Abstract
Purpose
Whether the association between diabetic kidney disease (DKD) and diabetic retinopathy (DR) in patients with type 2 diabetes mellitus (T2DM) is leveraged by anemia remains unclear. This study is to evaluate the joint effect of DKD and anemia on DR.
Methods
Data were collected from electronic medical records of 1389 patients with T2DM in the Yiwu Central Hospital of Zhejiang Province from 2018 to 2019. Based on retinal examination findings, patients were classified as without diabetic retinopathy (non-DR), non-proliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR). Odds ratio (OR) from multinomial logistic regression models adjusting for potential risk factors of DR were used to evaluate associations of DKD, renal function measures, and anemia with risk of NPDR and PDR. Path analysis was performed to help understand the association of DKD and hemoglobin (Hb) with DR.
Results
The study included 901 patients with non-DR, 367 patients with NPDR and 121 patients with PDR. Both high DKD risk and abnormal renal function measures were significantly associated with PDR. Anemia was associated with increased risk of NPDR (OR = 1.75, 95% confidence interval [CI] = 1.18–2.58) and PDR (OR = 3.71, 95% CI = 2.23–6.18). DKD severity and anemia had joint effect on NPDR (OR = 2.29, 95% CI = 1.32–3.96) and PDR (OR = 11.31, 95% CI = 5.95–21.51). These associations were supported by path analysis.
Conclusions
DKD severity, abnormal estimated glomerular filtration rate (eGFR), and urinary albumin/creatinine ratio (UACR) were associated with increased risk of DR in patients with T2DM, and anemia had joint effect on these associations. Improving Hb level may decrease the risk of DR in patients with T2DM.
Keywords: diabetic retinopathy, kidney disease, anemia, hemoglobin, joint effect
Diabetic retinopathy (DR) is one of the most common microvascular complications of type 2 diabetes mellitus (T2DM). The DR commonly progresses from mild non-proliferative diabetic retinopathy (NPDR) to severe NPDR, and even to proliferative diabetic retinopathy (PDR),1 which can lead to vision loss and blindness. At present, glycated hemoglobin (HbA1c), chronic hyperglycemia, body mass index (BMI), and systolic blood pressure (SBP) have been well recognized as significant modifiable risk factors for DR.2
Patients with T2DM are also frequently accompanied by diabetic kidney disease (DKD). Previous studies have reported the association between DKD and DR in patients with T2DM.3–5 However, the pathological link between the two microvascular complications has not been well established. For instance, urinary albumin/creatinine ratio (UACR) was significantly associated with DR risk among patients with T2DM, but the association of estimated glomerular filtration rate (eGFR) was not observed.6 It is generally known that an absolute or relative decrease of erythropoietin (EPO) induced by renal dysfunction was a key factor contributing to the development of anemia in patients with chronic kidney disease (CKD).7 A few studies have found that anemia is an independent risk factor for microvascular complications and cardiovascular disease in patients with T2DM.8–11 Therefore, we hypothesized that anemia may be a potential driving factor to DR ensue DKD. To test this hypothesis, we evaluated the independent effect of anemia and renal function on DR, and tested whether anemia and renal function had joint effect on DR. These analyses might provide useful information for the prevention and management of DR.
Materials and Methods
Participants
This is a retrospective cross-section study. The data were extracted from the electronic medical records of patients with T2DM admitted between 2018 and 2019 to Yiwu Central Hospital, Zhejiang Province, China. Inclusion criteria of the study were: (1) a diagnosis of T2DM; (2) a fundoscopic examination for DR performed by an experienced retinal ophthalmologist; and (3) data of blood and urine tests were available. Exclusion criteria included: (1) BMI > 40 kg/m2 or BMI < 15 kg/m2; (2) severe hypertension: SBP > 200 mm Hg or diastolic blood pressure (DBP) > 120 mm Hg; and (3) severe dyslipidemia: total cholesterol > 10 mmol/L or triglyceride > 15 mmol/L. The study has been approved by the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Definitions and Relevant Variable Management
The diagnosis of DR was made by an experienced retinal ophthalmologist after a dilated fundus examination according to the guidelines of the International Council of Ophthalmology (ICO).12,13 NPDR was defined as microaneurysms, intraretinal hemorrhages, hard exudates, cotton-wool spots, or macular edema but no evidence of retinal or iris neovascularization. Proliferative DR (PDR) was defined as neovascularization on the optic disc, retina, or iris, with or without vitreous hemorrhage or prior pan-retinal photocoagulation. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg, or current use of antihypertensive medications.14 Hyperlipidemia was defined as cholesterol > 5.5 mmol/L, or triglyceride > 2.0 mmol/L, or current use of lipid-lowering medications.15 Based on the standard in China, anemia was defined as hemoglobin (Hb) levels ≤ 120 g/L for men and ≤ 110 g/L for women.16 To explore the dose-response association between Hb levels and DR, Hb was categorized into four levels (< 120 g/L, 120 to approximately 139.9 g/L, 140 to approximately 159.9 g/L, and ≥ 160 g/L) in males and four levels (< 110 g/L, 110 to approximately 119.9 g/L, 120 to approximately 139.9 g/L, and ≥ 140 g/L) in females, respectively. Risks of DKD were stratified into four risk groups according to the Kidney Disease: Improving Global Outcomes (KDIGO) Guideline based on eGFR and albuminuria: no DKD or low risk, moderate risk, high risk, very high risk.17 The degree of albuminuria was classified as follows: normal to mild degree (UACR < 30 mg/g), moderate degree (30 mg/g ≤ UACR ≤ 300 mg/g), and severe degree (UACR > 300 mg/g). The eGFR category was classified into six groups: normal, mildly decreased, mildly to moderately decreased, moderately to severely decreased, severely decreased, and kidney failure (≥ 90, 60–89.9, 45–59.9, 30–44.9, 15–29.9, and <15 mL/min/1.73 m2). Detailed methodology is presented in Supplementary Figure S1. We combined high risk and very high risk into the high/very high group and combined no DKD or low risk and moderately increased risk into the low/medium group owing to small sample sizes and similar effect to DR. Biochemical analysis were conducted at the Yiwu Central Hospital, Zhejiang Province, China, using an automated chemistry analyzer (Architect c16000 analyzer; Abbott, Tokyo, Japan).
In this study, there were some missing data in BMI (16.4%) and UACR (8.2%), and less than a few missing data on Hb, HbA1c, and eGFR (all < 0.3%). In the statistical analysis, subjects with missing data on Hb, HbA1c, or eGFR (n = 7) were excluded from analysis. For the missing data of BMI and UACR, we performed multiple imputations of missing data based on predictive mean matching (PMM) by the MICE package in R software.18 In these imputations, UACR was log-transformed to achieve normality before imputation, and the multiple imputation models included age, sex, duration of diabetes, BMI, hypertension, hyperlipidemia, SBP, HbA1c, serum creatinine (SCr), UACR, total cholesterol, and high-density lipoprotein cholesterol (HDL-C) as predictors.
Statistical Analysis
Patients characteristics were presented as means ± standard deviation (SD) or medians (Q1 and Q3) for continuous variables and as frequency (n) and percentages (%) for categorical variables. Differences in the distribution of categorical variables among the three groups (non-DR, NPDR, and PDR) were compared using the χ2 test for categorical variables and using the Kruskal-Walls test for skewed continuous data or analysis of variance (ANOVA) for normal distributed data. Multinomial logistic regression models were used to assess associations of anemia and DKD with DR. All logistic regression models were adjusted for the potential confounders: age, gender, duration of diabetes, BMI, HbA1c, hypertension, hyperlipidemia, and medication on diabetes.
Path analysis, an extension of regression analysis, was also conducted to explore the association of anemia and renal function measures with DR. Based on the findings from previous research and the hypothesis of this study, six independent variables were included in the original path analysis model, including HbA1c, mean arterial pressure (MAP), Hb, UACR, eGFR, and DR. For the path analysis, UACR with abnormal distribution was log-transformed to achieve normality. The values were standardized using the method of Z-score before path analysis. Path analyses included four regression equations (DR ∼ Hb + UACR + MAP; Hb ∼ UACR + eGFR + HbA1c; UACR ∼ HbA1c + MAP; and eGFR ∼ HbA1c) using the weighted least squares mean and variance adjusted (WLSMV) estimator with the “lavaan” package in R software. For evaluating goodness of model fitness, several different fit indices were considered: root mean square error of approximation (RMSEA), standardized root mean square residual (SRMR), Goodness of Fit Index (GFI), Adjusted Goodness of Fit Index (AGFI), Non-Normed Fit Index (NNFI), and the Comparative Fit Index (CFI). The model was judged as having a good fit when RMSEA < 0.10, CFI, GFI, AGFI, and NNFI all were > 0.90, and SRMR was < 0.90.19
The results from multiple imputation analysis were pooled into a final point estimate plus standard error by Rubin's rules (Supplementary Fig. S2).18 As sensitivity, statistical analyses of the observed data (without imputation) were also performed (Supplementary Table S4 and Supplementary Table S5).
R software (version 3.4.3) was used for all analyses and P < 0.05 was considered statistically significant.
Results
Patients Characteristics
The study included 1410 patients with T2DM, after excluding 7 patients with T2DM with missing data on Hb, HbA1c, or eGFR and 14 patients with abnormal BMI, extremely severe hypertension, or severe dyslipidemia, a total of 1389 patients were included into analysis, including 901 (64.9%) with non-DR, 367 (26.4%) with NPDR, and 121 (8.7%) with PDR. Characteristics of patients among the three groups significantly differed in gender, duration of diabetes, medication on diabetes, blood pressure, total cholesterol, Hb, blood urea nitrogen (BUN), eGFR, SCr, and UACR (all P < 0.05; Table 1). The prevalence of anemia was significantly higher in the PDR group than the NPDR and non-DR groups (34.7%, 16.6%, and 9.9%, respectively, P < 0.001). The proportion of high/very high DKD risk also were significantly higher in the PDR group than that in the NPDR and non-DR groups (47.1%, 22.9%, and 17.9%, P < 0.001). There was no significant difference in age, BMI, HbA1c, triglyceride, HDL-C, LDL-C, or the history of hyperlipidemia or hypertension among the three groups (see Table 1).
Table 1.
Characteristics of Patients with T2DM by the Status of DR (n = 1389)
| Non-DR | NPDR | PDR | ||
|---|---|---|---|---|
| Variables | (n = 901) | (n = 367) | (n = 121) | P Value* |
| Mean ± SD/median (Q1, Q3) | ||||
| Age, y | 59.7 ± 12.9 | 60.6 ± 11.4 | 58.9 ± 10.5 | 0.348 |
| BMI, kg/m2 | 24.6 ± 3.5 | 24.9 ± 3.4 | 24.6 ± 3.2 | 0.533 |
| Duration of diabetes, y | 8.3 ± 6.4 | 12.1 ± 6.4 | 13.9 ± 7.9 | <0.001 |
| SBP, mm Hg | 130.6 ± 18.1 | 136.5 ± 19.0 | 144.8 ± 22.8 | <0.001 |
| DBP, mm Hg | 78.7 ± 10.9 | 79.7 ± 10.4 | 82.2 ± 12.7 | 0.003 |
| MAP, mm Hg | 96.0 ± 11.6 | 98.6 ± 11.6 | 103.1 ± 14.9 | <0.001 |
| Total cholesterol, mmol/L | 4.1 ± 1.1 | 4.0 ± 1.1 | 4.5 ± 1.4 | 0.001 |
| Triglyceride, mmol/L | 1.4 (0.9, 2.1) | 1.4 (1.0, 2.0) | 1.4 (1.1, 2.2) | 0.557 |
| HDL-C, mmol/L | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.486 |
| LDL-C, mmol/L | 2.2 ± 0.9 | 2.2 ± 0.8 | 2.4 ± 0.9 | 0.080 |
| HbA1c, % | 9.2 ± 2.5 | 9.4 ± 2.2 | 9.5 ± 2.3 | 0.308 |
| Hb, g/L | 134.6 ± 18.2 | 129.0 ± 18.5 | 123.6 ± 19.3 | <0.001 |
| SCr, µmol/L | 63.2 (53.8, 75.9) | 63.3 (53.1, 76.7) | 68.0 (57.3, 90.7) | 0.004 |
| BUN, mmol/L | 5.7 ± 1.8 | 6.0 ± 2.0 | 6.5 ± 2.2 | <0.001 |
| eGFR, mL/min/1.73m² | 122.2 ± 34.6 | 118.6 ± 36.6 | 106.9 ± 38.5 | <0.001 |
| UACR, mg/g, n (%) | 69.0 (34, 175) | 87.0 (44.0, 265.5) | 218.0 (57.0, 1127.0) | <0.001 |
| Gender: male | 520 (57.7) | 177 (48.2) | 63 (52.1) | 0.007 |
| Medication on diabetes | <0.001 | |||
| No | 91 (10.1) | 13 (3.5) | 4 (3.3) | |
| Oral medication | ||||
| Combine insulin positive | 496 (55.1) | 152 (41.4) | 41 (33.9) | |
| Oral medication | 314 (34.9) | 202 (55.0) | 76 (62.8) | |
| Hypertension | 432 (48.0) | 181 (49.3) | 69 (57.0) | 0.171 |
| Hyperlipidemia | 192 (21.3) | 62 (16.9) | 21 (17.4) | 0.157 |
| Anemia | 89 (9.9) | 61 (16.6) | 42 (34.7) | <0.001 |
| DKD severity | <0.001 | |||
| Low/medium | 740 (82.1) | 283 (77.1) | 64 (52.9) | |
| High/very high | 161 (17.9) | 84 (22.9) | 57 (47.1) | |
DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated hemoglobin; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio. Values in boldface type indicate statistical significance.
P value was estimated using χ2 for comparing proportions, ANOVA for comparing means, and the Kruskal-Walls test for comparing medians.
Associations of DKD Severity and Renal Function Measures with DR
In the multivariate analysis, severity of DKD was significantly associated with an increased risk of PDR (adjusted OR = 2.80, 95% CI = 1.78–4.42), but were not associated with the risk of NPDR (adjusted OR = 1.11, 95% CI = 0.80–1.55). Similarly, abnormal UACR (> 300 mg/g) and eGFR (< 60 mL/min/1.73m²) were significantly associated with an increased risk of PDR (adjusted OR = 2.93, 95% CI = 1.86–4.63; adjusted OR = 3.09, 95% CI = 1.43–6.70), but was not associated with the risk of NPDR (adjusted OR = 1.18, 95% CI = 0.84–1.65; and adjusted OR = 1.12, 95% CI = 0.55–2.26; Table 2).
Table 2.
Associations of Anemia and Renal Function Measures with the Risk of DR* (n = 1389)
| NPDR (n = 367) | PDR (n = 121) | ||||||
|---|---|---|---|---|---|---|---|
| Variables† | N | n (%) | Adjusted OR (95% CI)‡ | P Value | n (%) | Adjusted OR (95% CI)‡ | P Value |
| Anemia§ | |||||||
| − | 1197 | 306 (25.6) | Ref | 79 (6.6) | Ref | ||
| + | 192 | 61 (31.8) | 1.75 (1.18, 2.58) | 0.005 | 42 (21.9) | 3.71 (2.23, 6.18) | <0.001 |
| DKD severityǁ | |||||||
| Low/medium | 1087 | 283 (26.0) | Ref | 64 (5.9) | Ref | ||
| High/very high | 302 | 84 (27.8) | 1.11 (0.80, 1.55) | 0.524 | 57 (18.9) | 2.80 (1.78, 4.42) | <0.001 |
| eGFRǁ | |||||||
| ≥ 60 | 1330 | 351 (26.4) | Ref | 103 (7.7) | Ref | ||
| < 60 | 59 | 16 (27.1) | 1.12 (0.55, 2.26) | 0.758 | 18 (30.5) | 3.09 (1.43, 6.70) | 0.004 |
| UACRǁ | |||||||
| ≤ 300 | 1100 | 285 (25.9) | Ref | 65 (5.9) | Ref | ||
| > 300 | 289 | 82 (28.4) | 1.18 (0.84, 1.65) | 0.337 | 56 (19.4) | 2.93 (1.86, 4.63) | <0.001 |
DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio.
These 4 variables were analyzed separately.
Units of variables: eGFR in mL/min/1.73m²; UACR in mg/g.
Adjusted for age, sex, duration of diabetes, BMI, HbA1c, hypertension, hyperlipidemia, and medication on diabetes.
Additionally adjusted for DKD.
Additionally adjusted for anemia.
The Dose-Response Association Between Hb Levels and DR
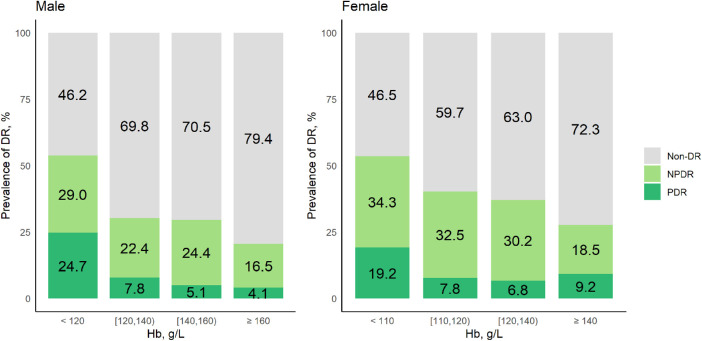
Figure 1 presents the DR prevalence at five Hb levels in male and female patients with T2DM, respectively. Higher prevalence of NPDR and PDR were observed in both males and females patients with anemia. The proportions of PDR were 24.7% in men with Hb levels < 120 g/L and 19.2% in women with Hb levels < 110 g/L. For men with Hb levels between 120 g/L and 140 g/L and women with Hb levels between 110 g/L and 120 g/L, the proportion of PDR was 7.8%. In multivariate analysis (see Table 2), anemia was significantly associated with an increased risk of NPDR (OR = 1.75, 95% CI = 1.18–2.58) and PDR (OR = 3.71, 95% CI = 2.23–6.18).
Figure 1.
Prevalence of DR at five different levels of Hb.
The Joint Effect of Anemia and DKD Severity on the Risk of DR
To evaluate the joint effect of anemia and DKD severity on the risk of DR, we grouped patients into 4 groups based on the status of the 2 variables, including 992 with low or medium DKD risk and normal Hb levels, 205 with high or very high DKD risk only, 95 with anemia only, and 97 with both high or very high DKD risk and anemia. The prevalence rate of PDR (30.9%) was highest among patients with both high or very high DKD risk and anemia, which was much higher than that in patients with high or very high DKD risk only (13.2%), with anemia only (12.6%), and with low or medium DKD risk and normal Hb levels (5.2%). In multivariate analysis, compared with patients with low or medium DKD risk and normal Hb levels, patients with high or very high DKD risk and normal Hb levels had an increased risk of PDR (OR = 2.71, 95% CI = 1.59–4.63, P < 0.001) and patients with low or medium DKD risk and anemia also had an increased risk of PDR (OR = 3.49, 95% CI = 1.68–7.23, P = 0.001), whereas patients with both high or very high DKD risk and anemia had significantly higher risk of PDR (OR = 11.59, 95% CI = 6.12–21.95, P < 0.001) and NPDR (OR = 2.38, 95% CI = 1.38–4.09, P = 0.002). It suggests their joint effect on PDR. The similar joint association were observed between eGFR or UACR and anemia (detailed in Table 3).
Table 3.
The Joint Effects of Anemia and Renal Function Measures with the Risk of DR* (n = 1389)
| NPDR (n = 367) | PDR (n = 121) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables† | N | n (%) | Adjusted OR (95% CI)‡ | P Value§ | n (%) | Adjusted OR (95% CI)‡ | P Value§ | |
| DKD | Anemia | |||||||
| Low/medium | − | 992 | 256 (25.8) | Ref | 52 (5.2) | Ref | ||
| High/very high | − | 205 | 50 (24.4) | 0.99 (0.68, 1.45) | 0.954 | 27 (13.2) | 2.71 (1.59, 4.63) | <0.001 |
| Low/medium | + | 95 | 27 (28.4) | 1.42 (0.85, 2.35) | 0.181 | 12 (12.6) | 3.49 (1.68, 7.23) | 0.001 |
| High/very high | + | 97 | 34 (35.1) | 2.38 (1.38, 4.09) | 0.002 | 30 (30.9) | 11.59 (6.12, 21.95) | <0.001 |
| P for trend | 0.003 | <0.001 | ||||||
| eGFR | Anemia | |||||||
| ≥ 60 | − | 1174 | 301 (25.6) | Ref | 75 (6.4) | Ref | ||
| < 60 | − | 23 | 5 (21.7) | 0.90 (0.30, 2.66) | 0.845 | 4 (17.4) | 3.35 (0.98, 11.45) | 0.054 |
| ≥ 60 | + | 156 | 50 (32.1) | 1.71 (1.14, 2.57) | 0.010 | 28 (18.0) | 4.27 (2.49, 7.32) | <0.001 |
| < 60 | + | 36 | 11 (30.6) | 2.24 (0.92, 5.48) | 0.076 | 14 (38.9) | 13.33 (5.29, 33.57) | <0.001 |
| P for trend | 0.003 | <0.001 | ||||||
| UACR | Anemia | |||||||
| ≤ 300 | − | 1002 | 256 (25.6) | Ref | 52 (5.2) | Ref | ||
| > 300 | − | 195 | 50 (25.6) | 1.10 (0.75, 1.61) | 0.640 | 27 (13.9) | 2.98 (1.75, 5.10) | <0.001 |
| ≤ 300 | + | 98 | 29 (29.6) | 1.53 (0.93, 2.52) | 0.095 | 13 (13.3) | 3.80 (1.87, 7.74) | <0.001 |
| > 300 | + | 94 | 32 (34.0) | 2.29 (1.32, 3.96) | 0.003 | 29 (30.9) | 11.31 (5.95, 21.51) | <0.001 |
| P for trend | 0.002 | <0.001 | ||||||
DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio.
The 4 variables were analyzed separately.
Units of variables: SCr in µmol/L; BUN in mmol/L; eGFR in mL/min/1.73m²; and UACR in mg/g.
Adjusted for age, sex, duration of diabetes, BMI, HbA1c, hypertension, hyperlipidemia, and medication on diabetes.
P for multiplicative interaction > 0.05.
Path Analysis
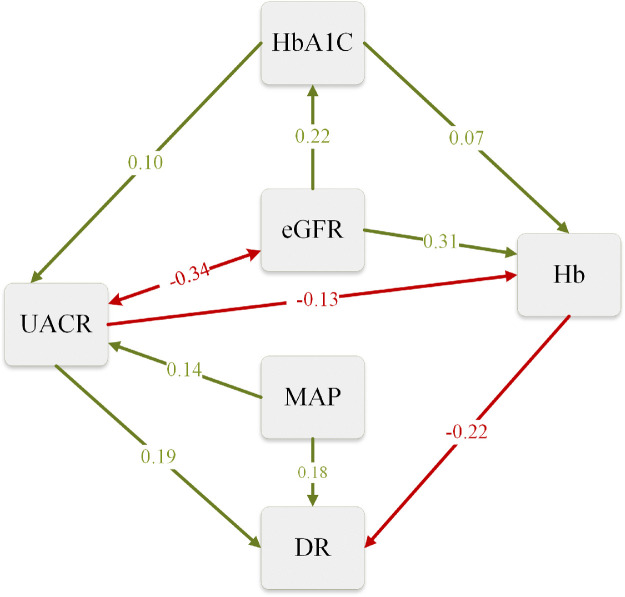
In the original path analysis model (model 1), the path coefficients relating eGFR and HbA1c to DR and the coefficient relating MAP to eGFR were small and nonsignificant (details in Supplementary Table S1 and Supplementary Fig. S3). After removing the nonsignificant variables from model 1, the model (model 2) achieved with a good fit (Supplementary Table S2 and Supplementary Table S3). The results from model 2 path analysis are illustrated in Figure 2.
Figure 2.
The diagram of the path analysis. Path diagram of the reduced model (model 2). DR, diabetic retinopathy (categorical variable); MAP, mean arterial pressure; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio. Green arrows indicate significant positive effects and red arrows indicate significant negative effects. One-headed arrows represent a causal effect of one variable on another (i.e. a path). Double-headed arrows link variables with correlated errors. The width of the arrows reflects the magnitude of standardized path coefficients. The values shown were standardized regression coefficient.
As shown in Figure 2, a significant positive association was found between eGFR and UACR, which was indicated by the red double arrow. Moreover, Hb levels were directly and negatively associated with the risk of DR. There were two paths from UACR to DR: a path from UACR to DR directly (direct path) and another indirect path from UACR to Hb to DR (indirect path). However, eGFR only had an indirect path to DR through Hb. From the path diagram, Hb likely served as a mediator for the associations of UACR and eGFR with the risk of DR.
Discussion
This cross-sectional study assessed associations of DKD severity and anemia with DR among patients with T2DM. The study found that anemia, DKD, and higher UACR levels were all significantly associated with the increased risk of PDR. More importantly, the DKD and UACR had joint effect with anemia on the risk of DR.
Both DKD and DR are common microvascular complications in patients with T2DM20 and have been extensively studied. Consistent with findings from most previous studies, our study findings suggested that high risk of DKD was significantly associated with the increased risk of PDR in the Chinese patients with T2DM.3–6,21,22 Furthermore, our study also explored the impact of abnormal renal function measure levels (eGFR < 60 mL/min/1.73m², UACR > 300 mg/g) on the risk of DR. Both high risk of DKD and abnormal renal function measures levels were not associated with the risk of NPDR significantly, but increased the risk of PDR. One of the surprising findings of this study was that path analysis results demonstrating paths from the two renal function measures to DR are likely to be diverse. There were two paths from UACR to DR: a path from UACR to DR directly (direct path) and another path from UACR to Hb to DR (indirect path). However, the path diagram demonstrated eGFR probably only had an indirect effect on the risk of DR, suggesting the effect of eGFR appeared to be substantially weaker than UACR. These findings were consistent with the results from most of the previous studies. For example, a previous survey indicated that proteinuria, not decreased eGFR, was more significantly associated with DR in a Korean population with T2DM.5 Another 7-year longitudinal study also showed that UACR had a greater predictive power for predicting the development and progression of DR than eGFR among patients with T2DM.6 A population-based prospective cohort study also found that UACR had a stronger association with DR than the eGFR, although both were significant risk factors for DR.23 Man et al.24 found a significant association between eGFR and DR after adjusting for UACR, however, the effect of eGFR and UACR on DR were not compared in this study. The association between eGFR and the risk of DR warrants further investigation.
A novel investigation of this study was to determine whether the association between DKD severity and DR were stronger in patients with anemia. Consistent with the previous findings,9,11 we found anemia was significantly associated with NPDR and PDR even after adjustment for DKD severity, suggesting anemia was an independent risk factor for DR. More importantly, we found that patients with T2DM with either anemia (OR = 3.5) or high risk of DKD (OR = 2.7) had an increased risk of PDR, whereas patients with T2DM with both anemia and high risk of DKD had much higher risk of PDR (OR = 11.59) than patients with T2DM without neither anemia nor high DKD risk, suggesting strong joint effect between DKD severity and anemia on DR. Similar joint effects were found between renal function measures of abnormality and anemia on DR. The path analysis suggested that UACR abnormality could promote the development of DR directly and indirectly by lowering the Hb levels. However, eGFR indirectly affects the risk of DR through decreased Hb levels, and reduction in eGFR only explains a small portion of increased risk of DR. Therefore, we speculated that the joint effect between anemia and DKD was likely explained by different paths for the effect of anemia and DKD on DR. The joint effect can be also demonstrated in Supplementary Figure 4.
Several previous studies demonstrated that significant decrease in Hb level was frequent among patients with microalbuminuria.25,26 The association may be explained by the mechanism that significant losses of transferrin in heavy proteinuria may result in iron-deficiency anemia27; in addition, endothelial dysfunction and microvascular damage in the renal tubulointerstitium play a crucial role in the pathogenesis of albuminuria, which can impair the production and release of EPO and in turn lead to anemia.10 Beyond this, hyperglycemia in patients with T2DM impaired erythropoietin secretion by tubulointerstitial cells and the hypoxic environment likely contributed to the anemia. Anemia is thought to exacerbate the progression of retinal hypoxia and in turn leads to a failure of vasodilatory response and subsequent neovascularization. In addition, retinal hypoxia could also induce the synthesis of vascular endothelial growth factors to exacerbate the progression of DR in patients with T2DM.7,10,28 Such results were also shown by Li et al.29 They found that anemia, independent of kidney disease, was associated with the progression of DR, through controlling for kidney disease, which might also suggest the existence of the mechanism described above during diabetic retinopathy progression.
Although the findings from our study should be interpreted with caution, our results suggest a potential health benefit of improving Hb levels among patients with T2DM. “Standards of Medical Care in Diabetes - 2020” recently developed by the American Diabetes Association (ADA) recommended that effective control of blood pressure, lipid profile, and glucose level could be useful to reduce the risk or slow the progression of DR. However, Hb was not included in this recommendation.20 Hb is a common index of blood tests and is easily obtained in regular medical checkups. Moreover, a pilot randomized trial indicates that compared with usual care, ferric citrate coordination complex treatment for patients with advanced CKD (eGFR ≤ 20 mL/min/1.73 m2) to normalize Hb levels resulted in significantly lower incidence of the composite end point of death, provision of dialysis, or transplantation.30 Hence, more attention should be paid to the change in Hb levels among patients with T2DM, particularly those accompanied with DKD, for the prevention and management of DR. Our findings also found a significantly strong association between DKD and PDR, but a weaker association between DKD and NPDR. Could correcting anemia delay or stop the progression from NPDR to PDR? Further studies are needed to unveil this question. Based on the findings of our article, we recommend that patients with diabetes, especially those with higher DKD risk or anemia, should regularly check their eyes to detect early retinal changes, even in the absence of DR symptoms. It benefits the prevention and early management of DR in patients with T2DM.
Our study had several limitations. First, because of the nature of the cross-sectional study, we could not establish the causality of the associations observed in this study. More detailed analysis, especially true for well-designed cohort studies, is still needed to validate these findings and disentangle the underlying mechanisms. Second, the standard diagnosis DKD requires the presence of an abnormality in kidney function persisting for more than 3 months.31 However, like some other studies,32 our study used one-time point renal function measures to define DKD severity, which may cause the misclassification of DKD. Finally, this study is based on the patients from one single hospital of China, limiting the generalizability of our study findings. Future multicenter cohort studies are needed to validate our conclusions.
In conclusion, the findings of this study supported the association of renal function measures and DKD with DR in patients with T2DM. It indicated that reduced Hb levels may increase the risk of DR in patients with T2DM. In addition, the presence of both anemia and high risk of DKD was associated with a higher risk of DR. Hence, clinicians should pay attention to abnormal Hb levels and improving Hb level may benefit for DR in patients with T2DM.
Supplementary Material
Acknowledgments
The authors thank the support from the faculties in Yiwu Central Hospital of Zhejiang Province.
This study was funded by the Chinese National Natural Science Foundation (81973055), the National Key Research and Development Program of China (2016YFC1305301), and the Fundamental Research Funds for the Central Universities.
Author Contributions: J.Y.W., X.X., and Y.X.Y. conceived this study. R.J.W, X.Y.W., and J.Y.W. facilitated patient recruitment and provided clinical inputs. X.X. analyzed the data. J.Y.W., X,X., W.L.L., S.T.S., M.J.M., B.L.S., S.J.W., Y.S., and Y.X.Y. discussed the data analyses and interpreted the results. X.X. wrote the first draft of the manuscript. B.L.S., S.J.W., J.Y.W., W.L.L., S.T.S., M.J.M., Y.S., and Y.X.Y. critically revised the manuscript, approved the final manuscript for publication and agreed to act as guarantors of the work.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure: J. Wang, None; X. Xin, None; W. Luo, None; R. Wang, None; X. Wang, None; S. Si, None; M. Mo, None; B. Shao, None; S. Wang, None; Y. Shen, None; X. Chen, None; Y. Yu, None
References
- 1. No authors listed Diabetic retinopathy. Nat Rev Dis Primers. 2016; 2: 16013. [DOI] [PubMed] [Google Scholar]
- 2. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016; 44(4): 260–277. [DOI] [PubMed] [Google Scholar]
- 3. Lin HT, Zheng CM, Wu YC, et al.. Diabetic retinopathy as a risk factor for chronic kidney disease progression: a multicenter case (-) control study in Taiwan. Nutrients. 2019; 11(3): 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park HC, Lee YK, Cho A, et al.. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS One. 2019; 14(7): e0220506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park YH, Shin JA, Han JH, Park YM, Yim HW. The association between chronic kidney disease and diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008-2010. PLoS One. 2015; 10(4): e0125338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen YH, Chen HS, Tarng DC. More impact of microalbuminuria on retinopathy than moderately reduced GFR among type 2 diabetic patients. Diabetes Care. 2012; 35(4): 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collister D, Komenda P, Hiebert B, et al.. The effect of erythropoietin-stimulating agents on health-related quality of life in anemia of chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2016; 164(7): 472–478. [DOI] [PubMed] [Google Scholar]
- 8. Kaiafa G, Kanellos I, Savopoulos C, et al.. Is anemia a new cardiovascular risk factor? Int J Cardiol. 2015; 186: 117–124. [DOI] [PubMed] [Google Scholar]
- 9. Traveset A, Rubinat E, Ortega E, et al.. Lower hemoglobin concentration is associated with retinal ischemia and the severity of diabetic retinopathy in type 2 diabetes. J Diabetes Res. 2016; 2016: 3674946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas MC. Anemia in diabetes: marker or mediator of microvascular disease? (review). Nat Clin Pract Nephrol. 2007; 3(1): 20–30. [DOI] [PubMed] [Google Scholar]
- 11. Lee MK, Han KD, Lee JH, et al.. High hemoglobin levels are associated with decreased risk of diabetic retinopathy in Korean type 2 diabetes. Sci Rep. 2018; 8(1): 5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong TY, Sun J, Kawasaki R, et al.. Guidelines on diabetic eye care: the International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology. 2018; 125(10): 1608–1622. [DOI] [PubMed] [Google Scholar]
- 13. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010; 376(9735): 124–136. [DOI] [PubMed] [Google Scholar]
- 14. Beaney T, Burrell LM, Castillo RR, et al.. May Measurement Month 2018: a pragmatic global screening campaign to raise awareness of blood pressure by the International Society of Hypertension. Eur Heart J. 2019; 40(25): 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen TT, Kawasaki R, Wang JJ, et al.. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009; 32(11): 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Wu Z, Chen Y, et al.. Increased prevalence and incidence of anemia among adults in transforming rural China: two cross-sectional surveys. BMC Public Health. 2015; 15: 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kidney Disease Improving Global Outcomes Working Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. 2013; Available from: https://kdigo.org/guidelines/ckd-evaluation-and-management/.
- 18. van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011; 1(3): 2011. [Google Scholar]
- 19. Barrett P. Structural equation modelling: adjudging model fit. Pers Individ Dif. 2007; 42(5): 815–824. [Google Scholar]
- 20. American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes - 2020. Diabetes Care. 2020; 43(suppl 1): S135–S151. [DOI] [PubMed] [Google Scholar]
- 21. Tapp RJ, Shaw JE, Harper CA, et al.. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003; 26(6): 1731–1737. [DOI] [PubMed] [Google Scholar]
- 22. Jeng CJ, Hsieh YT, Yang CM, et al.. Diabetic retinopathy in patients with diabetic nephropathy: development and progression. PLoS One. 2016; 11(8): e0161897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romero-Aroca P, Baget-Bernaldiz M, Navarro-Gil R, et al.. Glomerular filtration rate and/or ratio of urine albumin to creatinine as markers for diabetic retinopathy: a ten-year follow-up study. J Diabetes Res. 2018; 2018: 5637130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Man RE, Sasongko MB, Wang JJ, et al.. The association of estimated glomerular filtration rate with diabetic retinopathy and macular edema. Invest Ophthalmol Vis Sci. 2015; 56(8): 4810–4816. [DOI] [PubMed] [Google Scholar]
- 25. Thomas MC, Tsalamandris C, MacIsaac RJ, Jerums G. The epidemiology of hemoglobin levels in patients with type 2 diabetes. Am J Kidney Dis. 2006; 48(4): 537–545. [DOI] [PubMed] [Google Scholar]
- 26. Han JS, Lee MJ, Park KS, et al.. Albuminuria as a risk factor for anemia in chronic kidney disease: result from the Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). PloS One. 2015; 10(10): e0139747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prinsen BH, de Sain-van der Velden MG, Kaysen GA, et al.. Transferrin synthesis is increased in nephrotic patients insufficiently to replace urinary losses. J Am Soc Nephrol. 2001; 12(5): 1017–1025. [DOI] [PubMed] [Google Scholar]
- 28. Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol. 2009; 5(4): 204–210. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Yu Y, VanderBeek BL. Anaemia and the risk of progression from non-proliferative diabetic retinopathy to vision threatening diabetic retinopathy. Eye. 2020; 34(5): 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Block GA, Block MS, Smits G, et al.. A pilot randomized trial of ferric citrate coordination complex for the treatment of advanced CKD. J Am Soc Nephrol. 2019; 30(8): 1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019; 322(13): 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 395(10225): 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.