of rescue medication was at the discretion of the treating physician. Patients who used rescue medication were required to complete all planned visits through the end of the trial.
Assessments
Efficacy and safety assessments were performed on days 1 and 4 and at weeks 1, 2, 4, and 8; a follow-up phone call safety assessment was performed at week 9. At each visit, efficacy was assessed by standard ophthalmic examination procedures, and response to treatment was graded according to standardized scales previously described 25 for anterior chamber cell count, anterior chamber flare, limbal injection, hypopyon, peripheral anterior synechiae, keratic precipitates, and posterior synechiae. In addition, ocular pain, lacrimation, photophobia, and blurry vision were measured by using a visual analog scale (VAS, scale 0 mm = no symptoms to 100 mm = worst possible symptoms). A combined VAS was calculated from the sum of the individual scores (scale 0–400 mm). Time to treatment success was defined as the number of days required to reach and sustain anterior chamber cell clearing (grade 0)25 without the use of rescue medication. Visual acuity was assessed with spectacle and/or pinhole correction by using a standard eye chart (Snellen or Early Treatment Diabetic Retinopathy Study [ETDRS] based on investigator discretion). Safety was evaluated by IOP measurements, funduscopic examination, optical coherence tomography, corneal pachymetry, and adverse event (AE) assessments. The AEs were recorded from the time that written informed consent was received through week 9.
Statistical analysis
Efficacy analyses were performed on the intention-to-treat (ITT) population, defined as all randomized patients who satisfied inclusion and exclusion criteria. Safety analyses were performed on the safety population, defined as all randomized patients who received at least 1 dose of treatment, regardless of the number of assessments completed. Descriptive statistics were used to summarize demographic data, baseline characteristics, patient disposition and the primary reasons for discontinuation, the proportion of patients receiving rescue medication, and safety variables. Descriptive statistics were also tabulated for observed scores by visit for the following end points: anterior chamber cell grade, anterior chamber flare grade, limbal injection, hypopyon, peripheral anterior synechiae, keratic precipitates, posterior synechiae, ocular pain, lacrimation, photophobia, and blurry vision. A Kaplan–Meier analysis was used to assess time to cure without rescue therapy; patients who discontinued for reasons other than use of rescue therapy were censored, and patients who used rescue medications were right-censored. Pairwise comparisons to the prednisolone group were made by using the log-rank test, and the proportion of treatment successes was reported. Pairwise comparisons to the prednisolone group were also made by using Fisher's exact test. A noninferiority test at week 8 was performed for anterior chamber cell grade by using a mixed-effect model for repeated measures and a noninferiority bound of 0.5 (10% of the 5-point anterior chamber cell grade scale), consistent with previous reports.26 Last-observation-carried-forward (LOCF) imputations were performed for missing data, and visual acuity scores were converted to LogMAR equivalent scores. Post hoc inference testing was performed for the least squares (LS) mean difference between treatment groups for anterior chamber cell grade change from baseline.
Results
Patients
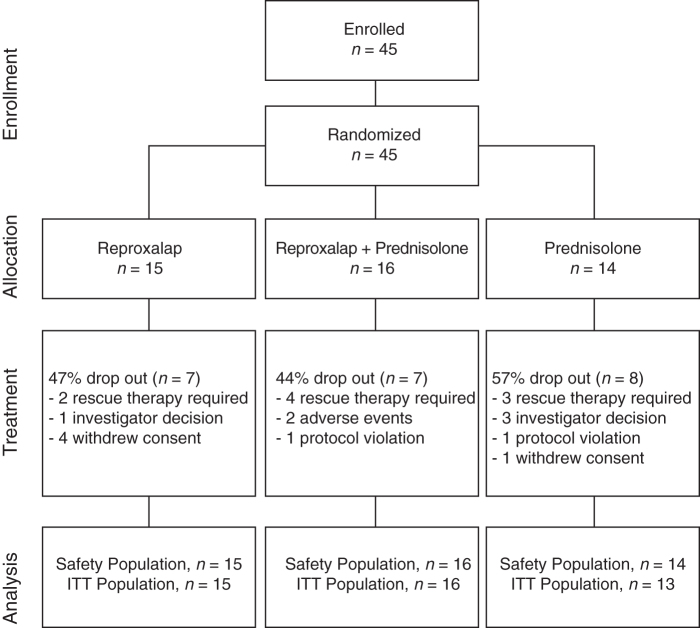
A total of 45 patients provided informed consent, received at least one dose of study drug, and were included in the safety population (Fig. 1). Of these patients, 1 was disqualified retroactively owing to an undisclosed history of malignancy, resulting in an ITT population of 44 patients. The total proportion of discontinuations was highest in the prednisolone group compared with the reproxalap and the combination groups. Overall, the most common reason for discontinuation was use of rescue therapy. The overall mean (standard deviation [SD]) age of patients in the ITT population was 45.5 (15.6) years, and 70% were female (Table 1). The baseline mean (SD) anterior chamber cell grade was lower in the reproxalap group compared with the anterior chamber cell grade in the other 2 treatment groups. In contrast, the baseline mean (SD) anterior chamber flare grade and VAS symptom score were lower in the combination group compared with the reproxalap group and the prednisolone group. No differences were observed in baseline visual acuity or IOP between treatment groups.
FIG. 1.
Patient disposition (CONSORT) for analysis populations. ITT, intention-to-treat.
Table 1.
Demographics and Baseline Characteristics (Intention-to-Treat Population)
| Treatment group | Reproxalapa (n = 15) | Combination Reproxalapa + Prednisoloneb (n = 16) | Prednisoloneb (n = 13) |
|---|---|---|---|
| Age, mean (SD), years | 46.8 (13.8) | 48.0 (19.5) | 41.1 (11.8) |
| Sex, n (%) | |||
| Female | 10 (67) | 14 (88) | 7 (54) |
| Race, n (%) | |||
| Asian | 1 (7) | 0 | 0 |
| Black or African American | 5 (33) | 4 (25) | 2 (15) |
| White | 8 (53) | 12 (75) | 11 (85) |
| Other | 1 (7) | 0 | 0 |
| Ethnic origin, n (%) | |||
| Hispanic or Latino | 3 (20) | 0 | 0 |
| Not Hispanic or Latino | 12 (80) | 16 (100) | 13 (100) |
| AC cell grade | 1.5 (0.5) | 1.9 (0.8) | 1.8 (0.9) |
| AC flare grade | 1.0 (0.9) | 0.6 (0.6) | 1.1 (0.9) |
| Limbal injection, n (%) | |||
| Grade 0 | 8 (53) | 8 (50) | 3 (23) |
| Grade 1 | 4 (27) | 6 (38) | 5 (38) |
| Grade 2 | 3 (20) | 2 (13) | 4 (31) |
| Grade 3 | 0 | 0 | 1 (8) |
| Posterior synechiae, n (%) | |||
| Grade 0 | 12 (80) | 9 (56) | 10 (77) |
| Grade 1 | 1 (7) | 2 (13) | 1 (8) |
| Grade 2 | 2 (13) | 4 (25) | 2 (15) |
| Grade 3 | 0 | 1 (6) | 0 |
| Keratic precipitates, n (%) | |||
| Grade 0 | 8 (53) | 10 (63) | 8 (26) |
| Grade 1 | 5 (33) | 4 (25) | 3 (23) |
| Grade 2 | 2 (13) | 2 (13) | 1 (8) |
| Grade 3 | 0 | 0 | 1 (8) |
| Peripheral anterior synechiae, n (%) | |||
| Grade 0 | 15 (100) | 15 (94) | 13 (100) |
| Grade 1 | 0 | 0 | 0 |
| Grade 2 | 0 | 0 | 0 |
| Grade 3 | 0 | 0 | 0 |
| Hypopyon, n (%) | |||
| Grade 0 | 15 (100) | 16 (100) | 12 (92) |
| Grade 1 | 0 | 0 | 1 (8) |
| VAS total symptom score, mm | 120 (52.5) | 82 (48.7) | 120 (54.3) |
| Ocular pain, median (range) | 15 (0–75) | 9 (0–74) | 29 (0–95) |
| Lacrimation, median (range) | 12 (0–75) | 2 (0–40) | 3 (0–71) |
| Photophobia, median (range) | 41 (0–81) | 21 (0–80) | 44 (0–97) |
| Blurry vision, median (range) | 19 (0–79) | 2 (0–76) | 31 (0 – 69) |
| Visual acuity LogMAR score | 0.2 (0.2) | 0.2 (0.2) | 0.1 (0.2) |
| IOP, mm Hg | 15 (4.4) | 14 (2.7) | 13 (4.9) |
Data are presented as mean (SD) unless otherwise noted.
Reproxalap ophthalmic drops 0.5%.
Prednisolone 1% ophthalmic solution.
AC, anterior chamber; IOP, intraocular pressure; ITT, intention-to-treat; SD, standard deviation; VAS, visual analog scale.
Efficacy analysis
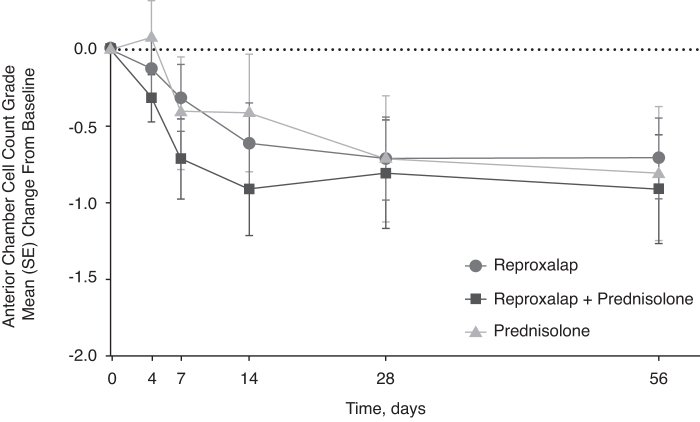
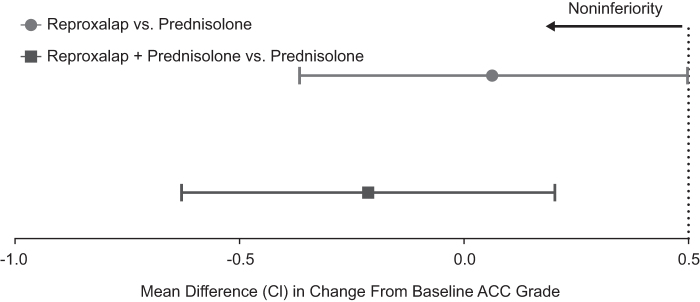
All treatment groups demonstrated a reduction in anterior chamber cell count at week 1 that continued to decrease to week 4 and was maintained through week 8 (Fig. 2), and there were no significant differences between groups. At week 8, reproxalap treatment and combination treatment were noninferior to prednisolone treatment (Fig. 3).
FIG. 2.
Change from baseline anterior chamber cell (ITT population) was similar across groups. The last observation was carried forward for any patients who did not complete the trial. Error bars represent standard error of the mean (SE).
FIG. 3.
Reproxalap monotherapy and reproxalap + prednisolone therapy were statistically noninferior to corticosteroid monotherapy (ITT population). A mixed-effect model for repeated measures was performed at week 8 to assess anterior chamber cell grade reduction noninferiority at an upper bound of 0.5, as previously reported.26 Error bars represent 95% confidence interval (CI). ACC, anterior chamber cell.
The proportion of patients with NAU who experienced treatment success was similar across groups (Table 2). Mean time to treatment success was lowest in the reproxalap group (18 days) compared with the combination group (36 days) and the prednisone group (21 days). Kaplan–Meier estimates for cure rates at the end of the trial were similar: 43%, 50%, and 46% for the reproxalap, combination, and prednisolone groups, respectively. No differences were observed in log-rank assessments across groups.
Table 2.
Efficacy Assessments (Intention-to-Treat Population)
| Reproxalapa (n = 15) | Combination Reproxalapa + Prednisoloneb(n = 16) | Prednisoloneb (n = 13) | |
|---|---|---|---|
| Treatment success measurements | |||
| Treatment success, n (%) | 6 (40) | 7 (44) | 6 (46) |
| Time to treatment success, mean (SD) days | 18 (13) | 36 (23) | 21 (22) |
| Kaplan–Meier estimated cure rate at 8 weeks, % | 43 | 50 | 46 |
| Log-rank P value vs. prednisolone | NS | NS | – |
| Slit lamp examinations at week 8 | |||
| AC flare (grade 0), n (%) | 9 (60) | 11 (69) | 6 (46) |
| Limbal injection (grade 0), n (%) | 8 (53) | 13 (81) | 8 (62) |
| Posterior synechiae (grade 0), n (%) | 13 (87) | 9 (56) | 10 (77) |
| Keratic precipitation (grade 0), n (%) | 10 (67) | 12 (75) | 8 (62) |
| Peripheral anterior synechiae (grade 0), n (%) | 15 (100) | 16 (100) | 13 (100)c |
| Hypopyon (grade 0), n (%) | 15 (100) | 16 (100) | 12 (92)d |
| Change in VAS score (mm) from baseline to week 8 | |||
| Ocular pain, median (range) | 0 (−44 to 55) | –1 (−71 to 14) | 0 (−95 to 50) |
| Lacrimation, median (range) | 0 (−72 to 100) | 0 (−39 to 21) | 0 (−8 to 29) |
| Photophobia, median (range) | –8 (−50 to 70) | –9 (−80 to 81) | –19 (−88 to 57) |
| Blurry vision, median (range) | –5 (−66 to 45) | 0 (−37 to 52) | –1 (−61 to 58) |
Reproxalap ophthalmic drops 0.5%.
Prednisolone 1% ophthalmic solution.
One patient had grade 1 peripheral anterior synechiae at baseline that by week 4 had improved to grade 0 with improvement persisting through week 8.
One patient presented with grade 1 hypopyon at baseline but improved to grade 0 by week 8.
NS, not significant.
At baseline, most patients had grade 0 (34%) or grade 1 (45%) flare; the proportion of patients with grade 0 flare increased after initiation of study treatment in all groups. At week 8, more patients in the reproxalap group and combination group had grade 0 flare than patients in the prednisolone group (Table 2). No significant differences were seen in flare grade between treatment groups at any time point. By week 8, the combination group had the highest proportion of patients at grade 0 limbal injection compared with the reproxalap and prednisolone groups. In contrast, more patients in the reproxalap group had grade 0 posterior synechiae at week 8 compared with the other groups, which remained unchanged from baseline. At week 8, the proportion of patients with grade 0 keratic precipitation, grade 0 hypopyon, or grade 0 peripheral anterior synechiae were similar between treatment groups. Similarly, at week 8, there were no marked differences in ocular pain, lacrimation, photophobia, or blurry vision across all treatment groups.
Safety analysis
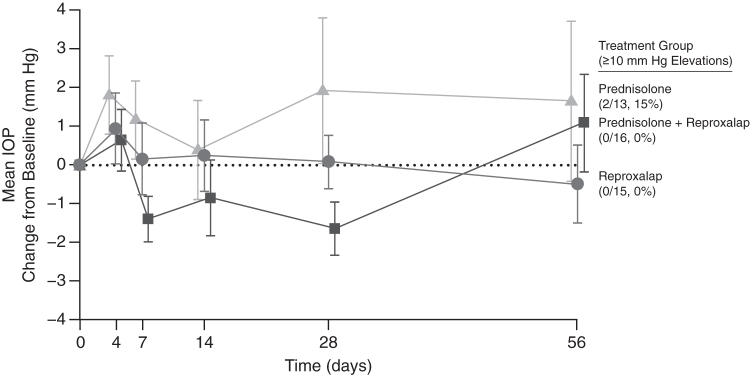
The most common treatment-related AE deemed possibly or definitely related to reproxalap treatment was eye irritation (Table 3). All AEs were considered mild to moderate in severity and resolved spontaneously. No serious AEs were observed. No patients in the prednisolone group reported any treatment-related AEs. Importantly, however, mean IOP elevation was greater in prednisolone-treated patients than in reproxalap-treated patients (Fig. 4). Two prednisolone-treated patients experienced elevations in IOP of more than 10 mm Hg, whereas IOP elevations in reproxalap-treated patients were less than 10 mm Hg. No significant changes in LogMAR visual acuity or corneal pachymetry were observed.
Table 3.
Treatment-Emergent Adverse Events Possibly or Definitely Related to Study Treatment (Safety Population)
| Reproxalapa (n = 15) | Reproxalapa + Prednisoloneb(n = 16) | Prednisoloneb (n = 14) | |
|---|---|---|---|
| Any AE | 4 (27) | 4 (25) | 0 |
| Preferred term | |||
| Eye irritation | 3 (20) | 2 (13) | 0 |
| Eye pain | 1 (7) | 1 (6) | 0 |
| Headache | 1 (7) | 1 (6) | 0 |
| Eye pruritus | 1 (7) | 0 | 0 |
| Macular edema | 0 | 1 (6) | 0 |
| Ocular hyperemia | 0 | 1 (6) | 0 |
Data are presented as n (%).
Reproxalap ophthalmic drops 0.5%.
Prednisolone 1% ophthalmic solution.
AE, adverse event.
FIG. 4.
Change from baseline in intraocular pressure (mm Hg, safety population) was numerically higher in the prednisolone group. Intraocular pressure elevations of ≥10 mm Hg were observed in 2 patients in the prednisolone group, whereas no elevations of ≥10 mm Hg were observed in the reproxalap or reproxalap + prednisolone groups. Error bars represent the standard error of the mean (SE). IOP, intraocular pressure.
Discussion
We provide the first clinical evidence of the efficacy of reproxalap, a topical ophthalmic RASP inhibitor, for the treatment of NAU. Consistent with long-standing reports of corticosteroid-induced ocular hypertension that occurs even after short courses of administration,27–30 2 of 29 (7%) corticosteroid-treated patients in the present trial showed >10 mm Hg increases in IOP. In contrast to the corticosteroid groups, average IOP did not increase in the reproxalap group. Thus, reproxalap may represent a novel class of immunomodulatory agents with potential to treat uveitis and other ocular inflammatory diseases without the known toxicities of topical corticosteroids. The activity of reproxalap was statistically noninferior to that of corticosteroid therapy in patients with mild-to-moderate NAU, and thus reproxalap may represent an alternative to corticosteroid treatment for uveitis and potentially other forms of ocular inflammation. The findings merit further study in larger clinical trials.
The toxicity of RASP in biological systems has been recognized for decades,31 and RASP have been widely associated with inflammatory disease, particularly that of the eye.10–15 Although other compounds have been described as binding specific RASP in vitro, in some cases through energetically unfavorable and complex multistep reactions,32 to our knowledge, no reports have previously described the pharmacologic potential of an irreversible and universal RASP inhibitor.
Although we are unaware of recent guidelines for the treatment of NAU, the Optometric Clinical Practice Guideline on Anterior Uveitis from 1994 advises the administration of topical prednisolone 1% 4 times daily or more frequently, depending on disease severity.33 On average, in the present trial, where prednisolone was administered 4 times daily and patients with baseline anterior chamber cell grade of 4+ were excluded, the reduction in anterior chamber cell grade and the proportion of patients with anterior chamber cell grade 0 were ∼50% lower than in other trials. In other trials, prednisolone was administered 8–16 times per day and then tapered, patients with baseline anterior chamber cell grades of 4+ were included, and the definition of grade 0 ranged from <4 or 5 cells (versus 0 or 1 cells in the present trial).26,34 Thus, treatment of NAU in patients with anterior chamber cell grades 1, 2, or 3 may benefit from prednisolone administration that is more frequent than 4 times daily. However, prednisolone is associated with a 9.5%–11% increase in the proportion of patients with an IOP of ≥10 mm Hg,26,30 and frequent administration of prednisolone may also lead to a rise in the prevalence of ocular hypertension.
The pathophysiologic complexity of immune-mediated disease often requires multiple therapeutic agents to treat inflammatory conditions. The novel mechanism of reproxalap may be useful in settings where combination therapy can be applied. In the present trial, the benefit of adding twice-daily prednisolone to reproxalap therapy was not clearly demonstrated; however, the trial was not statistically powered to measure the additive effects of prednisolone and reproxalap relative to either drug alone. Further, a floor effect as a result of the baseline anterior chamber cell grade of 1.7 in the present trial may have been approached in the reproxalap monotherapy group such that additional benefit from twice-daily prednisolone may have been difficult to detect. Nonetheless, given that NAU and other forms of uveitis are critical conditions that can lead to blindness and given the toxicity of topical ocular corticosteroid monotherapy, study of active agents in combination with standard or lower doses of corticosteroid is warranted.
Certain limitations of the study design should be considered when interpreting the results of this first randomized, comparator-controlled clinical trial of an RASP inhibitor for NAU. Efficacy analyses in this study were performed on the ITT population, of which ∼50% of patients discontinued study treatment, due to either per-protocol rescue or de facto rescue whereby patients were returned to the standard of care at the discretion of the investigator or request of the patient. In such cases, the LOCF imputation method was used to account for missing data. It is noted that the LOCF method has the potential to both introduce bias when estimating the treatment effect and underestimate the variability of the estimated results. Although the discontinuation rates were similar across treatment groups and the treatment effect was observed to be comparable between groups, an underestimate of the variability due to LOCF imputation could affect the statistical power of post hoc noninferiority analyses. For other types of analyses, such as the Kaplan-Meier method used to analyze treatment success in this study, noncompletion was considered equivalent to treatment failure; thus, no bias was introduced into the treatment success analysis based on the ITT/LOCF methodology.
An association between NAU and systemic autoimmune and autoinflammatory conditions is well established, and patients with uveitis-associated systemic diseases were included in this study. Nonetheless, due to the small sample size of this study and multitude of systemic diseases associated with NAU, it was not possible to perform statistical analysis of this covariate. Future randomized, controlled trials may be conducted in larger populations of NAU patients to explore interactions between specific systemic diseases and the response to reproxalap treatment.
Despite having ∼15 patients per treatment arm and lacking prospective statistical powering to detect differences between treatment groups, reproxalap demonstrated noninferiority to a standard-of-care topical corticosteroid. This trial supports the applicability of RASP inhibition as a novel therapeutic approach in the treatment of corticosteroid-responsive ocular inflammation, and it also supports the therapeutic potential of reproxalap to treat NAU and other forms of ocular inflammation as an alternative to traditional corticosteroid therapy or as a corticosteroid-sparing agent.
Acknowledgments
This trial was supported by Aldeyra Therapeutics, Inc., Lexington, MA. The authors would like to thank Aldeyra Therapeutics, Inc., for sponsoring this article and the medical writing support that was provided by Caroline Walsh Cazares, PhD, of JB Ashtin, who provided editorial support and assisted in implementing author revisions.
Author Disclosure Statement
K.J.M. has served as a consultant to Aldeyra Therapeutics, and is a co-inventor on a patent assigned to Aldeyra Therapeutics.
D.C. is an employee of and an investor in Aldeyra Therapeutics.
D.S.C. has received grants from Mallinckrodt; is a consultant for Aldeyra, Dompé, and Mallinckrodt; has received honoraria from AbbVie and Mallinckrodt; and has received travel reimbursement from AbbVie, Dompé, and Santen.
C.S.F. has received financial support from Aciont, Alcon, Aldeyra, Bausch & Lomb, Clearside, Biomedical, Dompé,, Eyegate Pharma, Mallinckrodt, Novartis, pSivida, and Santen; is an investor in Eyegate Pharma; is a consultant for Aldeyra, Allakos, Bausch & Lomb, Eyegate Pharma, Genentech, Novartis, and pSivida; and has received fees for lectures and services for speaker bureaus for Alcon, Allergan, and Mallinckrodt.
J.S. has received financial support for consultancy and research from Aldeyra, AbbVie, and Bausch & Lomb.
T.C.B. is an employee of Aldeyra Therapeutics and an investor in Novadigm Therapeutics, Springbank Pharmaceuticals, Evoke Pharma, and Aldeyra Therapeutics, and is an inventor on patents for Aldeyra Therapeutics.
Funding Information
Financial support was provided by Aldeyra Therapeutics, Inc.
References
- 1. McGhee C.N., Dean S., and Danesh-Meyer H.. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 25:33–55, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Islam N., and Pavesio C.. Uveitis (acute anterior). BMJ Clin. Evid. 2010:0705, 2010 [PMC free article] [PubMed] [Google Scholar]
- 3. Wu G.S., Walker J., and Rao N.A.. Effect of deferoxamine on retinal lipid peroxidation in experimental uveitis. Invest. Ophthalmol. Vis. Sci. 34:3084–3089, 1993 [PubMed] [Google Scholar]
- 4. Bosch-Morell F., Roma J., Puertas F.J., Marin N., Diaz-Llopis M., and Romero F.J.. Efficacy of the antioxidant ebselen in experimental uveitis. Free Radic. Biol. Med. 27:388–391, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Augustin A.J., Loeffler K.U., Sekundo W., Grus F.H., and Lutz J.. Effects of systemically applied allopurinol and prednisolone on experimental autoimmune uveitis. Graefes Arch. Clin. Exp. Ophthalmol. 237:508–512, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Augustin A.J., Spitznas M., Sekundo W., et al. Effects of allopurinol and steroids on inflammation and oxidative tissue damage in experimental lens induced uveitis: a biochemical and morphological study. Br. J. Ophthalmol. 80:451–457, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao N., Lan F., He R.R., and Kurihara H.. Protective effects of bilberry (Vaccinium myrtillus L.) extract against endotoxin-induced uveitis in mice. J. Agric. Food Chem. 58:4731–4736, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Demir D., Yilmaz T., Ilhan N., Yekeler H., Aydemir O., and Kükner A.S.. Protective role of alpha-tocopherol on retinal injury in experimental uveitis in guinea pigs. Pathophysiology. 13:75–79, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Yilmaz A., Yildirim O., Tamer L., et al. Effects of caffeic acid phenethyl ester on endotoxin-induced uveitis in rats. Curr. Eye Res. 30:755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Turk A., Aykut M., Akyol N., et al. Serum anti-carbonic anhydrase antibodies and oxidant-antioxidant balance in patients with acute anterior uveitis. Ocul. Immunol. Inflamm. 22:127–132, 2014 [DOI] [PubMed] [Google Scholar]
- 11. Bozkurt M., Yuksel H., Em S., et al. Serum prolidase enzyme activity and oxidative status in patients with Behcet's disease. Redox Rep. 19:59–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katta A.V., Katkam R.V., and Geetha H.. Lipid peroxidation and the total antioxidant status in the pathogenesis of age related and diabetic cataracts: a study on the lens and blood. J. Clin. Diagn. Res. 7:978–981, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balci M., Sahin S., Mutlu F.M., Yagci R., Karanci P., and Yildiz M.. Investigation of oxidative stress in pterygium tissue. Mol. Vis. 17:443–447, 2011 [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar D.M., and Agarwal N.. Oxidative stress in glaucoma: a burden of evidence. J. Glaucoma. 16:334–343, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Verdejo C., Marco P., Renau-Piqueras J., and Pinazo-Duran M.D.. Lipid peroxidation in proliferative vitreoretinopathies. Eye (Lond). 13:183–188, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Higdon A., Diers A.R., Oh J.Y., Landar A., and Darley-Usmar V.M.. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 442:453–464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kauppinen A., Niskanen H., Suuronen T., Kinnunen K., Salminen A., and Kaarniranta K.. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells—implications for age-related macular degeneration (AMD). Immunol. Lett. 147:29–33, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Kalariya N.M., Ramana K.V., Srivastava S.K., and van Kuijk F.J.. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp. Eye Res. 86:70–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sapkota M., DeVasure J.M., Kharbanda K.K., and Wyatt T.A.. Malondialdehyde-acetaldehyde (MAA) adducted surfactant protein induced lung inflammation is mediated through scavenger receptor a (SR-A1). Respir. Res. 18:36, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halilovic A, Brady T., and MacDonald S.. ADX-103, a novel small molecule aldehyde sequestering agent, decreases retinal edema and inflammation in a rat model of diabetic macular edema. Invest. Ophthalmol. Vis. Sci. 59:198, 2018 [Google Scholar]
- 21. Mandell K.J., Young S.L., and Brady T.C.. The aldehyde trap NS2 reduces ocular inflammation in an endotoxin-induced model in rats. Invest. Ophthalmol. Vis. Sci. 56:3095, 2015. 25813998 [Google Scholar]
- 22. Gibson D.J. Mandell K., Young SL, and Brady TC. The aldehyde trap NS2 mitigates dense haze in a rabbit model of photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 56:3931, 2015 [Google Scholar]
- 23. Clark D, Ousler G.W., Hollander D., and Brady T.. A randomized, double-masked, parallel-group, phase 2a dry eye disease clinical trial to evaluate the safety and efficacy of topical ocular ADX-102, a novel aldehyde sequestering agent. Invest. Ophthalmol. Vis. Sci. 59:1967, 2018 [Google Scholar]
- 24. Gomes P.J., Brady T., Hollander D., and Clark D.. A randomized, multi-center, double-masked, vehicle-controlled, parallel-group phase 2b allergic conjunctivitis clinical trial of topical ocular ADX-102, a novel aldehyde sequestering agent. Invest. Ophthalmol. Vis. Sci. 59:5571, 2018 [Google Scholar]
- 25. Foster C.S., Davanzo R., Flynn T.E., McLeod K., Vogel R., and Crockett R.S.. Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J. Ocul. Pharmacol. Ther. 26:475–483, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Sheppard J.D., Toyos M.M., Kempen J.H., Kaur P., and Foster C.S.. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Invest. Ophthalmol. Vis. Sci. 55:2993–3002, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker B. Intraocular pressure response to topical corticosteroids. Invest. Ophthalmol. 4:198–205, 1965 [PubMed] [Google Scholar]
- 28. Urban R.C. Jr., and Dreyer E.B.. Corticosteroid-induced glaucoma. Int. Ophthalmol. Clin. 33:135–139, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Armaly M.F. Statistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of response. Invest. Ophthalmol. 4:187–197, 1965 [PubMed] [Google Scholar]
- 30. Sheppard J.D., Comstock T.L., and Cavet M.E.. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv. Ther. 33:532–552, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esterbauer H., Schaur R.J., and Zollner H.. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11:81–128, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Colzani M., De Maddis D., Casali G., Carini M., Vistoli G., and Aldini G.. Reactivity, selectivity, and reaction mechanisms of aminoguanidine, hydralazine, pyridoxamine, and carnosine as sequestering agents of reactive carbonyl species: a comparative study. ChemMedChem. 11:1778–1789, 2016 [DOI] [PubMed] [Google Scholar]
- 33. Alexander K.L., Dul M.W., Lalle P.A., Magnus D.E., and Onofrey B.. Optometric clinical practice guideline for the care of the patient with anterior uveitis. American Optometric Association; 1994. Revised March 1999. Available at: www.aoa.org/documents/optometrists/CPG-7.pdf [Google Scholar]
- 34. The Loteprednol Etabonate US Uveitis Study Group. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Am. J. Ophthalmol. 127:537–544, 1999 [DOI] [PubMed] [Google Scholar]