Abstract
Background:
Cups are more frequently revised than stems after uncemented total hip arthroplasty, which warrants the development of cup surfaces that provide long-lasting, stable fixation. Large heads have become popular with the aim of reducing dislocation rates, but they generate greater frictional torque that may compromise cup fixation. We aimed to investigate (1) if a novel porous titanium surface provides superior cup fixation when compared with a porous plasma spray (PPS) surface and (2) if the use of the largest possible head compromises cup fixation when compared with a 32-mm head.
Methods:
Ninety-six patients were randomized to receive either a cup with a porous titanium coating (PTC) or a cup with PPS. A second randomization was performed to either the largest possible (36 to 44-mm) or a 32-mm head in metal-on-vitamin-E-infused polyethylene bearings. Roentgen stereophotogrammetric analysis (RSA) examinations were obtained postoperatively at 3, 12, and 24 months. The primary outcome was proximal cup migration when comparing the 2 cup surfaces and also when comparing the largest possible head with the 32-mm head. The patients were followed for 2 years.
Results:
The median (and interquartile range) proximal cup migration was 0.15 mm (0.02 to 0.32 mm) for the PTC cup and 0.21 mm (0.11 to 0.34 mm) for the PPS cup. The largest possible head had a proximal cup migration of 0.15 mm (0.09 to 0.31 mm), and the 32-mm head had a proximal cup migration of 0.20 mm (0.04 to 0.35 mm). There were no significant differences between the cup surface (p = 0.378) or the head size (p = 0.693) groups.
Conclusions:
Early cup fixation was not superior with the novel PTC cup; the use of the largest possible head (36 to 44 mm) did not compromise early cup fixation.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Porous plasma spray (PPS) is a well-established surface that is used in uncemented cups with very good long-term survival1,2. However, cups are still revised more frequently than stems in isolated component revision following aseptic loosening3,4. In order to enhance cup fixation, a porous titanium coating (PTC) with a rougher structure has been introduced for acetabular cups5,6. However, it remains uncertain whether this coating provides superior cup fixation.
Large heads have increased in popularity because they seem to provide greater stability in total hip arthroplasty (THA)7,8. However, the use of ≥36-mm heads in metal-on-cross-linked polyethylene (XLPE) THA has demonstrated a lower survival rate when compared with 32-mm heads in long-term reports9,10. Besides increased volumetric polyethylene wear11, increased frictional torque, which is generated by large heads12 (especially in metal-on-vitamin-E-infused polyethylene [MoVEPE] bearings)13, has been suggested as the mechanism of failure. The increased frictional torque could be transmitted to the cup-bone interface and compromise cup fixation14, but, to our knowledge, this hypothesis has not yet been tested clinically. Roentgen stereophotogrammetric analysis (RSA) is a method that can predict future implant failure as early as 2 years postoperatively15,16. Therefore, we designed a randomized controlled trial (RCT) that was aimed at answering the following 2 questions: (1) Does a PTC cup provide superior early cup fixation compared with a PPS cup in terms of decreased 2-year RSA cup migration? (2) Does the use of the largest possible head (36 to 44 mm) compromise early cup fixation with MoVEPE THA when compared with a 32-mm head in terms of increased 2-year RSA cup migration?
Materials and Methods
Study Design
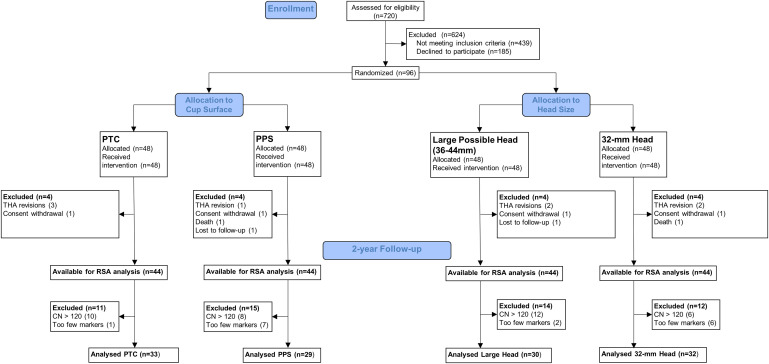
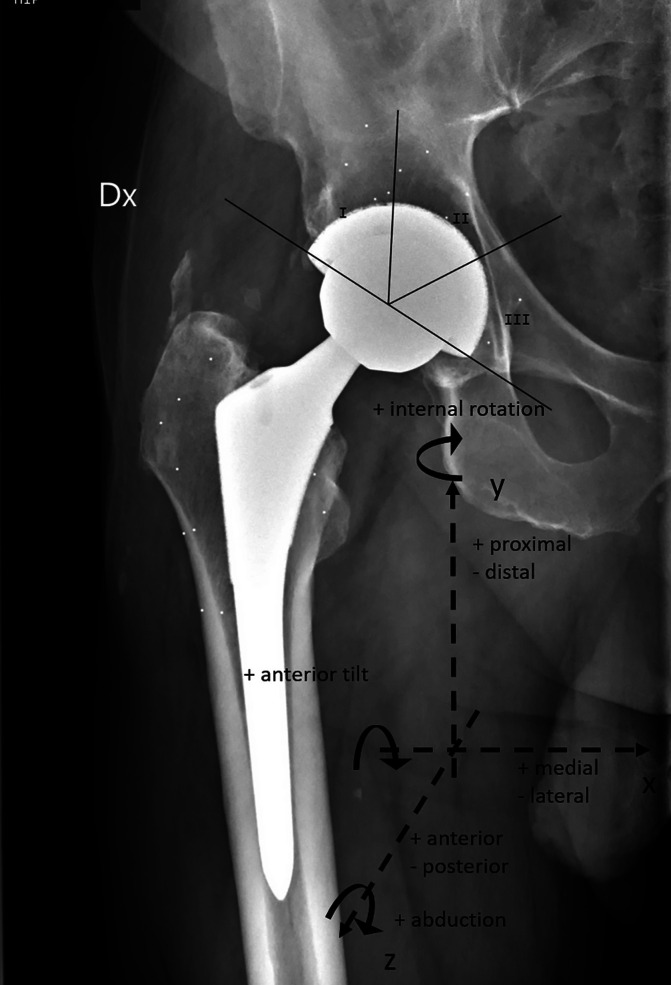
We conducted a single-blinded RCT at 2 international centers. Following eligibility screening (Table I), 96 patients were enrolled and randomly allocated into 2 groups at a 1:1 ratio according to the cup surface and into 2 groups at a 1:1 ratio according to the head size that they would receive. Either PTC or PPS was used for the cup surface. The head size was either the largest possible size between 36 and 44 mm that could fit in the thinnest insert available for the specific cup size or a 32-mm head. Forty-eight patients received a PTC cup and 48 received a PPS cup (control group). Similarly, 48 patients received the largest possible head and 48 received a 32-mm head (control group) (Fig. 1). When comparing the PTC cups with the PPS cups, the primary outcome was proximal cup migration along the y axis. Secondary outcomes were the presence of periacetabular radiolucencies and patient-reported outcome measures (PROMs). When comparing the largest possible head with the 32-mm head, primary outcomes were proximal cup migration along the y axis and cup rotation around the x axis (Fig. 2).
Fig. 1.
Flowchart showing how the final number of patients available for RSA analysis at the 2-year follow-up was determined. PTC = porous titanium coating (OsseoTi), PPS = porous plasma spray, THA = total hip arthroplasty, RSA = roentgen stereophotogrammetric analysis, and CN = condition number.
Fig. 2.
Anteroposterior view of a postoperative radiograph of a right hip. The coordinate system of the RSA examination is presented with the definition of translations along and rotations around the x, y, and z axes. Proximal cup migration was defined as proximal translation along the y axis. Radiographs were examined for radiolucencies in each Charnley-DeLee zone (I-III).
TABLE I.
Eligibility Criteria for Enrollment*
| Inclusion Criteria | Exclusion Criteria |
|
|
OA = osteoarthritis, THA = total hip arthroplasty, and ASA = American Society of Anesthesiologists.
The study was conducted according to the Consolidated Standards of Reporting Trials (CONSORT)17 and was approved by the ethical review board at each center. All of the patients provided informed consent prior to inclusion. The study was registered at ClinicalTrials.gov (NCT02316704).
Sample Size Calculation
A difference in 2-year proximal cup migration of 0.2 mm between the PTC and PPS cups was considered clinically relevant16. The expected standard deviation [SD] was 0.3 mm18. At least 36 patients in each group were required to detect a significant difference with 80% power (2 independent-samples t tests). The enrollment of 48 patients in each group ensured sufficient power despite a 20% rate of dropout.
Implants and Patient Care
At the time of surgery, the acetabulum and the proximal aspect of the femur were each marked with at least six 0.8-mm tantalum markers. All of the implants were provided by Zimmer Biomet. The G7 acetabular cup system19, with limited preplugged holes but without any screw fixation, was used in the 50 to 64-mm sizes. Its coating was either OsseoTi or PPS. OsseoTi is a novel PTC with a consistent porosity of 475 µm; it is made of Ti-6Al-4V alloy using additive manufacturing (3-dimensional [3D] printing) and is designed to provide a rougher 3D structure that mimics cancellous bone and therefore enhances osseointegration20. PPS is a blasted surface that is produced by high-speed projection of semimolten titanium powder that adheres to the cup and creates a meshed surface with variable pore distribution (100 to 1,000 µm)21. All of the patients received the neutral E1 antioxidant-infused polyethylene insert, which has an apical thickness varying from 14.7 to 4.7 mm and can accommodate head sizes from 32 to 44 mm. The insert was marked with at least six 1.0-mm tantalum markers. The Echo Bi-Metric stem with a modular CoCrMo alloy head was used. Follow-up occurred at 3, 12, and 24 months.
Randomization, Allocation, and Data Collection Process
Computer-generated randomization was utilized to create 2 blocks of envelopes (1 for each of the centers that was used in the RCT). Each block contained 48 envelopes allocating patients to PTC and the largest possible head (12), PTC and a 32-mm head (12), PPS and the largest possible head (12), and PPS and a 32-mm head (12). This ensured that the type of cup surface was balanced across the 2 head-size groups and vice versa. The envelopes were closed and shuffled. They were opened either during surgery, after the acetabulum was reamed (center 1), or 1 week before surgery during templating (center 2) to ensure that at least a 50-mm cup, which can accommodate a 36-mm head, could be used. Patients were blinded to their treatment. Unblinded research administrators and the attending orthopaedic surgeons collected all of the data prospectively.
RSA and Radiolucency Analysis
Baseline plain and RSA radiographs were acquired on postoperative day 1. Follow-up RSA examinations were acquired at 3, 12, and 24 months postoperatively in a standardized way (Table II). All of the RSA radiographs were analyzed at center 1 with the model-based RSA software (RSACore version 4.2; Leiden University Medical Center). At least 3 markers with adequate scatter in the acetabulum, corresponding to a condition number (CN) of ≤120 and a mean error of rigid-body fitting of ≤0.35, were required to establish a valid reference bone model. A 3D model of each cup, derived from reverse engineering, was fitted using contour detecting and 3 position-optimization algorithms. Translations were calculated as the movement of the model’s center of mass (CoM) in relation to the reference bone model, and rotations were calculated as movement around the CoM point. Postoperative examinations provided the reference of bone and 3D models. The patients underwent radiography in duplicate at the 3-month follow-up. The RSA precision was calculated by multiplying the critical t value by the SD of the double measurements from zero.
TABLE II.
Standardization of RSA Examinations at Each Center
| Characteristic | Center 1 | Center 2 |
| Patient position | Supine (operated leg parallel to the y axis of the cage) | Supine (operated leg parallel to the y axis of the cage) |
| Angle between the ceiling-mounted radiographic RSA tubes | 46° | 42° |
| Source-to-image detector distance | 150 cm | 160 cm |
| Type of uniplanar calibration cage | CarbonBox 021 (MEDIS Medical Imaging Systems) | Cage 77(RSA Biomedical) |
| Image detector | Carestream DRX-1 | Canon CXDI-50RF |
Radiographs obtained at the 1 and 2-year follow-up appointments were reviewed by 2 successive observers for signs of radiolucency using mdesk software (version 3.6.7.0; RSA Biomedical). A radiolucency was present if the maximal vertical distance between the acetabulum and the cup was ≥0.5 mm, extending to >50% of the Charnley-DeLee zone22 (Fig. 2). Any radiolucencies that were present on the immediate postoperative radiographs were defined as gaps. A gap that was no longer present in a subsequent radiograph was defined as filled, and, if a gap was still present but had not increased by >1 mm in thickness, it was defined as persistent. Progression of radiolucency was defined as an increase in thickness by ≥1 mm or extension to adjacent zones.
PROMs
PROMs were used to measure hip function, activity level, and health-related quality of life (HRQoL) preoperatively and at the time of the follow-up appointments. Hip function was measured with the Oxford Hip Score (OHS: 0 to 48) and the Harris hip score (HHS: 0 to 100). HRQoL was measured with the 3-level EuroQol-5 Dimensions (EQ-5D) score (0 to 1) and the EQ-5D-VAS (visual analog scale) score (0 to 100). Activity level was measured with the University of California Los Angeles (UCLA) Activity Scale (1 to 10).
Statistics
Categorical data were described in absolute numbers and percentages. The z test or the Fisher exact test, where applicable, was used for comparison of categorical data. Numerical data were described with medians and interquartile ranges. The significance of cup migration within each group was assessed with the Wilcoxon signed-rank test. The Mann-Whitney U test was used to compare numerical data among the groups. The level of significance was set to 0.05. Statistical analyses were performed with SPSS software (version 26; IBM).
Results
Participant Flow
Patients were enrolled between December 2014 and February 2017 and were followed for 2 years between January 2017 and March 2019. They had similar baseline demographics and surgery characteristics for cup surface and head size (Table III). By the 2-year follow-up, 8 patients had dropped out: 2 had withdrawn their consent (1 PTC cup with the largest head size and 1 PPS cup with a 32-mm head), 1 died of lung cancer (PPS cup with a 32-mm head), 1 did not come for radiographic examination (PPS cup with the largest head size), and 4 had been revised. Of those THAs that had been revised, 2 (both PTC cups with a 32-mm head) were due to dislocation, 1 was due to stem subsidence (PPS cup with the largest head size), and 1 was due to periprosthetic femoral fracture (PTC cup with the largest head size). There were no revisions due to cup loosening.
TABLE III.
Baseline Characteristics Stratified for Cup Surface and Head Size*
| Characteristic | Cup Surface | Head Size | ||
| PTC (N = 48) | PPS (N = 48) | Largest (N = 48) | 32 mm (N = 48) | |
| Women (no. [%]) | 20 (42) | 23 (48) | 21 (42) | 22 (46) |
| Median age (IQR) (yr) | 63 (55-69) | 63 (58-69) | 62 (55-67) | 65 (59-71) |
| Median BMI (IQR) (kg/m2) | 27 (24-29) | 28 (25-30) | 27 (26-29) | 26 (24-30) |
| ASA group (no. [%])† | ||||
| 1 | 21 (44) | 21 (44) | 23 (48) | 19 (40) |
| 2 | 23 (48) | 24 (50) | 21 (44) | 26 (54) |
| 3 | 4 (8) | 2 (4) | 3 (6) | 3 (6) |
| Head size (no. [%]) | ||||
| 32 mm | 24 (50) | 24 (50) | 0 (0) | 48 (100) |
| 36 mm | 5 (10) | 6 (13) | 11 (23) | 0 (0) |
| 40 mm | 18 (38) | 16 (33) | 34 (71) | 0 (0) |
| 44 mm | 1 (2) | 2 (4) | 3 (6) | 0 (0) |
| Cup surface (no. [%]) | ||||
| PTC | 48 (100) | 0 (0) | 24 (50) | 24 (50) |
| PPS | 0 (0) | 48 (100) | 24 (50) | 24 (50) |
PTC = porous titanium coating, PPS = porous plasma spray, IQR = interquartile range, BMI = body mass index, and ASA = American Society of Anesthesiologists.
Data about the ASA grade were missing in 1 patient who had received a PPS cup and the largest possible head.
RSA Cup Migration: PTC Versus PPS
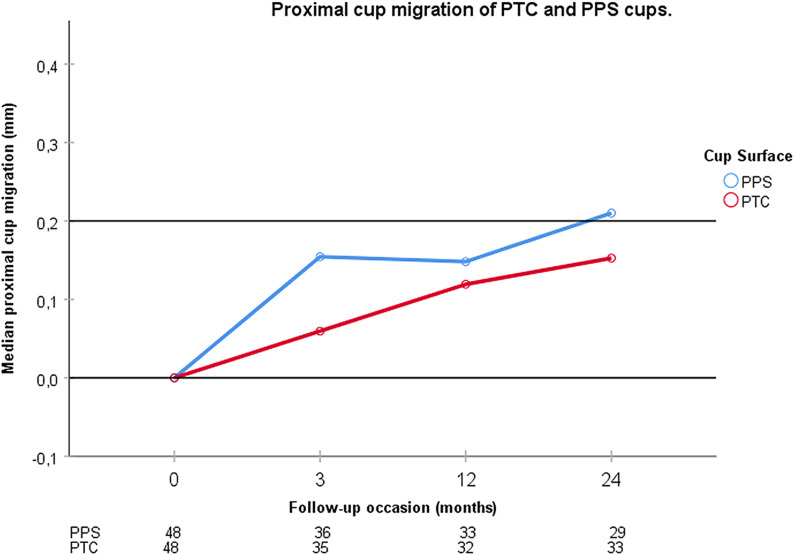
The RSA precision for proximal cup migration was 0.2 mm. Of the 88 patients who were available for follow-up at 2 years, 26 could not be analyzed due to a CN of >120 or the presence of <3 visible markers in the acetabulum. Thus, 33 patients with PTC cups and 29 with PPS cups were available for analysis. Both types of cups migrated significantly. The median 2-year proximal cup migration was 0.15 mm (interquartile range, 0.02 to 0.32 mm; p < 0.001) for the PTC cups and 0.21 mm (0.11 to 0.34 mm; p < 0.001) for the PPS cups (Fig. 3). The difference between them was not significant (p = 0.378); 45% (15 of 33) of the patients with PTC cups and 52% (15 of 29) with PPS cups had values of >0.2 mm (p = 0.624).
Fig. 3.
Proximal cup migration for PTC (OsseoTi) and PPS G7 acetabular cups is shown at the 3-month, 1-year, and 2-year follow-ups. The table below the graph shows the number of analyzed patients at each follow-up. The median PPS cup migration exceeded 0.2 mm at 2 years and was bigger than the median cup migration of PTC cups, but the difference between them did not reach significance at any follow-up.
RSA Cup Migration: Largest Head Versus 32-mm Head
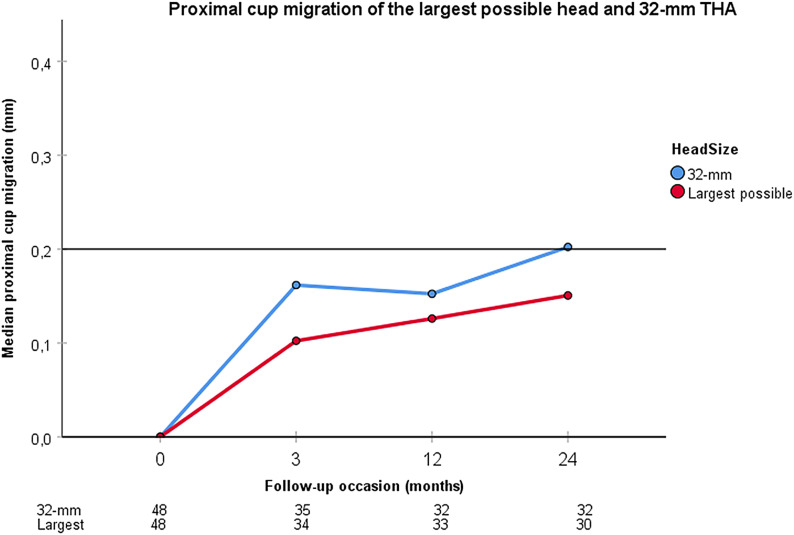
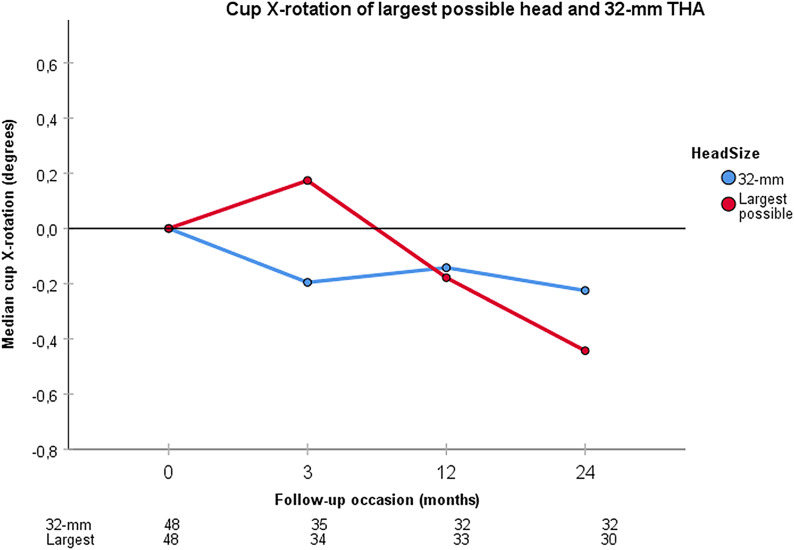
At the 2-year follow-up, 30 patients with the largest possible head could be compared with 32 patients with a 32-mm head. The median proximal cup migration was 0.15 mm (interquartile range, 0.09 to 0.31 mm) for THA with the largest possible head, and 0.20 mm (0.04 to 0.35 mm) for THA with a 32-mm head (Fig. 4). The cups of both groups migrated significantly (p < 0.001), but their difference was not significant (p = 0.693). Of the THAs with the largest possible head, 43% (13 of 30) had values of >0.2 mm compared with 53% (17 of 32) of THAs with a 32-mm head (p = 0.441). The precision for cup X-rotation was 1.11°. THAs with the largest possible head demonstrated a posterior cup tilt of 0.44° (−0.20° to 0.84°; p = 0.023) and THAs with a 32-mm head demonstrated a posterior cup tilt of 0.23° (−0.02° to 0.77°; p = 0.031) (Fig. 5). Their difference was not significant (p = 0.622).
Fig. 4.
Proximal cup migration for the largest possible head and a 32-mm head used during THA is shown at the 3-month, 1-year, and 2-year follow-ups. The table below the graph shows the number of analyzed patients at each follow-up. The 32-mm head tended toward greater values of proximal cup migration, but there were no significant differences between the 2 head size groups at any follow-up.
Fig. 5.
Cup X-rotation for the largest possible head and a 32-mm head used during THA is shown at the 3-month, 1-year, and 2-year follow-ups. The table below the graph shows the number of analyzed patients at each follow-up. Both head sizes demonstrate a significant negative X-rotation (posterior tilt) at 2 years. There was a tendency for greater posterior tilt for the largest possible head used during THA at 2 years, but the difference was not significant compared with a 32-mm head used during THA.
Radiolucencies Around the Cup
Gaps were observed in 8 patients in the PTC group and in 4 patients in the PPS group (Table IV). By the 2-year follow-up appointments, 2 in the PTC group and 1 in the PPS group with gaps had dropped out. Of the remaining 10 gaps (9 patients), 5 had filled in and 1 had progressed in the PTC group, while all 4 in the PPS group had filled in (Table V). At the 1-year follow-up, new radiolucencies were observed in 2 patients in the PTC group and in 4 patients in the PPS group (Table VI). At the 2-year follow-up, all 7 of the radiolucencies that had been observed in these 6 patients at 1 year were absent (Table VII), while 6 new radiolucencies in the PTC group and 4 in the PPS group had appeared.
TABLE IV.
Comparison of Gaps Between the 2 Cup Surfaces*
| PTC (N = 48) | PPS (N = 48) | P Value† | |||||
| With Gaps | Without Gaps | Ratio | With Gaps | Without Gaps | Ratio | ||
| Total | 8 | 40 | 8:40 | 4 | 44 | 4:44 | 0.355 |
| Zone 1 | 3 | 45 | 3:45 | 0 | 48 | 0:48 | 0.242 |
| Zone 2 | 5 | 43 | 5:43 | 2 | 46 | 2:46 | 0.435 |
| Zone 3 | 0 | 48 | 0:48 | 3 | 45 | 3:45 | 0.242 |
PTC = porous titanium coating, and PPS = porous plasma spray. The values are given as the number of patients.
Fisher exact test.
TABLE V.
Gap Progression at the 1 and 2-Year Follow-ups*
| Comparison | PTC† | PPS‡ | ||||
| Filled | Persistent | Progressed | Filled | Persistent | Progressed | |
| A. Between postop. and 1-year radiographs | ||||||
| Zone 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| Zone 2 | 2 | 1 | 0 | 2 | 0 | 0 |
| Zone 3 | 0 | 0 | 0 | 1 | 2 | 0 |
| Total | 5 | 1 | 0 | 3 | 2 | 0 |
| B. Between postop. and 2-year radiographs | ||||||
| Zone 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| Zone 2 | 2 | 0 | 1 | 2 | 0 | 0 |
| Zone 3 | 0 | 0 | 0 | 2 | 0 | 0 |
| Total | 5 | 0 | 1 | 4 | 0 | 0 |
PTC = porous titanium coating, and PPS = porous plasma spray. The values are given as the number of gaps.
6 zones in 6 patients.
5 zones in 4 patients for A; 4 zones in 3 patients for B.
TABLE VI.
Comparison of Radiolucencies Between the 2 Cup Surfaces at the 1 and 2-Year Follow-ups*
| Comparison | PTC | PPS | P Value† | ||||
| With Radiolucencies | Without Radiolucencies | Ratio | With Radiolucencies | Without Radiolucencies | Ratio | ||
| 1-year follow-up | |||||||
| Total no. of patients | 2 | 41 | 2:41 | 4 | 43 | 4:43 | 0.679 |
| Zone 1 | 1 | 42 | 1:42 | 0 | 47 | 0:47 | 0.478 |
| Zone 2 | 2 | 41 | 2:41 | 4 | 43 | 4:43 | 0.679 |
| Zone 3 | 0 | 43 | 0:43 | 0 | 47 | 0:47 | ‡ |
| 2-year follow-up | |||||||
| Total no. of patients | 6 | 38 | 6:38 | 4 | 40 | 4:40 | 0.739 |
| Zone 1 | 0 | 44 | 0:44 | 2 | 42 | 2:42 | 0.494 |
| Zone 2 | 3 | 41 | 3:41 | 2 | 42 | 2:42 | 1 |
| Zone 3 | 3 | 41 | 3:41 | 0 | 44 | 0:44 | 0.241 |
PTC = porous titanium coating, and PPS = porous plasma spray. The values are given as the number of patients; a patient may have radiolucencies in more than 1 zone.
Fisher exact test.
P value could not be calculated.
TABLE VII.
Progression of Radiolucencies Between the 1 and 2-Year Radiographs*
| Zone | PTC† | PPS‡ | ||||
| Filled | Persistent | Progressed | Filled | Persistent | Progressed | |
| Zone 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Zone 2 | 2 | 0 | 0 | 4 | 0 | 0 |
| Zone 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 3 | 0 | 0 | 4 | 0 | 0 |
PTC = porous titanium coating, and PPS = porous plasma spray. The values are given as the number of gaps.
3 zones in 2 patients.
4 zones in 4 patients.
PROMs
At baseline, 94 patients had completed the OHS PROM, 95 had completed the HHS PROM, 94 had completed the EQ-5D PROM, 92 had completed the EQ-5D-VAS PROM, and 94 had completed the UCLA PROM. At 2 years, the numbers were 89, 87, 89, 87, and 88, respectively. No clinically relevant differences were found in any PROM between patients with PTC and PPS cups (Table VIII).
TABLE VIII.
PROMs at Baseline and at 3, 12, and 24 Months Postoperatively*
| PROM | Baseline | 3 Months | 12 Months | 24 Months | ||||||||
| PTC† | PPS† | P Value | PTC† | PPS† | P Value | PTC† | PPS† | P Value | PTC† | PPS† | P Value | |
| OHS | 23 (16-28) | 22 (17-26) | 0.384 | 38 (35-45) | 42 (32-45) | 0.584 | 45 (40-48) | 46 (44-48) | 0.323 | 45 (40-48) | 47 (42-48) | 0.171 |
| HHS | 57 (48-65) | 58 (43-67) | 0.8 | 91 (79-100) | 93 (81-100) | 0.584 | 98 (90-100) | 100 (95-100) | 0.439 | 99 (94-100) | 100 (100-100) | 0.026 |
| EQ-5D score | 0.723 (0.648-0.772) | 0.723 (0.660-0.768) | 0.753 | 0.935 (0.824-0.985) | 0.883 (0.794-1) | 0.667 | 0.969 (0.834-1) | 0.969 (0.824-1) | 0.701 | 0.969 (0.846-1) | 0.969 (0.851-1) | 0.889 |
| EQ-5D-VAS | 51 (40-75) | 60 (37-72) | 0.644 | 85 (80-91) | 84 (72-90) | 0.852 | 86 (70-94) | 87 (70-95) | 0.674 | 86 (70-95) | 90 (70-99) | 0.172 |
| UCLA | 4 (3-7) | 5 (3.5-7) | 0.446 | 6 (4-7) | 6 (4-7) | 0.838 | 7 (4-8) | 7 (5.5-8.5) | 0.756 | 7 (5-8) | 6.5 (6-8.5) | 0.902 |
PROMs = patient-reported outcome measures, PTC = G7 cup with OsseoTi porous titanium coating, PPS = G7 cup with porous plasma spray surface, OHS = Oxford Hip Score, HHS = Harris hip score, EQ-5D = EuroQol-5 Dimensions score, VAS = visual analog scale, and UCLA = University of California Los Angeles Activity Scale.
The values are given as the median, with the interquartile range in parentheses.
Discussion
We investigated whether a novel PTC surface provides more stable early cup fixation when compared with PPS. We found a lower proximal cup migration with the PTC, but the difference (0.06 mm) did not reach significance and could therefore not be generalized. We also investigated if using the largest possible head with MoVEPE THA could jeopardize early cup fixation because of the presumed increased frictional torque when compared with a 32-mm head. We found a lower proximal cup migration but a greater posterior tilt when the largest possible head was used in THA. However, the differences between the head size groups were small (0.05 mm and 0.21°, respectively) and not significant. Whether these differences have any clinical relevance could not be assessed in this report because no complications related to cup loosening have yet been observed.
Our study has certain limitations. There were 15 more dropouts than we had accounted for, but our study maintained a reasonable statistical sensitivity for detecting differences in proximal cup migration (minimum of 0.22 mm) among the groups because the SD of our measurements was much lower (0.1 mm) than the 0.3 mm that was used in sample size calculation. Moreover, the suggested threshold of 0.2 mm refers to the 2-year cup migration within the same group, not the difference among the groups. Therefore, if a 2-year cup migration of 0.2 mm (SD, 0.3 mm) within a group is considered predictive for long-term cup survival16, 21 patients in each group are enough to detect it with 80% power (Wilcoxon signed-rank test). Despite having many dropouts, our study was still sufficiently powered to detect such a 2-year migration. The markers in the polyethylene, hidden by large heads in uncemented cups, were not visible in the majority of the RSA examinations. Thus, only a model-based approach could be used. The precision of this markerless technique was initially reported as comparable with traditional marker-based RSA23; however, more recent studies have reported an inferior precision, especially regarding rotation24,25. Approximately half of the proximal cup migration values were below the precision of the RSA examinations. A subgroup analysis, including only patients with 2-year RSA measurements that were above the method’s precision, still showed no difference in proximal cup migration between the cup surfaces or the head sizes (Table IX). Finally, the inclusion of only 2 different types of cups from the same manufacturer makes our results on cup migration related to head size less generalizable for other cup designs.
TABLE IX.
Subgroup Analysis of RSA Proximal Cup Migration at 2 Years After Exclusion of Patients with Values Smaller than the Precision of the RSA Examination*
| Subgroup Analysis | Whole Sample | |||||||
| Experimental Group | Control Group | Δ† | P Value‡ | Experimental Group | Control Group | Δ† | P Value‡ | |
| Cup surface | PTC | PPS | PTC | PPS | ||||
| Proximal cup migration§ (mm) | 0.34 (0.29 to 0.43) | 0.34 (0.29 to 0.40) | 0 | 0.967 | 0.15 (0.02 to 0.32) | 0.21 (0.11 to 0.34) | −0.06 | 0.378 |
| Sample size | 15 | 15 | 33 | 29 | ||||
| Head size | Largest | 32 mm | Largest | 32 mm | ||||
| Proximal cup migration§ (mm) | 0.32 (0.28 to 0.35) | 0.34 (0.29 to 0.41) | −0.02 | 0.563 | 0.15 (0.09 to 0.31) | 0.20 (0.04 to 0.35) | −0.05 | 0.693 |
| Sample size | 13 | 17 | 30 | 32 | ||||
| Posterior tilt§# (deg) | 0.42 (−1.4 to 2.39) | 1.37 (−0.06 to 1.55) | −0.95 | 0.755 | 0.44 (−0.20 to 0.84) | 0.23 (−0.02 to 0.77) | 0.21 | 0.622 |
| Sample size | 6 | 8 | 30 | 32 | ||||
PTC = porous titanium coating, and PPS = porous plasma spray. Results for the whole sample are also presented for comparison.
Difference between medians.
Mann-Whitney U test.
The values in the 2 groups are given as the median, with the interquartile range in parentheses.
Reverse X-rotation.
The 2-year proximal cup migration for PTC cups was below the 0.2-mm threshold that has been suggested as predictive for implant failure16 in subsequent years. In other reports, the early migration of PTC cups has varied. Salemyr et al. compared 2 different PTC cups and reported 2-year proximal migration of 0.24 and 0.38 mm, respectively26. Sillesen et al. reported a 3-year proximal migration of a PTC cup from 0.17 to 0.62 mm in 2 different centers27. The difference was attributed to diversity in the reaming technique and screw placement between the centers. In our study, the proximal cup migration did not differ between the 2 centers (p = 0.369), which both applied 1 mm of underreaming and no screws. The 2-year proximal migration was even smaller for the novel PTC cup in our study compared with the above-mentioned cups and the PPS cups, but the difference was not large enough to reach significance. Thus, the PTC surface seems to provide early cup fixation that is as stable as the PPS surface.
To our knowledge, the association between large heads and cup fixation has not been previously studied in clinical trials. In an vitro study, Meneghini et al. reported an increased frictional torque when larger metal heads were combined with XLPE, as well as when VEPE was used13. Scholl et al. also reported an increased frictional torque when larger metal and ceramic heads were combined with XLPE12. Jahnke et al. reported increased translational and rotational micromotions of uncemented cups when increased frictional torque was applied14. In our study, proximal cup migration was more likely with the largest possible head, but posterior tilt was more likely with a 32-mm head. Nevertheless, the differences could not be generalized. Our interpretation is that cup movement did not differ between the groups according to head size. Thus, our results do not support the hypothesis that the use of the largest possible metal head affects early cup fixation.
Our secondary outcomes comprised periacetabular radiolucencies and PROMs. There were twice as many patients with gaps in the group with the PTC cups as in the group with the PPS cups. This may be due to the rougher surface of the PTC cup, which requires a harder impaction in the underreamed acetabulum. However, gaps did not seem to compromise cup fixation because all except for 1 filled in. The presence of gaps with highly porous cups varies widely in the literature, with a reported prevalence of ≥20%, but the majority are usually filled in by the time of final follow-up28. Lindgren et al. compared a different PTC cup with another PPS cup and reported a higher risk for radiolucencies and patient-reported pain at the 5-year follow-up for the PTC cup29. In our study, neither the number of radiolucencies nor the PROMs differed between cup designs.
We conclude that the early cup fixation provided by the novel PTC surface was equal to rather than superior to the PPS surface. The use of the largest possible head (ranging from 36 to 44 mm) in MoVEPE bearings does not seem to compromise early cup fixation. The safety and potential superiority of the novel PTC surface need to be evaluated in longer-term reports regarding clinical outcome before its introduction in THA. Longer-term RCTs assessing polyethylene wear and dislocation rates, in addition to cup fixation, are needed to evaluate the safety of large heads.
Footnotes
Investigation performed at Copenhagen University Hospital Hvidovre, Copenhagen, Denmark, and the Department of Orthopaedics, Sahlgrenska University Hospital, Mölndal, Sweden
Disclosure: Zimmer Biomet, the manufacturer of the implants used in the study, provided institutional research support for the recruitment of research subjects. Zimmer Biomet played no role in the data analysis or manuscript preparation. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (including relationships with Zimmer Biomet) and “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work (http://links.lww.com/JBJSOA/A229).
References
- 1.Crawford DA, Berend KR, Adams JB, Lombardi AV. Survival of a second-generation porous plasma-sprayed acetabular component at minimum 15-year follow-up. J Surg Orthop Adv. 2019. Spring;28(1):31-4. [PubMed] [Google Scholar]
- 2.Berend KR, Adams JB, Morris MJ, Lombardi AV, Jr. Three-year results with a ringless third-generation porous plasma sprayed acetabular component in primary total hip arthroplasty. Surg Technol Int. 2017. Jan 10;30:295-9. [PubMed] [Google Scholar]
- 3.Swedish Hip Arthroplasty Register. Annual report 2018. 2019. Accessed 2020 Oct 2. https://registercentrum.blob.core.windows.net/shpr/r/Arsrapport_2018_Hoftprotes_final_web-rJgg8LvkOB.pdf [Google Scholar]
- 4.Danish Hip Arthroplasty Register. Annual report. 2019. Accessed 2020 Oct 2. http://danskhoftealloplastikregister.dk/wp-content/uploads/2019/09/DHR-%C3%A5rsrapport-2019_til-offentligg%C3%B8relse-1.pdf [Google Scholar]
- 5.Small SR, Berend ME, Howard LA, Rogge RD, Buckley CA, Ritter MA. High initial stability in porous titanium acetabular cups: a biomechanical study. J Arthroplasty. 2013. Mar;28(3):510-6. Epub 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 6.Della Valle CJ, Mesko NW, Quigley L, Rosenberg AG, Jacobs JJ, Galante JO. Primary total hip arthroplasty with a porous-coated acetabular component. A concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2009. May;91(5):1130-5. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SS, Lees D, James P, Serrano-Pedraza I, Partington PF, Muller SD, Meek RM, Reed MR. Lower rates of dislocation with increased femoral head size after primary total hip replacement: a five-year analysis of NHS patients in England. J Bone Joint Surg Br. 2011. Jul;93(7):876-80. [DOI] [PubMed] [Google Scholar]
- 8.Goel A, Lau EC, Ong KL, Berry DJ, Malkani AL. Dislocation rates following primary total hip arthroplasty have plateaued in the Medicare population. J Arthroplasty. 2015. May;30(5):743-6. Epub 2014 Nov 26. [DOI] [PubMed] [Google Scholar]
- 9.National Joint Registry for England. Wales, Northern Ireland and the Isle of Man.16th annual report. 2019. Accessed 2020 Oct 2. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf [Google Scholar]
- 10.Australian Orthopaedic Association National Joint Replacement Registry. 20th annual report. 2019. https://aoanjrr.sahmri.com/documents/10180/668596/Hip%2C+Knee+%26+Shoulder+Arthroplasty/c287d2a3-22df-a3bb-37a2-91e6c00bfcf0 [Google Scholar]
- 11.Lachiewicz PF, Soileau ES, Martell JM. Wear and osteolysis of highly crosslinked polyethylene at 10 to 14 years: the effect of femoral head size. Clin Orthop Relat Res. 2016. Feb;474(2):365-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholl L, Longaray J, Raja L, Lee R, Faizan A, Herrera L, Thakore M, Nevelos J. Friction in modern total hip arthroplasty bearings: effect of material, design, and test methodology. Proc Inst Mech Eng H. 2016. Jan;230(1):50-7. [DOI] [PubMed] [Google Scholar]
- 13.Meneghini RM, Lovro LR, Wallace JM, Ziemba-Davis M. Large metal heads and vitamin E polyethylene increase frictional torque in total hip arthroplasty. J Arthroplasty. 2016. Mar;31(3):710-4. Epub 2015 Sep 30. [DOI] [PubMed] [Google Scholar]
- 14.Jahnke A, Schroeder S, Fonseca Ulloa CA, Ahmed GA, Ishaque BA, Rickert M. Effect of bearing friction torques on the primary stability of press-fit acetabular cups: a novel in vitro method. J Orthop Res. 2018. Oct;36(10):2745-53. Epub 2018 Jun 13. [DOI] [PubMed] [Google Scholar]
- 15.Klerken T, Mohaddes M, Nemes S, Kärrholm J. High early migration of the revised acetabular component is a predictor of late cup loosening: 312 cup revisions followed with radiostereometric analysis for 2-20 years. Hip Int. 2015. Sep-Oct;25(5):471-6. Epub 2015 Apr 27. [DOI] [PubMed] [Google Scholar]
- 16.Pijls BG, Nieuwenhuijse MJ, Fiocco M, Plevier JWM, Middeldorp S, Nelissen RGHH, Valstar ER. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survivalstudies. Acta Orthop. 2012. Dec;83(6):583-91. Epub 2012 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010. Mar 23;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou ZK, Li MG, Börlin N, Wood DJ, Nivbrant B. No increased migration in cups with ceramic-on-ceramic bearing: an RSA study. Clin Orthop Relat Res. 2006. Jul;(448):39-45. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer-Biomet. G7® Acetabular System: surgical technique. 2019. Accessed 2020 Oct 2. https://www.zimmerbiomet.com/content/dam/zimmer-biomet/medical-professionals/000-surgical-techniques/hip/2336.2-GLBL-en_G7%20Surgical%20Technique.pdf [Google Scholar]
- 20.Zimmer-Biomet. OsseoTi® Porous Metal Technology. 2019. Accessed 2020 Oct 2. https://www.zimmerbiomet.com/medical-professionals/common/our-science/osseoti-porous-metal.html [Google Scholar]
- 21.Biomet Orthopaedics. Focus on fixation. PPS® Porous Plasma Spray. 2020. Accessed 2020 Oct 2. http://www.biomet.gr/userfiles/files/Technologies/Focus-on-Fixation-PPS.pdf [Google Scholar]
- 22.Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, O’Keefe TJ, Stulberg SD, Sutherland CJ. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2- to 5-year results. J Arthroplasty. 2005. Apr;20(3):369-78. Epub 2005 Apr 06. [DOI] [PubMed] [Google Scholar]
- 23.Valstar ER, Spoor CW, Nelissen RG, Rozing PM. Roentgen stereophotogrammetric analysis of metal-backed hemispherical cups without attached markers. J Orthop Res. 1997. Nov;15(6):869-73. [DOI] [PubMed] [Google Scholar]
- 24.Nebergall AK, Rader K, Palm H, Malchau H, Greene ME. Precision of radiostereometric analysis (RSA) of acetabular cup stability and polyethylene wear improved by adding tantalum beads to the liner. Acta Orthop. 2015;86(5):563-8. Epub 2015 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baad-Hansen T, Kold S, Kaptein BL, Søballe K. High-precision measurements of cementless acetabular components using model-based RSA: an experimental study. Acta Orthop. 2007. Aug;78(4):463-9. [DOI] [PubMed] [Google Scholar]
- 26.Salemyr M, Muren O, Eisler T, Bodén H, Chammout G, Stark A, Sköldenberg O. Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty. A randomised controlled trial. Int Orthop. 2015. May;39(5):823-32. Epub 2014 Oct 22. [DOI] [PubMed] [Google Scholar]
- 27.Sillesen NH, Greene ME, Nebergall AK, Nielsen PT, Laursen MB, Troelsen A, Malchau H. Three year RSA evaluation of vitamin E diffused highly cross-linked polyethylene liners and cup stability. J Arthroplasty. 2015. Jul;30(7):1260-4. Epub 2015 Feb 18. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S, Issa K, Kapadia BH, Pivec R, Khanuja HS, Mont MA. Highly-porous metal option for primary cementless acetabular fixation. What is the evidence? Hip Int. 2013. Nov-Dec;23(6):509-21. Epub 2013 Sep 3. [DOI] [PubMed] [Google Scholar]
- 29.Lindgren V, Galea VP, Nebergall A, Greene ME, Rolfson O, Malchau H; Multicenter Writing Committee. Radiographic and clinical outcomes of porous titanium-coated and plasma-sprayed acetabular shells: a five-year prospective multicenter study. J Bone Joint Surg Am. 2018. Oct 3;100(19):1673-81. [DOI] [PubMed] [Google Scholar]