Abstract
Objective:
This preliminary study aimed to evaluate the antimicrobial activity, flow and radiopacity of endodontic sealers with nanostructured silver vanadate decorated with silver nanoparticles (AgVO3).
Methods:
The minimum inhibitory concentration (MIC) of AgVO3 was evaluated against Enterococcus faecalis, Pseudomonas aeruginosa and Escherichia coli. Specimens were prepared from the following endodontic sealers: AH Plus (DENTSPLY DeTrey GmbH, Konstanz, Germany), Sealapex (Sybron Endo, Orange, CA, USA), Sealer 26 (DENTSPLY, Petrópolis, Brazil) and Endofill (DENTSPLY, Petrópolis, Brazil), with concentrations of 0%, 2.5%, 5% and 10% of AgVO3. Agar diffusion was used to evaluate the materials after 48 hours and 7 days (n=6). Flow (n=6) and radiopacity (n=9) were evaluated. The data were analysed by analysis of variance (ANOVA) and the Tukey honestly significant difference (HSD) (α=0.05).
Results:
The MIC of AgVO3 was 500 μg/mL for E. faecalis and 31.25 μg/mL for P. aeruginosa and E. coli. The AgVO3 did not influence the antimicrobial activity of AH Plus against E. faecalis (P>0.05) but did promote this activity for Sealapex (P<0.01). Moreover, this activity increased for Endofill from 2.5% and for Sealer 26 from 5% (P<0.05). Against P. aeruginosa, only AH Plus and Endofill 10% inhibited zone formation (P<0.01). The antimicrobial activity of Endofill increased from 2.5% against E. coli (P<0.01). Sealapex 5% and 10% (P<0.01), Sealer 26 10% and AH Plus promoted antimicrobial activity against E. coli. An increase in the zone of inhibition occurred between 48 hours and 7 days in the Sealapex 10% and Endofill 5% groups against E. coli. The flow of AH Plus and Endofill decreased with the increase of AgVO3 (P<0.05), and the flow of Sealer 26 and Sealapex was not affected (P>0.05). The radiopacity of AH Plus increased with AgVO3 (P<0.05). Endofill 5% and 10% did not differ from the control Endofill (P>0.05). The incorporation of AgVO3 did not influence the radiopacity of Sealer 26 (P>0.05). The incorporation of 2.5% and 5% AgVO3 reduced the radiopacity of Sealapex (P<0.05).
Conclusion:
Adding AgVO3 may increase the antimicrobial effect of endodontic sealers without major changes in their physicochemical properties.
Keywords: Antimicrobial agents, endodontics, nanotechnology, radiography dental digital
HIGHLIGHTS.
AgVO3 promoted antimicrobial activity against E. faecalis, P. aeruginosa and E. coli.
Antibacterial activity against E. faecalis and E. coli was verified for Sealapex with AgVO3.
Antibacterial activity against P. aeruginosa was verified for AH Plus and Endofill with 10% of AgVO3.
AgVO3 not affect the flow of Sealer 26 and Sealapex and increased the radiopacity of AH Plus.
INTRODUCTION
The success of endodontic treatment depends on controlling the root canal microbiota with appropriate chemical-mechanical preparation, canal obturation and coronal sealing (1-4). This control is difficult because of the complexity of the root canal anatomy (5).
Virulence factors determine bacterial resistance and the persistence of infection after chemical-mechanical preparation (4, 6). Gram-negative anaerobic bacteria and Gram-positive bacteria such as Enterococcus faecalis are prevalent in endodontically treated teeth with periapical lesions (1, 6).
Endodontic sealers are used to seal the irregularities of the root canal, and adding antimicrobial agents to these filling materials can help reduce the number of microorganisms and eradicate the infection (4, 7). However, not all sealers have this property, making the development of new materials with antimicrobial activity promising (1-4).
The antimicrobial properties of silver nanoparticles (AgNPs) are known (8-10). Bactericidal action takes place through their interaction with the thiol groups of enzymes in the metabolism of the bacterial cell, causing cell death; however, AgNPs are thermodynamically unstable and tend to agglomerate (9-11). The development of nanostructured silver vanadate decorated with AgNPs (AgVO3) can prevent agglomeration. Dental acrylic resins incorporating this nanomaterial have demonstrated antimicrobial activity against the microorganisms P. aeruginosa, C. albicans, S. mutans and S. aureus (12).
The physical properties of materials are keys to good performance. Changes in the composition of endodontic sealers with the nanomaterial must not compromise these properties, for example, the flow that ensures the filling of root canals and the radiopacity that differentiates the surrounding structures such as bone and dentin and the analysis of the extent and quality of the filling (13-16).
Thus, this preliminary study aimed to evaluate the antimicrobial activity, flow and radiopacity of endodontic sealers with nanostructured silver vanadate decorated with silver nanoparticles. The null hypothesis was that the addition of AgVO3 would not promote antimicrobial activity against E. faecalis, P. aeruginosa and E. coli or change the flow or radiopacity of the sealers.
MATERIALS AND METHODS
Synthesis of nanostructure silver vanadate decorated with agnps
Nanostructured silver vanadate decorated with silver nanoparticles (AgVO3) was synthesized through a precipitation reaction between silver nitrate (AgNO3, 99.8%; Merck KGaA, Darmstadt, Germany) and ammonium metavanadate (NH4VO3, 99%; Merck KGaA, Darmstadt, Germany) according to the methodology described by Holtz et al. (8, 9). Initially, 1.3569 g of AgNO3 and 0.9736 g of NH4VO3 were each dissolved in 200 mL of distilled water. The solutions were stirred separately on a 65°C heated surface for 10 minutes. Next, the silver nitrate solution was added in drops from a burette into the ammonium metavanadate solution under constant stirring at 65°C. The precipitate obtained was washed with distilled water and absolute ethanol several times, filtered, and then dried on a vacuum line for 10 hours. The morphology of the material obtained was analysed by scanning transmission electron microscopy (STEM) (Magellan 400L; FEI Company, Hillsboro, OR, USA) to confirm the presence of AgNPs on the surface of the crystals formed.
Minimum inhibitory concentration
The amounts of Minimum Inhibitory Concentration (MIC) of AgVO3 were determined for the microorganisms E. faecalis (ATCC 29212), P. aeruginosa (ATCC 27853) and E. coli (ATCC 25922) (American Type Culture Collection, Manassas, VA, USA), using the method of successive dilutions described by the Clinical and Laboratory Standards Institute (CLSI), in 96-well cell culture plates (TPP, Trasadingen, Unterklettgau, Switzerland) and performed in duplicate (17). The plates were prepared with the culture media specific to each microorganism, supplemented with decreasing concentrations of AgVO3. Each well was inoculated with the microorganism to be tested.
For each microorganism, 10 concentrations of the antimicrobial agent and two control groups were obtained, one positive with microorganism and one negative with AgVO3. The plates were incubated at 37°C for 24 hours in a microbiological oven. Microbial growth was measured by assessing the turbidity of the culture visible to the naked eye.
Sample preparation
Four endodontic sealers, Sealer 26 (DENTSPLY, Petrópolis, Brazil), Sealapex (Sybron Endo, Orange, CA, USA), Endofill (DENTSPLY, Petrópolis, Brazil) and AH Plus (DENTSPLY DeTrey GmbH, Konstanz, Germany), were modified by adding AgVO3 in three concentrations: 2.5%, 5% and 10% and the control group (without the antimicrobial agent). For the modified specimens, the AgVO3 powder was added proportionally to the powder or base paste of the endodontic sealer (m/m). Next, the mixture was incorporated into the liquid or catalyst paste following the manufacturer’s instructions; this was also done for the control group.
A polyvinyl chloride (PVC) tube of 25 mm diameter and 4 mm height was filled with condensation silicone (Zetalabor; Zhermack SpA, Badia Polesine, Italy). The PVC tube was inserted with 4 metal matrices of 4 mm diameter and 3 mm height. After the silicone had set, the matrices were removed forming cavities into which the endodontic sealer was inserted. The specimens remained in the oven at 37°C for 7 and 14 days (Sealapex), until completely set. The specimens were then polished with Sof-Lex sanding discs (3M ESPE, Saint Paul, MN, USA) in two particle sizes: fine and super fine.
Microbiological analysis
The agar diffusion method was used to determine the inhibitory effect against the species E. faecalis (ATCC 29212), P. aeruginosa (ATCC 27853) and E. coli (ATCC 25922).
The microorganisms were obtained from fresh cultures and transferred to test tubes with phosphate-buffered saline (PBS) solution. The inoculum was standardized by measuring optical density using a spectrophotometer with an absorbance reading of between 0.08 and 0.10 at a wavelength of 625 nm (108 CFU/mL of bacteria).
The media cultures Mueller-Hinton agar and tryptone soya agar were prepared and sterilized according to the manufacturer’s instructions and dispensed into sterile Petri plates of 90 mm2 to obtain an 8-mL base layer. Six specimens of each group, sterilized with ethylene oxide, were placed over the solidified layer base in their respective plates using sterile tongs, and a 12-mL aliquot of the inoculum incorporated into the culture medium was distributed onto the plates to cover the specimens.
The Petri dishes were incubated at 37°C for 48 hours in a microbiological stove. After this period, the zones of inhibition formed around the specimens were measured using a dry tip compass and a millimetre ruler. The samples were transferred to the propagation growth media, incubated under the same conditions and remeasured after 7 days. For each specimen, the zone of inhibition was determined in millimetres in three distinct regions.
Flow
The flow was evaluated in six samples within each group. Each sample was placed between two glass plates, the lower weighing 300 g and the higher weighing 560 g, for 1 minute. Next, the upper plate was removed, and the diameter of the disks formed by the sample was measured three times using a dry tip compass and a millimetre ruler.
Radiopacity
The radiopacity of nine specimens from each group was evaluated, and five radiographic images were obtained with an image digital X-ray machine (Dabi Atlante Indústrias Médico Odontológicas Ltda., Ribeirão Preto, Brazil) with a VistaScan digital sensor (Dΰrr Dental AG, Bietigheim-Bissingen, Germany) composed of a phosphor plate and 16-bit system. The specimens were placed on the sensor and an aluminium scale range (2-16 mm in increments of 2 mm) (18, 19). The X-ray unit was operated at 70 kV and 10 A with a 0.1-second exposure time at a distance of 30 cm from the focal point.
Using ImageJ software (NIH-Scion Corporation, Bethesda, MD, USA), the average grayscale value of regions of interest (ROI) of 30´30 pixels (±1) was measured on images of the specimens and aluminium scale. This procedure was repeated 5 times for each sample and for each degree of the aluminium scale. The average grayscale values measured on the specimens were converted into aluminium millimetres (mmAl) (18, 19).
Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Statistical analysis
For the statistical analysis, the parametric analysis of variance (ANOVA) test was performed, followed by the multiple comparison test of the Tukey honestly significant difference (HSD) test with a significance level of 5% (α=0.05). The statistical analysis was performed using the Statistical Package for Social Sciences software v17.1 (SPSS Inc.; Chicago, IL, USA).
In this study there was no participation, direct or indirect, of humans or animals so it Ethics Committee Approval and Informed Consent were not necessary.
RESULTS
The AgVO3 consisted of nanowires with an average diameter of 150 nm and micrometre-order length. The wires were coated with 25-nm semispherical metallic silver particles (Ag) (Figure 1).
Figure 1.
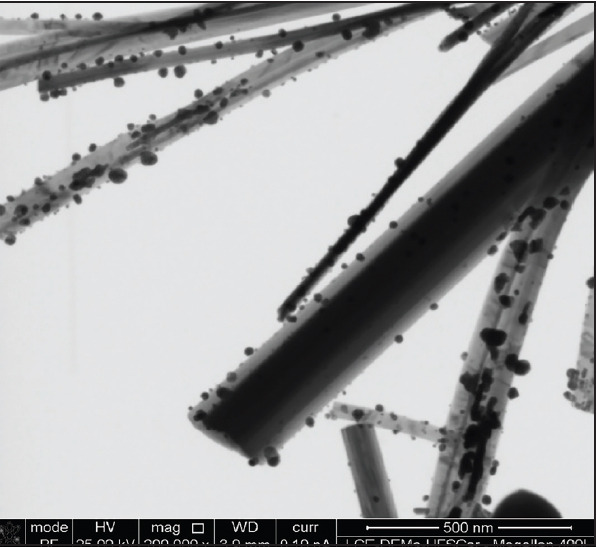
Scanning transmission electron microscopy image of AgVO3 treated with AgNPs (×200.000 magnification)
MIC
Table 1 shows the MIC results of the AgVO3. The MIC value compared with the strains of P. aeruginosa and E. coli was 31.25 μg/mL, and for E. faecalis, it was 500 μg/mL. These values
TABLE 1.
Microorganisms used in the microbiological experiments and Minimum Inhibitory Concentration (MIC) of AgVO3*
| Microorganisms | Source | Features | MIC |
|---|---|---|---|
| Enterococcus faecalis | ATCC1 29212 | Gram-positive coco, facultative aerobic | 500 μg/mL |
| Pseudomonas aeruginosa | ATCC 27853 | Gram-negative rod, aerobic | 31.25 μg/mL |
| Escherichia coli | ATCC 25922 | Gram-negative rod, facultative anaerobic | 31.25 μg/mL |
AgVO3: nanostructured silver vanadate decorated with silver nanoparticles 1 American Type Culture Collection
demonstrate the antimicrobial efficacy of the silver vanadate against Gram-positive and Gram-negative species.
Microbiological analysis
Table 2 shows the average diameter of the inhibition zones (in mm) of the endodontic sealers incorporated with different concentrations of AgVO3 against E. faecalis, P. aeruginosa and E. coli and the standard deviation and differences among groups.
TABLE 2.
Mean and standard deviation (SD) of the zone of inhibition formed against E. faecalis, P. aeruginosa and E. coli (mm)
| Microorganisms | Time | [AgVO3* ] | AH Plus | Endofill | Sealer 26 | Sealapex |
|---|---|---|---|---|---|---|
| Enterococcus faecalis | 48 h | 0% | 7.83±1.38a | 7.22±0.62a | 7.52±0.40a | 0±0a |
| 7 days | 7.61±1.43a | 7.33±0.80a | 7.38±0.40a | 0±0a | ||
| 48 h | 2.5% | 8.05±0.88a | 13.33±0.83b | 7.25±0.69a | 12.44±1.03b | |
| 7 days | 7.88±0.88a | 13.16±0.81b | 7.05±0.46a | 12.63±0.98b | ||
| 48 h | 5% | 7.52±0.73a | 17.25±0.90c | 9.25±0.41b | 15.86±0.98c | |
| 7 days | 7.19±0.71a | 16.94±0.82c | 9.47±0.41b | 16.22±1.11c | ||
| 48 h | 10% | 8.69±1.00a | 15.55±0.43d | 12.02±0.67c | 18.52±1.00d | |
| 7 days | 7.86±1.01a | 15.63±0.35d | 12.08±0.62c | 18.38±1.00d | ||
| Pseudomonas aeruginosa | 48 h | 0% | 0±0a | 0±0a | 0±0a | 0±0a |
| 7 days | 0±0a | 0±0a | 0±0a | 0±0a | ||
| 48 h | 2.5% | 0±0a | 0±0a | 0±0a | 0±0a | |
| 7 days | 0±0a | 0±0a | 0±0a | 0±0a | ||
| 48 h | 5% | 0±0a | 0±0a | 0±0a | 0±0a | |
| 7 days | 0±0a | 0±0a | 0±0a | 0±0a | ||
| 48 h | 10% | 5.83±0.52b | 9.66±0.35b | 0±0a | 0±0a | |
| 7 days | 6.30±0.24b | 9.72±0.47b | 0±0a | 0±0a | ||
| Escherichia coli | 48 h | 0% | 0±0a | 13.58±0.60a | 0±0a | 0±0a |
| 7 days | 0±0a | 13.86±0.67a | 0±0a | 0±0a | ||
| 48 h | 2.5% | 0±0a | 14.94±0.25b | 0±0a | 0±0a | |
| 7 days | 0±0a | 14.80±0.30b | 0±0a | 0±0a | ||
| 48 h | 5% | 0±0a | 15.52±0.41b | 0±0a | 7.30±0.54b | |
| 7 days | 0±0a | 15.66±0.33c | 0±0a | 7.13±0.49b | ||
| 48 h | 10% | 5.77±0.49b | 17.58±0.73d | 6.00±0b | 7.62±0.15b | |
| 7 days | 5.80±0.43b | 17.66±0.59d | 6.00±0b | 8.16±0.49c |
Same letters represent statistical similarity in the same column. The statistical correlation between different microorganisms has not been evaluated
AgVO3: nanostructured silver vanadate decorated with silver nanoparticles
The incorporation of AgVO3 did not increase the antimicrobial activity of AH Plus against E. faecalis; the zone of inhibition formed by groups modified with AgVO3 showed results similar to those of the control (P>0.05). The antimicrobial activity of Endofill increased with 2.5% AgVO3 (P<0.05). The largest zone of inhibition was observed with the addition of 5% AgVO3 (P<0.05). An increase in the zone of inhibition of Sealer 26 occurred with 5% AgVO3 (P<0.05). The incorporation of AgVO3 promoted antimicrobial activity in Sealapex, increasing the zone of inhibition in proportion to the concentration of AgVO3 (P<0.01).
Against P. aeruginosa, only AH Plus and Endofill with 10% AgVO3 inhibited zone formation (P<0.01). Endofill showed antimicrobial activity against E. coli, and this activity increased proportionally with the incorporation of 2.5%, 5% and 10% AgVO3 (P<0.01). The concentrations of 5% and 10% AgVO3 added to Sealapex (P<0.01) and the 10% added to Sealer 26 and AH Plus promoted antimicrobial activity for these sealers against E. coli. Regarding the time factor, an increase occurred in the zone of inhibition against E. coli between 48 hours and 7 days in group Sealapex with 10% AgVO3 and group Endofill with 5% AgVO3.
Flow and radiopacity
The flow of AH Plus and Endofill reduced in proportion to the concentration of AgVO3 (P<0.05). The incorporation of AgVO3 did not influence the flow of Sealer 26 or Sealapex, independent of the concentration (P>0.05) (Table 3).
TABLE 3.
Mean and standard deviation (SD) of the flow and radiopacity of the sealers incorporated with different concentrations of AgVO3* (mm) (mmAl equivalent)
| Test | [AgVO3*] | AH Plus | Endofill | Sealer 26 | Sealapex |
|---|---|---|---|---|---|
| Flow | 0% | 36.2±0.13a | 45.2±0.39a | 43.6±0.63a | 31.1±0.11a |
| 2.5% | 30.2±0.15ab | 38.1±0.15b | 40.0±0.26a | 27.1±0.28a | |
| 5% | 24.6±0.18b | 37.5±0.66b | 45.3±0.10a | 27.4±0.07a | |
| 10% | 18.2±0.08c | 35.6±0.21b | 42.4±0.34a | 25.1±0.05a | |
| Radiopacity | 0% | 4.73±0.22a | 5.00±0.12a | 3.91±0.19a | 4.63±0.10a |
| 2.5% | 4.90±0.05b | 4.52±0.10b | 4.20±0.07ab | 4.46±0.11b | |
| (+3.6%) | (-9.6%) | (+7.4%) | (-3.7%) | ||
| 5% | 4.88±0.07ab | 5.07±0.14a | 3.83±0.41ac | 4.41±0.13b | |
| (+3.2%) | (+1.4%) | (-2%) | (-4.8%) | ||
| 10% | 4.93±0.08b | 5.01±0.10a | 4.26±0.29ab | 4.64±0.11a | |
| (+4.2%) | (+0.2%) | (+8.9%) | (+0.2%) |
Same letters represent statistical similarity in the same column. The statistical correlation between tests has not been evaluated. Numbers in parentheses represent variation in average radiopacity values in comparison with respective control groups
AgVO3: nanostructured silver vanadate decorated with silver nanoparticles
The radiopacity values obtained for each group are displayed in Table 3. Average values ranged between 3.83 and 5.07 mmAl. In general, control groups without AgVO3 had the lowest radiopacity values. However, Endofill 2.5% and Sealapex 2.5% and 5% had lower radiopacity than their controls. The variation in the radiopacity values of the specimens with the addition of AgVO3 ranged between +8.9% and -9.6%.
DISCUSSION
Endodontic sealers can have an antimicrobial effect depending on their chemical composition (1). The main components of Endofill sealer are zinc oxide and eugenol, which are bactericidal (20). Epoxy resin-based AH Plus can release formaldehyde or monomer residuals in the polymer materials (1). Its antimicrobial effect may be related to the release of bisphenol. A diglycidyl ether during the polymerization process (21). The antimicrobial activity of calcium hydroxide-based material, such as found in Sealer 26 and Sealapex, depends on ionization, which releases hydroxide ions and increases pH (22). A pH greater than 9 can inactivate enzymes in the cell membrane of the microorganism, which results in a loss of biological activity of the cytoplasmic membrane or destruction of phospholipids and unsaturated fatty acids (22, 23).
In this preliminary study aimed at incorporating AgVO3 in endodontic sealers, the antimicrobial activity was evaluated by the agar diffusion method, once this test is used to evaluate the antimicrobial activity of filling materials in vitro (1, 22). Relevant factors of standardization related to the agar medium, incubation, the contact between material and agar and the solubility of sealers must be considered (22). In this study, the inoculum was standardized with a spectrophotometer (108 CFU/mL of bacteria). In addition, the agar medium completely enveloped the specimens, ensuring contact between the microorganism and sealer. The incubation temperature was always 37°C. The standardization of these factors allowed us to exclude some variables existing in vivo (22). The results are an indication of the antimicrobial properties of the sealer.
However, the size of the inhibition zones does not indicate the absolute antimicrobial effect because the size of these zones may be affected by the solubility of the sealers (1).
Sealapex did not completely set, even with an additional 14 days in the oven. So, some specimens dissolved during the microbiological test, preventing the measurement of the inhibition zone. These specimens were considered ‘Missing’ for the statistical analysis.
Studies have shown an increase in the antimicrobial activity of endodontic sealers mixed with antimicrobial agents. The study of Gjorgievska et al. (1) evaluated the antimicrobial activity of five endodontic sealers (RoekoSeal, Endomethasone N, N2, Apexit Plus, AH Plus) incorporated with 2% benzalkonium chloride and 2% cetylpyridinium chloride against Streptococcus mutans, Lactobacillus casei and Actinomyces viscosus using the agar diffusion method. Adding these substances enhanced the antimicrobial effects of the sealers. Baer and Maki (4) added amoxicillin to three endodontic sealers (Pulp Canal Sealer EWT, AH Plus and RealSeal SE) to evaluate the antimicrobial effect against E. faecalis. The authors found that sealers mixed with amoxicillin inhibited the growth of E. faecalis more than sealers without amoxicillin. Hiraishi et al. (24) evaluated the antimicrobial effect of experimental chlorhexidine (containing polymethyl methacrylate-PMMA) mixed with Super- Bond Sealer (methacrylate-based sealer) against E. faecalis, with chlorhexidine concentrations of 1%, 2% and 3%. The authors concluded that incorporating 2% and 3% chlorhexidine in the PMMA-based sealer had a long-lasing antimicrobial effect against E. faecalis.
E. faecalis is virulent in chronic periapical lesions because it is resistant to antimicrobial agents such as calcium hydroxide (25). Root canals infected with these bacteria usually remain asymptomatic, with advanced periapical lesions, because they do not respond to conventional endodontic therapy (26). In this study, Endofill, Sealer 26 and Sealapex showed an increase in antimicrobial activity with the addition of AgVO3 against E. faecalis, and AH Plus showed activity that is inherent to the control group, thus these sealers may act as potential agents for the eradication of this microorganism. The Sealer 26 and Sealapex sealers are based on calcium hydroxide, and despite the resistance of the bacteria to the material, their antimicrobial action associated with AgVO3 was effective.
The Endofill sealer with the addition of 5% AgVO3 presented significantly more antimicrobial activity against E. faecalis than Endofill with 10% AgVO3. Based on the observation of the dispersion of different concentrations of AgVO3, during the manipulation of sealers and through the analysis of the image of the surface of the specimens in Confocal Laser Microscope LEXT OLS4000® (Olympus, Tokyo, Japan), the results suggest that more agglomerations between particles of AgVO3 may occur depending on the concentration (Figure 2). The contact surface between the particulates of AgVO3 and the sealer is reduced, thus reducing the antimicrobial activity with the highest concentration.
Figure 2.
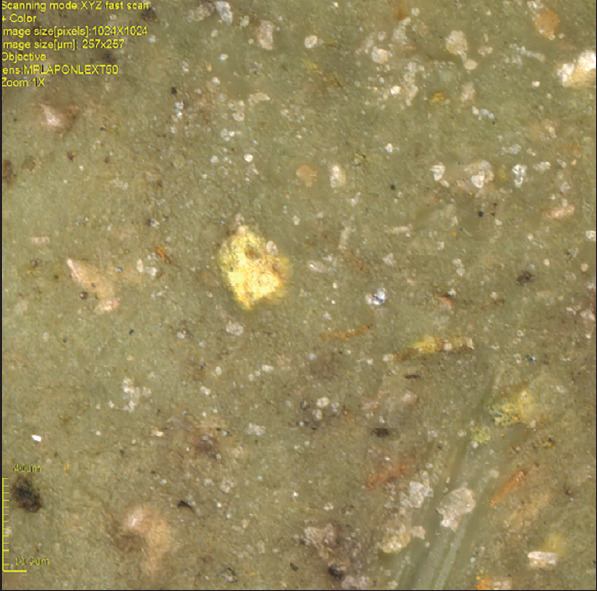
Confocal laser microscopic image of the surface of the Endofill incorporated with 10% of AgVO3 (×1024 magnification)
P. aeruginosa is also found in persistent periapical infections and is resistant to conventional endodontic treatments such as sodium hypochlorite-based irrigation solution at 5.25%, high concentrations of salts, colorants, antiseptics, including chlorhexidine digluconate and some antibiotics. Its growth is not usually inhibited by the endondontic sealers commonly used (27). In this study, AH Plus and Endofill exhibited antimicrobial activity against P. aeruginosa at a concentration of 10% AgVO3 despite its high resistance.
Endodontic infections are polymicrobial, and the canal is susceptible to secondary infections if it is accessed repeatedly because of pain or inadequate treatment (28). Facultative anaerobic microorganisms such as E. coli are found in between 10% and 20% of infected canals. They interact with strict anaerobic microorganisms and alter the nutritional relationships and oxygen tension determining the survival of these microorganisms (22). Thus, the antimicrobial activity of the tested sealers was also evaluated against E. coli, and the addition of AgVO3 at a concentration of 10% for AH Plus, Sealer 26 and Sealapex demonstrated antimicrobial activity against these bacteria, as did the addition of the 5% concentration to Sealapex. Endofill increased its antimicrobial activity against E. coli with the incorporation of AgVO3.
The sealers showing no inherent antimicrobial capacity with the addition of AgVO3 exhibited this activity against the tested microorganism. For example, Sealapex showed an increased inhibitory effect against E. faecalis with increasing concentrations of the nanomaterial. AH Plus and Endofill exhibited antimicrobial activity at a concentration of 10% AgVO3 against P. aeruginosa; AH Plus and Sealer 26 exhibited antimicrobial activity at a concentration of 10% AgVO3 against E. coli; and Sealapex exhibited this property for the concentrations of 5% and 10%.
The results showed that adding AgVO3 improved the antimicrobial properties of the endodontic sealers tested. Additionally, the MIC values of this study showed that this nanomaterial may be used as an antimicrobial agent and could inhibit the growth of the tested microorganisms.
The flow of the endodontic sealers is adequate to fill the accessory canals and voids between the gutta-percha cones. Although the flow allows the filling of irregularities, when increased, it can result in apical extrusion and injury to the periapical tissues (7).
The Sealer 26 and Sealapex sealers exhibited adequate flow values within the standards set by specification number 57 of the American National Standard/American Dental Association (ASNI/ADA) (2000) and International Organization for Standardization (ISO) 6876/2001, which state that the discs must measure at least 20 mm in diameter (7, 14, 15). The addition of different concentrations of AgVO3 did not influence the flow of these sealers.
The Endofill sealer also exhibits adequate values within the standard set by the ASNI/ADA (2000) specification, despite the flow reduction. AH Plus also exhibited decreased flow depending on the concentration of the nanomaterial; concentrations of 10% did not meet the ASNI/ADA (2000) specification.
Dental diagnosis relies largely on radiology to identify and distinguish intraoral material from adjacent anatomic structures; therefore endodontic sealers should be radiopaque (29). The ANSI/ADA (2000) specification for 57 states that the minimum radiopacity for endodontic sealers must be equivalent to 3 mmAl and that the sealers should be more radiopaque than bone or dentine (19). In this study, all the endodontic sealers, with different concentrations of AgVO3, showed radiopacity values higher than those recommended by ANSI/ADA (2000).
In general, the addition of AgVO3 improved or maintained the radiopacity of the endodontic sealers compared with controls without AgVO3, except for Endofill 2.5% and Sealapex 2.5% and 5%. In such situations, although lower values of radiopacity were observed than their respective controls, the differences ranged between 2% and 9.6%. Such small differences are unlikely to have a clinical effect, especially considering that all groups presented radiopacity values well above the minimum recommended. One possible explanation for these differences may be related to the uneven dispersion and agglomeration of the material. The small areas evaluated in the specimen (30×30 pixels) may have selected regions with lower grayscale density in some specimens.
The results of this study indicate the promise of using AgVO3 as an antibacterial additive for endodontic sealer, which may benefit patients with a need for endodontic treatment by reducing the need for retreatment. However, for clinical application, the biocompatibility of these modified materials needs to be evaluated. Artal et al. (30) has demonstrated that silver vanadate nanowires decorated with silver nanoparticles can be toxic to Daphinia similis as a function of silver (Ag) release.
CONCLUSION
Adding AgVO3 may increase the antimicrobial effect of en0dodontic sealers without major changes in their physicochemical properties.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Peer-review: Externally peer-reviewed.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – A.C.R.; Design – A.C.R., A.B.V.T.; Supervision – A.C.R.; Funding - A.C.R., A.B.V.T., M.A.S. C.L.V., C.O.S.; Materials - A.B.V.T., C.L.V., D.T.C., M.A.S..; Data collection &/or processing – A.B.V.T., C.L.V., C.O.S.; Analysis and/or interpretation – A.B.V.T., D.T.C., C.O.S.; Literature search – A.B.V.T., D.T.C.; Writing – A.B.V.T., C.L.V.; Critical Review – C.O.S., D.T.C., M.A.S., A.C.R.
REFERENCES
- 1.Gjorgievska E, Apostolska S, Dimkov A, Nicholson JW, Kaftandzieva A. Incorporation of antimicrobial agents can be used to enhance the antibacterial effect of endodontic sealers. Dent Mater. 2013;29(3):29–34. doi: 10.1016/j.dental.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Slutzky-Goldberg I, Slutzky H, Solomonov M, Moshonov J, Weiss EI, Matalon S. Antibacterial properties of four endodontic sealers. J Endod. 2008;34(6):735–8. doi: 10.1016/j.joen.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35(7):1051–5. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Baer J, Maki JS. In Vitro evaluation of the antimicrobial effect of three endodontic sealers mixed with amoxicillin. J Endod. 2010;36(7):1170–3. doi: 10.1016/j.joen.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 5.de Pablo OV, Estevez R, Péix Sánchez M, Heilborn C, Cohenca N. Root anatomy and canal configuration of the permanent mandibular first molar:a systematic review. J Endod. 2010;36(12):1919–31. doi: 10.1016/j.joen.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 6.Chavez de Paz LE. Redefining the persistent infection in root canals:possible role of biofilm communities. J Endod. 2007;33(6):652–62. doi: 10.1016/j.joen.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Arias-Moliz MT, Ruiz-Linares M, Cassar G, Ferrer-Luque CM, Baca P, Ordinola-Zapata R, et al. The effect of benzalkonium chloride additions to AH Plus sealer. Antimicrobial, physical and chemical properties. J Dent. 2015;43(7):846–54. doi: 10.1016/j.jdent.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Holtz RD, Souza-Filho AG, Brocchi M, Martins D, Duran N, Alves OL. Development of nanostructured silver vanadates decorated with silver nanoparticles as a novel antibacterial agent. Nanotechnology. 2010;21(18):185102. doi: 10.1088/0957-4484/21/18/185102. [DOI] [PubMed] [Google Scholar]
- 9.Holtz RD, Lima BA, Souza-Filho AG, Brocchi M, Alves OL. Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomedicine. 2012;8(6):935–40. doi: 10.1016/j.nano.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Marambio-Jones C, Hoek EM. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 2010;12(5):1531–51. [Google Scholar]
- 11.Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89(11):1175–86. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 12.Castro DT, Holtz RD, Alves OL, Watanabe E, Valente ML, Silva CH, et al. Development of a novel resin with antimicrobial properties for dental application. J Appl Oral Sci. 2014;22(5):442–9. doi: 10.1590/1678-775720130539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardes RA, de Amorim Campelo A, Junior DS, Pereira LO, Duarte MA, Moraes IG, et al. Evaluation of the flow rate of 3 endodontic sealers:Sealer 26, AH Plus, and MTA Obtura. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(1):47–9. doi: 10.1016/j.tripleo.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M. Physical properties of 5 root canal sealers. J Endod. 2013;39(10):1281–6. doi: 10.1016/j.joen.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Duarte MA, Ordinola-Zapata R, Bernardes RA, Bramante CM, Bernardineli N, Garcia RB, et al. Influence of calcium hydroxide association on the physical properties of AH Plus. J Endod. 2010;36(6):1048–51. doi: 10.1016/j.joen.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Bahar J, Alsalim S, Dayem RN. A novel Nano-calcium Carbonate-polyurethane- based root canal obturation material:synthesis and evaluation of some physical properties. Int Arab J Dent. 2013;4(3):98–102. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 6th ed. Wayne: CLSI; 2003. [Google Scholar]
- 18.Guerreiro-Tanomaru JM, Duarte MA, Gonçalves M, Tanomaru-Filho M. Radiopacity evaluation of root canal sealers containing calcium hydroxide and MTA. Braz Oral Res. 2009;23(2):119–23. doi: 10.1590/s1806-83242009000200005. [DOI] [PubMed] [Google Scholar]
- 19.Tanomaru JM, Cezare L, Gonçalves M, Tanomaru Filho M. Evaluation of the radiopacity of root canal sealers by digitization of radiographic images. J Appl Oral Sci. 2004;12(4):355–7. doi: 10.1590/s1678-77572004000400019. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz K, Moynihan M, Liu M, Kim S. Biologic properties of eugenol and zinc oxide-eugenol. Oral Surg Oral Med Oral Pathol. 1992;73(6):729–37. doi: 10.1016/0030-4220(92)90020-q. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Shen Y, Haapasalo M. Dentin extends the antibacterial effect of endodontic sealers against Enterococcus faecalis biofilms. J Endod. 2014;40(4):505–8. doi: 10.1016/j.joen.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 22.Leonardo MR, da Silva LA, Tanomaru-Filho M, Bonifácio KC, Ito IY. In vitro evaluation of antimicrobial activity of sealers and pastes used in endodontics. J Endod. 2000;26(7):391–4. doi: 10.1097/00004770-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Estrela C, Sydney GB, Bammann LL, Felippe Junior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995;6(2):85–90. [PubMed] [Google Scholar]
- 24.Hiraishi N, Yiu CK, King NM, Tay FR. Antibacterial effect of experimental chlorhexidine-releasing polymethyl methacrylate-based root canal sealers. J Endod. 2009;35(9):1255–8. doi: 10.1016/j.joen.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Portenier I, Haapasalo H, Orstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type-I collagen, and heat-killed microbial whole cells. J Endod. 2002;28(9):634–7. doi: 10.1097/00004770-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Reynaudaf Geijersstam A, Sorsa T, Stackelberg S, Tervahartiala T, Haapasalo M. Effect of Efaecalis on the release of serine proteases elastase and cathepsin G, and collagenase-2 (MMP-8) by human polymorphonuclear leukocytes (PMNs) Int Endod J. 2005;38(9):667–77. doi: 10.1111/j.1365-2591.2005.01011.x. [DOI] [PubMed] [Google Scholar]
- 27.Phee A, Bondy-Denomy J, Kishen A, Basrani B, Azarpazhooh A, Maxwell K. Efficacy of bacteriophage treatment on Pseudomonas aeruginosa biofilms. J Endod. 2013;39(3):364–9. doi: 10.1016/j.joen.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Tronstad L. Recent development in endodontic research. Scand J Dent Res. 1992;100(1):52–9. doi: 10.1111/j.1600-0722.1992.tb01809.x. [DOI] [PubMed] [Google Scholar]
- 29.Gu S, Rasimick BJ, Deutsch AS, Musikant BL. Radiopacity of dental materials using a digital X-ray system. Dent Mater. 2006;22(8):765–70. doi: 10.1016/j.dental.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Artal MC, Holtz RD, Kummrow F, Alves OL, Umbuzeiro Gde A. The role of silver and vanadium release in the toxicity of silver vanadate nanowires toward daphnia similis. Environ Toxicol Chem. 2013;32(4):908–12. doi: 10.1002/etc.2128. [DOI] [PubMed] [Google Scholar]


