Abstract
Background:
Risk of fetal alcohol spectrum disorder (FASD) is not based solely on the timing and level of prenatal alcohol exposure (PAE). The effects of teratogens can be modified by genetic differences in fetal susceptibility and resistance. This is best illustrated in twins.
Objective:
To compare the prevalence and magnitude of pairwise discordance in FASD diagnoses across monozygotic twins, dizygotic twins, full-siblings, and half-siblings sharing a common birth mother.
Methods:
Data from the Fetal Alcohol Syndrome Diagnostic & Prevention Network clinical database was used. Sibling pairs were matched on age and PAE, raised together, and diagnosed by the same University of Washington interdisciplinary team using the FASD 4-Digit Code. This design sought to assess and isolate the role of genetics on fetal vulnerability/resistance to the teratogenic effects of PAE by eliminating or minimizing pairwise discordance in PAE and other prenatal/postnatal risk factors.
Results:
As genetic relatedness between siblings decreased from 100% to 50% to 50% to 25% across the four groups (9 monozygotic, 39 dizygotic, 27 full-sibling and 9 half-sibling pairs, respectively), the prevalence of pairwise discordance in FASD diagnoses increased from 0% to 44% to 59% to 78%. Despite virtually identical PAE, 4 pairs of dizygotic twins had FASD diagnoses at opposite ends of the fetal alcohol spectrum—Partial Fetal Alcohol Syndrome versus Neurobehavioral Disorder/Alcohol-Exposed.
Conclusion:
Despite virtually identical PAE, fetuses can experience vastly different FASD outcomes. Thus, to protect all fetuses, especially the most genetically vulnerable, the only safe amount to drink is none at all.
Keywords: fetal alcohol spectrum disorder, twins, genetics, prenatal alcohol exposure
Introduction
The effects of teratogens can be modified by genetic differences in fetal susceptibility and resistance [1]. Fetal alcohol syndrome (FAS) is a permanent birth defect syndrome caused by maternal consumption of the teratogen alcohol during pregnancy. FAS is characterized by growth deficiency, a unique cluster of minor facial anomalies and central nervous system (CNS) structural and/or functional abnormalities. Not all fetuses exposed to and damaged by prenatal alcohol exposure (PAE) have FAS. The physical, cognitive, and behavioral deficits observed among individuals with PAE are not dichotomous, that is either normal or clearly abnormal. Rather, the outcomes all range along separate continua from normal to clearly abnormal [2]. This full range of outcomes caused by PAE is called Fetal Alcohol Spectrum Disorders (FASD). Diagnoses like FAS, Partial FAS (PFAS), Static Encephalopathy/Alcohol Exposed (SE/AE) and Neurobehavioral Disorder/Alcohol Exposed (ND/AE) fall broadly under the umbrella of FASD [2,3].
Fetal risk of damage from PAE is not just dependent on the timing, frequency, and quantity of exposure. Fetal alcohol spectrum disorders (FASD) are caused by a complex interaction of genes and environment, and are regulated by both parental and fetal genes [4]. Fetal genetics influences a fetus’ vulnerability to the teratogenic effects of PAE [5]. In a 1993 study of 16 monozygotic and dizygotic twin pairs, Streissguth and Dehaene [6] reported 100% pairwise concordance in FASD diagnoses among monozygotic twin pairs, while dizygotic twins were only 64% concordant. The outcomes of that study strongly suggested that genetic loci regulate susceptibility to, or resistance against FASD. This 1993 study was conducted prior to the creation of rigorous FASD diagnostic systems. Patients were diagnosed as FAS or Fetal Alcohol Effects. If two fetuses exposed to identical levels of alcohol can experience vastly different FASD outcomes, this would have important implications for public health messaging and the setting of exposure thresholds in FASD diagnostic guidelines.
The purpose of this study was to compare the prevalence and magnitude of pairwise discordance in FASD diagnoses across four groups of sibling pairs: monozygotic twins, dizygotic twins, full-siblings, and half-siblings sharing a common birth mother. All sibling pairs had virtually identical or reportedly similar levels of PAE, were raised together, were diagnosed by the same interdisciplinary team using the 4-Digit Diagnostic Code and were identical or similar in age at the time of diagnosis. This sibling-pair design sought to more fully assess and isolate the role of genetics on fetal vulnerability to the teratogenic effects of PAE by eliminating or minimizing pairwise discordance in age, PAE and other prenatal and postnatal risk factors.
Specific Aims:
To determine if the prevalence of FASD diagnostic discordance was higher among dizygotic twin pairs than among monozygotic twin pairs.
To determine if the prevalence of FASD diagnostic discordance increases as the proportion of genome shared between sibling-pairs decreases across the four study groups: monozygotic twins, dizygotic twins, full-siblings, and half-siblings sharing a common birth mother.
To document the greatest magnitude of FASD diagnostic discordance observed between twin pairs with virtually identical PAE. Can twins with virtually identical PAE present at opposite ends of the fetal alcohol spectrum?
To estimate the proportion of phenotypic variance in FASD diagnoses due to genetic factors (heritability).
Methods
A retrospective study was conducted using data collected from twin and sibling pairs that received a FASD diagnostic evaluation at the University of Washington Fetal Alcohol Syndrome Diagnostic & Prevention Network (FASDPN).
FASD Diagnostic Method:
FASD diagnoses were rendered using the FASD 4-Digit Diagnostic Code. It is described in full by Astley [2, 7, 8]. Briefly, the 4 digits of the FASD 4-Digit Code reflect the magnitude of expression of the 4 key diagnostic features of FASD, in the following order: 1) growth deficiency, 2) FAS facial phenotype, 3) CNS structural/functional abnormalities, and 4) PAE (Figure 1). The magnitude of expression of each feature is ranked independently on a 4-point Likert scale, with 1 reflecting complete absence of the FASD feature and 4 reflecting a strong “classic” presence of the FASD feature. Each Likert rank is specifically case defined. There are a total of 102 4-Digit Codes that fall broadly under the umbrella of FASD. These codes cluster under four clinically meaningful FASD diagnoses: fetal alcohol syndrome (FAS) (4-Digit Code Diagnostic Categories A, B): Partial FAS (PFAS) (Diagnostic Category C); Static Encephalopathy/Alcohol-Exposed (SE/AE) (Diagnostic Categories E, F); and Neurobehavioral Disorder/Alcohol-Exposed (ND/AE) (Diagnostic Categories G, H) (Figure 1). Individuals that did not meet criteria for one of these FASD diagnostic classifications were identified in this study as Not FASD/Alcohol-Exposed (Diagnostic Categories I, J).
Figure 1. FASD 4-Digit Diagnostic Code.
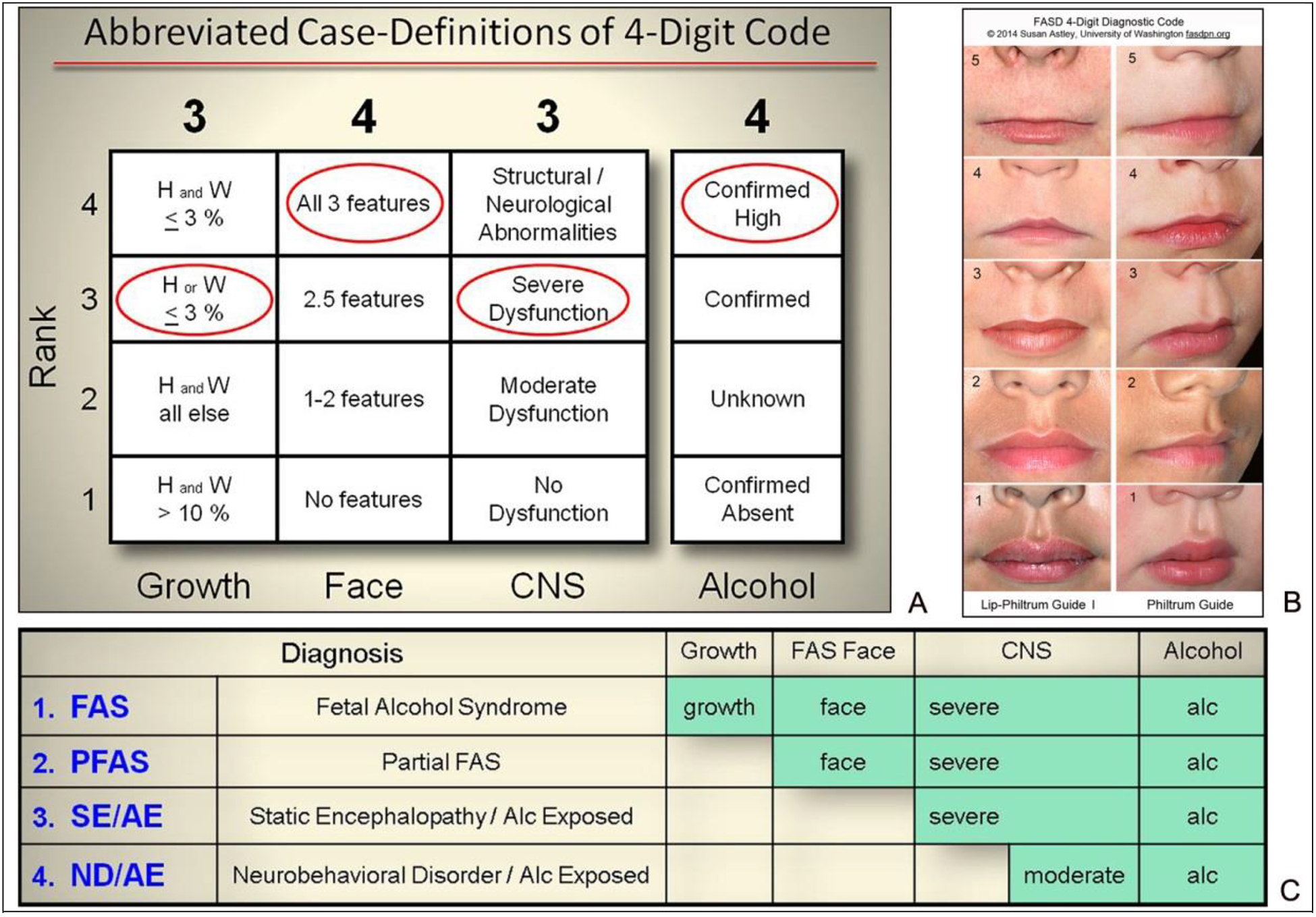

A) Abbreviated case-definitions for the fetal alcohol spectrum disorder (FASD) 4-Digit Code [2]. The 4-Digit Code 3434 is one of 12 4-Digit Codes that fall under the diagnostic category FAS. B) The Rank 4 FAS facial phenotype requires 3 features: 1) palpebral fissure lengths 2 or more standard deviations below the mean; 2) a smooth philtrum (Rank 4 or 5 on the University of Washington Lip-Philtrum Guide); and 3) a thin upper lip (Rank 4 or 5 on the University of Washington Lip-Philtrum Guide). C and D) The 4-Digit Code produces four diagnostic subgroups under the umbrella of FASD: FAS (Diagnostic Categories A, B), PFAS (Diagnostic Category C), SE/AE (Diagnostic Categories E, F), and ND/AE (Diagnostic Categories G, H). Abbreviations: CNS: central nervous system; H: height percentile; W: weight percentile.
Study Groups:
The sibling pairs were partitioned into four study groups: 1. monozygotic twins, 2. dizygotic twins, 3. full-siblings, and 4. half-siblings sharing a common birth mother. Monozygotic twin pairs share virtually 100% of their genome. Dizygotic and full-sibling pairs share, on average, 50% of their genome. Half-sibling pairs with a common birth mother share, on average, 25% of their genome [9].
Data from all twin and sibling pairs that met the following inclusion criteria were used in this study:
Sibling pairs were monozygotic twins, dizygotic twins, full-siblings, or half-siblings sharing the same birth mother.
Both siblings received an FASD diagnostic evaluation at an FASDPN clinic by the same interdisciplinary team using the 2004 FASD 4-Digit Diagnostic Code.
Siblings did not present with another genetic syndrome.
Age at diagnosis could range from newborn to adult. Effort was made to select pairs that both fit into one of three age ranges at the time of diagnosis (0–3 years, 4–8 years, 9 or more years). This minimized the chance that FASD diagnostic contrasts between pairs may be due to one sibling being too young to fully assess or comparably assess brain function.
All siblings had confirmed PAE. Twin pairs, by definition, had virtually identical PAE. Full sibling pairs and half-sibling pairs had to have concordant Alcohol Ranks (e.g., both siblings had to have Rank 3 alcohol exposure or both had to have Rank 4 alcohol exposure).
Effort was made to select siblings raised together who experienced identical or similar other prenatal and postnatal risk factors.
Siblings could be of any gender or race.
Data Set:
All data collected during an FASD diagnostic evaluation at the FASDPN are entered into the FASDPN database with patient consent and University of Washington Human Subjects Division approval. Approximately 3,000 patients have been evaluated in the clinic to date. The data document patient demographics, PAE, all other reported prenatal and postnatal adverse exposures and events and measures of growth, facial features, and structural and/or functional brain abnormalities used to derive the FASD 4-Digit Code. These data are recorded on three standardized diagnostic forms: the New Patient Information Form, FASD Diagnostic Form, and FAS Facial Photographic Analysis Report posted on the FASDPN website www.FASDPN.org [2, 8].
Key data used in this study included the patient’s FASD 4-Digit Code, FASD diagnostic category (FAS, PFAS, SE/AE, ND/AE and Not FASD/AE), and their Growth, Face, CNS and Alcohol Ranks (Figure 1). The CNS Rank in the 4-Digit Code serves two purposes: 1) Ranks 1 through 4 document the probability of underlying CNS structural abnormality (Rank 1: unlikely; Rank 2: possible; Rank 3: probable; and Rank 4: definite). 2) Ranks 1 through 3 also document the magnitude of CNS dysfunction as measured using standardized neuropsychological tools (Rank 1: no dysfunction; Rank 2: moderate dysfunction; and Rank 3 severe dysfunction). The CNS functional Ranks 1–3 introduced by the 4-Digit Code were case-defined to predict increasing likelihood of structural CNS abnormality—a predictive correlation that was subsequently confirmed through magnetic resonance imaging [7]. To distinguish these two CNS measures in the current study, they are labeled CNS1–4 and CNS1–3. PAE is ranked by the 4-Digit Code on a 4-point Likert scale (Figure 1A). Only subjects with Rank 3 or Rank 4 PAE were enrolled in this study. An Alcohol Rank 4 is assigned when PAE is confirmed and reported to be high risk (generally high peak blood alcohol concentrations delivered at least weekly in early pregnancy). An Alcohol Rank 3 is assigned when PAE is confirmed, but the amount reported is low to moderate risk (designated as PAE Rank 3b in this study) or the details on the amount and timing are unknown (designated as PAE Rank 3a in this study). The information used to generate the Alcohol Rank is presented on page 8 of the 4-Digit Code FASD Diagnostic Form [2]. In preparation for a FASD diagnostic evaluation, efforts are made to document the quantity, frequency and timing of maternal alcohol use before and during the index pregnancy. Although 99.5% of patients evaluated at the FASDPN clinic have confirmed PAE, only 30 to 40% have quantity, frequency and/or timing of exposure detailed in their records [10]. Recall error and reporting bias likely impact the accuracy of this more detailed information, therefore the more global measure of PAE “Alcohol Rank” was used as the primary measure of PAE risk in this study. In addition to the risk posed by PAE, measures of other prenatal and postnatal risks were also used in this study. Other prenatal risk factors documented in the FASDPN clinical database include poor prenatal care, pregnancy complications, presence of other syndromes/genetic abnormalities, and prenatal exposure to other substances (e.g., medications, tobacco, illicit drugs, and/or other teratogens). The 4-Digit Code ranks the magnitude of these other prenatal risks in a single composite measure labeled “Other Prenatal Risks Rank”. Rank 1 equals no risk; Rank 2 equals unknown risk; Rank 3 equals some risk; and Rank 4 equals high risk. Rank 4 is assigned when there is exposure to another teratogen (e.g., Dilantin) or when another syndrome or genetic condition is present (e.g., down syndrome, Fragile X, etc.). Rank 3 is assigned to all other prenatal risks. Postnatal risk factors documented in the FASDPN database include perinatal complications, number of home placements, physical and/or sexual abuse, neglect, and trauma. The 4-Digit Code ranks the magnitude of these other postnatal risks in a single composite measure labeled “Other Postnatal Risks Rank”. Rank 1 equals no risk; Rank 2 equals unknown risk; Rank 3 equals some risk; and Rank 4 equals high risk. Rank 4 is used to note severe postnatal circumstances that have been shown to have a significant adverse effect on development in most instances. Examples include physical/sexual abuse, multiple home placements, trauma, and severe neglect) [2]. Rank 3 is used to note conditions akin to those in Rank 4, but the circumstances are less severe.
Statistical Analyses:
The primary focus of the study was to compare the prevalence of discordant FASD diagnoses between sibling pairs across the 4 study groups. Descriptive statistics (means, SD, and proportions expressed as valid percentages (e.g., subjects with missing data are not included in the denominator)) were used to profile the demographic and clinical outcomes of the 4 study groups. The chi-square test with tests for linear trend was used to compare proportions between the study groups. One-way ANOVA was used to compare outcomes measured on a continuous scale between the study groups. Two-tailed p-values < 0.05 were interpreted as statistically significant.
Heritability is formally defined as the proportion of phenotypic variation that is caused by genotypic variation in a population. FASD is not a genetic disorder, but fetal genetics appears to modify the teratogenic effects of PAE [5, 6]. Heritability has historically been estimated from studies of twins. Monozygotic twin pairs share essentially 100% of their genome. Dizygotic twin pairs share, on average, 50% of their genome. If a trait appears to be more similar in monozygotic twins than in dizygotic twins (when the twin pairs were raised together in the same environment), genetic factors likely play an important role in determining (or modifying) that trait. By comparing a trait in monozygotic twins versus dizygotic twins, one can calculate an estimate of its heritability. Heritability estimates range from 0% to 100%. A heritability close to 0% indicates that almost all of the variability in a trait is due to environmental factors (e.g., PAE and other prenatal and postnatal risk factors), with very little influence from genetic differences. A heritability estimate close to 100% indicates that almost all of the variability in a trait comes from genetic differences, with very little contribution from variability in environmental factors. When a phenotype is determined by a combination of genetic and environmental factors, heritability will be somewhere between 0% and 100%. Comparing discordance for monozygotic versus dizygotic twins allows an indirect estimate of the importance of genetic factors in producing the phenotype. Heritability estimates based on twin discordance studies can be simplistically viewed as: percent heritability = ((dizygotic discordance minus monozygotic discordance)/dizygotic discordance)*100 [9, 11]. It is important to understand that heritability does not indicate what proportion of a trait is determined by genes and what proportion is determined by environment. A heritability of 80% does not mean that a trait is 80% caused by genetic factors; it means that 80% of the variability in the trait in a population is due to genetic differences. Heritability measures the fraction of variation between individuals in a population that is due to their genotypes.
Results
Demographic and Clinical Profiles of the Four Study Groups
The study selection criteria generated 84 sibling pairs broken into four study groups (monozygotic twins, dizygotic twins, full siblings and half sibling sharing the same birth mother) with key factors that defined and differentiated the groups (Table 1).
Table 1.
Key factors that defined and differentiated the 4 study groups.
| Features shared between sibling pairs | Study Groups | |||
|---|---|---|---|---|
| 1. Monozygotic twins | 2. Dizygotic twins | 3. Full-siblings | 4. Half-siblings with the same birth mother | |
| 9 pairs | 39 pairs | 27 pairs | 9 pairs | |
| Birth mother | identical | identical | identical | identical |
| Birth father | identical | identical | identical | different |
| Genome shared | ~100% | ~50% | ~50% | ~25% |
| Prenatal alcohol exposure | virtually identical | virtually identical | 100% same Rank | 100% same Rank |
| Other prenatal risks | virtually identical | virtually identical | 100% same Rank | 88% same Rank |
| Siblings raised together | 100% | 100% | 96% | 100% |
| Other postnatal risks | 100% same Rank | 87% same Rank | 83% same Rank | 75% same Rank |
| Matched in age within one of 3 age-ranges (0–3; 4–8, 9+ years) | 100% | 100% | *93% | *89% |
2 pairs of full-siblings and 1 pair of half-siblings had one sibling that was in a younger age category. In each of these 3 pairs, the younger sibling had the more severe FASD diagnostic outcome.
The demographic and FASD diagnostic profiles of the study sample (Table 2) were highly representative of the entire FASDPN clinic population (n = 3,000) from which it was selected [10]. The gender and age distributions were comparable between the 4 study groups. Race was 100% concordant across all 84 twin/sibling pairs. The number of full and half sibling pairs included in the study may appear smaller than one would expect from a patient population of 3,000. A number of factors inherent in the FASDPN clinical dataset limited the number of full and half sibling pairs available for inclusion in the study. In general, 85% of the patients evaluated by the FASDPN are in foster/adoptive care—no longer living with their birth parents. Confirmation of full or half sibling status requires knowledge of both birth parents’ names. This is typically available on only half of the FASDPN patient population. Of the 54% (n = 1,617) with birth parent names available, 8% (n = 129) were siblings (full or half). Seventy-two of the 129 full and half siblings met the inclusion criteria for the study. The primary reason siblings failed to meet the study’s inclusion criteria were they were too different in age (e.g., infant vs adolescent) at the time of their FASD evaluation to draw valid conclusions regarding the concordance/discordance of their diagnoses.
Table 2.
Comparison of demographic and FASD clinical profiles between the four study groups
| Demographic and Clinical Characteristics | Monozygotic | Dizygotic | Full-siblings | Half-siblings | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 18 (9 pairs) | N = 78 (39 pairs) | N = 54 (27 pairs) | N = 18 (9 pairs) | N = 168 (84 pairs) | ||||||
| Gender (N pairs; valid %) | ||||||||||
| female-female | 4 | 44.4 | 10 | 25.6 | 7 | 25.9 | 2 | 22.2 | 23 | 27.4 |
| male-male | 5 | 55.6 | 10 | 25.6 | 11 | 40.7 | 4 | 44.4 | 30 | 35.7 |
| Mixed gender | 0 | 0.0 | 19 | 48.7 | 9 | 33.3 | 3 | 33.3 | 31 | 36.9 |
| Overall proportion of female subjects | 8/18 | 44.4 | 37/78 | 47.4 | 23/54 | 42.6 | 7/18 | 38.9 | 75/168 | 44.6 |
| Race (N; valid %) | ||||||||||
| Caucasian | 4 | 22.2 | 32 | 41.0 | 32 | 59.3 | 14 | 77.8 | 82 | 48.8 |
| African American | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Native American | 0 | 0.0 | 8 | 10.3 | 2 | 3.7 | 1 | 5.6 | 11 | 6.5 |
| Hispanic | 0 | 0.0 | 4 | 5.1 | 6 | 11.1 | 0 | 0.0 | 10 | 6.0 |
| Mixed race: combinations of above races | 14 | 77.8 | 34 | 43.6 | 14 | 25.9 | 3 | 16.7 | 65 | 38.7 |
| Age at Diagnosis (years) (N, valid %) | ||||||||||
| 0–3.9 | 4 | 22.2 | 30 | 38.5 | 17 | 31.5 | 2 | 11.1 | 53 | 31.5 |
| 4–8.9 | 12 | 66.7 | 28 | 35.9 | 22 | 40.7 | 9 | 50.0 | 71 | 42.3 |
| 9–19.7 | 2 | 11.1 | 20 | 25.6 | 15 | 27.8 | 7 | 38.9 | 44 | 26.2 |
| Sibling pairs raised together (N pairs; valid %) | ||||||||||
| yes | 9 | 100.0 | 39 | 100.0 | 26 | 96.3 | 9 | 100.0 | 83 | 98.8 |
| Prenatal Alcohol Exposure: 4-Digit Alcohol Rank (N, valid %) | ||||||||||
| Rank 3a: Exposure confirmed, amount unknown. | 8 | 44.5 | 30 | 38.5 | 26 | 48.1 | 6 | 33.3 | 70 | 41.7 |
| Rank 3b: Exposure confirmed, amount low to mod. | 4 | 22.2 | 0 | 0.0 | 4 | 7.3 | 0 | 0.0 | 8 | 4.8 |
| Rank 4: Confirmed and level high | 6 | 33.3 | 48 | 61.5 | 24 | 44.4 | 12 | 66.7 | 90 | 53.5 |
| Other Prenatal Risks: 4-Digit Rank (N, valid %) | ||||||||||
| Rank 1: no risk | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rank 2: unknown risk | 2 | 11.1 | 8 | 10.3 | 0 | 0.0 | 2 | 11.1 | 12 | 7.1 |
| Rank 3: moderate risk | 16 | 88.9 | 70 | 89.7 | 54 | 100.0 | 15 | 83.3 | 155 | 92.3 |
| Rank 4: high risk | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | 1 | 0.6 |
| Postnatal Risks: 4-Digit Rank (N, valid %) | ||||||||||
| Rank 1: no risk | 0 | 0.0 | 2 | 2.6 | 1 | 1.9 | 0 | 0.0 | 3 | 1.8 |
| Rank 2: unknown risk | 2 | 11.1 | 0 | 0.0 | 5 | 9.3 | 1 | 5.6 | 8 | 4.8 |
| Rank 3: moderate risk | 6 | 33.3 | 37 | 47.4 | 31 | 57.4 | 4 | 22.2 | 78 | 46.4 |
| Rank 4: high risk | 10 | 55.6 | 39 | 50.0 | 17 | 31.5 | 13 | 72.2 | 79 | 47.0 |
| FASD Diagnoses (N, valid %) | ||||||||||
| FAS | 4 | 22.2 | 1 | 1.3 | 2 | 3.7 | 1 | 5.6 | 8 | 4.8 |
| PFAS | 0 | 0.0 | 5 | 6.4 | 3 | 5.6 | 2 | 11.1 | 10 | 6.0 |
| SE/AE | 8 | 44.5 | 15 | 19.2 | 12 | 22.2 | 5 | 27.8 | 41 | 24.4 |
| ND/AE | 4 | 22.2 | 48 | 61.5 | 23 | 42.6 | 9 | 50.0 | 84 | 50.0 |
| Sentinel Physical Findings/AE | 0 | 0.0 | 0 | 0.0 | 4 | 7.4 | 1 | 5.6 | 4 | 2.4 |
| Not FASD/AE | 2 | 11.1 | 9 | 11.5 | 10 | 18.5 | 0 | 0.0 | 21 | 12.5 |
The matching criteria used to select twin and sibling pairs effectively minimized pairwise discordance in PAE and other prenatal and postnatal risk factors (Table 3). By definition, PAE was 100% concordant (virtually identical) between the monozygotic and dizygotic twin pairs. The Other Prenatal Risk Rank was 100% concordant between the monozygotic, dizygotic and full-sibling pairs, and 87.5% concordant between the half-sibling pairs. The Postnatal Risk Rank was highly concordant across all 4 groups (monozygotic: 100%; dizygotic: 87.2%; full-sibling: 91.3% and half-sibling: 75.0%), but did decrease linearly as the proportion of genome shared between siblings decreased.
Table 3.
Matching criteria effectively minimized pairwise discordance in PAE and other prenatal and postnatal risk factors.
| Prenatal and Postnatal Risks | Monozygotic | Dizygotic | Full-siblings | Half-siblings | ||||
|---|---|---|---|---|---|---|---|---|
| N = 18 (9 pairs) | N = 78 (39 pairs) | N = 54 (27 pairs) | N = 18 (9 pairs) | |||||
| N Pairs | valid% | N Pairs | valid% | N Pairs | valid% | N Pairs | valid% | |
| Prenatal Alcohol Exposure (PAE): 4-Digit Code Rank | ||||||||
| Concordant Ranks between sibling pairs | ||||||||
| Overall | 9 | 100.0 | 39 | 100.0 | 27 | 100.0 | 9 | 100.0 |
| Rank 1: Confirmed absence of PAE | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rank 2: PAE unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rank 3a: PAE confirmed, amount unknown | 4 | 44.5 | 15 | 38.5 | 13 | 48.1 | 3 | 33.3 |
| Rank 3b: PAE confirmed, amount low to moderate | 2 | 22.2 | 0 | 0.0 | 2 | 7.3 | 0 | 0.0 |
| Rank 4: PAE confirmed, amount high | 3 | 33.3 | 24 | 61.5 | 12 | 48.6 | 6 | 66.7 |
| Discordant Ranks between sibling pairs | ||||||||
| Overall | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Other Prenatal Risks: 4-Digit Code Rank | ||||||||
| Concordant Ranks between sibling pairs | ||||||||
| Overall | 9/9 | 100.0 | 39/39 | 100.0 | 27/27 | 100.0 | 8/9 | 88.9 |
| Valid Overall (excluding pairs with unknown risk) | 8/8 | 100.0 | 35/35 | 100.0 | 27/27 | 100.0 | 7/8 | 87.5 |
| Rank 1: no risk | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rank 2: unknown risk | 1 | 11.1 | 4 | 10.3 | 0 | 0.0 | 1 | 11.1 |
| Rank 3: some risk | 8 | 88.9 | 35 | 89.7 | 27 | 100.0 | 7 | 77.8 |
| Rank 4: high risk | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Discordant Ranks between sibling pairs | ||||||||
| Overall | 0/9 | 0.0 | 0/39 | 0.0 | 0/27 | 0.0 | 1/9 | 11.1 |
| Valid Overall (excluding pairs with unknown risk) | 0/9 | 0.0 | 0/39 | 0.0 | 0/27 | 0.0 | 1/8 | 12.5 |
| Rank 4: high risk – Rank 3: some risk | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 11.1 |
| Postnatal Risks: 4-Digit Code Rank | ||||||||
| Concordant Ranks between sibling pairs | ||||||||
| Overall | 9/9 | 100.0 | 34/39 | 87.2 | 21/27 | 77.8 | 6/9 | 66.7 |
| Valid Overall (excluding pairs with unknown risk) | 8/8 | 100.0 | 34/39 | 87.2 | 20/23 | 91.3 | 6/8 | 75.0 |
| Rank 1: no risk | 0 | 0.0 | 1 | 2.6 | 0 | 0.0 | 0 | 0.0 |
| Rank 2: unknown risk | 1 | 11.1 | 0 | 0.0 | 1 | 3.7 | 0 | 0.0 |
| Rank 3: some risk | 3 | 33.3 | 16 | 41.0 | 13 | 48.1 | 0 | 0.0 |
| Rank 4: high risk | 5 | 55.6 | 17 | 43.6 | 7 | 26.0 | 6 | 66.7 |
| Discordant Ranks between sibling pairs | ||||||||
| Overall | 0/9 | 0.0 | 5/39 | 12.8 | 6/27 | 22.2 | 3/9 | 33.3 |
| Valid Overall (excluding pairs with unknown risk) | 0/8 | 0.0 | 5/39 | 12.8 | 3/23 | 13.0 | 3/8 | 25.0 |
| Rank 3: some risk – Rank 2: unknown risk | 0 | 0.0 | 0 | 0.0 | 2 | 7.4 | 1 | 11.1 |
| Rank 3: some risk – Rank 1: no risk | 0 | 0.0 | 0 | 0.0 | 1 | 3.7 | 0 | 0.0 |
| Rank 3: some risk – Rank 4: high risk | 0 | 0.0 | 5 | 12.8 | 2 | 7.4 | 2 | 22.2 |
| Rank 4: high risk – Rank 2: unknown risk | 0 | 0.0 | 0 | 0.0 | 1 | 3.7 | 0 | 0.0 |
Abbreviations: PAE: prenatal alcohol exposure
Specific Aims 1 and 2: Pairwise concordance/discordance of FASD diagnostic outcomes
FASD diagnoses (FAS, PFAS, SE/AE, ND/AE, Not FASD/AE) were 100% concordant between monozygotic twin pairs, but only 56.4% concordant among dizygotic twin pairs (Table 4). This closely mirrored the proportion of the genome shared between twin pairs (monozygotic 100%; dizygotic 56%).
Table 4.
Prevalence of FASD diagnostic concordance and discordance between twin and sibling pairs.
| Concordance and Discordance in FASD Outcomes between Twin and Sibling Pairs | 1. Monozygotic | 2. Dizygotic | 3. Full-siblings | 4. Half-siblings (maternal) | ||||
|---|---|---|---|---|---|---|---|---|
| N = 18 (9 pairs) | N = 78 (39 pairs) | N = 54 (27 pairs) | N = 18 (9 pairs) | |||||
| N pairs | Valid % | N pairs | Valid % | N pairs | Valid % | N pairs | Valid % | |
| Pairwise FASD Diagnoses (FAS, PFAS, SE/AE, ND/AE, Not FASD/AE) | ||||||||
| Concordant outcomes between sibling pairs | ||||||||
| Total concordant pairs | 9 | 100.0 | 22 | 56.4 | 11 | 40.7 | 2 | 22.2 |
| FAS-FAS | 2 | 22.2 | 0 | 0.0 | 0 | 40.7 | 0 | 0.0 |
| PFAS-PFAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| SE/AE-SE/AE | 4 | 44.4 | 4 | 10.3 | 2 | 7.4 | 0 | 0..0 |
| ND/AE-ND/AE | 2 | 22.2 | 16 | 41.0 | 6 | 22.2 | 2 | 22.2 |
| Not FASD/AE-Not FASD/AE | 1 | 11.2 | 2 | 5.1 | 3 | 11.1 | 0 | 0.0 |
| Discordant outcomes between sibling pairs | ||||||||
| *Total discordant pairs | 0 | 0.0 | 17 | 43.6 | 16 | 59.3 | 7 | 77.8 |
| FAS-PFAS | 0 | 0.0 | 1 | 2.6 | 0 | 0.0. | 0 | 0.0 |
| FAS-SE/AE | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 11.1 |
| # FAS-ND/AE | 0 | 0.0 | 0 | 0.0 | 2 | 7.4 | 0 | 0.0 |
| PFAS-SE/AE | 0 | 0.0 | 0 | 0.0 | 1 | 3.7 | 1 | 11.1 |
| # PFAS-ND/AE | 0 | 0.0 | 4 | 10.3 | 2 | 7.4 | 1 | 11.1 |
| SE/AE-ND/AE | 0 | 0.0 | 7 | 17.5 | 5 | 18.5 | 4 | 44.4 |
| SE/AE-Not FASD/AE | 0 | 0.0 | 0 | 0.0 | 2 | 7.4 | 0 | 0.0 |
| ND/AE-Not FASD/AE | 0 | 0.0 | 5 | 12.8 | 2 | 7.4 | 0 | 0.0 |
| Not FASD/AE-Not FASD/AE | 0 | 0.0 | 0 | 0.0 | 2 | 7.4 | 0 | 0.0 |
| Pairwise FASD Diagnostic Features | ||||||||
| Discordant outcomes between sibling pairs | ||||||||
| Growth Ranks 1–4 | ***1 | 11.1 | 17 | 43.6 | 10 | 37.0 | 4 | 44.4 |
| **Face Ranks 1–4: Total discordant pairs | 0 | 0.0 | 10 | 25.6 | 8 | 29.6 | 5 | 55.6 |
| Face Rank 1 vs 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Face Rank 2 vs 4 | 0 | 0.0 | 1 | 2.6 | 1 | 3.7 | 2 | 22.2 |
| Face Rank 3 vs 4 | 0 | 0.0 | 0 | 0.0 | 2 | 7.4 | 0 | 0.0 |
| CNS Ranks 1–4: probability of structural abnormality (none, possible, probable, definite) | ***1 | 11.1 | 17 | 43.6 | 14 | 51.9 | 5 | 55.6 |
| Alcohol Ranks 3–4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| CNS Functional Ranks 1–3: (no, moderate, severe dysfunction) | 0 | 0.0 | 17 | 43.6 | 10 | 37.0 | 5 | 55.6 |
| Microcephaly (head circumference <= 3rd percentile) | ***1 | 11.1 | 2 | 5.1 | 5 | 18.5 | 3 | 33.3 |
| Seizure disorder | 0 | 0.0 | 4 | 10.3 | 1 | 3.7 | 1 | 11.1 |
Linear trend across 4 study groups: MH Chi2:
10.7, p 0.001;
5.1, p 0.02.
One twin pair had discordant growth Ranks in their 4-Digit Codes (3244-1244). Another pair had discordant CNS structural Ranks (1243–1233) because only one twin presented with microcephaly. Their CNS functional Ranks, however, were both Rank 3. These contrasts in a single component of the 4-Digit Code did not result in discordant FASD diagnostic classifications. Both twin pairs had concordant diagnoses of SE/AE.
Large contrast in pairwise FASD diagnostic outcomes.
The prevalence of pairwise concordance in FASD diagnoses decreased significantly and linearly as the proportion of genome shared between siblings decreased across the 4 study groups (100% among 9 monozygotic twin pairs sharing 100% of their genome; 56.4% among 39 dizygotic twin pairs sharing 50% of their genome; 41.7% among 27 pairs of full-siblings sharing 50% of their genome; and 22.2% among 9 pairs of half-siblings sharing 25% of their genome) (Chi2 linear trend = 1.7, p = 0.001) (Table 4, Figure 2).
Figure 2. Twin/Sibling Pairwise Concordance in FASD outcomes and prenatal/postnatal risks.
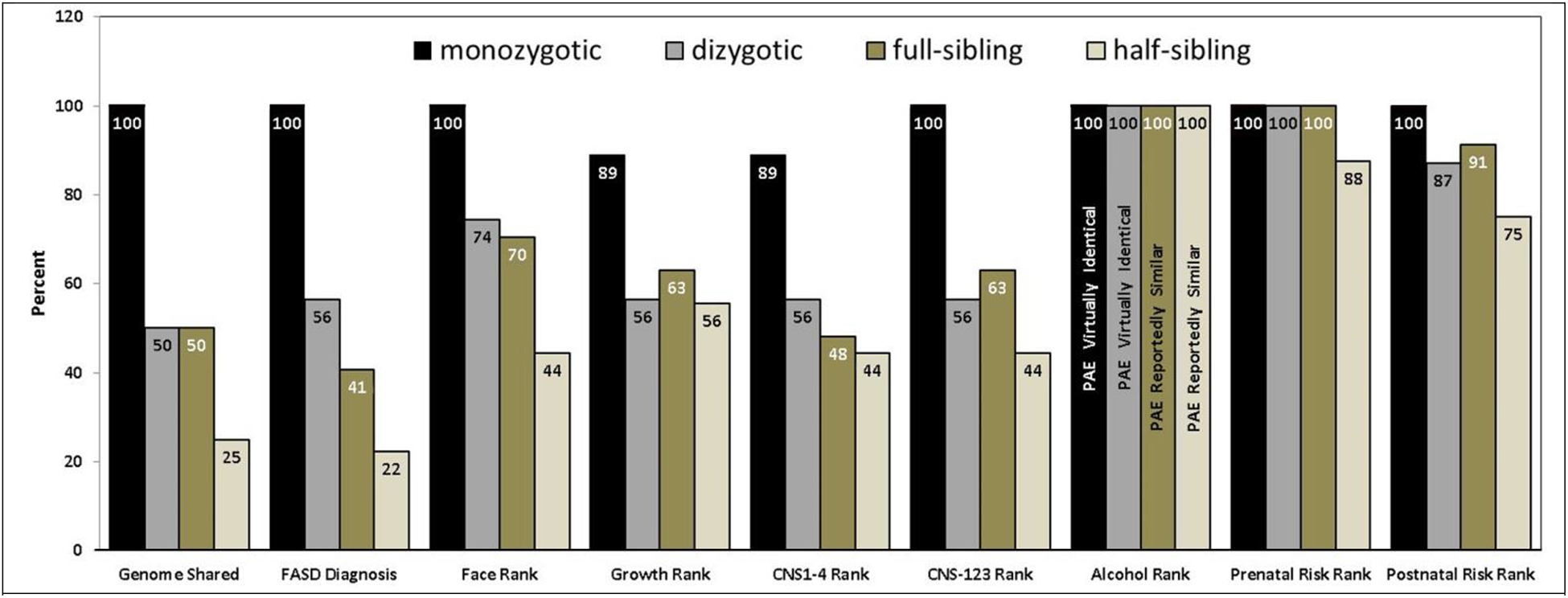
Monozygotic twins, dizygotic twins, full siblings and half siblings share 100%, 50%, 50% and 25% of their genome respectively as depicted by the first set of bars. If fetal genetics is modifying the teratogenic impact of PAE, the pattern of pairwise concordance reflected in the bars for each FASD outcome will more closely resemble the pattern of bars for Genome Shared than the patterns of bars reflecting pairwise concordance in Alcohol Rank, other Prenatal Risks or Postnatal Risks. The bar patterns across all FASD outcomes are far more reflective of the pattern of bars for Genome Shared than the pattern of bars for Alcohol Rank, Prenatal Risk Rank or Postnatal Risk Rank. Although the bar pattern for Postnatal Risk Rank resembles the bar pattern for Face Rank, discordance in postnatal risk factors cannot be contributing to discordance in Face Rank because only prenatal factors can impact facial morphology. Since the FAS facial phenotype, as defined by the 4-Digit Code, is so specific to (caused only by) PAE, the most compelling evidence supporting the role genetics plays in modifying the teratogenic impact of PAE is illustrated in how highly correlated the bar patterns are between Genome Shared and Face Rank and how poorly correlated the bar patterns are between Face Rank and Alcohol Rank (especially between monozygotic and dizygotic twins with virtually identical PAE).
When looking at the sub components that define FASD (growth deficiency, FAS facial phenotype, and CNS structural and/or functional abnormalities), the prevalence of pairwise discordance in the 4-Digit Rank for each of these components increased across the 4 groups as the proportion of genome shared between siblings decreased (Table 4, Figure 2). It is interesting to note that the prevalence of pairwise discordance in the Face Rank was the only component that increased significantly and linearly as the proportion of genome shared between siblings across the four groups decreased. The Rank 4 FAS facial phenotype, as defined by the 4-Digit Code, is the only component of FASD that is confirmed to be specific to (caused only by) PAE [7]. Other prenatal and postnatal risks can impact growth and brain development, but only PAE can cause the FAS facial phenotype. Thus, even though the prevalence of pairwise discordance in the Postnatal Risk Rank increased significantly across the 4 groups (monozygotic: 0%, dizygotic: 12.8%, full-sibling: 16.7%, and half-sibling: 25.0%) (Table 3, Figure 2), discordance in postnatal risk factors cannot influence the pairwise discordance observed in Face Ranks. It is also interesting to note that the proportion of twin and sibling pairs within each study group that have the same (concordant) Face Ranks closely matches the proportion of the genome shared between the twin and sibling pairs within each study group (Figure 2).
There was no evidence in this study population that gender influenced the severity or pairwise discordance of FASD diagnostic outcomes. The severity of the FASD diagnosis was comparable between males and females across the entire study sample (n = 168) (Chi2 = 5.4, p = 0.14).
Among the 19 dizygotic twins with discordant genders:
The female had a more severe FASD outcome than the male in 31.6% of the pairs (6/19)
The male had a more severe FASD outcome than the female in 26.3% of the pairs (5/19)
The male and female had the same FASD outcome in 42.1% of the pairs (8/19)
Specific Aim 3: Magnitude of FASD diagnostic discordance between twin pairs
FASD Diagnostic Discordance:
Since there was 100% diagnostic concordance between monozygotic twin pairs, this analysis focused on the 39 dizygotic twin pairs. Despite virtually identical PAE, 4 of the 39 dizygotic twin pairs had FASD diagnostic contrasts as large as PFAS vs ND/AE (Table 4). The sibling in each sibling pair with PFAS experienced severe CNS functional and/or structural abnormalities (i.e., the 3rd digit in their 4-Digit Code was Rank 3 or 4) while their co-twin experienced low to moderate CNS dysfunction (i.e., the 3rd digit in their 4-Digit Code was Rank 2). Two of these large contrasts occurred among same-sex dizygotic twins, (4334 vs 4324, 1434 vs 1224) and two occurred among mixed-gender dizygotic twins (4344 vs 2324, 1343 vs 1123). The four twin pairs had virtually identical PAE, were raised together, and had reportedly comparable prenatal and postnatal experiences (e.g., prenatal tobacco exposure, prenatal exposure to illicit drugs, multiple home placements, neglect, and/or physical/sexual abuse). In other words, their contrasts in FASD outcomes would appear to better-explained by their discordant genetic vulnerability to PAE, than their discordant environmental influences.
Four full-sibling pairs and one half-sibling pair also experienced large contrasts in FASD diagnoses (FAS vs ND/AE: 4-Digit Codes: 3443 vs 1223 and 3343 vs 1123) and PFAS vs ND/AE: 4-Digit Codes: 2434 vs 2324; 1443 vs 1323 and 2324 vs 4344) (Table 4). And like the dizygotic twins described above, these five sibling pairs had the same Prenatal Alcohol Ranks, were raised together, and had reportedly comparable prenatal and postnatal experiences (e.g., prenatal tobacco exposure, prenatal exposure to illicit drugs, multiple home placements, neglect, and/or physical/sexual abuse). Once again, their contrasts in FASD outcomes would appear to better-explained by their discordant genetic vulnerability to PAE, than their discordant environmental influences.
FAS Facial Phenotype Discordance:
The 4-Digit Code ranks the magnitude of expression of the FAS facial phenotype on a 4-point Likert scale (Rank 1: absent; Rank 2: mild; Rank 3: moderate; Rank 4: severe) (Figure 1A). As the proportion of genome shared between siblings decreased from 100% to 50% to 50% to 25% across the four groups (9 monozygotic, 39 dizygotic, 27 full-sibling and 9 half-sibling pairs, respectively), the prevalence of pairwise discordance in the FAS Facial Rank increased from 0.0% to 25.6% to 29.6% to 55.6% (Table 4). The prevalence of concordance in Facial Rank across the four groups closely followed the proportion of genome shared between siblings across the four groups (Figure 2).
Since the Rank 4 FAS facial phenotype, as defined by the 4-Digit Code, is the only FASD physical feature confirmed to be highly specific to (caused only by) PAE [7] it is interesting to document the prevalence of pairwise discordance (if any) involving the Rank 4 FAS facial phenotype (Table 4).
Two of the nine monozygotic twin pairs presented with the Rank 4 FAS facial phenotype. Both twins in each pair presented with concordant Rank 4 faces. Three of the 39 dizygotic twin pairs presented with the Rank 4 FAS facial phenotype; two pairs presented with concordant Rank 4 facial phenotypes and one pair presented with discordant Face Ranks (Rank 4 vs Rank 2). One twin in the discordant dizygotic twin pair presented with PFAS and a Rank 4 FAS facial phenotype. All three facial features of FAS were present—short PFLs, smooth philtrum and thin upper lip (mean PFL z-score −3.5, philtrum smoothness Rank 5, upper lip thinness Rank 4). In contrast, the co-twin presented with ND/AE and a Rank 2 Facial Phenotype. Only one of the three FAS facial features was present—a thin upper lip (mean PFL z-score −1.8, philtrum smoothness Rank 3, upper lip thinness Rank 4).
The Rank 4 facial phenotype was also observed among full-siblings and half-siblings (Table 4). Three of the 27 full-sibling pairs presented with the Rank 4 facial phenotype—all three pairs were discordant (Face Ranks 2 vs 4 and Face Ranks 3 vs 4). The Rank 4 facial phenotype was also observed in two of the 9 half-sibling pairs—both pairs had discordant Face Ranks (Face Ranks 2 vs 4). Even though the Alcohol Ranks were concordant for each of these five full-sibling and half-sibling pairs, this does not ensure that the day-to-day level of PAE was identical between each sibling pair.
Specific Aim 4: Heritability
Comparing pairwise discordance in FASD outcomes for monozygotic versus dizygotic twins allows an indirect estimate of the importance of genetic factors in modifying the teratogenic effects of PAE in this study sample. Percent heritability (((dizygotic discordance minus monozygotic discordance)/dizygotic discordance)*100) for different FASD outcomes in the current study were as follows:
-
FASD diagnosis (FAS, PFAS, SE/AE, ND/AE, Not FASD/AE)
((0.436 – 0.00) / 0.436)*100 = 100%
-
FAS Facial Rank:
((0.256 – 0.0) / 0.256)*100 = 100%
-
Growth Rank
((0.436 – 0.111)/0.436)*100 = 74.5%
-
CNS 1–4 Rank
((0.436 – 0.111)/0.436)*100 = 74.5%
Monozygotic twin pairs in this study had virtually identical genomes and virtually identical PAE. Under those genetic-environmental conditions, their FASD diagnoses and FAS facial phenotype Ranks were identical (0% discordant). In contrast, the dizygotic twin pairs in this study had virtually identical PAE, but shared only 50% of their genomes. Under those genetic-environmental conditions, 43.6% of the twin pairs had discordant FASD diagnoses and 25.6% had discordant FAS Facial phenotype Ranks. Based on these discordance rates for the monozygotic and dizygotic twin pairs, heritability estimates for the FASD diagnosis and the FAS Facial Rank were both 100%. In other words, essentially all of the discordance observed between twin pairs for these two outcomes appears to be due to differences in their genotypes, not differences in their environmental risk factors.
Heritability estimates for the Growth Rank and the CNS 1–4 Rank were both 74.5%, signifying that the Growth and CNS Ranks were determined by a combination of genetic and environmental factors. Although the prenatal environmental factor PAE was virtually identical between the twin pairs, PAE was not the only environmental risk factor in this study that could adversely impact growth and CNS development. Other prenatal risk factors like prenatal exposure to tobacco and other illicit drugs can impact growth and CNS development, but they, like PAE were virtually identical between the twin pairs. Thus, it is unlikely that these other prenatal risk factors explain the discordance in growth and CNS development observed between the twin pairs. On the other hand, postnatal environmental risk factors like neglect, abuse, and multiple home placements can adversely impact a child’s growth and CNS development and did vary slightly between the dizygotic twin pairs—5 of the 39 dizygotic pairs had discordant Postnatal Risk Ranks (Table 3). But a much higher number of dizygotic pairs had discordant Growth Ranks (n = 17) and discordant CNS 1–4 Ranks (N = 17) (Table 4). More specifically, not all 5 dizygotic pairs with discordant Postnatal Risk Ranks had discordant Growth or CNS 1–4 Ranks. Only 2 of the 5 pairs had discordant Growth Ranks and only 3 of the 5 pairs had discordant CNS 1–4 Ranks. Stated another way, only 2 (12%) of the 17 dizygotic pairs with discordant Growth Ranks had discordant Postnatal Ranks, and only 3 (18%) of the 17 dizygotic pairs with discordant CNS1–4 Ranks had discordant Postnatal Ranks. Thus, as the heritability estimates suggest, variations in the Growth and CNS 1–4 Ranks appeared to be influenced by both genetic and environmental factors. Overall, the heritability estimates generated for the growth (74.5%), FAS face (100%) and CNS (74.5%) components of FASD are reflective of the fact that growth and CNS development are susceptible to a multitude of prenatal and postnatal environmental risk factors, whereas the FAS facial phenotype is highly specific to early prenatal exposure to alcohol.
If fetal genotype is modifying the teratogenic impact of PAE, the prevalence of pairwise concordance across FASD outcomes would more closely reflect the percent of genome shared between sibling pairs than the pairwise concordance of PAE and other prenatal and postnatal risk factors. This is illustrated graphically In Figure 2 (and Table 4). Monozygotic twins, dizygotic twins, full siblings and half siblings share on average 100%, 50%, 50% and 25% of their genome respectively as depicted by the first set of bars. The pattern of pairwise concordance reflected in the bars for each FASD outcome more closely resemble the pattern of bars for Genome Shared than the patterns of bars reflecting pairwise concordance in Alcohol Rank, other Prenatal Risks, or Postnatal Risks. Since the FAS facial phenotype, as defined by the 4-Digit Code, is so highly specific to (caused only by) PAE, the most compelling evidence supporting the role genetics plays in modifying the teratogenic impact of PAE is illustrated in how highly correlated the bar patterns are between Genome Shared and Face Rank-and how poorly correlated the bar patterns are between Face Rank and Alcohol Rank (especially between monozygotic and dizygotic twin pairs with virtually identical PAE).
Discussion
Fetal genotype modifies the teratogenic effects of PAE:
The outcomes of this study provide conclusive evidence that fetal genotype can modify the teratogenic effects of PAE. When twin pairs with virtually identical PAE were genetically identical, their FASD diagnoses were identical. When twin pairs with virtually identical PAE were genetically different, their FASD diagnoses were often different (44% presented with discordant FASD diagnoses). And when their diagnoses were discordant, the magnitude of discordance was extreme 10% of the time. For example, four of the 39 pairs of dizygotic twins were born at opposite ends of the fetal alcohol spectrum (PFAS and ND/AE), despite virtually identical PAE. Finally, as the proportion of genome shared between siblings decreased from 100% to 50% to 50% to 25% across the four study groups (monozygotic, dizygotic, full-sibling and half-sibling pairs respectively), the prevalence of pairwise discordance in FASD diagnoses increased linearly from 0% to 44% to 59% to 78%.
The prevalence of pairwise concordance in FASD diagnoses observed in our 48 twin pairs (monozygotic 100%, dizygotic 56%) was comparable to the prevalence of concordance observed in 16 twin pairs (monozygotic 100%, dizygotic 64%) reported by Streissguth and DeHaene [6] back in 1993—the only other FASD twin group study published to date. The 16 twin pairs (5 monozygotic and 11 dizygotic) were born to alcohol-abusing mothers from two countries (the United States and France). The study population included 11 Caucasian and 5 Native American twin pairs ranging in age from 1.5 to 30 years. The study was conducted prior to the creation of rigorous, case-defined FASD diagnostic systems. Patients were diagnosed as FAS or Fetal Alcohol Effects (FAE) in accordance with gestalt approach to diagnosis published by Clarren and Smith in 1978 [12]. Thirty-nine percent had FAS, 19% had FAE and 42% were alcohol exposed but unaffected. The higher concordance observed in their dizygotic twin pairs will be due in part to the fact that concordance in a study using only two FASD diagnoses (FAS and FAE) will always be higher than concordance in a study using 4 FASD diagnoses (FAS, PFAS, SE/AE, and ND/AE). Over the years, the outcomes of ten additional twin pairs with PAE have been published as single-case studies [13–20]. Formal FASD diagnostic evaluations were rarely conducted, but the clinical descriptions of the twin pairs were consistent with monozygotic twin pairs having more concordant outcomes than dizygotic twin pairs.
Similar to humans, evidence of genetic modification of FASD outcomes also come from animal studies. For example, a study by Debelak and Smith [21] examined 11 genetic strains of chick embryos following ethanol exposure during early neurulation and found that the strains could be classified into very sensitive, moderately sensitive, or insensitive to ethanol-induced apoptosis of cranial neural crest cells, which give rise to facial structures. Comprehensive reviews on the genetics of FASD are presented by Mead and Sarkar [4] and Eberhart and Parnell [5].
Discordance in the FAS facial phenotype:
It is interesting to note the rather high prevalence of pairwise discordance (43.6%) in FASD diagnoses among dizygotic twins in this study, despite virtually identical PAE. It is clear that discordance in PAE does not explain their discordant FASD diagnoses, but are there factors other than PAE that may explain the discordance? FASD is characterized by growth deficiency, a specific cluster of minor facial anomalies, and abnormal CNS structure and/or function. Only the Rank 4 FAS facial phenotype, as defined by the 4-Digit Code, is confirmed to be specific to (caused only by) PAE [7]. The FAS facial phenotype is not caused by other prenatal and postnatal risk factors. In contrast, growth deficiency and CNS structural/functional abnormalities can be caused by a multitude of other prenatal and postnatal risk factors. Despite efforts to minimize postnatal contrasts between the twin pairs in this study (Table 3, Figure 1), some of the pairwise discordance in growth and CNS outcomes used to generate the FASD diagnoses is likely explained, in part, by discordant postnatal risk factors.
Documenting the prevalence of discordance (if any) for the Rank 4 FAS facial phenotype is of particular interest because the Rank 4 FAS facial phenotype is so highly specific to PAE [7]. If identical PAE can result in discordant Rank 4 facial phenotypes between twin pairs, this would further strengthen the evidence that genes are modifying the teratogenic impact of PAE. Since the FAS facial phenotype requires PAE in a very narrow window of time (during the gastrulation period of fetal development) [22, 23], the only pairwise discordance in Rank 4 facial phenotypes that would be meaningful (that could be validly interpreted) would be among monozygotic and dizygotic twin pairs—the only two groups where the timing of PAE can be confirmed to be virtually identical on a day-to-day basis between twin pairs.
Two of the nine monozygotic twin pairs presented with Rank 4 FAS facial phenotypes. Both twins in each pair presented with concordant Rank 4 faces. Three of the 39 dizygotic twin pairs presented with Rank 4 FAS facial phenotypes; two pairs presented with concordant Rank 4 faces and one pair presented with discordant Face Ranks (Rank 4 vs Rank 2). The twin pair with discordant Face Ranks presented as follows: Twin 1: PFAS, Face Rank 4, all three of the FAS facial features (mean PFL z-score −3.5, philtrum smoothness Rank 5, upper lip thinness Rank 4). Twin 2: ND/AE, Face Rank 2, only 1 of the 3 FAS facial features—a thin upper lip (mean PFL z-score −1.8, philtrum smoothness Rank 3, upper lip thinness Rank 4). The work by Das et al., [24], presented below, provides a compelling genetic explanation for why Face Ranks were always concordant among monozygotic twins, but occasionally discordant among dizygotic twins.
Das et al., [24] reported a significant gene-environment interaction explaining variation in facial morphology associated with ethanol use in pregnancy. Genetic diversity in ethanol metabolizing enzymes occurs in the general population. Ethanol is metabolized to acetaldehyde by two enzyme systems: the microsomal ethanol oxidizing system and alcohol dehydrogenase (ADH) [25, 26]. The presence of the ADH1B*3 allele has been found to be protective for offspring neurodevelopmental and growth outcome after maternal ethanol consumption in pregnancy [27]. In 2004, Das et al [24] demonstrated that among African American women and their offspring, the presence of an ADH1B*3 allele was protective for the effects of maternal ethanol ingestion during pregnancy on infant facial formation. The protective effect demonstrated was present with the allele present in only the mother, only the infant, or both the mother and the infant. Exposure to ethanol and absence of the ADH1B*3 allele in both the mother and infant resulted in significant reductions in three facial measurements obtained from infant facial photographs-palpebral fissure length, inner canthal distance and the distance from the bridge of the nose to the bottom of the upper lip. Based on the findings of Das et al., [24] one could speculate that discordant FAS Face Ranks could occur in dizygotic twins (as observed in our study) if the ADH1B*3 allele was absent in the mother and one twin, but present in the other twin. Based on the same line of reasoning, one would expect monozygotic twins to always present with concordant Face Ranks (as was observed in our study). Replication of the Das et al., study using a study population of monozygotic and dizygotic twins with PAE would greatly advance our understanding how the ADH1B*3 allele modifies the teratogenic impact of PAE.
Implications for public health messaging and setting FASD diagnostic exposure thresholds:
Despite virtually identical PAE, 4/39 (10%) dizygotic twin pairs had FASD diagnostic contrasts as large as PFAS vs ND/AE. The four twin pairs had virtually identical PAE, were raised together, and had reportedly identical prenatal and postnatal experiences (e.g., prenatal tobacco, illicit drug exposure, home placements, physical/sexual abuse). In other words, their contrasts in FASD outcomes would appear to better-explained by their discordant genetic vulnerability to PAE, than their discordant environmental influences. These 4 twin pairs provide powerful evidence that what may be construed in public health messaging (and some FASD diagnostic guidelines [28–30] as a safe level of exposure for one fetus, may very well place another fetus at significant risk. Not only can the same level of PAE cause strikingly different outcomes in two fetuses, but PAE reportedly below the threshold of exposure required by some FASD diagnostic guidelines can result in full FAS (See Figure 9 in Astley, et al., [31]). Thus, as stated by the U.S. Centers for Disease Control and Prevention [32] “There is no guaranteed safe level of alcohol use at any time during pregnancy. Fetal alcohol spectrum disorders are completely preventable if a woman does not drink during pregnancy.”
Potential Limitations
Zygosity Classification:
Twins were classified as monozygotic or dizygotic for this study based on clinical and social service records shared with the FASDPN clinic at the time of their FASD evaluation. It is unknown how many twin pairs had zygosity confirmed through DNA genotyping. While there remains a small chance that one or more twin pairs in this study have their zygosity misclassified, a study of 578 twin pairs conducted by the National Academy of Sciences found parent report of zygosity was confirmed accurate by DNA genotyping over 95% of the time [33]. When misclassification occurred, it was most likely to occur among monozygotic twins who were not strikingly similar in appearance and thus incorrectly classified as dizygotic. This direction of error would lead to more conservative estimates of heritability. In the current study, all monozygotic twins looked identical and all dizygotic twins were easily distinguished from one another.
Do twins share identical prenatal environments?
Not necessarily. It is for that reason the prenatal environments and PAE shared between our 48 twin pairs is described throughout this study as virtually identical. How twins experience the prenatal environment depends, in part, on chorionicity, i.e., whether twins share a single chorion (monochorionic) or have separate chorions (dichorionic). Monozygotic twins can be mono- or dichorionic, whereas dizygotic twins are dichorionic [34]. The chorionicity of the 9 monozygotic twin pairs in the current study was unknown. The chorion is the outer-most fetal membrane that contains the amnion/amniotic sac. The amnion is the thin inner-most fetal membrane that protects the embryo/fetus and contains amniotic fluid. The chorion connects the amnion, amniotic sac, and the fetus to the placenta and contributes to placental development. Thus, if twins share a chorion (e.g., are monochorionic) they will share a single placenta, whereas twins with separate chorions (e.g., dichorionic) develop individual placentas. Dizygotic twins are dichorionic, since they form from two separately fertilized eggs. Among Caucasian populations, total twinning rates were estimated at 15–16 per 1,000 in 2003 [35]. In Caucasian populations, monozygotic twins comprise ~26% of all twins. For Caucasian populations about 17% of all twin pairs are monozygotic-monochorionic, ~9% are monozygotic-dichorionic and ~74% are dizygotic-dichorionic. All twins can be expected to have many kinds of in-utero differences, such as placental flow in monozygotic twins and the amount of microchimerism in dizygotic twins [36]. The greatest risk associated with monochorionic placentation is related to the structure of blood vessels. One twin typically has better placement and therefore receives more of the nutrients [34]. The placenta also functions as a barrier, allowing small molecules (e.g., gases, nutrients, waste material, antibodies) to pass between mother and child through passive transport [37, 38]. Other small molecules that can impact fetal development (e.g., some maternal hormones like cortisol; bacteria; teratogens such as alcohol) can also be diffused through the placenta [37, 39]. Unequal placental sharing is a major cause of fetal growth discordance in monozygotic twins [40]. It is interesting to note that one of the few occurrences of discordant outcomes between monozygotic twins in the current study was discordant growth in one twin pair (Growth Rank 3 versus Growth Rank 1). Both twins had concordant weight percentiles (ranging from the 20th to 40th percentiles) at birth and 2 years of age. Height percentiles, however, were significantly discordant. One twin was significantly shorter (1st and 10th percentiles at birth and 2 years of age) than the other (20th and 60th percentiles at birth and 2 years of age). All in all, while prenatal environments, including level of PAE, are not necessarily 100% identical between twin pairs, the near perfect match between percent of genome shared and FASD diagnostic concordance (monozygotic twins: 100% genome shared and 100% FASD concordance; dizygotic twins: 50% genome shared, 56% FASD concordance) suggests the prenatal -environments and PAE levels in our 48 twin pairs were virtually identical.
Conclusions
Not all fetuses are equally vulnerable to the adverse effects of prenatal alcohol exposure. Risk is not just dependent on timing and level of exposure. Fetal genetics plays an important role. As demonstrated in this study, despite virtually identical prenatal alcohol exposures, two fetuses can experience vastly different FASD outcomes. So which fetus is genetically vulnerable? We currently have no way of knowing. Thus, to protect all fetuses, especially the most genetically vulnerable, the only safe amount to drink is none at all.
Acknowledgements
The Washington State FASDPN has been supported over the past 27 years by the following institutions: Centers for Disease Control and Prevention (1992-1997); Western Washington Chapter of the National March of Dimes Birth Defects Foundation (1995); Washington State Department of Social and Health Services, Division of Alcohol and Substance Abuse (1997-present); National Institute of Alcohol Abuse and Alcoholism (2000-2003); the Chavez Memorial Fund (2002-present) and the Center on Human Development and Disability, University of Washington (1993 to present).
Footnotes
Ethical approval
This study was approved by the University of Washington Human Subjects Division.
Contributor Information
Susan J. Astley Hemingway, Department of Epidemiology and Department of Pediatrics, University of Washington, Seattle, WA USA..
Julia M. Bledsoe, Department of Pediatrics, University of Washington, Seattle, WA, USA..
Allison Brooks, Department of Epidemiology, University of Washington, Seattle, WA USA..
Julian K. Davies, Department of Pediatrics, University of Washington, Seattle, WA, USA..
Tracy Jirikowic, Department of Rehabilitation Medicine, University of Washington, Seattle, WA, USA..
Erin M. Olson, College of Education, University of Washington, Seattle, WA, USA..
John C. Thorne, Department of Speech and Hearing Sciences, University of Washington, Seattle, WA, USA..
References
- 1.Wilson JG. Current Status of Teratology: General principles and mechanisms derived from animal studies In Wilson JG, Fraser FC (eds): “Handbook of Teratology, Vol. 1, General Principles and Etiology” London: Plenum Press, London, pp 47–74, 1977 [Google Scholar]
- 2.Astley SJ. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code, 3rd ed., Seattle WA, University of Washington Publication Services; 2004. Available from: http://depts.washington.edu/fasdpn/pdfs/guide04.pdf [Google Scholar]
- 3.Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol exposed individuals: Introducing the 4-Digit Diagnostic Code. Alcohol Alcohol. 2000;35:400–410. [DOI] [PubMed] [Google Scholar]
- 4.Mead EA and Sarkar DK. Fetal alcohol spectrum disorders and their transmission through genetic and epigenetic mechanisms, Frontiers in Genetics, 2014;5:1–10. DOI: 10.3389/fgene.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhart JK, Parnell SE. The genetics of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2016;40(6)1154–1165, DOI: 10.1111/acer.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streissguth AP, Dehaene P. Fetal alcohol syndrome in twins of alcoholic mothers: concordance of diagnosis and IQ. Am J Med Genet. 1993;47:857–861. [DOI] [PubMed] [Google Scholar]
- 7.Astley SJ. Validation of the fetal alcohol spectrum disorder (FASD) 4-Digit Diagnostic Code. J Popul Ther Clin Pharmacol. 2013;20(3):e416–467. [PubMed] [Google Scholar]
- 8.Astley SJ. FAS Facial Photographic Analysis Software [computer program] Version 2.1 Seattle: University of Washington; 2016. https://depts.washington.edu/fasdpn/htmls/face-software.htm. [Google Scholar]
- 9.Neale MC, Maes HHM. (2004) Methodology for genetic studies of twins and families. Kluwer Academic Publisher B.V. Dordrecht, The Netherlands. [Google Scholar]
- 10.Astley SJ. Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington State Fetal Alcohol Syndrome Diagnostic & Prevention Network. Canadian Journal of Clinical Pharmacology, Vol 17 (1) Winter 2010:e132–e164: March 26, 2010. [PubMed] [Google Scholar]
- 11.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era – concepts and misconceptions. Nature Review Genetics, 2008;9:255–266. DOI: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 12.Clarren SK, Smith DW The fetal alcohol syndrome. N Engl J Med 1978;298:1063–1067. [DOI] [PubMed] [Google Scholar]
- 13.Chasnoff IJ. Fetal alcohol syndrome in twin pregnancy. Acta Genet Med Gemellol (Roma). 1985;34(3–4):229–232. [DOI] [PubMed] [Google Scholar]
- 14.Christoffel KK, Salafsky I. Fetal alcohol syndrome in dizygotic twins. J Pediatrics. 1975;87(6):963–967. [DOI] [PubMed] [Google Scholar]
- 15.Crain LS, Fitzmaurice NE, Mondry C. Nail dysplasia and fetal alcohol syndrome: case report of a heteropaternal sibship. Am J Dis Child. 1983;137:1069–1072. [DOI] [PubMed] [Google Scholar]
- 16.Lesure JF. L’embryofetopathie alcoolique a ile de la Reunion: un drame social. Rev Pediatr. 1988;24:265–271. [Google Scholar]
- 17.Miller M, Israel J, Cuttone J. Fetal alcohol syndrome. J Pediatr Ophthalmol Strabismus 1981;18:6–15. [DOI] [PubMed] [Google Scholar]
- 18.Palmer RH, Ouelette EM, Warner L, Leichtman SR. Congenital malformations in offspring of a chronic alcoholic mother. Pediatrics 1974;53:490–494. [PubMed] [Google Scholar]
- 19.Riese ML. Maternal alcohol and pentazocine abuse: Neonatal behavior and morphology in an opposite-sex twin pair. Acta Genet Med Gemello (Roma) 1989;38:49–56. [DOI] [PubMed] [Google Scholar]
- 20.Riikonen RS. Difference in susceptibility to teratogenic effects of alcohol in discordant tins exposed to alcohol during the second half of gestation. Pediatric Neurology 1994;11(4):332–336. [DOI] [PubMed] [Google Scholar]
- 21.Debelak KA, Smith SM. Avian genetic background modulates the neural crest apoptosis induced by ethanol exposure. Alcohol Clin Exp Res. 2000;24:307–314. [PubMed] [Google Scholar]
- 22.Sulik KK. Critical periods for alcohol teratogenesis in mice, with special reference to the gastrulation stage of embyogenesis Mechanisms of alcohol damage in utero. Ciba Found Symp 1984;105:124–141. [DOI] [PubMed] [Google Scholar]
- 23.Astley SJ, Magnuson SI, Omnell LM, Clarren SK. Fetal alcohol syndrome: Changes in craniofacial form with age, cognition, and timing of ethanol exposure in the Macaque. Teratology 1999;59;163–172. [DOI] [PubMed] [Google Scholar]
- 24.Das UG, Cronk CE, Martier SS, Simpson PM, McCarver DG. Alcohol Dehydrogenase 2*3 Affects Alterations in Offspring Facial Morphology Associated With Maternal Ethanol Intake in Pregnancy, Alcohol Clin Exp Res. 2004;28(10)1598–1606, DOI: 10.1097/01.ALC.0000141816.14776.97. [DOI] [PubMed] [Google Scholar]
- 25.Crabb DW, Bosron WF, Li TK. Ethanol metabolism. Pharmacol Ther. 1987;34:59–73. [DOI] [PubMed] [Google Scholar]
- 26.Ehrig T, Bosron WF, Li TK. Alcohol and aldehyde dehydrogenase. Alcohol Alcohol 1990;25:105–116. [DOI] [PubMed] [Google Scholar]
- 27.McCarver DG, Thomasson HR, Martier SS, Sokol RJ, Li T. Alcohol dehydrogenase-2*3 allele protects against alcohol-related birth defects among African Americans. J Pharmacol Exp Ther 1977;283:1095–1101. [PubMed] [Google Scholar]
- 28.Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, et al. Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ 2015, DOI 10.1503/cmaj.141593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, et al. 2016 Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics. 2016;138(2):e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petryk SC, Ekeh J, Pandey M. The prenatal alcohol history – it is hard to get and it matters how we define it 7th International Conference on FASD Research: Results and Relevance: Session D3. March 1–4, 2017. Vancouver BC, Canada: [accessed 2019, December 12] Available from: http://interprofessional.ubc.ca/files/2017/03/D3a-Petryk.pdf [Google Scholar]
- 31.Astley SJ, Bledsoe JM, Davies JK, Thorne JC. Comparison of the FASD 4-Digit Code and Hoyme et al. 2016 FASD diagnostic guidelines. Advances in Pediatric Research 2017;4(13):1–26 DOI: 10.12715/apr.2017.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Fetal alcohol Spectrum Disorders. [accessed 2019, December 12] Available from: https://www.cdc.gov/ncbddd/fasd/features/key-finding-understanding-health-messages.html
- 33.Reed T, Plassman BL, Tanner CM, Dick DM, Rinehart SA, Nichols WC. Verification of self-report of zygosity determined via DNA testing in a subset of the NAS-NRC Twin Registry 40 years later. Twin Res Hum Genet. 2005;8(4):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marceau K, McMaster MTB, Smith TF, Daams JG, van Beijsterveldt CEM, Boomsma DI, Knopik VS. The prenatal environment in twin studies: A review on chorionicity. Behav Genet 2016;46;286–303. DOI: 10.1007/s10519-016-9782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoekstra C, Zhao ZZ, Lambalk CB, Willemsen G, Martin NG, Boomsma DI et al. Dizygotic twinning. Hum Reprod Update 2008;14(1):37–47. DOI: 10.1093/humupd/dmm036. [DOI] [PubMed] [Google Scholar]
- 36.Hall JG. Twinning. Lancet 2003, 362(9385):735–743. doi: 10.1016/S0140-6736(03)14237-7. [DOI] [PubMed] [Google Scholar]
- 37.Page KR. The physiology of the human placenta. Kings Lynn and Guilford Biddles Ltd, London, 1993. [Google Scholar]
- 38.Schneider H Placental transport function. Reprod Fertil Dev 1991;3(4):345–353. DOI: 10.1071/RD9910345. [DOI] [PubMed] [Google Scholar]
- 39.van der Aa E, Peereboom-Stegeman C, Noordhoek J, Gribnau FJ, Russel FM. Mechanisms of drug transfer across the human placenta. Pharm World Sci 1998;20(4):139–148. DOI: 10.1023/A:1008656928861. [DOI] [PubMed] [Google Scholar]
- 40.Chang YL. Monochorionic twin with selective intrauterine growth restriction. [Review]. J Med Ultrasound. 2008;16(3):194–201. DOI: 10.1016/S0929-6441(08)60048-X. [DOI] [Google Scholar]


