Summary
Galectins are soluble lectins that participate in many physiological and pathological functions. Since they can act extracellularly, the use of the recombinant protein is a recurrent strategy for studying their biological functions. Here, we provide a general protocol for the production of Galectins and their isolated or chimeric domains. We take advantage of their lectin activity and the 6xHis-tag addition for purification, thus obtaining a highly pure and active Galectin to use in both in vitro and in vivo assays.
For complete details on the use and execution of this protocol, please refer to Cattaneo et al. (2011), Tribulatti et al. (2012), and Prato et al. (2020).
Subject Areas: Cell Biology, Molecular Biology, Protein Biochemistry, Protein expression and purification
Graphical Abstract

Highlights
-
•
We present a protocol for obtaining highly pure recombinant Galectins
-
•
This protocol can be adapted for several Galectin family members with high yield
-
•
Recombinant galectins retain their lectin activity as assessed by hemagglutination
-
•
Recombinant Galectins are endotoxin-free and can be used in cell culture assays
Galectins are soluble lectins that participate in many physiological and pathological functions. Since they can act extracellularly, the use of the recombinant protein is a recurrent strategy for studying their biological functions. Here, we provide a general protocol for the production of Galectins and their isolated or chimeric domains. We take advantage of their lectin activity and the 6xHis-tag addition for purification, thus obtaining a highly pure and active Galectin to use in both in vitro and in vivo assays.
Before You Begin
Cloning Galectin DNA Coding Sequence in an Expression Plasmid
Timing: n/a
-
1.Use the molecular biology technique of choice to clone the DNA sequence containing the open reading frame encoding the Galectin of interest in an expression vector for bacteria with the following characteristics:
-
a.N-terminal poly-histidine tag (6xHis)
-
b.Resistance to Ampicillin
-
c.IPTG-inducible expression using the regulatory sequences of a commercially available plasmid.
-
a.
For example, our designed optimized encoding sequence of human Gal-8 isoform M (hGal-8M) was sent for synthesis to GenScript and then cloned in the pTrcHisB expression plasmid.
Note I: Galectin DNA codons should be optimized for expression in bacteria (this is already included in the GenScript gene synthesis services).
Note II: We recommend propagating and maintaining the plasmid encoding Galectin insert in an adequate Escherichia coli strain such as TOP10 or DH5α.
Obtaining Galectin-Expressing E. coli
Timing: 2 days
-
1.
Transform by heat shock 100 μl of Cl2Ca-competent E. coli BL21 strain with 1 μg of the plasmid containing Galectin insert. To this, incubate the mix of bacteria with DNA on ice for 20 min. Then, place the bottom of the tube in a 42°C water bath for 30 s, immediately followed by 2 min-incubation on ice. Then, add 0.9 mL of LB and incubate for 1 h at 37°C and shaking at 250 rpm. In parallel, also transform bacteria with the empty plasmid as an expression control.
-
2.
Grow transformed bacteria for 18–20 h at 37°C in LB agar plates with Ampicillin.
-
3.Pilot expression:
-
a.Inoculate a single E. coli recombinant colony into 5 mL of terrific broth (TB) with Ampicillin in a 50 mL-conical tube.Optional: Screen several colonies to decide which clone has the maximal Galectin expression.
-
b.Grow for 18–20 h at 37°C with shaking at 250 rpm.
-
c.Transfer 100 μL of 18–20 h culture to 5 mL of TB in a 50 mL-conical tube and reserve the remaining culture at 4°C.
-
d.Grow for 2 h at 37°C with shaking at 250 rpm.
-
e.Add 1 mM IPTG and incubate for another 3 h at 37°C with shaking at 250 rpm.Optional: Save 1 mL aliquot before IPTG addition, centrifuge, and conserve the pellet and supernatant refrigerated or at −20°C. This is an alternate expression control in case of do not have the empty plasmid.
-
f.Centrifuge 3 mL of the culture for 15 min at 14,000 rpm and 4°C and discard the supernatant.
-
g.Resuspend the pellet in 200 μL of 1× PBS plus 2 mM EDTA and 1 mM PMSF.
-
h.Lyse the resuspended bacterial pellet by soaking the sonicator’s small horn/tip during 30 s and setting the power at 15 watts.
-
i.Centrifuge for 10 min at 14,000 rpm and 4°C.
-
j.Reserve the supernatant and resuspend the pellet in 200 μL of 8 M urea.
-
k.Run an aliquot from the resuspended pellet (5 μL), the supernatant (10 μL), and the Low Molecular Weight (LMW) protein ladder (5 μL) in 10% SDS-PAGE. Stain the gel with Coomassie blue, using the protocol provided by the manufacturer, and verify the presence of a differential band of the expected size in the induced insert-containing clone. If different colonies were screened, decide which clone has the major expression of the recombinant protein in the soluble fraction (Figure 1).
-
a.
Note: In the above-provided protocol, recombinant Galectins are purified from the soluble fraction of bacterial lysate. In the pilot expression, however, the induced protein is usually obtained in the insoluble fraction due to the particular induction conditions and does not necessarily correlate with the scaled-up expression performed at 18°C.
-
4.Glycerol stocks
-
a.Mix gently 500 μL of the reserved culture (point 2c) corresponding to the chosen clone (point 2k) with 500 μL of sterile glycerol.
-
b.Save immediately at −70°C.
-
c.For each purification, inoculate and streak the bacteria from −70°C glycerol stock on an LB agar plate with Ampicillin.
-
a.
Note: Galectin-transformed bacteria glycerol stocks can be stored for at least two years at −70°C. If after that period some differences of protein expression are experienced, a new E. coli transformation should be accomplished.
Figure 1.
Analysis of Recombinant hGal-8 Pilot Expression by Coomassie Blue-Stained SDS-PAGE
A differential band of 36 kDa for Gal-8 M isoform (Gal-8M) and of 40 kDa for the L isoform (Gal-8L) can be observed on insoluble fractions. pTrcHisB corresponds to bacteria transformed with the empty plasmid (negative control). SN, supernatant. P, pellet. M, molecular weight marker.
Galectin Expression in E. coli
Timing: 3 days
-
5.Starter culture
-
a.Transfer a single colony of transformed bacteria (we used pTrcHisB-hGal-8M-transformed E. coli BL-21 strain) to a sterile 50 mL-conical tube containing 5 mL of LB with Ampicillin.
-
b.Incubate for 18–20 h at 37°C with shaking at 250 rpm.
-
a.
-
6.Induction of protein expression
-
a.Expand the starter culture by dilution 1/100 into a sterile 2 L-baffled Erlenmeyer flask containing 500 mL of TB plus Ampicillin.
-
b.Incubate for 3–4 h at 37°C with shaking at 250 rpm until OD600 nm= 1.
-
c.Low the temperature of the culture by refrigeration at 4°C for 2–4 h.
-
d.Add 0.25 mM IPTG and incubate 18–20 h at 18°C with shaking at 250 rpm.
-
a.
Note: For Gal-3, 1 mM IPTG should be added instead for a proper expression induction. We have observed for this particular Galectin that protein yield is very low when IPTG concentrations are below 1 mM.
-
7.Cell collection
-
a.Centrifuge the culture for 15 min at 7,500 rpm and 4°C.
-
b.Discard the supernatant and freeze the bacterial pellet at −70°C.
-
a.
Pause Point: Bacterial pellet can be stored at −70°C for several months.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial Strains | ||
| E. coli BL21 | Merck | Cat#GE27-1542-01 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Isopropyl-β-D-thio-galactopyranoside (IPTG) | Sigma-Aldrich | Cat#I6758 |
| Lysozyme from chicken egg white | Sigma-Aldrich | Cat#L6876 |
| Deoxyribonuclease I from bovine pancreas (DNase) | Sigma-Aldrich | Cat#D5025 |
| D-Lactose monohydrate (Lactose) | Sigma-Aldrich | Cat#61339 |
| Triton X-100 solution | Sigma-Aldrich | Cat#93443 |
| PMSF | Sigma-Aldrich | Cat#P7626 |
| Dulbecco’s PBS, without Ca2+ and Mg2+, powder | Sigma-Aldrich | Cat#59331C |
| Tris-HCl | Sigma-Aldrich | Cat#93363 |
| Tris base | Sigma-Aldrich | Cat#93362 |
| Imidazole | Sigma-Aldrich | Cat#56749 |
| NaCl | Sigma-Aldrich | Cat#S7653 |
| Tryptone | Sigma-Aldrich | Cat#T2559 |
| Yeast extract | Sigma-Aldrich | Cat#Y1625 |
| Ampicillin | Sigma-Aldrich | Cat#A8351 |
| Terrific Broth, modified | Sigma-Aldrich | Cat#T0918 |
| Glycerol | Sigma-Aldrich | Cat#G5150 |
| NaOH | Sigma-Aldrich | Cat#S8045 |
| Bovine serum albumin (BSA) | Sigma-Aldrich | Cat#05470 |
| Glycerol (suitable for cell culture) | Sigma-Aldrich | Cat#G2025 |
| Sodium azide | Sigma-Aldrich | Cat#71289 |
| Pyrogen-free water, suitable for parenteral administration | Tecsolpar | n/a |
| Ethanol | Merck | Cat#100983 |
| EDTA | Sigma-Aldrich | Cat#1614239 |
| Iodoacetamide | Sigma-Aldrich | Cat#I6125 |
| Coomassie Brilliant blue R | Sigma-Aldrich | Cat#B7920 |
| Unstained SDS-PAGE standards, low range (LMW) | Bio-Rad | Cat#1610304 |
| Acrylamide/Bis 19:1 | Invitrogen | Cat#AM9022 |
| SDS | Merck | Cat#436143 |
| TEMED | Thermo Fisher Scientific | Cat#17919 |
| Ammonium persulfate | Sigma-Aldrich | Cat#A3678 |
| Critical Commercial Assays | ||
| α-Lactose-Agarose | Sigma-Aldrich | Cat#L7634 |
| HisTrap™ Fast Flow | GE-Healthcare | Cat#GE17-5319-01 |
| Pierce™ High Capacity Endotoxin Removal Resin | Thermo Fisher Scientific | Cat#88272 |
| QCL-1000TM Endpoint Chromogenic LAL Assay | Lonza | Cat#50-647U |
| Deposited Data | ||
| Human Gal-8 M isoform | GenBank | NM_201543.3 |
| Human Gal-8 L isoform | GenBank | NM_006499.4 |
| Bovine Gal-8 | GenBank | NM_001045954.2 |
| Mouse Gal-8S isoform | GenBank | AK004782.2 |
| Mouse Gal-8 L isoform | GenBank | EF524570.1 |
| Human Gal-3 | GenBank | NM_002306.4 |
| Mouse Gal-3 | GenBank | NM_001145953.1 |
| Human Gal-1 | GenBank | NM_002305.4 |
| Mouse Gal-1 | GenBank | NM_008495.2 |
| Recombinant DNA | ||
| pTrcHisB expression vector | Thermo Fisher Scientific | Cat#V36020 |
| Other | ||
| 0.45 μm syringe filters | Corning | Cat#431220 |
| 0.22 μm syringe filters | Corning | Cat#431219 |
| Ice bucket | Thomas Scientific | n/a |
| 2-mL bed Poly-Prep Chromatography Columns | Bio-Rad | Cat#7311550 |
| Spectra/Por 6 Dialysis tubing, 3.5K MWCO, 18 mm, 33 ft | Thomas Scientific | Cat#3787J84 |
| Branson 450 analog sonifier | Branson | n/a |
| Ecotron thermostatic orbital shaker | Infors HT | n/a |
| Sorvall RC6 high-speed refrigerated centrifuge | Thermo Fisher Scientific | n/a |
| NanoDrop One spectrophotometer | Thermo Fisher Scientific | A38190 |
| Peristaltic pumpa | Cytiva, formerly GE Healthcare Life Sciences | 18111091 |
Alternatively, HisTrap Fast Flow columns can be used with syringes and adequate adaptors if a peristaltic pump is not available.
Materials and Equipment
Culture Broth
Luria Bertani Broth (LB)
10 g/L tryptone,
5 g/L yeast extract,
5 g/L NaCl
Terrific Broth (TB)
47.6 g/L terrific broth,
8 mL/L glycerol
LB Agar Plate
Prepare LB agar containing Ampicillin in Petri dishes. The plates can be wrapped with film foil and stored at 4°C for up to 2 weeks.
Ampicillin
Prepare a stock solution of 100 mg/mL in deionized water (1,000× solution). Filter-sterilize through a 0.22 μm syringe filter. Store the stock solution in aliquots at −20°C. To prepare media, add Ampicillin to a final concentration of 100 μg/mL to the cooled broth.
Buffers
Pyrogen-free PBS
Dissolve PBS powder following the manufacturer’s instructions using pyrogen-free water.
Lysis Buffer
| Reagent | Final Concentration (mM) | Amount (mL) |
|---|---|---|
| Phenylmethylsulfonyl fluoride (PMSF, 100 mM stock in isopropanol), add immediately before use | 1 | 0.3 |
| Triton X-100 | 0.5% | 0.3 |
| 1× PBS | n/a | 29.4 |
| Total | n/a | 30 |
IMAC pH 8.8 Buffer (For Column Equilibrating and Washing)a
| Reagent | Final Concentration (mM) | Amount (mL) |
|---|---|---|
| Tris buffer pH 8.8 (1 M) | 20 | 0.4 |
| NaCl (5 M) | 50 | 2 |
| Imidazole (1 M) | 30 | 0.6 |
| Pyrogen-free H2O | n/a | 17 |
| Total | n/a | 20 |
IMAC pH 8.8 Buffer (For Column Seeding)a
| Reagent | Final Concentration (mM) | Amount (mL) |
|---|---|---|
| Tris buffer pH 8.8 (1 M) | 20 | 0.4 |
| NaCl (5 M) | 50 | 2 |
| Imidazole (1 M) | 30 | 0.6 |
| Sample | n/a | n/a |
| Pyrogen-free H2O, to complete final 20 mL | n/a | n/a |
| Total | n/a | 20 |
IMAC pH 6.8 Buffera
| Reagent | Final Concentration (mM) | Amount (mL) |
|---|---|---|
| Tris buffer pH 6.8 (1 M) | 20 | 0.2 |
| NaCl (5 M) | 500 | 1 |
| Imidazole (1 M, stock in Tris buffer pH 6.8 20 mM) | 100 | 1 |
| Pyrogen-free H2O | n/a | 7.8 |
| Total | n/a | 10 |
Fresh new IMAC buffer must be prepared for each purification.
TBS Saline Buffer
| Reagent | Final Concentration (mM) | Amount |
|---|---|---|
| Tris-HCl pH 7.6 (1 M) | 30 | 30 mL |
| NaCl (5 M) | 500 | 100 mL |
| Pyrogen-free H2O | n/a | 920 mL |
| Total | n/a | 1 L |
Preparing and Saving Columns
Lactosyl-Sepharose
-
1.Before using:
-
a.On the first-time, pack 1 mL of resin into an empty 2 mL bed-column.
-
b.Before each use wash and equilibrate with 15 mL of PBS plus 1 mM PMSF.
-
a.
-
2.After using:
-
a.Wash with 10 mL TBS saline buffer.
-
b.Store in TBS containing 0.1% sodium azide at 2°C–8°C.
-
a.
HisTrap Fast Flow
-
1.Before using:
-
a.Wash the hose of the column with 50 mL of 1 M NaOH followed by 100 mL of pyrogen-free water.
-
b.Check that pH is around 5.5 before connecting the hose to the column.
-
c.Wash the HisTrap column with 10 mL of pyrogen-free water.
-
d.Equilibrate with 5 mL of IMAC pH 8.8 buffer.
-
a.
-
2.After using:
-
a.Wash with 15 mL TBS saline buffer.
-
b.Store in 20% ethanol at 2°C–8°C.
-
a.
Pierce High-Capacity Endotoxin Removal
-
1.Before using:
-
a.On the first use, degas the resin slurry and pack 0.5 mL on an empty 2 mL bed-column.
-
b.Wash with 5 mL of pyrogen-free water.
-
c.Equilibrate with 5 mL of TBS saline buffer.
-
a.
-
2.After using:
-
a.Regenerate the resin by incubating with 5 mL of 0.2 M NaOH during 18–20 h at 20°C–24°C OR 5 mL of 0.2 M NaOH in 95% ethanol for 1–2 h at 20°C–24°C.
-
b.Wash with 5 mL of 2 M NaCl.
-
c.Wash with 5 mL of pyrogen-free water.
-
d.Store in 20% ethanol at 2°C–8°C.
-
a.
Step-By-Step Method Details
This protocol describes in detail the expression and purification of mouse Gal-8 L isoform. Different Galectins and their derivatives that have been successfully purified using this method are listed in Table 1, and the plasmids are available upon request. Comments and notes highlight differences in the expression and/or purification steps between these Galectins and Gal-8. For any other given Galectin, the provided methodology constitutes a useful base protocol in which additional modifications can be included in order to raise protein yield
Table 1.
List of Recombinant Galectins Successfully Purified by Following This Protocol
| Galectin | Reference | Molecular Weight (kDa) |
|---|---|---|
| Human Gal-8 M isoform | (Etulain et al., 2014) | 36 |
| Human Gal-8 L isoform | (Etulain et al., 2014) | 40.4 |
| Human Gal-8N69 | (Cattaneo et al., 2011) | 36 |
| Human Gal-8C233 | (Cattaneo et al., 2011) | 36 |
| Mouse Gal-8 S isoform | (Tribulatti et al., 2007) | 36 |
| Mouse Gal-8 L isoform | (Tribulatti et al., 2007) | 37 |
| Mouse Gal-8 N-CRD | (Romaniuk et al., 2010, Cattaneo et al., 2011) | 21 |
| Mouse Gal-8 C-CRD | (Romaniuk et al., 2010, Cattaneo et al., 2011) | 18 |
| Mouse Gal-8 N-CRD-N-CRD | (Romaniuk et al., 2010, Cattaneo et al., 2011) | 39.9 |
| Mouse Gal-8 C-CRD-C-CRD | (Romaniuk et al., 2010, Cattaneo et al., 2011) | 34 |
| Bovine Gal-8 | Unpublished | 40.1 |
| Human Gal-3 | (Etulain et al., 2014) | 30 |
| Mouse Gal-3 | (Tribulatti et al., 2012) | 30 |
| Human Gal-1 | (Etulain et al., 2014) | 14.5 |
| Mouse Gal-1 | (Tribulatti et al., 2012) | 14.5 |
| Mouse Gal-1-8-1 | (Tribulatti et al., 2012) | 34.2 |
Protein Purification
Timing: 2 days
In this part of the protocol, bacteria cells are lysed, and the Galectin-containing extract is subjected to two sequential steps of protein purification for a high purity yield. The first step consists of a lactose-affinity chromatography, taking advantage of the Galectin’s specific lectin activity. Then, the eluate is subjected to a Ni2+-affinity chromatography taking advantage of the His-tag presence in the recombinant protein. Finally, imidazole is removed from protein preparation by dialysis, and endotoxins are eliminated using a poly-L-lysine resin.
-
1.Cell Lysis
-
a.Unfreeze the pellet at 20°C–24°C until it starts to melt. Transfer immediately to ice.
 CRITICAL: Maintain the sample and buffers on ice.
CRITICAL: Maintain the sample and buffers on ice. -
b.Resuspend the pellet in 30 mL of lysis buffer.
-
c.Add 100 μg/mL of lysozyme.
-
d.Wait until lysis is evident by observing an increased viscosity due to the release of DNA. Albeit sufficient lysis can be observed immediately after pellet resuspension, it usually takes from 15 to 30 min, and occasionally, up to 1 h. Then add 10 μg/mL DNase.
 CRITICAL: A complete lysis is required to obtain a high yield of recombinant protein.
CRITICAL: A complete lysis is required to obtain a high yield of recombinant protein. -
e.Distribute the sample into two tubes and proceed to sonicate by soaking the sonicator’s small horn/tip into the sample, setting the power at 15 watts. Perform five cycles of 30 s alternating the tubes and maintaining on ice between each sonication cycle.Alternatives: In the absence of an ultrasonic cell disruptor, incubation with DNase should be extended. Besides, the addition of Magnesium may be required in order to efficiently disrupt the DNA.
-
f.Remove cell debris by centrifugation at 40,000 rpm for 45 min at 4°C.Alternatives: In the absence of an ultracentrifuge, cell debris can be removed by two consecutive centrifugation cycles at 20,000 rpm for 30 min at 4°C.
-
g.Filter the supernatant through a 0.45 μm-pore membrane with a syringe.
-
a.
-
2.Lactose-affinity chromatography
-
a.Seed the soluble filtered extract into a pre-equilibrated 1 mL Lactosyl-Sepharose column.
-
b.Wash the column with 20 mL of ice-cold PBS plus 1 mM PMSF.
-
c.Elute with 10 mL of ice-cold pyrogen-free PBS plus 1 mM PMSF and 100 mM lactose.
 CRITICAL: Perform steps 2a to 2c by placing the column inside the refrigerator or in a cooled room.
CRITICAL: Perform steps 2a to 2c by placing the column inside the refrigerator or in a cooled room. -
d.Collect the elution in 1 mL-fractions.
-
e.Read the absorbance of each fraction at 280 nm using a nano-spectrophotometer to check the presence of the recombinant protein. Troubleshooting 1
-
a.
Alternatives: If a 280 nm spectrophotometer is not available, a Bradford reaction can be performed to identify the elution peak.
Optional: Check purity and integrity of Gal-8 in a 10% SDS-PAGE (Figure 2). Use 15% SDS-PAGE for Gal-1 and 12% SDS-PAGE for Gal-3.
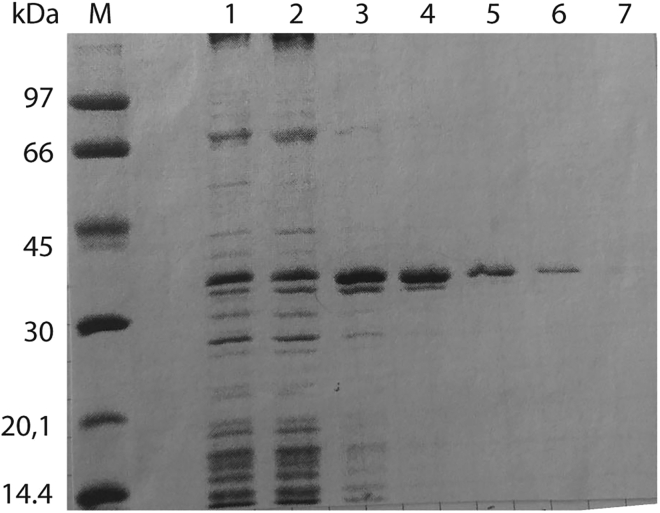
Figure 2.
Lactosyl-Sepharose Purification of Recombinant Mouse Gal-8 Isoform L (Gal-8L)
Aliquots from lactosyl-Sepharose-eluted fractions (1–7) were solved by 10% SDS-PAGE and stained with Coomassie blue. Fractions 1–4 were pooled for subsequent IMAC purification. M, molecular weight marker.
Pause Point: Eluates can be stored up to 2 days on the refrigerator until IMAC purification.
-
3.Metal-affinity chromatography
 CRITICAL: Keep all buffers and samples on ice. All buffers must be prepared with pyrogen-free water. If glass labware is used, it should be previously decontaminated at 180°C for 3 h.
CRITICAL: Keep all buffers and samples on ice. All buffers must be prepared with pyrogen-free water. If glass labware is used, it should be previously decontaminated at 180°C for 3 h.-
a.Pool the protein-containing fractions and dilute 1/5 with IMAC pH 8.8 buffer (Example: 4 mL of the sample plus 16 mL of IMAC pH 8.8 buffer, please see buffer preparation).
-
b.Seed the sample into the pre-equilibrated HisTrap column at a 0.5 mL/min rate.
-
c.Wash the column with 15 mL of IMAC pH 8.8 buffer.
-
d.Elute the protein with IMAC pH 6.8 buffer, collecting fractions of 1 - 1.5 mL.
-
e.Read the absorbance at 280 nm in a nano-spectrophotometer using an aliquot of each fraction to check for protein presence. Use the elution buffer as blank.
-
f.Dialyze during 18–20 h protein-containing fractions at a rate of 1/1000 (at least) in Tris-saline buffer at 4°C and mild agitation.
-
a.
Alternatives: Bradford’s reaction can also be used to verify protein-containing fractions.
Alternatives: Dialysis can be performed in two consecutive steps using less buffer; for example, two 1/250 dilutions.
Note: If biologically active Gal-1 is required, there is a need to prevent the formation of intramolecular disulfide bridges that hampers the correct homodimerization of this lectin, thus leading to gGalectin activity loss (Guardia et al., 2014). This can be achieved by a protein alkylation reaction with iodoacetamide. As the dialysis step may not be sufficient to eliminate iodoacetamide traces, it is recommended to perform this reaction between the purification steps. To do this, invert steps 2 and 3 as follows: perform first steps 3b–3e, seeding the extract into the pre-equilibrated IMAC column. Then, incubate the eluted protein for 18–20 h at 4°C in the presence of 0.1 M iodoacetamide. Next, dilute the alkylated lectin with PBS and executed steps 2a–2e. To finish, dialyze the Gal-1-containing fractions and continue with step 4 of the Protocol.
-
4.Endotoxin removal
-
a.Seed 1 mL containing 1–2 mg of the dialyzed Galectin into a 0.5 mL Pierce High-Capacity Endotoxin removal resin.
-
b.Collect the pass-through and seed it again for four consecutive times.
-
c.Collect the final eluted protein.
-
d.Measure endotoxins with the technique/kit of choice to make sure all endotoxins were removed. We use Endpoint Chromogenic LAL Assay. Endotoxin content is usually less than 0.06 UE/mL. Troubleshooting 2
-
a.
Note: Poly-L-lysine can be regenerated five times after the first use.
Protein Preparation
Timing: 1 day
Recombinant protein purity and specific activity are checked in this part of the protocol. Also, proper storage conditions to preserve Galectin integrity and function are described here.
-
5.Protein storage
-
a.Add 10% glycerol (suitable for cell culture) to stabilize the eluted protein and store up to 2 months at 4°C. Troubleshooting 3
-
b.For longer time conservation, add 50% glycerol and store at −20°C. Perform a hemagglutination assay (see step 7) to check the activity before its use.
-
a.
-
6.Protein analysis
-
a.Determine protein concentration by measuring absorbance at 280 nm using a nano-spectrophotometer.
-
b.Check purity and integrity of Gal-8 in a 10% SDS-PAGE.
-
a.
Note: Use 15% SDS-PAGE for Gal-1 and 12% SDS-PAGE for Gal-3.
-
7.Protein activity: hemagglutination assay (see scheme in Figure 3)
-
a.Add 1 mL of PBS to 100 μl of freshly obtained citrate-anticoagulated mouse blood and centrifuge at 3,500 rpm for 15 min at 4°C.
-
b.Discard the supernatant and mix the pellet (40 μL approximately) with 960 μl of PBS plus 1% BSA (PBS-BSA) thus obtaining a 4% v/v suspension of fresh mouse red blood cells (RBC).
-
c.Make two-fold serial dilutions of Gal-8 in PBS-BSA on a 96 well round-bottom plate, starting with 144 to 1.125 μg/mL on a final volume of 50 μL. Use one well with PBS-BSA without Gal-8 as a hemagglutination negative control.
-
d.Carefully add 50 μL of RBC suspension to each well without mixing to avoid bubble formation (just shake the plate by hand slightly at the end). Then, incubate the plate on a straight surface for 1 h at 20°C–24°C without moving. Gal-8 activity is visualized by the presence of a homogenous RBC mesh covering the well vs. an RBC button.
-
a.
Figure 3.
Step-by-Step Scheme of the Hemagglutination Assay
RBC, red blood cells. PBS-BSA: 1× PBS plus 1% BSA.
Expected Outcomes
After Lactosyl-Sepharose purification, the elution peak is usually observed in fractions 2–3 and about 30 mg of total protein is obtained in those fractions.
After IMAC purification, the eluted protein is found mostly in fractions 3 and 4 and about 5–20 mg of total protein is generally obtained in those fractions. Recombinant protein yields can vary among different Galectins but are nearly always in the order of mg/mL. As an example, an SDS-PAGE resolution of IMAC Gal-8-eluted fractions is shown in Figure 4.
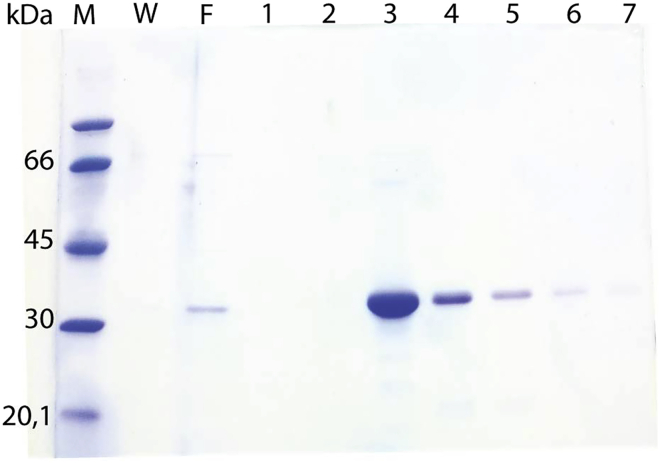
Figure 4.
IMAC Purification of Recombinant Mouse Gal-8 Isoform L (Gal-8L)
Aliquots from IMAC-eluted fractions (1–7) were solved by 10% SDS-PAGE and stained with Coomassie blue. Fractions 3 and 4 were pooled and dialyzed. M, molecular weight marker. W, wash. F, flow-through.
The expected hemagglutination activity for each Galectin and derivatives is depicted in Table 2. Results from a hemagglutination assay using recombinant Gal-8 are shown in Figure 5 as an example.
Table 2.
Suggested Concentration Range to Perform Hemagglutination Assay for Different Galectins and the Expected Activity
| Galectin | Recommended Dilution Range | Expected Activity |
|---|---|---|
| Gal-8 | 144–1.125 μg/mL | 18–4.5 μg/mL |
| Gal-3 | 400−12.5 μg/mL | 100−50 μg/mL |
| Gal-1 | 100–3.12 μg/mL | 25–12.5 μg/mL |
| Mouse Gal-8 N-CRD | 168–10.5 μg/mL | 42 μg/mL |
| Mouse Gal-8 C-CRD | n/a | None |
| Mouse Gal-8 N-N | 40–0.625 μg/mL | 5 μg/mL |
| Mouse Gal-8 C-C | 72–1.125 μg/mL | 18 μg/mL |
| Human Gal-8N69 | 72–0.56 μg/mL | 9 μg/mL |
| Human Gal-8C233 | 288–18 μg/mL | 72 μg/mL |
| Mouse Gal-1-8-1 | 50–1.56 μg/mL | 6.25 μg/mL |
Figure 5.
Determination of the Hemagglutination Activity of Recombinant Mouse Gal-8 Isoform L (Gal-8L)
Hemagglutination assay using half-decreasing concentrations of Gal-8 (from 144 to 1.125 μg/mL). Red blood cell agglutination is evident until a concentration of 18 μg/mL of the recombinant lectin. (-) corresponds to the negative control (without Gal-8).
Limitations
The present protocol was optimized for obtaining Galectin-8 and was successfully adapted for obtaining other Galectins such as Gal-1 and Gal-3 (Tribulatti et al., 2012). However, different conditions for the expression and purification of other recombinant Galectins different from Gal-1, Gal-3, and Gal-8, may be required and must be established in each case.
Troubleshooting
Problem 1
Low yield of the recombinant Galectin after purification (expected yield is above 4 mg of protein).
Potential Solution
-
1.
Make sure that the lysis step is performed correctly. Do not add the DNase nor sonicate the extract until the viscosity increases considerably. Wait as long as necessary and consider adding extra lysozyme and placing the extract at 20°C–24°C (outside the ice bucket) for a short period.
-
2.
Make sure that the columns are correctly regenerated and suitable for use. If necessary, recharge the IMAC column with nickel following the manufacturer's instructions. Prepare fresh IMAC buffers from Tris 1 M stocks and check the pH.
-
3.
Augment the concentration of IPTG to improve the induction of protein expression.
Problem 2
Endotoxins are still present in protein preparation after the Poly-L-Lysine step.
Potential Solution
Regenerate the Poly-L-Lysine resin and repeat step 4, seeding the protein once again.
Be sure to use pyrogen-free solutions all along the purification process. Do not reuse plastic ware and heat all glass labware before use. Carefully clean or replace the peristaltic pump tubing. It is a good idea to reserve and save tubing for these assays if the peristaltic pump is used for other purposes.
If the problem persists, consider using a new resin.
Problem 3
When Galectin concentration is >5 mg/mL, solubility may decrease during the storage at 4°C, resulting in protein precipitation.
Potential Solution
-
1.
To avoid precipitation, maintain the protein in 0.5 M NaCl at a concentration below 5 mg/mL.
-
2.
To clarify the protein solution, centrifuge at 15,000 rpm and 4°C for 20 min. Then, determine the protein new concentration and repeat the hemagglutination assay to check the lectin activity.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. María Virginia Tribulatti (vtribulatti@iib.unsam.edu.ar).
Materials Availability
No new material was generated as part of this study.
Data and Code Availability
No new code or data were generated as part of this study.
Acknowledgments
C.A.P., J.C. and V.C. are fellows, and M.V.T. and O.C. are researchers from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; Argentina). This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; Argentina) (grant numbers PICT 2015-2587, PICT 2017-0630, PICT 2018-01694).
Author Contributions
C.A.P., J.C., V.C., O.C., and M.V.T. conceived and wrote the protocol.
Declaration of Interests
The authors declare no competing interests.
Contributor Information
Cecilia Arahí Prato, Email: arahi.prato@gmail.com.
María Virginia Tribulatti, Email: vtribulatti@iib.unsam.edu.ar.
References
- Cattaneo V., Tribulatti M.V., Campetella O. Galectin-8 tandem-repeat structure is essential for T-cell proliferation but not for co-stimulation. Biochem. J. 2011;434:153–160. doi: 10.1042/BJ20101691. [DOI] [PubMed] [Google Scholar]
- Etulain J., Negrotto S., Tribulatti M.V., Croci D.O., Carabelli J., Campetella O., Rabinovich G.A., Schattner M. Control of angiogenesis by galectins involves the release of platelet-derived proangiogenic factors. PLoS One. 2014;9:e96402. doi: 10.1371/journal.pone.0096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia C.M., Caramelo J.J., Trujillo M., Méndez-Huergo S.P., Radi R., Estrin D.A., Rabinovich G.A. Structural basis of redox-dependent modulation of galectin-1 dynamics and function. Glycobiology. 2014;24:428–441. doi: 10.1093/glycob/cwu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato C.A., Carabelli J., Campetella O., Tribulatti M.V. Galectin-8 Enhances T cell Response by Promotion of Antigen Internalization and Processing. iScience. 2020;23:101278. doi: 10.1016/j.isci.2020.101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk M.A., Tribulatti M.V., Cattaneo V., Lapponi Maria J., Molinas Felisa C., Campetella O., Schattner M. Human platelets express and are activated by galectin-8. Biochem. J. 2010;432:535–547. doi: 10.1042/BJ20100538. [DOI] [PubMed] [Google Scholar]
- Tribulatti M.V., Figini M.G., Carabelli J., Cattaneo V., Campetella O. Redundant and antagonistic functions of galectin-1, -3, and -8 in the elicitation of T cell responses. J. Immunol. 2012;188:2991–2999. doi: 10.4049/jimmunol.1102182. [DOI] [PubMed] [Google Scholar]
- Tribulatti M.V., Mucci J., Cattaneo V., Aguero F., Gilmartin T., Head S.R., Campetella O. Galectin-8 induces apoptosis in the CD4(high)CD8(high) thymocyte subpopulation. Glycobiology. 2007;17:1404–1412. doi: 10.1093/glycob/cwm104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new code or data were generated as part of this study.