Abstract
Coronary artery calcium (CAC) is a highly specific marker for coronary atherosclerosis. The CAC Consortium, a multicenter, retrospective, real-world cohort study, was established to investigate the association between CAC and long-term, cause-specific mortality. This review summarizes findings from CAC Consortium studies published between 2016 and 2020, aiming to demystify CAC as a clinical decision-guiding tool and push the limits of who might benefit from CAC in clinical practice. CAC has been shown to effectively stratify cardiovascular risk across ethnicities irrespective of age, sex, and risk factor burden. In comparison to other widely used risk scores, CAC appears to be most consistent in its ability to add to cardiovascular disease (CVD) event prediction. Beyond risk stratification, CAC has been shown to identify high-risk patient subgroups. While currently recommended only for patients at borderline or intermediate risk by the American College of Cardiology/American Heart Association (10-year atherosclerotic CVD event risk, 5% to < 20%), CAC scoring may also provide value in select young patients aged 30–49 years and in low-risk patients with a family history. While new studies emphasize that patients with a CAC greater than or equal to 1000 be considered a distinct patient group, a CAC of 0 has additionally emerged to be a reliable negative risk factor, identifying patients at low risk of both CVD and non-CVD mortality.
© RSNA, 2020
Summary
Coronary artery calcium is the single most reliable predictor for long-term, cause-specific mortality across patient subgroups based on age, ethnicity, sex, and risk factor burden, proving its crucial role in cardiovascular risk stratification once more.
Essentials
■ CAC is an effective tool for risk stratification in adults irrespective of age, ethnic background, or sex.
■ CAC of 0 is a strong negative risk factor and “de-risks” patients.
■ CAC greater than or equal to 1000 is a strong indicator of markedly higher CVD risk.
■ CAC is the most reliable element in cardiovascular risk stratification in terms of precision of predictive ability.
Introduction
Coronary artery calcium (CAC) scanning and its use in cardiovascular disease (CVD) risk prediction and shared clinical decision making has evolved over the past three decades since Agatston et al first described non–contrast-enhanced, electrocardiographically gated CT as an effective tool to quantify CAC in 1990 (1). Long-term population-based observational studies such as the Multi-Ethnic Study of Atherosclerosis with 6814 asymptomatic men and women (2) and the Heinz Nixdorf RECALL Study with 4200 study participants (3) have consistently produced convincing evidence of a strong association between CAC and cardiovascular outcomes in asymptomatic individuals. To build upon the paradigm shift caused by these cohort studies, the CAC Consortium sought to study key patient subgroups and further identify the role of CAC in predicting cause-specific mortality. Lessons learned from the CAC Consortium will be the topic of this review.
In 2016, an observational cohort study, the CAC Consortium, was assembled, involving 66 636 asymptomatic adult participants free of known CVD at baseline. Participants were enrolled between 1991 and 2010 from four study sites in the United States, contributing CAC testing data after physician referral for clinical CVD risk stratification. The primary objective of the CAC Consortium was to study the association between CAC and long-term cause-specific mortality, including coronary heart disease (CHD), CVD, and non-CVD mortality (cancer, pulmonary disease, gastrointestinal disease, or other). The CAC Consortium remains the largest CAC cohort to date.
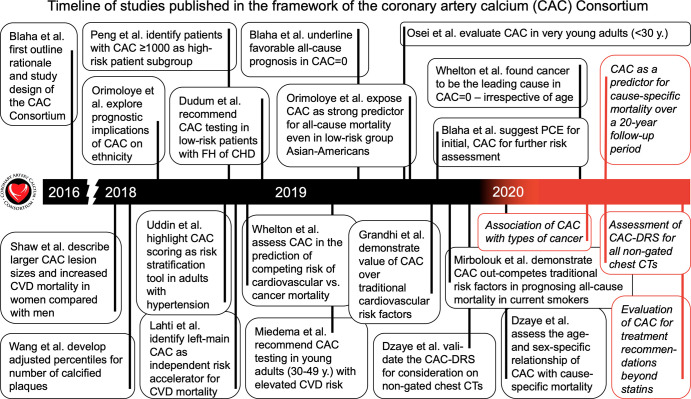
Since the introduction of the rationale and details on the study design (4), multiple studies published from the CAC Consortium have achieved key secondary objectives (Fig 1), which included examining the association between cause-specific mortality and CAC in understudied subgroups based on age, sex, ethnicity, and cardiovascular risk factors. Moreover, our secondary objectives encompassed studying the association between cause-specific mortality and CAC characteristics, including CAC volume, lesion size, density, and regional distribution.
Figure 1:
Timeline of studies published in the framework of the CAC Consortium (in black) and future directions in the field of CAC scoring (in red). CAC = coronary artery calcium, CAC-DRS = Coronary Artery Calcium Data and Reporting System, CHD = coronary heart disease, CVD = cardiovascular disease, FH = family history, PCE = Pooled Cohort Equation.
Studies of the association between CAC and cause-specific mortality in previously underrepresented subgroups based on age, ethnicity and sex (5–9), risk factor burden (10–12), and CAC burden and distribution (13–15) repeatedly showed a high predictive ability of CAC as a marker for subclinical atherosclerosis. Additionally, a key study identified a distinct subgroup of patients with a CAC greater than or equal to 1000 who are at high risk and could thus benefit from the most aggressive form of therapeutic intervention (14). On the other end of the spectrum, a study solidified the notion that a CAC of 0 could be established as a reliable negative risk factor, implying stable low 12-year rates of CVD mortality (15). By comparing the CAC score with traditional risk factors (16) or other risk scores (17), CAC proved to be the strongest parameter in the prognostic value of risk models toward cause-specific mortality. In addition, the CAC Consortium proved innovative in providing insights into the use of CAC in the prediction of competing long-term risks of CVD versus cancer mortality (18). Last, using data from the CAC Consortium, the CAC Data and Reporting System (CAC-DRS) enhanced risk stratification for cause-specific mortality by including the number of vessels with CAC (19).
Given the evidence, CAC can be considered a valuable clinical measure of arterial aging and overall “biologic age” that can change clinical management. In this review, we report on the lessons learned from the largest retrospective clinical cohort of CAC scoring yet assembled, with direct relevance to practicing radiologists and cardiologists using cardiac CT for risk stratification.
Age
Data on CAC in young adults are generally limited. For instance, Tota-Moharaj et al described the association between CAC and all-cause mortality at the extremes of age. With a study population of 8143 and a mean follow-up of 5.6 years ± 2.6 (standard deviation), they found younger patients (< 45 years) to have a twofold (if CAC 100–400) and 10-fold (if CAC > 400) increase in all-cause mortality rate compared with older patients (≥ 75 years) free of CAC (20).
From the CAC Consortium, Miedema et al assessed all-cause and cause-specific mortality among 22 346 individuals aged 30–49 years over a longer mean follow-up period of 12.7 years ± 4.0 (5). As was shown previously, CAC increased with age, and thus, CAC was relatively low in this setting compared with higher age groups. 34.4% had a CAC greater than 0, while 7.2% of the participants even had a CAC greater than 100. However, CHD mortality was 10-fold higher among individuals with a CAC greater than 100 (0.69 per 1000 person-years; 95% CI: 0.41, 1.16) compared with individuals with a CAC of 0 (0.07 per 1000 person-years; 95% CI: 0.04, 0.12). These findings highlight the emerging potential value of CAC in detecting patients at higher risk earlier in life than previously recognized.
Osei et al sought to push the lower age limit of CAC testing further, assessing the role of CAC in very young adults aged younger than 30 years, a unique patient subgroup no other cohort has previously assessed. In these patients referred for CAC testing due to presence of risk factors, 13% of the 373 participants of the CAC Consortium had a CAC greater than 0. They also found CAC to be associated with traditional risk factor burden: CAC was prevalent in 9.7%, 14.9%, and 34.4% of participants with 1, 2, and 3 or more risk factors, respectively. CAC was strongly correlated with an early onset of risk factors and may be of important investigational value even in this very young patient group (6).
Sex
Compared with men, women experience fewer CVD events (21). However, the fatality rate remains critical and makes CVD a major public health issue in women (22). Fundamental causal explanations about the pathophysiologic mechanisms and underlying causes remain unanswered (23). The most frequent cause of coronary thrombosis is plaque rupture, and hence, pathobiologic sex differences have been hypothesized, such as a difference in size of coronary arteries between men and women (24,25).
In the CAC Consortium, across age groups, women had a lower CAC prevalence than men, and within CAC subgroups, women had fewer calcified lesions and a lower CAC volume. CVD mortality among women and men with a CAC of 0 was similar. Yet, a disproportionately higher CVD mortality (1.3-fold higher) was observed in women compared with men when coronary calcium was present. CAC measures including number and size of CAC lesions improve prediction of CVD mortality. Comparing asymptomatic women and men from the CAC Consortium with greater than five CAC lesions, women with CAC lesions larger than 15 mm3 had a 2.2-fold higher CVD mortality than men. This is further aggravated when comparing the relative hazard for women (hazard ratio [HR], 35.10; 95% CI: 17.40, 70.79) and men (HR, 19.56; 95% CI: 13.13, 29.13) with more than 25 CAC lesions to women and men free of CAC lesions. This also adds credence to the hypothesis of pathobiologic sex differences insofar that multivessel CAC elevates CVD mortality risk more in women than in men. Importantly, CAC density was associated with CVD mortality in men but not in women (9). These findings emphasize the need to assess parameters such as calcium density and volume independently in their predictive ability of subclinical atherosclerosis, particularly with regards to sex. Raising awareness to more severe implications of a high CAC in women should see more emphasis in the future.
Ethnicity
CAC is a strong predictor of CHD beyond traditional risk factors irrespective of ethnic group (26). Evaluating CAC among ethnic subgroups is particularly relevant because traditional risk scores perform poorly in minorities, and CAC appears to emerge as the single most reliable parameter to estimate long-term CVD event rate across ethnicities. Despite being a standard for risk assessment in primary prevention, the Pooled Cohort Equation (PCE) frequently overestimates risk in sociodemographic subgroups (27). Conversely, the risk tends to be underestimated in Hispanic individuals and overestimated in Asian individuals, revealing how ethnicity-based differences are inadequately captured by the PCE. Thus, recalibration of the PCE has been recommended for specific ethnicities (28).
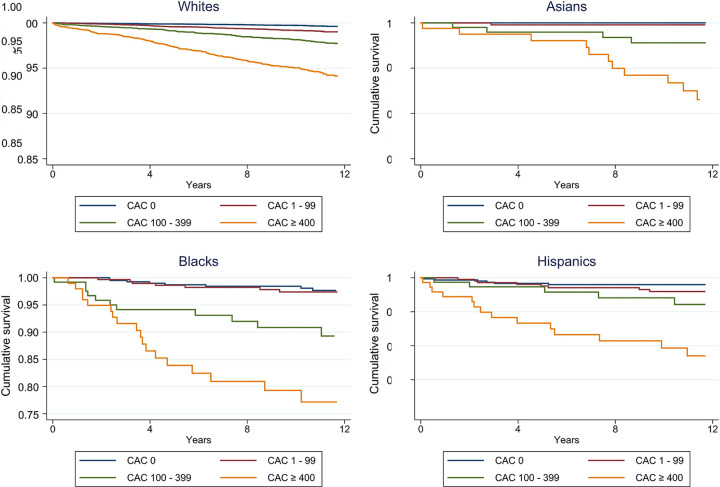
With the aim to assess cause-specific mortality among ethnic subgroups, Orimoloye et al sought to further understand the prognostic value of CAC based on data from the CAC Consortium, including 977 Black, 1349 Hispanic, 1621 Asian, and 38 277 White individuals (7). Within each ethnicity, a clear pattern of greater HRs with increasing CAC score could be detected (Fig 2). White individuals with a CAC greater than or equal to 400 had a risk of CVD mortality four times higher than White individuals with a CAC of 0 (subdistribution hazard ratio [sHR], 4.2; 95% CI: 3.0, 6.0). Using White individuals with a CAC of 0 as the reference, Black individuals with a CAC greater than or equal to 400 (sHR, 17.2; 95% CI: 9.3, 31.8) and Hispanic individuals with a CAC greater than or equal to 400 (sHR, 10.7; 95% CI: 5.7, 19.9) showed even higher sHR, a CAC of greater than or equal to 400 being a strong indicator of significantly elevated risk (7). These findings show the difference in risk implications of CAC in minorities, raising awareness toward selective use of CAC to enhance CVD risk assessment.
Figure 2:
Race/ethnicity-specific Kaplan-Meier curves for cardiovascular disease mortality by CAC group. CAC = coronary artery calcium. (Reprinted, with permission, from reference 7.)
Orimoloye et al also found a disproportionately higher incidence of CVD and all-cause mortality among Black individuals, closely followed by Hispanic individuals, across CAC categories including a CAC of 0, possibly indicating remaining health care disparities. Even after multivariable adjustment, accounting for study site, age, sex, and risk factors assessed, Black and Hispanic individuals had greater CVD and all-cause mortality risk compared with White individuals. In contrast, Asian individuals had the lowest all-cause mortality rates across CAC categories (7). Previously, the Mediators of Atherosclerosis in South Asians Living in America study with 893 study participants has pointed toward substantial differences in cardiovascular risk assessment among diverse populations, including South Asian Americans (29). The 1621 asymptomatic Asian Americans in the CAC Consortium were found to be a relatively low-risk group. Nevertheless, CAC strongly predicted cause-specific and all-cause mortality beyond traditional risk factors in this patient subgroup as reflected by a three times higher all-cause mortality in Asian individuals with a CAC greater than or equal to 400 compared with Asian individuals free of CAC (HR, 3.3; 95% CI: 1.3, 8.6) (8).
Baseline Hypertension
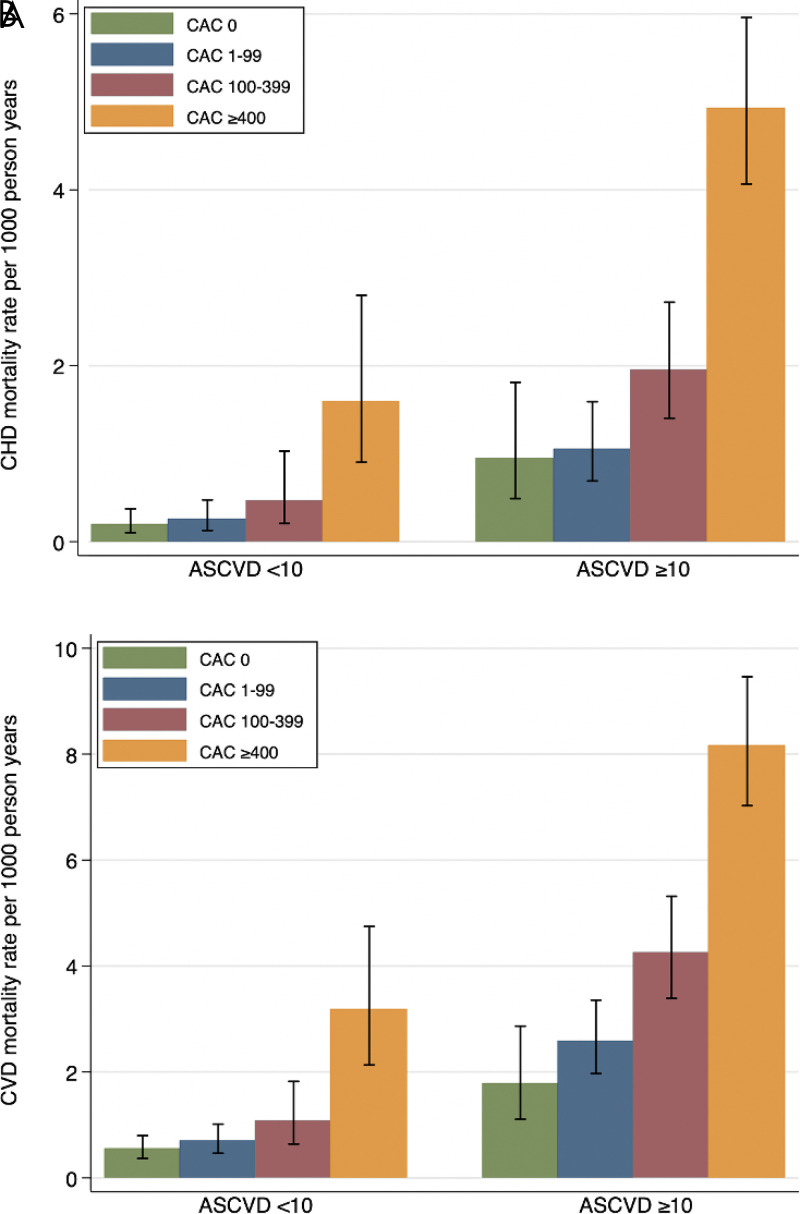
Shemesh et al demonstrated an association of CAC with long-term mortality in patients with hypertension. Although with a smaller cohort (423 participants compared with 16 167 participants of the CAC Consortium with baseline hypertension), they recommend the use of CAC scoring for risk assessment in patients with hypertension (30). Uddin et al sought to further assess the implication of CAC and distinguish risk by cause-specific mortality using data from the CAC Consortium. Mortality rates were observed to increase with rising CAC scores, both CHD mortality (CAC 100–399: HR, 1.88; 95% CI: 1.04, 3.40; and for CAC ≥ 400: HR, 4.16; 95% CI: 2.34, 7.39) and CVD mortality (CAC 100–399: HR, 1.93; 95% CI: 1.31, 2.83; and for CAC ≥ 400: HR, 3.51; 95% CI: 2.40, 5.13). Mortality rate (per 1000 person-years) also increased with CAC score when stratified by atherosclerotic CVD (ASCVD) risk group (Fig 3). These findings highlight the value of CAC in risk stratification in a large, long-term follow-up cohort of patients with baseline hypertension (11).
Figure 3:
Mortality rates (per 1000 person-years) for CHD and CVD among patients with hypertension by CAC group. ASCVD = atherosclerotic cardiovascular disease, CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease. (Reprinted, with permission, from reference 11.)
Current Smokers
Atherosclerotic changes in the aorta and the coronary arteries are greatest among heavy smokers and least in nonsmokers (31). McEvoy et al showed an association between smoking status, CAC, and all-cause mortality, adding to the present knowledge that smokers with CAC had an increased all-cause mortality compared with smokers without CAC (32).
Schulman-Marcus et al also described an association between smoking status, CAC, and all-cause mortality. Over a 15-year follow-up period, they established the potential benefit of identifying smokers with any CAC and in this way, allowing for aggressive risk factor reduction or initiation of other forms of preventive therapy (33).
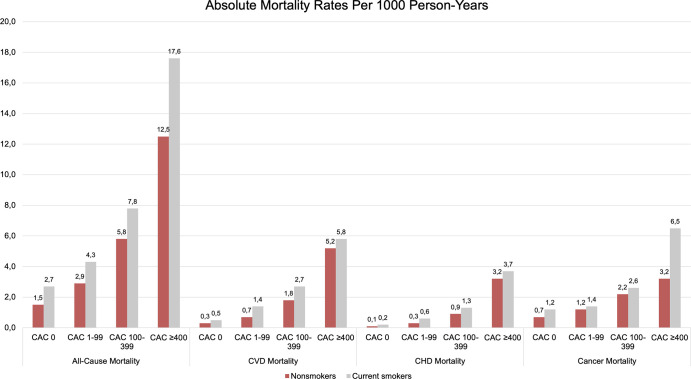
Implications of CAC on cause-specific mortality among smokers have remained less clear, even from the vast evidence established thus far. Mirbolouk et al found that current smokers showed more than a twofold increase in CVD mortality even in the absence of CAC (sHR, 2.10; 95% CI: 1.17, 3.79) compared with nonsmokers with no CAC. This sHR is almost equivalent to the CVD mortality risk of nonsmokers with a CAC of 100–399 (sHR, 2.13; 95% CI: 1.57, 2.90), relative to nonsmokers with a CAC of 0 (Fig 4). The authors further point toward the predictive ability of CAC in non-CVD outcomes, as reflected by an almost two times higher cancer mortality risk in smokers with a CAC greater than or equal to 400 compared with smokers free of CAC (sHR, 1.85; 95% CI: 1.07, 3.22). These findings highlight the heterogeneous risk in smokers and that the concept of a CAC of 0 as a negative risk factor should not be relied on in this patient group (12).
Figure 4:
Cause-specific mortality rates (per 1000 person-years) within CAC groups and by smoking status. CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease. (Adapted, with permission, from reference 12.)
Traditional Risk Factors
Risk assessment algorithms, including the PCE, are still heavily based on traditional CVD risk factors in estimating 10-year risk of CVD events. Despite the value and simple usage of age and traditional risk factors, mischaracterization and subsequent over- or underestimation of risk has been reported and identified as a possible opportunity for enhancement (27).
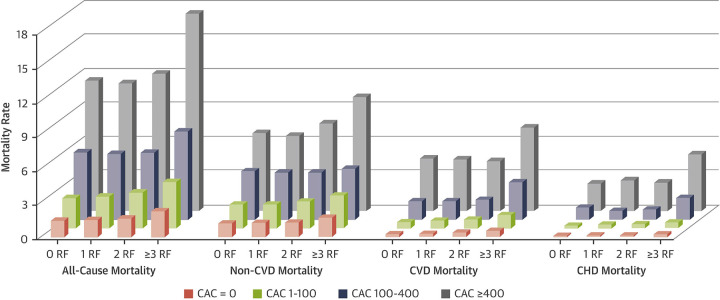
Interestingly, Grandhi et al found that across the spectrum of traditional CVD risk factors, CAC proved to be a more reliable predictor of long-term, all-cause mortality. Additionally, a CAC of 0 was found to be associated with a low CHD and CVD risk over a median follow-up of 12 years, irrespective of risk factor burden at baseline (Fig 5) (16).
Figure 5:
All-cause and cause-specific mortality rate (per 1000 person-years) by baseline CAC and risk factor burden. CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease, RF = risk factor. (Reprinted, with permission, from reference 16.)
Other Risk Scores
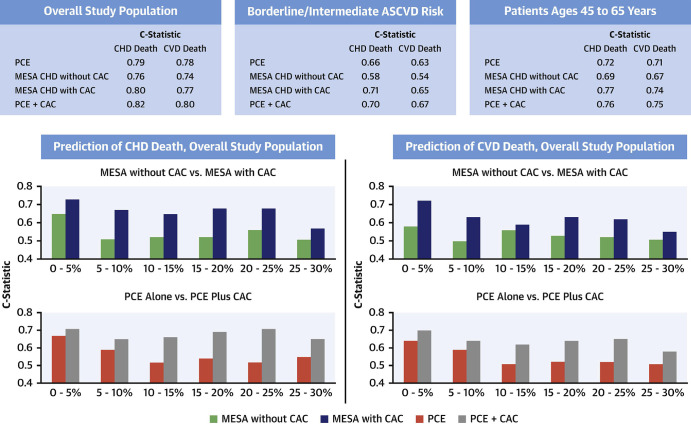
Currently, both the 2018 American College of Cardiology/American Heart Association (ACC/AHA) Multisociety Guideline on the Management of Blood Cholesterol as well as the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease recommend consideration of CAC for further risk assessment among individuals at intermediate risk, after evaluation using the PCE. Borderline and intermediate risk are defined as 10-year ASCVD risk of 5% to less than 7.5% and 7.5% to less than 20%, respectively (34,35). Treatment recommendations are traditionally explicitly risk based. Accurate risk prediction plays a key role in primary prevention and optimal therapy choice, particularly in individuals at borderline and intermediate risk who want to be involved toward shared decision making. Blaha et al first assessed how key modern risk scores, the PCE alone, the Multi-Ethnic Study of Atherosclerosis (MESA) risk score with and without CAC, and a combination of PCE plus CAC, compare in their predictive ability toward fatal CHD and CVD events measured within the CAC Consortium (17).
Among individuals with borderline or intermediate 10-year ASCVD risk, two findings were notable: (a) The MESA risk score with CAC had a significantly higher predictive ability than the MESA without CAC, and (b) the PCE could be improved in its predictive ability by adding CAC. MESA with CAC in this patient subgroup showed the best discriminative ability for CHD outcome, compared with PCE plus CAC, PCE alone, and MESA without CAC (C-statistic, 0.71; 0.70; 0.66; and 0.58, respectively). The PCE plus CAC showed the best discriminative ability for CVD outcome, compared with MESA with CAC, PCE alone, and MESA without CAC (0.67; 0.65; 0.63; and 0.54, respectively). These findings clearly elucidate the value of adding CAC to the equation in consistently discriminating the true risk for patients at borderline or intermediate 10-year ASCVD risk (Fig 6).
Figure 6:
Discriminative ability for the prediction of CVD events by 5% ASCVD risk strata groups. ASCVD = atherosclerotic CVD, CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease, MESA = Multi-Ethnic Study of Atherosclerosis, PCE = Pooled Cohort Equation. (Reprinted, with permission, from reference 17.)
In summary, Blaha et al support the use of the PCE for initial risk assessment as recommended by the 2019 ACC/AHA guideline on the primary prevention of CVD (35). Beyond that, they make a case for the usage of novel risk assessment tools combining current risk predictors (as was performed here using the PCE) with CAC for the prediction of CVD events (17). This approach may prove beneficial in optimizing future risk assessment in clinically referred patients, as CAC has shown to be the critical factor in risk-discriminative ability for both CHD and CVD outcome among currently available risk scores.
Competing Risks
In the United States, CVD and cancer remain the leading causes of death (36). By assessing competing long-term risks of CVD versus cancer mortality over a median follow-up of 12.4 years, Whelton et al presented two key lessons based on the CAC Consortium: First, cancer was the leading cause of death when CAC was 0 and accounted for 50% of deaths in this group. Second, patients with CAC greater than or equal to 300 had a risk of CVD mortality almost fourfold higher than patients with a CAC of 0 (sHR, 3.68; 95% CI: 2.90, 4.67) and a risk of cancer mortality 1.3-fold higher (sHR, 1.30; 95% CI: 1.07, 1.58) (18).
The leading cause of death in patients with a CAC of 0 is cancer, irrespective of age or sex (37). CHD and CVD mortality were low in this patient group at 0.3 and 0.1 per 1000 person-years for women and men, respectively. CVD overtook cancer as the leading cause of death at CAC greater than or equal to 400. Among this group with a CAC score greater than or equal to 400, women showed higher rates of cancer and CVD compared with men. For women, a U-shaped relationship for CVD versus cancer mortality was seen, whereas this relationship was found to be exponential for men (Fig 7). These findings highly suggest a synergistic use of CAC for risk prediction and subsequent prevention strategies of both CVD and cancer (38).
Figure 7:
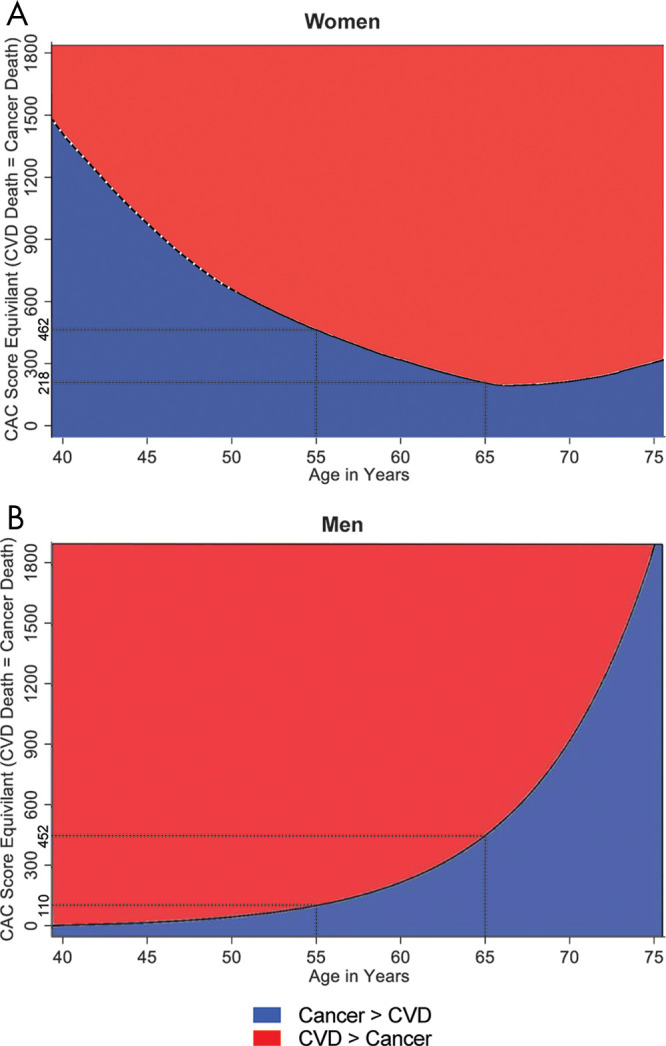
CAC score at which the rate of CVD and cancer mortality are equal for A, women and, B, men. Note U-shaped versus exponential relationship for CVD versus cancer mortality in women and men, respectively. CAC = coronary artery calcium, CVD = cardiovascular disease. (Reprinted, with permission, from reference 38.)
CAC of 0
In studies discussed so far, results have established how CAC is associated with higher risk of CHD, CVD, cancer, and all-cause mortality. How then does interpretation of CAC play out on either extreme of the spectrum, in asymptomatic individuals with a baseline CAC of 0 as well as in those with a very high CAC score?
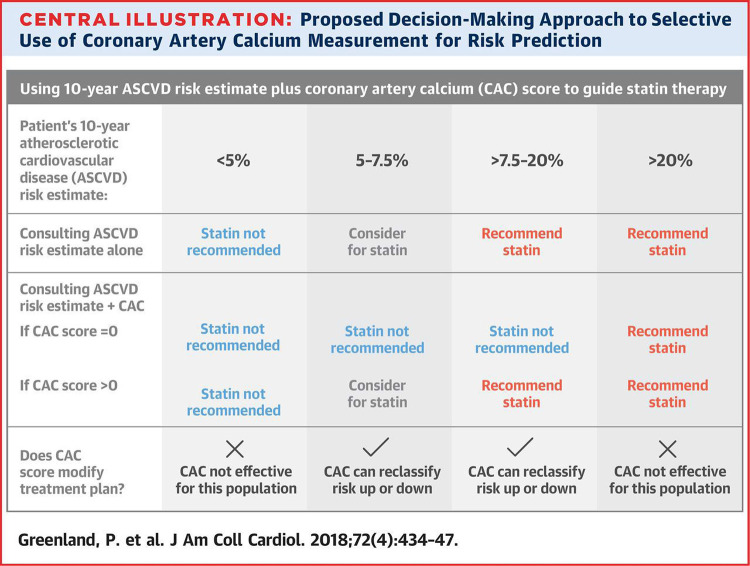
One of the key lessons from the CAC Consortium was the appreciation for the prognostic value implied by a CAC of 0. These participants were identified as a population group with favorable all-cause prognosis and stable low 12-year rates of CVD mortality (15). In contrast, even those individuals with a minimal CAC of 1–10 had a 1.4-fold increased risk of death from any cause as compared with a CAC of 0 (15), particularly in younger patients. Subsequently, identifying truly low-risk patients has strengthened the concept of “negative risk factors,” implying a diagnostic result that substantially downgrades risk (39). This finding has been acknowledged by the most recent 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease, stating that the value of statin therapy in this specific patient population may indeed be limited (35). On the basis of the mounting evidence favoring CAC as a risk stratification tool, and particularly a CAC of 0 as a negative risk factor, Greenland et al have distilled the current knowledge into an approach for selective use of CAC scoring to guide shared decision making in the primary prevention of CVD (Fig 8) (40).
Figure 8:
Guideline-based decision-making approach in primary prevention of CVD. Note CAC of 0 can reclassify risk in patients at borderline or intermediate CVD risk. (Reprinted, with permission, from reference 40.)
CAC Greater than or Equal to 1000
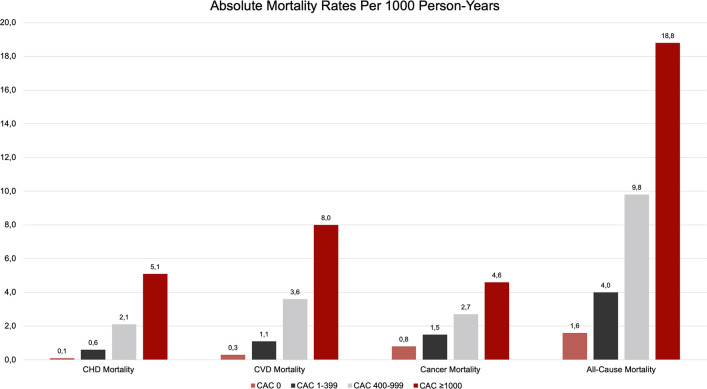
Having established a CAC of 0 as a highly specific negative risk predictor, Peng et al hypothesized a CAC score at the other end of the spectrum to be associated with very high rates of CHD, CVD, and all-cause mortality. In the CAC Consortium, 52.4% of those with a CAC score greater than or equal to 1000 (2869 of 66 636 = 4.3% of CAC Consortium) had four-vessel CAC and 43.9% had three-vessel CAC. Mean estimated total area was 6.91 cm2 ± 3.65 and thus substantially greater than those with lower CAC scores (CAC 400–999, 2.54 cm2 ± 0.7; and for CAC 1–399, 0.37 cm2 ± 0.41). Patients with an extreme CAC score (CAC ≥ 1000) tended to have more dispersed calcifications than those with lower CAC scores, with four of five (79.3%) patients revealing concomitant thoracic artery calcium and almost half (45.7%) of the patient group showing aortic valve calcium (14).
Patients with a CAC greater than or equal to 1000 additionally showed increased hazard ratios for all causes of mortality: fivefold (95% CI: 3.92, 6.48), 6.8-fold (95% CI: 4.74, 9.73), 1.6-fold (95% CI: 1.23, 1.95), and 2.9-fold (95% CI: 2.53, 3.31) risk of CHD, CVD, cancer, and all-cause mortality, respectively, compared with patients free of CAC. A CAC greater than or equal to 1000 was also indicative of an increased risk of CVD mortality (HR, 1.71; 95% CI: 1.41, 2.08) and all-cause mortality (HR, 1.51; 95% CI: 1.33, 1.70) compared with those with CAC scores 400–999. Patients with a CAC greater than or equal to 1000 could be identified as a high-risk, highly clinically relevant subgroup for which the most aggressive treatment options should be considered (Fig 9) (14).
Figure 9:
Cause-specific mortality rate (per 1000 person-years) by CAC group. CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease. (Adapted, with permission, from reference 14.)
Distribution of CAC
CAC distribution within the coronary tree was found to be of incremental value by the third-generation Framingham Offspring Study (41) and has raised awareness toward CAC distribution–dependent outcome. As early as 1989, involvement of the left main (LM) coronary artery had been linked to a poor prognosis (42).
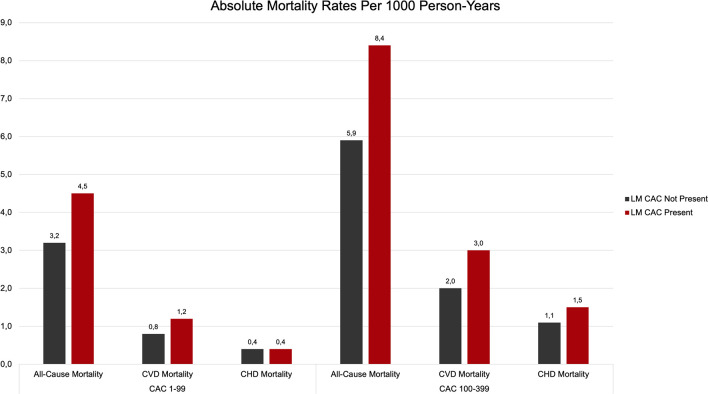
Lahti et al found that participants of the CAC Consortium with LM CAC had a significantly higher mean CAC score compared with patients without LM CAC (647 vs 205, respectively). This was also reflected in the greater proportions of very high CAC in patients with LM involvement; 43% of the LM CAC patient group compared with only 13% of patients without LM CAC had CAC greater than or equal to 400. Patients with LM CAC were also much more likely to present with a higher number of vessels with CAC. More than half of patients with LM CAC showed four-vessel CAC whereas none in the group without LM CAC presented with this pathobiologic feature.
Age-, sex-, and risk factor–adjusted HR showed a 1.3-fold increase in CVD mortality in patients with LM CAC (HR, 1.30; 95% CI: 1.10, 1.50) compared with those without LM CAC, even after adjusting for total CAC. Overall, a graded increase by CAC group and presence of LM CAC in CHD, CVD, and all-cause mortality could be detected, suggesting that the presence of LM CAC may prove useful as a marker for advanced coronary artery disease and subsequent aggressive preventive therapy beyond the traditional overall Agatston CAC score (Fig 10) (13).
Figure 10:
CHD, CVD, and all-cause mortality rates (per 1000 person-years) by presence or absence of left main CAC (LM CAC). CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease. (Adapted, with permission, from reference 13.)
CAC Data and Reporting System
In the context of the CAC Consortium and in light of increasing evidence of implications of CAC distribution, Dzaye et al have suggested a novel dual-reporting system be adopted based on CAC score and CAC distribution (19). This scoring system goes beyond relying solely on the Agatston score and takes into account the number of vessels with calcified plaques. In 2017, the Society of Cardiovascular Computed Tomography (SCCT) and the Society of Thoracic Radiology (STR) proposed the CAC-DRS as a way to standardize communication regarding CAC findings on non–contrast-enhanced CT scans (43). Dzaye et al have evaluated CAC-DRS for the first time for its validity in prognostic significance by assessing the association between CAC-DRS groups and CHD, CVD, and all-cause mortality (19).
CAC-DRS categories are defined as Ax/Ny, where A represents the Agatston score group (where A0, A1, A2, and A3 represent CAC of 0, CAC of 1–99, CAC of 100–299, and CAC ≥ 300, respectively), and N represents the number of vessels affected by CAC, ranging from 0 to 4 for the major epicardial coronary arteries. By nature, certain categories proved to be more common than others; rare phenotypes included A3/N1 and A1/N4, both of which made up less than 0.5% of the patient subcohort. Patients in the highest risk category (ie, A3/N4) had a significantly higher mortality rate than A0 patients (15.4 vs 1.2 deaths per 1000 person-years). Overall, CAC-DRS categories proportionately increased with CHD, CVD, and all-cause mortality (Fig 11) and thus strongly support the recommendations by the 2016 guidelines for CAC scoring of non–contrast-enhanced chest CT scans jointly produced by the SCCT/STR. They propose evaluation and reporting of CAC “on all noncontrast chest CT examinations” as a Class I recommendation, and that CAC at least “be estimated as none, mild, moderate or severe”; it may also be reasonable to perform more detailed CAC scoring on all non–contrast-enhanced chest CT examinations, evaluated as a Class IIb recommendation (19,43).
Figure 11:
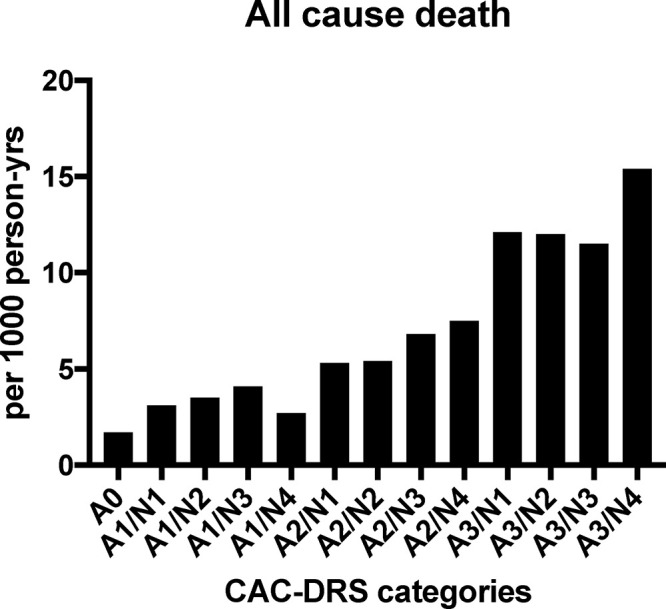
Cumulative all-cause mortality rate (per 1000 person-years) by CAC-DRS categories. CAC-DRS = Coronary Artery Calcium Data and Reporting System, Ax = Agatston score, Ny = number of vessels affected by CAC, yrs = years. (Reprinted, with permission, from reference 19.)
Outlook
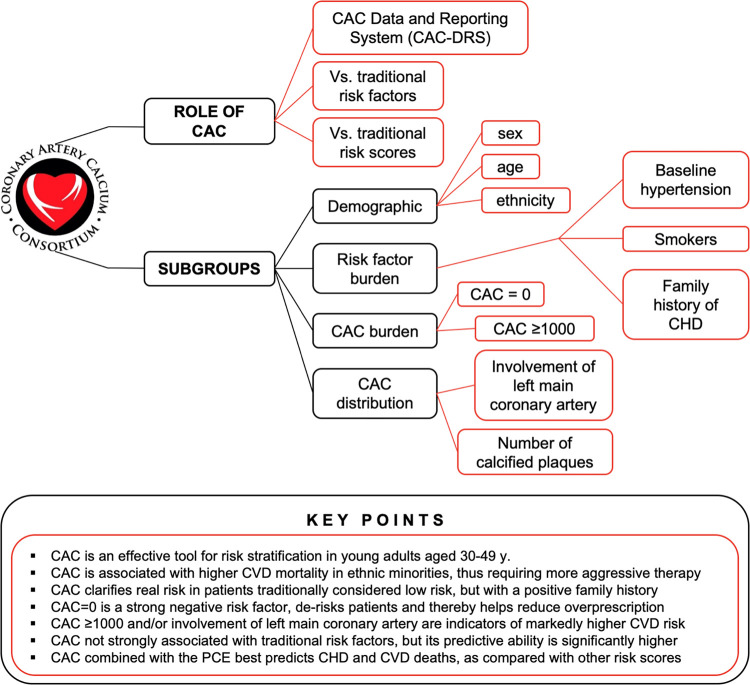
Thus far, the CAC Consortium has provided valuable insights into subgroups on the basis of age, ethnicity, sex, CAC and risk factor burden, and others. CAC is able to not only predict risk similarly to contemporary risk scores, but also markedly enhance them when adding to the traditional algorithms. In fact, combining risk scores with CAC data has proven to give unprecedented access to a more precise layer of modern clinical decision making. With this in mind, what are the next steps in CAC research and, in particular, the CAC Consortium?
To further assess the predictive ability of CAC in an even broader population, we intend to evaluate the implications of CAC scoring over an even longer follow-up of a mean of 20 years and more. Beyond treatment recommendations for statins, we plan to validate the use of CAC scoring for treatment goals regarding nonstatin LDL–lowering, as well as blood pressure therapy. Building on what has been established by Shaw et al (9), we intend to further analyze parameters including CAC density, volume, and area and what role they play in predicting all-cause and cause-specific mortality. Evidence has emerged that CAC density is inversely associated with CHD and CVD risk (44).
As the predictive ability of CAC was assessed in subgroups including smokers and patients with hypertension, the role of CAC in other populations considered at elevated risk remains less clear. Chronic conditions such as diabetes and dyslipidemia, as well as combination of CVD risk factors, have not yet been assessed. It is important to assess long-term, cause-specific mortality in these subgroups, to determine what implications CAC, particularly a CAC of 0 and very high CAC scores, have in these subpopulations.
Conclusions
The CAC Consortium strengthens the argument for a more extensive use of CAC in clinical practice. Based on the largest CAC scoring cohort yet assembled, CAC has shown to be a consistent and reliable predictor of all-cause, CHD, CVD, and even non-CVD mortality across subgroups based on sex or race and patient groups at all traditional cardiovascular risk levels (Fig 12).
Figure 12:
Insights gained and lessons learned from the CAC Consortium by role of CAC and subgroups evaluated. Across a wide spectrum of clinically relevant subgroups, CAC was confirmed as the most sensitive marker for subclinical atherosclerosis and can thus guide the decision-making process toward early intervention and reduction of long-term CHD, CVD, and all-cause mortality. CAC = coronary artery calcium, CHD = coronary heart disease, CVD = cardiovascular disease, PCE = Pooled Cohort Equation, y = years.
CAC offers more than advice on treatment recommendations: As a negative risk factor, a CAC of 0 de-risks patients and gives certainty regarding a favorable all-cause prognosis. This concept of “the power of zero” has been referred to repeatedly over the past years, implying the tremendous benefit gained from such score assuring both doctor and patient that no benefit from statins would be derived (45,46). On the other hand, patients with a very high CAC of greater than or equal to 1000 should be considered a distinct patient subgroup that benefits from early and aggressive pharmacotherapy and possibly other therapeutic interventions.
In patients with low risk and a family history of CHD, CAC is a reliable predictor of all-cause and cause-specific mortality. CAC should thus be considered more broadly than in the past. The 2016 SCCT/STR guidelines recommend documentation of CAC on all non–contrast-enhanced chest examinations, which is highly relevant to practicing radiologists (43). The CAC-DRS has shown to offer a standardized approach in quantifying CAC and may thus prove helpful implementing these recommendations (19). Overall, CAC is the most reliable tool to stratify cardiovascular risk across patient subgroups. If enabled on a more widespread basis, CAC can contribute its share to the kind of precision medicine that we intend to deliver to our patients of today and tomorrow.
Disclosures of Conflicts of Interest: S.A. disclosed no relevant relationships. S.M.I.U. disclosed no relevant relationships. A.D.O. disclosed no relevant relationships. O.H.O. disclosed no relevant relationships. M.J.B. Activities related to the present article: disclosed money paid to author’s institution from National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI). Activities not related to the present article: disclosed money paid to author from Amgen, Sanofi, Regeneron, Novartis, Novo Nordisk, Bayer, Gilead, Zogenix, and Tricida for consultancy, advisory board, and mock AdCom preparation; disclosed grants/grants pending to author’s institution from NIH/NHLBI, Food and Drug Administration, American Heart Association, Aetna Foundation, and Amgen Foundation. Other relationships: disclosed no relevant relationships. O.D. disclosed no relevant relationships.
Abbreviations:
- ACC/AHA
- American College of Cardiology/American Heart Association
- ASCVD
- atherosclerotic CVD
- CAC
- coronary artery calcium
- CAC-DRS
- CAC Data and Reporting System
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- HR
- hazard ratio
- LM
- left main
- MESA
- Multi-Ethnic Study of Atherosclerosis
- PCE
- Pooled Cohort Equation
- SCCT/STR
- Society of Cardiovascular Computed Tomography/Society of Thoracic Radiology
- sHR
- subdistribution HR
References
- 1.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 2.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 3.Schmermund A, Möhlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J 2002;144(2):212–218. [DOI] [PubMed] [Google Scholar]
- 4.Blaha MJ, Whelton SP, Al Rifai M, et al. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr 2017;11(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miedema MD, Dardari ZA, Nasir K, et al. Association of Coronary Artery Calcium With Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw Open 2019;2(7):e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osei AD, Uddin SMI, Dzaye O, et al. Predictors of coronary artery calcium among 20-30-year-olds: The Coronary Artery Calcium Consortium. Atherosclerosis 2020;301:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orimoloye OA, Budoff MJ, Dardari ZA, et al. Race/Ethnicity and the Prognostic Implications of Coronary Artery Calcium for All-Cause and Cardiovascular Disease Mortality: The Coronary Artery Calcium Consortium. J Am Heart Assoc 2018;7(20):e010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orimoloye OA, Banga S, Dardari ZA, et al. Coronary artery calcium as a predictor of coronary heart disease, cardiovascular disease, and all-cause mortality in Asian-Americans: The Coronary Artery Calcium Consortium. Coron Artery Dis 2019;30(8):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw LJ, Min JK, Nasir K, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J 2018;39(41):3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudum R, Dzaye O, Mirbolouk M, et al. Coronary artery calcium scoring in low risk patients with family history of coronary heart disease: Validation of the SCCT guideline approach in the coronary artery calcium consortium. J Cardiovasc Comput Tomogr 2019;13(3):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uddin SMI, Mirbolouk M, Kianoush S, et al. Role of Coronary Artery Calcium for Stratifying Cardiovascular Risk in Adults With Hypertension. Hypertension 2019;73(5):983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirbolouk M, Kianoush S, Dardari Z, et al. The association of coronary artery calcium score and mortality risk among smokers: The coronary artery calcium consortium. Atherosclerosis 2020;294:33–40. [DOI] [PubMed] [Google Scholar]
- 13.Lahti SJ, Feldman DI, Dardari Z, et al. The association between left main coronary artery calcium and cardiovascular-specific and total mortality: The Coronary Artery Calcium Consortium. Atherosclerosis 2019;286:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ³1,000: Results From the CAC Consortium. JACC Cardiovasc Imaging 2020;13(1 Pt 1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaha MJ, Cainzos-Achirica M, Dardari Z, et al. All-cause and cause-specific mortality in individuals with zero and minimal coronary artery calcium: A long-term, competing risk analysis in the Coronary Artery Calcium Consortium. Atherosclerosis 2020;294:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandhi GR, Mirbolouk M, Dardari ZA, et al. Interplay of Coronary Artery Calcium and Risk Factors for Predicting CVD/CHD Mortality: The CAC Consortium. JACC Cardiovasc Imaging 2020;13(5):1175–1186. [DOI] [PubMed] [Google Scholar]
- 17.Blaha MJ, Whelton SP, Al Rifai M, et al. Comparing Risk Scores in the Prediction of Coronary and Cardiovascular Deaths: Coronary Artery Calcium Consortium. JACC Cardiovasc Imaging 2020. 10.1016/j.jcmg.2019.12.010. Published online January 9, 2020. [DOI] [PMC free article] [PubMed]
- 18.Whelton SP, Al Rifai M, Dardari Z, et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC Consortium. Eur Heart J Cardiovasc Imaging 2019;20(4):389–395. [DOI] [PubMed] [Google Scholar]
- 19.Dzaye O, Dudum R, Mirbolouk M, et al. Validation of the Coronary Artery Calcium Data and Reporting System (CAC-DRS): Dual importance of CAC score and CAC distribution from the Coronary Artery Calcium (CAC) consortium. J Cardiovasc Comput Tomogr 2020;14(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults,45 years old and elderly adults.75 years old. Eur Heart J 2012;33(23):2955–2962. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB. The Framingham Study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med 2002;5(2):27–37. [PubMed] [Google Scholar]
- 22.Zhang Y. Cardiovascular diseases in American women. Nutr Metab Cardiovasc Dis 2010;20(6):386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley KE, Geraci SA. Ischemic heart disease in women. South Med J 2013;106(7):427–433. [DOI] [PubMed] [Google Scholar]
- 24.Sheifer SE, Canos MR, Weinfurt KP, et al. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J 2000;139(4):649–653. [DOI] [PubMed] [Google Scholar]
- 25.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47(8 Suppl):C13–C18. [DOI] [PubMed] [Google Scholar]
- 26.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358(13):1336–1345. [DOI] [PubMed] [Google Scholar]
- 27.DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162(4):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the Atherosclerotic Cardiovascular Risk Equation in a Large Contemporary, Multiethnic Population. J Am Coll Cardiol 2016;67(18):2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandula NR, Kanaya AM, Liu K, et al. Association of 10-year and lifetime predicted cardiovascular disease risk with subclinical atherosclerosis in South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. J Am Heart Assoc 2014;3(5):e001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shemesh J, Motro M, Morag-Koren N, et al. Coronary artery calcification predicts long-term mortality in hypertensive adults. Am J Hypertens 2011;24(6):681–686. [DOI] [PubMed] [Google Scholar]
- 31.Strong JP, Richards ML, McGill HC Jr, Eggen DA, McMurry MT. On the association of cigarette smoking with coronary and aortic atherosclerosis. J Atheroscler Res 1969;10(3):303–317. [DOI] [PubMed] [Google Scholar]
- 32.McEvoy JW, Blaha MJ, Rivera JJ, et al. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovasc Imaging 2012;5(10):1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulman-Marcus J, Valenti V, Hartaigh BÓ, et al. Prognostic utility of coronary artery calcium scoring in active smokers: a 15-year follow-up study. Int J Cardiol 2014;177(2):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(24):3168–3209 [Published correction appears in J Am Coll Cardiol 2019;73(24):3234–3237.]. [DOI] [PubMed] [Google Scholar]
- 35.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 37.Whelton SP, Rifai MA, Marshall CH, et al. Coronary Artery Calcium and the Age-Specific Competing Risk of Cardiovascular Versus Cancer Mortality: The Coronary Artery Calcium Consortium. Am J Med 2020. 10.1016/j.amjmed.2020.02.034. Published online April 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzaye O, Al Rifai M, Dardari Z, et al. Coronary Artery Calcium as a Synergistic Tool for the Age- and Sex-Specific Risk of Cardiovascular and Cancer Mortality: The Coronary Artery Calcium Consortium. J Am Heart Assoc 2020;9(8):e015306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaha MJ, Blankstein R, Nasir K. Coronary Artery Calcium Scores of Zero and Establishing the Concept of Negative Risk Factors. J Am Coll Cardiol 2019;74(1):12–14. [DOI] [PubMed] [Google Scholar]
- 40.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018;72(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferencik M, Pencina KM, Liu T, et al. Coronary Artery Calcium Distribution Is an Independent Predictor of Incident Major Coronary Heart Disease Events: Results From the Framingham Heart Study. Circ Cardiovasc Imaging 2017;10(10):e006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor HA, Deumite NJ, Chaitman BR, Davis KB, Killip T, Rogers WJ. Asymptomatic left main coronary artery disease in the Coronary Artery Surgery Study (CASS) registry. Circulation 1989;79(6):1171–1179. [DOI] [PubMed] [Google Scholar]
- 43.Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr 2017;11(1):74–84 [Published correction appears in J Cardiovasc Comput Tomogr 2017;11(2):170.]. [DOI] [PubMed] [Google Scholar]
- 44.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasir K. Message for 2018 Cholesterol Management Guidelines Update: Time to Accept the Power of Zero. J Am Coll Cardiol 2018;72(25):3243–3245. [DOI] [PubMed] [Google Scholar]
- 46.Budoff MJ, Blankstein R, Nasir K, Blaha MJ. Power of zero stronger than “soft” plaque. J Cardiovasc Comput Tomogr 2020;14(3):279. [DOI] [PubMed] [Google Scholar]