Supplemental Digital Content is available in the text.
Keywords: checkpoint inhibitor, combination therapy, expanded access program, ipilimumab, nivolumab, overall survival
Abstract
CheckMate 218, a North American expanded access program (EAP), investigated nivolumab plus ipilimumab in patients with advanced melanoma. Safety and efficacy, including 2-year survival in clinically relevant patient subgroups, are reported. Eligible patients were aged ≥18 years with unresectable stage III/IV melanoma, an Eastern Cooperative Oncology Group performance status of 0/1, and no prior checkpoint inhibitors. Patients received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 cycles (induction) followed by nivolumab 3 mg/kg every 2 weeks (maintenance) until progression or unacceptable toxicity or a maximum of 48 weeks. Safety and overall survival (OS) data were collected. This EAP included 754 treated patients from the USA (n = 580) and Canada (n = 174). Median follow-up time was 17.8 months. All-grade and grade 3–4 treatment-related adverse events were reported in 96% and 53% of patients and led to treatment discontinuation in 36% and 26% of patients, respectively. OS rates at 12 and 24 months were 82% [95% confidence interval (CI) 79–84] and 70% (95% CI 66–74), respectively. Twenty-four-month OS rates were 63% in patients aged ≥75 years, 56% in patients with elevated lactate dehydrogenase levels, 73% in patients with BRAF wild-type tumors, 70% in patients with BRAF mutant tumors, and 56% in patients with mucosal melanoma. In this EAP, nivolumab plus ipilimumab demonstrated high survival rates and safety outcomes consistent with those from randomized clinical trials, further supporting the use of this combination for advanced melanoma across multiple subgroups.
Introduction
Treatment for advanced melanoma has been dramatically transformed over the past decade with the introduction of immune checkpoint inhibitors into clinical practice [1,2]. These novel immunotherapies offer the potential for durable responses and prolonged survival, with manageable toxicities, in patients with otherwise limited treatment options. Historically, advanced melanoma has been associated with a dismal prognosis, with a median overall survival (OS) of 1 year or less [3]. However, the outlook for these patients has substantially improved, with median OS now being extended to more than 5 years with the combined use of the immune checkpoint inhibitors nivolumab [an anti-programmed death 1 (PD-1) antibody] and ipilimumab [an anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody] [4].
Nivolumab, given alone or in combination with ipilimumab, is indicated for the first-line treatment of patients with advanced melanoma [5,6]. Combination therapy with ipilimumab and nivolumab became the focus of clinical investigation based on the scientific rationale that PD-1 and CTLA-4 inhibit antitumor immunity via distinct molecular pathways [7]. The combination of nivolumab plus ipilimumab has been shown to be more effective than ipilimumab alone for treatment-naive patients with advanced melanoma in randomized clinical trials (RCTs), albeit with a higher frequency of treatment-related grade 3–4 adverse events (AEs) compared with nivolumab or ipilimumab monotherapy [4,8–10]. In a randomized, phase 2 study in treatment-naive patients with advanced melanoma (CheckMate 069), nivolumab plus ipilimumab treatment demonstrated significant improvement compared with ipilimumab alone in objective response rate (ORR) and median progression-free survival [8,9]. Moreover, in a randomized, phase 3 study in treatment-naive patients with advanced melanoma (CheckMate 067), nivolumab plus ipilimumab or nivolumab alone compared with ipilimumab alone showed significant improvement in median OS at a minimum follow-up of 60 months [4]. Five-year OS rates were 52% with the combination, 44% with nivolumab monotherapy, and 26% with ipilimumab monotherapy [4].
CheckMate 218 (ClinicalTrials.gov identifier: NCT02186249) is an expanded access program (EAP) that provided patients access to investigational nivolumab to be used in combination with commercially supplied ipilimumab in North American patients with unresectable stage III or stage IV melanoma. Access was provided from the start of the EAP on 12 August 2014, until regulatory approval of the combination was granted on 1 October 2015, in the USA and on 31 October 2016, in Canada. This EAP included 754 patients, among whom 580 were treated in the USA and 174 were treated in Canada. OS data from earlier time points in the EAP were previously reported for the combined USA and Canadian cohorts [11], and updated data specifically on the Canadian cohort has recently been published [12]. Updated safety and OS data for the overall EAP population (USA + Canada), with a median follow-up of 17.8 months, are reported here.
Patients and methods
Patients
Patients included in this EAP were ≥18 years of age, had a diagnosis of unresectable stage III or IV metastatic melanoma per the American Joint Committee on Cancer staging system (seventh edition) [13], had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and did not receive prior anti–CTLA-4 or anti–PD-1 treatment. Prior systemic treatment for localized or metastatic disease, including BRAF and/or MEK inhibitors, was allowed. Exclusion criteria were active (symptomatic) or untreated brain metastases or leptomeningeal metastases, a life expectancy <6 weeks, autoimmune disease, conditions requiring systemic corticosteroids or other immunosuppressive medications within 14 days of drug administration, and the requirement for other systemic antineoplastic therapy while receiving nivolumab.
Expanded access program design
In this EAP, investigational nivolumab was used in combination with commercially supplied ipilimumab. All investigators had previous experience with the administration of nivolumab and ipilimumab, either as monotherapy or in combination. Nivolumab (intravenously over 60 minutes at 1 mg/kg) and ipilimumab (intravenously over 90 minutes at 3 mg/kg) were administered for 4 doses every 3 weeks during the induction phase. Single-agent nivolumab (intravenously over 60 minutes at 3 mg/kg every 2 weeks) was subsequently administered during the maintenance phase until disease progression or unacceptable toxicity or until a maximum of 48 weeks from the first nivolumab monotherapy dose, whichever occurred first (Supplementary Fig. S1, Supplemental digital content 1, http://links.lww.com/MR/A249). Patients in Canada who discontinued the combination because of toxicity were allowed to restart nivolumab monotherapy if toxicities had resolved and after consultation with the medical monitor. Following approval of the combination therapy in the USA, this EAP was closed in the USA, and patients who were still on treatment were transitioned to the commercial supply of nivolumab. At that time, patients continued to be enrolled into the Canadian cohort. The EAP was later closed in Canada when a safety analysis determined patient safety was consistent with that across the nivolumab program, with some of the patients who were exhibiting clinical benefit at EAP completion being provided nivolumab through a post-EAP drug access program funded by Bristol Myers Squibb. Study completion (last patient, last visit) was 7 November 2017, and the study is now operationally closed.
Median follow-up was defined as the time between randomization date and the last known date alive (for patients who were alive) or death date (for patients who had died). Results reported here reflect the final database lock for this EAP.
Assessments
Data from safety parameters, which included AEs, physical examination findings, ECOG PS, and laboratory results, were collected according to health authority regulations beginning at cycle 1 and were recommended for monitoring until 100 days after the discontinuation of therapy. AEs were evaluated between the first dose and 30 days after the last dose of therapy. Severity of AEs was determined according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Serious AEs were defined as those that resulted in death, were life-threatening, resulted in persistent or significant disability/incapacity, or required intervention or hospitalization.
OS was defined as the time between treatment start and death from any cause. The protocol recommended collection of survival data for up to 5 years from the first nivolumab monotherapy dose. However, the EAP was closed early when a safety analysis determined patient safety was consistent with that of the broader nivolumab development program. Response data (e.g. ORR and progression-free survival) were not collected in this EAP.
Statistical analyses
Statistical analyses for collected safety and OS data were descriptive; no formal hypothesis testing was performed. Given that the primary objective of the EAP was to provide access to therapy, the overall sample size was determined based on the number of patients who met the inclusion criteria and enrolled in the EAP.
Expanded access program oversight
This EAP was conducted in accordance with Good Clinical Practice and was in compliance with the protocol. Prior to initiation of the EAP, the protocol was approved by the institutional review board or independent ethics committee at each EAP site. All patients provided written informed consent. This EAP is registered at www.clinicaltrials.gov as NCT02186249.
Results
Patient disposition, baseline characteristics and treatment
Overall, 849 patients were enrolled at 64 sites (which treated at least one patient) throughout the USA and Canada; 754 patients were treated (Fig. 1). The final database lock occurred on 24 January 2018, and median follow-up time was 17.8 months (range 0.3–36.1). All patients discontinued treatment [277 (37%) patients discontinued treatment in the maintenance phase]. The most common reasons for discontinuation were EAP-related drug toxicity in 308 patients (41%) and disease progression in 130 patients (17%).
Fig. 1.
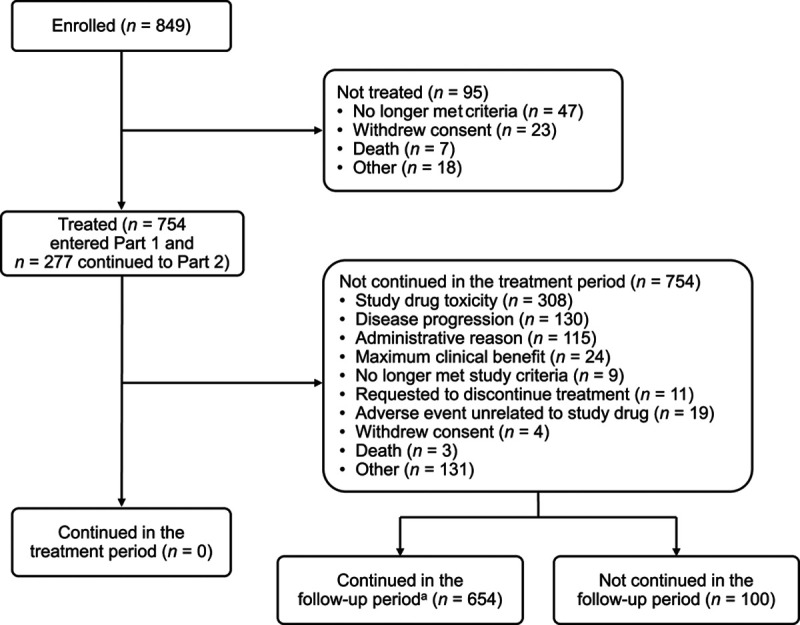
Patient disposition. Part 1 was the induction phase with nivolumab plus ipilimumab; part 2 was the maintenance phase with nivolumab monotherapy. aAfter the EAP end, still being followed for adverse events. EAP, expanded access program.
Patient demographics and baseline characteristics for the 754 treated patients are shown in Table 1. Median age was 58 years (range 17–87). The majority of patients were male [478 (63%)], had a history of cutaneous melanoma [590 (78%)], were diagnosed with stage IV disease [643 (85%)], and were treatment-naive [532 (71%)]. Patients with BRAF mutant tumors comprised less than half of the EAP population [329 (44%) patients]. Prior systemic anticancer therapies had been used in 130 (17%) and 97 (13%) patients in the adjuvant and metastatic settings, respectively. In total, 222 (29%) patients received prior systemic therapy, including targeted therapy reported as dabrafenib [70 (9%) patients], trametinib [60 (8%) patients], and vemurafenib [27 (4%) patients], with most patients receiving dabrafenib and trametinib as one regimen.
Table 1.
Patient demographics and baseline characteristics
| Nivolumab plus ipilimumab (N = 754) | |
|---|---|
| Age | |
| Median, years (range) | 58 (17–87) |
| ≥65 years, n (%) | 219 (29) |
| ≥75 years, n (%) | 59 (8) |
| Sex, n (%) | |
| Male | 478 (63) |
| Female | 276 (37) |
| Region, n (%) | |
| USA | 580 (77) |
| Canada | 174 (23) |
| ECOG PS, n (%) | |
| 0 | 520 (69) |
| 1 | 234 (31) |
| Subtype of melanoma, n (%) | |
| Cutaneous | 590 (78) |
| Mucosal | 47 (6) |
| Uveal | 38 (5) |
| Acral | 8 (1) |
| Other | 69 (9) |
| BRAF mutation status, n (%) | |
| Mutant | 329 (44) |
| Wild-type | 321 (43) |
| Not reported | 104 (14) |
| Disease stage at EAP entry, n (%) | |
| III | 97 (13) |
| IV | 643 (85) |
| Unknown | 14 (2) |
| M stage at EAP entry, n (%) | |
| M0, M1A, M1B | 321 (43) |
| M1C | 392 (52) |
| Unknown | 41 (5) |
| Brain metastases at initial diagnosis, n (%) | |
| Yes | 19 (3) |
| No | 602 (80) |
| Unknown | 132 (18) |
| Not reported | 1 (<1) |
| Baseline LDH, n (%) | |
| ≤ULN | 493 (65) |
| >ULN | 239 (32) |
| >2 × ULN | 72 (10) |
| Not performed or reported | 22 (3) |
| Number of prior therapies, n (%) | |
| 0 | 532 (71) |
| 1 | 109 (14) |
| 2 | 73 (10) |
| ≥3 | 40 (5) |
| Time from prior therapy to first dose date, n (%)a | |
| <6 months | 145 (19) |
| ≥6 months | 75 (10) |
| Not reported | 534 (71) |
EAP, expanded access program; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Percentages based on patients who received prior therapies.
The median number of nivolumab doses for the induction and maintenance phases combined was four (range 1–39). The number of patients who received four induction doses was 317 (42%) for nivolumab and 310 (41%) for ipilimumab; for three doses, the numbers were 178 (24%) and 183 (24%), respectively, for two doses the numbers were 151 (20%) and 152 (20%), and for one dose the numbers were 108 (14%) and 109 (14%). A total of 277 (37%) patients continued on to receive maintenance nivolumab monotherapy. Among all patients, 95 (13%) patients received >10 doses of nivolumab in the maintenance phase.
Safety
AEs are summarized in Table 2. Treatment-related AEs (TRAEs) of any grade occurred in 723 (96%) patients, with the most common being fatigue [364 (48%) patients], diarrhea [303 (40%) patients], nausea [236 (31%) patients], pruritus [193 (26%) patients], increased aspartate aminotransferase [186 (25%) patients], maculopapular rash [182 (24%) patients], and increased alanine aminotransferase [182 (24%) patients]. Grade 3–4 TRAEs were observed in 400 (53%) patients; the most common were diarrhea [70 (9%) patients], increased alanine aminotransferase [69 (9%) patients], colitis [58 (8%) patients], increased lipase [56 (7%) patients], and increased aspartate aminotransferase [55 (7%) patients].
Table 2.
Adverse event summarya
| Nivolumab plus ipilimumab (N = 754) |
||
|---|---|---|
| Any grade, n (%) |
Grade 3–4, n (%) |
|
| Any-cause adverse event | 748 (99) | 485 (64) |
| Any treatment-related adverse event | 723 (96) | 400 (53) |
| Treatment-related adverse events in ≥5% of patients | ||
| Fatigue | 364 (48) | 19 (3) |
| Diarrhea | 303 (40) | 70 (9) |
| Nausea | 236 (31) | 11 (1) |
| Pruritus | 193 (26) | 4 (1) |
| Increased aspartate aminotransferase | 186 (25) | 55 (7) |
| Maculopapular rash | 182 (24) | 27 (4) |
| Increased alanine aminotransferase | 181 (24) | 69 (9) |
| Decreased appetite | 145 (19) | 2 (<1) |
| Pyrexia | 138 (18) | 5 (1) |
| Rash | 125 (17) | 6 (1) |
| Vomiting | 114 (15) | 7 (1) |
| Headache | 111 (15) | 8 (1) |
| Arthralgia | 105 (14) | 4 (1) |
| Hypothyroidism | 96 (13) | 2 (<1) |
| Increased lipase | 90 (12) | 56 (7) |
| Generalized pruritus | 84 (11) | 3 (<1) |
| Colitis | 80 (11) | 58 (8) |
| Abdominal pain | 75 (10) | 6 (1) |
| Myalgia | 71 (9) | 2 (<1) |
| Chills | 69 (9) | 0 |
| Dry mouth | 59 (8) | 0 |
| Cough | 58 (8) | 0 |
| Hyperthyroidism | 58 (8) | 4 (1) |
| Pruritic rash | 58 (8) | 0 |
| Increased amylase | 55 (7) | 18 (2) |
| Pneumonitis | 49 (6) | 11 (1) |
| Decreased weight | 47 (6) | 1 (<1) |
| Increased blood alkaline phosphatase | 45 (6) | 3 (<1) |
| Dyspnea | 44 (6) | 3 (<1) |
| Hypophysitis | 44 (6) | 7 (1) |
| Autoimmune hepatitis | 42 (6) | 30 (4) |
| Vitiligo | 38 (5) | 1 (<1) |
| Any treatment-related adverse events leading to discontinuation of treatment | 270 (36) | 198 (26) |
| Treatment-related adverse events in ≥5% of patients leading to discontinuation of treatment | ||
| Colitis | 57 (8) | 47 (6) |
| Diarrhea | 50 (7) | 36 (5) |
| Increased alanine aminotransferase | 39 (5) | 31 (4) |
Includes adverse events reported between first dose and 30 days after last dose of EAP therapy.
Treatment discontinuation due to any-grade and grade 3–4 TRAEs occurred in 270 (36%) and 198 (26%) patients, respectively. Serious TRAEs of any grade, grade 3–4, and grade 5 occurred in 258 (34%) patients, 204 (27%) patients, and one (<1%) patient, respectively. The most frequent grade 3–4 treatment-related select AEs (defined as those associated with an immune-mediated mechanism) involved the gastrointestinal [120 (16%) patients] and the hepatic systems [118 (16%) patients; Table 3].
Table 3.
Select (with a potential immunologic etiology) treatment-related adverse events in ≥5% of patients
| Nivolumab plus ipilimumab (N = 754) | ||
|---|---|---|
| Any grade, n (%) |
Grade 3–4, n (%) |
|
| Skin | 497 (66) | 45 (6) |
| Pruritus | 193 (26) | 4 (1) |
| Maculopapular rash | 182 (24) | 27 (4) |
| Rash | 125 (17) | 6 (1) |
| Generalized pruritus | 84 (11) | 3 (<1) |
| Pruritic rash | 58 (8) | 0 |
| Vitiligo | 38 (5) | 1 (<1) |
| Gastrointestinal | 331 (44) | 120 (16) |
| Diarrhea | 303 (40) | 70 (9) |
| Colitis | 80 (11) | 58 (8) |
| Hepatic | 266 (35) | 118 (16) |
| Increased aspartate aminotransferase | 186 (25) | 55 (7) |
| Increased alanine aminotransferase | 181 (24) | 69 (9) |
| Increased blood alkaline phosphatase | 45 (6) | 3 (<1) |
| Autoimmune hepatitis | 42 (6) | 30 (4) |
| Endocrine | 223 (30) | 22 (3) |
| Hypothyroidism | 96 (13) | 2 (<1) |
| Hyperthyroidism | 58 (8) | 4 (1) |
| Hypophysitis | 44 (6) | 7 (1) |
| Pulmonary | 50 (7) | 11 (1) |
| Pneumonitis | 49 (6) | 11 (1) |
| Renal | 38 (5) | 13 (2) |
Of 754 total patients, immune-modulating medications (IMMs) for managing AEs were required by 600 (80%) patients for any-grade AEs, with 332 (44%) patients requiring them for grade 3–4 AE management; of patients with an AE, these proportions were 80% (600/748) and 68% (332/485), respectively (Supplementary Table S1, Supplemental digital content 1, http://links.lww.com/MR/A249). The most common IMM class was systemic corticosteroids, which were administered to 571 (76%) patients. Immunosuppressive agents were administered to 92 (12%) patients, and included infliximab in 67 patients (mainly used to treat colitis), mycophenolic acid in 24 patients (mainly used to treat certain steroid-refractory immune-mediated AEs such as hepatitis, or as a ‘steroid-sparing’ agent), and azathioprine in two patients (mainly to reduce the symptoms of rheumatoid arthritis; Table S2, Supplemental digital content 1, http://links.lww.com/MR/A249).
AEs of any cause were similar between patients <65 years of age (n = 535) and ≥65 years of age (n = 219). Grade 3–4 AEs of any cause were reported in 351 (66%) and 134 (61%) patients, respectively; the most common grade 3–4 AEs were increased alanine aminotransferase (11%) and diarrhea (10%) in patients <65 years of age and diarrhea (9%) and colitis (7%) in patients ≥65 years of age.
As of the clinical database lock, deaths were reported for 190 (25%) of the 754 treated patients [160 (21%) for disease progression, 7 (1%) for EAP-related drug toxicity, 13 (2%) for other reasons, and 10 (1%) for unknown or unreported reasons]. A total of 64 deaths occurred within 100 days after the last dose; six deaths were deemed to be treatment-related (one each attributed to septic shock, myocardial infarction, drug-induced liver injury, sepsis, myocarditis, and colitis).
Efficacy
With a median follow-up of 17.8 months, median OS was not reached for the overall EAP group of 754 patients, and 564 patients (75%) were censored (Fig. 2). Twelve-month, 18-month, and 24-month survival rates were 82% [95% confidence interval (CI) 79–84], 74% (95% CI 71–78), and 70% (95% CI 66–74), respectively. Among the 477 patients who discontinued treatment during the induction phase (i.e. prior to the maintenance phase), 12-month, 18-month, and 24-month survival rates were 75% (95% CI 71–79), 67% (95% CI 63–72), and 65% (95% CI 60–70), respectively.
Fig. 2.
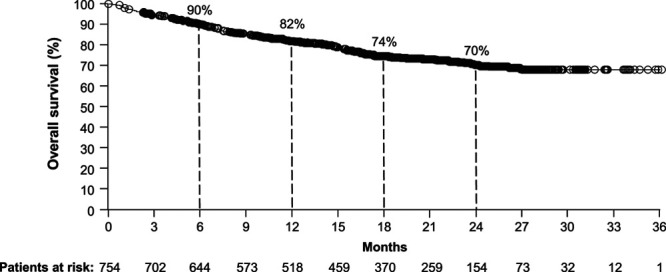
Survival outcomes in the overall population. Kaplan–Meier curves are shown for the overall population (190 events/754 patients) with a median OS not available (minimum follow-up not reached). OS, overall survival.
OS outcomes in key subgroups are presented in Fig. 3a–h. Twenty-four-month OS rates were similar between patients <65 years of age [71% (95% CI 66–75)] and ≥65 years of age [69% (95% CI 61–75); Fig. 3a] and between patients with BRAF wild-type [73% (95% CI 67–78)] and mutant tumors [70% (95% CI 63–75); Fig. 3f]. Twenty-four-month OS rates were numerically different between known prognostic subgroups, including patients with an ECOG PS of 0 [77% (95% CI 72–81)] and an ECOG PS of 1 [56% (95% CI 48–63); Fig. 3d]; patients with LDH levels that were ≤upper limit of normal (ULN; 77% (95% CI 72–81)], >ULN [56% (95% CI 49–63)], and >2 × ULN [39% (95% CI 26–53); Fig. 3e]; and patients with metastasis (M0)/M1A/M1B stage disease [78% (95% CI 72–82)] and M1C stage disease [65% (95% CI 59–70); Fig. 3g]. There were also numerical differences in 24-month OS rates between other patient subgroups, including male [75% (95% CI 70–80)] and female patients [62% (95% CI 54–68); Fig. 3c], and patients with cutaneous [72% (95% CI 68–76)], mucosal [56% (95% CI 37–71)], and uveal [44% (95% CI 24–63)] melanoma (Fig. 3h).
Fig. 3.
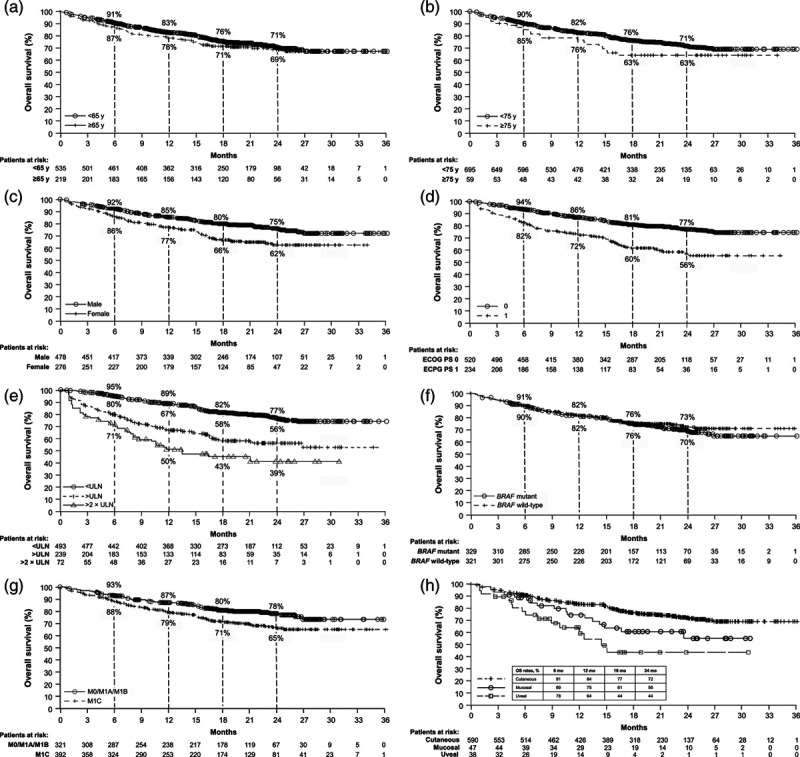
OS outcomes in key subgroups. Kaplan–Meier curves are shown by age, with a median OS of NR for patients aged <65 years (127 events/535 patients) and NR for patients aged ≥65 years (63 events/219 patients) (a); by age, with a median OS of NR for patients aged <75 years (169 events/695 patients) and NR (95% CI: 16.5–NR) for patients aged ≥75 years (21 events/59 patients) (b); by gender, with a median OS of NR for male patients (100 events/478 patients) and NR for female patients (90 events/276 patients) (c); by ECOG PS status, with median OS of NR for patients with PS 0 (103 events/520 patients) and NR (95% CI 21.4–NR) for patients with PS 1 (87 events/234 patients) (d); by LDH levels, with a median OS of NR for ≤ULN (93 events/493 patients), NR (95% CI 21.0–NR) for >ULN (93 events/239 patients), and 11.6 (95% CI 8.0–NR) for >2 × ULN (38 events/72 patients) (e); by BRAF status, with a median OS of NR for mutant BRAF status (84 events/329 patients) and NR for wild-type BRAF status (75 events/321 patients) (f); by M stage, with a median OS of NR for M0/M1A/M1B disease (64 events/321 patients) and NR for M1C disease (113 events/392 patients) (g); and by melanoma subtype, with a median OS of NR for cutaneous melanoma (142 events/590 patients), NR (95% CI 14.9–NR) for mucosal melanoma (17 events/47 patients), and 14.5 (95% CI 8.9–NR) for uveal melanoma (16 events/38 patients) (h). CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; NR, not reached; OS, overall survival; ULN, upper limit of normal.
Discussion
CheckMate 218, a large North American EAP, provided access to investigational nivolumab to be used in combination with commercially available ipilimumab for patients with advanced melanoma prior to regulatory approval of the combination. Outcomes with nivolumab plus ipilimumab in this EAP were consistent with findings from the registrational RCTs, CheckMate 069 and CheckMate 067 [4,8–10], with a large population of patients and less stringent inclusion criteria than CheckMate 067 with respect to the number of prior therapies and melanoma subtypes allowed. Unlike the RCTs, this EAP solely utilized investigators who had previous experience with the administration of nivolumab and ipilimumab, either as monotherapy or in combination.
The patient population in this EAP differed in certain respects from those in the RCTs [8,10]. For example, in contrast to CheckMate 067, which exclusively enrolled untreated patients, this EAP included patients who had previously received systemic treatment, including BRAF/MEK inhibitors in patients with BRAF mutant disease. As a result, the EAP population had a greater proportion of patients with BRAF mutant disease (44% or more) than the study group treated with the combination in CheckMate 067 (32%). In addition, this EAP enrolled patients with rarer subtypes of melanoma, including 38 patients with uveal melanoma and 47 patients with mucosal melanoma. Patients with uveal melanoma were excluded from CheckMate 067 and, while patients with mucosal melanoma were enrolled in that study, data for these patients were limited. However, the proportions of patients with elevated baseline LDH levels were similar in the EAP (32%) and the study group receiving the combination in CheckMate 067 (36%).
Safety results from this EAP were consistent with those from the RCTs [4,8–10]. For instance, grade 3–4 TRAEs occurred in 53% of patients in this EAP and in 59% of patients treated with the combination in CheckMate 067 [4]. TRAEs of any grade led to treatment discontinuation in 36 and 42% of patients receiving the combination in this EAP and in CheckMate 067, respectively [4]. Diarrhea and increased alanine aminotransferase were the most frequent grade 3–4 TRAEs in the EAP (each, 9%); these were also among the most common in CheckMate 067, reported at similar rates [4]. In this EAP, IMMs for managing AEs were required by 35% of patients for AEs of less than grade 3 and by 44% of patients for grade 3–4 AEs, suggesting that lower-grade immune-mediated AEs can have a clinically meaningful impact that requires therapeutic intervention. Six treatment-related deaths (1%, equal to those reported for patients treated with nivolumab plus ipilimumab in CheckMate 067 [4]) were reported, and no new safety signals were identified.
Nivolumab plus ipilimumab was associated with high survival rates in this EAP, and these findings were consistent with those from the RCTs [4,8–10,14]. For the overall EAP group, the 24-month survival rate was 70%. Interestingly, 24-month OS rates in this EAP were similar in patients with BRAF wild-type and mutant disease (73% vs. 70%), suggesting that BRAF mutation status may not be predictive of survival outcomes with the combination. In CheckMate 067, the 24-month OS rate was 64% in patients treated with the combination [14], and there appeared to be a long-term survival benefit in patients with BRAF mutant disease treated with the combination compared with those treated with nivolumab monotherapy [5-year OS rate: 60% vs. 46%; unstratified hazard ratio 0.70 (95% CI 0.46–1.05)] [4]. Male patients in this EAP exhibited numerically greater survival outcomes than female patients, consistent with a recent systematic review of treatment with immune checkpoint inhibitors [15]. Survival rates were similar for patients aged ≥65 and <65 years, suggesting that the combination is effective in both older and younger patients. The efficacy of the combination in this EAP was further evidenced by 24-month OS rates of >55% in patients with poor prognostic characteristics (e.g. an ECOG PS of 1, elevated LDH level, or M1C stage disease).
This EAP also included patients with rare melanoma subtypes, such as uveal and mucosal melanoma, who typically face a poor prognosis [16,17]. Mucosal melanoma represents 1–4% of all melanomas [16], and more than 40% of patients with this form of melanoma are diagnosed with metastatic disease [18]. In the metastatic setting, median OS for mucosal melanoma has been reported to be approximately 9 months [19]. Among patients with mucosal melanoma in this EAP, median OS was not reached, and the 12-month OS rate was 75%. Uveal melanoma comprises 3–5% of all cases of melanoma, and up to one-half of patients with this form of the disease develop metastases [20]. For metastatic uveal melanoma, the median OS has been reported to be approximately 10 months, with a 12-month OS rate of 43% [17]. Among patients with uveal melanoma in this EAP, the median OS was approximately 15 months, and the 12-month OS rate was 64%. Findings with uveal melanoma in this EAP are supported by results from phase 2 studies in Spain (n = 50; median follow-up, 7.1 months) and the USA (n = 35; median follow-up, 13.9 months) in which nivolumab plus ipilimumab demonstrated clinical activity in patients with metastatic uveal melanoma, with a median OS of 12.7 and 19.1 months, respectively [21,22]. While the results presented here for mucosal and uveal melanoma are interesting, these patients comprised a small proportion of the EAP (6% and 5%, respectively).
In this EAP, 37% of patients continued on to receive maintenance nivolumab monotherapy, compared with 47% of patients in CheckMate 067, with only 13% receiving >10 doses of maintenance nivolumab [10]. The results from this EAP suggest that patients who experience significant AEs and meet discontinuation criteria can still obtain benefit.
This EAP has several important limitations. The EAP was observational in nature, with the primary purpose being to provide early access to nivolumab for use in combination with ipilimumab. Results for OS should be interpreted with caution, particularly at the later time points, because of the high proportion of early censoring resulting from the EAP closure and the short minimum follow-up time. In addition, there was a lack of information collected on therapies given after EAP treatment, which could have had an impact on the observed OS.
The results of CheckMate 218, a large EAP, add to the growing clinical database of information supporting the use of combination nivolumab plus ipilimumab in the treatment of advanced melanoma. This EAP provides additional safety and efficacy information for patients with advanced melanoma treated with this combination, including patients with various melanoma subtypes. Results of this EAP are consistent with findings from the registrational RCTs and support the use of nivolumab plus ipilimumab in a broad patient population with advanced melanoma.
Acknowledgements
We thank the patients and investigators who participated in the CheckMate 218 EAP. We would also like to thank Ute Dugan at Bristol Myers Squibb for her contributions to the design of the protocol. We also acknowledge ONO Pharmaceutical Company, Ltd. (Osaka, Japan) for contributions to nivolumab development and Dako (Santa Clara, CA), an Agilent Technologies, Inc. company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay. Professional medical writing and editorial assistance were provided by Mark Palangio and Michele Salernitano at Ashfield Healthcare Communications, funded by Bristol Myers Squibb.
This EAP was supported by Bristol Myers Squibb (Princeton, NJ, USA).
Hogg D, Chapman PB, Sznol M, et al. Overall survival (OS) analysis from an expanded access program (EAP) of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (MEL). Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, 2–6 June 2017 (abstract 9522).
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Conflicts of interest
F.S.H.: research funding: Bristol Myers Squibb (Inst), Novartis; consulting or advisory role: 7 Hills Pharma, Aduro, Apricity, Bayer, Bicara, Compass Therapeutics, Genentech/Roche, Kairos, Partners Therapeutics, Pfizer, Pionyr, Psioxus, Therapeutics Rheos, Sanofi, Surface, Takeda, Torque, Verastem; stock ownership: Pionyr, Torque. P.B.C.: research funding: NCI/Core Grant P30 CA008748, Pfizer; consulting or advisory role: Cell Medica, Merck, Scancell. M.S.: consulting or advisory role: Abbvie, Adaptimmune, Agonox, Allakos, Almac, Anaeropharma, Arbutus, Array, AstraZeneca/Medimmune, Bristol Myers Squibb, Baxalta-Shire, Biodesix, Celldex, Chugai-Roche, Genentech-Roche, Genmab, Genocea, GI Innovation, Gritstone, Hinge, Immunocore, Incyte, Innate Pharma, Inovio, Janssen/Johnson & Johnson, Kyowa-Kirin, Lilly, Lion Biotechnologies (Iovance), Merck USA, Modulate Therapeutics, Molecular Partners, Nektar, Newlink Genetics, Novartis, Omniox, Pfizer, Pieris, Pierre-Fabre, Seattle Genetics, Symphogen, Theravance, Torque, Vaccinex; stock ownership: Actym, Adaptive Biotechnologies, Amphivena, Intensity, Torque. C.D.L.: consulting or advisory role: Immunocore; research funding: Bristol Myers Squibb, Dynavax, Genentech, Immunocore, Merck, Novartis; travel expenses: Bristol Myers Squibb. R.G.: consulting or advisory role: Amgen, Array, Bristol Myers Squibb, GlaxoSmithKline, Genentech-Roche, Incyte, NewLink Genetics, Novartis; research funding: Amgen, Array, Bristol Myers Squibb, Boston Biomedical, Checkmate Pharmaceuticals, GlaxoSmithKline, Genentech-Roche, Incyte, Merck, Nektar, NewLink Genetics, Novartis, Syndax, Takeda, Tesaro. M.S.: honoraria: Bristol Myers Squibb, Merck, Novartis, Sanofi Genzyme. R.K.: consulting or advisory role: Bristol Myers Squibb, Array, Novartis, Immunocore; research funding: Merck, Regeneron. W.S.: consulting or advisory role: Bristol Myers Squibb, Merck, Novartis, Iovance, Regeneron; research funding: Bristol Myers Squibb, Merck, Novartis. M.A.: consulting or advisory role: Acceleron Pharma, Aduro Biotech, Agenus, Array BioPharma, Arrowhead Pharmaceuticals, Boehringer, Ingelheim, Bristol Myers Squibb, Eisai, Exelixis, Genentech, Glactone Pharma, Immunocore, Iovance Biotherapeutics, Merck, Newlink Genetics/Pharmatech, Novartis, Oncolys BioPharma, Pfizer, Surface, Werewolf Pharma, X4 Pharma; research funding: Bristol Myers Squibb (Inst); honoraria: Bristol Myers Squibb. D.R.S.: grants and/or other financial support from Abbvie, Acerta Pharma, Aeglea Biotherapeutics, Amgen, ARMO BioSciences, Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celldex, Clovis Oncology, Daiichi Sankyo, EMD Serono, Evelo Therapeutics, Foundation Medicine, G1 Therapeutics, Genentech/Roche, Genzyme, GlaxoSmithKline, GRAIL, Illumina, Intuitive Surgical, Ipsen, Lilly, Merck, Millennium, Moderna Therapeutics, Nektar, Neon Therapeutics, Novartis, Oncogenex, Pfizer, PharmaMar, Precision Oncology, Purdue Pharma, Spectrum Pharmaceuticals, Sysmex, Takeda, Tesaro, Transgene, TRM Oncology, University of Texas Southwestern Medical Center – Simmons Cancer Center. A.P.: consulting or advisory role: Array, Bristol Myers Squibb, Regeneron, Seattle Genetics; research funding: Regeneron (Inst), Bristol Myers Squibb (Inst), Merck (Inst). J.M.: consulting or advisory role: Bristol Myers Squibb, Celgene, EMD Serono, Merck, Novartis, Roche; research funding: Merck. K.B.K.: consulting or advisory role: Array BioPharma, Bristol Myers Squibb, Genentech, Novartis, Seattle Genetics, Astellas; research funding: Bristol Myers Squibb (Inst); honoraria: Bristol Myers Squibb, Merck, Novartis, Seattle Genetics, Astellas, Array BioPharma, Genentech. S.E.: consulting or advisory role: Bristol Myers Squibb Canada, EMD Serono, Genzyme Canada, Merck Canada, Novartis. N.I.K.: research funding: Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, HUYA Bioscience International, Merck, Novartis, Regeneron; consulting or advisory role: Array BioPharma, AstraZeneca, Bristol Myers Squibb, EMD Serono, HUYA Bioscience International, Genentech, Immunocore, Merck, Regeneron; stock ownership: Bellicum Pharmaceuticals, Mazor Robotics, TransEnterix; Honoraria: Bristol Myers Squibb. W.v.D.: employee: Bristol Myers Squibb. M.L.: stock ownership: Bristol Myers Squibb; employee: Bristol Myers Squibb. D.H.: research funding: EMD Serono; consulting or advisory role: Amgen, Bristol Myers Squibb, EMD Serono, Merck, Novartis, Roche. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.melanomaresearch.com.
References
- 1.Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Investig Dermatol. 2017; 10:325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018; 2:CD011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011; 16:5–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019; 381:1535–1546 [DOI] [PubMed] [Google Scholar]
- 5.Opdivo [package insert]. 2019, Princeton, NJ: Bristol-Myers Squibb Company [Google Scholar]
- 6.Yervoy [package insert]. 2019, Princeton, NJ: Bristol-Myers Squibb Company [Google Scholar]
- 7.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013; 14:1212–1218 [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015; 372:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016; 17:1558–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015; 373:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogg D, Chapman PB, Sznol M, Lao CD, Gonzalez R, Daniels GA, et al. Overall survival (OS) analysis from an expanded access program (EAP) of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (MEL).; Presented at the American Society of Clinical Oncology Annual Meeting; Chicago, IL, June 2–6, 2017 (abstract 9522)
- 12.Hogg D, Monzon JG, Ernst S, Song X, McWhirter E, Savage KJ, et al. Canadian cohort expanded-access program of nivolumab plus ipilimumab in advanced melanoma. Curr Oncol. 2020; 27:204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017; 377:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018; 19:737–746 [DOI] [PubMed] [Google Scholar]
- 16.Yde SS, Sjoegren P, Heje M, Stolle LB. Mucosal melanoma: a literature review. Curr Oncol Rep. 2018; 20:28. [DOI] [PubMed] [Google Scholar]
- 17.Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019; 30:1370–1380 [DOI] [PubMed] [Google Scholar]
- 18.Lian B, Cui CL, Zhou L, Song X, Zhang XS, Wu D, et al. The natural history and patterns of metastases from mucosal melanoma: an analysis of 706 prospectively-followed patients. Ann Oncol. 2017; 28:868–873 [DOI] [PubMed] [Google Scholar]
- 19.Kuk D, Shoushtari AN, Barker CA, Panageas KS, Munhoz RR, Momtaz P, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist. 2016; 21:848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017; 101:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piulats Rodriguez JM, De La Cruz Merino L, Espinosa E, Alonso Carrión L, Martin Algarra S, López-Castro R, et al. Phase II multicenter, single arm, open label study of nivolumab in combination with ipilimumab in untreated patients with metastatic uveal melanoma. Annals Oncol. 2018; 29suppl 8viii442–viii466 [Google Scholar]
- 22.Pelster M, Gruschkus SK, Bassett R, Gombos DS, Shephard M, Posada L, et al. Phase II study of ipilimumab and nivolumab (ipi/nivo) in metastatic uveal melanoma (UM). J Clin Oncol. 2019; 37suppl9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


