Abstract
Purpose
To determine whether multicolor scanning laser ophthalmoscopy (MC-SLO) was better than color fundus photography (CFP) to enhance residents and specialists’ preoperative decision-making and intraoperative performance on the epiretinal membrane (ERM).
Methods
Consecutive patients with idiopathic ERM were recruited prospectively. All the patients underwent MC-SLO and CFP imagings and were randomized into MC-SLO (n = 20) and CFP (n = 20) groups. Preoperatively, residents and specialists were required to have ERM delineation and select an optimal location for initial ERM peeling independently, based on the MC-SLO (MC-SLO group) or CFP (CFP group) images. Intraoperative optical coherence tomography (iOCT) was introduced to evaluate the accuracy.
Results
Preoperatively, residents and specialists acted more effectively in ERM delineation and selection of initial grasping location in the MC-SLO group (both P < 0.001). In the MC-SLO group, higher resident–specialist agreements were achieved in ERM delineation (P = 0.002) and selection of initial grasping location (P = 0.035). The iOCT revealed greater interobserver (iOCT–resident and iOCT–specialist) agreements of ERM delineation in MC-SLO group (P < 0.001 and = 0.027, respectively). Surgeons acted more effectively on completely peeling the ERM in the MC-SLO group (P < 0.001).
Conclusions
MC-SLO provided a better visual reference for residents and specialists in ERM delineation and the selection of an initial grasping location for the surgery, compared with CFP.
Translational Relevance
MC-SLO is able to help surgeons achieve better intraoperative performance and shorten the learning process for residents.
Keywords: multicolor scanning laser ophthalmoscopy, color fundus photography, epiretinal membrane, intraoperative optical coherence tomography, pars plana vitrectomy
Introduction
Epiretinal membrane (ERM) is a vitreomacular disorder of cellular proliferation on the inner retinal surface, with its typical presentation of metamorphopsia with or without visual impairment.1,2 The understanding of ERM has increased significantly with the introduction and rapid advancement of optical coherence tomography (OCT). OCT has become the gold standard for the definite diagnosis of ERM.2,3
Pars plana vitrectomy is the optimal treatment for ERM when patients have metamorphopsia and/or vision decrease.4,5 Two main tasks of ERM surgery are the delineation of the ERM border and the decision of where to begin engaging and peeling off the ERM. This process is usually challenging for surgeons, especially for residents with insufficient ERM peeling experiences.2,6–8
To improve ERM surgical management, preoperative assessment and planning might be an important step before surgeons and patients entering the operating theatre. Conventionally, color fundus photography (CFP) and OCT images have acted as the main references for the diagnosis and preoperative assessment of ERM. However, CFP reflects limited information of ERM borders and thickness landscapes owing to the low contrast and resolution.9,10 OCT offers cross-sectional ERM images that differ considerably from the en face views of ERM seen by surgeons with microscope.
Recently, multicolor scanning laser ophthalmoscopy (MC-SLO) has been demonstrated to have certain advantages in the fundus photography of various vitreoretinal diseases.9–15 The Spectralis MultiColor SLO (Heidelberg Engineering, Heidelberg, Germany) uses blue (488 nm), green (515 nm), and infrared (820 nm) excitation laser lights.10 It provides higher contrast fundus photography by the suppression of light scatter and reveals retinal findings at varying depths by compiling the reflectance patterns of the above three scanning lasers into a single en face image.10,14
We assume that the border and thickness delineation of ERM presented by MC-SLO may assist surgeons more than conventional CFP in the surgical management of ERM. Thus, the current study was performed to determine whether MC-SLO could act better than CFP on supporting surgeons to have better preoperative decision-making and intraoperative performance in ERM surgery. Intraoperative OCT (iOCT) was introduced into the evaluation system to strengthen objectivity of the current study, because it helps surgeons to identify the starting points for ERM peeling and provides real-time feedback in a cross-sectional view.16–19
Methods
Ethics Statement and Subjects
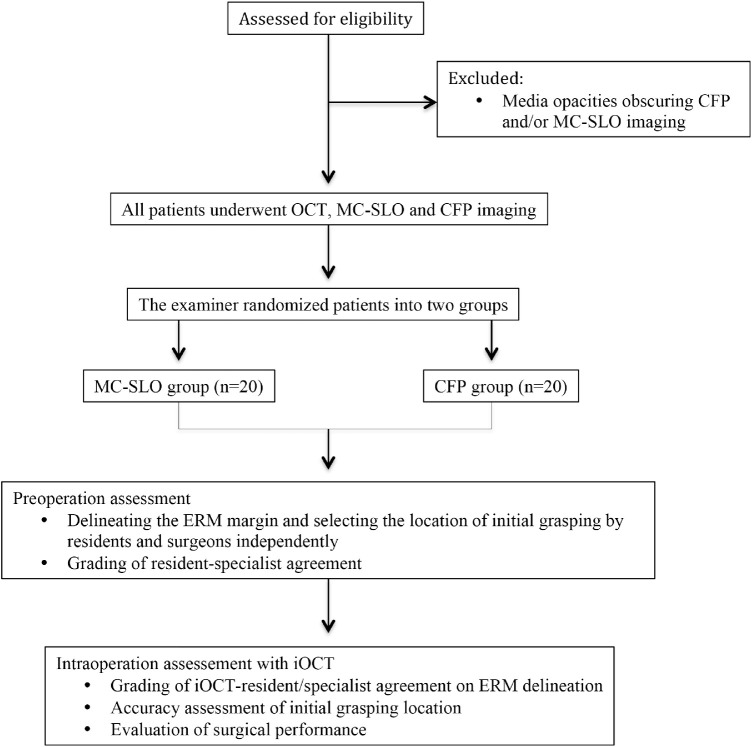
This study was approved by the Institutional Review Board of Zhongshan Ophthalmic Center, affiliated to Sun Yat-sen University (Guangzhou, China), and performed in accordance with the World Medical Association's Declaration of Helsinki. Written informed consent was obtained from each subject. Inclusion criteria were patients with idiopathic ERM confirmed by OCT (Spectralis OCT; Heidelberg Engineering) imaging and having symptoms of metamorphopsia and/or vision decrease. Idiopathic ERM was defined as the existence of irregular and hyperreflective bands above the inner retinal surface detected by at least one slice of the OCT macula-scanning image and having no associated ocular abnormality.15 Exclusion criteria were media opacities (significant cataracts or vitreous floaters) obscuring fundoscopy with CFP and/or MC-SLO. All eligible patients underwent comprehensive ophthalmologic examinations during the whole length of the study, including Snellen best-corrected visual acuity (BCVA), noncontact tonometry, slit-lamp microscopy, dilated fundus photography (MC-SLO and CFP), and OCT imaging (Fig. 1).
Figure 1.
Flowchart of the study. Eligible patients were randomized into MC-SLO and CFP groups. Preoperatively, residents and specialists delineated the ERM margins and select the initial grasping location in both MC-SLO and CFP groups, and were graded for resident–specialist agreement. Intraoperatively, assessments were made with iOCT for the iOCT–resident and iOCT–specialist agreement on ERM delineation, accuracy of initial grasping location, and surgical performance.
Retinal Imagings of OCT, MC-SLO, and CFP
Retinal imagings of OCT, MC-SLO, and CFP were obtained by a trained examiner (K.Y.) for each subject on the same day (1 day before the scheduled surgery). OCT and MC-SLO images were obtained simultaneously using the combined Spectralis OCT instrument (Heidelberg Engineering) with a resolution of 768 × 768 pixels and an angle of 30°. The system's built-in technologies (confocal scanning laser, active eye tracking, and noise reduction) were adapted to achieve high-quality MC-SLO images. CFP was obtained using a nonmydriatic fundus camera (VISUCAM 200; Carl Zeiss Meditec AG, Jena, Germany). A 30° image of the macula was obtained from each subject with the autofocus standard picture setting.
After OCT, MC-SLO, and CFP images were obtained, the examiner and an experienced ophthalmologist (Z.Z.) collaborated using the devices’ system-provided methods to moderately adjust the brightness and contrast of all obtained images for sufficient clarity. All images (merged MC-SLO ophthalmoscopy, CFP, and OCT) were then printed in color for the next step in the study assessment.
Patients’ Visual Perception of OCT, MC-SLO, and CFP
After obtaining the images and printing them, the examiner randomly displayed printed images to each related patient, providing a brief description of the examination results on each image. To assess the visual perception of the patients from each of the three images, patients completed a 5-point Likert scale with a single item for each image (i.e., The above image I am reading is informative enough and has definitely convinced me that my examined eye has ERM: 1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree).
Preoperative Decision-Making of ERM Margin and Where to Start the Initial Membrane Peeling
All eligible patients were randomized into two groups (groups MC-SLO and CFP) with a 1:1 ratio by the examiner (K.Y.). For patients in the MC-SLO group, only the MC-SLO images were provided to the residents (M.L. and Y.S., both with ERM surgical experience of less than 1 year) and experienced surgeons (Y.W. and S.Z., both with ERM surgical experience of more than 10 years). For patients in the CFP group, only the preoperative CFP images were provided to the residents and experienced surgeons.
The residents and experienced surgeons were required to delineate the whole ERM margin and decide where to start the initial membrane peeling on the printed images. The principle for the delineation of the ERM margin was to independently line out the ERM margin based on individual ERM demarcation experience as well as the change of color and texture on the printed MC-SLO or CFP printed images (Fig. 2). The principle for the residents and surgeons to decide where to start the initial membrane peeling was to independently select a specific point of the ERM margin where they considered the easiest to create the free edge for complete ERM peeling, also based on their experience of ERM peeling and the change of color and texture on the printed MC-SLO or CFP images (Fig. 3).
Figure 2.
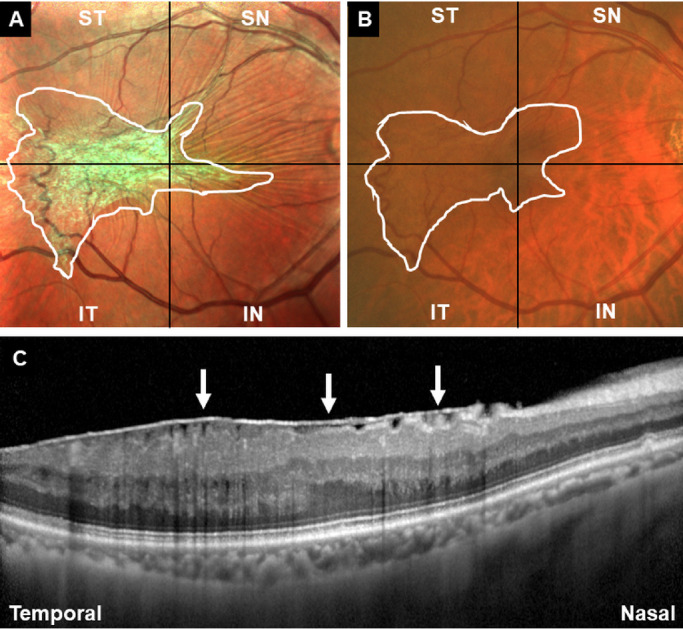
Representative case on how to have preoperative decision making of ERM demarcation in the MC-SLO group (A) and the CFP group (B). The residents and experienced surgeons were required to have delineation of the whole ERM margin based on the MC-SLO and CFP images (white lines in A and B). (C) OCT image required when having MC-SLO imaging supported the definite diagnosis of ERM (white arrows) in the patient.
Figure 3.
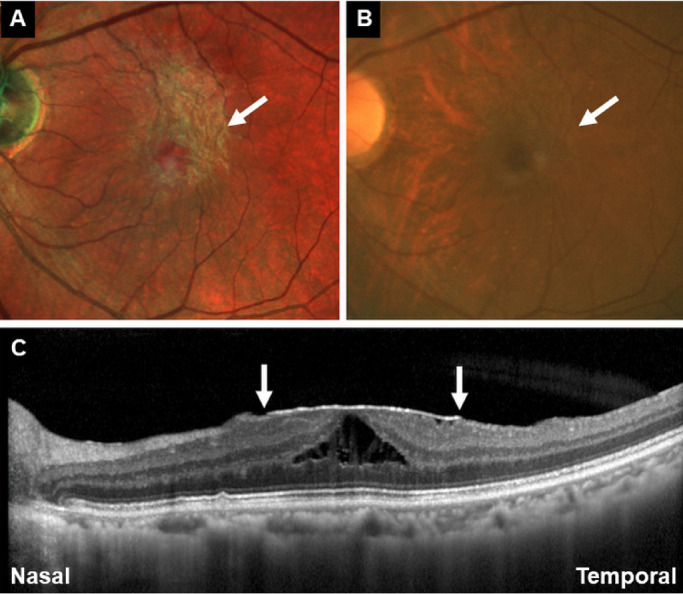
Representative case on how to have preoperative decision making of where to start the initial membrane peeling in the MC-SLO group (A) and the CFP group (B). The residents and experienced surgeons were required to decide where they considered the easiest to create the free edge for complete ERM peeling (white arrows in A and B). (C) OCT images were acquired when having MC-SLO imaging revealed the ERM margins (white arrows).
Because all the residents and surgeons had been habituated to peel off ERM with end-grasping forceps in a centripetal fashion, they were all apt to select the point where it seemed to be sharply and completely identifiable. The time cost (from when the color images were demonstrated to the participators until all the required answers were made) of the residents and surgeons to have delineation of ERM and make a decision as to where to have the initial grasping of ERM were recorded (in seconds), respectively.
After that, graders (K.Y. and L.W.) collected all the lined-out images to grade the interobserver (residents vs surgeons) agreement in terms of ERM delineation and selection of location of initial grasping. When the graders have different opinions, another grader with more experience (Z.Z.) was invited to resolve the conflict after a group discussion.
For interobserver agreement grading of ERM delineation, both MC-SLO and CFP images were subdivided into four quadrants (superotemporal, inferotemporal, superonasal, and inferonasal), centering on the central fovea of macula (Fig. 2). When the delineation of ERM from the residents and surgeons achieved significant agreement in one of the quadrants (nonoverlap distance in every 30° falling within one-half of the papillary diameter), the graders added one score into the table and finally summarized the interobserver agreement score from 0 to 4 (0 = no significant agreement achieved in any of the four quadrants; 4 = significant agreement achieved in all of the four quadrants). For interobserver agreement grading of the selection of initial grasping, the graders independently made a score from 1 to 3 (1 = definitely not nearby or the same location with the one decided by each other; 2 = not the same location but within the distance of the optic disc's diameter of the same eye; 3 = the same location with neglectable distance).
Intraoperative Agreement and Performance Assessment
Intraoperative OCT (CALLISTO eye; Carl Zeiss Meditec AG) was applied for the objective and definite judgement of ERM delineation and location selection of initial grasping. Standard three-port 27G pars plana vitrectomy (Constellation Vitrectomy System; Alcon Laboratories, Fort Worth, TX) and end-grasping forceps (Grieshaber Revolution DSP ILM forceps; Alcon Laboratories) were used in each subject to have vitreous removal and ERM peeling.
After vitreous cortex removal, iOCT was introduced to achieve as accurate as possible of a result from the residents and surgeons’ preoperative judgements based on the color print images. For delineation of ERM, the iOCT operator (Z.Z.) navigated the iOCT scanning along the track of delineation, which were outlined by the residents and surgeons, respectively. The correct ERM margin was defined as where there was more remarkable and obvious elevation of the retina's inner surface when compared with the nearby area when it was identified on iOCT.
In the same way as discussed elsewhere in this article, when the delineation of ERM from the residents and surgeons achieved significant agreement with iOCT in one of the quadrants, the graders added one score into the table and finally summarized the interobserver agreement score from 0 to 4 (0 = no significant agreement achieved in any of the four quadrants; 4 = significant agreement achieved in all of the four quadrants).
After the grading of interobserver agreement of ERM delineation. The surgeons (S.Z. and Y.W.) checked the accuracy of preoperative decision-making of the location of initial grasping of ERM. The iOCT operator (Z.Z.) navigated the iOCT scanning to the specific locations where were preoperatively selected by the residents and surgeons (Fig. 4). When the selected points differed, the iOCT operator and surgeons were required to verify the accuracy of both. The accuracy of location-selection was defined as true only when fulfill both of the two following items: (1) where there was most obvious and remarkable margin when compared with the nearby inner retina surface and (2) the surgeons were able to create a free edge for continuous ERM peeling after at most two attempts, and without the assistance of vital dye or obvious damage to the underlying retinal neurosensory layer.
Figure 4.
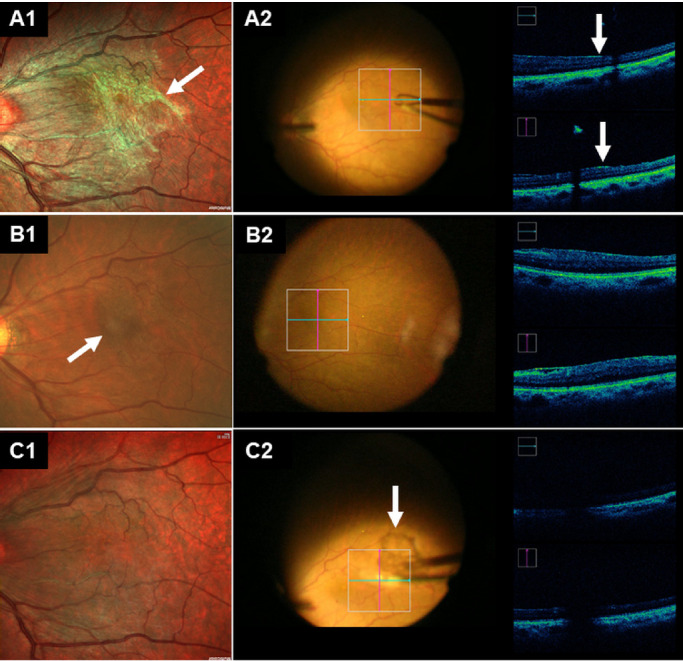
Representative case on how to have intraoperative agreement assessment in the MC-SLO group (A) and the CFP group (B). All the fundus photographs (A1, B1, and C1) have been rotated 180° to appear the same with the surgeons’ viewing under microscope when performing the ERM surgery. (A) In the MC-SLO group, the location to have initial peeling has been pointed out preoperatively (white arrow in A1), iOCT imaging revealed the selected location was true, as there was more remarkable and obvious elevation of the retina's inner surface (white arrows in A2). (B) In the CFP group, the location to have peeling has been pointed out preoperatively (white arrows in B1), iOCT imaging revealed the selected location was false, as there was no remarkable and obvious elevation of the retina's inner surface. (C) MC-SLO image taken 1 day postoperatively (C1) and iOCT imaging taken intraoperatively (C2) revealed complete peeling off of the ERM (arrows in C2).
Details of the surgery in both groups were recorded, including the total time cost of ERM peeling, extra use of vital dye (0.5% indocyanine green solution) to assist in the visualization of the ERM margin, the number of preretinal petechial hemorrhages, and iatrogenic retinal surface tear caused by the forceps when having the ERM peeled.
Follow-ups After Surgery
On the first day after the surgery, each patient was examined to confirm whether there were postoperative complications. If there were no serious postoperative complications, all the patients were discharged 1 day after the surgery. Follow-up examinations were scheduled at 1 week and 1 month after the surgery. If necessary, additional visits were scheduled.
Statistical Analysis
All Snellen visual acuity values were converted to the logarithm of the minimum angle of resolution for statistical analyses. All data were analyzed using the SPSS 22.0 statistical software (SPSS Inc., IBM, Somers, NY). A P value of less than 0.05 was considered statistically significant.
Results
General Characteristics of the Study Eyes
Forty patients (40 eyes) were included in the study, with 20 in the MC-SLO group and 20 in the CFP group. There was no statistically significant difference in terms of gender, age, BCVA, Gass grading, or lens status between the MC-SLO group and the CFP group (all P > 0.05). The general characteristics of patients in both groups are summarized in Table 1.
Table 1.
General Characteristics of Group MC-SLO (n = 20) and Group CFP (n = 20)
| MC-SLO Group | CFP Group | P Value | |
|---|---|---|---|
| Gender (male:female) | 9:11 | 7:13 | 0.748* |
| Age (years) | 63.3 ± 8.2 | 63.4 ± 7.9 | 0.984† |
| Preoperative logMAR BCVA | 0.55 ± 0.27 | 0.56 ± 0.21 | 0.608‡ |
| Gass grade | 0.445§ | ||
| 0 | 2 | 3 | |
| 1 | 7 | 10 | |
| 2 | 11 | 7 | |
| Lens status (phakic: pseudophakic) | 15: 5 | 17: 3 | 0.695* |
logMAR, logarithm of the minimal angle of resolution.
Fisher's exact test.
Unpaired t-test.
Unpaired Mann-Whitney U test.
χ2 analysis.
Preoperative Decision-Making
As for the time cost of delineation of ERM and decision of initial grasping location, the experienced surgeons were superior to the residents in both MC-SLO and CFP groups (both P < 0.001). Meanwhile, both the residents and specialists acted more effectively in the delineation of ERM and decision of initial grasping location in the MC-SLO group (both P < 0.001). Significantly higher resident–specialist agreement scores were achieved in the MC-SLO group in terms of ERM delineation (P = 0.002) and initial grasping location selection (P = 0.035).
Intraoperative Assessment and Performance
The introduction of iOCT revealed greater interobserver (iOCT–resident and iOCT–specialist) agreement scores for ERM delineation in the MC-SLO group (P < 0.001 and P = 0.027 for residents and specialists, respectively). The specialists achieved higher iOCT identified agreement scores than the residents in both the MC-SLO and CFP groups (P = 0.028 and P < 0.001, respectively).
Additionally, the iOCT revealed that the residents achieved greater accuracy in the selection of an initial grasping location in the MC-SLO group than in the CFP group (P = 0.025). Although without statistical significance (P = 0.451), the specialists achieved a higher ratio of selection accuracy in the MC-SLO group than in the CFP groups. There was no statically significant difference of selection accuracy between the residents and specialists in the MC-SLO group (P = 0.695). However, the residents achieved lower accuracy in the CFP group than the specialists did, although without statistical significance (P = 0.056).
In terms of intraoperative performance, the surgeons acted more effectively to have complete ERM peeled in the MC-SLO group than in the CFP group (P < 0.001). Meanwhile, the use of vital dye (indocyanine green) to assist ERM peeling was also lower than that in the CFP group, although without statistical significance (P = 0.182). There was no statistically significant difference in the number of petechial hemorrhages between the two groups (P = 0.231). However, the number of iatrogenic retinal surface tears seemed to be lower in the MC-SLO group (P = 0.031).
Postoperative Follow-up
Partial or complete anatomic resolution was observed in each of the included subjects at the last follow-up examinations (no less than 3 months after the initial surgery). No serious postoperative complications occurred during the follow-up period. Both the MC-SLO and CFP groups achieved significant improvement of logarithm of the minimum angle of resolution BCVA at the final follow-up (both P < 0.001). There was no statistically significant difference in the final logarithm of the minimum angle of resolution BCVA between the MC-SLO group and CFP group (P = 0.436). The main preoperative and intraoperative outcomes are summarized in Table 2.
Table 2.
Main Preoperative and Intraoperative Outcomes in Group MC-SLO (n = 20) and Group CFP (n = 20)
| MC-SLO Group | CFP Group | P Value | |
|---|---|---|---|
| Preoperative | |||
| Time to delineate ERM (sec) | |||
| Residents | 73.4 ± 19.4 | 146.0 ± 50.8 | <0.001* |
| Specialists | 50.6 ± 12.3 | 122.4 ± 46.2 | <0.001* |
| P value | <0.001† | <0.001† | |
| Time to decide initial grasping location (sec) | |||
| Residents | 46.2 ± 13.5 | 75.9 ± 24.1 | <0.001‡ |
| Specialists | 40.3 ± 10.3 | 58.0 ± 16.3 | <0.001‡ |
| P value | <0.001§ | <0.001§ | |
| Resident-specialist agreement score | |||
| Delineation of ERM | 3.2 ± 0.9 | 2.2 ± 1.0 | 0.002‡ |
| Initial grasping location | 2.1 ± 0.8 | 1.6 ± 0.7 | 0.035‡ |
| Intraoperative | |||
| Delineation agreement score | |||
| Residents | 3.2 ± 0.9 | 2.0 ± 1.0 | <0.001‡ |
| Specialists | 3.6 ± 0.6 | 3.1 ± 0.8 | 0.027‡ |
| P value | 0.028§ | <0.001§ | |
| Accuracy of initial grasping location selection | |||
| Residents (true:false) | 15:5 | 7:13 | 0.025¶ |
| Specialists (true:false) | 17:3 | 14:6 | 0.451¶ |
| P value | 0.695¶ | 0.056¶ | |
| ERM peeling duration (sec) | 576.0 ± 115.6 | 760.7 ± 159.7 | <0.001* |
| Vital dye usage (yes: no) | 1: 19 | 5: 15 | 0.182¶ |
| Number of petechial hemorrhages | 2.5 ± 1.9 | 3.3 ± 2,5 | 0.231‡ |
| Number of iatrogenic retinal surface damage | 0.9 ± 0.9 | 1.7 ± 1.2 | 0.031‡ |
| Final logMAR BCVA | 0.30 ± 0.17 | 0.34 ± 0.15 | 0.436* |
logMAR, logarithm of the minimal angle of resolution.
Unpaired t-test.
Paired t-test.
Unpaired Mann-Whitney U test.
Paired Mann-Whitney U test
Fisher's exact test.
Discussion
The current study revealed the ability of MC-SLO to help residents and surgeons have preoperative disease education and decision-making in ERM patients. It was also determined that MC-SLO might have the potential to enhance surgeons’ intraoperative performance by providing more exact visual information about the ERM demarcation. To the best of our knowledge, the study is the first to investigate the potential use of MC-SLO in the surgical management of ERM. Additionally, iOCT was introduced into the evaluation system to strengthen objectivity of the current study.
MC-SLO Enables Higher Efficiency and Resident–Specialist Agreement in Preoperative ERM Delineation
In the current study, both residents (P < 0.001) and specialists (P < 0.001) acted more effectively in ERM delineation (Fig. 1) in the MC-SLO group. Meanwhile, the specialists were superior to the residents in both MC-SLO (P < 0.001) and CFP (P < 0.001) groups. A recent study by Terasaki et al.15 found that MC-SLO could be used for more effective and efficient screening for the presence of an ERM than CFPs. Consistent with that finding, the current study revealed that MC-SLO could provide clearer visual information to residents and specialists when they were involved in the preoperative decision-making.
MC-SLO Helps Residents to Improve Their Accuracy in Selecting Initial ERM Grasping Locations
As we know, there are several advantages of examining ERM with MC-SLO compared with CFP. MC-SLO can be performed through undilated pupils and is less affected by media opacities.17 MC-SLO is better able to be detect and precisely demarcate ERM.20,21 More important, MC-SLO was superior on presenting the retinal folds created by ERM, primarily owing to the green channel present in the combination-pseudocolor en face image.10,14 For this characteristic of MC-SLO, the pattern of ERM can be clearly viewed not only in the plane, but also stereoscopically. Therefore, we performed MC-SLO preoperatively to help determine the initial grasping locations (Fig. 2), which is critical for the peeling process in the surgery. We then compared the accuracy of the selection between using MC-SLO and CFP (Fig. 3) in both the residents’ and the specialists’ groups.
With clear information from MC-SLO, residents overcame the experience barrier, made objective and correct judgements preoperatively, and thus shortened the learning curve for this surgery. The accuracy of the residents was comparable with that of the specialists when using MC-SLO (P = 0.695), and was significantly higher than when using CFP (P = 0.025). However, the difference of the accuracy between using MC-SLO and CFP was not significant in specialists (P = 0.451). It is worth mentioning that there were certain false decisions in both residents and specialists groups when using MC-SLO. In these cases, the stereoscopic changes of ERM were not prominent, that is, flat ERMs, for which the starting points needed to be judged during the peeling process.
MC-SLO Helps Surgeons to Improve Their Intraoperative Performance
By performing MC-SLO preoperatively, intraoperative performance was improved, although the finial BCVA was not significantly different (Table 2). The ERM peeling duration was significantly shorter (P < 0.001), and there was significantly less iatrogenic retinal surface tear (P = 0.031). Moreover, there was less vital dye usage intraoperatively (1:19 in the MC-SLO group vs. 5:15 in the CFP group; P = 0.182), and fewer petechial hemorrhages (2.5 ± 1.9 in the MC-SLO group vs. 3.3 ± 2.5 in CFP group; P = 0.231); however, these differences were not significant, probably owing to the limited sample size. These improvements might have affected visual qualities, which could be investigated further by using microperimetry, multifocal electroretinograms, and other methods.
The Advantages of MC-SLO in the Management of ERM Surgery Compared with iOCT and En Face OCT
Because iOCT allows tomographic visualization of the ERM during peeling, it enables peeling without staining in 31% to 63% of cases.22,23 Moreover, the real-time visual feedback of iOCT enhances surgical performance, for example, by showing retinal traction during peeling.16,18,19,24–29 Nonetheless, iOCT is relatively new for us, and, owing to its high cost, it would not be always available in some of institutions. In addition, the edge of ERM has to be analyzed by continuous iOCT video in a cross-sectional view for peeling to start, whereas it could be detected with just one shot on MC-SLO with an en face view. For this reason, we believed that MC-SLO is an effective and efficient substitution for iOCT in the ERM surgery.
En face OCT could reveal the retinal folds as radiating striae in black and white,30,31 that is, in a two-dimensional view. However, the thickness landscape of ERM, which is an important indicator for the grasping location in the surgery, could only be observed on MC-SLO by the combination-pseudocolor image.
Strengths, Limitations, and Prospects
The strengths of this study include the perspective study design, objective evaluation system, high-quality imaging, and experienced surgeons. Limitations include the limited sample size, the lack of long-term follow-up, and a single evaluation index for the postoperative visual function. Nevertheless, this study is the first to investigate the potential use of MC-SLO in the surgical management of ERM. Our data support that MC-SLO is an effective and efficient tool for the ERM surgery, provided a better visual reference for residents and specialists in ERM delineation and selection of an initial grasping location, and helped surgeons to achieve better intraoperative performance.
Acknowledgments
All the authors have no proprietary interest with the study. The present manuscript has been read and approved by all the authors. Zhaotian Zhang and Miaoling Li contributed equally to the article.
Disclosure: Z. Zhang, None; M. Li, None; Y. Sun, None; Y. Wei, None; S. Zhang, None
References
- 1. Wise GN. Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am J Ophthalmol. 1975; 79: 349–347. [DOI] [PubMed] [Google Scholar]
- 2. Wickham L, Konstantinidis L, Wolfensberger TJ. Epiretinal membranes, vitreoretinal traction, and cystoid macular edema. In: Schachat AP, (Eds.) Ryan's Retina, 6th ed. Amsterdam: Elsevier; 2018: 2194–2212. [Google Scholar]
- 3. Stevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol. 2016; 10: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sandali O, El Sanharawi M, Basli E, et al.. Epiretinal membrane recurrence: incidence, characteristics, evolution, and preventive and risk factors. Retina. 2013; 33: 2032–2038. [DOI] [PubMed] [Google Scholar]
- 5. Dawson SR, Shunmugam M, Williamson TH. Visual acuity outcomes following surgery for idiopathic epiretinal membrane: an analysis of data from 2001 to 2011. Eye (London, England). 2014; 28: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson TH. Macular pucker and vitreomacular traction. In: Williamson TH, (ed.) Vitreoretinal Surgery, 2nd ed. Berlin: Springer; 2008: 239–254. [Google Scholar]
- 7. Yadav H. Epiretinal membranes. In: Narendran V, Kothari AR, (Eds.) Principles and Practice of Vitreoretinal Surgery, 1st ed. New Delhi: Jaypee Brothers; 2014: 423–428. [Google Scholar]
- 8. Kuhn F. Working with membranes. In: Kuhn F. (Ed.) Vitreoretinal Surgery: Strategies and Tactics, 1st ed. Berlin: Springer; 2016: 283–316. [Google Scholar]
- 9. Ben Moussa N, Georges A, Capuano V, Merle B, Souied EH, Querques G. MultiColor imaging in the evaluation of geographic atrophy due to age-related macular degeneration. Br J Ophthalmol. 2015; 99: 842–847. [DOI] [PubMed] [Google Scholar]
- 10. Feng Henry L, Sharma S, Stinnett S, Asrani S, Mruthyunjaya P. Identification of posterior segment pathology with en face retinal imaging using multicolor confocal scanning laser ophthalmoscopy. Retina. 2019; 39: 972–979. [DOI] [PubMed] [Google Scholar]
- 11. Terasaki H, Sonoda S, Kakiuchi N, Shiihara H, Yamashita T, Sakamoto T. Ability of multicolor scanning laser ophthalmoscope to detect non-glaucomatous retinal nerve fiber layer defects in eyes with retinal diseases. BMC Ophthalmol. 2018; 18: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaprinis K, Bobat H, De Salvo G. Multicolor imaging in combined hamartoma of the retina and retinal pigment epithelium. Eye (London, England). 2018; 32: 1478–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saurabh K, Roy R, Sinharoy S, Shah D, Nangia P. Measurement of size of pigmented choroidal nevus: superiority of multicolor imaging compared to conventional color fundus photography. Indian J Ophthalmol. 2018; 66: 1501–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kilic Muftuoglu I, Bartsch DU, Barteselli G, Gaber R, Nezgoda J, Freeman WR. Visualization of macular pucker by multicolor scanning laser imaging. Retina. 2018; 38: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terasaki H, Sonoda S, Shiihara H, et al.. More effective screening for epiretinal membranes with multicolor scanning laser ophthalmoscope than with color fundus photographs. Retina. 2020; 40: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 16. Leisser C, Hackl C, Hirnschall N, et al.. Visualizing macular structures during membrane peeling surgery with an intraoperative spectral-domain optical coherence tomography device. Ophthalmic Surg Lasers Imaging Retina. 2016; 47: 328–332. [DOI] [PubMed] [Google Scholar]
- 17. Tan AC, Fleckenstein M, Schmitz-Valckenberg S, Holz FG. Clinical application of multicolor imaging technology. Ophthalmologica. 2016; 236: 8–18. [DOI] [PubMed] [Google Scholar]
- 18. Ehlers JP, Modi YS, Pecen PE, et al.. The DISCOVER Study 3-year results: feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery. Ophthalmology. 2018; 125: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ung C, Miller JB.. Intraoperative optical coherence tomography in vitreoretinal surgery. Semin Ophthalmol. 2019; 34: 312–316. [DOI] [PubMed] [Google Scholar]
- 20. Huo YJ, Yang LL, Wei WB. Diagnostic performance of laser retinal imaging in the epiretinal membrane. Zhonghua Yan Ke Za Zhi. 2016; 52: 836–839. [DOI] [PubMed] [Google Scholar]
- 21. Song JH, Moon KY, Jang S, Moon YR. Comparison of multicolor fundus imaging and colour fundus photography in the evaluation of epiretinal membrane. Acta Ophthalmol. 2019; 97: e533–e539. [DOI] [PubMed] [Google Scholar]
- 22. Falkner-Radler CI, Glittenberg C, Gabriel M, Binder S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina. 2015; 35: 2100–2106. [DOI] [PubMed] [Google Scholar]
- 23. Leisser C, Hirnschall N, Palkovits S, Doeller B, Kefer K, Findl O. Intraoperative optical coherence tomography-guided membrane peeling for surgery of macular pucker: advantages and limitations. Ophthalmologica. 2019; 241: 234–240. [DOI] [PubMed] [Google Scholar]
- 24. Leisser C, Hirnschall N, Hackl C, et al.. Risk factors for postoperative intraretinal cystoid changes after peeling of idiopathic epiretinal membranes among patients randomized for balanced salt solution and air-tamponade. Acta Ophthalmol. 2018; 96: e439–e444. [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez-Cortes JH, Estudillo AR, Sanchez-Ramos JA, Bages-Rousselon Y, Fernandez-Mar M, Mohamed-Hamsho J. Anatomical changes of full-thickness macular hole documented by microscope-integrated spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2018; 49: e105–e111. [DOI] [PubMed] [Google Scholar]
- 26. Tao J, Wu H, Chen Y, et al.. Use of iOCT in vitreoretinal surgery for dense vitreous hemorrhage in a Chinese population. Current Eye Res. 2019; 44: 219–224. [DOI] [PubMed] [Google Scholar]
- 27. Lytvynchuk LM, Falkner-Radler CI, Krepler K, et al.. Dynamic intraoperative optical coherence tomography for inverted internal limiting membrane flap technique in large macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2019; 257: 1649–1659. [DOI] [PubMed] [Google Scholar]
- 28. Agarwal A, Gupta V.. Intraoperative optical coherence tomography and proportional reflux hydrodissection-guided pars plana vitrectomy for complex severe proliferative diabetic retinopathy. Indian J Ophthalmol. 2020; 68: 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorusso M, Micelli Ferrari L, Cicinelli MV, et al.. Feasibility and safety of intraoperative optical coherence tomography-guided short-term posturing prescription after macular hole surgery. Ophthalmic Res. 2020; 63: 18–24. [DOI] [PubMed] [Google Scholar]
- 30. Pavlidis M, Georgalas I, Körber N. Determination of a new parameter, elevated epiretinal membrane, by en face oct as a prognostic factor for pars plana vitrectomy and safer epiretinal membrane peeling. J Ophthalmol. 2015; 2015: 838646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bae K, Choi JH, Kim KT, Kang SW. En-face optical coherence tomography in patients with epiretinal membrane: intuitive method for predicting functional outcomes. Retina. 2019; 40: 1972–1979. [DOI] [PubMed] [Google Scholar]