Abstract
Purpose:
This phase 1 study aimed to determine the safety, tolerability and recommended phase 2 dose (RP2D) of crizotinib in combination with cytotoxic chemotherapy for children with refractory solid tumors and ALCL.
Methods:
Pediatric patients with treatment refractory solid tumors or ALCL were eligible. Using a 3+3 design, crizotinib was escalated in 3 dose levels; 165, 215, or 280 mg/m2/dose BID. In Part A, patients received crizotinib oral solution (OS) in combination with topotecan and cyclophosphamide (topo/cyclo); in Part B, crizotinib OS was administered with vincristine and doxorubicin (vcr/dox). In parts C and D, patients received topo/cyclo in combination with either crizotinib formulated capsules (FC) or microspheres (cMS), respectively. Crizotinib pharmacokinetic evaluation was required.
Results:
Forty-four eligible patients were enrolled, 39 were evaluable for toxicity. Parts A and B were terminated due to concerns regarding palatability and tolerability of the OS. In Part C, crizotinib, FC 215 mg/m2/dose BID, in combination with topo/cyclo was tolerated. In Part D, the maximum tolerated dose (MTD) was exceeded at 165 mg/m2/dose of crizotinib cMS. Pharmacokinetics of crizotinib in combination with chemotherapy were similar to single agent crizotinib and exposures were not formulation dependent.
Conclusions:
The RP2D of crizotinib FCs in combination with cyclophosphamide and topotecan was 215 mg/m2/dose BID. The oral solution of crizotinib was not palatable in this patient population. Crizotinib cMS was palatable, however, patients experienced increased toxicity that was not explained by the relative bioavailability or exposure and warrants further investigation.
Keywords: pediatric, oncology, phase 1, crizotinib, chemotherapy combinations
Introduction
The anaplastic lymphoma kinase (ALK) oncogene is aberrantly expressed in a subset of human malignancies. ALK is an orphan receptor tyrosine kinase first identified as part of the t(2;5) chromosomal translocation associated with most anaplastic large cell lymphomas (ALCL) and a subset of t-cell non-Hodgkin lymphomas.[1] ALK and its mutant translocation with the nucleophosmin gene (NPM-ALK) results in a constitutively active ALK receptor tyrosine kinase[1,2]. Germline mutations in ALK are a major cause of hereditary neuroblastoma and can be somatically acquired in up to 10% of sporadic cases. An additional 4% of high-risk neuroblastoma cases carry ALK amplification[3]. Additionally, an oncogenic role for full-length ALK has been described in thyroid cancer and rhabdomyosarcoma[4,5]. Given this biologic relevance, ALK is an exciting tractable target for ALCL, neuroblastoma and other solid tumors.
Crizotinib is an orally bioavailable, selective small molecular inhibitor of c-Met/HGFR, ROS1, and ALK receptor tyrosine kinases. In preliminary biochemical screens, crizotinib inhibited HGF-stimulated or constitutive total tyrosine phosphorylation of wild type c-Met/HGFR with a mean IC50 value of 11 nM across a panel of human tumor cell lines[6]. In preclinical murine models of ALCL, administration of crizotinib (100 mg/kg/day) resulted in complete regression of tumors within 15 days of crizotinib initiation. In murine neuroblastoma models, xenografts that express R1275Q-ALK, one of the two most commonly occurring ALK mutations, were highly sensitive to crizotinib exposure [6].
In preclinical studies, crizotinib in combination with topotecan/cyclophosphamide showed synergistic activity in neuroblastoma cell lines harboring the most common ALK mutations. Importantly, synergistic activity has been observed in cell lines previously shown to be crizotinib resistant when the drug is combined with chemotherapy. Combination therapy with crizotinib/topotecan/cyclophosphamide was evaluated in SH-SY5Y xenografts, which harbor the second most frequent ALK mutation (F1174L). Although initially effective, topotecan/cyclophosphamide treatment did not result in a sustained response. However, the combination of crizotinib with topotecan/cyclophosphamide resulted in rapid and sustained tumor regressions. Mice treated with chemotherapy plus crizotinib showed significantly improved EFS and decreased tumor growth rates. Complete responses were maintained for an additional 24 weeks after cessation of treatment. The combination of crizotinib and chemotherapy was also studied in Felix-PDXs, which harbor the third most common ALK mutation in neuroblastoma (R1245C). Similar to SH-SY5Y, these xenografts have been shown to display de novo resistance to crizotinib. Treatment with crizotinib alone or topotecan/cyclophosphamide alone resulted in a brief tumor growth delay, however complete responses were achieved and maintained in Felix-PDX treated with the chemotherapy plus crizotinib [7].
The recommended phase 2 dose (RP2D) of single agent crizotinib in pediatric patients with recurrent or refractory solid tumors was 280 mg/m2/dose BID in the Children’s Oncology Group (COG) Phase 1 Consortium Trial (ADVL0912, NCT00939770)[8]. Dose limiting toxicities (DLTs) during cycle 1 were neutrophil count decrease (1/21 patients receiving 165 mg/m2/dose), dizziness and intracranial hemorrhage (1/11 patients receiving 215 mg/m2/dose), elevated hepatic transaminases and neutropenia (2/6 patients receiving 365 mg/m2/dose). In patients (n=17) who received crizotinib, 280 mg/m2/dose BID, no cycle 1 DLTs were observed; subsequent cycle DLTs included neutrophil count decrease, limb edema, diarrhea and skin infections[8]. Common non-dose limiting, non-hematological toxicity included nausea (65%), vomiting (58%), ALT elevation (62%), AST elevation (56%), diarrhea (41%), fatigue (31%) anorexia (22%), abdominal pain (18%). Overall, the pharmacokinetic parameters were similar to those reported in adults[9]. Crizotinib was well tolerated as a single agent and objective tumor responses were seen in patients with ALCL, inflammatory myofibroblastic tumor and neuroblastoma with activating ALK mutations[8].
The tolerability, toxicity profile, and activity of single agent crizotinib, as well as the preclinical data demonstrating that crizotinib synergizes with chemotherapy provided rationale for evaluation of crizotinib in combination with chemotherapy [7,10] The primary objective of this trial was to estimate the RP2D or maximum tolerated dose (MTD), toxicity profile and pharmacokinetics of crizotinib administered twice daily in combination with either topotecan and cyclophosphamide (topo/cyclo) or vincristine and doxorubicin (vcr/dox).
Materials and Methods
Patient Eligibility
Patients >12 months or <22 years of age with recurrent or refractory solid tumors, including lymphomas and excluding CNS tumors, were eligible. Histologic verification of malignancy was required and patients needed to have either measurable or evaluable disease. Other eligibility criteria included a Karnofsky/Lanksy performance score ≥ 50. Patients must have recovered from acute side effects of prior therapy. Intervals from prior therapy included: > 21 days for myelosuppressive chemotherapy, > 7 days for biologic agents, > 42 days for immunotherapy, ≥ 21 days for monoclonal antibodies. Intervals from hematopoietic growth factor support were, > 7 days for short-acting and > 14 days for long acting factors. For prior radiation, > 14 days was required for local palliative radiation, > 150 days for prior total body irradiation, craniospinal XRT or radiation to ≥ 50% of the pelvis, > 42 days for other substantial bone marrow radiation or therapeutic 131I-MIBG. In addition, ≥ 84 days from stem cell transplant or rescue without evidence of active graft vs. host disease and ≥ 42 from autologous stem cell infusion after 131I-MIBG therapy were required. Patients must not have received prior therapy with crizotinib. Adequate renal function (age-adjusted normal serum creatinine, or GFR ≥ 70 ml/minutes/1.73m2); adequate liver function (total bilirubin ≤ 1.5 X ULN, ALT ≤ 110, serum albumin ≥ 2 g/dL); adequate cardiac function (QTc ≤ 480 msec) and BSA ≥ 1.07m2 for patients receiving the formulated capsules and ≥ 0.43m2 for patients receiving the microsphere formulation were required. Adequate bone marrow function, defined as absolute neutrophil count (ANC) ≥ 1,000/mm3 and transfusion independent platelet count ≥ 100,000 mm3 were also required.
Exclusion criteria included women who were pregnant or breastfeeding; uncontrolled infection; concurrent use of other investigational agents, anticancer agents, chronic use of CYP3A4 inducers, inhibitors or substrates with narrow therapeutic indices, agents to prevent organ rejection post-transplant and chronic systemic corticosteroids. Proton pump inhibitors (PPIs) were prohibited for patients enrolling on the crizotinib microsphere formulation (cMS) as this formulation requires an acidic environment for the microsphere’s to be released and absorbed.
This trial was approved by the Institutional Review Board of participating sites and conducted in compliance with ethical standards. All patients or their legal guardians signed a document of informed consent and assent was obtained according to institutional guidelines.
Drug Administration
Crizotinib was supplied by Pfizer Inc and administered orally twice daily on days 1–21 of each 21-day cycle. The starting dose was 165 mg/m2/dose with. In order to balance the potential overlapping toxicities of crizotinib and cytotoxic chemotherapy with ensuring an effective dose being delivered, a starting dose of 165 mg/m2dose was chosen, with planned dose escalations to 215 mg/m2/dose and 280 mg/m2/dose. This starting dose is known to be sufficient for ALCL but is a de-escalation from the single agent MTD. The cytotoxic chemotherapy, cyclophosphamide (250 mg/m2/dose), topotecan (0.75 mg/m2/dose), vincristine (1.5 mg/m2/dose, maximum dose 2 mg) and doxorubicin (45 mg/m2/dose) were obtained from commercial supplies and administered intravenously. These regimens were chosen based on known activity in many pediatric malignancies including ALCL, neuroblastoma and sarcomas. For patients on Parts A, C and D, topotecan and cyclophosphamide were administered on days 1–5 of each cycle. For patients on Part B of the study, vincristine and doxorubicin were administered on day 1 of each cycle. Supportive care included dexrazoxane administered prior to doxorubicin and initiation of a myeloid growth factor (filgrastim or biosimilar or pegylated filgrastim) 24–48 hours after the completion of cytotoxic chemotherapy and continued until the post-nadir ANC was ≥ 2,000/mm3. Three crizotinib formulations were utilized during the study. Oral solution (OS, 25 mg/mL) was used in Parts A and B, formulated capsules (FC, 150 mg, 200 mg, 250 mg) were used in Part C, and microspheres (cMS, 20 mg, 40 mg, 120 mg) were evaluated in Part D. All administered doses were based on body surface area (BSA). A BSA-based dosing nomogram was used to prescribe FC and cMS.
Study Design
The goals of this study were to understand the toxicity and pharmacokinetics of these novel combinations as well to select of the most optimal formulation of crizotinib for use in pediatric patients. With this, the primary objectives of the study were to: (1) estimate the RP2D or MTD of crizotinib administered orally, twice daily in combination with topo/cyclo or vcr/dox in children with refractory/relapsed solid tumors or ALCL, (2) define and describe the toxicities of crizotinib in combination with topo/cyclo or vcr/dox and (3) characterize the pharmacokinetics of crizotinib in children with relapsed/refractory cancer when combined with either topo/cyclo or vcr/dox. To achieve this, four parts of the study were conducted. Parts A (crizotinib OS + topo/cyclo) and B (crizotinib OS + vcr/dox) of the study accrued simultaneously. Parts C (crizotinib FC + topo/cyclo) and D (crizotinib cMS + topo/cyclo) were added when data from Parts A and B suggested that the crizotinib OS was not palatable and there was concern that the adverse events were related to the taste of the OS rather than dose or drug related toxicities. In Parts C and D, only the topo/cyclo chemotherapy backbone in combination with crizotinib was evaluated because the combination of crizotinib with a chemotherapy regimen that included doxorubicin was being evaluated in a COG pilot study for ALCL (ANHL12P1, NCT01979536). All parts of the study independently followed a 3+3 dose escalation design. Monitoring for regimen-related toxicity included physical examinations (weekly during cycle 1, then prior to subsequent cycles), weekly serum chemistries and complete blood counts (twice weekly during cycle 1, then weekly). Supportive cares including anti-emetics were allowed and recommendations masking the flavor of the crizotinib OS were provided.
Toxicity was graded according to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAEv4; http://ctep.cancer.gov). Hematological DLT was defined as neutropenia or thrombocytopenia that precluded the initiation of the next cycle of therapy within 7 days of the scheduled start date. Grade 3 or 4 febrile neutropenia was not considered a DLT because it is an expected toxicity with the cytotoxic chemotherapy backbones. Non-hematological DLT was defined as any grade 3 or 4 non-hematologic toxicity attributed to the investigational agent with the exception of grade 3 nausea and vomiting of < 3 days duration, grade 3 liver enzyme elevation that resolved to levels that met initial eligibility criteria within 7 days of study drug interruption, grade 3 or 4 fever < 5 days duration, grade 3 infection < 5 days duration, grade 3 hypophosphatemia, hypokalemia, hypocalcemia, or hypomagnesemia responsive to oral supplementation. In addition, QTc prolongation > 500 ms that persisted despite correction of serum electrolytes or any grade 2 non-hematologic toxicity that persisted for ≥ 7 days and was considered sufficiently medically significant or intolerable by patients that it required treatment interruption were considered dose limiting. The MTD was the highest dose at which fewer than one-third of patients experienced a DLT during cycle 1 of therapy.
Disease evaluations were performed after the first cycle and then every other cycle until the fifth cycle and every third cycle thereafter. Tumor response was reported using the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) or MIBG response for patients with neuroblastoma and MIBG avid disease only.[11,12]
Taste Questionnaires
Taste Questionnaires to assess the acceptability of each of the crizotinib formulations were completed during each cycle of therapy for consenting patients.[13,14] The questions asked for each formulation are presented in Supplemental Table 1.
Pharmacokinetics
Patients on Parts A, B and C of the study had plasma samples for crizotinib concentration measurements obtained prior to the first dose on day 1 of cycle 1 and at steady state, defined as being between days 15 and 21 of cycle 1. Steady state samples were drawn pre-dose (12 hours after the last dose) and then 1 hr, 2 hrs, 4 hrs and 6–8 hrs after the morning dose on that given day. Patients on Part D of the study had plasma samples obtained prior to the first dose on day 1 of cycle 1 and prior to the morning dose on day 2. The first dose on day 1 was given in the evening so that a 12-hour trough level could be obtained on day 2. In addition, samples were collected at steady state following the same schedule as used in Parts A-C.
Plasma crizotinib concentrations were quantified by Covance Bioanalytical Services (Indianapolis, IN) using a previously described, validated high-pressure liquid chromatography (HPLC)-tandem mass spectrometry method with a lower limit of quantification of 0.2 ng/mL[15]. Crizotinib plasma concentration-time data were analyzed using the NCA (non-compartmental analysis) module of the Phoenix Software Package (Certara, Princeton, NJ). To estimate the steady state AUC for the 12 hr dosing interval (AUCtau), which is equivalent to AUC0−∞ after a single dose, the trough concentration prior to the dose was also used as the 12-hr post-dose concentration as previously described[9]. For comparison across dose levels, PK parameters were normalized to a crizotinib FC dose of 215 mg/m2/dose. Relative bioavailability was calculated as the dose normalized median AUCtau of crizotinib OS or cMS divided by the median normalized AUCtau of crizotinib FC 215 mg/m2/dose.
RESULTS
Of 46 patients enrolled between April 29, 2013 and July 14, 2017, 44 were eligible and 39 were fully evaluable for toxicity. Both of the ineligible patients did not complete the required investigations for eligibility assessment within the appropriate timeline. Characteristics of eligible patients are shown (Table 1).
Table 1.
Patient Characteristics for eligible Patients (N=44)
| Characteristic | Part A | Part B | Part C | Part D |
| (n=10) | (n=9) | (n=16) | (n=9) | |
| Age (years) Median (Range) | 14.5 (4 – 21) | 11 (6 – 21) | 16.5 (9 – 21) | 14 (1 – 18) |
| Number (%) | Number (%) | Number (%) | Number (%) | |
| Sex | ||||
| Male | 5 (50) | 5 (56) | 8 (50) | 5 (56) |
| Female | 5 (50) | 4 (44) | 8 (50) | 4 (44) |
| Race | ||||
| White | 6 (60) | 9 (100) | 13 (81) | 7 (78) |
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| American Indian or Alaska Native | 1 (10) | 0 (0) | 0 (0) | 1 (11) |
| Black or African American | 3 (30) | 0 (0) | 3 (19) | 0 (0) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (11) |
| Ethnicity | ||||
| Non-Hispanic | 10 (100) | 6 (67) | 13 (81) | 7 (78) |
| Hispanic | 0 (0) | 3 (33) | 3 (19) | 1 (11) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (11) |
| Diagnosis | ||||
| Adrenal cortical carcinoma | 1 (11) | |||
| Alveolar rhabdomyosarcoma | 1 (10) | 1 (11) | 1 (6) | 1 (11) |
| Carcinoma NOS | 1 (10) | 1 (6) | ||
| Desmoplastic small round cell tumor | 1 (10) | 1 (11) | ||
| Ewings sarcoma family of tumors | 4 (40) | 1 (11) | 3 (19) | 2 (22) |
| Ganglioneuroblastoma | 1 (10) | |||
| Hepatocellular carcinoma | 1 (6) | |||
| Hepatocellular carcinoma, fibrolamellar | 1 (6) | 1 (11) | ||
| Epithelial Myoepithelial Carcinoma | 1 (11) | |||
| Wilms tumor | 1 (11) | 1 (6) | 1 (11) | |
| Neuroblastoma, | 1 (10) | 1 (11) | 2 (13) | 1 (11) |
| Osteosarcoma | 1 (10) | 2 (22) | 2 (13) | 1 (11) |
| Renal cell carcinoma | 1 (6) | |||
| Rhabdomyosarcoma | 1 (11) | 1 (6) | ||
| Synovial sarcoma | 1 (6) | |||
| Undifferentiated sarcoma | 1 (6) | 1 (11) | ||
| Prior Therapy | ||||
| Prior Chemotherapy Regimens (N=number patient) | (N=10) | (N=9) | (N=16) | (N=9) |
| Median (Range) Number of Regimens | 2 (1 – 6) | 3 (1 – 6) | 3 (1 – 6) | 1 (1 – 3) |
| Prior Radiation Therapy Courses ((N=number patient) | (N=8) | (N=6) | (N=13) | (N=5) |
| Median (Range) Number of Courses | 1 (1 – 3) | 1.5 (1 – 4) | 1 (1 – 2) | 1 (1 – 2) |
Maximum Tolerated Dose Determination
The MTD and RP2D of crizotinib in combination with cytotoxic chemotherapy was determined to be 215 mg/m2/dose BID. Cycle 1 DLTs are shown (Table 2). In part A (crizotinib OS + topo/cyclo), one of six patients experienced dose limiting grade 3 nausea at dose level 1 (165 mg/m2/dose); one of three evaluable patients had dose limiting nausea (grade 3) and dehydration (grade 3) at dose level 2 (215 mg/m2/dose). In part B (crizotinib OS + vcr/dox) no DLTs were observed at dose level 1 or dose level 2, however, at dose level 3 (crizotinib 280 mg/m2/dose BID) the first two evaluable patients experienced DLTs of grade 3 nausea in one patient and grade 3 dehydration and grade 4 QTc prolongation in the other patient. Parts A and B were closed to accrual as there were sufficient data to suggest that despite the use of strategies to mask taste as well as the use of multiple antiemetics including 5HT3 inhibitors, diphenhydramine, lorazepam, scopalamine patch and phenergan, the OS was not palatable; taste and palatability of crizotinib OS were likely contributing to the adverse events and tolerability of the combination. To eliminate the impact of the palatability of the OS, Part C (crizotinib FC + topo/cyclo) was open to accrual using the crizotinib formulated capsules at the starting dose level of 165 mg/m2/dose BID; no DLTs were observed in the initial 3 evaluable patients. At dose level 2 (215 mg/m2/dose BID) one of 3 patients experienced DLTs of grade 3 esophageal pain and grade 3 hematuria. Of note, one of these patients was ultimately deemed to be inevaluable as they did not receive the required percent of protocol therapy. An additional 3 patients enrolled at this dose level and none of these patients experienced DLTs. The crizotinib dose was escalated to 280 mg/m2/dose BID and two of three evaluable patients experiencing DLTs of grade 3 blurred vision and grade 3 diplopia in one and grade 3 hypotension in the other. Therefore, this dose exceeded the MTD. Three additional patients were enrolled to dose level 2 on the PK expansion cohort with one patient experiencing a DLT of grade 3 dysphagia. Overall at dose level 2 in Part C, 2 of 8 evaluable (9 enrolled) patients experienced DLT. Thus, the MTD and RP2D of crizotinib FC in combination with topo/cycle was determined to be 215 mg/m2/dose BID.
Table 2.
DLTs Summary
| Crizotinib Dose | Part | Dose Level | Number of Patients Entered | Number of Patients Evaluable | Number of Patients with DLT | DLT Description |
|---|---|---|---|---|---|---|
| 165 mg/m2/dose BID | A |
Crizotinib OS: 165 mg/m2/dose BID, D1–21; Cyclophosphamide: 250 mg/m2/dose, D1–5 Topotecan: 0.75 mg/m2/dose, D1–5 |
6 | 6 | 1 | Nausea |
| B |
Crizotinib OS: 165 mg/m2/dose BID, D1–21; Vincristine: 1.5 mg/m2/dose (Max 2 mg), D1 Doxorubicin: 45 mg/m2/dose, D1 |
4 | 3 | 0 | ||
| C |
Crizotinib FC: 165 mg/m2/dose BID, D1–21; Cyclophosphamide - 250 mg/m2/dose, D1–5 Topotecan - 0.75 mg/m2/dose, D1–5 |
4 | 3 | 0 | ||
| D |
Crizotinib cMS: 165 mg/m2/dose BID, D1–21; Cyclophosphamide - 250 mg/m2/dose, D1–5 Topotecan - 0.75 mg/m2/dose, D1–5 |
6 | 5 | 3 | Platelet count decreased (3) Neutrophil count decreased (2) Diarrhea (1); Dehydration (1) Hypokalemia (1) |
|
| 215 mg/m2/dose BID | A |
Crizotinib OS: 215 mg/m2/dose BID, D1–21; Cyclophosphamide: 250 mg/m2/dose, D1–5 Topotecan: 0.75 mg/m2/dose, D1–5 |
4 | 3 | 1 | Nausea (1) Dehydration (1) |
| B |
Crizotinib OS: 215 mg/m2/dose BID, D1–21; Vincristine: 1.5 mg/m2/dose (Max 2 mg), D1 Doxorubicin: 45 mg/m2/dose, D1 |
3 | 3 | 0 | ||
| C |
Crizotinib FC: 215 mg/m2/dose BID, D1–21; Cyclophosphamide - 250 mg/m2/dose, D1–5 Topotecan - 0.75 mg/m2/dose, D1–5 |
9 | 8 | 2 | Esophageal pain (1) Hematuria (1) |
|
| D |
Crizotinib cMS: 215mg/m2/dose BID, D1–21; Cyclophosphamide - 250 mg/m2/dose, D1–5 Topotecan - 0.75 mg/m2/dose, D1–5 |
3 | 3 | 3 | Neutrophil count decreased (1) Sepsis (1) Syncope (1) |
|
| 280 mg/m2/dose BID | B |
Crizotinib OS: 280 mg/m2/dose BID, D1–21; Vincristine: 1.5 mg/m2/dose (Max 2 mg), D1 Doxorubicin: 45 mg/m2/dose, D1 |
2 | 2 | 2 | Nausea (1) Dehydration (1) QTc Prolongation (1) |
| C |
Crizotinib FC: 280 mg/m2/dose BID, D1–21; Cyclophosphamide - 250 mg/m2/dose, D1–5 Topotecan - 0.75 mg/m2/dose, D1–5 |
3 | 3 | 2 | Blurred vision (1) Diplopia (1) Hypotension (1) |
In order to assess the tolerability of crizotinib formulations appropriate for young children, we evaluated crizotinib microspheres (cMS). In Part D (crizotinib cMS + topo/cyclo) the crizotinib starting dose was the RP2D (215 mg/m2/dose BID) from Part C. All three evaluable patients experienced DLTs: grade 4 neutrophil count decreased, grade 4 sepsis and grade 3 syncope. The dose was de-escalated to 165 mg/m2/dose BID and three out of five evaluable patients (6 enrolled, 1 inevaluable) experienced DLTs of grade 3 or 4 platelet count decreased (3), grade 4 neutrophil count decreased (2), grade 4 dehydration (1), grade 4 diarrhea (1) and grade 4 hypokalemia (1). In addition, delays in starting cycle 2 due to prolonged thrombocytopenia ranged from 12 to > 21 days. One patient had grade 4 thrombocytopenia precluding the start of cycle 2 for > 21 days and was removed from protocol treatment due to this toxicity. Thus, the MTD was exceeded when crizotinib cMS 165 mg/m2/dose BID was administered in combination with topo/cyclo. No further dose levels were investigated.
Additional Toxicities
All hematologic and non-hematologic toxicities throughout all cycles that were grade ≥ 3 and related to protocol therapy are described in supplemental table 2a for parts A,B,C and 2b for part D. Overall, Grade 1 and 2 toxicities previously known to be related to crizotinib included creatinine increase in 41%, blurred vision in 36%, QTc prolongation in 8%, and thromboembolic events in 5%. Grade 1 and 2 pulmonary toxicities including infection, pleural effusion and pulmonary edema were noted in 3% of patients, however there were no reports of pneumonitis.
Palatability Assessment
Results from the taste questionnaire overall suggested that crizotinib OS was not palatable. Specifically, of the 14 respondents (age median: 12 years, range: 4–21 years) during week 1 of treatment, 71.4% reported that they disliked the medication very much and 85.7% reported not liking the flavor of the medicine. Of the 14 respondents for the FC questionnaire (age median: 17 years, range: 9–21 years), 50% reported the capsules to be very easy to swallow, 43% okay to swallow and 7% were not sure. No patients reported the capsules to be difficult to swallow. Of the 7 patient respondents for the cMS questionnaire (age median: 13.5 years, range: 1–21 years), 1 (14%) liked the medicine a little, 2 (28.6%) neither liked or dislike the medicine, 2 (28.6%) disliked it a little and 2 (28.6%) disliked it very much. In terms of the ease of administration, 3 (42.9%) reported the cMS to be somewhat easy to administer, whereas one respondent each reported it to be very difficult, somewhat difficult and very easy to administer.
Pharmacokinetics
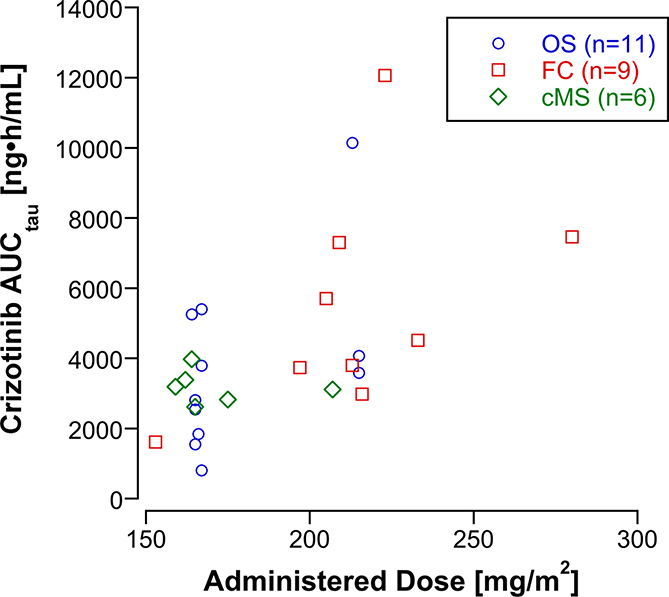
Individual subject (n=26) and a summary of crizotinib peak plasma concentrations (Cmax) and steady-state AUCtau by dose level and formulation are listed in Table 3. The steady-state AUCtau was highly variable. At the 215 mg/m2 dose level, the crizotinib exposure (AUCtau), ranged 4-fold, from 2,980 to 12,100 ng•h/mL. Despite this substantial inter-subject variability, the steady-state AUCtau appears to increase in proportion to the dose (Figure 1). Subjects receiving formulated capsules had higher steady state AUCtau but also received higher doses of crizotinib. The interpatient relative bioavailability of the microsphere formulation and oral solution were 93%. The steady state AUCtau in the three subjects who received the microsphere formulation and experienced a DLT were comparable to the steady state AUCtau of subjects who received similar doses of the oral solution and FC and did not experience a DLT.
TABLE 3:
Steady State Pharmacokinetics in Subjects Receiving Crizotinib Oral Solution (OS), Formulated Capsules (FC), or Microspheres (cMS)
| Crizotinib (mg/m2/dose) BID | Crizotinib Formulation | Subject Number | Part | Age (y) | Sex | BSA (m2) | Crizotinib Dose (mg/dose) | Actual Crizotinib Dose (mg/m2/dose) | C1 DLT | Cmax (ng/mL) | AUCtau (ng•h/mL) | CL/F (ml/min/m2) | AUCtau (ng•h/mL) Normalized to FC 215 mg/m2/dose | Bioavailability Relative to FC 215 mg/m2/dose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 165 | OS | 1 | B | 7 | F | 1.02 | 170 | 167 | 87 | 811 | 3420 | 1044 | ||
| 3 | A | 21 | M | 1.59 | 265 | 167 | 512 | 5402 | 500 | 6955 | ||||
| 5 | A | 19 | M | 2.08 | 345 | 166 | 209 | 1842 | 1530 | 2386 | ||||
| 6 | A | 14 | M | 1.65 | 275 | 167 | 405 | 3793 | 730 | 4883 | ||||
| 7 | B | 21 | M | 2.27 | 375 | 165 | 198 | 1549 | 1770 | 2018 | ||||
| 8 | B | 13 | M | 1.44 | 238 | 165 | 303 | 2544 | 1080 | 3315 | ||||
| 9 | A | 4 | M | 0.67 | 110 | 164 | 564 | 5257 | 530 | 6892 | ||||
| 10 | A | 14 | F | 1.2 | 198 | 165 | 289 | 2811 | 980 | 3663 | ||||
| Mean (%CV) | 14 | 354 (41%) | 3001 (56%) | 1320 (73%) | 3894 (56%) | |||||||||
| Median (range) | 14 (4–21) | 303 (87–564) | 2678 (811–5402) | 1030 (500–3420) | 3489 (1044–6955) | 94% | ||||||||
| 165 | FC | 25 | C | 15 | M | 1.63 | 250 | 153 | 150 | 1612 | 1590 | 2265 | Insufficient data | |
| 165 | cMS | 41 | D | 17 | F | 1.82 | 300 | 165 | yes | 265 | 2616 | 1050 | 3409 | |
| 42 | D | 2 | F | 0.57 | 100 | 175 | 270 | 2823 | 1040 | 3678 | ||||
| 43 | D | 15 | M | 1.51 | 240 | 159 | 335 | 3192 | 830 | 4159 | ||||
| 44 | D | 14 | M | 1.34 | 220 | 164 | yes | 356 | 3976 | 690 | 5181 | |||
| Mean (%CV) | 12 | 307 (15%) | 3152 (19%) | 900 (19%) | 4101 (21%) | |||||||||
| Median (range) | 14 (2–17) | 303 (265–356) | 3008 (2616–3976) | 940 (690–1050) | 3892 (3409–5181) | 93% | ||||||||
| 215 | OS | 12 | B | 19 | M | 2.08 | 447 | 215 | 357 | 3588 | 1000 | 3588 | ||
| 13 | B | 6 | M | 0.82 | 175 | 213 | 1000 | 10146 | 350 | 10241 | ||||
| 14 | B | 6 | F | 0.78 | 168 | 215 | 427 | 4071 | 880 | 4071 | ||||
| Mean (%CV) | 10 | 595 (59%) | 5935 (62%) | 740 (46%) | 5967 (62%) | |||||||||
| Median (range) | 6 (6–19) | 427 (357–1000) | 4071 (3588–10146) | 880 (350–1000) | 4071 (3588–10241) | 98% | ||||||||
| 215 | FC | 26 | C | 16 | F | 1.62 | 350 | 216 | 375 | 2976 | 1210 | 2962 | ||
| 27 | C | 20 | M | 2.15 | 450 | 209 | 719 | 7299 | 480 | 7509 | ||||
| 29 | C | 9 | M | 1.29 | 300 | 233 | 489 | 4515 | 860 | 4166 | ||||
| 30 | C | 18 | M | 1.64 | 350 | 213 | 424 | 3797 | 940 | 3833 | ||||
| 31 | C | 14 | F | 1.27 | 250 | 197 | 418 | 3739 | 880 | 4081 | ||||
| 35 | C | 11 | F | 1.22 | 250 | 205 | 665 | 5702 | 600 | 5980 | ||||
| 36 | C | 20 | M | 2.02 | 450 | 223 | 1100 | 12061 | 310 | 11628 | ||||
| Mean (%CV) | 15 | 599 (43%) | 5727 (55%) | 750 (41%) | 5737 (52%) | |||||||||
| Median (range) | 16 (9–20) | 489 (375–1100) | 4515 (2976–12061) | 860 (310–1210) | 4166 (2962–11628) | 100% | ||||||||
| 215 | cMS | 38 | D | 10 | M | 1.16 | 240 | 207 | 303 | 3110 | 1110 | 3230 | ||
| 39 | D | 18 | F | 1.73 | 280 | 162 | yes | 294 | 3386 | 800 | 4494 | |||
| Mean (CV%) | 14 | 298 (2%) | 3248 (6%) | 955 (23%) | 3862 (23%) | |||||||||
| Median (range) | 10, 18 | 294, 303 | 3110, 3386 | 800, 1110 | 3230, 4494 | 93% | ||||||||
| 280 | FC | 32 | C | 13 | M | 1.25 | 350 | 280 | 853 | 7463 | 625 | 5730 | 99% | |
F=female; M=male; BSA= body surface area; C1=cycle 1; DLT= Dose limiting Toxicity; AUCtau Normalized to 215 mg/m2/dose = (measured AUCtau *215mg/m2/dose)/Actual dose (mg/m2/dose); CL/F = apparent clearance.
Figure 1:
Administered dose (actual dose in mg divided by the body surface area) of Crizotinib vs. the AUCtau at steady state according to drug formulation (OS, oral solution; FC, formulated capsules; cMS, microspheres).
Response
Amongst the 44 patients who were evaluable for response, 36 had measurable disease. The response evaluable patients remained on protocol therapy from 1–23 cycles. Objective responses, defined as complete or partial using RECIST v 1.1 and confirmed by follow up imaging and central review were demonstrated in 7 patients (Table 4). These included 3 children with Ewing sarcoma family tumors and one patient each with: osteosarcoma, neuroblastoma, renal cell carcinoma and epithelial-myoepithelial carcinoma. Examples of responses include a patient on Part A (crizotinib OS at 165 mg/m2/dose + topo/cyclo) with Ewing sarcoma who had complete resolution of FDG uptake on PET/CT, achieving a complete response. In addition, a patient with neuroblastoma and PET/CT evaluable disease enrolled on Part B (crizotinib OS at 165 mg/m2/dose + vcr/dox) had complete resolution of FDG uptake constituting a complete response lasting through cycle 13 when the patient completed protocol therapy. A partial response was achieved by two patients on Part A (crizotinib OS + topo/cyclo), one patient on Part B and two patients on Part C (crizotinib FC + topo/cyclo). Stable disease of longer than 6 months was confirmed by central review in a total of three patients (one on Part A, two on Part D) with the following diseases: Ewing sarcoma family tumor (N=1), undifferentiated sarcoma (N=1) and neuroblastoma (N=1). The part D patient with undifferentiated sarcoma and prolonged stable disease over 6 cycles had a site reported partial response by RECIST criteria however due to the lack of follow-up confirmation imaging, this was classified as stable disease.
Table 4.
Treatment responses including prolonged stable disease
| Tumor Histology | Part of Study | Crizotinib dose (mg/m2/dose) | Response | Prior exposure to cytotoxic agents received |
|---|---|---|---|---|
| Ewing Sarcoma | A | 165 | PR | YES |
| Primitive neuroectodermal tumor (PNET) | A | 165 | Prolonged SD (8 cycles) | NO |
| PNET/Ewing Sarcoma Family | A | 165 | CR | NO |
| Neuroblastoma | B | 165 | CR | YES |
| Epithelial-myoepithelial carcinoma | B | 215 | PR | Unknown |
| Osteosarcoma | C | 215 | PR | NO |
| Renal cell carcinoma | C | 280 | PR | NO |
| Ewing Sarcoma | C | 165 | PR | NO |
| Neuroblastoma | D | 165 | Prolonged SD (10 cycles) | YES |
| Undifferentiated sarcoma | D | 215 | Prolonged SD (6 cycles) | NO |
Discussion
To our knowledge this was the first in human clinical trial combining crizotinib with conventional chemotherapy and thus, the primary goal of this trial was to administer the highest tolerable dose of crizotinib in combination with standard doses of cytotoxic chemotherapy to children with refractory/relapsed solid tumors or ALCL. Ultimately, we established the RP2D as crizotinib FC 215 mg/m2/dose administered orally, twice daily, in combination with topotecan (0.75 mg/m2/dose) and cyclophosphamide (250 mg/m2/dose) administered intravenously on days 1 to 5 of a 21-day cycle with myeloid growth factor support. In order to maximize the potential for clinical benefit of tyrosine kinase inhibition, crizotinib was escalated whereas the doses of cytotoxic agents remained constant. The backbone cytotoxic regimens were selected based on defined toxicity profiles and broad activity in childhood cancer, including, neuroblastoma, sarcoma, and ALCL.
An additional goal of the study was to assess the tolerability of crizotinib formulations that would be appropriate for dosing and administration in young children. The formulation of crizotinib had a significant impact on the toxicity profile and determination of the MTD/RP2D of crizotinib in combination with cytotoxic chemotherapy. In the phase 1 trial of single agent crizotinib for children with relapsed solid tumors, nine children received OS. In that study, a palatability questionnaire was not employed. Patients did however identify taste as an issue but there was no significant concern for tolerability.[9,8] In the current study, crizotinib OS was not tolerable in combination with topo/cyclo possibly related to poor palatability contributing to gastrointestinal toxicity including nausea and dehydration. Crizotinib appeared to be better tolerated when combined with vcr/dox, however, dose escalation beyond 215 mg/m2/dose BID was not pursued because this combination was being evaluated in a randomized trial in patients with ALCL (NCT01979536) and further safety data was not needed. Unfortunately, the MTD of cMS in combination with topo/cyclo was exceeded at both dose levels (215 and 165 mg/m2/dose BID) due to both hematological and non-hematological DLTs in all patients.
The pharmacokinetics of crizotinib were highly variable, however, the dose normalized exposure among patients receiving OS, cMS and FC was similar. In this small cohort of patients, the intra-patient variability in exposure appeared to be less for cMS (CV< 25%) compared to FC (CV=62%) or OS (CV=56%). The interpatient relative bioavailability of the OS and cMS to FC is 93% and is similar to bioequivalence 99% (90% CI 92%, 108%) of the oral formulations in adults[15]. PK for crizotinib in combination with chemotherapy was similar to PK for single agent crizotinib. For single agent crizotinib 215 mg/m2/dose BID (n=5), steady state mean AUCtau was 5630 ng•h/mL and Cmax was 601 ng/mL compared to the AUCtau of 5366 ng•h/mL and Cmax of 548 ng/mL in 12 subjects who received crizotinib 215 m/m2/dose BID in combination with chemotherapy on this trial [9]. Although younger children may tolerate the OS better than older children and adolescents, the cMS formulation was developed to provide acceptable dosing precision in young children and address the issues of palatability of the OS. However, the crizotinib cMS formulation in combination with cyclo/topo was intolerable due to toxicity at the 165 mg/m2/dose BID level. Based on the limited sample size and pharmacokinetic data, bioavailability, peak concentration, and exposure do not appear to account for the increased frequency of DLTs. Taste questionnaires indicate that cMS was palatable. Thus, it is difficult to explain the increased frequency of toxicity seen with the cMS formulation. The most plausible explanation for the variation in toxicity based on formulation is that our sample size is small and it is very possible this finding is related to chance only and not truly increased toxicity of the cMS formulation. Given concerns about the inability to inhibit ALK at doses lower than 165 mg/m2/dose BID, a lower dose level was not investigated and this formulation will not be advanced for this patient population. [16]
In vitro and in vivo preclinical data demonstrate that crizotinib is synergistic with chemotherapy and when combined with chemotherapy, crizotinib resistance can be overcome[7].
Given the primary aim of this trial was to establish the MTD/RP2D of crizotinib in combination with chemotherapy for patients that might benefit from target inhibition of this multi-target TKI, this trial did not include biomarker selection or tumor molecular profiling to interrogate the relationship of these findings to response. In this population of heavily pre-treated patients, objective responses were observed in seven patients with prolonged stable disease observed in an additional 3 patients. These patients had a variety of tumor histologies with the large majority having a sarcoma. Most, but not all, of the patients had not had prior exposure to the conventional chemotherapy agents received on this trial, presumably because the agents are not thought to be efficacious for their disease histology. Thus, is it likely that crizotinib, or at least the combination of crizotinib and chemotherapy, contributed to the response. Given crizotinib is not solely an ALK inhibitor, it is certainly possible that inhibition of other genes such as C-MET have impacted tumors that typically are not associated with ALK aberrations.
Integration of ALK inhibition with chemotherapy is of upmost importance for diseases driven by ALK aberrations, including ALCL and the subset neuroblastoma harboring ALK mutations or amplification. Similar to studies in adults, this study demonstrates the challenges of combining crizotinib with other anticancer therapy[17–21]. However, the safety, tolerability, and pharmacokinetic data for crizotinib formulations is valuable to the continuing efforts to combine crizotinib with chemotherapy. Crizotinib FC at 165 mg/m2/dose BID in combination with multiagent chemotherapy is being evaluated in an ongoing randomized trial in patients with ALCL (ANHL12P1, NCT01979536). In addition, the efficacy of crizotinib when used with standard of care therapy for ALK aberrant high-risk neuroblastoma is currently being evaluated in a COG Phase 3 trial for high-risk neuroblastoma (ANBL1531, NCT03126916). This trial utilizes crizotinib FC or OS at 215 mg/m2/dose BID in combination with chemotherapy to evaluate the role of ALK inhibition in patients with newly diagnosed high-risk neuroblastoma with ALK aberrations. Given that the oral solution has not been studied in combination with all the chemotherapy agents and therapeutic modalities utilized in this trial, very close early safety monitoring is being undertaken.
Supplementary Material
Acknowledgments:
Funding Information:
Pediatric Early Phase Clinical Trials Network (PEP-CTN) UM1CA228823; Cookies for Kids’ Cancer Foundation; Pfizer; Dr. Reid was supported in part by Grant P30 CA015083 from the National Cancer Institute (NCI).
Footnotes
Disclosure of potential conflicts of interest: The authors have no conflicts of interest to disclose.
CLINICAL TRIAL REGISTRY:
The trial is registered as NCT01606878 at Clinicaltrials.gov.
“Compliance with Ethical Standards” Statement:
The authors have no conflict of interest to disclose. This trial was approved by the Institutional. Review Board of participating sites and conducted in compliance with ethical standards. All patients or their legal guardians signed a document of informed consent and assent was obtained according to institutional guidelines.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Mosse YP, Wood A, Maris JM (2009) Inhibition of ALK signaling for cancer therapy. Clin Cancer Res 15 (18):5609–5614. doi: 10.1158/1078-0432.CCR-08-2762 [DOI] [PubMed] [Google Scholar]
- 2.Pulford K, Lamant L, Espinos E, Jiang Q, Xue L, Turturro F, Delsol G, Morris SW (2004) The emerging normal and disease-related roles of anaplastic lymphoma kinase. Cell Mol Life Sci 61 (23):2939–2953. doi: 10.1007/s00018-004-4275-9 [DOI] [PubMed] [Google Scholar]
- 3.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM (2008) Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455 (7215):930–935. doi: 10.1038/nature07261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins DS, Gupta AA, Rudzinski ER (2014) What is new in the biology and treatment of pediatric rhabdomyosarcoma? Curr Opin Pediatr 26 (1):50–56. doi: 10.1097/MOP.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonhomme B, Godbert Y, Perot G, Al Ghuzlan A, Bardet S, Belleannee G, Criniere L, Do Cao C, Fouilloux G, Guyetant S, Kelly A, Leboulleux S, Buffet C, Leteurtre E, Michels JJ, Tissier F, Toubert ME, Wassef M, Pinard C, Hostein I, Soubeyran I (2017) Molecular Pathology of Anaplastic Thyroid Carcinomas: A Retrospective Study of 144 Cases. Thyroid 27 (5):682–692. doi: 10.1089/thy.2016.0254 [DOI] [PubMed] [Google Scholar]
- 6.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G (2007) Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 6 (12 Pt 1):3314–3322. doi: 10.1158/1535-7163.MCT-07-0365 [DOI] [PubMed] [Google Scholar]
- 7.Krytska K, Ryles HT, Sano R, Raman P, Infarinato NR, Hansel TD, Makena MR, Song MM, Reynolds CP, Mosse YP (2016) Crizotinib Synergizes with Chemotherapy in Preclinical Models of Neuroblastoma. Clin Cancer Res 22 (4):948–960. doi: 10.1158/1078-0432.CCR-15-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosse YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, Rolland D, Balis FM, Maris JM, Weigel BJ, Ingle AM, Ahern C, Adamson PC, Blaney SM (2013) Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol 14 (6):472–480. doi: 10.1016/S1470-2045(13)70095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balis FM, Thompson PA, Mosse YP, Blaney SM, Minard CG, Weigel BJ, Fox E (2017) First-dose and steady-state pharmacokinetics of orally administered crizotinib in children with solid tumors: a report on ADVL0912 from the Children’s Oncology Group Phase 1/Pilot Consortium. Cancer Chemother Pharmacol 79 (1):181–187. doi: 10.1007/s00280-016-3220-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Wu B, Baruchel S (2017) Oral Metronomic Topotecan Sensitizes Crizotinib Antitumor Activity in ALK(F1174L) Drug-Resistant Neuroblastoma Preclinical Models. Transl Oncol 10 (4):604–611. doi: 10.1016/j.tranon.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45 (2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, Pearson AD, Cohn SL (2010) Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer 102 (9):1319–1326. doi: 10.1038/sj.bjc.6605621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mistry P, Batchelor H, project SP-U (2017) Evidence of acceptability of oral paediatric medicines: a review. J Pharm Pharmacol 69 (4):361–376. doi: 10.1111/jphp.12610 [DOI] [PubMed] [Google Scholar]
- 14.Aljebab F, Alanazi M, Choonara I, Conroy S (2018) Observational study on the palatability and tolerability of oral prednisolone and oral dexamethasone in children in Saudi Arabia and the UK. Arch Dis Child 103 (1):83–88. doi: 10.1136/archdischild-2017-312697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, O’Gorman M, Boutros T, Brega N, Kantaridis C, Tan W, Bello A (2015) Evaluation of crizotinib absolute bioavailability, the bioequivalence of three oral formulations, and the effect of food on crizotinib pharmacokinetics in healthy subjects. J Clin Pharmacol 55 (1):104–113. doi: 10.1002/jcph.356 [DOI] [PubMed] [Google Scholar]
- 16.Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, Plegaria JS, Cole K, Toporovskaya Y, Zhao H, Carpenter EL, Christensen JG, Maris JM, Lemmon MA, Mosse YP (2011) Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med 3 (108):108ra114. doi: 10.1126/scitranslmed.3002950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janne PA, Shaw AT, Camidge DR, Giaccone G, Shreeve SM, Tang Y, Goldberg Z, Martini JF, Xu H, James LP, Solomon BJ (2016) Combined Pan-HER and ALK/ROS1/MET Inhibition with Dacomitinib and Crizotinib in Advanced Non-Small Cell Lung Cancer: Results of a Phase I Study. J Thorac Oncol 11 (5):737–747. doi: 10.1016/j.jtho.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 18.Ou SI, Govindan R, Eaton KD, Otterson GA, Gutierrez ME, Mita AC, Argiris A, Brega NM, Usari T, Tan W, Ho SN, Robert F (2017) Phase I Results from a Study of Crizotinib in Combination with Erlotinib in Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer. J Thorac Oncol 12 (1):145–151. doi: 10.1016/j.jtho.2016.09.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broniscer A, Jia S, Mandrell B, Hamideh D, Huang J, Onar-Thomas A, Gajjar A, Raimondi SC, Tatevossian RG, Stewart CF (2018) Phase 1 trial, pharmacokinetics, and pharmacodynamics of dasatinib combined with crizotinib in children with recurrent or progressive high-grade and diffuse intrinsic pontine glioma. Pediatr Blood Cancer 65 (7):e27035. doi: 10.1002/pbc.27035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia MMGM, Losada EP, Velasco G, Alameda F, Alvaro T, Capellades J, Marquez-Martin A, Sarmiento B, Mesia Barroso C, Bruna Escuer J, Eugenia V, Hernandez-Lain A, Guzman M, Manuel Sepulveda J. (2018) GEINO 1402: A phase 1b dose-escalation study followed by an extension phase to evaluate safety and efficacy of crizotinib in combination with temozolomide (TMZ) and radiotherapy (RT) in patients with newly diagnosed glioblastoma (GB): Results of the dose-escalation. Journal of Clinical Oncology 36 (no. 15_suppl):2054–2054 [Google Scholar]
- 21.Shaw ATLS, Ramalingam SS, Bauer TM, Boyer MJ, Costa EC, Felip E, H JY, Hida T, Hughes BGM, Kin SW, Nishio M, Seto T, Ezeh P, Chakrabarti D, Wang J, Chang A, Fumagalli L, Solomon B. (2018) Avelumab (anti-PD-L1) in combination with crizotinib or lorlatinib in patinets with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. . Journal of Clinical Oncology 36 (15_suppl):9008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.