Abstract
This study investigated patterns of cortical organization in adolescents who had sustained a traumatic brain injury (TBI) during early childhood to determine ways in which early head injury may alter typical brain development. Increased gyrification in other patient populations is associated with polymicrogyria and aberrant development, but this has not been investigated in TBI. Seventeen adolescents (mean age = 14.1 ± 2.4) who sustained a TBI between 1–8 years of age, and 17 demographically-matched typically developing children (TDC) underwent a high-resolution, T1-weighted 3-Tesla magnetic resonance imaging (MRI) at 6–15 years post-injury. Cortical white matter volume and organization was measured using FreeSurfer's Local Gyrification Index (LGI). Despite a lack of significant difference in white matter volume, participants with TBI demonstrated significantly increased LGI in several cortical regions that are among those latest to mature in normal development, including left parietal association areas, bilateral dorsolateral and medial frontal areas, and the right posterior temporal gyrus, relative to the TDC group. Additionally, there was no evidence of increased surface area in the regions that demonstrated increased LGI. Higher Vineland-II Socialization scores were associated with decreased LGI in right frontal and temporal regions. The present results suggest an altered pattern of expected development in cortical gyrification in the TBI group, with changes in late-developing frontal and parietal association areas. Such changes in brain structure may underlie cognitive and behavioral deficits associated with pediatric TBI. Alternatively, increased gyrification following TBI may represent a compensatory mechanism that allows for typical development of cortical surface area, despite reduced brain volume.
Keywords: abnormal development, adaptive behavior, pediatric traumatic brain injury, structural neuroimaging
Introduction
Much is unknown about developmental mechanisms responsible for giving the human cerebral cortical mantle its characteristic appearance of outward folds and inward fissures, although experimental evidence indicates the fundamental role of specific progenitor cell types, cellular processes, and genetic programs on cortical folding,1 as well as the importance of mechanical forces.2 While the majority of folding in humans occurs between the 25th week of gestation to a postnatal age of 4 months,2 brain cortical configuration is continuously remodeled and re-organized during the first 2 decades of life, possibly as a result of myelination and continued synaptic modifications3 as it grows by radial expansion.4 While genetic causes of abnormal cortical development remain poorly understood, recent evidence suggests that cytoskeletal structural proteins (e.g., centrosomal and microtubule-related proteins) are important in cortical development, and that related genetic mutations may interfere with proper neuronal migration and result in cortical malformations and microcephaly.5
The extent to which folding patterns of the cerebral cortex may reflect brain function has been a challenging question for neuroanatomists for more than a century.2,6 Most striking are the severe cognitive deficits observed in congenital brain abnormalities such as lissencephaly, polymicrogyria, and pachygyria.2 Evidence from previous studies also suggests that neurobehavioral disorders may be associated with abnormal cortical morphology3 including developmental language disorder,7 autism,8 bipolar disorder and schizophrenia,9 neurofibromatosis type 1,10 William's syndrome,11 and temporal lobe epilepsy.12 It is plausible that significant life events during development (e.g., brain trauma, malnutrition, neurotoxicity, etc.) may also alter the normal maturational development of cortical folding. Indeed, animal models indicate that microgyria can be induced in rats by producing focal freeze lesions within key timeframes during early development,13 and such malformation may be associated with pronounced alterations of both architecture and physiology of the neocortex and thalamus, likely due to disturbances of underlying network connections.14 However, to our knowledge, the potential impact of early traumatic brain injury (TBI) on cortical folding has not been investigated to date.
The current study investigated patterns of cortical organization (gyrification) in adolescents who had sustained TBI in early childhood to determine ways in which early TBI may alter expected structural brain development. We hypothesized that cortical gyrification would be altered in individuals who had sustained TBI in early childhood, due to disrupted cortical maturation during key developmental periods. This was investigated using the local gyrification index (LGI), which is a metric applied to cortical reconstructions of data. Higher LGI values reflect regions of more extensive cortical folding. The LGI at a given vertex on the cortical mantle reflects the ratio of the cortex invaginated within the sulci relative to the extent of outer, visible cortex; thus, the LGI is directly dependent on the amount and size of the sulci. Maximal LGI values are typically located in the vicinity of the Sylvian fissure, which reflects the large amount of insular cortex hidden within the Sylvian fissure.15 Other moderately high LGI maxima are located in sulci that appear during early development of the fetal cortex (intraparietal sulcus, superior temporal sulcus, and in the vicinity of the parieto-occipital sulcus and calcarine sulcus),16 which is another indication that LGI may be closely associated with neural development.15
The extant literature documents chronic aspects of not only medical and health status, but also cognitive/academic, emotional/behavioral, and family/social outcomes following pediatric TBI.17 Frontal and temporal lobe brain regions are particularly vulnerable to injury as a result of TBI,18 and these regions are also implicated as neural substrates of social information processing and social behavioral regulation.19 Social behavior changes over time as typically developing children mature; similarly, brain regions implicated in social behavior (including anterior cingulate, ventromedial, orbitofrontal, and dorsolateral prefrontal cortex, among other brain regions) change with maturation.20 Therefore, we hypothesized that individuals with a history of TBI would likely receive lower ratings on the Vineland-II Adaptive Behavior Scales, which are measures of personal and social skills utilized for daily living. We further hypothesized that abnormal cortical folding would be related to behavioral and social skills, as assessed using the Vineland-II Adaptive Behavior Scales.
Methods
The data used in the present study were part of a research program on pediatric TBI that was conducted at Baylor College of Medicine and the University of Arkansas for Medical Sciences. Institutional Review Board approval at these institutions was obtained for the current study.
Participants
Participants included 22 children ages 10–18 who had previously been hospitalized within 24 h after sustaining complicated (abnormal day-of-injury CT finding) mild, moderate, or severe TBI in early childhood (mean age at injury = 4.1 ± 2.0 years; range = 1–8 years). Injury severity was defined by the lowest post-resuscitation Glasgow Coma Scale (GCS)21 score obtained at the time of the injury, where those with GCS scores between 13–15, 9–12, and 3–8 are considered to have suffered from mild, moderate, and severe injuries, respectively. Participants were adolescents at the time of imaging (mean age at evaluation = 13.5 ± 2.3 years). Only patients with closed head injury (i.e., acceleration/deceleration, or impact with a blunt object) were recruited to increase homogeneity in pathophysiology. Patients injured as a result of non-accidental trauma (as indicated by medical chart notes) were excluded due to increased risk for repeated head trauma. Participants meeting these criteria were identified by a review of medical records, and the families were contacted to explain the study and enrollment procedures. Twenty typically developing children (TDC) who were demographically similar to the TBI patients (mean age at evaluation = 13.6 ± 2.3) were recruited by community advertising.
In addition to recruiting participants with comparable distributions of age and sex in the TBI and TDC groups, recruitment of the TDC participants was conducted to ensure that a Socioeconomic Composite Index (SCI) based on occupational status of the parent, annual household income, and years of maternal education were comparable to that of the children with TBI. The SCI was derived according to the guidelines provided by Yeates and colleagues,22 where z-scores correspond to socioeconomic status (SES) relative to the average of the entire sample. This socioeconomic background information was provided by the parent via questionnaire. Handedness ratio was determined using the Edinburgh Handedness Inventory.23
The informed consent form was approved by the Institutional Review Boards, and parents or legal guardians of the participants provided informed consent. The participants were fluent in English, had no pre-existing major developmental disorder (e.g., autism or mental deficiency), and had not subsequently been hospitalized for brain injury. Of the recruited participants, 34 participants (17 TBI, 17 TDC) had imaging data that were of sufficient quality to be retained in the present analysis. Reasons for exclusion due to poor scan quality included severe motion artifact/poor image resolution (one case), extensive lesions that interfered with cortical reconstruction (two cases), cortical reconstruction errors that were not able to be corrected by editing (one case) and failure of the LGI processing in FreeSurfer (one case); refer to Supplementary Table S1 for details regarding cases with lesions. Demographic details for the present sample of 34 participants are provided in Table 1, and injury characteristics, with initial findings on CT, are provided for each TBI participant in Table 2.
Table 1.
Demographic and Clinical Characteristics of Participants
| |
TBI (n = 17) |
TDC (n = 17) |
χ2 | df | p | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Sex | 0.13 | 1 | 0.714 | ||||
| Male | 11 | 65 | 12 | 71 | |||
| Female | 6 | 35 | 5 | 29 | |||
| Ethnicity | 1.11 | 2 | 0.574 | ||||
| African American | 0 | 0 | 1 | 6 | |||
| Caucasian | 12 | 71 | 12 | 71 | |||
| Hispanic/Latino | 5 | 29 | 4 | 24 | |||
| M | SD | M | SD | t | df | p | |
| Age at evaluation | 14.1 | 2.4 | 14.0 | 2.3 | 0.14 | 32 | 0.888 |
| Age at injury | 3.8 | 2.0 | |||||
| Years post-injury | 9.9 | 2.9 | |||||
| Glasgow Coma Scale | 8.5 | 3.6 | |||||
| Handedness ratio | 81.8 | 32.1 | 91.2 | 21.8 | 1.00 | 31 | 0.324 |
| SCI z-score | –0.03 | 0.83 | 0.22 | 0.71 | 0.95 | 32 | 0.351 |
TBI, traumatic brain injury; TDC, typically-developing children; SCI, Socioeconomic Composite Index.22
Table 2.
Injury Characteristics of Participants with Traumatic Brain Injury
| Subject | Sex | Age at injury | TSI | Injury mechanism | GCS | Day-of-injury CT results |
||
|---|---|---|---|---|---|---|---|---|
| Primary injury | Hemi | Location | ||||||
| 1 | F | 3 | 7 | MVA (pass) | 3 | SF | R | Temporal |
| 2 | M | 2 | 9 | MVA (ped) | 8 | SF | R | Temporal |
| L | Parietal | |||||||
| L | Basilar | |||||||
| 3 | M | 3 | 15 | BFT | 9 | CBH | R | Cerebellar |
| 4 | M | 5 | 8 | Fall | 5 | EDH | R | Frontal |
| R | Parietal | |||||||
| 5 | M | 4 | 8 | BFT | 10 | SDH | R | Parietal |
| 6 | F | 8 | 5 | RVA | 12 | CC | B | Occipital |
| 7 | M | 5 | 9 | Fall | 13 | PCH | L | Parietal |
| 8 | F | 4 | 14 | MVA (pass) | 3 | HC | R | Temporal |
| 9 | F | 4 | 10 | MVA (pass) | 11 | SAH | R | Lateral ventricle |
| M | Fourth ventricle | |||||||
| 10 | M | 2 | 9 | Fall | 13 | IVH | R | Choroid plexus |
| R | Lateral ventricle | |||||||
| 11 | M | 5 | 6 | RVA | 7 | SDH | L | Along posterior fossa near cisterna magna |
| 12 | M | 2 | 13 | Fall | 8 | SAH | L | Temporal |
| 13 | F | 4 | 13 | Fall | 8 | EDH | L | Occipital |
| 14 | M | 8 | 6 | MVA (pass) | 3 | DAI | R | Temporal |
| R | Basal ganglia | |||||||
| L | Frontal | |||||||
| 15 | M | 1 | 15 | MVA (ped) | 8 | Basilar SF | B | Occipital |
| 16 | M | 3 | 12 | Fall | 15 | IVH | B | Occipital horns |
| 17 | F | 2 | 9 | Fall | 8 | Basilar SF | L | Cranial fossa |
TSI, time-since-injury (years); GCS, Glasgow Coma Scale score (initial); Hemi, hemisphere; F, female; M, male; MVA, motor vehicle accident (pass, passenger, ped, pedestrian); BFT, blunt force trauma; RVA, recreational vehicle accident; SF, skull fracture; CBH, cerebellar hematoma; EDH, epidural hematoma; SDH, subdural hematoma; CC, cortical contusion; PCH, parenchymal hematoma; HC, hemorrhagic contusion; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; DAI, diffuse axonal injury; R, right; L, left; B, bilateral; M, midline.
Behavioral, cognitive, and social functioning
The Vineland Adaptive Behavior Scales, 2nd Edition (Vineland-II),24 is a measure of personal and social skills utilized for daily living. Adaptive behavior consists of the individual's ability to perform daily self-care activities and interact well with others. Appropriate adaptive behavior is based on expected levels of behavior for the individual's same age peer group. Ratings of adaptive behavior reflect the typical performance rather than the potential maximum ability of the individual. The Vineland-II consists of four behavioral domains: Communication, Daily Living Skills, Socialization, Motor Skills (typically administered for children ages 5 years or younger, or for older children with suspected deficits in this domain), and an optional Maladaptive Behavior domain. An Adaptive Behavior Composite score is generated based on the ratings for the individual's ability to perform tasks in the various behavioral domains and is likely the best indicator of overall functional level. Vineland-II standard scores have a mean of 100 and standard deviation of 15. A subset of the participants with adequate neuroimaging data (16 TBI, 12 TDC) were rated by one of their parents on the Vineland-II questionnaire at the time of testing.
The participants were also administered cognitive measures, including the California Verbal Learning Test—Children's version (CVLT-C; a measure of word-list learning and delayed recall) and select subtests of the Delis-Kaplan Executive Function System (D-KEFS). Specifically, the D-KEFS Color Word Interference Test Inhibition/Switching scaled scores and D-KEFS Verbal Fluency Category Switching were included as measures of cognitive flexibility. Performance on these measures was previously reported for this data set.25,26 However, the current study examines performance on these cognitive measures in relation to brain gyrification, as this was not within the scope of the previous studies. With the exception of one TBI participant who did not have D-KEFS Color Word Interference Test scores, all cognitive test score data was collected from all participants.
Magnetic resonance image acquisition and processing
Patients were scanned at the Brain Imaging Research Center at the Psychiatric Research Institute of the University of Arkansas for Medical Sciences (7 TBI, 7 TDC) or at Texas Children's Hospital (10 TBI, 10 TDC) on Philips 3T Achieva (Philips, Cleveland, OH) scanners. Both locations used scanners that have similar platforms and the same software release to increase the compatibility of the data across sites. Volumetric T1-weighted magnetic resonance imaging (MRI) scans were acquired using a 32-channel head coil with the following acquisition parameters: 170 contiguous 1-mm thick slices, matrix size = 256 × 256 mm, repetition time = 6.95 msec, echo time = 3.13 msec, field of view = 256 × 256 mm. All scans were evaluated by a board-certified neuroradiologist with specific expertise in pediatrics, who also recorded all observed lesions.
Cortical reconstruction, including segmentation and volumetric analysis, was performed using the FreeSurfer structural image analysis tools (v.5.1.0; http://surfer.nmr.mgh.harvard.edu), as described previously.27,28 Results for each subject were visually inspected, and manual editing was performed to optimize accuracy, when necessary. Once the three-dimensional cortical reconstruction was complete, the LGI technique15 was utilized in order to quantify the gyrification of the cortex over the extent of the whole cortical surface. Smoothing was performed using a 5-mm full-width half-maximum Gaussian kernel prior to statistical analysis. White matter volumes for each participant were also derived from FreeSurfer using well-described standard pipelines for cortical segmentation and parcellation. This is a surface-based, region of interest-based metric that includes all white matter within the cerebrum.
Statistical analysis
Two-tailed independent t-tests were used to examine group differences on demographic variables, such as age at evaluation (years), handedness ratio, and SES, as measured by the SCI for household income, occupational status of the parent, and maternal years of education. Two-tailed independent t-tests also were used to examine group differences on white matter volume. The data for all 34 participants were included in this analysis. Chi-squared analysis was used to examine group differences in sex, ethnicity, and study site.
Two-tailed independent t-tests were also performed to examine group differences on the Vineland-II Composite Scores. Within the TBI group, Pearson correlations were used to evaluate the relationship between injury severity (i.e., GCS) and standard scores on the parent-rated Vineland-II Communication, Daily Living Skills, Socialization, and Adaptive Behavior Composite scales. Supratentorial brain volume (controlling for intracranial volume) was compared between groups using analysis of covariance (ANCOVA). The threshold for statistical significance was set at p < 0.05, and Cohen's d was utilized as a measure of effect size for between-group comparison, where d ≥ |0.20|, |0.50|, and |0.80| was interpreted as small, medium, and large, respectively.29
Cortical surface-based statistical maps were created by fitting a general linear model at each surface vertex for the following: 1) LGI differences between groups; 2) surface area; 3) the relation of LGI to the Vineland-II Composite Scores within the entire sample; 4) the relation of LGI to cognitive measures (i.e., CVLT-C Total Learning T-scores, CVLT-C Delayed Recall scaled scores, D-KEFS Color Word Interference Test Inhibition/Switching scaled scores, and D-KEFS Verbal Fluency Category Switching scaled scores) within the entire sample; and 5) the relation between LGI and injury severity (i.e., GCS) within the TBI group. A Monte Carlo simulation30 was used to perform cluster-wise correction for multiple comparisons (vertex-wise threshold of p < 0.05; 10,000 iterations). Cluster-wise probabilities are reported, which represent the likelihood of finding a maximum cluster of the same size or larger during simulation. A negative sign was used when correcting for multiple comparisons for the between-group LGI comparisons, given the observed uncorrected results and prior findings from lesion studies in rodents.13 Absolute signs were used for all other corrections for multiple comparisons.
To assess for other brain abnormalities that may be present in patients with TBI, two-tailed independent t-tests were used to examine group differences in white matter volume. Additionally, within the entire sample, Pearson correlations were used to evaluate the relationship between white matter volume and standard scores on parent-rated Vineland-II scales that demonstrated a significant relationship with LGI.
Finally, to assess whether study site or observable lesions affected the LGI results, we conducted the between-group comparison of LGI two additional times as supplementary analyses—with study site included as a covariate, and excluding two additional participants with lesions that may have subtly affected cortical reconstruction (in contrast to the two participants that were originally excluded from the analyses due to significant interference of lesions on cortical reconstruction; Supplementary Table S1).
Results
The participants in the TBI and TDC groups were well matched for demographic factors including age at evaluation, sex, ethnicity, SCI, and handedness, and there was no relationship between participant group and study site (χ2 = 0.00, p = 1.00). Parent ratings on the Vineland-II Composite Scores did not significantly differ between the two groups, although there was a medium-large effect size for higher ratings for the TDC group on the Communication domain, suggesting that they demonstrated better communication skills, on average (Table 3). Further, correlational analyses demonstrated a significant positive relationship between TBI severity (i.e., GCS) and Vineland-II Communication domain scores (r = 0.62, p = 0.011), suggesting that communication skill decreases with increasing injury severity. Moderate effect sizes were also observed for higher standard scores in the TDC group on the Vineland-II Socialization domain and Adaptive Behavior Composite; however, no relationships were found between TBI severity and standard scores on these Vineland-II scales or that of the Daily Living Skill domain.
Table 3.
Vineland-II Adaptive Behavior Scale Scores (Parent Informant)
| Domain | TBI (n = 16) |
TDC (n = 12) |
t(26) | p | d | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Socialization | 96.0 | 11.2 | 101.9 | 6.7 | 1.62 | 0.117 | 0.64 |
| Communication | 96.9 | 16.3 | 108.2 | 12.5 | 1.99 | 0.057 | 0.78 |
| Daily Living Skills | 100.9 | 13.7 | 105.3 | 10.3 | 0.91 | 0.370 | 0.36 |
| Adaptive Behavior Composite | 98.1 | 14.5 | 105.8 | 8.0 | 1.67 | 0.107 | 0.66 |
All scores are reported as standard scores with a mean of 100 and a standard deviation of 15; Cohen's d ≥ |0.20|, |0.50|, and |0.80| indicate a small, medium, and large effect size, respectively.29
TBI, traumatic brain injury; TDC, typically-developing children; M, mean; SD, standard deviation.
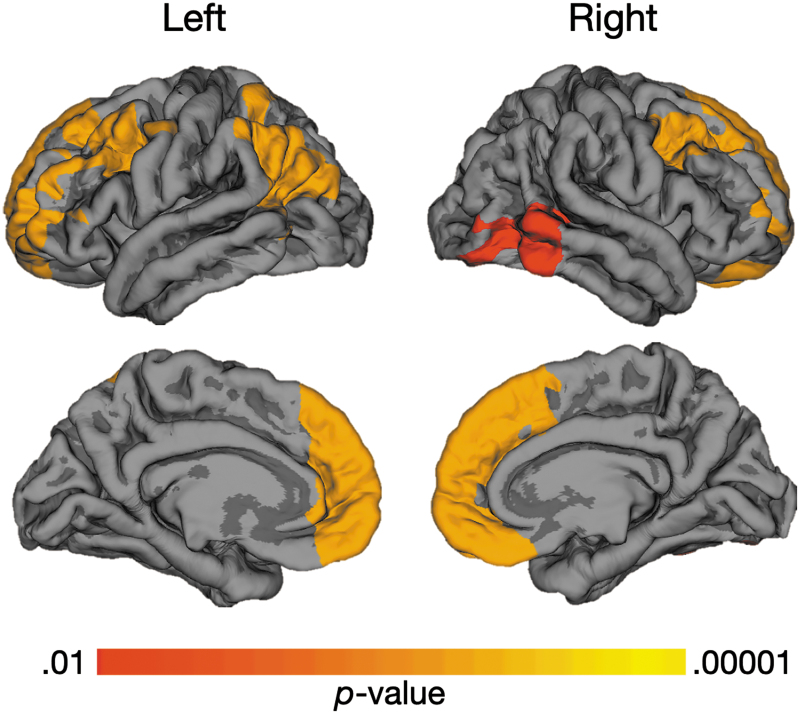
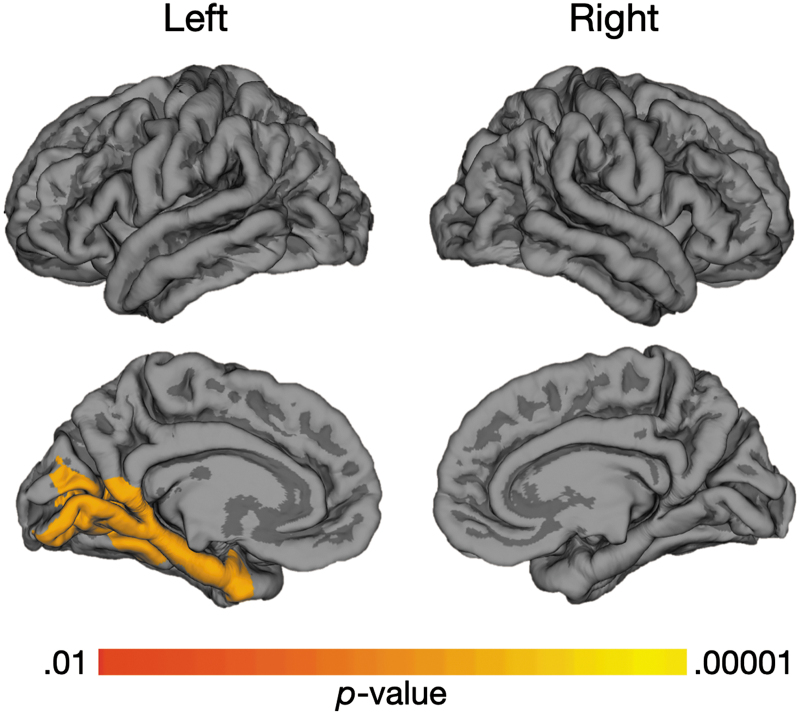
In the analysis of LGI between groups, a total of 149,926 vertices were examined for the right hemisphere, and 149,955 vertices were examined for the left hemisphere. In relation to the TDC group, participants with TBI demonstrated significantly increased LGI in several regions, including left parietal association areas, bilateral dorsolateral and medial frontal areas, and the right posterior temporal gyrus (Fig. 1). The increased LGI in these regions suggests the presence of more extensive folding in these regions in the TBI group. No regions of increased LGI were seen in the TDC group, relative to those with TBI.
FIG. 1.
Comparison of Local Gyrification Index (LGI) between traumatic brain injury (TBI) and typically developing child (TDC) groups. Warm color highlights regions of significant difference in LGI between groups of adolescents with TBI versus those in the TDC group, where increased LGI is demonstrated in the TBI group. The most significant differences appear in yellow in the bilateral medial and dorsolateral frontal, left parietal, and right temporal areas of the cortical surface, which are among the latest to develop. Color image is available online.
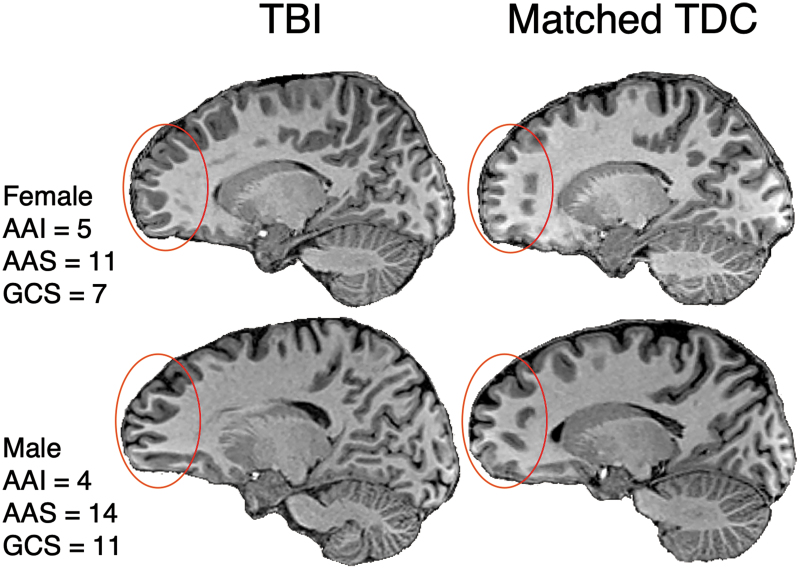
As examples, Figure 2 compares T1-weighted MRI scans of two of the children in the present cohort who sustained a severe injury at young ages with two age-, sex-, and SES-matched peers in the TDC group. Of note, there is an absence of focal lesions, and the white matter appears to be relatively intact in both cases; however, the depth of the cortical folding and the shape of the gyri are different in the children with TBI.
FIG. 2.
Examples of gyrification patterns in two adolescents several years after pediatric TBI compared with two demographically-matched adolescents in the TDC comparison group on conventional T1-weighted imaging. Note the irregular pattern and seemingly deeper gyrification in the medial aspects of the frontal lobes (regions within the overlaid red circle) in the children with TBI versus their age-, sex-, and SES-matched controls. TBI, traumatic brain injury; TDC, typically developing child; AAI, age at injury; AAS, age at scan; GCS, Glasgow Coma Scale score. Color image is available online.
The supplementary between-group analysis, in which the effects of study site were controlled for, demonstrated very similar results as those presented above, where an absence of findings in the right posterior temporal region was the only exception (Supplementary Fig. S1). Additionally, the supplementary analysis that was performed excluding two additional TBI participants with lesions that may have subtly affected cortical reconstruction also revealed very similar results to the original between-group comparison, with the exception that the LGI group differences did not extend as far into the right dorsolateral frontal and right medial inferior frontal regions (Supplementary Fig. S2).
ANCOVA revealed no difference in cortical surface area or white matter volume between the two groups; however, a comparison of supratentorial brain volume (controlling for intracranial volume) indicated that the TDC group had significantly larger brain volume (1150.0 ± 104.4 cm3) relative to the TBI group [1134.6 ± 100.9 cm3), F(2, 33) = 40.197, p < 0.001].
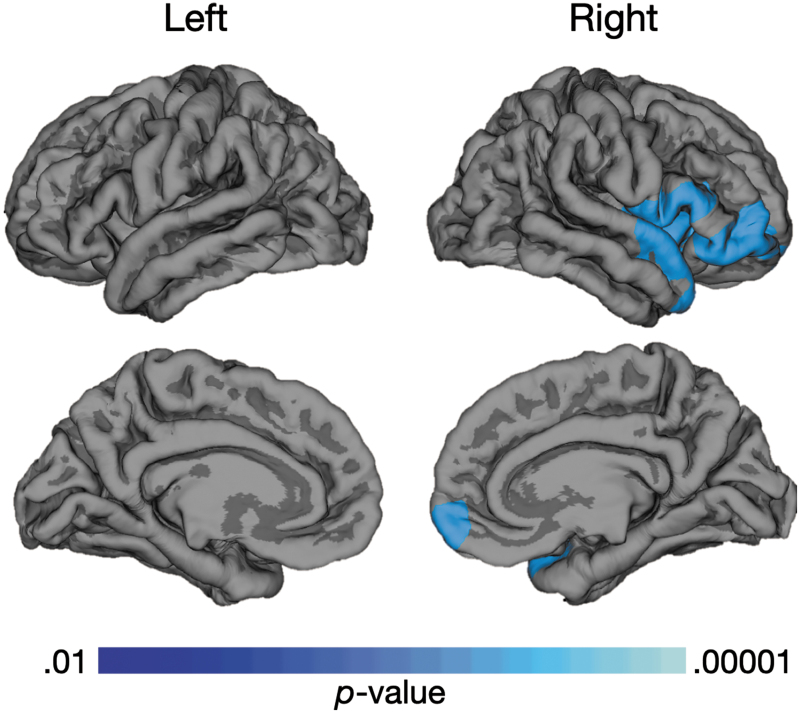
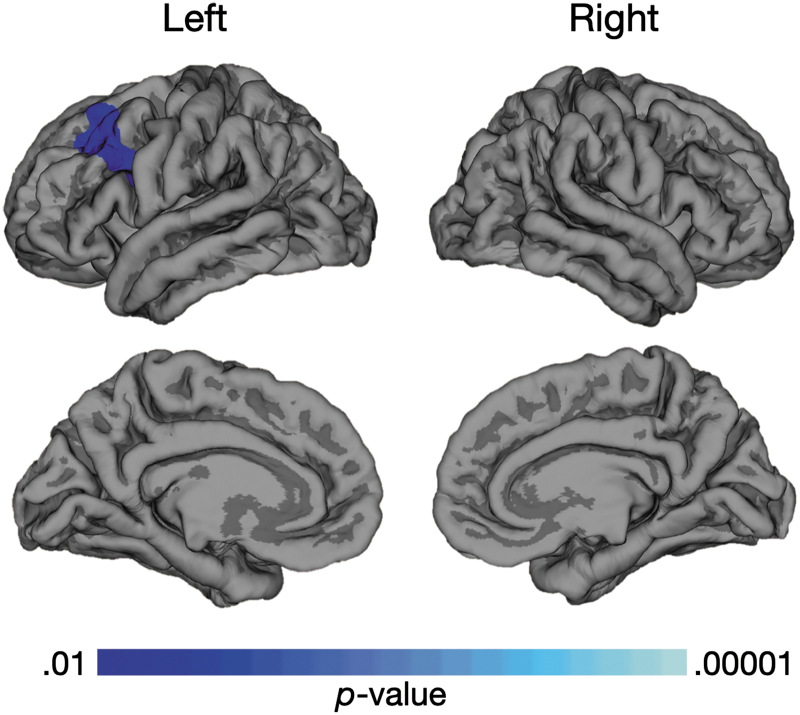
Vineland-II scores were collected from a total of 16 participants in the TBI group and 12 in the TDC group. The statistical brain map of the correlation between LGI and Vineland-II Socialization domain scores (Fig. 3) demonstrates a negative correlation in right dorsolateral, superior temporal, and medial prefrontal regions; thus, lower Socialization scores are associated with increased gyrification. There were no regions with positive correlations with Socialization, and no significant correlations between LGI and other Vineland-II domain or Adaptive Behavior Composite standard scores. There also was no significant correlation between white matter volume and the Vineland-II Socialization score. Additionally, no correlation was found between LGI and CVLT-C scores or performance on the Category Switching trial of the D-KEFS Verbal Fluency test; however, a negative correlation was found between LGI in the left rostral middle frontal region (p < 0.005) and performance on the Inhibition/Switching trial of the D-KEFS Color-Word Interference Test, such that increased gyrification was associated with lower test scores (Fig. 4).
FIG. 3.
Relationship between Local Gyrification Index (LGI) and parent-rated Vineland-II Socialization domain scores. Blue regions are indicative of significant negative correlation between increased LGI in the right medial, ventrolateral frontal, and superior temporal brain regions and decreased Socialization scores on the parent-rated Vineland–II Adaptive Behavior Scale in both groups combined (16 traumatic brain injury, 12 typically developing children). Color image is available online.
FIG. 4.
Relationship between Local Gyrification Index (LGI) and inhibition/switching scaled scores on the Delis-Kaplan Executive Function System (D-KEFS) Color-Word Inhibition Test. Blue color highlighting is indicative of a negative correlation between increased LGI in the left rostral middle frontal region and poorer inhibition/switching performance in both groups combined (16 traumatic brain injury, 17 typically developing children). Color image is available online.
Finally, injury severity in the TBI group was positively correlated with LGI in left medial temporal and occipital regions (p = 0.0001), such that higher GCS scores (i.e., less severe injuries) were associated with increased gyrification in these regions (Fig. 5). No negative correlations were found between injury severity and LGI in any region.
FIG. 5.
Relationship between Local Gyrification Index (LGI) and injury severity (i.e., Glasgow Coma Scale [GCS]) in adolescents with traumatic brain injury (n = 17). Warm color highlighting is indicative of a positive correlation between increased GCS and increased LGI, suggesting that less severe injury (i.e., higher GCS) is associated with increased gyrification in the left medial temporal and occipital regions. Color image is available online.
Discussion
It is well-known that dynamic changes in brain organization take place over the course of the human lifespan, with cortical volume increasing during childhood and decreasing during adolescence, and white matter volume increases that appear to continue into the third decade of life.31 Cortical re-organization has also been observed throughout the lifespan, and it may be facilitated under conditions of early development and in response to neural insult by means of neuroplasticity.32 Higher LGI in other patient populations is associated with polymicrogyria and aberrant development, but this is the first indication of altered cortical organization as manifest by increased LGI following pediatric TBI. The current findings suggest an altered pattern of expected development in cortical organization in the TBI group, with changes in late-developing frontal and parietal association areas. Such changes in brain structure may underlie cognitive and behavioral deficits associated with TBI sustained in early childhood. Therefore, cortical gyrification patterns may reflect the effect of biological processes that underlie cognitive functioning,15 and disruptions of these biological processes may contribute to deficits in cognitive functioning. Indeed, neurobehavioral changes in rodent models of microgyria have been observed,33 including increased susceptibility to learning and memory deficits, auditory temporal processing, and working memory deficits.34,35
We found increased gyrification in regions implicated in social cognition, social behavior, and emotional regulation, including ventromedial, orbital and medial prefrontal cortex, anterior cingulate, dorsolateral prefrontal cortex, anterior superior temporal cortex, and parietal association cortex.36–42 According to brain-based models of social cognitive development, these brain regions and others undergo gradual maturation, beginning with regions such as the fusiform gyrus, which subserve basic social functions such as facial recognition and identification of emotions; regions such as the medial prefrontal and superior temporal cortices regions mature later, gradually forming a network supporting more complex social cognitive processes such as empathy, theory of mind and social problem solving.41,42 The maturational processes include morphological development, myelination, synaptogenesis, and later pruning of synapses of the prefrontal cortex, in addition to protracted, linear development of white matter within the anterior brain regions.20
In the current study, although the group level mean scores were within an average range, the parent-rated Vineland-II scores demonstrated a meaningful effect size for higher functioning in the TDC group on the Communication domain, along with somewhat lower, moderate effect sizes for the Socialization domain and Adaptive Behavior Composite. These observations suggest that even after several years of recovery and development, children who acquired a TBI at a young age may display poorer communication and social skills compared with their age-matched peers. Lower Vineland-II Socialization scale parent ratings also correlated with increased LGI in the right dorsolateral prefrontal and anterior superior temporal regions. The uncinate fasciculus connects the anterior temporal lobe with the orbitofrontal cortex,43 and though its function is poorly understood, the uncinate is thought to be involved in emotional processing, memory, language functions, and social cognition.44 The uncinate fasciculus in the right hemisphere, specifically, has been shown to play an important role in the emotional empathy network.45 Therefore, our results suggest that increased LGI in the cortical regions connected by the uncinate may be related to the deficits in communication and social/emotional functions observed in our sample of children with TBI.
From a different perspective, the Vineland-II scores of the TBI group were within normal range despite having sustained severe, generally diffuse injuries during a period of rapid brain development. Taken together with their reduced supratentorial brain region volume, increased LGI may represent a compensatory mechanism. Consistent with theoretical models of cognitive and social cognitive development,46,47 which integrate brain region development and environmental factors, the children who sustained early TBI were raised in families with socioeconomic resources that did not differ significantly from the TDC group; consequently, we would not expect that recovery and acquisition of cognitive and social cognitive abilities was further disrupted by a disadvantageous environment in the TBI group.
Previously, some aspects of executive functioning were observed to be stronger in the TDC group than the TBI group in this cohort, including performance on the D-KEFS Color Word Interference (Inhibition/Switching Trial),26 as well as was a medium effect size (but no significant difference) in delayed verbal memory performance.25 In the current study, lower performance on D-KEFS Color Word Interference (Inhibition/Switching Trial) correlated with increased LGI in the left rostral middle frontal cortex. Of note, gray matter volume of this region also correlates with performance on this task,48 and executive functioning in general is often associated with frontal lobe integrity. However, verbal fluency (category switching) and verbal memory performance were not correlated with LGI in any brain region, suggesting that cortical folding is not a good predictor of performance in all cognitive domains. Further, given that the correlation between LGI and performance on the Color-Word Interference test was seen in the left rostral middle frontal region, whereas that between LGI and social skill was seen in right medial, ventrolateral frontal, and superior temporal brain regions, bi-directional causation between cognitive and social skill deficit is not likely. Given the current findings, further study of the relationship between LGI and cognitive functioning is warranted.
In some respects, the observed finding of greater cortical folding and the association of a negative correlation between cortical folding and functioning may seem counterintuitive, given the expectation that greater cortical folding (presumably representing increased complexity of the underlying neural circuitry) would instead be associated with greater intelligence or higher functioning.49 However, there is evidence that cortical folding during normal development typically appears to decrease with age in adolescence,50–52 during a period of time that is associated with increases in cognitive skills and functional level. Specifically, in healthy children, developmental decreases in brain surface complexity appear first in the occipital lobes and move anteriorly as they mature into young adulthood.52 This appears to be consistent with the results of the current study, which found alterations in LGI in medial frontal, dorsolateral frontal, and parietal association areas, all of which are among the last regions to develop in childhood and adolescence.53 Accordingly, we would not expect to find increased LGI following later onset of TBI during the preadolescent age range when reduction of cortical folding is typically ongoing. This view is consistent with evidence that severe TBI is more disruptive when it occurs at an early stage of brain maturation.54,55
The inferior aspects of the frontal and temporal lobes are known to be the most common location of cortical contusions following TBI due to their proximity to the irregular inner surface of the skull, which moors the dural covering of the brain under normal circumstances but becomes a vulnerability to injury under high-impact conditions.18 However, the pattern of increased cortical gyrification in the current study is less likely to be related to deformation of the cortex as a result of the injury itself, particularly as the regions of increased cortical gyrification overlap only partially with inferior frontal and temporal lobes. It is more likely that the alteration in gyrification occurred as a pathological and/or compensatory developmental response to injury.
Given that there was no significant difference in cortical surface area between the two groups, despite reduced brain volume in the TBI group, the present results suggest that an increase in gyrification might allow for the development of increased cortical surface area as a compensatory strategy. This interpretation of the increased LGI in the early TBI group is broadly consistent with studies of pre-term and very pre-term infants, where MRIs performed at term demonstrated increased surface area in various brain regions, including the right and left parietal regions, which predicted higher scores on the Bayley-III Cognitive and Language scales, respectively, when measured at 2 years of age.56 In contrast to surface area, the same study found that LGI was predictive of Bayley scores in fewer brain regions, and the direction of the correlation was inconsistent.56 In the present study we postulate that higher LGI had compensated for the lower supratentorial brain volume in the early TBI group, who were imaged during adolescence; their cognitive functioning at the time of imaging is also consistent with this view.
Alternatively, a possible mechanistic explanation for the increased gyrification has been observed in William's syndrome, where small microvascular infarcts may affect gyral folding during early cortical development.11 This can have implications for altered cortical folding in pediatric TBI as well, given that microvascular alterations have been observed following TBI, likely in association with secondary hypoxic/ischemic events.57
Limitations of the current study include the small sample size, which restricted our ability to explore other factors such as sex differences. This would be of interest for future research since evidence from rat studies suggests that sex differences might exist in relation to microgyria and neurobehavioral functioning.58 Another limitation was the retrospective nature of the current study, with heterogeneity in age at injury and post-injury time intervals. Larger studies that compared groups of children who sustained TBI at different stages of development have found that trajectories of cognitive functioning differ depending on age and severity of TBI; severe injury during infancy had persistent, adverse effects on intellectual development even 30 months later, whereas an injury of comparable severity occurring later in childhood had a more favorable recovery.59 A recent study reported similar results, as communication skills declined from 3 to 12 months post-injury in infants, whereas performance improved over this time period in toddlers.54 Severe TBI during infancy often has diffuse effects, which disrupt development of brain regions and networks at a time when cortical maturation and myelination are rapidly advancing. These effects interfere with learning cognitive skills and often result in persistent cognitive impairment relative to age expectation; however, long-term follow up over a decade has not confirmed whether cognitive deficits emerge or worsen when the brain injured child reaches the age when the cognitive ability in question is typically established.60
Future studies may further explore the nature of developmental changes in cortical organization over time, which will require greater control over post-injury time intervals. The use of rating scales of behavior is also less than ideal, as ratings may be more subjective than standardized neuropsychological testing; however, subjective rating scales often provide information that may not be fully captured in neuropsychological testing. In the future, the relation of neuropsychological performance to LGI should also be further explored, especially in longitudinal studies. Finally, similar future studies are necessary to further elucidate the consistency of our findings, the relation of these changes to injury at various levels of the severity continuum, and the mechanism underlying our observations.
Conclusions
In summary, brain maturation includes both cortical thinning (starting at about the age of puberty, ostensibly through synaptic pruning) as well as the re-organization of gyrification. Higher LGI in other patient populations is associated with polymicrogyria and aberrant development, but this is the first indication of altered cortical organization following pediatric TBI. The current results suggest that increased gyrification may occur in response to TBI in early childhood, with changes in late-developing frontal and parietal association areas. Such changes in brain structure may underlie cognitive, behavioral, and social deficits associated with early TBI. The specific mechanism and function of increased cortical folding in response to early pediatric TBI remains unclear; however, results of the present study suggest that increased gyrification following early TBI may represent pathological development and/or a compensatory mechanism that allows for typical development of cortical surface area, despite reduced brain volume. Longitudinal imaging and neuropsychological assessment of early and later pediatric TBI cohorts would elucidate the mechanisms underpinning LGI.
Supplementary Material
Acknowledgments
We wish to express our gratitude to our participants and their family members. We also wish to express our gratitude for the invaluable assistance of Matthew Spruiell.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Information
The study was funded by the National Institutes of Health grant R21 NS065937 (“Trauma to Developing Brain: Model Refinement and Therapeutic Intervention”; Levin, PI and Noble, PI).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Borrell V. (2018). How cells fold the cerebral cortex. J. Neurosci. 38, 776–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kroenke C.D. and Bayly P.V. (2018). How forces fold the cerebral cortex. J. Neurosci. 38, 767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanton R.E., Levitt J.G., Thompson P.M., Narr K.L., Capetillo-Cunliffe L., Nobel A., Singerman J.D., McCracken J.T., and Toga A.W. (2001). Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 107, 29–43 [DOI] [PubMed] [Google Scholar]
- 4. Rakic P. (2000). Radial unit hypothesis of neocortical expansion. Novartis Found. Symp. 228, 30–42 [DOI] [PubMed] [Google Scholar]
- 5. Poirier K, Lebrun N, Broix L, Tian G., Saillour Y., Boscheron C., Parrini E., Valence S., Pierre B.S., Oger M., Lacombe D., Geneviève D., Fontana E., Darra F., Cances C., Barth M., Bonneau D., Bernadina B.D., N'guyen S., Gitiaux C., Parent P., des Portes V., Pedespan J.M., Legrez V., Castelnau-Ptakine L., Nitschke P., Hieu T., Masson C., Zelenika D., Andrieux A., Francis F., Guerrini R., Cowan N.J., Bahi-Buisson N., and Chelly J. (2013). Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 45, 639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welker W. (1990). Why does the cortex fissure and fold? A review of determinants of gyri and sulci, in: Cerebral Cortex, Jones E.G. and Peters A. (eds). Penum: New York, NY, pps. 3–136 [Google Scholar]
- 7. Jackson T. and Plante E. (1996). Gyral morphology in the posterior Sylvian region in families affected by developmental language disorder. Neuropsychol. Rev. 6, 81–94 [DOI] [PubMed] [Google Scholar]
- 8. Kates W.R., Ikuta I., Burnette C.P. (2009). Gyrification patterns in monozygotic twin pairs varying in discordance for autism. Autism Res. 2, 267–278 [DOI] [PubMed] [Google Scholar]
- 9. McIntosh A.M., Moorhead T.W., McKirdy J., Hall J., Sussmann J.E.D., Stanfield A.C., Harris J.M., Johnstone E.C., and Lawrie S.M. (2009). Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr. Scand. 119, 192–198 [DOI] [PubMed] [Google Scholar]
- 10. Violante I.R., Ribeiro M.J., Silva E.D., and Castelo-Branco M. (2013). Gyrification, cortical and subcortical morphometry in neurofibromatosis type 1: an uneven profile of developmental abnormalities. J. Neurodev. Disord. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmitt J.E., Watts K., Eliez S., Bellugi U., Galaburda A.M., and Reiss A.L. (2002). Increased gyrification in Williams syndrome: evidence using 3D MRI methods. Dev. Med. Child Neurol. 44, 292–295 [DOI] [PubMed] [Google Scholar]
- 12. Oyegbile T., Hansen R., Magnotta V., O'Leary D., Bell B., Seidenberg M., and Hermann B.P. (2004). Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology 18, 729–737 [DOI] [PubMed] [Google Scholar]
- 13. Threlkeld S.W., McClure M.M., Rosen G.D., and Fitch R.H. (2006). Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 1109, 22–31 [DOI] [PubMed] [Google Scholar]
- 14. Rosen G.D., Burstein D., and Galaburda A.M. (2000). Changes in efferent and afferent connectivity in rats with induced cerebrocortical microgyria. J. Comp. Neurol 418, 423–440 [PubMed] [Google Scholar]
- 15. Schaer M., Cuadra M.B., Tamarit L., Lazeyras F., Eliez S., and Thiran J.P. (2008). A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging 27,161–170 [DOI] [PubMed] [Google Scholar]
- 16. Garel C., Chantrel E., Brisse H., Elmaleh M., Luton D., Oury J.F., Sebag G., and Hassan M. (2001). Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. A.J.N.R. Am. J. Neuroradiol. 22, 184–189 [PMC free article] [PubMed] [Google Scholar]
- 17. Babikian T., Merkley T., Savage R.C., Giza C.C., and Levin H. (2015). Chronic aspects of pediatric traumatic brain injury: review of the literature. J. Neurotrauma 32, 1849–1860 [DOI] [PubMed] [Google Scholar]
- 18. Bigler E.D. (2007). Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology 21, 515–531 [DOI] [PubMed] [Google Scholar]
- 19. Porcelli S, Van Der Wee N., van der Werff S., Aghajani M., Glennon J.C., van Heukelum S., Mogavero F., Lobo A., Olivera F.J., Lobo E., Posadas M., Dukart J., Kozak R., Arce E., Ikram A., Vorstman J., Bilderbeck A., Saris I., Kas M.J., and Serretti A. (2019). Social brain, social dysfunction and social withdrawal. Neurosci. Biobehav. Rev. 97, 10–33 [DOI] [PubMed] [Google Scholar]
- 20. Yeates K.O., Bigler E.D., Dennis M., Gerhardt C.A., Rubin K.H., Stancin T., Taylor H.G., Vannatta K. (2007). Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol. Bull. 133, 535–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness. a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 22. Yeates K.O., Taylor H.G., Drotar D., Wade S.L., Klein S., Stancin T., and Schatschneider C. (1997). Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J. Intl. Neuropsychol. Soc. 3, 617–630 [PubMed] [Google Scholar]
- 23. Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 [DOI] [PubMed] [Google Scholar]
- 24. Sparrow S., Cicchetti D., and Balla D. (2005). Vineland Adaptive Behavior Scales, Second edition. AGS Publishing: Circle Pines, MN [Google Scholar]
- 25. Lindsey H.M., Lalani S.J., Mietchen J, Gale S.D., Wilde E.A., Faber, MacLeod M.C., Hunter J.V., Chu Z.D., Aitken M.E., Ewing-Cobbs L., and Levin H.S. (2019). Acute pediatric traumatic brain injury severity predicts long-term verbal memory performance through suppression by white matter integrity on diffusion tensor imaging. Brain Imaging Behav. 2019 May 27; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faber J., Wilde E.A., Hanten G., Ewing-Cobbs L., Aitken M.E., Yallampalli R., MacLeod M.C., Mullins S.H., Chu D., Li X., Hunter J.V., Noble-Haeusslein L., and Levin H.S. (2016). Ten-year outcome of early childhood traumatic brain injury: diffusion tensor imaging of the ventral striatum in relation to executive functioning. Brain Inj. 30, 1635–1641 [DOI] [PubMed] [Google Scholar]
- 27. Bigler E.D., Abildskov T.J., Wilde E.A., McCauley S.R. Li X., Merkley T.L, Fearing MA., Newsome M.R., Scheibel R.S., Hunter J.V., Chu Z., and Levin H.S. (2010). Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage 50, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 28. McCauley S.R., Wilde E.A., Merkley T.L., Schnelle K.P., Bigler E.D., Hunter J.V., Chu Z., Vásquez A.C., and Levin H.S. (2010). Patterns of cortical thinning in relation to event-based prospective memory performance three months after moderate to severe traumatic brain injury in children. Dev. Neuropsychol. 35, 318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Lawrence Erlbaum: Hillsdale, N.J [Google Scholar]
- 30. Hagler D.J. Jr., Saygin A.P., and Sereno M.I. (2006). Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33, 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Courchesne E., Chisum H.J., Townsend J., Cowles A., Covington J., Egaas B., Harwood M., Hinds S., and Press G.A. (2000). Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216, 672–682 [DOI] [PubMed] [Google Scholar]
- 32. Johansson BB. (2004). Brain plasticity in health and disease. Keio J. Med. 53, 231–246 [DOI] [PubMed] [Google Scholar]
- 33. Fitch R.H., Breslawski H., Rosen G.D., and Chrobak J.J. (2008). Persistent spatial working memory deficits in rats with bilateral cortical microgyria. Behav. Brain Funct. 4, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herman A.E., Galaburda A.M., Fitch R.H., Carter A.R., and Rosen G.D. (1997). Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cereb. Cortex 7, 453–464 [DOI] [PubMed] [Google Scholar]
- 35. Threlkeld S.W., Hill C.A., Szalkowski C.E., Truong D.T., Rosen G.D., and Fitch R.H. (2012). Effects of test experience and neocortical microgyria on spatial and non-spatial learning in rats. Behav. Brain Res. 235, 130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hiser J. and Koenigs M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol. Psychiatry 83, 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hughes B.L. and Beer J.S. (2012). Orbitofrontal cortex and anterior cingulate cortex are modulated by motivated social cognition. Cereb. Cortex 22, 1372–1381 [DOI] [PubMed] [Google Scholar]
- 38. Jenkins L.M., Andrewes D.G., Nicholas C.L., Drummond K.J., Moffat B.A., Phal P., Desmond P., and Kessels R.P. (2014). Social cognition in patients following surgery to the prefrontal cortex. Psychiatry Res. 224, 192–203 [DOI] [PubMed] [Google Scholar]
- 39. Xi C, Zhu Y, Niu C, Zhu C., Lee T.M.C., Tian Y., and Wang K. (2011). Contributions of subregions of the prefrontal cortex to the theory of mind and decision making. Behav. Brain Res. 221, 587–593 [DOI] [PubMed] [Google Scholar]
- 40. Beauchamp M.H. (2017). Neuropsychology's social landscape: common ground with social neuroscience. Neuropsychology 31, 981–1002 [DOI] [PubMed] [Google Scholar]
- 41. Ryan N.P., van Bijnen L., Catroppa C., Beauchamp M.H., Crossley L., Hearps S., and Anderson V. 3 (2016). Longitudinal outcome and recovery of social problems after pediatric traumatic brain injury (TBI): Contribution of brain insult and family environment. Int. J. Dev. Neurosci. 49, 23–30 [DOI] [PubMed] [Google Scholar]
- 42. Beauchamp M.H. and Anderson V. (2010). SOCIAL: an integrative framework for the development of social skills. Psychol. Bull. 136, 39–64 [DOI] [PubMed] [Google Scholar]
- 43. Catani M., Howard R.J., Pajevic S., and Jones D.K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17, 77–94 [DOI] [PubMed] [Google Scholar]
- 44. Catani M. and Thiebaut de Schotten M. (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132 [DOI] [PubMed] [Google Scholar]
- 45. Oishi K., Faria A.V., Hsu J., Tippett D., Mori S., and Hillis A.E. (2015). Critical role of the right uncinate fasciculus in emotional empathy. Ann. Neurol. 77, 68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fine J.G. and Sung C. (2014). Neuroscience of child and adolescent health development. J. Couns. Psychol. 61, 521–527 [DOI] [PubMed] [Google Scholar]
- 47. Johnson M.H., Grossmann T., and Cohen Kadosh K. (2009). Mapping functional brain development: Building a social brain through interactive specialization. Dev. Psychol. 45, 151–159 [DOI] [PubMed] [Google Scholar]
- 48. Pa J., Possin K.L., Wilson S.M., Quitania L.C., Kramer J.H., Boxer A.L., Weiner M.W., and Johnson J.K. (2010). Gray matter correlates of set-shifting among neurodegenerative disease, mild cognitive impairment, and healthy older adults. J. Int. Neuropsychol. Soc. 16, 640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hofman M.A. (2014). Evolution of the human brain: when bigger is better. Front. Neuroanat. 8, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klein D., Rotarska-Jagiela A., Genc E., Sritharan S., Mohr H., Roux F., Han C.E., Kaiser M., Singer W., and Uhlhaas P.J. (2014). Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One 9, e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., and Giedd J.N. (2011). How does your cortex grow? J. Neurosci. 31, 7174–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Su S., White T., Schmidt M., Kao C.Y., and Sapiro G. (2013). Geometric computation of human gyrification indexes from magnetic resonance images. Hum Brain Mapp 34, 1230–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., and Thompson P.M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 101, 8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keenan H.T., Presson A.P., Clark A.E., Cox C.S., and Ewing-Cobbs L. (2019). Longitudinal developmental outcomes after traumatic brain injury in young children: are infants more vulnerable than toddlers? J. Neurotrauma 36, 282–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keenan H.T., Clark A.E., Holubkov R., Cox C.S., and Ewing-Cobbs L.. Psychosocial and executive function recovery trajectories one year after pediatric traumatic brain injury: the influence of age and injury severity. J. Neurotrauma 35, 286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kline J.E., Illapani V.S.P., He L., Altaye M., Logan J.W., and Parikh N.A. (2019). Early cortical maturation predicts neurodevelopment in very preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2019 Nov 8; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park E., Bell J.D., Siddiq I.P., and Baker A.J. (2009). An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 575–584 [DOI] [PubMed] [Google Scholar]
- 58. Rial D., Xikota J.C., Miozzo A., Cruz V.E., Prediger R.D., and Walz R. (2009). Differential gender-related susceptibility to learning and memory deficits in mice submitted to neonatal freezing microgyria model. Brain Res. Bull. 79, 177–181 [DOI] [PubMed] [Google Scholar]
- 59. Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2005). Functional plasticity or vulnerability after early brain injury? Pediatrics 116, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 60. Anderson V., Godfrey C., Rosenfeld J.V., and Catroppa C. (2012). Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics 129, e254–e261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.