Abstract
Objective:
To evaluate the positive predictive value (PPV) of endometrial BCL-6 overexpression as a noninvasive screening test endometriosis in patients undergoing in vitro fertilization (IVF).
Methods:
Retrospective cohort study at a university-affiliated private practice. Inclusion criteria were reproductive age females currently undergoing IVF with a diagnosis of unexplained infertility or unexplained recurrent pregnancy loss. Those with endometrial BCL-6 overexpression underwent laparoscopic surgery with an indication for treatment of suspected endometriosis. The primary outcome was the PPV of endometrial BCL-6 testing to surgically diagnose endometriosis. Statistical analysis was performed using SPSS v.25.0.
Results:
Seventy-five patients met inclusion criteria for our study. The PPV of BCL-6 testing for endometriosis was 96%. Of those patients without endometriosis, 100% had other inflammatory pelvic pathologies, which were diagnosed and treated at the time of laparoscopy.
Conclusions:
Endometrial BCL-6 overexpression has a high PPV for diagnosing endometriosis and can help identify a patient population that may require surgical treatment before embryo transfer.
Keywords: Endometriosis, BCL6, Unexplained Infertility, Recurrent Pregnancy Loss, In Vitro Fertilization
INTRODUCTION
Endometriosis is a progressive, estrogen-dependent, chronic inflammatory disease affecting approximately 10% of reproductive age women, and over 200 million women worldwide.1–3 It is an ancient disease described as early as 1855 BC by Egyptians as “suffocation of the womb” long before the histologic diagnosis of ectopic endometrial-like glands and stroma was described by Karl von Rokitansky in 1860.4 Endometriosis has been reported in every organ in the human body, with clinically significant extragenital endometriosis leading to genitourinary, gastrointestinal, thoracic, and/or nervous system dysfunction.5–8 Patients with endometriosis have almost double the risk of developing ovarian cancer, and endometriosis treatment can decrease the risk of malignancy.9
Affected women can present with dysmenorrhea, non-menstrual pelvic pain, ovulatory pain, dyspareunia, dyschezia, and/or changes to bowel or bladder function which can be exacerbated during menses.10 Symptom severity varies from debilitating to mild, with up to 25% of women being completely asymptomatic.11 Some women may only present with unexplained infertility, with an increased suspicion for endometriosis arising only after multiple failed in vitro fertilization (IVF) treatments.12 If there is no treatable cause of infertility, which occurs after documentation of at least one patent fallopian tube, evidence of ovulation, and a normal semen analysis in the male partner, women are given the diagnosis of unexplained infertility.13 In fact, endometriosis is a leading cause of unexplained infertility, accounting for up to 50 – 80% of women struggling to conceive.3,14 According to 2017 Society for Assisted Reproductive Technology data, only 2.5% of IVF cycles are performed in patients diagnosed with endometriosis, leading us to believe that endometriosis is grossly underdiagnosed in the setting of infertility.15,16
Numerous theories exist to explain how endometriosis can affect fertility, including distorted pelvic anatomy, abnormal utero-tubal transport, ovulatory dysfunction, altered cell-mediated immunity, decreased oocyte quality, dyssynchronous oocyte maturation, and altered endometrial receptivity.10,17–20 Currently, progesterone resistance and altered endometrial receptivity describe the leading mechanisms behind endometriosis-related implantation failure. It is well established there is altered progesterone signaling in the endometrium of women with endometriosis, with downregulation of progesterone receptors and upregulation of estrogen receptors causing a shift from secretory to proliferative endometrium.20–22 Adequate progesterone levels and endometrial receptor expression are needed for endometrial stabilization, embryo implantation, and maintenance of pregnancy; any mechanism that interferes with progesterone signaling can be detrimental to this process.
Endometriosis has been associated with aberrant cellular and humoral immunity.23 B-cell chronic lymphocytic leukemia/lymphoma 6 (BCL-6) is a protein encoded by a proto-oncogene present on chromosome 3 (3q27.3) that stimulates inflammatory cytokines such as interleukin-6 (IL-6), IL-8, and IL-17 in the peritoneal fluid of women with endometriosis.10,18,19,22,24 Peritoneal cytokine upregulation leads to a cascading effect of inflammation, neovascularization, fibrosis, and adhesion formation characteristic of the endometriosis phenotype.19,22 Additionally, IL-6 is directly involved in upregulating endometrial transcription factor Signal Transducer and Activator of Transcription 3 and BCL-6 expression.22,25 In the endometrium, BCL-6 forms a complex that binds to and inactivates regulators of the progesterone pathway, leading to progesterone resistance, aberrant decidualization, implantation failure, and recurrent miscarriages in women with endometriosis.18,25
Recently, there has been a push for the development of noninvasive screening and diagnostic tests to accurately identify patients with endometriosis; however, a surgical diagnosis consisting of laparoscopy with or without histologic confirmation remains the gold standard.26 Histologic diagnosis can be challenging as endometriosis can present at different stages of disease. While it is classically defined as the presence of ectopic endometrial-like glands and stroma, endometriosis can also be associated with hemosiderin-laden macrophages, foreign body giant cells, and proliferation of fibrous adhesive connective tissue.27 Additionally, endometriosis can present solely as smooth muscle cells and fibrosis in certain scenarios such as deep infiltrating endometriosis in the rectovaginal septum, which can be challenging when counseling patients about the differences between visual and histologic findings for endometriosis.28
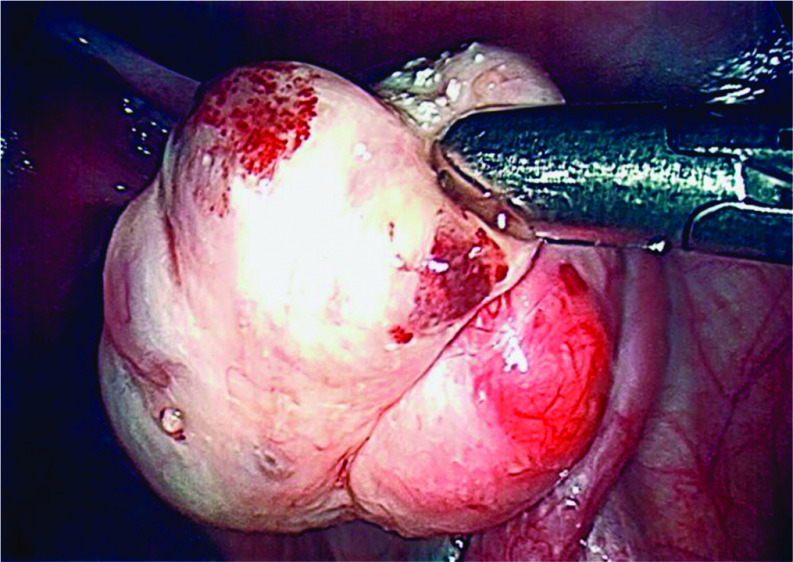
Histologic diagnosis of endometriomas can also be difficult depending on endometrioma subtype as they are treated differently in patients desiring to preserve fertility. Type I endometriomas arise from endometrial-like tissue implanted on the ovarian cortex (Figure 1). They are treated by brushing or washing off the lesions or superficially desiccating the capsule to minimize adverse effects on ovarian reserve.29–31 Type II endometriomas arise from functional cysts that are invaded by endometrial-like implants. Type IIA and IIB endometriomas are associated with less than 50% invasion of endometriosis within the cyst wall and are easier to excise while minimizing effects on ovarian reserve. Type IIC endometriomas have greater than 50% invasion and a greater amount of fibrosis which makes them even more difficult to remove during fertility-sparing surgery.29–31 Treatment of type IIC endometriomas involves superficially desiccating the cyst capsule to preserve ovarian reserve.30–32 In the setting of infertility, we do not recommend cystectomy and recommend aspiration, irrigation, or superficial desiccation of endometriomas.30–32
Figure 1.
Visually Diagnosed Type I Endometriomas.
An endometriosis screening test called ReceptivaDxTM (CiceroDx, Huntington Beach, CA) has been developed to detect endometrial BCL-6 overexpression in asymptomatic women with unexplained infertility or recurrent pregnancy loss.2 It also detects beta-3 integrin expression, a cell adhesion molecule needed for successful implantation.14,22,25 Positive endometrial BCL-6 testing, defined as an HSCORE >1.4, has been associated with poor IVF outcomes and recurrent miscarriage.3,18,25 By treating the underlying cause of endometrial inflammation secondary to endometriosis, whether medically or surgically, studies have shown improvement in subsequent live birth rates (LBR) (50 –76%) when compared to controls (7.4%).3,12 This landmark study reported that 93.8% of patients that tested positive for BCL-6 had laparoscopic findings of endometriosis.2
We sought to confirm the positive predictive value (PPV) of endometrial BCL-6 overexpression in identifying endometriosis in women with unexplained infertility. Given that BCL-6 expression is upregulated by IL-6 and its overexpression is a marker of inflammation, we also evaluated the prevalence of other inflammatory pathologies including endosalpingiosis, hydrosalpinges, and leiomyomas, which could impact fertility.
METHODOLOGY
This is a retrospective cohort study of patients who underwent laparoscopic surgery with or without robotic-assistance for diagnosis and treatment of endometriosis at a university-affiliated, high-volume gynecologic surgery referral center. Our study was deemed to be IRB exempt by Pearl IRB (Protocol #20-CNEZ-101). Charts were reviewed retrospectively for patient demographics, diagnoses, and clinical outcomes, and this data was extracted from our center’s electronic medical record system. Patient data was de-identified and stored in an encrypted database for data analysis.
Our inclusion criteria were reproductive age females currently undergoing IVF with endometrial BCL-6 overexpression who underwent laparoscopic surgery for treatment of suspected endometriosis between February 2018 through January 2020. Patients were evaluated by their chosen reproductive endocrinology and infertility (REI) specialist, and patients diagnosed with unexplained infertility, history of failed IVF, or recurrent pregnancy loss (defined by the American Society for Reproductive Medicine [ASRM] criteria as two or more documented pregnancy losses by ultrasound or pathology) were offered endometrial biopsy with ReceptivaDxTM. All women underwent endometrial biopsy to test for BCL-6 and beta-3 integrin in the secretory phase of their natural cycle or 5 – 10 days after progesterone supplementation in a mock cycle.2 Endometrial biopsies were considered abnormal with BCL-6 HSCORE >1.4 per manufacturer recommendations.2 Abnormal beta-3 integrin expression was not considered in the clinical management of patients with endometriosis and was not included in this study. We documented the prevalence of endometritis in our patient population based on the presence of plasma cells or diagnosed endometritis on endometrial biopsy. Women with positive BCL-6 testing desiring surgical management for suspected endometriosis were referred to our center for surgical consultation. BCL-6 negative patients were not included in this study.
Patients underwent operative laparoscopy with or without robotic assistance for treatment of endometriosis by a single surgeon, and all visible endometriosis was treated with excision except for superficial endometriomas. All specimens were sent to pathology to confirm the diagnosis of endometriosis. Pathology was deemed positive for endometriosis when ectopic endometrial-like glands or stroma were present. Specimens with hemosiderin-laden macrophages were not considered diagnostic. As BCL-6 overexpression is not specific to endometriosis-related inflammation, other potential causes of inflammation included in pathology reports were noted, including fibrosis, endosalpingiosis, hydrosalpinx, and leiomyomas.
Patients were routinely seen in our office at 1 and 6 months postoperatively for endometriosis follow-up. Statistical analysis was performed using SPSS v.25.0.
RESULTS
A total of 75 patients met inclusion criteria. Patient demographics are described in Table 1. Average age of patients at the time of consultation was 36.9, with an average body mass index of 23. In patients with endometrial BCL-6 overexpression, the PPV for histology-proven plus visually diagnosed type I and IIC endometriomas was 96%. Three-quarters of patients (74.7%, n = 56) had a histologically confirmed diagnosis, while 21.3% were diagnosed visually through the presence of ovarian endometriotic implants (n = 16). At time of BCL-6 testing, 10.7% of patients (n = 8) had endometritis on endometrial biopsy and were subsequently treated with antibiotics. Five patients with endometritis on BCL-6 testing had histology-confirmed endometriosis while the other three had visually diagnosed superficial ovarian endometriosis. The prevalence of other gynecologic pathologies found at time of surgery is described in Table 2.
Table 1.
Patient Characteristics
| Age (years) | 36.91 ± 4.18 [range 29 – 47] |
| Body Mass Index (kg/m2) | 23.31 ± 3.46 [range 17 – 36] |
| Nulliparous | 74.67% (n = 56) |
| Time trying to conceive (years) | 2.19 ± 1.60 [range 0 – 10] |
| History of failed in vitro fertilization | 64.0% (n = 48) |
| History of recurrent pregnancy loss | 22.67% (n = 17) |
Table 2.
Prevalence of Histology-Confirmed Gynecologic Pathology During Laparoscopic Surgery
| Prevalence % (n) | |||
|---|---|---|---|
| Diagnosis | Endometriosis (96%, n = 72) | No Endometriosis (4%, n = 3) | Total (n = 75) |
| Endometriosis | 100 (72) | — | 96.00 (72) |
| Hemosiderin-laden macrophages | 51.39 (37) | 66.67 (2) | 52.00 (39) |
| Endosalpingiosis | 2.78 (2) | 33.33 (1) | 4.00 (3) |
| Hydrosalpinx | 2.78 (2) | 33.33 (1) | 4.00 (3) |
| Uterine leiomyoma | 33.33 (24) | 0.00 (0) | 32.00 (24) |
| Adenomyosis | 1.39 (1) | 0.00 (0) | 1.33 (1) |
| Fibrosis/Adhesions | 98.61 (71) | 100.00 (3) | 98.67 (74) |
Among women who did not have visually or histologically confirmed endometriosis (n = 3), all had other pathologies which could result in endometrial BCL-6 overexpression. Pathologic specimens showed fibrosis or adhesions in 100% (n = 3) and hemosiderin-laden macrophages in 66.7% (n = 2) of patients. Hydrosalpinx and endosalpingiosis were both found in the same patient. The majority of the patients included in this study were nulliparous (74.7%) with an average of 2.2 years trying to conceive prior to initial surgical consultation. Women with at least 6 months of postoperative follow-up were assessed for reproductive outcomes (n = 40), resulting in a clinical pregnancy rate (CPR) of 90.0%.
DISCUSSION
Inflammation and aberrantly expressed inflammatory cytokines are associated with the pathophysiology of endometriosis. The pelvic inflammatory environment derived from endometriosis is known to be associated with subfecundity and recurrent pregnancy loss. While women experiencing infertility and pregnancy loss do not typically seek surgical diagnosis of endometriosis for fertility alone, persistent endometriosis could affect the success rate of advanced fertility treatment such as IVF. Diagnosing endometriosis and other inflammatory processes is simply the first step, with treatment ultimately needed for many patients struggling to conceive.
According to ASRM, there is a high pretest probability (approximately 50%) of patients with unexplained infertility having endometriosis.10 Perhaps the prevalence of endometriosis is much higher, and endometrial BCL-6 testing may help determine which patients are at high risk for endometriosis and other inflammatory pathologies, and may benefit from surgical treatment.12 In our study, we found the PPV of BCL-6 testing to be as high as 96% for the diagnosis of endometriosis, similar to previously reported rates.2
Endometrioma excision has been associated with improved conception rates, both through assisted reproductive technology (ART) or spontaneous conception.20 Type IIA and IIB endometriomas can be easily separated from the surrounding ovarian cortex without damaging the primordial and preantral follicles.29–31 The true prevalence of large endometriomas are likely underrepresented in our study as patients with ultrasound findings suggestive of endometrioma were referred for surgical management without BCL-6 testing. Overall, during laparoscopy 92% of women had a visual diagnosis of endometriomas, which were surgically treated. We believe that the differences in surgical treatment of endometriomas account for the discrepancy in histology-confirmed endometriosis in our cohort.
Three women had BCL-6 overexpression without confirmed endometriosis, but all of these women had other inflammatory pathologies that could explain their positive test results. Chronic diseases and prior infections can cause fibrosis and mild inflammatory states.33 As part of the normal healing process, tissue damage recruits inflammatory cytokines, many of which are profibrotic. IL-6 is a major inflammatory cytokine needed to produce peritoneal fibrosis.33 In our study, all (100%) patients without proven endometriosis had fibrosis or fibrous adhesions on pathology. Although causation cannot be inferred, fibrosis can be a reason for the elevated BCL-6 seen in these patients.
Leiomyomas are associated with inflammation and infertility, and up to 87% of reproductive age women undergoing surgical management for symptomatic fibroids have been found to have coexisting endometriosis at the time of surgery.1 In our study, a total of 24 women (33.3%) had uterine fibroids, all of which showed histological or visual evidence of endometriosis. While it is not fully understood how different International Federation of Gynecology and Obstetrics (FIGO) classes of fibroids influence inflammation and infertility, there are a few published studies that help explain their impact. One study found that FIGO type 0-3 fibroids can lead to altered endometrial cytokine expression and endometrial receptivity.34 Leiomyomas are caused by inflammatory mediators, including IL-6, and fibroblasts which deposit extracellular matrix, leading to a state of inflammation, neovascularization, tissue remodeling, and growth.35 A study comparing myometrial progenitor cells to fibroid progenitor cells found that fibroid cells secrete a statistically higher level of chronic inflammatory cytokines compared to myometrial cells.36 This could describe a possible mechanism for the elevated BCL-6 observed in these women.
Previously reported studies have shown improved CPR and LBR following medical or surgical treatment of endometriosis.3,12,37 Although not a primary endpoint of our study, reproductive outcomes in women with at least 6 months of postoperative follow-up was assessed. A total of 40 women were included in this timeframe, with 36 documented clinical pregnancies, giving a clinical pregnancy rate (CPR) of 90.0%. Most patients (84%) conceived through IVF. There were ten live births (27.8%), twenty-five ongoing pregnancies (69.4%), and one miscarriage (2.8%). All of these pregnancies were conceived within 1 year of their surgery. It is important to note that our CPR is not solely the result of treatment of endometriosis, as prior studies have reported, but includes treatment of other coexisting pelvic pathologies which were often excluded in these studies. After a review of the literature, one study of women undergoing surgical management after BCL-6 testing had a CPR of 61.9%, similar to our study, with an LBR of 52.4%; however, the study period encompassed 7 years.3 Another study reported a 42.3% LBR for patients who conceived within 6 months following laparoscopic treatment in women with primarily stage 3 or 4 endometriosis.33 To our knowledge, the highest reported CPR following laparoscopic surgery was 76% in a study of 29 patients.12
To our knowledge, this report is the largest published cohort of BCL-6 positive patients with visually and histology-proven endometriosis. This allows for an estimation of the true prevalence of endometriosis in patients with endometrial BCL-6 overexpression, which may be helpful to physicians counseling patients pre-operatively regarding their risk of endometriosis. One study has evaluated the presence of hydrosalpinx at the time of laparoscopy in patients with endometrial BCL-6 overexpression, which was found to be 4.6%,2 similar to our reported rate. However, ours is the first study to also review other coexisting inflammatory pelvic pathologies. Additionally, our study is the first independent study to confirm the accuracy of the ReceptivaDxTM test as previous studies were at least, in part, partially funded.
There are several limitations to our study. Infertility is often a multifactorial disease, and patients presenting to an REI specialist may be found to have various inflammatory pelvic pathologies that could interfere with their ability to conceive. Our study is not designed to infer causation between BCL-6 testing and individual pelvic pathologies. We are unable to distinguish if BCL-6 overexpression was due to endometriosis or to alternative pelvic pathology in patients who had multiple factors for infertility. For example, 10.7% of patients had endometritis diagnosed on biopsy which could have also accounted for BCL-6 overexpression. However, all patients with endometritis at time of BCL-6 testing had either histologic diagnosis of endometriosis (n = 5) or type I/IIC endometriomas (n = 3).
There may have been selection bias in our study since only patients who considered surgical management were referred to our center. Other variables such as the severity of patient symptoms or planned timing to conceive could have affected patient decision-making to proceed with surgery and confounded our results. Since our sample consisted solely of patients with BCL-6 overexpression who desired surgical management for suspected endometriosis, no women with negative test results were included. This precluded the determination of the sensitivity, specificity, and negative predictive value (NPV) of BCL-6 testing in our patient population, however these have previously been reported in the literature.38 NPV of BCL-6 testing would be relevant during initial diagnostic work-up of infertility, as a high NPV could be used to reassure patients that surgery may not be indicated. While BCL-6 testing has a published sensitivity of 93% and specificity of 96% for visual or histological diagnosis of endometriosis, determining the test’s sensitivity and specificity for histology-confirmed endometriosis alone would also better define the potential role of surgery.38
Pregnancy outcomes were subject to reporting bias as patients were not routinely followed in our office for management of infertility and subsequent conception, possibly underestimating the number of pregnancies that occurred following surgical treatment. Due to the short follow-up period at our surgical practice (mean 6.6 months), we believe that long-term studies would have a more accurate representation of the true CPR and LBR. We could not assess the exact number of patients trying to conceive postoperatively, which would provide more accurate data regarding CPR. Additionally, our current data is only applicable in the infertility setting and may not be representative of endometriosis patients primarily presenting with pain symptoms.
Future studies should implement a longer follow-up period to evaluate CPR and reproductive outcomes and include patients with negative BCL-6 testing who opted for surgical management to determine parameters such as sensitivity, specificity, and NPV for histology-confirmed endometriosis. While studies have shown that medical or surgical treatment in BCL-6 positive patients improves LBR, it would be interesting to compare pre-operative and postoperative BCL-6 expression to assess if it could be used as a marker of treatment response prior to embryo transfer. In addition, future studies could include a cost-benefit analysis to examine if earlier detection and treatment of endometriosis results in fewer IVF cycles, quicker time to conception, and a higher LBR.
With advances in ART, many patients are proceeding with IVF prior to medical or surgical treatment of endometriosis. Often, patients can experience numerous IVF failures that can occur after the transfer of their highest quality embryos, leaving them with lower quality embryos or the need to undergo further IVF cycles to improve their chances of a successful pregnancy. When considering medical management of endometriosis in the setting of infertility, patients need to understand that treatment can suppress ovulation, and many women who have been struggling to conceive may not wish to delay conception any longer.10,40 The data regarding medical management of endometriosis for infertility is also conflicting. Contrary to previously reported data, a recent 2019 Cochrane review examining long-term gonadotropin-releasing hormone (GnRH) agonist therapy (3 months) prior to IVF/ICSI showed no benefit in LBR or miscarriage rates compared to no pretreatment in women with endometriosis.41
We recommend that patients with unexplained infertility be counseled regarding all their options to improve reproductive outcomes, including laparoscopy for diagnosis and treatment of endometriosis. During laparoscopic treatment of peritoneal endometriosis, we advocate for an excisional approach as opposed to ablation. Several studies have established the role of laparoscopy for treatment of endometriosis, showing that pro-inflammatory cytokines in the peritoneal fluid decrease following surgical excision of endometriotic lesions.22,23 A study evaluating 588 fresh embryo cycles revealed women with endometriosis had double the rate of miscarriage when compared to normal controls, highlighting the importance of treatment prior to embryo transfer even in women with mild disease.39 Furthermore, treatment prior to embryo transfer can improve endometrial receptivity defects in order to optimize implantation and maintenance of pregnancy.25
Endometrial BCL-6 testing provides a supplementary screening test with high PPV that can assist providers when counseling patients about all of their options including laparoscopic treatment of endometriosis. In our experience, couples with difficulty conceiving, history of failed IVF, and suspected endometriosis can achieve a clinical pregnancy following surgical treatment of endometriosis.37 This new information may help guide providers and patients undergoing IVF to consider laparoscopic evaluation for endometriosis and help these women achieve their reproductive goals.
Contributor Information
Camran Nezhat, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Stanford University Medical Center, Palo Alto, CA; University of California San Francisco, San Francisco, CA.
Anupama Rambhatla, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Stanford University Medical Center, Palo Alto, CA.
Catarina Miranda-Silva, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Coimbra University Hospital Center, Coimbra, Portugal.
Atena Asiaii, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Stanford University Medical Center, Palo Alto, CA.
Kimsa Nguyen, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA.
Aimee Eyvazzadeh, Dr. Aimee Eyvazzadeh M.D., Inc., San Ramon, CA.
Salli Tazuke, The Colorado Center for Reproductive Medicine – San Francisco. Menlo Park, CA.
Shruti Agarwal, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Stanford University Medical Center, Palo Alto, CA.
Sunny Jun, The Colorado Center for Reproductive Medicine – San Francisco. Menlo Park, CA.
Azadeh Nezhat, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Stanford University Medical Center, Palo Alto, CA; University of California San Francisco, San Francisco, CA.
Robert A. Roman, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Palo Alto, CA; Stanford University Medical Center, Palo Alto, CA.
References:
- 1.Nezhat C, Li A, Abed S, et al. Strong association between endometriosis and symptomatic leiomyomas. JSLS. 2016;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans-Hoeker E, Lessey BA, Jeong JW, et al. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci. 2016;23:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Likes CE, Cooper LJ, Efird J, et al. Medical or surgical treatment before embryo transfer improves outcomes in women with abnormal endometrial BCL6 expression. J Assist Reprod Genet. 2019;36:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fert Steril. 2012;98:S1–S62. [DOI] [PubMed] [Google Scholar]
- 5.Veeraswamy A, Lewis M, Mann A, Kotikela S, Hajhosseini B, Nezhat C. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449–466. [DOI] [PubMed] [Google Scholar]
- 6.Nezhat C, Falik R, McKinney S, King LP. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359–372. [DOI] [PubMed] [Google Scholar]
- 7.Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549–562. [DOI] [PubMed] [Google Scholar]
- 8.Nezhat C, Lindheim SR, Backhus L, et al. Thoracic endometriosis syndrome: a review of diagnosis and management. JSLS. 2019;23:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24:623–628. [DOI] [PubMed] [Google Scholar]
- 10.Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fert Steril. 2012;98:591–598. [DOI] [PubMed] [Google Scholar]
- 11.Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fert Steril. 2005;84:1574–1578. [DOI] [PubMed] [Google Scholar]
- 13.Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil Steril. 2020;113:305–322. [DOI] [PubMed] [Google Scholar]
- 14.Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31:109–124. [DOI] [PubMed] [Google Scholar]
- 15.National Summary Report [cited 2020. Feb 2]; Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2017.
- 16.Evans B, Stentz NC, Richter KS, et al. Mature follicle count and multiple gestation risk based on patient age in intrauterine insemination cycles with ovarian stimulation. Obstet Gynecol. 2020;135:1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nezhat C, Vang N, Tanaka PP, Nezhat C. Optimal management of endometriosis and pain. Obstet Gynecol. 2019;134:834–839. [DOI] [PubMed] [Google Scholar]
- 18.Almquist LD, Likes CE, Stone B, et al. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: a cohort study. Fertility and Sterility 2017;108:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn SH, Dhalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fert Steril. 2016;106:1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- 22.Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fert Steril. 2017;108:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2017;8:7138–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.“BCL6 gene” [cited 2020, Feb 3]; Available from National Institutes of Health: https://ghr.nlm.nih.gov/gene/BCL6.
- 25.Lessey BA, Young SL. What exactly is endometrial receptivity? Fert Steril. 2019;111:611–617. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal SK, Chapron C, Giudice LC, et al. Clinical diagnosis of endometriosis: a call to action. AJOG. 2019;220:354e.1–354e.12. [DOI] [PubMed] [Google Scholar]
- 27.Gerbie AB, Merrill JA. Pathology of endometriosis. Clin Obstec Gynecol. 1988;31:779–786. [DOI] [PubMed] [Google Scholar]
- 28.Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reprod. 2017;33:347–352. [DOI] [PubMed] [Google Scholar]
- 29.Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776. [PubMed] [Google Scholar]
- 30.Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improvingthe classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213. [DOI] [PubMed] [Google Scholar]
- 31.Falik RC, Li A, Farrimound F, Razavi GM, Nezhat C, Nezhat F. Endometriomas: classification and surgical management. OBG Management. 2017;29:39–43. [Google Scholar]
- 32.Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using combined technique of excisional (cystectomy) and ablative surgery. Fert Steril. 2010;94(1):28–32. [DOI] [PubMed] [Google Scholar]
- 33.Mack M. Inflammation and fibrosis. Matrix Biology. 2018;68-69:106–121. [DOI] [PubMed] [Google Scholar]
- 34.Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fert Steril. 2019;111:629–640. [DOI] [PubMed] [Google Scholar]
- 35.Ciebiera M, Wlodarczyk M, Zgliczynska M, et al. The role of tumor necrosis factor ɑ in the biology of uterine fibroids and related symptoms. Int J Mol Sci. 2018;19:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orciani M, Caffarini M, Biagini A, et al. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int. 2018;2018:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soriano D, Adler I, Bouaziz J, et al. Fertility outcome of laparoscopic treatment in patients with severe endometriosis and repeated in vitro fertilization failures. Fert Steril. 2016;106:1264–1269. [DOI] [PubMed] [Google Scholar]
- 38.“Resolving barriers to a successful pregnancy: uterine lining dysfunction and progesterone resistance” [cited 2020, March 3]; Available from ReceptivaDx: https://receptivadx.com/wp-content/uploads/2020/02/brochure_digital.pdf.
- 39.Pallacks C, Hirchenhain J, Krussel JS, Fehm TN, Fehr D. Endometriosis doubles odds for miscarriage in patients undergoing IVF or ICSI. Eur J Obstet Gynecol Reprod Biol. 2017;213:33–38. [DOI] [PubMed] [Google Scholar]
- 40.Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstetricia et Gynecologica Scandinavica. 2017;96:659–667. [DOI] [PubMed] [Google Scholar]
- 41.Georgiou EX, Melo P, Baker PE, et al. Long-term GnRH agonist therapy before in vitro fertilisation (IVF) for improving fertility outcomes in women with endometriosis (Review). Cochrane Database Syst Rev. 2019;11:CD013240. [DOI] [PMC free article] [PubMed] [Google Scholar]