Abstract
Recurrent aphthous ulcer (RAU) is a well-known painful, inflammatory disease with uncertain etiology for which local symptomatic therapy is only available. The aim of this study was to formulate and characterize muco-adhesive sponges containing a mixture of tenoxicam and miconazole nitrate to manage pain, inflammation and avoid candida infection that may accompany RAU due to poor oral hygiene. Two polymers at different concentrations were used to prepare sponges applying simple freeze-drying. Medicated chitosan (2%) sponges (mC2) showed acceptable physical appearance, surface pH (6.3 ± 0.042), porosity (25.7% ± 1.8), swelling index (5.7 ± 0.11), in-vivo and ex-vivo muco-adhesion time (115 min.±0.813 and 155 min.±1.537, respectively), ex-vivo muco-adhesion force (0.09 N ± 0.002) and scanning electron microscope (SEM) images. For concurrent clear-cut determination of tenoxicam and miconazole nitrate from mC2, a new UPLC method was developed and validated. mC2 sponges exhibited superior in-vitro drug release profiles where ∼100% of tenoxicam released within 5 min for fast pain relief with a more prolonged miconazole nitrate release. Furthermore, in-vivo animal study revealed that mC2 caused a significant decrease in the acetic acid-induced ulcer size in rats after 6 days of treatment (p < .0001) compared to negative and positive controls. Additionally, histopathological examination showed faster healing with complete restoration of the normal oral histology in rats. The present study concludes that chitosan sponge loaded with a combination of tenoxicam and miconazole nitrate could improve healing of RAU cases.
Keywords: Freeze-drying technique, chitosan, CMC, mucoadhesive buccal sponges, recurrent aphthous ulcer
1. Introduction
Recurrent aphthous ulcers (RAU) or aphthae are considered to be one of the most encountered insults that affect the oral cavity (Rogers, 1997; Stoopler & Musbah, 2013). They are painful oval-shaped sores in the oral cavity and tend to be reddish in color due to blood vessel dilatation (Baccaglini et al., 2011; Bruce & Rogers, 2003; Rogers, 2003). According to the morphology/size of lesions, RAU may be minor <10 mm or major >10 mm ulcers and with respect to the number of lesions it could be multiple (herpetiform). On the other hand, according to severityit is classified into simple or complex (Lehman & Rogers, 2016). Generally, the lesions are self-resolving within two weeks in most patients and re-occurring three to six times a year (Rogers, 1997). The exact etiology of RAU still not entirely clear. Vitamins and minerals deficiencies, blood disorders, GIT disorders, hormonal imbalance, stress and genetic predispositions (Rogers, 1977; Bruce & Rogers, 2003; Jurge et al., 2006; Scully & Porter, 2008; Gallo et al., 2009) were reported to be linked with an elevated risk of experiencing RAU (Scully & Porter, 2008). Additionally, drug-induced RAU could be evidenced during the long-term or chronic systemic use of some pharmacological classes like non-steroidal anti-inflammatory drugs (NSAIDs), anti-cholinergics and immuno-suppressants (i.e. methotrexate) where the ulcers diminish upon discontinuation of the triggering drug (Natah et al., 2004). Although some immuno-suppressants could trigger occurrence of aphthous ulcers, some other immune-suppressants and immune-modulators can be used to treat severe cases of RAU like systemic corticosteroids (i.e. prednisone) and thalidomide whose use is limited due to its side-effects (Belenguer-Guallar et al., 2014).
The pathogenesis of RAU is mainly governed by multiple immunological mechanisms. An inflammatory response is initiated by the erosion of the mucosal lining exposing the underlying epithelium followed by progressive desquamation (Alsaad & Ghazarian, 2005). Edematous swelling then occurs due to inflammatory cell infiltration and both the migration and the activation of T-cells and leukocytes (macrophages and mast cells). Thereafter, the elevated levels of interleukin 2 (IL-2) and tumor necrosis factor α (TNF-α) act as pivotal factors in the development of the inflammatory process (Jurge et al., 2006). Damage can then extend to reach the lamina propria which contains nerve endings and dilated blood vessels leading to pain, redness and bleeding in severe cases. Concomitant or/and secondary microbial infection can worsen the case and limit the healing process.
Appropriate treatment and management of patients suffering from RAU based on the precise diagnosis and proper investigation of the underlying causes and predispositions. Pharmacological treatments are mainly topical or/and systemic according to the severity of the case and the cause of the ulcers (Belenguer-Guallar et al., 2014). Treatments are primary directed toward the symptomatic relief and then managing associated systemic conditions if any (Patricia Ortiz Vega & Chimenos Küstner, 2002; Belenguer-Guallar et al., 2014). Regarding topical treatment, the main therapeutic classes of choice are antiseptics (like chlorhexidine) antimicrobials (like tetracycline, triclosan and miconazole nitrate), anti-inflammatories, analgesic drugs (like colchicine and NSAIDs) and local anesthesia (like lidocaine) (Lai et al., 2003; Dong et al., 2017; Hixon et al., 2017; Wang et al., 2017). NSAIDs would have a crucial role in the relief of pain, controlling the ongoing inflammatory process and promoting wound healing. Specifically, tenoxicam topical preparations were reported to reduce moderate to severe throat and buccal inflammations (Elhakim et al., 2000). These classes are administered in the form of oral gels or mouthwashes with frequent application every1–2 hours (Campisi et al., 1997; Schemel-Suárez et al., 2015). Additionally, topical glucocorticoids like triamcinolone acetonide and flucinolone acetonide are provided in ointment bases or hydro-alcoholic mouthwashes (Muñoz-Corcuera et al., 2009). Due to the pain and the discomfort that patient suffers during RAU he/she cannot maintain proper oral hygiene by good brushing or the use of mouth washes. Other studies discussed the relationship between oral hygiene and colonization of Candia species in oral cavity. It was found that 83.4% of patients with Candida poorly maintained oral hygiene (Muzurovic et al., 2012). Accordingly, patients with RAU are of increased risk of Candida infection due to poor oral hygiene and the use of antifungals will be beneficial as a prophylactic agent against Candida infection that may accompany RAU. Moreover, recent study revealed that Candida infections retard healing of ulcers and considered as a protractive factor of oral ulcers (Terai et al., 2018). In particular, miconazole nitrate and other azole antifungal agents were proved to treat mucosal candidiasis (Tejada et al., 2017). Moreover, the FDA approved the use of miconazole nitrate buccal tablets and gels for the management of oropharyngeal candida infections (Collins et al., 2011). Conclusively, a topical preparation that contains a combination between more than one pharmacological classes in the management of RAU would be beneficial in improving the awaited therapeutic outcomes.
Sponges are defined as dispersions of gas (usually air) in a solid matrix to yield a solid porous structure (Lai et al., 2003). They provided promising platforms for the delivery of different local as well as systemic drugs to the mucosal surface. Moreover, sponges act as ideal scaffolds for wound healing, tissue engineering and many orthodontic applications (Dong et al., 2017; Hixon et al., 2017; Wang et al., 2017). Sponges display plenty of advantages over other drug carriers. Owing to their porous network structures, sponges possess high surface-to-volume ratios which allow greater drug payloads (Ayensu et al., 2012). Additionally, they have the ability to maintain their swollen gel structures and hence, could be retained for prolonged time at the site of application compared to other local gels and semi-solids (Boateng et al., 2010). Generally, sponges are composed of polymers like alginates, cellulose derivatives, pectins, gelatin and chitosan and have been investigated for different applications. Polymers are selected according to the required sponge characteristics and its intended use. Furthermore, polymer favorable properties such as mechanical strength, chain flexibility, biocompatibility, biodegradability and muco-adhesiveness would also contribute to the decision of polymer selection (Freag et al., 2018).
To the best of our knowledge, there is no available formulation intended for the topical management of RAU that contains a combination of drugs of different therapeutic classes. Therefore, the aim of this work is to prepare muco-adhesive sponges with optimum properties containing tenoxicam together with miconazole nitrate as a prophylactic agent to simultaneously manage pain, inflammation and fungal candida infection that may accompany RAU by screening two different polymers (chitosan and carboxymethyl cellulose CMC) at different concentrations through the evaluation of their physical characters, in-vitro and in-vivo studies.
2. Materials AND methods
2.1. Materials and instruments
Tenoxicam and miconazole nitrate were generously provided by Egyptian International Pharmaceutical Industries Company (E.I.P.I.Co) (10th of Ramadan City, Egypt) and EVA pharmaceuticals (Cairo, Egypt), respectively. Chitosan (Ch) [20–190 kDa, degree of deacetylation 85%, viscosity 200–800 cps] was purchased from Sigma Chemical Company (Saint Louis, MO). Carboxymethyl cellulose (CMC) and glacial acetic acid were purchased from Fisher Scientific, U.K. %. Acetonitrile (ACN), methanol (MeOH), ammonium acetate were purchased from Sigma-Aldrich, Germany. All the chemicals were analytical grade except for methanol and acetonitrile were (High performance liquid chromatography) HPLC grade.
2.1.1. Instrumentation for the developed UPLC method
The ultra-performance liquid chromatography (UPLC) system utilized in this work was Thermo Fisher UHPLC Dionex Ultimate 3000 (Germering, Germany). The system consists of a pump (ISO-3100SD), autosampler (WPS 3000SL) and column thermostat (TCC-3000 SD). The detector was a Diode Array detector (DAD-3000 RS) (Germering, Germany). For data acquisition, the software utilized was Chromeleon 6.8 (Germering, Germany). The separation was carried out using AcclaimTM RSLC 120 C18 column 2.2 μm (2.1 × 100 mm), Thermo Fisher. The pH of the buffer was adjusted using Jenway pH-meter3310, Dunmow, Essex, United Kingdom. MilliQ water was prepared using a water purification system (Thermo scientific Barnstead Smart2Pure 3 UV, Hungary). For the degassing of the mobile phase and also to facilitate the drug extraction, an ultrasonicator (Elmasonic S60 h, Germany) was utilized. The dosage form vortexed by vortex mixer VelpScientifica, Europe.
2.2. Preparation of plain and medicated sponges
Six formulae of plain sponges were prepared using polymers CMC and Ch according to the composition listed in Table 1. A calculated amount of CMC was sprinkled into distilled water while Ch was sprinkled into 1% v/v acetic acid solution and stirred until reaching homogenous gels. The homogenous polymer dispersion was then kept at room temperature over night; to get rid of air bubbles. 80 µL of each homogenous gel were injected into each well (128 × 86 mm) of a plastic microtiter plate using a micropipette. Molded gels were refrigerated for 24 h prior to lyophilization. The molded gels were then freeze-dried using a lyophilizer (Martin Christ Alpha 1-2LD machine, Germany) at 40 mTorr at −45 °C for 4 hours (Hazzah et al., 2015). Then, the prepared plain sponges were characterized to select the polymers’ concentration that gives sponges with acceptable properties to proceed with drug loading.
Table 1.
Composition of prepared sponges.
| Formula code | Concentration of polymer solutions (% w/w) |
Distilled water (mL) | 1% acetic acid solution (mL) | |
|---|---|---|---|---|
| CMC | Ch | |||
| M0.5 | 0.5 | – | 30 | – |
| M1 | 1 | – | 30 | – |
| M2 | 2 | – | 30 | – |
| C0.5 | – | 0.5 | – | 30 |
| C1 | – | 1 | – | 30 |
| C2 | – | 2 | – | 30 |
*0.01% miconazole nitrate and 0.006% tenoxiam were added to the selected sponge.
The drug loading process was simple and straight forward. Calculated amounts of miconazole nitrate and tenoxicam were dissolved in both distilled water and 1% v/v acetic acid to produce final concentrations of 0.01% (w/v) of miconazole nitrate and 0.006% (w/v) of tenoxiam. Mixtures were then stirred on a magnetic stirrer until homogenous dispersions were obtained. Afterwards, CMC and Ch were respectively sprinkled into the previously prepared dispersions on a magnetic stirrer until reaching consistent gels. Then the process was continued as described for the plain sponges. After the characterization of the prepared medicated sponges of different polymers, the medicated sponge of optimum properties was selected to conduct further in-vivo animal and histopathological tests and studies.
2.3. Characterization of the prepared plain and medicated sponges
2.3.1. Physical appearance of the plain sponges
The prepared plain sponges were assessed regarding their shape, flexibility, brittleness and their ejection from the microtitre plates to assess their stickiness. Any formulation that failed this test would be excluded from further studies.
2.3.2. Surface pH
Each of the prepared plain and medicated sponges were put into 3 ml of simulated salivary fluid (SSF; pH 6.75) for 1 hour at ambient temperature. After swelling, pH of the surface was obtained using a pH meter (Darwish & Elmeshad, 2009).
2.3.3. Porosity
The porosity of the prepared plain and medicated sponges was determined in terms of total pore volume using the following Equation (Hazzah et al., 2015):
| (1) |
where the following equations were applied to calculate the theoretical volume before the lyophilization process and the practical volume of the sponge:
| (2) |
| (3) |
Knowing that m, ρ are the mass and the density of each polymer whereas, r is the radius of the well of the microtiter plate. Sponges’ heights or thicknesses were measured using a ruler and the density of polymer solutions was determined using a pycnometer. All measurements were made in triplicates to calculate the mean porosity (%) ± SD for each sponge.
2.3.4. Swelling index
Dry plain and medicated sponges were separately weighed (W1). Then, on 2% agar gel plates, sponges were placed individually and incubated at 37 ± 1 °C. After 2 min, the sponges were removed from the plates and a filter paper was used to drain any water leftover on the surface of the sponges. Afterwards, each sponge was weighed again (W2) and the following equation was applied. The swelling index (SI) for each sponge was determined in triplicate, and the mean ± SD was calculated (Patel et al., 2007).
| (4) |
2.3.5. In-vivo muco-adhesion time
All the in-vivo evaluations in this work that included human subjects were conducted according to the Helsinki agreement protocol for experiments involving humans (Association, 2001) and the code of conduct of the research ethics committee for experimental and clinical studies (Approval # CL-2007) at the Faculty of Pharmacy, The British University in Egypt (BUE).
The in-vivo muco-adhesion time study composed of 8 human volunteers (2 males and 6 females aged between 21 and 30) who approved to contribute to this study. Each human subject was randomly and blindly provided by a plain and a medicated sponge in order to place on the inner surface of the lower lips. They were then required to observe the time (min.) necessary for the sponge to completely detach and then recorded as in-vivo muco-adhesion time.
It is worth noting that an informed consent was obtained from all participants in this study after each subject had been adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail. The subjects were informed of the right to refuse to participate in the study or to withdraw consent to participate at any time without reprisal.
2.3.6. Ex-vivo muco-adhesion time of medicated sponges
The ex-vivo muco-adhesion time for the prepared medicated sponges was determined using chicken pouch membranes. On the side of glass beakers, the membranes were attached using cyanoacrylate glue. Thereafter, the sponges were lightly pressed for 1 min against the membranes to stick together usingfingertips.500 ml of SSF (PH 6.75) was then added to the beaker which is continuously stirred at 150 rpm with the temperature set to 37 °C aiming to mimic the movement of the mouth and the flow of the saliva (Perioli et al., 2007). The ex-vivo muco-adhesion time was expressed in terms of the time (min.) elapsed until complete erosion or detachment of the sponges from the membranes. All recordings were made in triplicates.
2.3.7. Ex-vivo muco-adhesion force of medicated sponge
The modified two-armed physical balance method was applied to determine the ex-vivo muco-adhesion force of the prepared medicated sponges (Freag et al., 2018). A chicken pouch membrane was attached to a glass slide which is fixed on the balance’s base using cyanoacrylate adhesive. The sponge was then fixed to the balance pan and then the balance pan was lowered carefully until the sponge surface came in contact with the chicken pouch membrane. A weight of 50 gm was added for 1 min to ensure the adhesion between them. A gradual amount of water was added carefully to the pan on the other arm of the balance. The total weight of water (W) that made the sponge to detach from the membrane was recorded as an indicator for the ex-vivo muco-adhesive force. The experiment was carried out in triplicate to get the mean muco-adhesion force ± SD.
The adhesion force was calculated in triplicates using the following equation:
| (5) |
where W is the weight of water in gm and g is the acceleration due to gravity.
2.3.8. UPLC analytical method development and validation
2.3.8.1. Standard Solutions preparation and chromatographic conditions
To prepare 1 mg/mL standard stock solutions, 25 mg of miconazole nitrate and tenoxicam standards were dissolved in 25 mL methanol, separately. Subsequently, they were sonicated for 5 min. The calibration curve concentrations were prepared by serial dilutions (5–500 µg/mL for miconazole nitrate and 5–200 µg/mL for tenoxicam) using methanol. The stock solutions; when not in use; were stored at 4 °C. All the quantification process during this work was performed using the optimized chromatographic conditions. The optimum chromatographic conditions were as follow: the mobile phase was a mixture of methanol: acetonitrile: 13 mM acetate buffer (pH 7.00) (50:20:30; v/v/v) at 0.4 mL/min flow rate. The temperature of the column oven was adjusted at 40 °C and the injection volume of the sample was 10 μL. The diode array detector was maintained at wavelength 223 nm.
2.3.8.2. Method validation
Validation was accomplished according to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (Branch, 2005) with respect to the following parameters.
The linearity of the method was determined by preparing serial dilutions: 5, 10, 20, 30, 50, 100, 150, 400 and 500 μg/mL for miconazole nitrate and 5, 10, 20, 30, 50, 100, 150, 200 μg/mL for tenoxicam. Each concentration was injected 3 times into the UPLC, under the optimum chromatographic conditions, mentioned earlier. Afterwards, the concentration was plotted versus the average peak areas in order to construct the calibration curve. From the linear regression analysis, the regression equation and the correlation coefficient were determined. The linearity of the method was evaluated using the square of the regression coefficient (R2). For the limit of quantification (LOQ), it is equivalent to the concentration of the analyte in which S/N is equal to 10; in which S is signal and N is noise. While for the limit of detection (LOD), it is equivalent to the concentration of the analyte in which S/N is equal to 3.
The precision of the method was determined using inter-day and intra-day precision. For the intra-day precision, it was evaluated by injecting 3 different concentrations; for each standard; into UPLC, each was injected three times on the same day. While for the inter-day precision, it was obtained by injecting the same concentrations three times on two consecutive days. The concentrations were 50, 150, 200 μg/mL for tenoxicam and 50, 150, 400 μg/mL for miconazole nitrate. The results were evaluated with respect to % relative standard deviation (%RSD).
The accuracy of the method was accomplished by comparing the concentration practically measured by the UPLC instrument; calculated from the regression equation; to the theoretical value. The results were evaluated with respect to % recovery (%R).
2.3.9. Determination of the drug content in the medicated sponges
For the preparation of the newly developed medicated sponge dosage form, 2 mL methanol was added to 1 sponge in an Eppendorf, then vortexed for 15 min and finally ultrasonicated for 15 min at room temperature. The solution was filtered using a 0.22 µm nylon syringe filter and injected into UPLC under the optimum chromatographic conditions. The concentration of each drug in the solution was determined using the pre-constructed calibration curves.
2.3.10. In-vitro drug release
The release behavior of each drug from the medicated sponges was studied by simple modifications on the method suggested by Phaechamud et al. (Phaechamud et al., 2015). Medicated sponges were suspended in test tubes each containing 2 ml of release medium (SSF pH 6.75 containing 2% tween 80) to ensure sink condition. Three test tubes were used for each medicated sponges (mC2 and mM1) and for each time interval (0, 5, 10, 20, 30, 60 and 120 min.). The test tubes were then placed into a beaker which was incubated at 37 °C and 120 rpm in a horizontal shaking water bath. After, 10 µL samples were taken from each test tube at each time interval and then were injected into UPLC to determine the area under the curve then the percent of drug release was obtained by using the preset calibration curve.
2.3.11. Visualization using scanning electron microscopy (SEM)
Visualization of surface contours and 3 D structure of the network of the plain M1 and C2 sponges and the selected medicated sponge was performed using scanning electron microscopy (SEM) (Jeol, JXA-840A, Tokyo, Japan). The sponge was firstly fixed on an aluminum holder and coated with gold using a sputter coater (Edwards S-105A, England) before being examined.
2.4. In-vivo animal study
2.4.1. Ulcer healing evaluation
Based on the carried evaluations, the prepared medicated sponges were selected for the in-vivo animal study which consisted of eighteen albino rats (weight 30–35 g) divided into three equal groups (control-plain-medicated). Group C: control group, they do not receive any intervention, Group P: Plain group, which receive Plain chitosan sponge (C2) and Group M: medicated group, which receive medicated chitosan sponge (mC2) containing mixture of miconazole nitrate and tenoxicam. The mice were fed on commercial pellet and water ad libitum throughout the study. The in-vivo study was executed following the ARRIVE guidelines and in accordance to the Helsinki declaration and the code of conduct of the research ethics committee for experimental and clinical studies (Approval # EX-2005) at the Faculty of Pharmacy at BUE.
First, ulcers were induced using filter papers (6 mm in diameter) soaked in 50% acetic acid, then put on the inner mucus membrane of the lips for 1 minute, then remove the filter paper (Karavana et al., 2011). Pictures of the induced ulcers were taken before any group could receive any interventions in order to measure their diameters (day 0). Both the P and M groups received plain and medicated sponges, respectively, once daily for six days and pictures of the ulcers were taken. The assessed ulcer healing parameters were the measurement of ulcer areas. The changes in ulcers were measured (mm2) using ImageFocus 4.0 software every day. The data of the in-vivo ulcers areas are presented as the mean area of each group for every day of the 6 days of the study ± SD. All measurements were recorded for further analysis.
2.4.2. Histopathological examinations with hematoxylin and eosin (H & E) stain
All rats were sacrificed by cervical dislocation then autopsy samples were taken from the lips of rats in different groups and fixed in 10% formaline saline, then treated as described by Bancroft and Stevens (1996). Samples was dehydrated using dilutions of ethanol then entrenched in paraffin and dried in hot air oven overnight. For histopathological investigation, tissue segments were cleared out of paraffin layers then placed on glass slides. Afterwards, H & E stain was added and the slides were examined under the light microscope
2.5. Statistical analysis
Characterization data for plain and medicated sponges (expressed as a percentage or mean (±SD)) as well as ulcer areas of different rat groups at day 6 were statistically compared using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test for post-hoc analysis. The difference between any groups would be considered statistically significant at a probability value of p < .05. All statistical analysis and regression analysis were performed using GraphPadPrism 7.0 software.
3. Results and discussion
3.1. Characterization of the prepared plain and medicated sponges
3.1.1. Physical appearance of the plain sponges
Plain sponges were prepared to select the most appropriate concentrations of each polymer to form the sponge. Accordingly, they were evaluated regarding satisfactory physical integrity and mechanical properties of the final lyophilized products. The majority of the prepared plain sponges showed acceptable physical properties except for M0.5 and M2 showed a complete failure with brittleness and stickiness to the walls of the wells in the microtitre plates, respectively thus they were excluded from further studies.
3.1.2. Surface pH, porosity%, swelling index, in-vivo & ex-vivo muco-adhesion time and ex-vivo muco-adhesion force
From Table 2 it can be seen that the surface pH of the obtained plain sponges ranged from 6.33 ± 0.01 to 6.79 ± 0.03. These results reflect acceptable pH values with respect to the salivary pH (5.5 − 7), accordingly, no irritation could be produced upon the application of these sponges to the oral mucosal lining. The heights of the prepared sponges vary owing to the different lyophilization behavior of polymers and their different concentrations. Accordingly, porosity (%) differs whereM1 was found to be the most porous sponge and the porosity slightly increases with the increase of Ch concentration from C0.5 to C2. However, C2 displayed the highest swelling index and it was noticed that, similar to porosity, the swelling index was directly proportional to Ch concentration. This is may be explained by increasing the amount of the interlinking fibers and hence the ability to accommodate more water with in the polymer matrix (Kassem et al., 2015). In the same context, sufficient swelling plays a crucial role in muco-adhesive preparations as it directly affects the uniformity of drug release, muco-adhesion and the ability to absorb exudates (Ismail et al., 2000).
Table 2.
Characterization of the prepared plain/medicated sponges.
| Parameter |
Muco-adhesion time (minutes) |
Ex-vivo muco-adhesion force (N) | ||||
|---|---|---|---|---|---|---|
| Formula | Surface pH | Porosity (%) | Swelling index | In-vivo | Ex-vivo | |
| Plain sponge | ||||||
| C0.5 | 6.36 ± 0.026 | 21 ± 1.2 | 0.48 ± 0.01 | 60 ± 0.547 | **NA | **NA |
| C1 | 6.35 ± 0.037 | 23.5 ± 0.9 | 3.92 ± 0.06 | 120 ± 1.1136 | **NA | **NA |
| C2 | 6.33 ± 0.01 | 26 ± 1.5 | 5.23 ± 0.09 | 116 ± 1.11 | **NA | **NA |
| M1 | 6.79 ± 0.03 | 35 ± 2.5 | 3.788 ± 0.1 | 15 ± 2.64 | **NA | **NA |
| *Medicated sponge | ||||||
| mC0.5 | 6.29 ± 0.091*** | 22 ± 0.88*** | 0.55 ± 0.04*** | 59 ± 1.62 *** | 90 ± 0.53 | 0.014 ± 0.0115 |
| mC1 | 6.26 ± 0.066*** | 23.8 ± 1.1*** | 3.68 ± 0.03*** | 123 ± 0.113*** | 136 ± 0.197 | 0.032 ± 0.0057 |
| mC2 | 6.30 ± 0.042*** | 25.7 ± 1.8*** | 5.70 ± 0.11*** | 115 ± 0.813*** | 155 ± 1.537 | 0.09 ± 0.002 |
| mM1 | 6.68 ± 0.12*** | 36.2 ± 1.7*** | 3.529 ± 0.8*** | 19 ± 0.48*** | 30 ± 0.1 | 0.06 ± 0.002 |
*Medicated sponge contains 10 mg% miconazole nitrate and 6 mg% tenoxiam. **NA means Not Applied experiment. *** Symbol means results insignificantly different (p > .05) from plain sponges.
Generally, Ch sponges (C0.5, C1 and C2) had much longer in-vivo muco-adhesion time compared to CMC sponge (M1) due to the cationic nature of Ch polymer. Chitosan chain contains positively charged amino groups which then interact with the negatively charged sulfate groups and sialic acid residues in mucin which is the main component in the mucousal linings. Consequently, this electrostatic interaction leads to strong muco-adhesion. Additionally, increasing Ch concentration from 0.5% to 1% significantly increased the muco-adhesion time (p < .05) which did not increase with more concentration. This could be accredited to increasing the amount of the positively charged amino groups which can interact with mucin (Abruzzo et al., 2012).
Data illustrated in Table 2 also discloses that the addition of miconazole nitrate and tenoxicam did not significantly affect (p > .05) either of the surface pH, porosity, the swelling indices and the in-vivo muco-adhesion time of the studied sponges. This observation should be restricted to the used concentration of both drugs. Additionally, in-vivo and ex-vivo muco-adhesion time showed good correlation, however, in-vivo muco-adhesion time was expectedly shorter than the ex-vivo muco-adhesion time due to the continuous washing with the saliva and the mouth movement in-vivo.
Regarding the ex-vivo muco-adhesion force, in general, Ch-medicated sponges (mC0.5, mC1 and mC2) possessed stronger adhesion to the chicken pouch membrane than CMC-medicated sponge (mM1). This could be explained by the favorable interaction between the positively charged amino groups in the Ch chains–which is absent in the CMC polymer- and the negatively charged mucin as illustrated above. Furthermore, results revealed that the concentration of Ch solution significantly affected the muco-adhesion force (p < .05) as it increased from 0.014 N ± 0.0115 (mC0.5) to 0.09 N ± 0.002 (mC2). Increasing Ch concentration means greater cationic amino groups density which in return leads to more interaction with the negatively charged mucin glycoprotein and thus leading to stronger muco-adhesion (Lehr et al., 1992; Abruzzo et al., 2012).
According to the findings discussed in this section, both mC2 and mM1 formulae were selected due to their promising properties in order to continue further experiments.
3.1.3. Chromatographic method development and validation
3.1.3.1. Method development
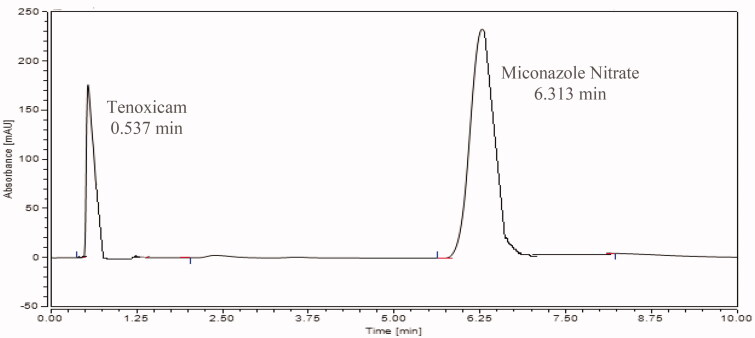
The analytical method was evaluated and validated as per ICH guidelines. The analytical conditions; including temperature, composition of the mobile phase, wavelength and flow rate for both drugs; were optimized. For the mobile phase composition, at first different ratios of water: methanol (50:50, 70:30 and 30:70; v/v); were investigated and they led to poor resolution. It is worth mentioning that replacing methanol with acetonitrile did not improve the resolution. After, a mixture of methanol, acetonitrile and 13 mM acetate buffer (pH = 7.00) was tested. Different ratios (45:45:10, 25:25:50, 15:15:70, 20:50:30 and 50:20:30; v/v/v) were tested, and the optimum ratio was found to be methanol: acetonitrile: 13 mM acetate buffer (pH= 7) (50:20:30; v/v/v). The optimum flow rate was set at 0.4 mL/min with an optimum temperature of 40 °C and higher temperature did not improve the resolution or had a dramatic effect on retention times of analytes. The stationary phase used was Acquity UPLC C18 column (2.1 × 100 mm, 2.2 μm). The optimum wavelength selected for miconazole nitrate and tenoxicam was 233 nm, based on their absorption spectrum measured by the diode array detector (DAD). Under these optimized conditions, the retention times were 0.540 min and 6.283 min for tenoxicam and miconazole nitrate, respectively.
3.1.3.2. Method validation
Linearity of the analytical method is the ability of a method to get the response directly proportional to sample concentration over a given range. It was found that for tenoxicam, the linearity ranged from 5 to 200 μg/mL. From the calibration curve, the regression equation was obtained where y = 0.9357x + 1.3436, the regression coefficient (R2) = 1, the slope was 0.9357and the intercept was 1.3436. As for miconazole nitrate, the method was linear at concentration range of 5–500 μg/mL. By applying linear regression analysis, the slope, the intercept and the regression coefficient were calculated from the regression equation y = 1.3037x + 0.4601 where the regression coefficient (R2) = 0.9999, the slope was 1.3037and the intercept was 0.4601. For miconazole nitrate and tenoxicam, the LOD and the LOQ were 1 and 5 μg/mL, respectively.
The precision measures whether the method is able to generate reproducible results or not. For tenoxicam, the inter-day precision, % RSD ranged from 1.30% to 2.00%, while for intra-day precision % RSD ranged from 0.21% to 1.85%. As for miconazole nitrate, the inter-day precision, % RSD ranged from 0.46% to 2.31%, and intra-day precision, % RSD ranged from 0.16% to 1.26%. The low% RSD indicate high precision of the method. The results of the precision are presented in Table 3.
Table 3.
Inter-day and Intra-day precision of tenoxicam and miconazole nitrate.
| Tenoxicam |
Miconazole Nitrate |
||||
|---|---|---|---|---|---|
| Concentration (μg/mL) | Inter-Day precision | Intra-Day precision | Concentration (μg/mL) | Inter-Day precision | Intra-Day precision |
| % RSD* | %RSD* | % RSD* | %RSD* | ||
| 50 | 2.00 | 1.85 | 50 | 2.31 | 1.26 |
| 150 | 1.30 | 0.26 | 150 | 0.69 | 0.16 |
| 200 | 1.38 | 0.21 | 400 | 0.46 | 0.41 |
*Average of 3 times.
The accuracy is the closeness of the results obtained from a method to the reference true values. The method was found accurate and this was confirmed by high % recovery for both miconazole nitrate and tenoxicam. For tenoxicam, % recovery was 98.05% to 101.71%. For miconazole nitrate, % recovery ranged from 95.27% to 102.10%. % RSD was determined for both drugs and ranged from 0.11 to 2.49 in case of tenoxicam 0.16 to 1.94 in case of miconazole nitrate. The results of the accuracy are presented in Table 4.
Table 4.
Accuracy of tenoxicam and miconazole nitrate.
| Tenoxicam |
Miconazole nitrate |
||||
|---|---|---|---|---|---|
| Theoretical concentration (ug/mL) |
% Recovery* | %RSD* | Theoretical concentration (ug/mL) |
% Recovery* | %RSD* |
| 5 | 99.42 | 1.06 | 5 | 101.86 | 1.39 |
| 10 | 98.05 | 2.34 | 10 | 97.07 | 0.38 |
| 20 | 98.24 | 1.74 | 20 | 100.42 | 1.92 |
| 30 | 99.50 | 2.49 | 30 | 102.10 | 1.75 |
| 50 | 101.71 | 1.85 | 50 | 95.27 | 1.26 |
| 100 | 99.78 | 0.11 | 100 | 99.71 | 0.56 |
| 150 | 100.31 | 0.26 | 150 | 101.53 | 0.16 |
| 200 | 99.80 | 0.21 | 400 | 100.34 | 0.41 |
| 500 | 99.69 | 1.94 | |||
*Average of 3 times.
3.1.4. Determination of the drug content
The drugs (miconazole nitrate and tenoxicam) were extracted as mentioned earlier in the experimental part. It is noteworthy that different solvents (methanol, acetonitrile, 10% acetic acid) were examined to extract both drugs from the prepared medicated sponges, however methanol was selected as it showed the highest % recovery, it could be contributed to the solubility of both drugs in it. As presented in Figure 1, the retention time (tR) of tenoxicam was 0.537 min and for miconazole nitrate was 6.313 min, under the optimum conditions. The % recovery was found to be 83% and 100.1% with % RSD 1.47 and 0.48 for tenoxicam and miconazole nitrate, respectively from mC2 while, the % recovery was 83.33% and 99.72% with % RSD 1.53 and 0.47 for tenoxicam and miconazole nitrate, respectively from mM1. The drug contents for tenoxicam in the medicated mC2 sponge and the mM1 sponge were found to be 49.8 µg/ml ± 1.26 and 50 µg/ml ± 0.96, respectively. On the other hand, the drug contents for miconazole nitrate were 100.1 µg/ml ± 2.06 and 99.7 µg/ml ± 1.77, respectively. The drug content was uniform between different sponges and did not deviate distinctly.
Figure 1.
Chromatogram for the newly developed medicated sponge under the optimum conditions.
3.1.5. In-vitro drug release
Generally, the drug release from polymeric sponges was reported to be governed by three main mechanisms which are: (1) liberation of the drug bounded to the surface of the sponge, (2) drug diffusion through the swollen gel matrix and (3) the erosion of the polymeric matrix (Agnihotri & Aminabhavi, 2004).
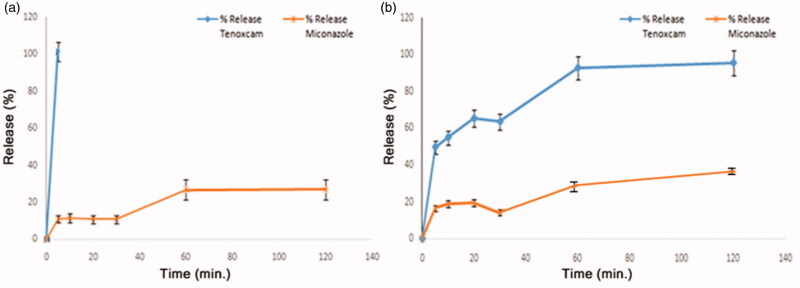
With respect to the in-vitro release profiles displayed in Figure 2, the release of the drugs found on the surface of the sponges mM1 and mC2 is reflected by the initial sharp release lines within the first 5 min then, a more prolonged release pattern for the bulk-dispersed drug in mM1 but not for mC2 as will be soon discussed. The initial fast release is attributed to the rapid dissolution of the surface-attached drug rather than that dispersed through the matrix. Afterwards, upon hydration the sponges are swollen into gel-like structures in which the path lengths through which the drugs should be diffused are increased and hence retard the release of the drugs. Moreover, the thick gel layer formed around the sponges hinders the penetration of the dissolution media to the inner bulk of the sponge and accordingly delays drug diffusion and erosion of the polymeric matrix. As a result drugs are release in more controlled and sustained patterns (Rodriguez et al., 2000).
Figure 2.
In-vitro release profiles of tenoxicam and miconazole nitrate from: (a) mC2 sponge and (b) mM1 sponge.
In Figure 2(a,b) the release of miconazole nitrate is more suppressed and sustained over a longer duration of time than that of tenoxicam. Only about 40% and 30% of miconazole nitrate was released after 120 min from mM1 and mC2, respectively. This may be owing to the higher molecular weight of miconazole nitrate (416.123 g/mole) that decreases its tendency for diffusion compared to that of tenoxicam (337.4 g/mole). Additionally, miconazole nitrate (pKa = 6.77) is less soluble in the release medium (SSF pH 6.75) and this weak solubility will also result in more sustained release. Furthermore, for the same reasons the total percentage of miconazole nitrate released at the end of the in-vitro release study (120 min) is much lower than that of tenoxicam.
On the other hand, the total amount of tenoxicam (100%) was recovered after 120 min from mM1 but within the first 5 min from mC2. As mentioned before, the preparation of mC2 was carried out in 1% acetic acid solution thus, during lyophilization all the amount of tenoxicam (pKa = 2.21) was rapidly precipitated to the bottom of the sponge. Subsequently, all the amount of tenoxicam will be found on one surface of the sponge leaving the bulk deprived of this acidic drug while miconazole nitrate is homogenously dispersed through the mC2 sponge. This uneven distribution of tenoxicam between upper and lower sponge surfaces of the sponge could be advantageous. Tenoxicam has a distinctive yellow color so, the drug-rich surface was easily differentiated. Upon application, the yellow-colored surface would be the one put in contact with the ulcers aiding in fast relief of the pain and inflammation. Contrarily, both miconazole nitrate and tenoxicam are homogenously distributed in mM1 sponge.
Conclusively, the release profiles for both drugs from mC2 will be beneficial in the management of RAU where the fast initial burst release of tenoxicam will lead to rapid onset of pain relief followed by a sustained miconazole nitrate release providing a prolonged anti-candidiasis effect for the protection against secondary fungal infection.
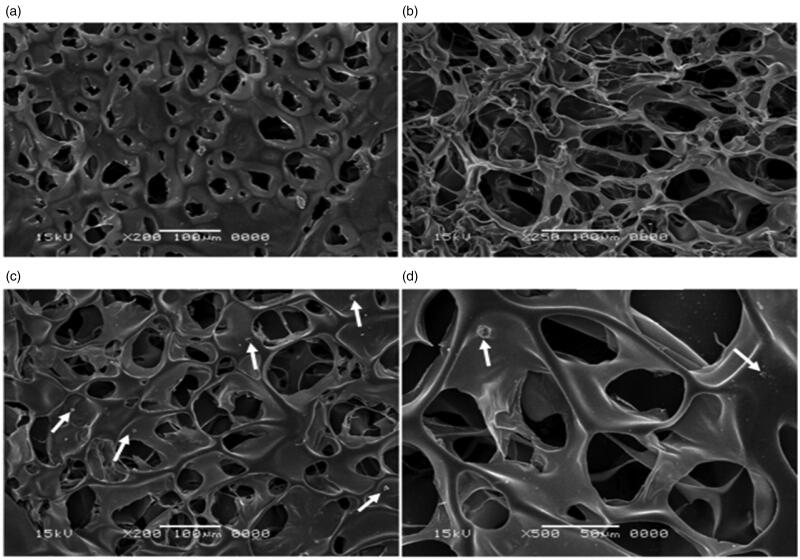
3.1.6. Scanning electron microscopy (SEM) images of selected plain polymers
The porous matrix and the interlinking fibers in the sponge network is clearly depicted by the SEM micrographs (Figure 3). They revealed that M1 possesses more porous structure than that of C2. This finding is closely correlated to the data obtained in the porosity test as previously explained. However, C2 displayed smoother texture than that of M1. Additionally, the SEM micrographs displayed in Figure 3 of medicated C2 [mC2] show the drugs’ crystals projecting out of the surface of the sponges reflecting the surface-linked drug molecules. The interlinking network structure is favorably preserved after the addition of the drug which in return will preserve its porosity and swelling properties and this confirms the data discussed under the Section 3.1.2.
Figure 3.
SEM micrographs of: (a) Ch plain sponge [C2], (b) CMC plain sponge [M1], medicated C2 sponge [mC2] at (c) magnification of 20,000× and (d) magnification of 50,000×.
3.2. Selection and characterization of the optimum medicated sponge
mC2 was selected for further in-vivo animal investigations. This is because chitosan displayed beneficial and superior properties such as a higher swelling index so it might have a high ability to absorb exudates from the inflamed area around the ulcer (Li et al., 2012; Liu et al., 2012), along with the reported intrinsic wound healing ability of chitosan (Arca & Şenel, 2008; Muzzarelli, 2009; Croisier & Jérôme, 2013). mC2 also possessed the highest muco-adhesion (i.e. time and force) that could be translated to increased contact time between the ulcerated areas and the medicated sponge giving prolonged drug action (Park et al., 1989).
3.3. In-vivo animal study
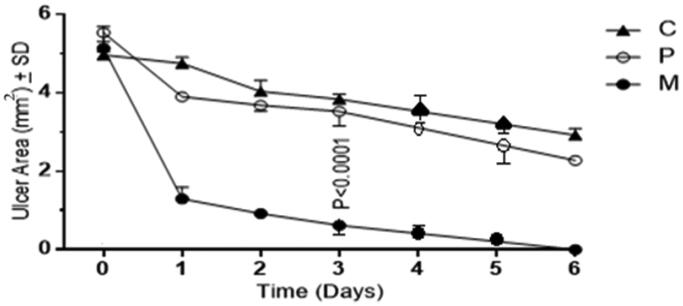
With reference to the curves in Figure 4, a significant and abrupt decrease (p < .0001) in ulcer areas is evidenced for the medicated group (M) with respect to both of the control (C) and the plain (P) groups in the first day after intervention. Starting on day 2 till the end of the course of the in-vivo study, the decrease in ulcers areas in group M continued at a slower rate until total disappearance of ulcers and inflammations reflecting complete healing and restoration of the normal buccal structure at day 6 which is further confirmed by the histopathological examination results in the next section.
Figure 4.
Ulcers’ area progression over six days for the control group (C), the plain group (P) and the medicated group (M).
The mild insignificant (p > .05) decrease in ulcers areas evidenced for group P than that of group C might be attributed to the pre-mentioned wound healing properties of chitosan. Chitosan promotes wound healing via different interesting mechanisms that include decrease hemorrhage (hemostatic) through triggering coagulation, deformation of erythrocytes and platelet activation (Lord et al., 2011; Gu et al., 2013; Nandi et al., 2013). Moreover, chitosan was believed to have the ability to potentiate angiogenesis by encouraging the expression of cytokines and growth factors, collagen production, fibroblast multiplication and integrin engagement (Arca & Şenel, 2008; Muzzarelli, 2009; Croisier & Jérôme, 2013).
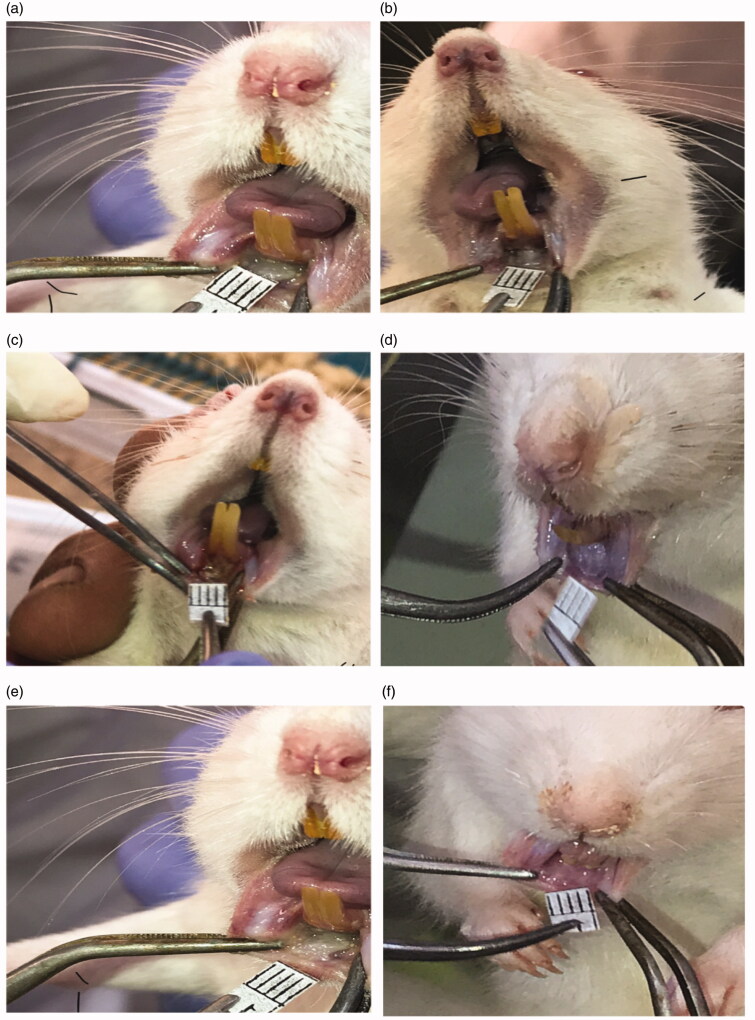
The pictures of animals displayed in Figure 5 additionally illustrate the sizes of the induced ulcers at day 0 and the decrease in ulcer sizes at day 6 for groups C, P and M. It is obvious that group M showed significant results reflected by the complete healing of the induced ulcer. Contrariwise, both of groups C and P showed minor decrease in ulcer size with greater decrease in the plain group due to the healing potentials of chitosan. Moreover, during visual inspection of oral cavity of rats, bleeding was observed in group C during the first two days of ulcer induction while bleeding was absent in groups P and M. This could be attributed to the pre-mentioned homeostatic activity of chitosan.
Figure 5.
Picture of animals showing induced ulcer at day 0 for: (a) control, (c) plain and (e) medicated groups and at day 6 for: (b) control, (d) plain and (f) medicated groups. The heads of the black arrows point at the ulcerated areas.
Therefore, chitosan might help to promote healing of ulcers in-vivo which makes the medicated chitosan sponge a potent and effective treatment option in the management of RAU.
3.4. Histopathological examinations
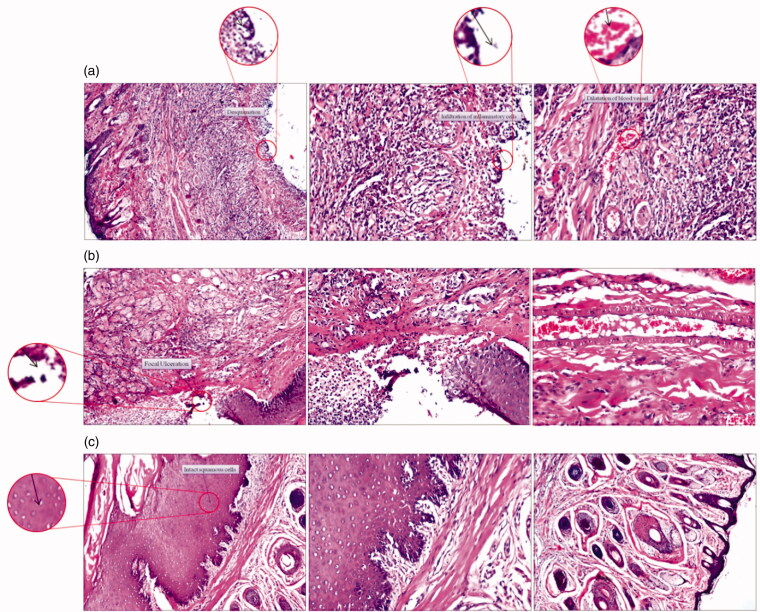
The histopathological examination of the lip and the oral cavity of the studied groups of rats after 6 days displayed in Figure 6 revealed the following:
Figure 6.
Representative photomicrographs (100x) of sections in the lips stained with H & E of (a) the control group of rats, (b) the group of rats receiving plain chitosan sponge and (c) the group of rats receiving medicated chitosan sponge.
3.4.1. Control group of rats (C)
3.4.1.1. Inner mucosal layer
Focal ulceration and desquamation of the mucosal layer with intense inflammation reflected by the presence of dilated blood vessels and massive inflammatory cell infiltration in the underlying lamina propria and submucosa were recorded in Figure 6(a).
3.4.1.2. Outer skin layer
The dermal layer showed inflammatory cell infiltration (Figure 6(a)).
3.4.2. Plain group of rats (P)
3.4.2.1. Inner mucosal layer
The lining mucosa showed minor healing signs reflected by smaller focal ulceration while the lamina propria was infiltrated by few inflammatory cells (Figure 6(b)) with congested blood vessels in the submucosa (Figure 6(b)). This minute progression may be ascribed to the aforementioned wound healing and hemostatic properties of chitosan.
3.4.2.2. Outer skin layer
The dermal layer still showing inflammatory cells infiltration as recorded in (Figure 6(b)).
3.4.3. Medicated group of rats (M)
3.4.3.1. Inner mucosal layer
Regarding Figure 6(c), the normal histological structure of the stratified squamous keratinized mucosal epithelium with lamina propria and the underlying submucosa and muscularis were reestablished with no histopathological alteration.
3.4.3.2. Outer skin layer
There were no observed histopathological changes in the oral mucosal tissue with total restoration of the normal structures of the epidermis and the underlying dermis with preserved sebaceous glands and intact hair follicles as displayed in Figure 6(c).
To this end, the data interpreted in this section ensure what was concluded in the in-vivo animal study that the medicated chitosan sponges (mC2) were significantly effective (p < .0001) to decrease ulcers sizes and to stop bleeding at ulcerated areas–revealed by visual inspection- in rats together with the rapid and complete restoration of normal oral cavity structures.
4. Conclusion
The prepared medicated chitosan sponge showed unique mechanical and physical properties with optimal muco-adhesion time and force. In addition to the intrinsic wound healing ability of chitosan, the in-vivo animal studies revealed that the medicated chitosan sponge induced complete restoration of the normal histology of the ulcerated areas in the studied animals.
Conclusively, chitosan sponges provided promising platforms for the concomitant delivery of both miconazole nitrate and tenoxicam in the management of RAU.
5. Limitations and future perspectives
Although muco-adhesive polymeric sponges possess a lot of merits, some challenges may limit its uses and applications. For instance, high drug doses cannot be incorporated into polymeric sponges otherwise high drug concentrations may alter the sponge’s physical properties like surface pH, porosity, swelling, muco-adhesion, etc. Further studies had to be carried out to investigate maximum drug loads. Additionally, irritating and bitter-taste drugs will serve as important barriers for patient acceptability. Finally, our future perspectives are directed toward conducting clinical trials for the verification of sponges’ performance in humans.
Acknowledgement
This whole work was kindly supported by Year 5 students of the Pharmaceutics Research Methodology Module at the Faculty of Pharmacy, The British University in Egypt (BUE). The profound gratitude was expressed for Nardeen Fawzy, Moamen Sherif, Monika Amgad, Esraa Hassan, Yasmeen Fawzy, Somaia Moustafa and Doaa Mohsen for their valuable contribution to the practical part of this work. Additionally, sincere thanks should be offered to teaching assistant Nada Hesham for fruitful participation in the practical part of the UPLC analysis. Lots of thanks to Year 5 students of the Analytical chemistry Research Methodology; Randa Essam Eldin, Nada Salah Eldin, Mohamed Ghaith, Alaa Eldin Abdulfattah, Ahmed Salama, Mohamed Osama and Eslam Gamil for their cooperation in the practical part of the UPLC method.
Author contributions
M.O. shared in designing the protocol of work, wrote the manuscript and performed the practical pharmaceutics experiments. C.L. performed practical pharmaceutics experiments. M.T. performed the UPLC practical parts. H.A.W. supervised, performed and wrote the UPLC parts. D.A.designed the protocol of work and supervised the whole work. M.M.E. performed the in-vivo practical part of the work. D.A. and M.M.E. reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Abruzzo A, Bigucci F, Cerchiara T, et al. (2012). Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr Polym 87:581–8. [DOI] [PubMed] [Google Scholar]
- Agnihotri SA, Aminabhavi TM. (2004). Controlled release of clozapine through chitosan microparticles prepared by a novel method. J Control Release 96:245–59. [DOI] [PubMed] [Google Scholar]
- Alsaad KO, Ghazarian D. (2005). My approach to superficial inflammatory dermatoses. J Clin Pathol 58:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arca HC, Şenel S. (2008). Chitosan based systems for tissue engineering part II: soft tissues. Fabad J Pharm Sci 33:211–6. [Google Scholar]
- Association WM. (2001). World medical association declaration of helsinki. ethical principles for medical research involving human subjects. Bull World Health Organ 79:373. [PMC free article] [PubMed] [Google Scholar]
- Ayensu I, Mitchell JC, Boateng JS. (2012). Development and physico-mechanical characterisation of lyophilised chitosan wafers as potential protein drug delivery systems via the buccal mucosa. Colloids Surfaces B Biointerfaces 91:258–65. [DOI] [PubMed] [Google Scholar]
- Baccaglini L, Lalla R, Bruce A, et al. (2011). Urban legends: recurrent aphthous stomatitis. Oral Dis 17:755–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. (1996). Theory and practice of histological techniques 5th Ed. Edinburgh: Churchill Livingstone. [Google Scholar]
- Belenguer-Guallar I, Jiménez-Soriano Y, Claramunt-Lozano A. (2014). Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent 6:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng JS, Auffret AD, Matthews KH, et al. (2010). Characterisation of freeze-dried wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int J Pharm 389:24–31. [DOI] [PubMed] [Google Scholar]
- Branch SK. (2005). Guidelines from the international conference on harmonisation (ICH). J Pharm Biomed Anal 38:798–805. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Rogers RS. (2003). Acute oral ulcers. Dermatol Clin 21:1–15. [DOI] [PubMed] [Google Scholar]
- Campisi G, Spadari F, Salvato A. (1997). Sucralfate in odontostomatology. Clin Exp Minerva Stomatol 46:297–305. [PubMed] [Google Scholar]
- Collins CD, Cookinham S, Smith J. (2011). Management of oropharyngeal candidiasis with localized oral miconazole therapy: efficacy, safety, and patient acceptability. Patient Prefer Adherence 5:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier F, Jérôme C. (2013). Chitosan-based biomaterials for tissue engineering. Eur Polym J 49:780–92. [Google Scholar]
- Darwish MK, Elmeshad AN. (2009). Buccal mucoadhesive tablets of flurbiprofen: characterization and optimization. Drug Discov Ther 3:181–189. [PubMed] [Google Scholar]
- Dong Y, Liu W, Lei Y, et al. (2017). Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mater Sci Eng C 70:371–7. [DOI] [PubMed] [Google Scholar]
- Elhakim M, Siam A, Rashed I, Hamdy MH. (2000). Topical tenoxicam from pharyngeal pack reduces postoperative sore throat. Acta Anaesthesiol Scand 44:733–6. [DOI] [PubMed] [Google Scholar]
- Freag MS, Saleh WM, Abdallah OY. (2018). Exploiting polymer blending approach for fabrication of buccal chitosan-based composite sponges with augmented mucoadhesive characteristics. Eur J Pharm Sci 120:10–9. [DOI] [PubMed] [Google Scholar]
- Gallo C. d B, Mimura MAM, Sugaya NN. (2009). Psychological stress and recurrent aphthous stomatitis. Clinics 64:645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Xie H, Huang C, et al. (2013). Preparation of chitosan/silk fibroin blending membrane fixed with alginate dialdehyde for wound dressing. Int J Biol Macromol 58:121–6. [DOI] [PubMed] [Google Scholar]
- Hazzah HA, Farid RM, Nasra MMA, et al. (2015). Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: development and characterization. Int J Pharm 492:248–57. [DOI] [PubMed] [Google Scholar]
- Hixon KR, Lu T, Sell SA. (2017). A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater 62:29–41. [DOI] [PubMed] [Google Scholar]
- Ismail FA, Napaporn J, Hughes JA, Brazeau GA. (2000). In situ gel formulations for gene delivery: release and myotoxicity studies. Pharm Dev Technol 5:391–7. [DOI] [PubMed] [Google Scholar]
- Jurge S, Kuffer R, Scully C, Porter SR. (2006). Number VI: recurrent aphthous stomatitis. Oral Dis 12:1–21. [DOI] [PubMed] [Google Scholar]
- Karavana SY, Sezer B, Güneri P, et al. (2011). Efficacy of topical benzydamine hydrochloride gel on oral mucosal ulcers: an in vivo animal study. Int J Oral Maxillofac Surg 40:973–8. [DOI] [PubMed] [Google Scholar]
- Kassem MAA, ElMeshad AN, Fares AR. (2015). Lyophilized sustained release mucoadhesive chitosan sponges for buccal buspirone hydrochloride delivery: formulation and in vitro evaluation. AAPS Pharm Sci Tech 16:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HL, Abu’Khalil A, Craig DQM. (2003). The preparation and characterisation of drug-loaded alginate and chitosan sponges. Int J Pharm 251:175–81. [DOI] [PubMed] [Google Scholar]
- Lehman JS, Rogers RS. (2016). Acute oral ulcers. Clin Dermatol 34:470–4. [DOI] [PubMed] [Google Scholar]
- Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. (1992). In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm 78:43–8.(92)90353-4. [Google Scholar]
- Li D, Li P, Zang J, Liu J. (2012). Enhanced hemostatic performance of tranexamic acid-loaded chitosan/alginate composite microparticles. Biomed Res Int 2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang H, Yin Y, et al. (2012). Controlled acetylation of water-soluble glucomannan from bletilla striata. Carbohydr Polym 89:158–62. [DOI] [PubMed] [Google Scholar]
- Lord MS, Cheng B, McCarthy SJ, et al. (2011). The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 32:6655–62. [DOI] [PubMed] [Google Scholar]
- Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. (2009). Oral ulcers: clinical aspects. a tool for dermatologists. Part I. acute ulcers. Clin Exp Dermatol 34:289–94. [DOI] [PubMed] [Google Scholar]
- Muzurovic S, Babajic E, Masic T, et al. (2012). The relationship between oral hygiene and oral colonisation with candida species. Med Arch 66:415–7. [DOI] [PubMed] [Google Scholar]
- Muzzarelli RAA. (2009). Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr Polym 76:167–82. [Google Scholar]
- Nandi SK, Kundu B, Basu D. (2013). Protein growth factors loaded highly porous chitosan scaffold: a comparison of bone healing properties. Mater Sci Eng C 33:1267–75. [DOI] [PubMed] [Google Scholar]
- Natah SS, Konttinen YT, Enattah NS, et al. (2004). Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg 33:221–34. [DOI] [PubMed] [Google Scholar]
- Park H, Amiji M, Park K. (1989). Mucoadhesive hydrogels effective at neutral PH. In Proc Int Symp Control Release Bioact Mater 16:217–8. [Google Scholar]
- Patel VM, Prajapati BG, Patel MM. (2007). Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS Pharm Sci Tech 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricia Ortiz Vega A, Chimenos Küstner E. (2002). Diagnóstico Diferencial de Las Úlceras Orales. Piel 17:119–27. [Google Scholar]
- Perioli L, Ambrogi V, Giovagnoli S, et al. (2007). Mucoadhesive bilayered tablets for buccal sustained release of flurbiprofen. AAPS Pharm Sci Tech 8:E20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaechamud T, Yodkhum K, Charoenteeraboon J, Tabata Y. (2015). Chitosan-aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater Sci Eng C 50:210–25. [DOI] [PubMed] [Google Scholar]
- Rodriguez CF, Bruneau N, Barra J, Alfonso D. (2000). Hydrophilic cellulose derivatives as drug delivery carriers: influence of substitution type on the properties. Handb Pharm Control release Technol 1–30. [Google Scholar]
- Rogers RS. (1977). Recurrent aphthous stomatitis: clinical characteristics and evidence for an immunopathogenesis. J Invest Dermatol 69:499–509. [DOI] [PubMed] [Google Scholar]
- Rogers RS. (1997). Recurrent aphthous stomatitis: clinical characteristics and associated systemic disorders. Semin Cutan Med Surg 16:278–83. [DOI] [PubMed] [Google Scholar]
- Rogers RS. (2003). Pseudo-Behçet’s disease. Dermatol. Clin 21:49–61. [DOI] [PubMed] [Google Scholar]
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. (2015). Oral ulcers: differential diagnosis and treatment. Med Clínica 145:499–503. [DOI] [PubMed] [Google Scholar]
- Scully C, Porter S. (2008). Oral mucosal disease: recurrent aphthous stomatitis. Br J Oral Maxillofac Surg 46:198–206. [DOI] [PubMed] [Google Scholar]
- Stoopler ET, Musbah T. (2013). Recurrent aphthous stomatitis. CMAJ 185:120706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada G, Piccirilli GN, Sortino M, et al. (2017). Formulation and in-vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater Sci Eng C 79:140–50. [DOI] [PubMed] [Google Scholar]
- Terai H, Ueno T, Suwa Y, et al. (2018). Candida is a protractive factor of chronic oral ulcers among usual outpatients. Jpn Dent Sci Rev 54:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Luo W, Li P, et al. (2017). Preparation and evaluation of chitosan/alginate porous microspheres/bletilla striata polysaccharide composite hemostatic sponges. Carbohydr Polym 174:432–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.