Abstract
Background
Central nervous system (CNS) malignancies are the most common solid tumors among children, and novel therapies are needed to help improve survival. Pomalidomide is an immunomodulatory agent that displays anti-angiogenic and cytotoxic activity making it an appropriate candidate to explore in pediatric CNS tumors.
Methods
A phase 1 first in pediatric trial of pomalidomide was conducted in children with recurrent, progressive and refractory CNS tumors. The primary objective was to determine the maximum tolerated dose (MTD) and/or recommended phase II dose (RP2D) when given orally once daily for 21 consecutive days of a 28-day cycle. Once the MTD was established, 12 additional patients were enrolled on expansion cohorts based on age and steroid use.
Results
29 children were enrolled and 25 were evaluable for dose limiting toxicity (DLT). The MTD was 2.6 mg/m2 (Dose level 2). Four DLTs were observed in three patients at dose level 3 (3.4 mg/m2) included grade 3 diarrhea, grade 3 thrombocytopenia, grade 3 lung infection and grade 4 neutropenia. The most common adverse events were grade 1 and 2 myelosuppression. One patient with an oligodendroglioma had stable disease for 9 cycles and a second patient with an anaplastic pleomorphic xanthoastrocytoma achieved a sustained partial response. Immunologic analyses suggested that pomalidomide triggers immunomodulation.
Conclusions
The MTD of pomalidomide is 2.6 mg/m2. It was well-tolerated, and immune correlates showed a serum immune response. These data led to an industry-sponsored phase 2 trial of pomalidomide monotherapy in children with recurrent brain tumors (NCT03257631).
Keywords: Brain tumor, pediatric, pomalidomide, central nervous system tumor, IMiD agent
Introduction
Central nervous system (CNS) tumors are the most common solid tumors among pediatric patients and often lead to significant mortality. Although the overall survival (OS) for children with CNS tumors is approximately 70% at 5 years, the survival for children with recurrent tumors is much worse.1 In addition, many children who survive their disease are left with significant late effects and morbidities, including seizure disorders, endocrine dysfunction, visual and hearing impairment, motor dysfunction, neurocognitive delay, decreased quality of life and risk of secondary malignancy, further emphasizing the need for novel effective therapies that minimize toxicities.2–4
A variety of treatment strategies have been employed for children with recurrent CNS tumors, including biologically targeted agents, high-dose chemotherapy with autologous hematopoietic cell rescue, re-irradiation, and anti-angiogenic agents; thus far, none have significantly improved outcome. Novel targeted therapies and immune modulating agents offer the potential for improved outcomes. One group of agents that possesses both anti-angiogenic and immune modulating properties are the immunomodulatory “-imide” drugs (IMiD), a group of drugs with structural characteristics similar to thalidomide.5,6 The IMiD agents have shown significant efficacy in adults with multiple myeloma and myelodysplastic syndrome.7–9 Lenalidomide and pomalidomide are more potent analogs of thalidomide possessing multiple pharmacologic properties and potential anti-tumor effects including anti-angiogenesis, immune-modulation and cytotoxic activity.5,6
Lenalidomide has previously been tested in a phase 1 clinical trial through the Pediatric Brain Tumor Consortium (PBTC) in children with recurrent CNS tumors. The primary toxicity was myelosuppression, and anti-tumor activity was observed.10 A separate Children’s Oncology Group phase 1 study of lenalidomide in children with relapsed solid tumors and myelodysplastic syndrome reported a significant immune response, including increased serum interleukin (IL)-2, IL-15, GM-CSF, NK cells, NK cytotoxicity and lymphokine activated killer cell cytotoxicity.11 There was also a significant decrease in the CD4+/CD25+ (Treg) population suggesting that a decrease in Tregs may augment or promote anti-tumor activity.
Pomalidomide is FDA-approved at a starting dose of 4 mg/day for adult patients with multiple myeloma who have received at least 2 prior therapies. Prior to this current study, it had not been tested in the pediatric population. Pomalidomide has a number of antitumor pharmacologic effects, including a direct antitumor effect as well as immunomodulatory and anti-angiogenic properties.5 It enhances T-cell and NK cell immunity, increases production of interferon-γ and IL-2 and demonstrates anti-fibrotic activity.12,13 It inhibits microvessel formation and endothelial cord formation in a dose-dependent manner, inhibits endothelial cell migration and invasion, and inhibits HIF-1α expression in hypoxic conditions.14 Although there is pharmacologic overlap with lenalidomide, there are data suggesting that they may utilize different targets. Notably, pomalidomide has demonstrated activity in lenalidomide-resistant cell lines suggesting a unique mechanism of action.15 In addition, adult patients with multiple myeloma resistant to lenalidomide have responded to pomalidomide.16
Compared to thalidomide, pomalidomide has demonstrated enhanced immunological effects in lab testing, including an approximately 500–2,000 times greater potency at stimulating the proliferation of T-cells.13,15 Also, there are murine data showing that pomalidomide crosses the blood-brain-barrier.17 There are clinical reports demonstrating pomalidomide’s effectiveness against myeloma meningitis, CNS myelomatosis and CNS lymphoma, showing clinical support of CNS penetration and efficacy.18–20 Finally, in lenalidomide-resistant multiple myeloma cell lines and patients, pomalidomide plus dexamethasone were synergistic, suggesting glucocorticoids may actually enhance and not diminish the effect of this immunomodulatory agent, which is important in patients with CNS tumors who often are on concomitant steroid therapy.20 For these reasons, including enhanced immunomodulatory effects, CNS penetration, activity in lenalidomide-resistant patients and synergy with glucocorticoids, there has been interest in exploring pomalidomide in treating pediatric CNS tumors.
The PBTC conducted a multicenter first in child, phase 1 dose escalation trial of pomalidomide for children with recurrent, progressive or refractory CNS tumors. The primary objectives were to determine the maximum tolerated dose (MTD) or recommended phase 2 dose (RP2D), of pomalidomide in this pediatric population. The secondary objectives were to explore the preliminary efficacy of pomalidomide as defined by radiographic response rate, duration of response, and progression-free survival (PFS) and to investigate the relationships among pomalidomide dose, exposure, radiographic response and changes in immune correlates. Funding for this study was provided by National Cancer Institute grants, P30CA008748 and UMICA081457.
Methods
Eligibility
Patients between 3 and 21 years of age with a recurrent, progressive or refractory CNS tumor were eligible. Refractory disease was specifically defined as the presence of persistent abnormality on conventional MRI imaging that is further distinguished by histology (tissue sample) or advanced imaging, or as determined by the treating physician and discussed with the principal investigator prior to enrollment. Patients were required to have a performance score (Lansky or Karnofsy) greater than or equal to 60. Patients were required to be able to swallow capsules, and due to capsule size and dosing requirements, have a body surface area (BSA) ≥ 0.55m2. Laboratory parameters required for eligibility at enrollment required the following: absolute neutrophil count ≥ 1000/mm3, platelet ≥ 100,000/mm3 (unsupported), hemoglobin ≥ 8 g/dl, total bilirubin ≤ 1.5 times the institutional upper limit of normal (ULN), ALT ≤ 3 times the institutional ULN, albumin ≥ 3 g/dl, serum creatinine with a normal range based on age and gender and oxygen saturation of ≥ 93% on room air. Patients with significant concurrent illness, including pulmonary, renal, hepatic or cardiac disease were excluded. Patients who were pregnant or breast feeding and those patients with a history of thromboembolic events were excluded. Concomitant steroid use was allowable during the dose escalation phase.
The institutional review board (IRB) of each participating PBTC institution approved the protocol prior to patient enrollment and continuing review was obtained as required by the PBTC and the local institutional IRB guidelines. Patients or their legal guardian provided written consent prior to enrollment; assent was obtained as appropriate.
Treatment and Dosing Schedule
The study was sponsored and pomalidomide provided by the National Cancer Institute’s Cancer Therapy Evaluation Program (CTEP). Patients received pomalidomide orally daily for 21 consecutive days followed by a 7-day rest period; one course was 28 days. Pomalidomide was supplied as 0.5 mg, 1 mg, 2 mg, 3 mg, and 4 mg hard gelatin capsules. Dosing was based on the patient’s actual BSA calculated at the beginning of each course of therapy. The dose prescribed was rounded to the nearest 0.5 mg based on the BSA and the minimum number of capsules required to achieve this dose. Dosing tables reflecting this approach were available within the protocol. Medication diaries were provided to families/patients to document adherence and missed doses, and these were reviewed prior to each subsequent course.
Once the MTD or RP2D was established, 4 expansion cohorts were to be opened at this MTD/RP2D. The expansion cohorts were divided based on age less than 12 y/o and greater than or equal to 12 y/o and based on whether patients were concomitantly taking “treatment” dose steroids or not. For purposes of these expansion cohorts, “treatment” steroid use was defined as doses greater than 0.75 mg/m2/day of dexamethasone or the equivalent. Doses less than this were considered physiologic replacement doses and these patients were eligible for the “no steroid” dose expansion strata.
Pharmacokinetics
The pharmacokinetic (PK) studies were mandatory. Serial whole blood samples (2 ml each) were collected at the following times: pre-dose, 0.5, 1, 2, 4 (± 1), 8 (± 2), and 24 (± 2) hours post-pomalidomide dose on day 1; one additional sample was obtained between days 3–21. Plasma concentrations of pomalidomide were determined by validated liquid chromatography mass spectrometry (LC-MS/MS) assay.21 Pharmacokinetic parameters of interest, such as apparent volume of the central compartment (Vc/F), elimination rate constant (Ke), half-life (t1/2), apparent oral clearance (CL/F), and area under the plasma concentration time curve (AUC) were estimated using compartmental methods. Dose proportionality were investigated by performing one-way analysis of variance (ANOVA) on dose-normalized AUC.
Immune Correlates
Immune correlates were optional. The following samples were collected in consenting patients: circulating cytokines (IL-2, IL12, IL-15, TNF-alpha and GMCSF), NK cells, including receptors KIR2DS4 and NKp46 natural cytotoxicity receptor (NKp46), LAMP-1, Granzyme B+ (GZMB) cells and regulatory T cells (CD4+CD25+Foxp3+ T cells and Tregs). Samples were collected at baseline (prior to the first dose of pomalidomide) and between Day 15–21 of course 1. The concentration (pg/ml) of cytokines (IL2, IL-12p40, IL12p70, TNF-alpha and GM-CSF) in serum samples were measured by ELISA. The immune cellular components (% of Tregs in total CD3+T cells; % of NK cell subsets in total NK cells) and percent of GZMB+ in total T-cells were evaluated in peripheral blood mononuclear cells using flow cytometry. All the procedures were performed according to the standard operating procedures of the University of Florida Brain Tumor Immunotherapy Program.
Statistical Design and Dose Defining
A “Rolling-6” phase I design was used to estimate the maximum tolerated dose (MTD) and/or recommended Phase II dose (RP2D). No intra-patient dose escalation was allowed.22 Dose level 1, which corresponds to 1.9 mg/m2, was the starting dose, and de-escalation to dose level 0, which corresponds to 1.3 mg/m2, was possible in the event that dose level 1 was too toxic. As per study design, levels were to be escalated to dose level 2 (2.4 mg/m2), and dose level 3 (3.6 mg/m2) as tolerated. Patients who completed all therapy during the dose-finding period but who failed to comply with all the specified clinical and laboratory monitoring requirements for the first course were considered not evaluable for estimating the MTD/RP2D and were replaced.
The dose–finding period was defined as the first course of therapy (day 1 through the start of cycle 2). Any pomalidomide-related adverse event during the first course of therapy that led to a dose reduction, significant dose delay or resulted in the permanent cessation of therapy was considered dose limiting. Non-hematologic dose-limiting toxicities (DLTs) included any of the following with at least a possible attribution to pomalidomide: any grade 4 non-hematological toxicity, any adverse event attributed to the study agent that is at least a grade 2 and required treatment interruption for > 3 days during the first 21 day treatment period, greater than 7 days delay in starting the subsequent course due to a non-hematological toxicity that does not meet requirements to start next course, grade 3 skin rash or any other grade 3 non-hematologic toxicity with the specific exclusion of grade 3 nausea and vomiting controlled by antiemetics and grade 3 hepatotoxicity that returned to grade ≤ 1 within 7 days of withholding drug and did not recur on re-challenge. Hematologic DLTs included the following: any grade 4 neutropenia that occurred during the 21-day dosing period, grade 4 neutropenia of ≥ 5 days duration that occurred during the rest period, any grade 3 or 4 thrombocytopenia that occurred during the 21 day dosing period, grade 3 or 4 thrombocytopenia with clinically significant bleeding at any time during DLT phase, grade 4 anemia, any grade of febrile neutropenia or greater than 7 day delay in starting the subsequent course due to neutropenia or thrombocytopenia that did not meet starting requirements.
Potential relationships between immune correlates and objective responses as well as immunologic correlates and steroid use were explored. A general linear model was utilized to evaluate a dose response relationship between the levels of immunologic correlates and the dose of pomalidomide. The longitudinal changes in these markers using mixed effects models were assessed and the associations with responses and progression-free survival (PFS) were explored in a descriptive fashion.
The data that support the findings of this study are available upon request from the PBTC Operations Center. These data are not publicly available due to privacy or ethical restrictions.
Results
Patient Characteristics
Twenty-nine eligible patients were enrolled between 7/24/2015 and 9/21/2016. Patient characteristics are listed in Table 1. The median age at study entry was 12.3 years (5.8–20.8), and 16/29 (55.2%) of patients were female. The median number of previous therapies was 4 (1–12) which included surgery, chemotherapy, other anticancer agents (biologic, antiangiogenic agents, for example) and radiation, either alone or in combination. Four patients were not evaluable for MTD estimation due to insufficient labs to monitor for DLTs (n=1) and progressive disease prior to completing DLT phase (n=3). Once the MTD was established, 12 additional patients were enrolled in the 4 expansion cohorts and treated at the MTD (2.6 mg/m2). One of 12 on the expansion cohort was not evaluable for characterization of DLTs due to early progressive disease.
TABLE 1:
Patient characteristics
| Number (N = 29) | ||
|---|---|---|
| at Diagnosis | at Study Entry | |
| AGE (Years) | ||
| Minimum | 5.4 | 5.8 |
| Maximum | 20.8 | 20.8 |
| Number | Percentage | |
| SEX | ||
| Males | 13 | 44.8 |
| Females | 16 | 55.2 |
| DIAGNOSIS | ||
| Anaplastic astrocytoma | 4 | 13.8 |
| Astrocytoma, NOS | 3 | 10.3 |
| Diffuse Brainstem Glioma | 4 | 13.8 |
| Ependymoma, NOS | 3 | 10.3 |
| Glioblastoma multiforme | 6 | 20.7 |
| Medulloblastoma | 2 | 6.9 |
| Meningioma, NOS | 1 | 3.4 |
| Oligodendroglioma, NOS | 2 | 3.4 |
| Pilocytic astrocytoma | 1 | 3.4 |
| Pineal parenchymal tumor | 1 | 3.4 |
| Supratentorial primitive neuroectodermal tumor | 2 | 6.9 |
| DOSE | ||
| Level 1 (1.9 mg/m2) | 6 | 20.7 |
| Level 2 (2.6 mg/m2) | 18 | 62.1 |
| Level 3 (3.4 mg/m2) | 5 | 17.2 |
MTD and Toxicity
Five DLTs were observed. Four were observed at dose level 3 (3.4 mg/m2) in three patients and included the following: grade 3 diarrhea (1), grade 3 thrombocytopenia (1), grade 3 lung infection (1) and grade 4 neutropenia (1); one patient had both the grade 3 lung infection and grade 3 thrombocytopenia. There was also 1 DLT (grade 4 thrombocytopenia) observed in a patient enrolled on the expansion cohort at the MTD, dose level 2 (2.6 mg/m2). The most common attributable adverse events included grades 1 and 2 lymphopenia, leukopenia, neutropenia, thrombocytopenia, anemia, fatigue and headaches (Table 2).
TABLE 2:
Adverse events (AEs) considered possible, probable or definitely attributable to pomalidomide seen in 10% or greater of patients (n=29). Number of events is listed with the number of patients in parentheses.
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Overall |
|---|---|---|---|---|---|---|
| Lymphopenia | 36 (17) | 17 (11) | 10 (7) | 1 (1) | 64 (22) | |
| Leucopenia | 36 (19) | 20 (9) | 4 (3) | 60 (20) | ||
| Neutropenia | 10 (7) | 24 (10) | 15 (8) | 3 (3) | 52 (18) | |
| Thrombocytopenia | 14 (11) | 4 (3) | 1 (1) | 3 (3) | 22 (15) | |
| Anemia | 9 (9) | 8 (5) | 3 (2) | 20 (12) | ||
| Fatigue | 10 (9) | 2 (2) | 3 (3) | 15 (13) | ||
| Headache | 7 (4) | 2 (2) | 3 (2) | 12 (6) | ||
| Hypocalcemia | 6 (5) | 3 (3) | 9 (6) | |||
| Alanine aminotransferase increased | 7 (7) | 1 (1) | 8 (8) | |||
| Creatinine increased | 8 (5) | 8 (5) | ||||
| Hypoalbuminemia | 6 (5) | 1 (1) | 7 (6) | |||
| Aspartate aminotransferase increased | 7 (7) | 7 (7) | ||||
| Skin and subcutaneous tissue disorders | 5 (4) | 1 (1) | 6 (4) | |||
| Hypokalemia | 5 (4) | 5 (4) | ||||
| Constipation | 3 (2) | 2 (2) | 5 (3) | |||
| Diarrhea | 4 (4) | 1(1) | 5 (5) | |||
| Vomiting | 3 (3) | 2 (2) | 5 (4) | |||
| Hyperkalemia | 3 (1) | 1 (1) | 4 (2) | |||
| Hypophosphatemia | 3 (3) | 1 (1) | 4 (4) | |||
| Metabolism and nutrition disorders | 4 (3) | 4 (3) |
Responses and Survival
All 29 eligible patients were evaluable for response. One patient with an oligodendroglioma had stable disease for 9 cycles before developing progressive disease (treated at the MTD, 2.6 mg/m2) and a second patient with a radiographic progressive anaplastic pleomorphic xanthoastrocytoma (treated at Dose level 1, 1.9 mg/m2), achieved a partial response (PR). PR was defined as greater than or equal to 50% reduction in tumor size by bi-dimensional measurement, as compared with the baseline measurements, on a stable or decreasing dose of corticosteroids, accompanied by a stable or improving neurologic examination, and maintained for at least 8 weeks. At initial diagnosis, this patient underwent a surgical resection followed by focal radiotherapy with concomitant temozolomide. The tumor was also noted to have a BRAFV600E mutation, common among anaplastic pleomorphic xanthoastrocytomas.23 Prior to enrollment on the current trial, this patient had advanced imaging, including MRI perfusion and MRI spectroscopy that were all consistent with an active high-grade glioma. This patient remained on treatment for 4+ years from initiation and also underwent resection of the non-progressive residual mass at approximately 3 years on therapy. Pathology revealed active tumor despite the radiographic response and, due to study completion, the patient was transitioned to compassionate pomalidomide use. In Table 1, this patient is included as an Astrocytoma (NOS) as there was no specific designation for sites to choose anaplastic pleomorphic xanthoastrocytoma when entering data. The median number of treatment courses for all patients was 2 (range: 1–50). Twelve-month PFS and overall-survival (OS) were 3.5+/−2.4% and 28.1+/−8.4%, respectively (Figure 2).
Figure 2:
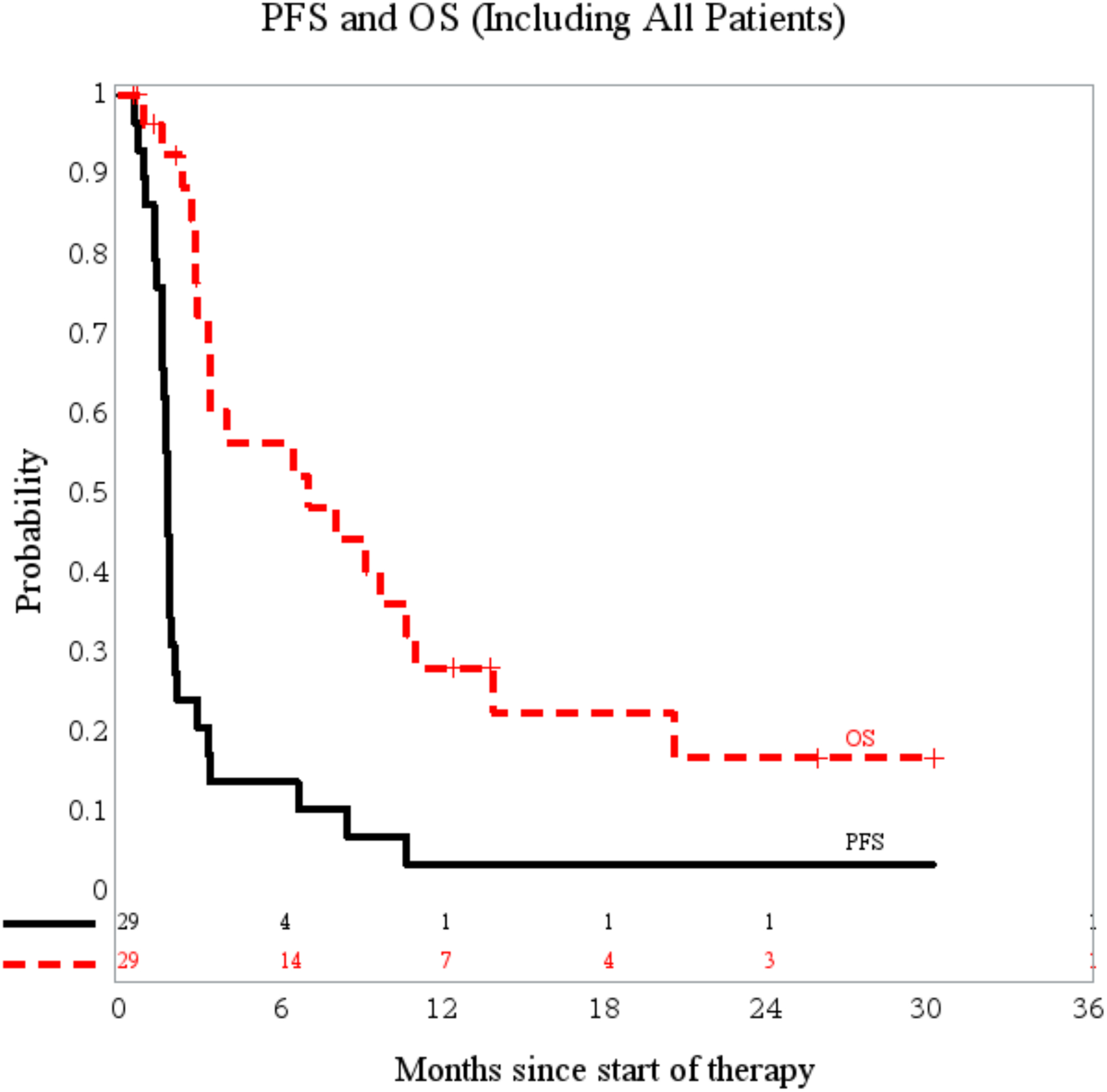
PFS and OS of all eligible patients enrolled (n=29)
Pharmacokinetics
Following a single oral dose of pomalidomide, the median Tmax was 2–4 hours post-dose, with values that ranged from 1.0 to 24.3 hours regardless of food intake. Estimates of half-life were similar among dosing group levels, ranging from 5 to 7 hours; the pharmacokinetic concentration-time curve for all individual patients treated at the MTD (2.6 mg/m2) is depicted in Figure 1. These data are similar to adult phase 1 data where the maximum-plasma concentration (Cmax) is reached within 2.5–3 hours, regardless of the dose.24 There were no clear trends in PK parameters among the differing age and steroid expansion strata at the MTD. Inter-individual variability was generally similar among dosing groups. Variability within a dose group appeared on par with differences between dose groups at times as reflected by wide confidence intervals (CI). The 90% CI for all exponents of the power model relating dose and exposure bounded unity was not contained within the prespecified interval; therefore, these data were considered inconclusive and the possibility of a proportional relationship between dose and exposure is not precluded.
Figure 1:
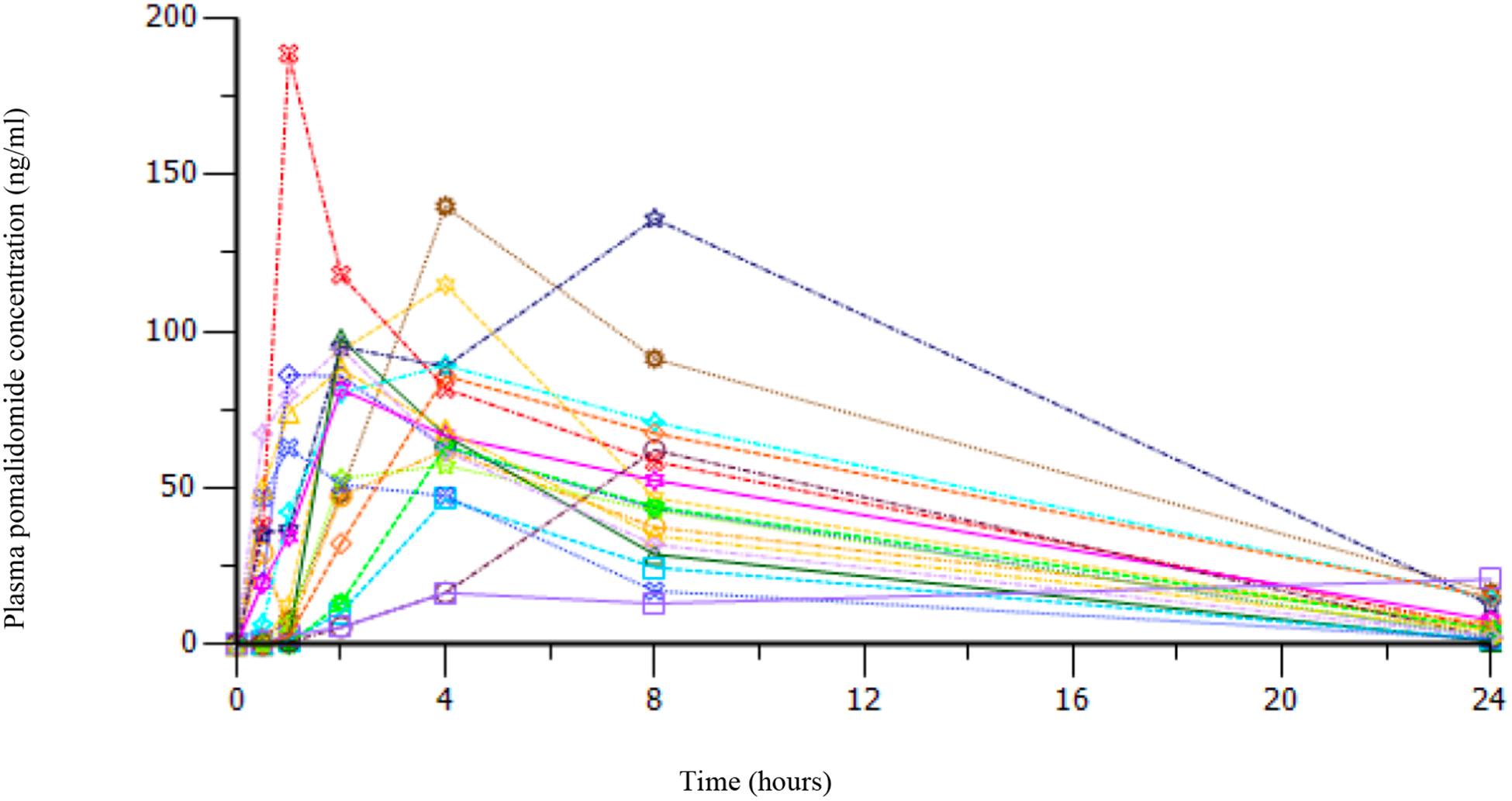
Pharmacokinetic concentration-time curve for individual patients treated at the MTD (2.6 mg/m2).
Immune Correlates
Twenty-four patients had immune correlates collected. These data were part of an exploratory objective and are descriptive in nature. Among all patients at all dose levels, there was enhancement of GZMB+ T cells (p=0.034), Tregs (p=0.0017), and NKp46 (p=0.040) levels at Day 15–21 when compared to baseline (Table 3). Also, upon further analyses, those patients with higher baseline Tregs had statistically longer overall survival (p=0.036). There were several other non-statistically significant trends noted regarding correlations with outcome including the following: those patients with higher baseline NKp46 NK cell levels trended towards a higher likelihood of progression (p=0.058); those patients with larger increases in NKp46 NK cells from baseline to day 15 had a lower likelihood of progression (p=0.083), and those patients with greater increases from baseline to Day 15 in Tregs also had longer overall survival (p=0.091). Other cytokine levels did not show statistically significant changes between baseline and Day 15–21 of the first cycle of pomalidomide in this patient cohort. The immune correlates in the 2 patients with SD and PR did not show any other unique or distinct patterns other than those described above for all patients. Limitations of these analyses are the relatively small sample size, heterogenous diagnoses and the examination of immune correlates only after the first course of treatment without information on longitudinal changes in immune responses.
TABLE 3:
Immune Correlates with statistically significant changes from baseline as compared to day 15–21.
| Immune Correlate | N | Min | Q1 | Median | Mean | Standard Dev | Q3 | Max | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Pre-dose GZMB+ T cells | 24 | 2.38 | 8.74 | 14.40 | 18.38 | 12.86 | 28.30 | 47.70 | |
| Day 15 GZMB+ T cells | 24 | 2.23 | 8.16 | 25.25 | 27.29 | 18.92 | 40.90 | 67.60 | |
| Change in GZMB+ T cells | 24 | −25.97 | −4.17 | 7.40 | 8.91 | 18.74 | 23.83 | 44.20 | 0.034 |
| Pre-dose Tregs | 24 | 0.28 | 2.32 | 3.77 | 4.35 | 2.82 | 5.63 | 10 | |
| Day 15 Tregs | 24 | 1.30 | 3.79 | 6.78 | 8.02 | 6.59 | 9.36 | 33 | |
| Change in Tregs | 24 | −5.13 | −0.19 | 2.77 | 3.67 | 5.89 | 6.30 | 23.03 | 0.0017 |
| Pre-dose NKp46+ NK cells | 24 | 1.82 | 5.67 | 13.25 | 16.26 | 13.36 | 22.35 | 57.50 | |
| Day 15 NKp46+ NK cells | 24 | 1.60 | 5.13 | 8.34 | 11.69 | 8.70 | 18.60 | 29.70 | |
| Change in NKp46+ NK cells | 24 | −37.20 | −7.56 | −3.36 | −4.56 | 11.91 | 0.88 | 25.82 | 0.040 |
Discussion
This is the first phase 1 study evaluating pomalidomide in pediatric patients, and the trial established that the MTD of pomalidomide in children with recurrent, progressive and refractory CNS tumors is 2.6 mg/m2. The DLTs that established this MTD were grade 3 diarrhea, grade 3 thrombocytopenia, grade 3 lung infection and grade 4 neutropenia. As with the other IMiD agents, pomalidomide was well tolerated with the majority of attributable toxicities being grade 1 and 2 myelosuppression, including leukopenia, lymphopenia, neutropenia, thrombocytopenia and anemia. Unlike lenalidomide, pediatric patients were not able to tolerate pomalidomide dosing higher than in adults.10 Pharmacokinetic parameters were similar to adults and estimates for the half-life was similar among the 3 unique dose levels.24 There were no clear consistent trends in pharmacokinetics attributable to stratification by age or steroid use at the MTD when the expansion cohorts were compared.
Although limited by sample size and sample collection at only 2 time points, the available immune correlates support that pomalidomide has significant immunomodulatory effects after a single cycle when comparing baseline (pre-treatment) to days 15–21 of treatment. The observed increases in granzyme, Tregs, and NKp46 are consistent with an immune activation state with possible compensatory regulatory T-cell expansion. Additional longitudinal collection of immune correlates in those patients who continued therapy beyond cycle 1 could have been more informative, but unfortunately, these collections were not part of this study. We also did not observe significant alteration in other plasma cytokine levels with pomalidomide administration which differs from other studies examining cytokine levels in pediatric cancer patients treated with lenalidomide at similar time points after one cycle of treatment.11,16 This may be due to differences in immune modulation in pediatric brain tumor patients compared to other patients with other solid tumors examined in the lenalidomide study through Children’s Oncology Group, or differences between immunomodulatory effects of lenalidomide compared to pomalidomide. While both drugs are of the same class of IMiD agents, differences in the binding of cellular targets have been described, as well as differential sensitivities have been observed in response to treatment in preclinical models and in multiple myeloma patients refractory to lenalidomide but sensitive to pomalidomide treatment.16,25 Studies of the immunomodulatory effects of pomalidomide in pediatric cancer patients in larger clinical trials are warranted to discern which pathways of the host immune response may be most important in anti-tumor activity.
Among the 29 eligible patients, 1 patient had stable disease for 9 cycles and a second patient achieved a PR and continued on therapy for 4+ years without recurrence prior to transitioning to compassionate use. Unfortunately, there were no identifiable patient, histologic or immune correlate characteristics that distinguished these 2 patients from others enrolled on the trial. While there may be tumor molecular and biologic characteristics that predict those with a better response to pomalidomide, none were observed in this trial. Although there were 2 responses noted in the phase 1 PBTC lenalidomide trial, these were seen in patients with low-grade glioma. Very few patients with low-grade glioma enrolled on this phase 1 pomalidomide trial due to a competing PBTC trial specifically for recurrent and progressive low-grade gliomas.10,26
Twenty-eight of the 29 eligible patients enrolled experienced an event, either progressive disease or death. The exception being the patient who achieved a PR and who continues, as of this writing, on compassionate use therapy. The immune correlates described here do suggest that pomalidomide induces a serum immune response; however, further evaluation alone or in combination with other cytotoxic and immunomodulatory drugs would be necessary to see if this immune response progresses during therapy and has any correlation to response.
These MTD, safety and preliminary efficacy data in the confines of this phase 1 trial were encouraging enough to lead to a phase 2 prospective pharmaceutical-sponsored monotherapy efficacy study for children among 4 recurrent CNS tumor strata: DIPG, high-grade glioma, ependymoma and medulloblastoma (NCT03257631). Also, these phase 1 data may serve as a basis for future combinatorial approaches of pomalidomide with other classic chemotherapies, immunotherapies or targeted agents.
Abbreviations:
- BSA
Body surface area
- CI
Confidence interval
- CNS
Central nervous system
- CTEP
Cancer Therapy Evaluation Program
- DLT
Dose limiting toxicity
- IL
Interleukin
- IRB
Institutional review board
- IMiD agent
Immunomodulatory agent
- MTD
Maximum tolerated dose
- OS
Overall survival
- PBTC
Pediatric Brain Tumor Consortium
- PFS
Progression-free survival
- PK
Pharmacokinetics
- RP2D
Recommended phase 2 dose
- ULN
Upper limit of normal
Footnotes
Conflict of Interest:
JF and KEW served on a pediatric advisory board for Celgene. IJD served as an unpaid consultant for Apexigen, served as a pediatric oncology steering committee member for AstraZeneca, pediatric advisory council member for Bristol-Myers Squibb, a consultant for Fennec and a pediatric advisory board member for Roche. GWR has previously consulted for Roche and Eli-Lilly. DAM is a co-founder of iOncologi, Inc., a biotechnology company specializing in immuno-oncology, holds patents that have been licensed to iOncologi, Inc., Annias Immunotherapeutics, Inc., Immunomic Therapeutics, Inc., and Celldex Therapeutics, Inc., and he has served as a consultant/advisor to Bristol-Meyers Squibb, Inc., Tocagen, Inc., Imvax, Inc., and Oncorus, Inc. All other authors report no conflict of interest.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol 2019; 21(Supplement_5): v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rey-Casserly C, Diver T. Late effects of pediatric brain tumors. Curr Opin Pediatr 2019; 31(6): 789–96. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich NJ. Neurologic sequelae of brain tumors in children. J Child Neurol 2009; 24(11): 1446–54. [DOI] [PubMed] [Google Scholar]
- 4.Roddy E, Mueller S. Late Effects of Treatment of Pediatric Central Nervous System Tumors. J Child Neurol 2016; 31(2): 237–54. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt M, Wasch R, Reinhardt H, Kleber M. Pomalidomide. Recent Results Cancer Res 2014; 201: 359–72. [DOI] [PubMed] [Google Scholar]
- 6.Weisel K, Kanz L. Lenalidomide. Recent Results Cancer Res 2014; 201: 347–57. [DOI] [PubMed] [Google Scholar]
- 7.Scott LJ, Lyseng-Williamson KA. Lenalidomide: a review of its use in the treatment of relapsed or refractory multiple myeloma. Drugs 2011; 71(5): 625–49. [DOI] [PubMed] [Google Scholar]
- 8.Richardson P, Mitsiades C, Laubach J, et al. Lenalidomide in multiple myeloma: an evidence-based review of its role in therapy. Core Evid 2010; 4: 215–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier SK, Hammond JM. Role of lenalidomide in the treatment of multiple myeloma and myelodysplastic syndrome. Ann Pharmacother 2006; 40(2): 286–9. [DOI] [PubMed] [Google Scholar]
- 10.Warren KE, Goldman S, Pollack IF, et al. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol 2011; 29(3): 324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg SL, Cairo MS, Russell H, et al. Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a Children’s Oncology Group Phase I Consortium report. J Clin Oncol 2011; 29(3): 316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 2009; 58(7): 1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgun G, Calabrese E, Soydan E, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 2010; 116(17): 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res 2009; 77(2): 78–86. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Gill N, Lentzsch S. Recent advances of IMiDs in cancer therapy. Curr Opin Oncol 2010; 22(6): 579–85. [DOI] [PubMed] [Google Scholar]
- 16.Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood 2011; 118(11): 2970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MMaT Y. Brain Microdialysis of Pomalidomide in Rats North American Regional ISSX Meeting; 2011; Atlanta, GA, USA; 2011. [Google Scholar]
- 18.Li Z, Qiu Y, Personett D, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One 2013; 8(8): e71754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertz MA. Pomalidomide and myeloma meningitis. Leuk Lymphoma 2013; 54(4): 681–2. [DOI] [PubMed] [Google Scholar]
- 20.Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma 2013; 54(4): 864–6. [DOI] [PubMed] [Google Scholar]
- 21.Piestansky J, Barath P, Majerova P, et al. A simple and rapid LC-MS/MS and CE-MS/MS analytical strategy for the determination of therapeutic peptides in modern immunotherapeutics and biopharmaceutics. J Pharm Biomed Anal 2020; 189: 113449. [DOI] [PubMed] [Google Scholar]
- 22.Onar-Thomas A, Xiong Z. A simulation-based comparison of the traditional method, Rolling-6 design and a frequentist version of the continual reassessment method with special attention to trial duration in pediatric Phase I oncology trials. Contemp Clin Trials 2010; 31(3): 259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonse R, Gupta T, Epari S, et al. Impact of WHO 2016 update of brain tumor classification, molecular markers and clinical outcomes in pleomorphic xanthoastrocytoma. J Neurooncol 2018; 136(2): 343–50. [DOI] [PubMed] [Google Scholar]
- 24.Gay F, Mina R, Troia R, Bringhen S. Pharmacokinetic evaluation of pomalidomide for the treatment of myeloma. Expert Opin Drug Metab Toxicol 2013; 9(11): 1517–27. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012; 26(11): 2326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 2019; 20(7): 1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


