Abstract
Heart failure (HF) is a major public health problem and a leading cause of hospitalization in western countries. Over the past decades, the goal has been to find the best method for monitoring congestive symptoms to prevent hospitalizations. Addressing this task through regular physician visits, blood tests, and imaging has proven insufficient for optimal control and has not decreased enough HF-related hospitalization rates. In recent years, new devices have been developed for this reason and CardioMEMS is one of the therapeutic monitoring options. CardioMEMS has shown to be effective in preventing and reducing HF hospitalizations in patients both with HF with reduced ejection fraction and HF with preserved ejection fraction. CardioMEMS’ versatility has made it a great option for pulmonary artery pressure monitoring, both during the coronavirus disease-19 (COVID-19) pandemic and when the clinic visits have (partially) resumed. CardioMEMS is the remote haemodynamic monitoring system with the most evidence-driven efficacy, and COVID-19 has put it in the spot as a centre-stage technology for HF monitoring. In a few months of the COVID-19 epidemic, CardioMEMS has grown to maturity, making it the new normal for high-quality, high-value remote HF care.
Keywords: Remote monitoring, Pulmonary artery pressure sensor, COVID-19
Heart failure hospitalizations—a major public health problem
Heart failure (HF) is a major public health problem and a leading cause of hospitalization in western countries. The prevalence of HF is approximately 1–2% in the adult population in developed countries, rising to ≥10% among people >70 years of age. Among people >65 years of age presenting to primary care with breathlessness on exertion, one in six will have unrecognized HF (mainly HF with preserved ejection fraction, HFpEF).1 Since the earliest cases of coronavirus disease-2019 (COVID-19) infection were reported, our care delivery systems have been challenged in unprecedented ways, affecting all patients with cardiovascular pathologies, but fundamentally those with HF.2
The aim of the management of HF is to maintain the pumping function of the heart and prevent progression to decompensated HF, which is the most common cause of hospitalization in HF patients. Improvements in treatments and their implementation have improved survival and reduced the hospitalization rate in patients with HF with reduced ejection fraction (HFrEF),1 although outcomes are still often unsatisfactory. The most recent European data (ESC-HF pilot study) demonstrate that 12-month all-cause mortality rates for hospitalized and stable/ambulatory HF patients were 17% and 7%, respectively, and the 12-month hospitalization rates were 44% and 32%, respectively.3 Over the past decades, the goal has been to find the best method for monitoring congestive symptoms to prevent hospitalizations. Addressing this task through regular physician visits, blood tests, and imaging has proven insufficient for optimal control and has not decreased enough HF-related hospitalization rates. Hopes were set on circulating biomarkers, but even N-terminal pro-brain natriuretic peptide, which is an important new diagnostic and prognostic tool, cannot reliably predict mortality or overall hospitalization rates.4 Numerous prognostic markers of death and/or HF hospitalization have been identified in patients with HF. More recently, several multivariable prognostic risk scores have been developed for different populations of patients with HF,5–7 and some are available as interactive online applications. Multivariable risk scores have been developed to help predict death in patients with HF, but in general, they remain less useful for the prediction of subsequent HF hospitalizations.6,7
Long before the COVID-19 pandemic altered our lives, remote monitoring emerged as a viable way to overcome the long interval between office visits, monitor patients remotely, and keep them safe by identifying disease progression in time to prevent hospitalization. Non-invasive methods of monitoring HF patients, such as consistent scheduled phone calls with patients and transfer of physiologic data through electronic devices, have been studied but have not had significant impact on mortality or hospitalization rates.8–12 Numerous clinical trials have been conducted to assess the impact of implantable cardiac electronic devices in the management of HF and early detection of congestive symptoms (e.g. intrathoracic impedance), but the results are equivocal and not precise enough to allow early detection of fluid retention and timely management.13–15 In sum, non-haemodynamic-based remote monitoring has not proven to reduce HF hospitalization.
Haemodynamic monitoring using CardioMEMS
Continuous monitoring of pulmonary artery pressure (PAP) could be used as an indicator of HF and would allow optimal monitoring and timely management before the occurrence of symptomatic, acutely decompensated HF.16 The CardioMEMS device was created for this purpose and approved by the Food and Drug Administration (FDA) in 2014 to help monitor PAP and send the data on daily basis to providers, allowing timely management of HF to help lower the hospitalization rate.17
The CardioMEMS is a wireless pressure-sensitive device that uses microelectromechanical systems (MEMS) technology. It consists of an implantable HF sensor, a delivery catheter, and an electronic monitoring unit. Using right heart catheterization, the device is implanted in the distal left posterior pulmonary artery. The device measures the pulmonary arterial pressure through ambient atmospheric pressure changes detected by its barometer. CardioMEMS does not have any batteries or leads and is powered by an external antenna and radiofrequency signals. The electronic unit transmits the PAP measurements daily. These data can be used by physicians to adjust the HF therapy (mainly through adjusting the diuretic dose) before congestive symptoms develop, ultimately resulting in lower hospitalization rates.18
The goal of the CHAMPION trial (CardioMEMS Heart Sensor Allows Monitoring of Pressures to Improve Outcomes in New York Heart Association (NYHA) Functional Class III Heart Failure Patients) was to determine if physicians could reduce HF hospitalizations by managing patient PAP using the CardioMEMS HF System.18 The trial, published almost a decade ago, met its primary efficacy endpoint of reduction in the rate of HF hospitalizations, with the treatment arm having 28% fewer HF hospitalizations compared with the control arm at 6 months. Men and women in the treatment group had similar HF hospitalization rates. The CHAMPION trial also met its secondary efficacy endpoints, with the treatment arm having lower PAP, fewer days in the hospital, and better quality of life compared with the control arm. Over the entire randomized follow-up in the 1.5-year trial, treatment-group patients had 33% fewer HF hospitalizations than control group patients. For every 100 patients treated, 23 HF hospitalizations were prevented per year.
The current recommendation for CardioMEMS use is in NYHA Class III HF patients who have been hospitalized for HF within 1 year prior to implantation. An important variable in the assessment of CardioMEMS’ effectiveness in reducing hospitalization is the 30-day rate of readmission. Adamson et al.19 showed that medications were changed more often in the treatment group compared with the control group and that the overall rate of hospitalization was 49% lower in the treatment than in the control group. It was also determined that all-cause 30-day readmissions were 58% lower in the treatment group. Subset analysis of the CHAMPION trial has shown that the sensor can also lead to better haemodynamic management of patients with preserved ejection fraction and lower rates of hospitalization when compared with standard therapy.20 A recently published post-approval study of 1200 patients in multiple practice settings demonstrated a 58% reduction in HF hospitalizations and a 28% reduction in all-cause hospitalizations during the year after implantation of CardioMEMS.21
An economic analysis of data from the CHAMPION trial showed an incremental cost-effectiveness ratio based on all-cause comprehensive management of HF patients of $29,593 per quality-adjusted life-year gained when the patients were managed with PAP monitoring;22 these analyses indicate that the CardioMEMS HF System is a cost-effective solution.
Singh et al.23 showed, before the COVID-19 pandemic invaded us, that despite its well-established beneficial clinical outcomes in the management of HF patients, adoption of CardioMEMS was slow and among their patient population only 21% of patients eligible for the device received it.
CardioMEMS and COVID-19
The COVID-19 pandemic has significantly impacted patients with HF and their care delivery systems. To reduce severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) transmission, health systems have largely transitioned to noncontact care delivery methods for ambulatory care. DeFilippis et al.24 indicate that such services may include telehealth visits, during which an audiovisual telecommunication system is used; virtual check-ins between the patient and provider via telephone or another device, which are conducted to decide whether additional services are needed; and electronic visits (‘e-visits’), which are communications between patients and providers through an online portal. Of note, despite the well-established feasibility and safety of these telemedicine systems for patients with HF, their use has not yet been reliably associated with a reduction in emergency department visits or hospitalizations.8–12 Importantly, the option for in-person clinic visits should remain available for patients without access to telemedicine services, high-risk patients (e.g. patients on continuous inotropes), and those for whom physical examination is critical for clinical decision-making.
Telemonitoring through PAP monitoring and biosensing devices has been adopted quickly during the COVID-19 pandemic to provide better assessment of HF clinical status, while maintaining social distancing through the performance of virtual visits. As pointed out by Abraham et al.,25 these systems accompanied by their dedicated cloud-based information management are no longer the future but the present of improved HF care. The authors suggest, as a first step for implementation, that high-risk patients at home and in settings like nursing homes might be the first recipients of such technology-driven remote HF assessment.
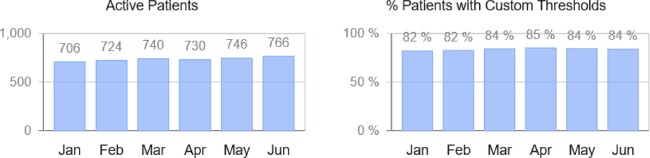
It is highly likely that these indications will expand quickly to lower-risk patients as both patients and physicians become more familiar with the added value of haemodynamic remote monitoring. Indeed, a remarkable increase in the number of CardioMEMS active patients has been observed in Europe, the Middle East, and Africa (EMEA) during the first semester of 2020, up from 706 active patients in January to 766 in June (Figure 1). Furthermore, during the high peak of the first wave of SARS-CoV-2 infections, a substantial decrease in hospitalizations was identified among HF patients with implanted CardioMEMS devices. Additional confounders during the pandemic must be taken in consideration, including patient fear and legally imposed restricted mobilisation, and it would be incorrect to attribute this entirely to CardioMEMS. Figure 2 illustrates the number of patients that required hospitalizations out of all active patients within EMEA included in Merlin.net data as of June 2020. Of note, CardioMEMS, as other telemedicine systems, can help to manage HF patients outside of the hospital setting during a pandemic but this should not be the exclusive recommendation, as discussed above.
Figure 1.
Active patients and percentage of patients meeting custom thresholds within EMEA included in Merlin.net data as of June 2020.
Figure 2.
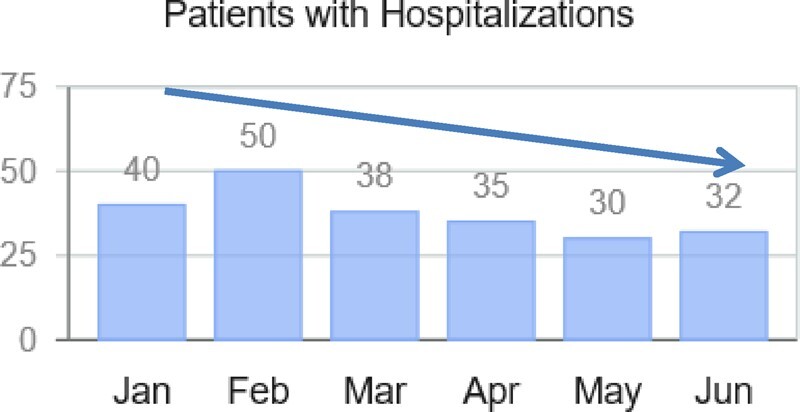
Number of patients that required hospitalization out of all active patients within EMEA included in Merlin.net data as of June 2020. Remarkably, despite the peak of the COVID-19 pandemic in Europe during the months of March, April, and May, a clear decrease in hospitalizations was observed.
Conclusions
To conclude, due to both the ever-increasing costs associated with health care admissions and the social distancing imposed by COVID-19, finding solutions for better monitoring of HF has become imperative. In recent years a series of new devices has been created for this reason and CardioMEMS is one of the therapeutic monitoring options. CardioMEMS has been shown to be effective in preventing and reducing HF hospitalizations in patients with HFrEF and HFpEF. CardioMEMS’ versatility has made it a great option for PAP monitoring, both during the pandemic and when clinic visits resume. CardioMEMS is the remote haemodynamic monitoring system with the most evidence-driven efficacy, and COVID-19 has put it in the spot as a centre-stage technology for HF monitoring. In a few months of the COVID-19 epidemic, CardioMEMS has grown to maturity more than it had in the past decade, making it the new normal for high-quality, high-value remote HF care.
Funding
This paper was published as part of a supplement supported by an educational grant from Abbott.
Conflict of interest: AB-G reports consultation and lecture fees from AstraZeneca, Abbott, Vifor, Novartis, Boehringer-Ingleheim, Roche Diagnostics, Critical Diagnostics.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Reza N, DeFilippis EM, Jessup M.. Secondary impact of the COVID-19 pandemic on patients with heart failure. Circ Heart Fail 2020;13:e007219. [DOI] [PubMed] [Google Scholar]
- 3. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L; on behalf of the Heart Failure Association of the European Society of Cardiology (HFA). EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 4. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes-Genis A, Mueller T, Richards M, Januzzi JL Jr; on behalf of the Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–731. [DOI] [PubMed] [Google Scholar]
- 5. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN; Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 6. Ouwerkerk W, Voors AA, Zwinderman AH.. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014;2:429–436. [DOI] [PubMed] [Google Scholar]
- 7. Lupón J, Simpson J, McMurray JJV, de Antonio M, Vila J, Subirana I, Barallat J, Moliner P, Domingo M, Zamora E, Bayes-Genis A.. Barcelona Bio-HF Calculator Version 2.0: incorporation of angiotensin II receptor blocker neprilysin inhibitor (ARNI) and risk for heart failure hospitalization. Eur J Heart Fail 2018;20:938–940. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM.. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD; Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873–1880. [DOI] [PubMed] [Google Scholar]
- 10. Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol 2005;45:1654–1664. [DOI] [PubMed] [Google Scholar]
- 11. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, De Marco T, Escarce JJ, Evangelista LS, Hanna B, Ganiats TG, Greenberg BH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M, Tong K, Fonarow GC; Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition-Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med 2016;176:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angermann CE, Störk S, Gelbrich G, Faller H, Jahns R, Frantz S, Loeffler M, Ertl G; Competence Network Heart Failure. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail 2012;5:25–35. [DOI] [PubMed] [Google Scholar]
- 13. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M; DOT-HF Investigators. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124:1719–1726. [DOI] [PubMed] [Google Scholar]
- 14. Brachmann J, Böhm M, Rybak K, Klein G, Butter C, Klemm H, Schomburg R, Siebermair J, Israel C, Sinha AM, Drexler H, OptiLink HF; Study Executive Board and Investigators. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF Study (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink). Eur J Heart Fail 2011;13:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, Clementy N, Amori V, Mangoni di S Stefano L, Burri H; MORE-CARE Study Investigators. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail 2017;19:416–425. [DOI] [PubMed] [Google Scholar]
- 16. Castro PF, Concepción R, Bourge RC, Martı´nez A, Alcaino M, Deck C, Ferrada M, Alfaro M, Perrone S.. A wireless pressure sensor for monitoring pulmonary artery pressure in advanced heart failure: initial experience. J Heart Lung Transplant 2007;26:85–88. [DOI] [PubMed] [Google Scholar]
- 17. Pour-Ghaz I, Hana D, Raja J, Ibebuogu UN, Khouzam RN.. CardioMEMS: where we are and where can we go? Ann Transl Med 2019;7:418–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 19. Adamson PB, Abraham WT, Stevenson LW, Desai AS, Lindenfeld J, Bourge RC, Bauman J.. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail 2016;9:e002600. [DOI] [PubMed] [Google Scholar]
- 20. Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, Costanzo MR, Henderson JD, Brett ME, Adamson PB, Stevenson LW; CardioMEMS Post-Approval Study Investigators. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS Post-Approval study. Circ Heart Fail 2020;13:e006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW.. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 22. Martinson M, Bharmi R, Dalal N, Abraham WT, Adamson PB.. Pulmonary artery pressure-guided heart failure management: US cost-effectiveness analyses using the results of the CHAMPION clinical trial. Eur J Heart Fail 2017;19:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh R, Varjabedian L, Kaspar G, Zughaib M.. CardioMEMS in a Busy Cardiology Practice: less than optimal implementation of a valuable tool to reduce heart failure readmissions. Cardiol Res Pract 2018;2018:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeFilippis EM, Reza N, Donald E, Givertz MM, Lindenfeld J, Jessup M.. Considerations for heart failure care during the COVID-19 pandemic. JACC Heart Fail 2020;8:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abraham WT, Fiuzat M, Psotka MA, O’Connor CM.. Heart failure collaboratory statement on remote monitoring and social distancing in the landscape of COVID-19. JACC Heart Fail 2020;8:692–694. [DOI] [PMC free article] [PubMed] [Google Scholar]