Abstract
Objective:
The purpose of this study was to determine the ability of a methacrylate resin dentin bonding agent to adhere to the dentin surfaces of prepared and conditioned root canals with either 32% phosphoric acid (PA) or 17% ethylenediaminetetraacetic acid (EDTA).
Methods:
Prior to the application of the methacrylate resin, the root canals of 54 intact, caries-free, single- rooted, de-crowned, extracted human maxillary incisor and canine teeth were endodontically prepared and conditioned with either 32% PA or 17% EDTA or with distilled water as the unconditioned control. The resin-treated roots were cross-sectioned at three levels and scanning electron microscope (SEM) imaged for circumferential views of the root canals at 60-90× magnification and site-specific views at 250× magnification, and then randomly coded for independent and blind evaluation by four calibrated examiners. The circumferential surface of the root canals that showed no resin adhesion were digitally measured and subtracted from the digitally measured total root canal circumference, and resin adhesion was expressed as a percentage of the circumference.
Results:
The mean percentages of resin adhesion were 97% for the PA group, 94% for the EDTA group, and 76% for the control group. There were statistically significant differences among the PA, EDTA, and control groups.
Conclusion:
Root canals conditioned with 32% PA or 17% EDTA had more resin adhesion than unconditioned root canals. Root canals conditioned with 32% PA had more resin adhesion than those conditioned with 17% EDTA.
Keywords: Adhesion, canals, conditioned, resin, root
HIGHLIGHTS.
Conditioning root canal dentin improved dentin adhesion.
Conditioning root canal dentin with phosphoric acid provided greater adhesion of resin than conditioning with ethylenediaminetetraacetic acid.
Enhanced adhesion of resin to root canal dentin conditioned with phosphoric acid could reduce the potential for micro-leakage in root canals obturated with resin sealers.
INTRODUCTION
An important therapeutic objective in endodontic treatment is to provide a fluid-tight seal in obturated root canals. Endodontic sealers in conjunction with semi-solid core materials play an important role in the accomplishment of this objective. The clinical concern for preventing micro-leakage and bacterial recontamination in obturated root canals, has led to the development and evaluation of resin-based root canal sealers that more effectively adhere to the dentin surfaces of prepared root canals and increase resistance to fluid permeation in obturated root canals (1-11).
Removal of the smear layer and demineralization of the dentin surface with acid conditioners are essential for resin adhesion (12-16). Root canal irrigating solutions acting as conditioners can affect the surface adhesion of resin sealers used in root canal obturation (17). Ethylenediaminetetraacetic acid (EDTA), a chelating agent commonly used to irrigate root canals prior to sealer placement, has been shown to effectively remove the smear layer from dentin surfaces in prepared root canals (18-21), but its ability to etch dentin is limited. Phosphoric acid (PA), an etching agent with its ability to remove the smear layer, open dentinal tubules, increase dentin permeability, de-mineralize dentin, and expose collagen fibrils of inter-tubular dentin, is a standard dentin conditioning agent used to maximize the adhesion of a resin to coronal dentin for bonded composite restorations (15, 16, 22-24). However, PA has not been considered as a root canal dentin conditioner for improving the adhesion of resin sealers, even though it has been demonstrated that composite resin fillings in cavity preparations conditioned with PA showed considerably lesser micro-leakage than those conditioned with EDTA (25).
Primers in dentin bonding systems are methacrylate resin monomers that provide a mechanism for bonding via copolymerization with resins when chemically or light cured (26). All-Bond2 is a dual-cure dentin universal bonding system that has been clinically used for over 20 years in restorative dentistry. Although combining procedural steps in dentin bonding systems have simplified its clinical application, the three-step process of etch, prime, and bond provides superior adhesion (27). Therefore, this adhesive system can adequately serve as an appropriate and effective prototypical agent for evaluating resin adhesion in prepared root canals conditioned with either PA or EDTA.
The purpose of this study was to evaluate and compare the extent of methacrylate resin adhesion in prepared root canals conditioned with either 32% PA or 17% EDTA or with distilled water as an unconditioned control.
MATERIALS AND METHODS
This study was approved as exempt by the Institutional Review Board of the University Health Science Center where this study was conducted.
Collection of teeth
Fifty-four intact, caries-free, single-rooted, extracted human maxillary incisors and canines that were collected according to the infection control protocol of a University College of Dentistry, were autoclave sterilized and stored in distilled water.
Preparation of root canals
The teeth were de-crowned with a high-speed diamond stone (Brasseler USA, Savannah, GA) resulting in roots with average lengths of 16 mm. The entire root canal of each root, from the canal orifice to the apical foramen, was instrumented using size 10, 15, and 20 stainless-steel hand K-Files (SybronEndo, Orange, CA) and further instrumented using size 20/06 to 40/06 EndoSequence nickel–titanium rotary instruments (Brasseler USA, Savannah, GA) in a crown-down manner to accomplish a final intra-canal apical instrument size of 40/06. During instrumentation, the root canals were copiously irrigated with 10 mL of a 5.25% sodium hypochlorite solution (Clorox Professional Products Co. Oakland, CA). The irrigating solution was delivered using a 5 cc plastic syringe with a 30 gauge needle loosely placed in the root canal to within 1 mm of the working length. The root canals were then irrigated, in the same manner, with 10 mL of distilled water to remove any residual chemical and debris. The roots were randomly divided into three groups: two groups of 22 specimens each (PA and EDTA) and one control group of 10 specimens. The specimens were then stored in distilled water at room temperature before conditioning and the application of the resin.
Conditioning of root canals
The PA group (22 specimens) was conditioned with 32% PA (Uni-Etch, Bisco Inc. Schaumburg, IL), the EDTA group (22 specimens) was conditioned with 17% EDTA (Endo-Cleanse, Roydent Dental Products, Rochester Hills, GA), and the control group (10 specimens) was sham-conditioned with distilled water. The root canals in each of the three groups were conditioned by irrigation with 10 mL of the designated conditioning solution for 30 s in the same manner as previously stated above. The conditioning solutions were allowed to remain in the root canals for another 30 s, and then, the root canals were suctioned to remove the remaining solution.
Application of resin
The resin application procedure for each tooth (dentin conditioning, primer application, and resin placement) was accomplished in sequence to avoid drying of the dentin and to maintain a moist dentin surface. All-Bond2 (Universal Bonding System, Bisco Inc. Schaumburg IL) was used as the adhesive resin agent according to the manufacturer’s instructions. All-Bond2 is a dual-cure bonding system consisting of N-tolyl glycine glycidyl methacrylate as primer A and biphenyl dimethacrylate in an acetone solution as primer B. Equal amounts of primers A and B were mixed together. Five consecutive coats of this mixture were placed into the root canals to within 1 mm of the apex using coarse paper points (Brasseler USA, Savannah, GA) and dried using a compressed air syringe for 10 s after each application. Equal amounts of D/E Resin and PreBond were mixed together. A 40/06 gutta-percha cone (Brasseler USA, Savannah, GA) completely covered with this mixture was placed into the root canal to within 1 mm of the apex, manipulated in a circular fashion for 10 s to thoroughly coat the walls, and then removed. The system was air-thinned with a compressed air syringe and was allowed to chemically cure for 10 min. The roots were then number coded for identification and stored in 100% humidity at 21°C for 24 h to ensure the chemical cure of the resin bonding system and prevent dehydration of the root specimens.
Preparation of cross-sectional root surfaces
Cross-sectional cuts were made in each root specimen with a slow-speed saw (Isomet, Buehler, Lake Bluff, IL) under water cooling conditions at 4, 8, and 12 mm from the coronal end of the 16 mm root specimen, giving rise to cross-sectional surfaces from the cervical, middle, and apical regions of the root, in which the coronal side of each cross-sectional cut was designated for evaluation. This yielded three representative cross-sectional surfaces for each root specimen. In this manner, a total of 162 cross-sectional surfaces, 66 in the PA group, 66 in the EDTA group, and 30 in the control group, were produced. However, two root sections in the PA group and two in the EDTA group fractured during sectioning and were discarded, leaving 64 cross-sections in each of these two groups, for a final total of 158 cross-sections for SEM examination. The cross-sectioned root surfaces were sequentially polished with 200, 400, 600, 800, and 1000 grit abrasive discs under tap water to remove the roughness created by the Isomet saw during sectioning. They were then wiped clean with wet cotton swabs to remove any residual debris and were thoroughly rinsed with 10 mL of continuous flowing distilled water forcefully delivered with a plastic syringe and 30-gauge needle. This protocol produced uniformly smooth and clean cross-sectional root surfaces that were suitable for imaging and examination under SEM magnification.
Preparation for SEM examination
The sectioned root specimens were air-dried, dehydrated, desiccated, and sputter-coated with palladium (Desk II Cold Sputter/Etch Unit, Denton Vacuum LLC, Moorestown, NJ). The sputter-coated cross-sectioned root surfaces were imaged under SEM (JSM5510, JEOL USA Inc. Peabody, MA) at 60–90× magnification for an overall view of the entire root canal circumference and at 250× magnification for a site-specific view of an area of resin adhesion on the root canal circumference. Each image was identified with regard to its specimen number, cross-sectional surface region (cervical, middle, and apical), and conditioning group. SEM digital images were then randomly coded and loaded into the VixWin Digital Imaging Software Program (Gendex Dental Systems, Des Plains, IL) for an independent and blind assessment of resin adhesion by four calibrated examiners. Examples of SEM cross-sectional circumferential and site-specific images for each group are shown in Figure 1a-e.
Figure 1.

a-f. Representative cross-sectional images for each group (control, EDTA, and PA). (a) Control group, apical circumferential view at 80× magnification (b) control group, apical site-specific view at 250× magnification (c) EDTA group, apical circumferential view at 80× magnification (d) EDTA group, apical site-specific view at 250× magnification (e) PA group, apical circumferential view at 85× magnification (f) and PA group, apical site-specific view at 250× magnification
Evaluation of resin adhesion
The root canal circumferences for each cross-sectional image at 60-90× magnification were measured in millimeters using 5-mm point-to-point ruler measurement segments of the Vix-Win Digital Imaging Software Program as shown in Figure 2a. In the same manner, the portions of root canal circumferences that showed no resin adhesion were measured as shown in Figure 2b and were subtracted from the root canal circumferences. This gave millimeter measurements of the portions of root canal circumferences that showed resin adhesion. The millimeter measurements were converted into microns based on a micron bar scale for each image, and the percentages of the root canal circumference that showed resin adhesion were calculated. The mean percentages of resin adhesion for the PA, EDTA, and control groups were then determined.
Figure 2.
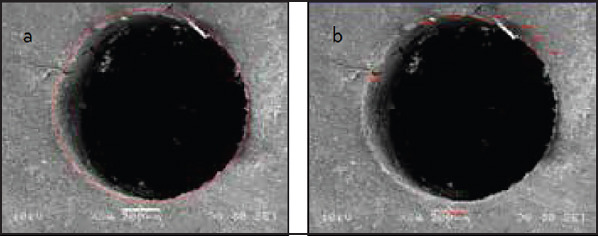
a, b. (a) Measurement of root canal circumference (b) and circumferential portions with no resin adhesion
Statistical analysis of resin adhesion
Statistical analysis was performed by Tukey-Kramer HSD, to test the measurements of resin adhesion observed versus the conditioners used, using JMP v 9.01 (SAS Inc., Carey, NC). The differences among the PA, EDTA, and control groups were statistically significant at P=0.05. The mean percentages of resin adhesion were 97% for the PA group, 94% for the EDTA group, and 76% for the control group (Figure 3). The results by treatment and observer, as illustrated in Figure 4, show that there was an agreement between data in the PA and EDTA groups, which was less for data in the control group, with the range for the observations of resin adhesion clustered at 80% to 100% for the PA group specimens, 70% to 100% for the EDTA group specimens, and scattered at 0% to 100% for the control group specimens 98 through 150 (Figure 4).
Figure 3.

Resin coverage expressed as mean percentages of total circumference
Figure 4.

Resin coverage by specimen, treatment, and observer
RESULTS
The differences among the treatment groups as measured by four observers were statistically significant at P=0.05). The root canals conditioned with 32% PA and 17% EDTA had more resin adhesion than the unconditioned root canals, and the root canals conditioned with 32% PA had more resin adhesion than those conditioned with 17% EDTA.
DISCUSSION
This study assessed and compared the methacrylate resin coverage in prepared root canals conditioned with either 17% EDTA or 32% PA. Dentin conditioning is an essential factor that facilitates the adhesion of a resin to dentin. A conditioning agent that aggressively alters the chemical composition and physical characteristics of dentin would provide a more receptive surface for resin adhesion. Since conditioning dentin surfaces with PA is an established technique for resin bonding in restorative dentistry (23, 24) and since resin-based sealers have gained prominence in root canal obturation (4-11), PA should be considered as a potential root canal conditioner for improving the adhesion of resin sealers to the dentin surfaces of prepared root canals during obturation.
Maxillary incisor and canine teeth were used in this investigation. To compensate for variations in the internal root canal morphology, the root canals of all teeth were prepared to the same final master apical rotary instrument size of 40/06. This canal preparation protocol produced root canal preparations that were very similar in apical canal size and overall canal taper. Therefore, variations in size and shape of root canals were minimized.
This comprehensive investigation examined 158 digital images of cross-sectioned root canals at three representative levels (apical, middle, and cervical) that were instrumented to a standard size of 40/06; conditioned with either 32% PA, 17% EDTA or distilled water as the unconditioned control; and treated with a methacrylate resin. It was hypothesized that the PA group would have higher root canal resin adhesion than the EDTA group. A standard protocol for root canal instrumentation and irrigation was followed before the conditioning agents (PA, EDTA or distilled water as the control) were applied after root canal preparation. Our concept was to compare the effects of PA and EDTA on their ability to enhance the adhesion of a resin after root canal preparation and before root canal filling. Therefore, all samples were instrumented in a standardized manner with rotary instruments and irrigated with 5.25% sodium hypochlorite, which represented contemporary methods for root canal cleaning, debridement, disinfection, and shaping, before the root canals were conditioned for comparison of adhesion after resin application. The results of this study showed that the PA group had statistically significant greater resin adhesion than the EDTA group and that conditioned root canals had statistically significant higher resin adhesion than unconditioned root canals.
The effects of PA on dentin surfaces in terms of its ability to remove the smear layer, expose dentinal tubules, and de-mineralize inter-tubular dentin have been thoroughly investigated (12-16). However, few studies that compared the effects of various endodontic irrigating solutions on root canal dentin, included PA in their analyses (28-31). A partial removal of the smear layer within the root canal was achieved with 10% PA, and complete removal was achieved with 32% PA (28). PA in combination with citric acid removed the smear layer and de-mineralized the surfaces of root canal dentin (29). Acid solutions, such as 15% EDTA, 15% citric acid, and 5% PA, used in combination with 2.5% sodium hypochlorite were effective in eliminating the smear layer and root canal debris (30). The least debris removal occurred with 5% PA, but this was attributed to the low concentration (30). The selection of a higher concentration of PA as the conditioning agent in this present study was based on the finding that 32% PA achieved complete smear layer removal in instrumented root canals and also on the results of studies that investigated the demineralization depth, micro-tensile bond strength, and composite resin adhesion to dentin, which showed that PA concentrations of 37% consistently produced high adhesive bond strengths (28, 32, 33).
As it has been shown that a low concentration of 5% PA effectively decalcified root canal dentin, it was suggested that etching dentin with higher concentrations of PA would cause more calcium ion extraction and hydroxyapatite re-precipitation (31). Although mineral re-precipitation of hydroxyapatite could occur with higher concentrations of PA, it has not been determined if this would have a significant effect on the quality of the adhesive bond.
The duration of agent application is another factor that can effect dentin conditioning. A study on the effects of PA etching on human dentin found that increasing the duration of application produced greater surface roughness independent of acid concentration (34). Investigations on dentin adhesive bonding have used high concentrations of PA-based conditioning agents in root canals for a 15 s duration (11, 35). However, it has been shown that by extending the etching duration of 37% PA to 30 s, a more consistent and predictable adhesive composite resin bond could be achieved (36).
The optimal duration for the application of EDTA has not been clearly established and varies in different studies. A 2-min application of EDTA was used in an investigation on the bonding of a resin endodontic sealer to root canal dentin, and a 3-min application of EDTA was used in an investigation on dentin conditioning for resin bonding (35, 37).
The duration for the application of the conditioning agents used in the present study was determined as follows: first, by taking into consideration what a reasonable application time would be for both conditioning agents to produce their desired effect and second, by keeping the duration of application the same for both conditioning agents to eliminate duration time as a variable.
In terms of the clinical application of using PA as a root canal conditioning agent, two important concerns should be addressed.
The first concern would be the possibility of apical extrusion of PA during root canal irrigation. Root canals are routinely irrigated with solutions that can cause adverse tissue reactions if extruded, particularly with sodium hypochlorite. However, irrigating solutions of any kind, whether acid or alkaline, should be delivered with care and caution to avoid apical extrusion. Every precaution in the delivery of irrigating solutions should be taken with any endodontic irrigating solution. These include using side-vented and loosely fitting syringe needles, slowly irrigating without creating any back pressure, and allowing the solution to freely flow out of the root canal. Clinicians should also follow these same principles when irrigating root canals with PA as a final dentin conditioning agent to ensure safe application.
The second concern would be the removal of adhesive resin root canal fillings if retreatment was indicated. Currently, root canals filled with gutta-percha and resin-type sealers are routinely retreated by removing the root canal filling materials with instruments and solvents. During this process of root canal re-instrumentation, the dentin surfaces would be further mechanically planed and prepared with hand and rotary instruments and irrigated to remove any remaining resin material before refilling. Although we anticipate better adhesion of a resin sealer to dentin conditioned with PA, we believe that the same canal preparation protocol of thorough re-instrumentation and irrigation will adequately remove resin filling materials in retreatment cases.
Based on the findings of this present investigation and other previous investigations that investigated the effects of conditioning agents on resin adhesion in root canals, the application of PA, as a root canal conditioning agent, should be further investigated and considered in endodontics (7, 11, 30, 31, 35).
CONCLUSION
Within the parameters of this study, it can be concluded that the dentin conditioners (EDTA and PA) increased the ability of a resin to adhere to dentin surfaces in prepared root canals and that there was greater resin adhesion in root canals conditioned with PA than in those conditioned with EDTA. Because dentin conditioning with PA was found to enhance resin adhesion in prepared root canals, it could effectively improve the seal in root canals obturated with resin sealers and thereby decrease the potential for micro-leakage. Further studies investigating the ability of PA and various other conditioning agents to produce a root canal dentin surface that favors optimal resin adhesion are indicated.
Acknowledgements:
The authors would like to thank Ms. Nancy Pecora for preparing and scanning the specimens for SEM examination, Dr. Jahanzeb Chaudhry for loading the digital images and Drs. Matthew Hillis, Ashwani Sharma, Valentine Emechete, Daniel Gano and Eric Hall for their valuable participation as research assistants.
Footnotes
Ethical Approval: This study was approved as exempt by the Institutional Review Board of the University Health Science Center where this study was conducted.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept - P.M.D.; Design - P.M.D., J.G.P., V.T.H., W.G.D.; Supervision - P.M.D., J.G.P.; Resource - W.G.D., V.T.H.; Materials - W.G.D., V.T.H.; Data Collection and/or Processing - P.M.D., J.G.P., W.G.D.; Analysis and/or Interpretation - P.M.D., J.G.P., W.G.D.; Literature Review - P.M.D., V.T.H.; Writer - P.M.D., W.G.D.; Critical Review - P.M.D., J.G.P., V.T.H., W.G.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: We gratefully acknowledge that this research was supported by a grant from the University of Tennessee, College of Dentistry Alumni Foundation.
REFERENCES
- 1.Marin-Bauza GA, Rashed-Junior FJ, Souza-Gabriel AE, Sousa-Neto MD, Miranda CE, Silva-Sousa YT. Physiochemical properties of methacrylate resin-based root canal sealers. J Endod. 2010;36(9):1531–6. doi: 10.1016/j.joen.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RS, Fransman R. Adhesive dentistry and endodontics:materials, clinical strategies and procedures for restoration of access cavities:a review. J Endod. 2005;31(3):151–65. doi: 10.1097/01.don.0000155222.49442.a1. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RS. Adhesive dentistry and endodontics:Part 2:bonding in the root canal system-the promise and the problems:a review. J Endod. 2006;32(12):1125–34. doi: 10.1016/j.joen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Doyle MD, Loushine RJ, Agee KA, Gillespie WT, Weller RN, Pashley DH, et al. Improving the performance of EndoRez root canal sealer with a dual-cured two-step self-etch adhesive I. Adhesive strength to dentin. J Endod. 2006;32(8):766–70. doi: 10.1016/j.joen.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Economides N, Kokorikos I, Kolokouris I, Panagiotis B, Gogos C. Comparative study of apical sealing ability of a new resin-based root canal sealer. J Endod. 2004;30(6):403–5. doi: 10.1097/00004770-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie WT, Loushine RJ, Weller RN, Mazzoni A, Doyle MD, Waller JL, et al. Improving the performance of EndoRez root canal sealer with a dualcured two-step self-etch adhesive II. Apical and coronal seal. J Endod. 2006;32(8):771–5. doi: 10.1016/j.joen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Gogos C, Economides N, Stavrianos C, Kolokouris I, Kokorikos I. Adhesion of a new methacrylate resin based sealer to human dentin. J Endod. 2004;30(4):238–40. doi: 10.1097/00004770-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Imai Y, Komabayashi T. Properties of a new injectable type of root canal filling resin with adhesiveness to dentin. J Endod. 2003;29(1):20–3. doi: 10.1097/00004770-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Leonard JE, Gutmann JL, Guo IY. Apical and coronal seal of roots obturated with a dentin bonding agent and resin. Int Endod J. 1996;29(2):76–83. doi: 10.1111/j.1365-2591.1996.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 10.Mannocci F, Ferrari M. Apical seal of roots obturated with laterally condensed gutta-percha, epoxy resin cement and dentin bonding agent. J Endod. 1998;24(1):41–4. doi: 10.1016/S0099-2399(98)80212-4. [DOI] [PubMed] [Google Scholar]
- 11.Perdigão J, Lopes MM, Gomes G. Interfacial adaptation of adhesive materials to root canal dentin. J Endod. 2007;33(3):259–63. doi: 10.1016/j.joen.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 13.Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin bonding mechanism. J Esthet Dent. 1991;3(4):133–8. doi: 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakabayashi N, Ashizawa M, Nakamura M. Identification of a resin-dentin hybrid layer in vital human dentin created in vivo:durable bonding to vital dentin. Quintessence Int. 1992;23(2):135–41. [PubMed] [Google Scholar]
- 15.Tagami J, Tao L, Pashley DH. Correlation among dentin depth, permeability and bond strength of adhesive resins. Dent Mater. 1990;6(1):45–50. doi: 10.1016/0109-5641(90)90044-f. [DOI] [PubMed] [Google Scholar]
- 16.Tao L, Pashley DH. Shear bond strength to dentin:effects of surface treatments, depth and position. Dent Mater. 1988;4(6):371–8. doi: 10.1016/S0109-5641(88)80052-6. [DOI] [PubMed] [Google Scholar]
- 17.Yurdagüven H, Tanalp J, Toydemir B, Mohseni K, Soyman M, Bayirli G. The effect of endodontic irrigants on the microtensile bond strength of dentin adhesives. J Endod. 2009;35(9):1259–63. doi: 10.1016/j.joen.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigant regimens. J Endod. 1987;13(4):147–157. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- 19.Goldman LB, Goldman M, Kronman JH, Lin PS. The efficacy of several irrigation solutions for endodontics:a scanning electron microscopy study. Oral Surg Oral Med Oral Pathol. 1981;52(2):197–204. doi: 10.1016/0030-4220(81)90319-4. [DOI] [PubMed] [Google Scholar]
- 20.Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS. The efficacy of several endodontic irrigating solutions:a scanning electron microscopy study:Part 2. J Endod. 1982;8(11):487–92. doi: 10.1016/s0099-2399(82)80073-3. [DOI] [PubMed] [Google Scholar]
- 21.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions:Part 3. J Endod. 1983;9(4):137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 22.Pradelle-Plasse N, Wenger F, Colon P. Effect of conditioners on dentin permeability using an impedence method. J Dent. 2002;30(5-6):251–7. doi: 10.1016/s0300-5712(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 23.De Munck K, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue:methods and results. J Dent Res. 2005;84(2):118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 24.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts B, Van Meerbeek B. Clinical effectiveness of contemporary adhesives:a systemic review of current clinical trials. Dent Mater. 2005;21(9):864–81. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Murry PE, Smyth TW, About I, Remusat R, Franquin JC, Smith AJ. The effect of etching on bacterial microleakage of an adhesive composite restoration. J Dent. 2002;30(1):29–36. doi: 10.1016/s0300-5712(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83(6):454–8. doi: 10.1177/154405910408300604. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong SR, Vargas MA, Fang Q, Laffoon JE. Microtensile bond strength of a total-etch 3-step, total-etch 2 step, self-etch 2-step and self-etch 1-step dentin bonding system through 15-month water storage. J Adhes Dent. 2003;5(1):47–56. [PubMed] [Google Scholar]
- 28.Garberoglio R, Becce C. Smear layer removal by root canal irrigants. Oral Surg Oral Med Oral Pathol. 1994;78(3):359–67. doi: 10.1016/0030-4220(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 29.Ayad MF. Effects of rotary instrumentation and different etchants on the removal smear layer on human dentin. J Prosthet Dent. 2001;85(1):67–72. doi: 10.1067/mpr.2001.112792. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Heredia M, Ferre-Luque CM, González-Rodriguez MP. The effectiveness of different acid irrigating solutions in root canal cleaning after hand and rotary instrumentation. J Endod. 2006;32(10):993–7. doi: 10.1016/j.joen.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Heredia M, Ferrer-Luque CM, González-Rodriguez MP, Martin-Peinado FJ, González-Lopez S. Decalcifying effect of 15% EDTA, 15% citric acid, 5% phosphoric acid, and 2.5% sodium hypochlorite on root canal dentine. Int Endod J. 2008;41(5):418–23. doi: 10.1111/j.1365-2591.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- 32.Nunes MF, Swift EJ Jr. Perdigão J. Effects of demineralization depth on microtensile bond strength to human dentin. J Adhesive Dent. 2001;3(2):137–43. [PubMed] [Google Scholar]
- 33.O'Keefe KL, Powers JM. Adhesion of resin composite core materials to dentin. Int J Prosthodont. 2001;14(5):451–6. [PubMed] [Google Scholar]
- 34.Mahmoud SH, Ahmed Mahmoud ME, Grawish KM, Mel- A, Zaher AR. Effects of phosphoric acid concentration and etching duration on enamel and dentin tissues of uremic patients receiving hemodylasis:an AFM Study. J Adhes Dent. 2012;14(3):215–21. doi: 10.3290/j.jad.a22421. [DOI] [PubMed] [Google Scholar]
- 35.Gorgos C, Stavrinos C, Kolokouris I, Papadoyannis I, Economides N. Shear bond strength of AH-26 root canal sealer to dentin using three dentin bonding agents. J Dent. 2003;31(5):321–6. doi: 10.1016/s0300-5712(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 36.Lopes GC, Baratieri CM, Baratieri LN, Monteiro S Jr, Cardosa Vieira LC. Bonding of cervical sclerotic dentin:effects of acid etching time. J Adhes Dent. 2004;6(1):19–23. [PubMed] [Google Scholar]
- 37.Blomlöf J, Cederlund A, Jonsson B, Ohlson NG. Acid conditioning combined with single-component and two-component dentin bonding agents. Quintessence Int. 2001;32(9):711–5. [PubMed] [Google Scholar]


