Abstract
Fine and ultra-fine particulate matter (PM) are major constituents of urban air pollution and recognized risk factors for cardiovascular diseases. This review examined the effects of PM exposure on vascular tissue. Specific mechanisms by which PM affects the vasculature include inflammation, oxidative stress, actions on vascular tone and vasomotor responses, as well as atherosclerotic plaque formation. Further, there appears to be a greater PM exposure effect on susceptible individuals with pre-existing cardiovascular conditions.
Keywords: particulate matter, air pollution, lungs, vascular tissue, endothelial cells, inflammation, vascular tone, oxidative stress, atherosclerotic plaque
Introduction
The 2004 American Heart Association statement and subsequent 2010 update on “Air Pollution and Cardiovascular Disease” concluded that air pollution exposure contributes to cardiovascular morbidity and mortality (Brook et al. 2004; 2010; Cohen et al. 2017). Short-term exposure to particulate matter (PM) immediately impacts cardiovascular health and long-term exposure reduces life expectancy by months to years (Brook et al. 2010; Chen et al. 2019). PM smaller than 10 μm in diameter (PM10) and fine PM (PM2.5 or smaller than 2.5 μm) are major constituents of urban air pollution and recognized as risk factors for mortality (Tsai et al. 2014; Pinichka et al. 2017; Burnett et al. 2018; Landrigan et al. 2018; Initiative 2019). Studies over a broad range of geographical regions indicate that each 10μg/m3 rise in ambient fine particulate matter (PM2.5) concentrations increases daily mortality rates by approximately 1–5% (Pope et al. 2002, Vodonos et al. 2018; Burnett et al. 2018; Initiative 2019; Lelieveld et al. 2019). The size and composition of PM, including water soluble inorganic ions such as sulfate, nitrate, ammonium, sodium, water insoluble particles such as black carbon, redox active trace elements and metals including copper, vanadium, chromium, manganese, iron and nickel, as well as organic compounds such as polycyclic aromatic hydrocarbons (PAHs), were shown to influence its potential toxicity (Schroeder et al. 1987, See et al. 2007, Zou et al. 2016, Forman and Finch 2018).
Although debate exists as to whether PM produces vascular dysfunction via direct particulate entry into the systemic circulation, or through release of mediators into the bloodstream via affected lung tissue (Donaldson et al. 2001, Utell et al. 2006; Robertson et al. 2012), defining potential modes and routes of PM entry is beyond the scope of this review. This article examines the current experimental and clinical literature to provide a comprehensive review focused on the effects of air pollution, specifically PM, on vascular inflammation, vessel tone, reactive oxygen species (ROS) generation, and atherosclerotic plaque formation (Figure 1 for visual depiction). A clear understanding of these relationships may help identify at-risk populations and determine targets for future interventions and/ or treatments.
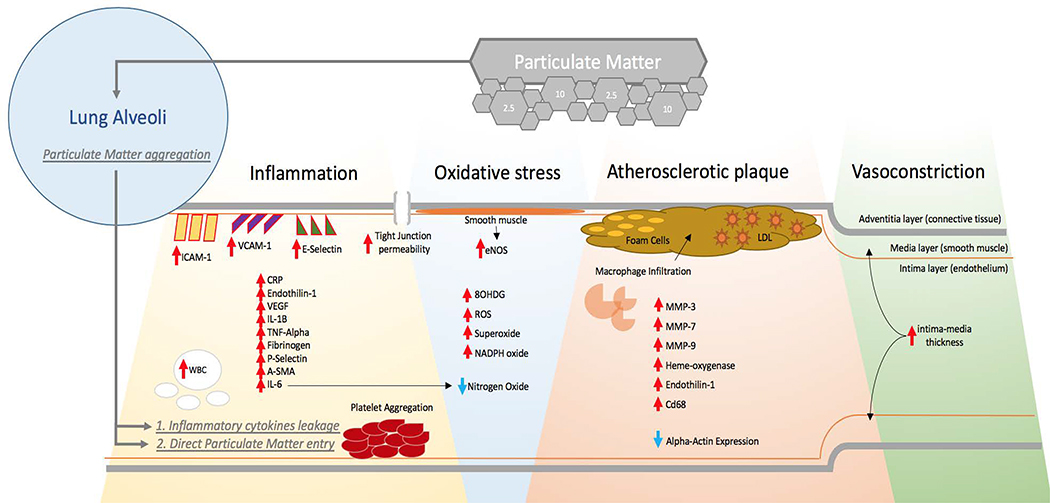
Figure 1.
Schematic of the Particulate Matter effects on Vasculature
Particulate matter (PM) effects the vasculature either via direct entry into the bloodstream or by inciting an inflammatory response in the lung alveoli that results in secondary cytokine leakage into the systemic circulation. PM exposure is associated with increased platelet aggregation, and elevated levels of WBCs, CRP, Endothilin-1, VEGF, IL-1B, TNF-alpha, Fibrinogen, P-Selectin, A-SMA, and IL-6 in the peripheral blood. PM activates endothelial adhesion molecules including ICAM-1, VCAM-1, and E-Selectin. Exposure decreases eNOS production in smooth muscles and generates oxidative stress through 8OHDG, ROS, superoxide, and NADPH Oxide production. At the endothelial cell level, PM alters tight junction permeability and causes BBB leakage. PM exposure promotes macrophage infiltration, foam cell formation, and elevations in LDL, MMP-3, MMP-7, MMP-9, Heme-oxygenase, Endothelin-1, and CD68. Changes in endothelial function secondary to PM exposure cause vasoconstriction, increases in intima-media thickness and impaired vascular tone.
Methods
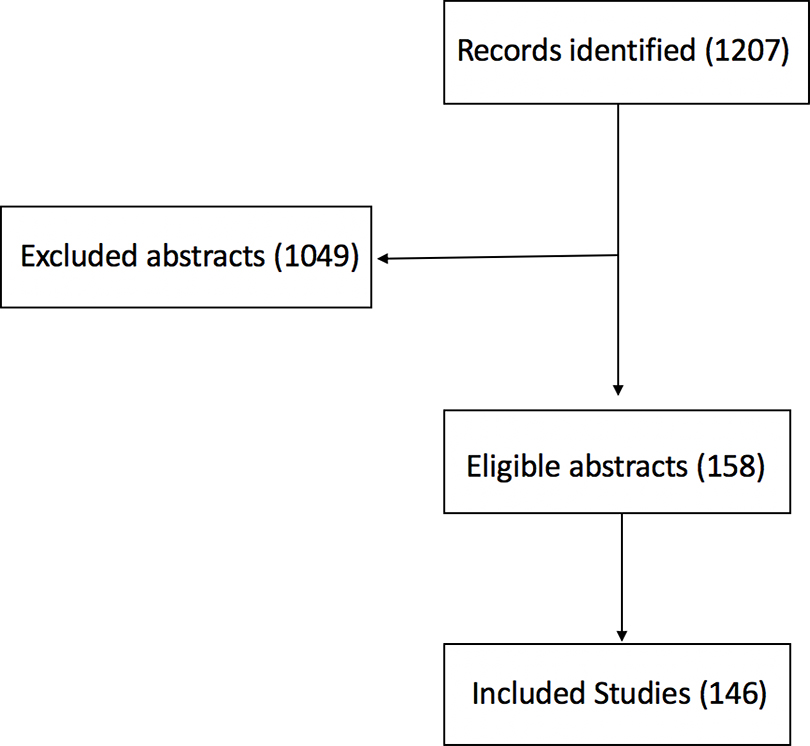
A keyword search of recent PubMed articles published between 2000 and 2020 was performed. The literature search rationale included published review articles that focused on: 1)global overviews of epidemiological and population studies relating PM effect to cardiovascular morbidity and mortality; 2) effects of air pollution constituents on cardiovascular health; 3) review studies that examine the negative effects of PM exposure on cardiac tissue, brain, and other organs; 4) reviews that provide an overview of the inflammatory, oxidative, vasoconstrictive, and atherosclerotic mechanisms of PM effects in the setting of cardiovascular disease. In addition, the literature search included original research articles that examined the relationship between air pollution and the following vascular processes: inflammation, vascular tone, oxidative stress, and atherosclerotic plaque formation (Table 1 for literature search terms used). Titles, abstracts, and full-text articles of potentially relevant studies were screened at different stages of the literature search (flow chart of literature search presented in Figure 2).
Table 1.
Search Criteria
| Section | Search terms |
|---|---|
| Particulate Matter and Vascular Inflammation | Air pollution arteries inflammation |
| Particulate matter arteries inflammation | |
| Air pollution inflammation cardiovascular endothelial cells | |
| Particulate matter inflammation cardiovascular endothelial cells | |
| Effect of Particulate Matter on Vascular Tone | Air pollution vascular tone |
| Particulate matter vascular tone | |
| Air pollution arterial tone | |
| Particulate matter arterial tone | |
| Effects of Particulate Matter on Oxidative Stress | Air pollution vasculature oxidative stress |
| Particulate matter vasculature oxidative stress | |
| Air pollution arteries oxidative stress | |
| Particulate matter arteries oxidative stress | |
| Effect of Particulate Matter on Atherosclerotic Plaque Formation | Air pollution atherosclerosis |
| Particulate matter atherosclerosis | |
| Air pollution and plaque formation | |
| Air pollution plaque remodeling | |
Figure 2:
Flow Chart of Literature Search
Study selection criteria
This review includes investigations that used in-vitro cell cultures of animal and human origins, animal subjects, healthy participants, and patients with relevant medical conditions. Study selection was not limited by age or gender. Studies with experimental exposures derived from ambient, vehicular, or combustion-related sources were included. Investigations that used cigarette smoke as exposure were excluded. Studies examining the effects of air pollution on carotid arteries, aorta, coronary arteries, arteries of the lung, cerebral arteries, and umbilical cord veins were considered for this review. The literature regarding systemic inflammation and circulating factors was included only when vascular effects were incorporated as outcomes. Selection of outcome variables was not limited. Eligible study designs included experimental research, clinical trials, epidemiology/ population studies, and crossover investigations. The literature search was not limited by study sample size or the impact factor of the study journal. Only articles written in English were included. The following data was extracted from each investigation: first author and publication year, study design, exposure type, sample size, sample characteristics, outcome measures, and study findings.
Survey of current vascular/cardiovascular published reviews: existing data and knowledge gaps (Table 2)
Table 2.
Current vascular/cardiovascular published reviews
| Author, Year | Title | Review Summery |
|---|---|---|
| Section A | ||
| (Alfaro-Moreno et al. 2007) | Particulate matter in the environment: pulmonary and cardiovascular effects | Respiratory effect of particulate matter, pre and postnatal effects of air pollution, lung function, allergic reactions in air pollution exposure and extrapulmonary transition of air pollution and the effect of Pm on cardiovascular morbidity and mortality |
| (Simkhovich, et al. 2008) | Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms | Size and composition of ambient particles, morbidity and mortality of PM in CVD in clinical studies. Pulmonary toxicity, ROS mechanisms in heart and lung tissue |
| (Franchini and Mannucci 2009) | Particulate air pollution and cardiovascular risk: short-term and long-term effects | Mechanisms of air pollution entry, clinical and epidemiological studies of short-term and long-term effect of PM on cardiovascular system morbidity and mortality |
| (Hassing et al. 2009) | Air pollution as noxious environmental factor in the development of cardiovascular disease | Short and long-term air pollution exposure general effects on CVD in the Netherlands |
| (Fang, et al. 2010) | A Systematic Review of Occupational Exposure to Particulate Matter and Cardiovascular Disease | Epidemiological data on Ischemic heart disease, cerebrovascular disease morbidity and mortality risks in occupational exposure to PM |
| (Sun, et al. 2010) | Cardiovascular effects of ambient particulate air pollution exposure | Cardiac events and rates of hospital admission and exposure to PM, cardiac function, effect of PM on heart rate variability, and hypertension |
| (Franchini and Mannucci 2011) | Thrombogenicity and cardiovascular effects of ambient air pollution | Pulmonary inflammation and direct entry of PM into the systemic circulation and population studies of short-term and long-term effect of PM on cardiovascular system morbidity and mortality |
| (Lippmann 2014) | Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: coherence and public health implications | PM toxicity and Air pollution related morbidity and mortality in cerebrovascular and cardiovascular diseases |
| (Cosselman, et al. 2015) | Environmental factors in cardiovascular disease | Epidemiological data on air pollution exposure and cardiovascular risks (Stroke, cardiac dysfunctions), effect of CVD of arsenic, cadmium, lead. Public health implications of PM exposure |
| (Nasser, et al. 2015) | Outdoor particulate matter (PM) and associated cardiovascular diseases in the Middle East | Regional characteristics of association of PM levels and incidence on CVD in the middle east |
| (Du et al. 2016) | Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence | Direct and indirect actions of PM on cardiovascular system, epidemiological data on cardiovascular mortality, ischemic heart disease, cardiac arrest, blood pressure and thrombosis in PM exposure |
| (Bourdrel, et al. 2017) | Cardiovascular effects of air pollution | Outdoor air pollution sources, epidemiological evidence for air pollution exposure and cardiovascular morbidity and mortality, coronary artery disease, other cardiac outcomes and air pollution exposure, effects of air pollution policies, review cites only clinical studies |
| (An, et al. 2018) | Impact of Particulate Air Pollution on Cardiovascular Health | Long-term and short-term cardiovascular effect of PM exposure (hospital admissions rate and hypertension), clinical studies describing PM modes of entry, dietary and pharmacological intervention |
| Section B | ||
| (Watkinson, et al. 2001) | Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter | Ozone metals and PM in cardiovascular models of rodents |
| (Gray, et al. 2015) | Respiratory and cardiovascular effects of metals in ambient particulate matter: a critical review | The effect of metal components of air pollution on the respiratory and CVD |
| (Luben, et al. 2017) | A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon | Exposure to PM and CVD risks with particular emphasis on the effects of black carbon. CVD related emergency department visits, hospital admissions and mortality are reviewed. |
| (Boovarahan and Kurian 2018) | Mitochondrial dysfunction: a key player in the pathogenesis of cardiovascular diseases linked to air pollution | Air pollution and non-communicable diseases, mitochondrial dysfunction in air pollution, relationship between carbon monoxide, nitric oxide, sulfur dioxide, hydrogen sulfide, heavy metals and cardiovascular disease. |
| (Kirrane, et al. 2019) | A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter | Comparison of Black Carbon and PM effects on CVD, including autonomic nervous system tone, heart rate, ischemia and repolarization abnormalities |
| Section C | ||
| (Sandhu, et al. 2005) | Ambient particulate matter, C-reactive protein, and coronary artery disease | Association of CRP and inflammatory markers with morbidity and mortality in cardiac and CVD outcomes in patients exposed to PM |
| (Frampton 2006) | Inflammation and airborne particles | Lung vascular inflammation and PM effect on cardiovascular system via lung inflammation |
| (Frampton 2007) | Does inhalation of ultrafine particles cause pulmonary vascular effects in humans? | Clinical studies of pulmonary inflammation and systemic effects and mode of PM entries |
| (Polichetti, et al. 2009) | Effects of particulate matter (PM(10), PM(2.5) and PM(1)) on the cardiovascular system | Association between PM and smoking with CVD morbidity and mortality and mechanisms of tissue inflammation and oxidative stress are discussed |
| (Nelin,et al. 2012) | Direct and indirect effects of particulate matter on the cardiovascular system | Direct and indirect effect of PM on hypertension, nervous system. Inflammatory responses and reactive oxygen species production in the lungs in PM exposure |
| (Grunig,et al. 2014) | Perspective: ambient air pollution: inflammatory response and effects on the lung’s vasculature | Effect of urban air pollution and cigarette smoke on the airways and lung inflammation, pulmonary hypertension, arterial remodeling by inflammatory immune response. |
| Section D | ||
| (Gonzalez-Flecha 2004) | Oxidant mechanisms in response to ambient air particles | Pro-oxidant properties of ambient particulate matter, mechanisms of CAPs induced oxidative stress and toxicity, cardiac effects of ambient air particles |
| (Huang and Ghio 2006) | Vascular effects of ambient pollutant particles and metals | Ambient pollutant particles and transition metal effects in vasoconstriction |
| (Nogueira 2009) | Air Pollution and Cardiovascular Disease | Oxidative stress literature in animals and humans discussed as a central pathway of endothelial dysfunction, heart rate variability, vasoconstriction, and hypertension in long term PM exposure. Short term PM exposure effect evaluated on myocardial and ischemic events and sudden death |
| (Miller, et al. 2012) | From particles to patients: oxidative stress and the cardiovascular effects of air pollution | Ultrafine particles and cardiovascular epidemiology, oxidative stress mechanisms in PM exposure and their effect on CVD development and progression |
| (Miller 2014) | The role of oxidative stress in the cardiovascular actions of particulate air pollution | Review of oxidative stress as a mediator of PM effect in CVD and potential interventions |
| (Kelly and Fussell 2015) | Linking ambient particulate matter pollution effects with oxidative biology and immune responses | Effects of PM on airways and pulmonary epithelium, cardiovascular system and oxidative potential of PM |
| (Lawal 2017) | Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways | Oxidative stress and inflammation in PM induced cardiovascular disease and atherosclerosis with emphasis on Nrf2 and AhR dependent regulatory pathways |
| (Yan, et al. 2016) | Inflammatory cell signaling following exposures to particulate matter and ozone | ROS formation and oxidative stress induced by PM and O3 exposure, EGRF and TLR signaling pathways |
| (Zanoli, et al. 2017) | A systematic review of arterial stiffness, wave reflection and air pollution | PM effect on arterial stiffness |
| Section E | ||
| (Gill, et al. 2011) | Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis | Air pollution effect on CVD based on the findings Multi-Ethnic Study of Atherosclerosis |
| (Moller, et al. 2016) | Atherosclerosis and vasomotor dysfunction in arteries of animals after exposure to combustion-derived particulate matter or nanomaterials | Air pollution effect on vascular vasomotor function and atherosclerotic plaque formation, with emphasis on different chemical components of air pollution |
| (Moller, et al. 2011) | Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis | Development of atherosclerosis, reduced vasoconstriction in animal and clinical models |
| (Prueitt, et al. 2015) | Evaluation of atherosclerosis as a potential mode of action for cardiovascular effects of particulate matter | PM effect on atherosclerosis and thrombosis in animal models |
| (Akintoye, et al. 2016) | Association between fine particulate matter exposure and subclinical atherosclerosis: A meta-analysis | Meta-analysis of carotid media intima thickness, arterial calcification, and ankle-brachial index as noninvasive measures of clinical and subclinical atherosclerosis |
| (Bai and Sun 2016) | Fine particulate matter air pollution and atherosclerosis: Mechanistic insights | Development of atherosclerotic plaque and mechanisms of PM mediated atherosclerosis |
| (Cao, et al. 2016) | Foam cell formation by particulate matter (PM) exposure: a review | Foam cell formation and development of atherosclerosis in PM exposure |
| Section F | ||
| (Brook 2008) | Cardiovascular Effects of Air Pollution | Characteristics of PM air pollution, epidemiology of cerebrovascular disease and PM, geography and sources of pollution, arrhythmias, heart failure, thrombosis and atherosclerosis in PM exposure in clinical studies |
| (Donaldson, et al. 2001) | Ambient Particle Inhalation and the Cardiovascular System: Potential Mechanisms | Ultrafine particles in urban air, Pathogenesis of PM particles, oxidative stress and modulation of Calcium in tissues, PM effect on epithelial permeability and atherosclerosis in cardiovascular health, and epidemiological evidence of systemic oxidative stress in susceptible populations |
| (Fiordelisi, et al. 2017) | The mechanisms of air pollution and particulate matter in cardiovascular diseases | Cardiac morbidity and mortality in epidemiological studies of PM exposure, modes of translocation into the cardiovascular system and pulmonary oxidative stress and inflammation, disturbance of autonomic nervous system, discussion of potential therapeutic approaches |
| (Franchini, et al. 2012) | Air pollution, vascular disease and thrombosis: linking clinical data and pathogenic mechanisms | Epidemiological cardiovascular mortality and morbidity related to PM exposure, experimental studies and pathophysiological mechanisms of cardiac and vascular function, hemostatic unbalance, ROS and atherosclerosis |
| (Franklin, et al. 2015) | Air pollution and cardiovascular disease | Epidemiologic studies of air pollution and secondary smoke effect on short-term and long-term CVD and mortality risks. Air pollution and exercise. biological pathways in PM effect on hypertension, insulin resistance, diabetes, atherosclerosis, coagulation and thrombosis, autonomic imbalance, and global burden of disease. |
| (Grahame and Schlesinger 2010) | Cardiovascular health and particulate vehicular emissions: a critical evaluation of the evidence | Epidemiological evidence of association between CVD and PM, Mechanistic evidence of PM effect on heart rate variability, cardiac arrhythmia, tissue inflammation, atherosclerosis, and blood pressure |
| (Langrish, et al. 2012) | Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms | Effect of PM on vasoconstriction, blood pressure, myocardial ischemia, heart rate variability, systemic inflammation, thrombosis, atherosclerosis in controlled human studies |
| (Martinelli et al. 2013) | Air particulate matter and cardiovascular disease: a narrative review | Measurements of PM, epidemiological studies linking PM to cardiovascular disease, autonomic and hemostatic system activation, pulmonary oxidative stress and inflammation. Effect of PM on susceptible populations. |
| (Newby et al. 2015) | Expert position paper on air pollution and cardiovascular disease | Air pollution related morbidity and mortality in cardiac diseases, venous thromboembolism, pulmonary inflammation and ROS, epigenetic changes, and air quality recommendations |
| (Shrey, et al. 2011) | Air pollutants: the key stages in the pathway towards the development of cardiovascular disorders | Review of mechanisms linking PM exposure to thrombosis, atherosclerosis, myocardial dysfunction, and cardiac autonomic control in CVD |
| (Wang, et al. 2017) | Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease | Routes of PM entry, neurotoxicity of PM and neurodegenerative disease, learning and memory impairment, neuroinflammation, behavioral changes, and oxidative stress |
| (Hamanaka and Mutlu 2018) | Particulate Matter Air Pollution: Effects on the Cardiovascular System | Air pollution composition and morbidity and mortality epidemiological data - clinical data only, effects of air pollution on inflammation in adipose tissues, oxidative stress and atherosclerosis, effect on blood pressure, metabolic syndrome and insulin resistance, epigenetic changes, and BMI role |
| (Vidale and Campana 2018) | Ambient air pollution and cardiovascular diseases: From bench to bedside | Air pollution composition and sources, pathways of air pollution entry into the system, atherosclerosis, thrombosis and coagulation pathways affected by air pollution, insulin resistance diabetes and metabolic syndrome, arterial hypertension, autoimmune imbalance |
| Section G | ||
| (Brook, et al. 2004) | Air Pollution and Cardiovascular Disease A Statement for Healthcare Professionals From the Expert Panel on Population and Prevention Science of the American Heart Association | Statement from Health Professionals from the Expert Panel on Population and Preventive Science of the American Heart Association: Ambient Air Pollution and Secondary smoke effect on and cardiovascular disease. Long- and short-term epidemiological health effect studies and at-risk populations, PM effect on congenital heart disease, biological mechanisms of pulmonary and systemic oxidative stress and inflammation |
| (Mills et al. 2007) | Air pollution and atherothrombosis | Pulmonary and systematic inflammation, effect PM on vascular inflammation atherosclerosis, vasomotor funcntion and plaque stability |
Over 50 published literature reviews describe the effects of air pollution on cardiovascular health and disease which are referenced in Table 2. The papers in Table 2 Section A provide global overviews of the epidemiological and population studies relating PM effect to cardiovascular morbidity and mortality. Reviews in Table 2 Section B examine the effects of air pollution constituents on cardiovascular health. Table 2 sections C-E reviews provide an overview of the distinct mechanisms of PM effects in the setting of cardiovascular disease. These mechanisms include inflammation (See Table 2 Section C), oxidative stress and vasoconstriction (See Table 2 Section D), and atherosclerosis (See Table 2 Section E). Table 2 Section F includes a large number of review studies that examine the adverse effects of PM exposure on cardiac tissue, brain, and other organs.
Although prior review articles described the effects of PM with respect to cardiovascular diseases, this review is unique in its reporting and analysis of recent evidence supporting a link between PM exposure and cardiovascular diseases attributed to actions specifically on vascular tissue. Several other reviews described inflammatory, oxidative, and atherosclerotic processes in vascular tissue associated with PM exposure, however the evidence is not from recent studies (Table 2 Section G). A more current single review by Rajagopala et al (2018) provides an overview of these processes in the context of cardioembolic and autonomic nervous system pathogenesis; however, an in-depth focus on vascular mechanisms was not the purpose of this paper. No apparent prior reviews focused exclusively on vascular tissue. The current review provides a detailed report and analysis of endothelium-specific inflammatory, vasomotor, oxidative, and atherosclerotic effects of air pollution, specifically fine and ultrafine PM. In addition, this review addresses the susceptibility of individuals with underlying cardiovascular conditions to adverse effects of PM from a vascular perspective.
Effect of particular matter on vascular inflammation (Table 3, Figure 1)
Table 3.
Particulate Matter and Vascular Inflammation: Literature Summary
| Authors, Year | Methods | Main Findings |
|---|---|---|
| Liu et al. 2018 | Mice exposed by inhalation to filtered or ambient air | Accumulation of CD45þ lymphocytes and CD68þ macrophages in pulmonary arterioles. TNF-a, IL-6 and IL-13 markedly elevated in lung tissues |
| Green et al. 2016 | Women enrolled in the multi-ethnic, longitudinal Study of Women’s Health Across the Nation (six sites) | Exposures to PM2.5 and ozone associated with adverse effects on inflammation and hemostatsis |
| Michikawa et al. 2016 | 2360 Japanese participants | Positive association of PM with hs-CRP and WBC count |
| Pope et al. 2016 | 24 healthy, non-smokers exposed to 3 periods of ambient PM | Elevated levels of circulating monocytes, T lymphocytes, iendothelial microparticles, sICAM-1, and sVCAM-1 |
| Dabass et al. 2016 | Adult NHANES trial participants from 2001 to 2008 | No overall association between PM2.5 and biomarkers of cardiovascular risk |
| Wolf et al. 2016 | 2,944 participants of the KORA (Cooperative Health Research in the Region Augsburg) | Individuals with prediabetes have larger effects due to PM exposure |
| Shisler et al. 2015 | Human coronary artery endothelial cells cultured with plasma from 6 healthy volunteers exposed to filtered air or diesel exhaust PM (100mg/m3) for 2 hours | Inflammatory pathways and cytokines upregulated both at 2 and 24 hours after DE exposure |
| Viehmann et al. 2015 | Heinz Nixdorf Recall Study, a German population-based prospective cohort of 4814 participants | Long-term exposure to PM associated with increased hs-CRP and platelets |
| Lanki et al. 2015 | Six adult cohorts from Central and Northern Europe | Living in close proximity to busy traffic associated with elevated CRP concentrations |
| Hampel et al. 2015 | European ESCAPE and TRANSPHORM multi-center projects | Increase in PM2.5 copper and PM10 iron were associated with increase in hsCRP |
| Siponen et al. 2015 | 52 ischemic heart disease patients | C-reactive protein, interleukin-12 and myeloperoxidase elevation in air pollution |
| Johannesson et al. 2014 | Panel study in healthy adults | CRP blood levels fluctuations in different dose PM exposures |
| Lee et al. 2014 | Community adults in a densely populated inner city neighborhood in Boston, Massachusetts | At ambient levels oxidative stress and systemic inflammation exacerbate cardiac autonomic responses to PM2.5 |
| Wang et al. 2013 | Rats exposed to intrathecal PM2.5 alone, PM2.5 and ozone, ozone alone, or fresh air | Increased blood plasma concentrations of endothelin-1 and lower serum VEGF result in inflammation and endothelial damage |
| Oppenheim et al. 2013 | Apolipoprotein (Apo) E−/− and C57Bl6 mice exposed to DE or filtered air (FA) | MVE-exposed Apo E−/− mice demonstrated BBB permeability, elevated ROS and MMP-2/ MMP-9 activity when compared to FA controls |
| Niu et al. 2013 | Female residents of Jinchang and Zhangye, China | The levels of CRP, IL-6, and VEGF were significantly higher in more polluted areas |
| Krishnan et al. 2013 | 17 individuals with metabolic syndrome (MetS) and 15 healthy subjects inhaled filtered air or diesel exhaust in two-hour different day sessions with a minimum 2-week washout period | short-term DE exposure resulted in cardiovascular events (hemoconcentration and thrombocytosis) |
| Rask-Madsen at al. 2013 | DM and glucose | DM and glucose intolerance are independently associated with vascular inflammation |
| Davel et al. 2012 | Wistar rats exposed to urban PM2.5 for two weeks | Decreased endothelial-dependent relaxation and eNOS expression with high local TNF-a levels |
| Channell et al. 2012 | hCAES obtained from healthy individuals exposed to filtered air, diesel exhaust PM (100mg/m3), or NO2 (500ppb) for 2 hours | Exposure to both diesel PM and NO2 leads to a proinflammatory response marked by increases ICAM-1, VCAM-1, and IL-8 |
| Lee et al. 2012 | Cells of the human monocytic leukemia cell line and human umbilical vein endothelial cells | mRNA and protein expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion mole- cule-1 (ICAM-1) were upregulated in HUVECs after incubation with exhaust particles |
| Robertson et al. 2012 | Wistar rats exposed to DEP or saline vehicle by intratracheal instillation and hind-limb blood flow | DEP instillation increased cell counts, total protein and IL-6 in BALF 6 h after exposure. Levels of IL-6 and TNFa were only raised in blood 24 h after DEP exposure |
| Guo et al. 2012 | Wistar rats with intratracheal PM10 exposure | Upregulation of endothelial mediators (ET-1 and eNOS) and inflammatory markers (IL-1, TNF-, COX-2, iNOS and ICAM-1) |
| Rich et al. 2012 | 125 healthy adults | CRP blood levels went down from 55% during the pre-Olympic period (high pollutant levels) to 46% during the Olympic games |
| Tsai et al. 2012 | Individuals exposed to short-term PM | Increased circulating levels of IL-1b, IL-6, and TNF-a |
| Huttunen et al. 2012 | 52 elderly individuals with history of ischemic heart disease | Upregulation of IL-12 and CRP within days of exposure |
| Cherng et al. 2011 | Coronary artery of rats exposed to diesel PM or filtered air | NOS uncoupling and generation of reactive oxygen species results in endothelial dysfunction and impaired vasodilation |
| Alexeeff et al. 2011 | 642 elderly men from the Veterans Administration (VA) Normative Aging Study | positive associations between air pollution and sICAM-1 for aver- ages of 4, 8, and 12 weeks |
| Hoffmann et al. 2009 | Population based prospective cohort study of 4,814 subjects | Elevated circulating hs-CRP and fibrinogen |
| Rudez et al. 2009 | 40 healthy volunteers | 13 consecutive CPR blood measurements over the throughout 1 year were not associated with 1 to 4-day changes in PM exposure levels |
| Knuckles et al. 2008 | Isolated arteries and veins of animals immersed in diesel PM | DE induced uncoupling of eNOS in-vivo and ex-vivo |
| Bräuner et al. 2008 | 29 subjects participated in a randomized, two-factor crossover study with or without biking exercise | Exposure to outdoor air pollution particles was not associated with systemic inflammation |
| Scapellato et al. 2007 | Systematic review of evidence | Activated leukocytes and endothelial PDGF and IL-8 regulate inflammation |
| Chuang et al. 2007 | 76 healthy young non-smokers in China | Variations in 1 and 3 day PM10 exposure levels were associated with changes in CRP blood concentrations |
| Yue et al. 2007 | Male coronary artery disease patients | Increases in CRP correlation with traffic-related particles and combustion-generated aerosols |
| O’Neill et al. 2007 | 92 Boston area residents with type 2 diabetes | Consistently positive association between air pollution and inflammatory markers |
| Saura et al. 2006 | Culture of human aortic endothelial cells | IL-6 inhibits eNOS via a Stat3 dependent mechanism, reducing NOS levels and leading to endothelial dysfunction |
| Kristovich et al. 2004. | Human endothelial cells treated with human monocyte derived macrophages exposed to sublethal particulate matter for 24 hrs | Upregulation of ICAM-1, VCAM-1, E-selectin on endothelial cells |
| Van Eeden et al. 2001 | 30 healthy males exposed to PM10 for 2 months | Increased levels of circulating of IL-1b, IL-6, GM-CSF, and TNF-a |
| Ridker et al. 2000 | Longitudinal women’s health study | CRP - a strong predictor of future cardiovascular events |
Experimental models
Vascular endothelium, which mediates vasodilation, inflammation, and platelet aggregation, is susceptible to the effects of PM (Saura et al. 2006, Knuckles et al. 2008, Cherng et al. 2011) It has long been recognized that systemic inflammation results in vascular endothelial dysfunction and triggers cardiovascular events (Scapellato and Lotti 2007). In rats, administration of intratracheal PM2.5 (3.2 mg/rat dose) twice a week for three weeks resulted in a 50% increase in plasma C-reactive protein (CRP) levels and a 20% elevation in plasma endothelin-1 levels (Wang et al. 2013). Following murine diesel exhaust particle (DEP) inhalation, pro-inflammatory cytokines were upregulated in the lungs (66% increase in tumor necrosis factor-alpha (TNF-α), 127% rise in interleukin-6 (IL-6), and 87% elevation in IL-13 over controls). In addition to observed inflammation in the lungs, apoptosis of endothelial cells on the pulmonary artery was observed by a 450% increase in α -smooth muscle actin (α -SMA - marker of apoptosis) in TUNEL assays (estimated from graph data) (Liu et al. 2018). Although experimental evidence supports PM exposure-induced endothelial inflammation, significant variability exists between studies. Rats exposed to 2-weeks of PM2.5 via inhalation exhibited decreased endothelial nitric oxide synthase (eNOS) expression with elevated TNF-α protein expression in pulmonary arteries. However, no marked changes were detected in systemic inflammatory markers, including plasma blood cell count, cytokine levels, and coagulation factors (Davel et al. 2012). These inconsistencies may relate to variation in animal age, exposure duration and/ or unique assay kits produced by different manufacturers (Wang et al. 2013). Further, variations in data exist based upon mouse strain and underlying genetic differences.
In-vitro cell culture studies also demonstrate the effects of particulate exposure on vascular inflammation. Primary human coronary artery endothelial cells (hCAECs) treated with blood plasma obtained from humans before and after exposure to 100 μg/m3 DEP or filtered air for 2 hr displayed 20% elevations in levels of vascular cellular adhesion molecule 1 (VCAM-1), and 10% rise in IL-8 following DEP exposure (Channell et al. 2012). In the same model, genomic analysis of hCAEC culture exposed to the plasma of subjects after DEP inhalation confirmed upregulation of inflammatory pathways related to ligand-receptor interactions directly on endothelial cells (Schisler et al., 2015). Two additional studies (Kristovich et al., 2004; Lee et al. 2012) demonstrated that in-vitro endothelial cell activation increased ICAM-1 (300% in both studies) and VCAM-1 (1000% and 230%, respectively) expression following administration of peripheral blood monocytes exposed to DEP through NFk-b activation. hCAEC methodologies allow for identification of receptors, ligands and mechanistic pathways that mitigate endothelial cell responses. However, in-vitro approaches often do not use inhalation/aerosol exposure for PM delivery, which may affect mechanisms of PM translocation into the system.
The relationship between systemic inflammation and cytokine expression is complicated not only by experimental design but also by heterogeneity in the inflammatory time course and PM dose effects. A single intratracheal dose of PM2.5 administration in rats resulted in a marked 1000% increase in BALF protein levels of IL-6 expression in pulmonary arteries despite no observed changes in BALF protein levels of CRP and TNF-α 6 hr after treatment. However, roughly 400% rise in plasma TNF-α and 200% rise in plasma IL-6 blood concentrations were noted at 24 hr after exposure when compared to 6 hr (estimated from graph data) (Robertson, Gray et al. 2012). Guo et al (2012) exposed Wistar rats to aerosolized PM10 at different concentrations (0.3,1, 3 or 10 mg/kg) for 15 days. Plasma endothelin-1 was elevated at all concentrations of PM10 (1.55, 1.62, 1.67, and 1.7 over controls for each concentration, respectively), and mRNA levels of IL-1 and ICAM-1 were increased at the 3mg/kg exposure dose (127 and 245% respectively compared to controls). Data suggest that PM exposure produced delayed systemic inflammation following the pulmonary response and overall inflammatory profile is dose dependent.
The precise inflammatory mechanisms resulting from PM exposure remain unclear. Experimental evidence suggests parallel endothelial dysfunction from inflammatory substrates and blood brain barrier (BBB) disruption. In particular, activation of the nitric oxide pathways through uncoupling of the endothelial nitric oxide enzyme and subsequent reduction in local expression of nitrogen oxide in the vascular endothelium is associated with reduction in tight junction protein expression (Saura et al., 2006). Mice exposed to mixed vehicle (gasoline and diesel engine) exhaust for 30 days were injected with the molecular tracer, sodium fluorescein, on the final day of exposure. Elevated levels of inflammatory biomarkers (iNOS by 300% and IL-1b by 200%) in cerebral tissue and arteries correlated with decreased levels of tight junction proteins, including a 200% fall in occludin and claudin-5. Elevated endothelial monolayer tracer transfer suggestive of a leaky BBB was observed in mice exposed to vehicle exhaust (Oppenheim et al. 2013). These findings indicated that vehicular pollutants might increase inflammation and endothelial monolayer permeability of peripheral vessels through modification of intracellular gaps and alterations in protein structure of endothelial tight junctions. Evidence suggests that PM-induced endothelial cell and vascular flow changes are multifactorial, which contribute to systemic inflammatory responses through circulating plasma proteins, decreased vascular tone, changes in endothelial cell dynamics, and BBB alterations.
Clinical Studies
Studies of healthy human subjects demonstrated changes in inflammatory biomarkers following air pollution exposure. Blood CRP levels are used clinically as an indicator of the presence and intensity of inflammation and were directly linked to cardiovascular health. In a longitudinal women’s health study, CRP was a reliable predictor of future cardiovascular events. This association was present in subgroups of women with no history of hyperlipidemia, hypertension, smoking, diabetes, or family history of coronary artery disease (CAD) (Mehta et al 2015). Blood CRP levels exhibit strong associations with PM exposure. Analysis of a German population-based cohort study of 4814 participants demonstrated that a 2.4μg/m3 increase in daily surface concentration of PM10 and PM2.5 was associated with a 5.4% elevation in plasma CRP (Viehmann et al. 2015). Similarly, in the Longitudinal Study of Women’s Health Across the Nation (2086 women), plasma CRP rose 21% per 10μg/m3 increase in PM2.5 (Green et al. 2016). Further, prolonged exposure to PM when residing in close proximity to a busy road (> 10,000 vehicles per day) was associated with a 10% elevation in blood CRP levels compared to living on a quiet, residential road (<1000 vehicles per day), as illustrated in a cross-sectional cohort study of 22,561 adults from Central and Northern Europe (Lanki et al. 2015). However, other investigators noted that the correlations between CRP and PM exposure are not straightforward. Hoffmann et al (2009) reported association between CRP levels and annual PM exposure lost significance after adjusting for daily PM exposure levels. This suggests that short-term variations in daily PM levels may impact long term PM-mediated effects.
To evaluate temporal effects of air pollution on inflammatory cytokines, a study of 2360 participants from a diverse Japanese population (participants aged 20 and above from 300 randomly selected districts from all 47 prefectures in Japan) examined short- and long-term effects of background concentration of suspended PM on serum CRP and WBC count (Michikawa et al. 2016). On the day of blood draw, high background concentrations of suspended particulate matter (sPM) and gaseous co-pollutant concentrations were associated with elevated serum WBC counts (113%), while 1-month average sPM concentrations were correlated with increased serum CRP levels (142%). Rich et al (2012) studied 125 healthy adults over the period of the Beijing Olympic Games and concluded that CRP blood levels diminished from 55% during the pre-Olympic period (high pollutant levels) to 46% during the Olympic games, when pollutant levels were strictly controlled. Similarly, Chuang et al. (2007) examined 76 healthy young non-smokers in China and found that variations in 1 and 3 day PM10 exposure levels associated with changes in CRP blood concentrations. However, in 40 healthy volunteers recruited in the Netherlands, 13 consecutive CPR blood measurements throughout 1 year were not correlated with 1 to 4-day changes in PM exposure levels (Rudez et al. 2009). Differences in exposure location, levels, and assessment accuracy need to be taken into account when interpreting clinical studies on PM exposure. It is clear that CRP is a key factor in PM-induced inflammatory responses. Blood CRP levels display more robust elevation trends in longer term studies, while short duration exposures with variable PM levels may not be sufficient to stimulate discernable CRP responses (Johannesson et al. 2014).
Blood samples from healthy human subjects exposed to 50 μg/m3 of PM2.5 over a 3-day period revealed elevations in circulating endothelial apoptosis microparticles (CD14, CD16, CD8, CD4) and T lymphocytes (Pope et al. 2016). With a 10 μg/m3 incremental rise in PM2.5 concentration there was an associated elevation in plasma endothelial adhesion molecules sICAM-1 and sVCAM-1. Further, a 5 μg/m3 increase in long-term ambient PM2.5 was associated with 6% higher IL-6 levels (Pope et al. 2016). In an analysis of participants in the population based CoLaus Swiss cohort study, short-term PM10 exposure (day of visit) induced significant effects on circulating inflammatory markers (van Eeden et al. 2001, Tsai et al. 2012). For every 10 μg/m3 elevation in PM10, IL-1β levels increased by 0.034 pg/mL, IL-6 by 0.036 pg/mL, and TNF-α by 0.024 pg/mL in the blood. In contrast, a study of healthy subjects exposed to particle-rich (PM10–2.5 and PM2.5) air while biking (cross-over study) demonstrated no significant differences in blood inflammatory biomarkers (CRP, fibrinogen, IL-6, TNF-α, lag time to copper-induced oxidation of plasma lipids and protein oxidation measured as 2-aminoadipic semialdehyde in plasma) compared to trials in which subjects were exposed to particle filtered air (Brauner et al. 2008).
Specific elemental PM components may directly influence levels of inflammatory mediators. Niu et al ( 2013) examined PM2.5 levels in Jinchang and Zhangye, China and noted personal and daily exposure levels along with concentrations of inflammatory biomarkers in female residents of each city. Data demonstrated PM2.5 levels to be comparable between the cities (47.4 and 54.5μg/m3, respectively), but nickel (8200%), copper (2600%), arsenic (1200%) and selenium (600%) levels to be higher in Jinchang. Plasma concentrations of CRP (3.44±3.46 vs. 1.55±1.13), IL-6 (1.65±1.17 vs. 1.09±0.60), and vascular endothelial growth factor (117.6±217.0 vs. 22.7±21.3) were significantly elevated in the Jinchang population, suggesting a relationship between elemental PM components and inflammation (Niu et al. 2013). Long-term exposure to transitional metals within ambient PM was associated with elevated inflammatory blood markers [5 ng/m3 increases in PM2.5 copper and 500 ng/m3 rise in PM10 iron associated with 6.3% and 3.6% elevation in hs-CRP, respectively]. Ten ng/m3 increases in PM2.5 zinc was associated with a 1.2% rise in hs-CRP (Hampel, Peters et al. 2015) (Table 2a).
There appears to be a more robust PM exposure effect in susceptible individuals or those with pre-existing cardiovascular conditions. Lee et al (2014) demonstrated that PM2.5 exposure is associated with increased heart rate and reduced heart rate variability in adults living in urban settings with preexisting systemic inflammation. An interquartile range rise in PM2.5 (13.6 μg/m3) was correlated with a reduction in standard deviation of night-time normal to normal heart rate intervals (8.4%, marker of autonomic function). Significantly greater decrease was noted in individuals with elevated blood WBC, platelet counts, serum CRP, plasma fibrinogen, and urinary 8-hydroxy-2-deoxyguanosine (8-OHdG) (Lee et al. 2014). In a nationally representative sample of 16,160 individuals in the United States with air pollution data modeled according to zip code, there were no significant relationships between higher PM2.5 concentration (mean 11.88 SD±0.37) and inflammatory biomarkers (blood CRP and WBC) in healthy subjects. However, in subgroups of individuals with CAD or diabetes there were positive correlations between PM2.5 levels and blood CRP and WBC values (Dabass et al. 2016). Similarly, in a study of 56 non-smoking subjects with CAD residing in an urban area in Germany, increased traffic-related and combustion generated PM10 and PM2.5 were associated with elevated plasma CRP levels (Yue et al. 2007). In a study of 52 patients with ischemic heart disease in Finland, PM2.5 exposure from traffic and biomass combustion sources was correlated with elevated blood CRP levels (Siponen et al. 2015). In the same cohort, personal photometer measures of PM exposure correlated with plasma CRP and IL-12 levels (Huttunen et al. 2012).
Patients with glucose intolerance and diabetes mellitus (DM) are also vulnerable to the adverse effects of air pollution (Li et al 2018). A study of 2944 patients with DM demonstrated an association between traffic-related air pollution (PM10, PM2.5, and NO2) and increased insulin (14.5%) (Wolf et al. 2016). In a cohort of 92 diabetic patients, changes in daily ambient levels of PM2.5 correlated with changes in plasma concentrations of ICAM-1 and VCAM-1. (O’Neill et al. 2007) Traffic-related air pollution exposure based upon geocoded address location in 642 elderly non-smoking individuals demonstrated that for averages of 4-, 8-, and 12-week exposures, black carbon levels (estimated by land use regression) were associated with elevated soluble plasma ICAM-1 concentrations. An interquartile range rise in 8-week black carbon exposure was associated with a 1.58% elevation in plasma ICAM-1. Subgroup analysis indicated that PM2.5 exposure exerted greater effects (interaction) on plasma ICAM-1 concentrations in diabetic individuals (Alexeeff et al. 2011). Krishnan et al (2013) conducted a cross-over study of 17 metabolic syndrome patients and 15 controls, in which subjects inhaled DEP or filtered air for two hr and then crossed over to the other exposure following a 2-week washout period. There was an increase in matrix metalloproteinase (MMP)-9, IL-10, and IL-1b in patients with metabolic syndrome 7 and 22 hr post-inhalation of DEP. Data suggest that while DM and glucose intolerance are independently associated with vascular inflammation (Wang et al. 2018); PM susceptibility may contribute to further vascular risk (Rask-Madsen and King 2013). Overall, epidemiological studies support an association between vascular diseases and both short-term spikes in PM levels as well as prolonged exposure to PM in urban settings. (See Table 2b). Although there is a large body of literature available on PM and vascular health, it is difficult to determine a precise mechanism of action due to the large number of associated inflammatory mediators and their complex interactions. Moreover, variability in individual exposure levels and PM compositions makes it particularly challenging to draw overarching conclusions from diverse PM exposure studies.
Effect of Particulate Matter on Vascular Tone (Table 4, Figure 1)
Table 4.
Effect of Particulate Matter on Vascular Tone: Literature Summary
| Authors, Year | Methods | Main Findings |
|---|---|---|
| Zanoli et al. 2017 | Review | Arterial stiffening |
| Mirowsky et al. 2017 | Monitored ozone and ambient PM2.5 in 13 patients with CAD. | Increase in LAEI, tPA, PAI-1, neutrophils, monocytes, IL-6, and TNF-alpha |
| Wauters et al. 2015 | 18 healthy male subjects exposed to DE or filtered air for 2 hours at rest, during dobutamine stress ECG, or exercise stress ECG w/ hypoxia (12% O2) | DE had no effect at rest or in hypoxic conditions. Serum ET-1, exhaled NO not increased after DE |
| Louwies et al. 2013 | Ambient PM10 exposure monitored in 84 healthy adults | Every 10μg/m3 increase in PM10 decreased (0.86μm decrease) central retinal artery and (0.93 μm) vein diameter |
| Pope et al. 2011 | 26 healthy young adults exposed to Filtered Air or PM | Decline in MVRI after increased ambient PM2.5 |
| Barath et al. 2010 | 18 healthy male volunteers exposed to DE or Filtered Air for 1 hour during intermittent exercise | Decreased vasodilatation to Ach, bradykinin, sodium nitroprusside, and verapamil |
| Lenters et al. 2010 | Monitored NO2, SO2, PM2.5 in young adults | Increase in pulse wave index, AIx with increased NO2, SO2 |
| LeBlanc et al. 2009 | Sprague-Dawley rats exposed to inhalational FA or TiO2 nanoparticles | Increased coronary arteriole tone, impaired FID, attenuated Ach and Ca(2+) |
| Lundbäck et al. 2009 | 12 healthy adults exposed to DE (350 μg/m3) or FA for 1 hour during moderate exercise | AP, AIx, and Tr increased 10 min post DE-exposure, increased stiffness of the radial artery |
| Tamagawa et al. 2008 | 31 female New Zealand White rabbits exposed to intratrachael PM10 or saline for 5 days or 4 weeks | PM10 increased lung and activated macrophages, IL-6, and reduced Ach dilation of carotid artery |
| Peretz et al. 2008 | 27 adults exposed to Filtered Air and DE | DE decreased BAd with dose-dependent response, increased plasma ET-1 |
| Rundell et al. 2007 | PM exposure in 16 athletes during exercise in locations with high or low PM air concentrations | Increased vasoconstriction, reduced post-exercise FMD in brachial artery, reduction in re-oxygenation post-exercise in high PM, not low PM |
| Briet et al. 2007 | 40 white male nonsmokers | Small artery reactive hyperemia increased with PM2.5 and PM10 |
| Törnqvist et al. 2007 | 15 healthy subjects exposed to diluted diesel exhaust or filtered air for 1 hour during intermittent exercise | A selective impairment of Ach-induced endothelium-dependent vasodilatation in forearm vessels |
| Frampton et al. 2006 | 40 healthy subjects and 16 subjects with asthma | Altered peripheral blood leukocyte and adhesion molecules expression, increased retention of leukocytes in the pulmonary vasculature in PM exposure |
| O’Neill et al. 2005 | 270 patients with or at risk of diabetes | Increased nitroglycerin-mediated reactivity, decreased vascular reactivity |
| Mills al. 2005 | 30 healthy men exposed to DE (300 μg/m3) or FA for 1 hr during intermittent exercise | DE attenuated vasodilation following bradykinin, Ach, sodium nitroprusside infusion. DE decreased tPA release following bradykinin |
| Nurkiewicz et al.,2004 | Sprague-Dawley rats intratracheally exposed to saline, TiO2 or ROFA. | ROFA increased PMNs, BAL albumin, BAL LDH |
| Brook et al. 2002 | 25 healthy adults exposed to PM or Filtered air | Increased brachial artery vasoconstriction |
Experiential Models
Vascular tone is regulated by both endothelial derived factors and smooth muscle. The vasodilatory effects attributed to PM exposure may be mediated by widespread endothelial dysfunction (Mirowsky et al. 2017). Sprague-Dawley rats exposed to TiO2 nano-particulates showed impairment of endothelium-dependent vasodilation in subepicardial coronary arterioles as evidenced by increases in spontaneous tone and blunted responses to flow-, acetyl choline (AcH) -, and Ca+-ionophore- induced vasodilation (LeBlanc et al. 2009). In another study Tamagawa et al. (2008) exposed male Wistar rats to 16 weeks of DE and reported increased levels of mRNA biomarker of endothelin-1, endothelin receptors A and B, and endothelial NO synthase in the aorta. Although no changes were observed in the heart ventricles following the same exposure, PM exposure through DE resulted in impairments of vascular tone. In addition, carotid arteries from white rabbits exposed to acute (5 days) or chronic (4 week) intratracheal PM10 demonstrated decreased endothelial-dependent AcH-mediated relaxation of the carotid artery by 34%, with no marked effect on endothelial-independent sodium nitroprusside(SNP)-mediated vasoconstriction (Tamagawa et al. 2008). In rats exposed to intratracheal administration of TiO2 or residual oil fly ash (average diameter 2.2um), Nurkiewicz et al (2004) demonstrated impairment in Ca2+ ionophore-induced endothelial-dependent arteriolar dilation in the spinotrapezius muscle, indicative of systemic microvascular dysfunction. Evidence indicates that the robust inflammatory response and endothelial activation following PM exposure may play a critical role in arterial stiffening (Zanoli et al. 2017).
Clinical Studies
Clinical studies in healthy adults suggest exposure to air pollution derived PM and subsequent arterial inflammation initiate dysregulated vasoconstriction (Louwies et al. 2013). Louwies et al (2013) demonstrated that each 10μg/m3 increase in PM10 exposure (averaged over the 24 hr prior to examination) was associated with a 0.93μm decrease in central retinal artery diameter. Inhalation of concentrated ambient fine particles (CAP; 150 μg /m3) plus ozone (120 ppb) produced significant brachial artery vasoconstriction (1100%, measured by diameter) compared to inhalation of filtered air (Brook et al. 2002). In a controlled exposure study, Wauters et al. (2015) found that healthy adults exposed to DE concentration of 300 μg/m3 for 2 hr exhibited a 40% elevation (as estimated from the raw data presented in study tables) in pulmonary vasomotor tone when undergoing stress tests An investigation that measured arterial stiffness in 12 healthy volunteers exposed to 350 μg /m3 DE or filtered air for one hr during moderate exercise found that acute exposure resulted in an immediate and transient rise in arterial stiffness (2.5 mmHg increase in arterial augmentation pressure and 7.8% elevation in augmentation index) (Lundback et al. 2009). Further, increased ambient NO2 and SO2 levels were associated with accelerated arterial-wall stiffening in young adults, as indicated by a 4.1% rise in pulse wave velocity and a 37.6% elevation in vascular augmentation index (Lenters et al. 2010). Short-term exposure studies indicate that alterations in vascular diameter as well as secondary markers of vascular tone closely follow PM concentration changes, which may play an important role in cardiovascular diseases such as hypertension. PM might interrupt vascular homeostasis through pro-inflammatory changes in the vascular wall, reducing endothelial dilatory capacity.
Vascular reactivity and arterial stiffness may occur in a time and dose dependent manner following PM exposure. Peretz et al (2008) demonstrated that healthy adults exposed to 200 μg/m3 DE for 2 hr exhibited decreased brachial artery diameters and increased plasma levels of endothelin-1 compared to adults exposed to only 100 μg /m3 DE or filtered air. Exposure to elevated levels of ambient PM2.5 was associated with a lower reactive hyperemia-peripheral arterial tonometry ratio in healthy young adults. However, no marked change was seen after acute 3 hr experimental exposure to combustion generated PM2.5. Findings suggest that prolonged exposure times are necessary to produce clinically significant results in healthy populations (Pope et al. 2011). Rundell et al (2007) exposed 16 healthy athletes to low number (inner campus location, 5309±1,942 particles cm−3) or high number (near major highway, 143,501±58,565 particles cm−3) PM1 concentrations during exercise. Flow-mediated brachial artery dilation (FMD) and forearm oxygen kinetics were measured before and after exercise. Significant vasoconstriction of brachial artery diameter (4% change) was found after exercise in high PM1, but not low PM1 concentrations (Rundell et al. 2007).
The decreased vascular compliance seen in conditions of PM exposure may be the consequence of a muted response to vasodilators. Brook et al (2002) noted in short-term exposure to CAP and ozone a resultant arterial vasoconstriction occurred without effects on endothelial-dependent flow-mediated vasodilation or endothelial-independent nitroglycerin-mediated vasodilation in healthy adults. However, Briet et al (2007) implicated PM in endothelial dysfunction where exposure to ambient nitrogen, sulfur, and carbon oxides, as well as PM2.5 and PM10 produced impairment of both large and small artery vasodilation, as evidenced by brachial artery endothelium-dependent flow-mediated dilatation and reactive hyperemia, respectively. There was no correlation with endothelium-independent glyceryl trinitrate dilatation of the brachial artery (Briet et al. 2007). One-hr exposure to dilute DE (300 μg /m3) in healthy individuals initiated reduction in vascular tone and endogenous fibrinolysis, with diminished responses to vasodilators and decreased release of tissue plasminogen activator (Mills et al. 2005). Inhalation of DE by healthy volunteers during exercise impairs the vasodilatory response to bradykinin, AcH, and SNP in forearm vessels. This impaired response to AcH-induced endothelial-dependent vasodilation in forearm vessels appears to persist for at least 24 hr after exposure (Tornqvist et al. 2007). In addition, healthy males exposed to 1 hr 250 μg /m3 DEP exhibited attenuated calcium channel-dependent vasodilation within 6 hr treatment (Barath et al. 2010). The previously described microvascular changes may be vessel specific or markers of more widespread cardiovascular disease, such as hypertension, in which vascular tone plays in important role. Taken together, researchers examining the effects of PM induced vasoconstriction suggest primary involvement of endothelial-dependent mechanisms with select investigations demonstrating the presence of endothelial-independent activation. The dose and time dependent manner by which PM activates the smooth muscle response and triggers endothelial dysfunction may play a role in the observed variation.
Those with underlying cardiovascular disease or risk factors may be vulnerable to the adverse effects of PM exposure on vascular tone, even at low levels. O’Neill et al (2005) suggested that ambient levels of PM2.5, black carbon, sulfates, and particle number were associated with decreased flow-mediated vascular wall reactivity (measured by percent brachial artery diameter change) in diabetic patients, but not individuals at risk. PM2.5 was correlated with nitroglycerin-mediated reactivity (−7.6%). Type I diabetes conferred greater vulnerability than type II diabetes (O’Neill et al. 2005). Similarly, Frampton (2006) attributed changes in heart rate variability in cardiovascular patients to endothelial activation and/ or vasoconstriction in the systemic circulation. Investigations on vascular tone suggest that PM promotes vasoconstriction in both healthy and diseased individuals by endothelial inflammation which, in turn, affects smooth muscle cell interactions and promotes dysregulation of vascular tone.
Effects of Particulate Matter on Oxidative Stress (Table 5, Figure 1)
Table 5.
Particulate Matter and Oxidative Stress: Literature Summary
| Authors, Year | Methods | Main Findings |
|---|---|---|
| Jantzen et al. 2018 | 23 healthy elderly subjects | CD34+KDR+ late endothelial progenitor cells decreased by 48% in the blood |
| Li et al. 2017 | Controlled human exposure study | PM2.5 increased markers of oxidative stress, including serum malondialdehyde, iso-prostaglandin F2α, superoxide dismutase, and 8-hydroxy-2-deoxyguanosine |
| Zhang et al. 2016a | 97 elderly subjects living in the Los Angeles area | Demonstrated associations among airway oxidative stress and inflammation with traffic-related air pollutants, ultrafine particles and transition metals |
| Zhang et al. 2016b | 93 elderly subjects living in the Los Angeles area | Reactive hyperemia index inversely associated with traffic related pollutants and other mobile-source components, as well as oxidative potential of transition metals and PM<2.5 |
| Liu et al. 2015 | Controlled human exposure study | Increased levels of urinary 8-OHdG in healthy nonsmoking adults exposed to coarse (2.5–10 μm) or ultrafine concentrated ambient particles |
| Wang et al. 2012 | Human lung EC monolayer | PM induces marked increases in vascular permeability via ROS-mediated calcium leakage |
| Huang et al. 2012 | 125 Beijing participants in the during-Olympic Games period | Change in PM caused decrease in levels of airway inflammation and urinary 8-OHdG |
| Miller et al. 2009 | Effects of DEP (10–100 microg/mL) in isolated rat aortic rings | DEP caused oxidative stress through oxygen-centered free radicals that reduce bioavailability of endothelium-derived NO |
| Hartz et al. 2008 | Isolated rat brain capillaries exposed to DEPs | DEPs, alters blood-brain barrier function through oxidative stress and proinflammatory cytokine production |
| Sun et al. 2008 | Sprague-Dawley rates exposed to PM2.5 | Upregulation of Rho/ROCK pathway, activated by reactive oxygen species, causes increased vascular tone |
| Nurkiewicz et al. 2006 | Rats intratracheally indussed with residual oil fly ash (ROFA) or titanium dioxide | Impaired endothelium-dependent dilation, PMNL adhesion, MPO deposition, and oxidative stress |
| Taniyama et al. 2003 | Review | ROS plays a central role in normal physiology, and contributes to vascular disease |
| Gurgueira et al. 2002 | Rat model of short-term concentrated ambient particle exposure | Tissue-specific increases in superoxide dismutase and catalase activity |
| Bai et al. 2001 | Human pulmonary artery endothelial cells exposed to diesel exhaust particles | Endothelial cell damage caused by DEP extracts is likely mediated by oxygen derived free radicals |
Experimental Studies
Reactive oxygen species produce endothelial cell apoptosis and promote monocyte adhesion through expression of VCAM-1 and ICAM-1 (Cook-Mills et al. 2011). Further, ROS impair endothelium-dependent vasorelaxation by inactivating nitric oxide (NO) (Meza et al. 2019). Miller et al (2009) demonstrated that aortic rings from rats exposed to DE exhibited an impaired response to AcH-induced endothelial-dependent vasorelaxation. Diesel exhaust particle exposure resulted in an oxidative stress response characterized by a 900% increase in oxygen free radicals (at 10 μg/ml DE) and a 36% decrease in NO (at 100 μg/ml DE).High DE exposure concentrations were used in this model, which may exaggerate the effects of DE on vascular tissue. ROS are known to induce both vascular smooth muscle cell (VSMC) apoptosis and proliferation and play an important role in VSMC migration (Taniyama and Griendling 2003). Gurgueira et al. (2002) exposed Sprague-Dawley rats to CAPs and reported significant oxidative stress levels in lung and heart tissues, determined as in situ chemiluminescence accompanied by 200% increases in lactate dehydrogenase (LDH). CAP exposure was also found to produce a tissue-specific activation of superoxide dismutase (SOD) and catalase (CAT). PM leads to generation of ROS but also activates mechanisms that mitigate their detrimental effects (Gurgueira et al. 2002).
Isolated rat brain capillaries exposed to DEP displayed a concentration-dependent increase in P-glycoprotein, an efflux transporter and key BBB mediator. Pretreatment of the capillaries with radical scavengers counters P-glycoprotein upregulation (Hartz et al. 2008). Hartz et al. (2008) suggested that PM may gain access to other organ systems in a similar manner: a marked elevation in vascular permeability was noted in human lung endothelium following traffic-generated PM (aerodynamic diameter 0.1–0.3 μm) exposure. The change in vascular integrity was attributed to ROS. Generation of ROS following PM exposure not only induces tight junction protein relocation from the cell periphery, but also leads to activation of the calcium-dependent caplain protease, which results in tight junction degradation and endothelial cell barrier disruption (Wang et al. 2012).
Sun et al (2008b) performed experiments in which Sprague-Dawley rats were exposed to PM2.5 or filtered air for 10 weeks and angiotensin-II was introduced during the final week of treatment in order to induce hypertension. The aortas of PM2.5 exposed animals showed 220% increased superoxide production accompanied by elevated NADPH oxidase expression in the smooth muscle cells. While PM2.5 itself did not markedly affect mean arterial pressure (MAP), these particles potentiated the effect of angiotensin-II by sensitizing the vasculature (Sun et al. 2008). In the same experiment model, data demonstrated that PM2.5-induced ROS production activated the Rho/ROCK pathway which increases vascular tone through Ca++ sensitization (Sun et al. 2008).
Human pulmonary artery endothelial cells are damaged by DE particle extracts through generation of oxygen-derived free radicals and NO (Bai et al. 2001). A similar effect was noted at the level of the microvasculature, Sprague-Dawley rats exposed to intratracheal instillation of residual oil fly ash with an average diameter of 2.2μm exhibited a rise in markers of leukocyte rolling and adhesion in the microvascular wall, accompanied by reduction in endothelium-dependent arteriolar dilation in the spinotrapezius muscle microvessels (Nurkiewicz et al. 2006). Wauters et al (2013) incubated human umbilical vein endothelial cells with the serum of healthy volunteers exposed to PM2.5 DE and found that enhanced ROS production correlated with total inhaled PM2.5 exposure. Acute experimental exposure to DE PM2.5 impaired AcH-induced vasodilation in skin microvasculature of healthy adults (Wauters et al. 2013). This investigation used a male-only cohort with a small sample size (n=12), limiting the generalizability of its results.
Clinical Studies
In elderly Los Angeles residents, elevated markers of airway inflammation (FeNO) and oxidative stress (malondialdehyde (MDA)) were associated with PM0.18, transition metals, and traffic-derived air pollutants, including black carbon (BC), carbon monoxide (CO), and nitrogen oxides (NOx) (Zhang et al 2016a). Reactive hyperemia index was inversely correlated with ambient PM2.5, BC, NOx, and CO. Zhang et al (2016b), in a similar cohort, detected altered microvascular endothelial function, characterized by the reactive hyperemia index of the brachial artery inversely associated with traffic related pollutant exposure (PM2.5, BC, NOx, CO) and other mobile-source components and tracers with high oxidative potential. Combined house dust (PM2.5 concentration of 275 μg/m3) and ozone (100 ppb) exposure enhanced ROS production capacity in granulocytes and monocytes (Jantzen et al. 2018). Following exposure, CD34+KDR+ late endothelial progenitor cell number decreased by 48% in the blood of elderly individuals … Exposure data was collected from central monitoring stations, which may introduce measurement error. Individual diets were not recorded, a potential confounding factor influencing vascular function. A controlled human exposure study demonstrated increased levels of urinary 8-OHdG in healthy nonsmoking adults exposed to coarse (2.5–10 μm) or ultrafine concentrated ambient particles (CAPs) (< 0.3 μm). Urinary MDA levels were elevated in those exposed to fine CAPs (0.15–2.5 μm) (Liu et al. 2015).
Li et al (2017) conducted a randomized, crossover trial in 60 college students and noted that systolic blood pressure (SBP) was 2.61% higher (95% Cl 0.39–4.79) when exposed to PM2.5 versus filtered air. For every 10 μg/m3 rise in PM2.5 exposure, SBP increased by 0.85% (95% Cl 0.10–1.62). Exposure to PM2.5 elevated markers of oxidative stress, including serum MDA, iso-prostaglandin F2α, SOD, and 8-OHdG compared to filtered air exposure. Compared to Sun et al (2008a), Li et al (2017) used a shorter exposure period (9 days versus 10 weeks). The exposure concentration of PM2.5 was greater in Li et al (2017) (46.8 μg/m3 daily average) than in Sun et al (2008a) (14.1 μg/m3 over the ten-week period).
Oxidative stress biomarkers were examined in 125 Beijing participants during-Olympic Games period, which coincided with strict air pollution reduction, and in pre-Olympic Games period, during which pollutant levels were high. There was a significant decrease ranging from −72.5 to −4.5% in levels of airway inflammation, measured by fractional exhaled NO testing and urinary 8-OHdG levels, respectively, between pre-Olympic games period and during-Olympic Games period (Huang et al. 2012)
Particulate matter exposure may enhance oxidative stress and result in endothelial dysfunction. Studies showed conflicting results on the effect of PM on blood pressure (Sun et al. 2008, Li et al. 2017). The blood group RH 1 is often used as a marker of vascular function (Zhang et al. 2016). However, increased sympathetic activity may significantly affect RH1 and this cannot be directly compared to other markers of vascular function, such as flow-mediated vasodilation. Exposure concentrations varied between the aforementioned studies, with some experimental models using elevated exposure concentrations (Miller et al. 2009, Wang et al. 2012). These results may overestimate the effect of PM on oxidative stress.
Effect of Particulate Matter on Atherosclerotic Plaque Formation (Table 6, Figure 1)
Table 6.
Particulate Matter and Atherosclerotic Plaque Formation: Literature Summary
| Authors, Year | Methods | Main Findings |
|---|---|---|
| Du et al. 2018 | ApoE−/− mice fed normal or high-fat chow and exposed to PM2.5 or filtered air for 8h/d, 7d/wk, for 16 wks. | PM2.5 increased aortic root plaques and plaque area, ox-LDL, LDL-C, apoplipoprotein B, and serum and aortic CD36. Decreased HDL-C, apolipoprotein A1 |
| Miller et al. 2017 | Healthy humans exposed to gold nanoparticles for 2h during exercise. Carotid endarterectomy patients exposed to gold 2x for 2h before surgery | Gold in blood and urine, excised carotid plaques Particles in blood and liver, with the smallest particles in the highest quantities |
| Bell et al., 2017 | MESA-Air participants | PM2.5 decreased HDL particle number but not HDL cholesterol. |
| Cao et al. 2016 | Review | Increased reactive oxygen species (ROS) or an adaptive response to oxidative stress in mediating particle-induced cell formation |
| Wang et al. 2016 | 4800 participants | Living < 150 m from major roadways increased CIMT, not CAC or AAC |
| Akintoye et al. 2016 | Meta-analysis investigating the effect of PM2.5 | PM2.5 increased CIMT, not arterial calcification or ABI |
| Kaufman et al. 2016 | PM2.5 and NOx measured in subjects aged 45–84 years | PM2.5 increased coronary calcification progression, no effect on CIMT |
| Ramanathan et al. 2016 | Healthy participants | PM2.5 significantly decreased the anti-oxidant capacity of HDL in participants with a higher pre-exposure anti-oxidant capacity |
| Keebaugh et al. 2015 | ApoE-/- mice exposed to CAP for 5 hr/day, 4 days/wk for 8 weeks | Increased plaque area and lipid content in brachiocephalic artery, increase serum MDA, decreased HRV |
| Cao et al. 2015 | Human THP-1 derived macrophages exposed to DEP | DEP increased intracellular reduced GSH, lipid droplet formation, and led to lysosomal dysfunction |
| Morales-Barcenas et al. 2015 | Airway epithelial A549 cells exposed to PM10 from industrial zone (IZ) or commercial zone (CZ) | PM10 increased protease activity and invasiveness. CZ PM10 increased MMP-2 after 24 hr exposure |
| Su et al 2015 | 689 adults | PM2.5, PM10, NO2, and NOx increased subclinical atherosclerosis |
| Du et al. 2015 | Human monocyte-derived macrophage and Chinese hamster ovary cell lines | Smaller HDL particles were the most efficient at removing cholesterol from macrophages. |
| Rao et al. 2014 | ApoE−/− and LDLR−/− exposed to PM2.5 or filtered air for 6 months | PM2.5 increased 7-ketocholesterol in plasma and aortic plaques; increased CD36 in macrophages in plaques |
| Armijos et al. 2014 | 287 healthy children | Increased CIMT in children <100 meters from the nearest heavily trafficked road compared to those living ≥ 200 meters away |
| Gan et al. 2014 | 509 healthy subjects | No associations between TRAP and progression of carotid atherosclerosis |
| Li et al. 2013 | LDLR−/− exposed to inhalational UFPs or FA for 5h/d, 3d/wk for 10 wks | Exposure to UFP decreased plasma HDL, paraoxonase activity, HDL anti-oxidant capacity. Increased ox-LDL, free oxidized fatty acids, triglycerides, TNF-alpha, plaque lesion, cross-sectional plaque area in aortic sinus |
| Chen et al. 2013 | ApoE−/− exposed to PM or filtered air | Increased serum total cholesterol, LDL, TNF-alpha, C-reactive protein. Increased aortic plaque area and TNF-alpha and IL-6 in BAL of PM mice |
| Toth et al. 2013 | Review | Low HDL-C identifies patients at elevated risk, and much investigation suggests that HDL may play a variety of antiatherogenic roles |
| Li et al. 2013 | Fat-fed low density lipoprotein receptor-null (LDLR⁻/⁻) mice exposed to filtered air (FA) or UFPs for 10 weeks | UFP exposure promoted proatherogenic lipid metabolism and reduced HDL antioxidant capacity |
| Miller et al. 2013 | ApoE−/− mice and wild-type mice exposed to (35 μL) DE or saline 2 days/wk for 4 wks. | DEP in both strains increased neutrophils in BAL and Nrf2 in liver |
| Wilker et al. 2013 | 380 elderly men | Black carbon increased CIMT |
| Lund et al. 2011 | ApoE−/− mice exposed to inhalational filtered air or whole engine emissions | Increased ox-LDL, vascular ROS, MMP-9, macrophage infiltration, endothelin-1 |
| Bai et al. 2011 | ApoE−/− mice exposed to (200 mg/m3) PM2.5 or FA, 6h/day, 5 days/wk for 7wks. | Increased oxidative stress (iNOS, nitrotyrosine, CD36) and lipid and DNA oxidation in PM2.5 mice |
| Kunzli et la. 2011 | Review | CIMT is a useful candidate for cross-sectional and longitudinal studies investigating the role of air pollution in atherogenesis |
| Campen et al. 2010 | ApoE−/− mice exposed to DE or FA for 6 h/day for 50 days. | DE increased MMP-9, ET-1 mRNA, collagen, and macrophages in DE vessels. |
| Tranfield et al. 2010 | Watanabe heritable hyperlipidemic rabbits exposed to instilled (5 mg) PM10 or saline for 2 days/wk for 4 wks. | Ultrastructural plaque analysis showed increased foam cells, fragmented or absent dense ECM, increased endothelial-foam cell contact |
| Bauer et al. 2010 | 3,380 adults | PM2.5 increased CIMT and atherosclerosis |
| Ying et al. 2009 | ApoE−/− mice exposed to CAPs PM2.5 or FA | Increased aortic expression of NADPH oxidase subunits, iNOS, superoxide generation, protein nitration |
| Floyd et al. 2009 | ApoE-/- mice exposed to FA or CAPs for 6 h/day, 5 days/wk for 5 months | CAPs-induced changes to inflammation, proliferation, cell cycle, hematological system, and cardiovascular pathways |
| Yatera et al. 2008 | Watanabe heritable hyperlipidemic rabbits exposed to intratracheal PM10 2x/wk for 1 month | PM10 increased the number of monocytes in the endothelium over plaques, in plaques, and in the smooth muscle beneath plaques |
| Sun et al. 2008 | Human bronchial epithelial cells, hSMCs, monocytes exposed PM2.5 and PM0.1. ApoE−/− exposed to PM2.5 or FA for 6 hr/day, 5 day/wk for 6 months | PM2.5 and UFP increased TF protein. PM2.5 increased TF and macrophages in plaques |
| Araujo et al. 2008 | ApoE−/− mice exposed to UFP, PM2.5, or FA 5 hrs/ day, 3 days/wk for total of 75 hrs. | UFP increased the number and size of atherosclerotic lesions infiltrated w/ foam cells, reduced HDL’s anti-inflammatory effect, and increased MDA and Nrf2 in the liver |
| Lund et al. 2007 | ApoE-/- mice exposed by inhalation to FA, PM whole exhaust or filtered exhaust | Increased aortic MMP-3, MMP-7, and MMP-9 mRNA, tissue inhibitor of MMP-2, ET-1 and HO-1. ROS in arteries, not plasma, of PM exposed mice |
| Sun et al. 2005 | 28 ApoE−/− exposed to PM2.5 or FA for 6 h/day, 5 days/wk for 6 mo. | PM2.5 increased vasoconstriction to phenylephrine and serotonin, attenuated Ach-vasodilation, increased iNOS, macrophage infiltration, ROS, and 3-nitrotyrosine |
| Miyata et al. 2003 | New Zealand White rabbits with abdominal aorta balloon injury followed by PM10 or saline exposure for 4 wks ± lovastatin(5mg/kg/day) | PM10 exposure accelerated balloon catheter induced plaque formation, increased intimal macrophages, and lipid accumulation |
Abbreviations: AP: Augmentation Pressure, AIx: Augmentation Index, Tr: time to wave reflection, FMD: flow-mediated dilation, BP: blood pressure, NMD: endothelial-independent nitroglycerin-mediated dilatation, MVRI: microvascular responsiveness index, OC: organic carbon, EC: elemental carbon, TiO2: titanium dioxide, SNP: sodium nitroprusside, FID: flow-induced dilation, ROFA: residual oil fly ash, BAL: bronchoalveolar lavage, MPO: myeloperoxidase, hSMCs: human smooth muscle cells, TF: tissue factor, CIMT: carotid intima-media thickness, TRAP: traffic-related air pollution, ABI: ankle-brachial index, CAC: coronary artery calcification, AAC: abdominal aortic calcification, MDA: malondialdehyde, CRP: c-reactive protein, CK: creatine kinase
Experimental Models
Air pollution derived PM exposure contributes to atherosclerotic plaque formation and progression in experimental models. The potential for deposition of nanoparticles (NP) in atherosclerotic plaques was suggested by Miller et al (2017) in ApoE knockout mice using intratracheal administration of engineered gold nanomaterial to simulate environmental NP. Similar findings were demonstrated in human studies. Patients undergoing carotid endarterectomy who inhaled AuNP prior to surgery subsequently possessed gold present in their carotid artery plaques. Further, PM2.5 exposure-induced oxidative stress was found to enhance atherosclerosis in mice (Ying et al. 2009). ApoE knockout mice exhibited elevated ROS levels (300% increases in inducible NOS), and greater atherosclerotic plaque areas following PM2.5 exposure.
Plaque formation starts in the presence of endothelial dysfunction when low density lipoprotein (LDL) particles enter the arterial wall and accumulate in the vascular intima. Accumulation eventually leads to the pathologic oxidation of LDL (ox-LDL). Multiple studies reported that PM exposure was correlated with increased levels of ox-LDL (Lund, Lucero et al. 2011, Li, Navab et al. 2013). ApoE knockout mice fed normal chow and exposed to a mixture of inhaled PM2.5 and PM10 exhibited a 43% rise in LDL. LDL and cholesterol values were further elevated in mice fed a high-fat diet. The exposed mice demonstrated lower serum antioxidant capacities and greater degrees of atherosclerosis, as evidenced by increased plaque area in cross-sectional slices of the ascending aorta (Chen et al. 2013, Du et al. 2018). Mice exposed to PM2.5 exhibit greater lipid content and 700% rise in plasma 7-ketocholesterol, a form of oxidative cholesterol, which correlates with increased plaque formation in both ApoE and LDL receptor (LDLR) knockout mice (Rao et al. 2014, Cao et al. 2016). Yatera et al (2008) found that PM10 exposure in heritable hyperlipidemic rabbits treated with BrdU-labeled monocytes showed monocyte adhesion to endothelium over plaques and increased deposition of monocytes in the plaques and below the plaques in smooth muscle. In addition, a 160% elevation in ICAM-1 and a 60% rise in VCAM-1 expression was observed in the plaque tissue of PM10 exposed rabbits (Yatera et al. 2008). Similar findings were demonstrated in mice (Sun et al. 2005; 2008b, Cao et al. 2016). Inhalational PM2.5 exposure was associated with increased lipid content, plaque area, and macrophage infiltration in plaques of ApoE−/− mice fed a high-fat diet. ApoE−/− mice exposed to concentrated ambient ultrafine particles (PM0.20) exhibited a 200% elevation in plaque area in the brachiocephalic artery compared to filtered air controls (Keebaugh et al. 2015). Molecular analysis of aortic plaques in ApoE−/− mice exposed to concentrated ambient air particles revealed a 325% upregulation of CD68 (a marker of macrophage infiltration) (Floyd et al. 2009), suggesting that PM alters gene expression in a manner that may promote atherosclerosis.
DEP exposure induced lipid droplet accumulation in macrophage cell lines in a concentration-dependent manner compared to controls 24 hr after exposure (Cao et al. 2015). Miyata et al (2013) noted that PM10 exposure in white rabbits was correlated with a 200% rise in macrophage accumulation in plaque area, increased foam cell generation, and accelerated plaque progression (300% elevation in intima/media ratio).
In airway epithelial cell cultures, PM10 from an urban commercial zone increased matrix metalloprotease-9 (MMP-9; by 23.2%) and matrix metalloprotease-2 (MMP-2; by 23.7%) activity 48 hr after incubation. There was no change in MMP-9 and MMP-2 mRNA at 48 hr, suggesting that mRNA decayed at an earlier time-point (Morales-Barcenas et al. 2015). Similarly, mixed whole engine exhaust exposure in ApoE−/− mice was related to upregulation of factors implicated in vascular remodeling and atherogenesis, including MMP-9, MMP-3, MMP-7, endothelin-1, and heme oxygenase-1 (Lund et al. 2007, Campen et al. 2010). Diesel exhaust exposure increased endothelin-1 mRNA by 100%, MMP-9 mRNA by 60%, and lipid peroxides by 300% in the aortas of ApoE−/− mice (Campen et al. 2005).
High density lipoprotein (HDL) is an antioxidant that reduces ox-LDL levels, counters atherogenesis, and removes cholesterol from macrophage foam cells present in plaque (Toth et al. 2013). The protective, anti-inflammatory effect of HDL was reduced in ApoE knockout mice exposed to PM2.5 (Araujo et al. 2008). Particulate size impacted both HDL function and atherosclerotic plaque volume. Exposure to ultra fine particles (particles < 0.18 μm) was associated with a 25% greater plaque volume and reduction in HDL-mediated protective capacity (measured by comparing the anti-inflammatory capacity of HDL against LDL-induced chemotaxis) when compared to fine particulate (<2.5 μm) exposure (Araujo et al. 2008). These findings corroborate a study in LDLR−/− mice, in which ultrafine PM exposure engendered atherogenic lipid metabolism and reduced antioxidant capacity (HDL) in fat-fed LDLR−/− mice (Li et al 2013). These mice also exhibited increased atherosclerotic lesion ratios and aortic cross sectional lesion areas (62% elevation in atherosclerotic lesion thickness and 220% rise in cross-sectional lesion area) following ultrafine PM treatment (Li et al. 2013). Air pollution derived PM also affects plaque stability. Aortic plaques from ApoE−/− mice exposed to PM demonstrated decreased alpha-actin expression by 360%, suggesting plaque instability (Floyd et al 2009). However, MMP-8, which is typically increased in rupture-prone plaques, was diminished in ApoE−/− mice exposed to concentrated ambient air particles. These plaques may not have progressed to the point of rupture. ApoE−/− mice tend to exhibit plaque rupture in the brachiocephalic artery, while Floyd et al (2009) analyzed the larger aortic plaques. Analysis of the brachiocephalic artery in ApoE−/− mice may further characterize the effects of particles on plaque stability. After PM10 exposure, Watanabe heritable hyperlipidemic rabbits exhibited a 250% increase in number of foam cells in atherosclerotic plaques and ultrastructural plaque alterations (Tranfield et al 2010). Changes included reduction and fragmentation of the subendothelial extracellular membrane, which contributes to plaque instability. Oropharyngeal aspirate instillation of DEP increased the size of plaques by 200% and number of plaques by 133% in the brachiocephalic arteries of ApoE−/− mice fed western diets to induce complex atherosclerotic plaques (Miller et al 2013). Exposure generated increased fibrous caps and plaque complexity with more buried fibrous layers. Bai et al (2011) examined aortic plaque composition in ApoE−/− mice following inhalational DEP exposure and noted 150 to 300% elevation in plaque lipid content, cellularity, foam cell formation, and smooth muscle cell content. Further, inducible NOS, CD36, and nitrotyrosine expression were increased by 1.5 to 200% in arterial wall plaques of DEP exposed animals. All these data are suggestive of plaque instability (Bai et al. 2011).
These studies suggest that air pollution contributes to atherosclerosis by increasing LDL levels, promoting macrophage infiltration into plaques, altering plaque stability, and decreasing antioxidant capacity of HDL. Particulate matter is suggested to alter gene expression in a way that promotes vessel remodeling, which may diminish plaque stability. Most experimental models investigated the effects of PM on atherogenesis in animals susceptible to atherosclerosis. These observations may be representative of air pollution-mediated effects on vulnerable populations. Those less susceptible to atherogenesis may exhibit less or none of the reported effects. Exposure concentrations and time-periods varied among the studies reviewed. Some exposures were at pollution levels similar to ambient air concentrations (Chen et al. 2013), while others were at higher levels (Sun et al. 2005). Increased exposure concentrations may lead to exaggerated effects on the vasculature that would not be observed clinically. The chemical composition of the exposures differed among studies, which may influence results.
Clinical studies
While the specific effects of PM exposure on different stages of atherosclerosis have been examined in animal models, clinical studies focused largely on associations between air pollution exposure and plaque formation/ progression. Carotid intimal-media thickness (CIMT) is a common marker for subclinical atherosclerosis. A population-based study of 3,380 subjects demonstrated that those in the 90th percentile of residential PM exposure had 0.028mm greater CIMT measurements than those in the 10th percentile (Bauer et al. 2010). Su et al (2015) demonstrated that long-term exposure to traffic-related PM2.5, PM10, as well as gaseous pollutants originating from traffic emissions (NO2 and NOx) was associated with increased CIMT (4.23% per 1.0 × 10−5/m rise in PM2.5 and 3.72% per 10 μg/m3 elevation in PM10) in 35–65 year old individuals. One-interquartile increase in 1-year average BC concentration was correlated with a 1.1% rise in CIMT (Wilker et al 2013). Analysis of African American and pediatric cohorts reported similar results, with increased CIMT measurements in those living in close proximity to a major roadway compared to individuals in more rural areas (Armijos et al. 2015; Wang et al. 2016). Estimating air pollution exposure by calculating the residential distance from a heavily trafficked road may lead to exposure misclassification. Residential traffic noise is a potential confounder and was not controlled for in these studies (Armijos et al 2015; Wang et al. 2016). Other studies failed to demonstrate significant relationships between PM exposure and increased CIMT, although positive trends were noted (Kunzli et al. 2011, Gan et al. 2014, Akintoye et al. 2016). A meta-analysis found no significant association between PM and elevated CIMT, although there was heterogeneity among the CIMT estimates (Akintoye et al. 2016) which may limit the study generalizability. To investigate the relationship between air pollution and cardiovascular health, the Multi-Ethnic Study of Atherosclerosis and Air Pollution estimated each individual’s air pollution exposure and measured CT evidence of coronary artery calcium in 6,795 participants aged 45–84 years over a 10-year period (Kaufman et al. 2016). For an increase of 5 μg/m3 of PM2.5 exposure, coronary artery calcium (CAC) progressed by 4.1 units per year (95% CI 1.4–6.8). An elevation of 40 parts per billion (ppb) of NOx exposure increased CAC by 4.8 units per year (95% CI 0.9–8.7). However, PM and NOx exposure were not correlated with intima-media thickness change (Kaufman et al. 2016). During the study period, levels of ambient PM2.5 were relatively low, at an annual average of 14.2 μg/m3, compared to air quality standards in the United States and the European Union (which allow for 12 μg/m3 and 25 μg/m3 PM2.5 per year, respectively). Data suggest that ambient PM2.5 exposure, at concentrations encountered worldwide, might be associated with atherosclerosis progression (Kaufman et al. 2016). A further analysis of the Multi-Ethnic Study of Atherosclerosis found that a rise of 5 μg/m3 PM2.5 over a 3-month period decreased HDL particle levels, but not HDL cholesterol, in the blood (Bell et al. 2017). Small HDL particles may be important in cholesterol efflux and a reduction may decrease the ability of HDL to remove cholesterol (Du et al. 2015). Short-term exposure to PM2.5 significantly lowered antioxidant capacity of HDL in participants with a higher pre-exposure antioxidant capacity (Ramanathan et al 2016).
Clinical studies investigating the relationship between air pollution and atherosclerosis yielded mixed results. There are several limitations in these investigations. Models used to estimate air pollution levels are susceptible to measurement error. Many are cross-sectional studies and are unable to describe a temporal relationship between PM exposure and atherogenesis. Further studies are needed to both clearly define the association and understand the mechanisms underlying PM-initiated atherosclerosis.
Summary and Conclusions
Experimental and clinical studies discussed in this review strongly implicate systemic and local endothelial cell inflammation and oxidative stress in the pathogenesis of vascular disease in conditions of PM exposure. Air pollution also affects atherosclerotic plaque formation, vascular tone, fibrinolysis, and platelet activity. Individuals with underlying cardiovascular diseases or diabetes may exhibit increased susceptibility.
Questions remain regarding the route and mechanisms by which PM exerts biologic effects discussed in this review on vascular tissue. Experimental and clinical particle model systems are limited by challenges in recapitulating and representing the high chemical and physical diversity of ambient PM. Relevant particle diameter sizes range from coarse (PM 2.5–10 μm) to fine (PM smaller than 2.5 μm), and ultrafine (PM smaller than approximately 200nm). Further, particles originate from a wide variety of sources including vehicular, biomass, meat cooking, sea salt, dust, and secondary photochemical formation (Forman and Finch 2018). These particulate substrates further undergo chemical transformations and conversions. Metal contents differentially affect free radical propagation and toxicity (Forman and Finch, 2018). For example, the hydroxyl radical (HO•) is highly reactive, with a half-life of approximately one nanosecond. Because of rapid reactions with molecules immediately around its production, the reactions are only to that local cell membrane domain. If HO• is produced in extracellular fluids, none of it reaches the cell surface. The production of HO• at the surface requires the presence of iron or another transition metal. Cell surface production of HO• produces a small amount of lipid peroxidation, which results in lipid raft disruption and calcium release. Subsequent cell signaling might increase production of pro-inflammatory cytokines (Premasekharan et al. 2011).
Experimental PM collection methods account for particulate composition, temporal/ seasonal variance, and modifications during storage and delivery. Synthetic experimental particulate models designed to recapitulate complex exposure-host interactions need to consider mechanisms of entry into the body, diffusion capabilities, movement of particles through membranes and barriers, interaction with immune mediators/ host defenses, and deposition within diverse tissues and target organ systems. The broad physical and chemical diversity of PM renders these challenges significant.
Clearly, PM impacts the cardiovascular system through multiple, diverse physiologic pathways. Further animal and human studies are needed to determine the specific air pollution constituents and PM sizes that impact the vasculature through each of these mechanisms. As exposure duration and onset age affect vascular responses to PM, toxicology studies may help establish differential vascular effects according to PM level, age, and temporal exposure patterns. Future investigations are needed to understand why specific populations remain more susceptible to the vascular effects of PM. These questions may be addressed by experiments that expand on the foundational information presented in this review. Improving air quality standards, reducing personal exposures, and redesigning engine and fuel technologies could all impact air quality and potentially mitigate the effects of air pollution on the cardiovascular system and human health.
Highlights.
We review the current literature and outline the effects of particulate matter (PM) on vascular tissue.
PM exposure induces inflammation, vasoconstriction, oxidative stress, and atherosclerotic plaque formation.
PM exposure is particularly detrimental to individuals with underlying cardiovascular conditions.
Further studies examining the vascular effects of specific PM compositions, concentrations, and particle sizes are warranted.
Acknowledgements
The authors are grateful for the valuable critical comments by Caleb E. Finch (USC) and Henry J. Forman (USC).
Funding
National Institute on Aging - PO1- 53-5177-5369
National Institute of Environmental Health Sciences - RO1- 53-5177-4936
Footnotes
Competing interests
No competing interests to report
Ethical Approval and Consent to participate
Not Applicable
Consent for publication
Not Applicable
Availability of supporting data
Not Applicable
References
- Akintoye E, Shi L, Obaitan I, Olusunmade M, Wang Y, Newman JD and Dodson JA (2016). Association between fine particulate matter exposure and subclinical atherosclerosis: A meta-analysis. Eur J Prev Cardiol 23: 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeff SE, Coull BA, Gryparis A, Suh H, Sparrow D, Vokonas PS and Schwartz J (2011). Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect 119: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Moreno E, Nawrot TS, Nemmar A and Nemery B (2007). Particulate matter in the environment: pulmonary and cardiovascular effects. Curr Opin Pulm Med 13: 98–106. [DOI] [PubMed] [Google Scholar]
- An Z, Jin Y, Li J, Li W and Wu W (2018). Impact of particulate air pollution on cardiovascular health. Curr Allergy Asthma Rep 18: 1–15. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ and Nel AE (2008). Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armijos RX, Weigel MM, Myers OB, Li WW, Racines M and Berwick M (2015). Residential exposure to urban traffic is associated with increased carotid intima-media thickness in children. J Environ Public Health 215: 713540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N, Kido T, Suzuki H, Yang G, Kavanagh TJ, Kaufman JD, Rosenfeld ME, van Breemen C and Eeden SF (2011). Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis 216: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y and Sun Q (2016). Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim Biophys Acta 1860: 2863–2868. [DOI] [PubMed] [Google Scholar]
- Bai Y, Suzuki AK and Sagai M (2001). The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: Role of active oxygen species. Free Radic Biol Med 30: 555–562. [DOI] [PubMed] [Google Scholar]
- Barath S, Mills NL, Lundback M, Tornqvist H, Lucking AJ, Langrish JP, Soderberg S, Boman C, Westerholm R, Londahl J, Donaldson K, Mudway IS, Sandstrom T, Newby DE and Blomberg A (2010). Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol 7: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Moebus S, Mohlenkamp S, Dragano N, Nonnemacher M, Fuchsluger M, Kessler C, Jakobs H, Memmesheimer M, Erbel R, Jockel KH, Hoffmann B and Group HNRSI (2010). Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol 56: 1803–1808. [DOI] [PubMed] [Google Scholar]
- Bell G, Mora S, Greenland P, Tsai M, Gill E and Kaufman JD (2017). Association of air pollution exposures with high-density lipoprotein cholesterol and particle number: The multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 37: 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boovarahan SR and Kurian GA (2018). Mitochondrial dysfunction: A key player in the pathogenesis of cardiovascular diseases linked to air pollution. Rev Environ Health 33: 111–122. [DOI] [PubMed] [Google Scholar]
- Bourdrel T, Bind MA, Bejot Y, Morel O and Argacha JF (2017). Cardiovascular effects of air pollution. Arch Cardiovasc Dis 110: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner EV, Moller P, Barregard L, Dragsted LO, Glasius M, Wahlin P, Vinzents P, Raaschou-Nielsen O and Loft S (2008). Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol 5: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briet M, Collin C, Laurent S, Tan A, Azizi M, Agharazii M, Jeunemaitre X, Alhenc-Gelas F and Boutouyrie P (2007). Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension 50: 970–976. [DOI] [PubMed] [Google Scholar]
- Brook RD (2008). Cardiovascular effects of air pollution. Clin Sci 115: 175–187. [DOI] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S and Silverman F (2002). Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105: 1534–1536. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr. and Tager I (2004). Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671. [DOI] [PubMed] [Google Scholar]
- Brook RD and Rajagopalan S (2010). Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep 12: 291–300. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L and Kaufman JD (2010). Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378. [DOI] [PubMed] [Google Scholar]
- Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA 3rd, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, Di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, van Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Quoc Thach T, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H and Spadaro JV (2018). Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci 115: 9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Babu NS, Helms GA, Pett S, Wernly J, Mehran R and McDonald JD (2005). Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE−/− mice. Toxicol Sci 88: 95–102. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD and McDonald JD (2010). Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(−/−) mice. Toxicol Appl Pharmacol 242: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Jantzen K, Gouveia AC, Skovmand A, Roursgaard M, Loft S and Moller P (2015). Automobile diesel exhaust particles induce lipid droplet formation in macrophages in vitro. Environ Toxicol Pharmacol 40: 164–171. [DOI] [PubMed] [Google Scholar]
- Cao Y, Long J, Ji Y, Chen G, Shen Y, Gong Y and Li J (2016). Foam cell formation by particulate matter (PM) exposure: A review. Inhal Toxicol 28: 583–590. [DOI] [PubMed] [Google Scholar]
- Channell MM, Paffett ML, Devlin RB, Madden MC and Campen MJ (2012). Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci 127: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Jia G, Wei Y and Li J (2013). Beijing ambient particle exposure accelerates atherosclerosis in ApoE knockout mice. Toxicol Lett 223: 146–153. [DOI] [PubMed] [Google Scholar]
- Chen CC, Chen PS & Yang CY (2019). Relationship between fine particulate air pollution exposure and human adult life expectancy in Taiwan. J Toxicol Environ Health A 82: 826–832 [DOI] [PubMed] [Google Scholar]
- Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR and Kanagy NL (2011). Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Perspect 119: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT and Tang CS (2007). The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 176: 370–376. [DOI] [PubMed] [Google Scholar]
- Cook-Mills JM, Marchese ME, and Abdala-Valencia H. 2011. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15:1607–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL and Forouzanfar MH (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study. Lancet 389: 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A and Kaufman JD (2015). Environmental factors in cardiovascular disease. Nat Rev Cardiol 12: 627–642. [DOI] [PubMed] [Google Scholar]
- Dabass A, Talbott EO, Venkat A, Rager J, Marsh GM, Sharma RK and Holguin F (2016). Association of exposure to particulate matter (PM2.5) air pollution and biomarkers of cardiovascular disease risk in adult NHANES participants. Int J Hyg Environ Health 219: 301–310. [DOI] [PubMed] [Google Scholar]
- Davel AP, Lemos M, Pastro LM, Pedro SC, de Andre PA, Hebeda C, Farsky SH, Saldiva PH and Rossoni LV (2012). Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 295: 39–46. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A and MacNee W (2001). Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect 109: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Jiang S, Zeng X, Zhang J, Pan K, Zhou J, Xie Y, Kan H, Song W, Sun Q and Zhao J (2018). Air pollution is associated with the development of atherosclerosis via the cooperation of CD36 and NLRP3 inflammasome in ApoE(−/−) mice. Toxicol Lett 290: 123–132. [DOI] [PubMed] [Google Scholar]
- Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L and Jessup W (2015). HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res 116: 1133–1142. [DOI] [PubMed] [Google Scholar]
- Du Y, Xu X, Chu M, Guo Y and Wang J (2016). Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis 8: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang SC, Cassidy A and Christiani DC (2010). A systematic review of occupational exposure to particulate matter and cardiovascular disease. Int J Environ Res Public Health 7: 1773–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G and Sorriento D (2017). The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev 22: 337–347. [DOI] [PubMed] [Google Scholar]
- Floyd HS, Chen LC, Vallanat B and Dreher K (2009). Fine ambient air particulate matter exposure induces molecular alterations associated with vascular disease progression within plaques of atherosclerotic susceptible mice. Inhal Toxicol 21: 394–403. [DOI] [PubMed] [Google Scholar]
- Forman HJ and Finch CE (2018). A critical review of assays for hazardous components of air pollution. Free Radic Biol Med 117: 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW (2006). Inflammation and airborne particles. Clin Occup Environ Med 5: 797–815. [DOI] [PubMed] [Google Scholar]
- Frampton MW (2007). Does inhalation of ultrafine particles cause pulmonary vascular effects in humans. Inhal Toxicol 19: 75–79. [DOI] [PubMed] [Google Scholar]
- Franchini M, Guida A, Tufano A and Coppola A (2012). Air pollution, vascular disease and thrombosis: Linking clinical data and pathogenic mechanisms. J Thromb Haemost 10: 2438–2451. [DOI] [PubMed] [Google Scholar]
- Franchini M and Mannucci PM (2009). Particulate air pollution and cardiovascular risk: short-term and long-term effects. Semin Thromb Hemost 35: 665–670. [DOI] [PubMed] [Google Scholar]
- Franchini M and Mannucci PM (2011). Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 118: 2405–2412. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Brook R and Arden Pope C 3rd (2015). Air pollution and cardiovascular disease. Curr Problem Cardiol 40: 207–238. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Allen RW, Brauer M, Davies HW, Mancini GB and Lear SA (2014). Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: A prospective cohort study. BMJ Open 4: 4–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill EA, Curl CL, Adar SD, Allen RW, Auchincloss AH, O’Neill MS, Park SK, Van Hee VC, Diez Roux AV and Kaufman JD (2011). Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Prog Cardiovasc Dis 53: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Flecha B (2004). Oxidant mechanisms in response to ambient air particles. Mol Aspects Med 25: 169–182. [DOI] [PubMed] [Google Scholar]
- Grahame TJ and Schlesinger RB (2010). Cardiovascular health and particulate vehicular emissions: A critical evaluation of the evidence. Air Qual Atmos Health 3: 3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DL, Wallace LA, Brinkman MC, Buehler SS and La Londe C (2015). Respiratory and cardiovascular effects of metals in ambient particulate matter: A critical review. Rev Environ Contam Toxicol 234: 135–203. [DOI] [PubMed] [Google Scholar]
- Green R, Broadwin R, Malig B, Basu R, Gold EB, Qi L, Sternfeld B, Bromberger JT, Greendale GA, Kravitz HM, Tomey K, Matthews K, Derby CA, Jackson EA, Green R and Ostro B (2016). Long- and short-term exposure to air pollution and inflammatory/emostatic markers in midlife women. Epidemiology 27: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G, Marsh LM, Esmaeil N, Jackson K, Gordon T, Reibman J, Kwapiszewska G and Park SH (2014). Perspective: ambient air pollution: inflammatory response and effects on the lung’s vasculature. Pulm Circ 4: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhu N, Guo Z, Li GK, Chen C, Sang N and Yao QC (2012). Particulate matter (PM10) exposure induces endothelial dysfunction and inflammation in rat brain. J Hazard Mater 213: 28–37. [DOI] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy GG and Gonzalez-Flecha B (2002). Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect 110: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB and Mutlu GM (2018). Particulate matter air pollution: Effects on the cardiovascular system. Front Endocrinol 9: 600–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel R, Peters A, Beelen R, Brunekreef B, Cyrys J, de Faire U, de Hoogh K, Fuks K, Hoffmann B, Huls A, Imboden M, Jedynska A, Kooter I, Koenig W, Kunzli N, Leander K, Magnusson P, Mannisto S, Penell J, Pershagen G, Phuleria H, Probst-Hensch N, Pundt N, Schaffner E, Schikowski T, Sugiri D, Tiittanen P, Tsai MY, Wang M, Wolf K, Lanki T and groups ETS (2015). Long-term effects of elemental composition of particulate matter on inflammatory blood markers in European cohorts. Environ Int 82: 76–84. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Block ML, Hong JS and Miller DS (2008). Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J 22: 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassing HC, Twickler TB, Kastelein JJ, Cramer MJ and Cassee FR (2009). Air pollution as noxious environmental factor in the development of cardiovascular disease. Netherland J Med 67: 116–121. [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Dragano N, Stang A, Mohlenkamp S, Schmermund A, Memmesheimer M, Brocker-Preuss M, Mann K, Erbel R and Jockel KH (2009). Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect 117: 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman-Strickland P, Diehl SR, Zhu P, Tong J, Gong J, Zhu T and Zhang J (2012). Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med 186: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC and Ghio AJ (2006). Vascular effects of ambient pollutant particles and metals. Curr Vasc Pharm 4: 199–203. [DOI] [PubMed] [Google Scholar]
- Huttunen K, Siponen T, Salonen I, Yli-Tuomi T, Aurela M, Dufva H, Hillamo R, Linkola E, Pekkanen J, Pennanen A, Peters A, Salonen RO, Schneider A, Tiittanen P, Hirvonen MR and Lanki T (2012). Low-level exposure to ambient particulate matter is associated with systemic inflammation in ischemic heart disease patients. Environ Res 116: 44–51. [DOI] [PubMed] [Google Scholar]
- Initiative IS-LDB (2019). The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: The Global Burden of Disease Study 2017. Lancet Planet Health 3: 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen K, Jensen A, Kermanizadeh A, Elholm G, Sigsgaard T, Moller P, Roursgaard M and Loft S (2018). Inhalation of house dust and zone alters systemic levels of endothelial progenitor cells, oxidative stress, and inflammation in elderly subjects. Toxicol Sci 163: 353–363. [DOI] [PubMed] [Google Scholar]
- Johannesson S, Andersson EM, Stockfelt L, Barregard L and Sallsten G (2014). Urban air pollution and effects on biomarkers of systemic inflammation and coagulation: a panel study in healthy adults. Inhal Toxicol 26: 84–94. [DOI] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR Jr., Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA and Watson KE (2016). Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet 388: 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AJ, Sioutas C, Pakbin P, Schauer JJ, Mendez LB and Kleinman MT (2015). Is atherosclerotic disease associated with organic components of ambient fine particles. Sci Total Environ 533: 69–75. [DOI] [PubMed] [Google Scholar]
- Kelly FJ and Fussell JC (2015). Linking ambient particulate matter pollution effects with oxidative biology and immune responses. Ann N Y Acad Sci 1340: 84–94. [DOI] [PubMed] [Google Scholar]
- Kirrane EF, Luben TJ, Benson A, Owens EO, Sacks JD, Dutton SJ, Madden M and Nichols JL (2019). A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ Int 127: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles TL, Lund AK, Lucas SN and Campen MJ (2008). Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol 230: 346–351. [DOI] [PubMed] [Google Scholar]
- Krishnan RM, Sullivan JH, Carlsten C, Wilkerson HW, Beyer RP, Bammler T, Farin F, Peretz A and Kaufman JD (2013). A randomized cross-over study of inhalation of diesel exhaust, hematological indices, and endothelial markers in humans. Part Fibre Toxicol 10: 3–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristovich R, Knight DA, Long JF, Williams MV, Dutta PK, and Waldman WJ (2004). Macrophage-mediated endothelial inflammatory responses to airborne particulates: impact of particulate physicochemical properties. Chem Res Toxicol 17:1303–1312. [DOI] [PubMed] [Google Scholar]
- Kunzli N, Perez L, von Klot S, Baldassarre D, Bauer M, Basagana X, Breton C, Dratva J, Elosua R, de Faire U, Fuks K, de Groot E, Marrugat J, Penell J, Seissler J, Peters A and Hoffmann B (2011). Investigating air pollution and atherosclerosis in humans: concepts and outlook. Prog Cardiovasc Dis 53: 334–343. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potocnik J, Preker AS, Ramesh J, Rockstrom J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K and Zhong M (2018). The Lancet Commission on pollution and health. Lancet 391: 462–512. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Bosson J, Unosson J, Muala A, Newby DE, Mills NL, Blomberg A and Sandstrom T (2012). Cardiovascular effects of particulate air pollution exposure: Time course and underlying mechanisms. J Intern Med 272: 224–239. [DOI] [PubMed] [Google Scholar]
- Lanki T, Hampel R, Tiittanen P, Andrich S, Beelen R, Brunekreef B, Dratva J, De Faire U, Fuks KB, Hoffmann B, Imboden M, Jousilahti P, Koenig W, Mahabadi AA, Kunzli N, Pedersen NL, Penell J, Pershagen G, Probst-Hensch NM, Schaffner E, Schindler C, Sugiri D, Swart WJ, Tsai MY, Turunen AW, Weinmayr G, Wolf K, Yli-Tuomi T and Peters A (2015). Air pollution from road traffic and systemic inflammation in adults: A cross-sectional analysis in the European ESCAPE project. Environ Health Perspect 123: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal AO (2017). Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett 270: 88–95. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Cumpston JL, Chen BT, Frazer D, Castranova V and Nurkiewicz TR (2009). Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J Toxicol Environ Health A 72: 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Huang SH, Yang YT, Cheng YW, Li CH and Kang JJ (2012). Motorcycle exhaust particles up-regulate expression of vascular adhesion molecule-1 and intercellular adhesion molecule-1 in human umbilical vein endothelial cells. Toxicol In Vitro 26: 552–560. [DOI] [PubMed] [Google Scholar]
- Lee MS, Eum KD, Fang SC, Rodrigues EG, Modest GA and Christiani DC (2014). Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution. Int J Cardiol 176: 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, Klingmuller K, Pozzer A, Poschl U, Fnais M, Daiber A and Munzel T (2019). Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 40: 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters V, Uiterwaal CS, Beelen R, Bots ML, Fischer P, Brunekreef B and Hoek G (2010). Long-term exposure to air pollution and vascular damage in young adults. Epidemiology 21: 512–520. [DOI] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H and Kan H (2017). Particulate matter exposure and stress hormone levels: A randomized, double-blind, crossover trial of air purification. Circulation 136: 618–627. [DOI] [PubMed] [Google Scholar]
- Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioutas C and Hsiai T (2013). Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res 54: 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tang K, Jin XR, Xiang Y, Xu J, Yang LL, Wang N, Li YF, Ji AL, Zhou LX and Cai TJ (2018). Short-term air pollution exposure is associated with hospital length of stay and hospitalization costs among inpatients with type 2 diabetes: A hospital-based study. J Toxicol Environ Health A 81: 819–829. [DOI] [PubMed] [Google Scholar]
- Lippmann M (2014). Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: Coherence and public health implications. Crit Rev Toxicol 44: 299–347. [DOI] [PubMed] [Google Scholar]
- Liu J, Ye X, Ji D, Zhou X, Qiu C, Liu W and Yu L (2018). Diesel exhaust inhalation exposure induces pulmonary arterial hypertension in mice. Environ Pollut 237: 747–755. [DOI] [PubMed] [Google Scholar]
- Liu L, Urch B, Poon R, Szyszkowicz M, Speck M, Gold DR, Wheeler AJ, Scott JA, Brook JR, Thorne PS and Silverman FS (2015). Effects of ambient coarse, fine, and ultrafine particles and their biological constituents on systemic biomarkers: A controlled human exposure study. Environ Health Perspect 123: 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwies T, Panis LI, Kicinski M, De Boever P and Nawrot TS (2013). Retinal microvascular responses to short-term changes in particulate air pollution in healthy adults. Environ Health Perspect 121: 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luben TJ, Nichols JL, Dutton SJ, Kirrane E, Owens EO, Datko-Williams L, Madden M and Sacks JD (2017). A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon. Environ Int 107: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AK, Knuckles TL, Obot Akata C, Shohet R, McDonald JD, Gigliotti A, Seagrave JC and Campen MJ (2007). Gasoline exhaust emissions induce vascular remodeling pathways involved in atherosclerosis. Toxicol Sci 95: 485–494. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC and Campen MJ (2011). The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am J Respir Crit Care Med 184: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundback M, Mills NL, Lucking A, Barath S, Donaldson K, Newby DE, Sandstrom T and Blomberg A (2009). Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol 6: 17–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli N, Olivieri O and Girelli D (2013). Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med 24: 295–302. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Wei J, and Wenger NK (2015). Ischemic heart disease in women: a focus on risk factors. Trends Cardiovasc Med 25: 140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza CA, La Favor JD, Kim DH, and Hickner RC (2019). Endothelial dysfunction: Is there a hyperglycemia-induced imbalance of NOX and NOS. Int J Mol Sci 20: 14–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa T, Okamura T, Nitta H, Nishiwaki Y, Takebayashi T, Ueda K, Kadota A, Fujiyoshi A, Ohkubo T, Ueshima H, Okayama A, Miura K and Group NDR (2016). Cross-sectional association between exposure to particulate matter and inflammatory markers in the Japanese general population: NIPPON DATA. Environ Pollut 213: 460–467. [DOI] [PubMed] [Google Scholar]
- Miller MR (2014). The role of oxidative stress in the cardiovascular actions of particulate air pollution. Biochem Soc Trans 42: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadoke PW and Newby DE (2009). Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ Health Perspect 117: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, McLean SG, Duffin R, Lawal AO, Araujo JA, Shaw CA, Mills NL, Donaldson K, Newby DE and Hadoke PW (2013). Diesel exhaust particulate increases the size and complexity of lesions in atherosclerotic mice. Part Fibre Toxicol 10: 15–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, Krystek P, Campbell CJ, Hadoke PWF, Donaldson K, Cassee FR, Newby DE, Duffin R and Mills NL (2017). Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11: 4542–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Shaw CA and Langrish JP (2012). From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol 8: 577–602. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T and Newby DE (2005). Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112: 3930–3936. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez MC, Soderberg S, Sandstrom T, Blomberg A, Newby DE and Donaldson K (2007). Air pollution and atherothrombosis. Inhal Toxicol 19: 81–89. [DOI] [PubMed] [Google Scholar]
- Mirowsky JE, Carraway MS, Dhingra R, Tong H, Neas L, Diaz-Sanchez D, Cascio W, Case M, Crooks J, Hauser ER, Elaine Dowdy Z, Kraus WE and Devlin RB (2017). Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ Health 16: 45–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata R, Hiraiwa K, Cheng JC, Bai N, Vincent R, Francis GA, Sin DD and Van Eeden SF (2013). Statins attenuate the development of atherosclerosis and endothelial dysfunction induced by exposure to urban particulate matter (PM10). Toxicol Appl Pharmacol 272: 1–11. [DOI] [PubMed] [Google Scholar]
- Moller P, Christophersen DV, Jacobsen NR, Skovmand A, Gouveia AC, Andersen MH, Kermanizadeh A, Jensen DM, Danielsen PH, Roursgaard M, Jantzen K and Loft S (2016). Atherosclerosis and vasomotor dysfunction in arteries of animals after exposure to combustion-derived particulate matter or nanomaterials. Crit Rev Toxicol 46: 437–476. [DOI] [PubMed] [Google Scholar]
- Moller P, Mikkelsen L, Vesterdal LK, Folkmann JK, Forchhammer L, Roursgaard M, Danielsen PH and Loft S (2011). Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis. Crit Rev Toxicol 41: 339–368. [DOI] [PubMed] [Google Scholar]
- Morales-Barcenas R, Chirino YI, Sanchez-Perez Y, Osornio-Vargas AR, Melendez-Zajgla J, Rosas I and Garcia-Cuellar CM (2015). Particulate matter (PM10) induces metalloprotease activity and invasion in airway epithelial cells. Toxicol Lett 237: 167–173. [DOI] [PubMed] [Google Scholar]
- Nasser Z, Salameh P, Nasser W, Abou Abbas L, Elias E and Leveque A (2015). Outdoor particulate matter (PM) and associated cardiovascular diseases in the Middle East. Int J Occup Med Environ Health 28: 641–661. [DOI] [PubMed] [Google Scholar]
- Nelin TD, Joseph AM, Gorr MW and Wold LE (2012). Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett 208: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Kunzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S and Storey RF (2015). Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Liberda EN, Qu S, Guo X, Li X, Zhang J, Meng J, Yan B, Li N, Zhong M, Ito K, Wildman R, Liu H, Chen LC and Qu Q (2013). The role of metal components in the cardiovascular effects of PM2.5. PLoS One 8: 83–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira JB (2009). Air pollution and cardiovascular disease. Rev Port Cardiol 28: 715–733. [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Castranova V and Boegehold MA (2004). Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect 112: 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V and Boegehold MA (2006). Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect 114: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES and Schwartz J (2007). Air pollution and inflammation in type 2 diabetes: A mechanism for susceptibility. Occup Environ Med 64: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES and Schwartz J (2005). Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation 111: 2913–2920. [DOI] [PubMed] [Google Scholar]
- Oppenheim HA, Lucero J, Guyot AC, Herbert LM, McDonald JD, Mabondzo A and Lund AK (2013). Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol 10: 1–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA and Kaufman JD (2008). Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect 116: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinichka C, Makka N, Sukkumnoed D, Chariyalertsak S, Inchai P and Bundhamcharoen K (2017). Burden of disease attributed to ambient air pollution in Thailand: A GIS-based approach. PLoS One 12: 189–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polichetti G, Cocco S, Spinali A, Trimarco V and Nunziata A (2009). Effects of particulate matter PM(10), PM(2.5) and PM(1) on the cardiovascular system. Toxicology 261: 1–8. [DOI] [PubMed] [Google Scholar]
- Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ and O’Toole T (2016). Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119: 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K and Thurston GD (2002). Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J Am Med Assoc 287: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA 3rd, Hansen JC, Kuprov R, Sanders MD, Anderson MN and Eatough DJ (2011). Vascular function and short-term exposure to fine particulate air pollution. J Air Waste Manage Assoc 61: 858–863. [DOI] [PubMed] [Google Scholar]
- Premasekharan G, Nguyen K, Contreras J, Ramon V, Leppert VJ and Forman HJ (2011). Iron-mediated lipid peroxidation and lipid raft disruption in low-dose silica-induced macrophage cytokine production. Free Radic Biol Med 51: 1184–1194. [DOI] [PubMed] [Google Scholar]
- Prueitt RL, Cohen JM and Goodman JE (2015). Evaluation of atherosclerosis as a potential mode of action for cardiovascular effects of particulate matter. Regul Toxicol Pharmacol 73: 1–15. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Al-Kindi SG and Brook RD (2018). Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 72: 2054–2070. [DOI] [PubMed] [Google Scholar]
- Ramanathan G, Yin F, Speck M, Tseng CH, Brook JR, Silverman F, Urch B, Brook RD and Araujo JA (2016). Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol 13: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q and Rajagopalan S (2014). CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res 115: 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C and King GL (2013). Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T and Zhang JJ (2012). Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. J Am Med Assoc 307: 2068–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S, Gray GA, Duffin R, McLean SG, Shaw CA, Hadoke PW, Newby DE and Miller MR (2012). Diesel exhaust particulate induces pulmonary and systemic inflammation in rats without impairing endothelial function ex vivo or in vivo. Part Fibre Toxicol 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudez G, Janssen NA, Kilinc E, Leebeek FW, Gerlofs-Nijland ME, Spronk HM, ten Cate H, Cassee FR and de Maat MP (2009). Effects of ambient air pollution on hemostasis and inflammation. Environ Health Perspect 117: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell KW, Hoffman JR, Caviston R, Bulbulian R and Hollenbach AM (2007). Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal Toxicol 19: 133–140. [DOI] [PubMed] [Google Scholar]
- Sandhu RS, Petroni DH and George WJ (2005). Ambient particulate matter, C-reactive protein, and coronary artery disease. Inhal Toxicol 17: 409–413. [DOI] [PubMed] [Google Scholar]
- Saura M, Zaragoza C, Bao C, Herranz B, Rodriguez-Puyol M and Lowenstein CJ (2006). Stat3 mediates interleukin-6 [correction of interelukin-6] inhibition of human endothelial nitric-oxide synthase expression. J Biol Chem 281: 30057–30062. [DOI] [PubMed] [Google Scholar]
- Scapellato ML and Lotti M (2007). Short-term effects of particulate matter: An inflammatory mechanism. Crit Rev Toxicol 37: 461–487. [DOI] [PubMed] [Google Scholar]
- Schisler JC, Ronnebaum SM, Madden M, Channell M, Campen M, and Willis MS (2015). Endothelial inflammatory transcriptional responses to an altered plasma exposome following inhalation of diesel emissions. Inhal Toxicol 27: 272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder WH, Dobson M, Kane DM and Johnson ND (1987). Toxic trace elements associated with airborne particulate matter: A review. J Air Pollut Control Assoc 37: 1267–1285. [DOI] [PubMed] [Google Scholar]
- See SW, Wang YH and Balasubramanian R (2007). Contrasting reactive oxygen species and transition metal concentrations in combustion aerosols. Environ Res 103: 317–324. [DOI] [PubMed] [Google Scholar]
- Shrey K, Suchit A, Deepika D, Shruti K and Vibha R (2011). Air pollutants: The key stages in the pathway towards the development of cardiovascular disorders. Environ Toxicol Pharmacol 31: 1–9. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT and Kloner RA (2008). Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol 52: 719–726. [DOI] [PubMed] [Google Scholar]
- Siponen T, Yli-Tuomi T, Aurela M, Dufva H, Hillamo R, Hirvonen MR, Huttunen K, Pekkanen J, Pennanen A, Salonen I, Tiittanen P, Salonen RO and Lanki T (2015). Source-specific fine particulate air pollution and systemic inflammation in ischemic heart disease patients. Occup Environ Med 72: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TC, Hwang JJ, Shen YC and Chan CC (2015). Carotid intima-media thickness and long-term exposure to traffic-related air pollution in middle-aged residents of Taiwan: A cross-sectional study. Environ Health Perspect 123: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Hong X and Wold LE (2010). Cardiovascular effects of ambient particulate air pollution exposure. Circulation 121: 2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC and Rajagopalan S (2005). Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. J Am Med Ass 294: 3003–3010. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Kirk RI, Wang A, Moatti D, Jin X, Lu B, Schecter AD, Lippmann M, Gordon T, Chen LC and Rajagopalan S (2008a). Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxicol 20: 127–137. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC and Rajagopalan S (2008b). Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol 28: 1760–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing L, Li Y, Laher I, Sin DD, Man SF and van Eeden SF (2008). Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol 295: L79–L85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y and Griendling KK (2003). Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T and Blomberg A (2007). Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med 176: 395–400. [DOI] [PubMed] [Google Scholar]
- Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D’Agostino RB Sr., Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M and Rader DJ (2013). High-density lipoproteins: A consensus statement from the National Lipid Association. J Clin Lipidol 7: 484–525. [DOI] [PubMed] [Google Scholar]
- Tranfield EM, van Eeden SF, Yatera K, Hogg JC and Walker DC (2010). Ultrastructural changes in atherosclerotic plaques following the instillation of airborne particulate matter into the lungs of rabbits. Can J Cardiol 26: 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai DH, Amyai N, Marques-Vidal P, Wang JL, Riediker M, Mooser V, Paccaud F, Waeber G, Vollenweider P and Bochud M (2012). Effects of particulate matter on inflammatory markers in the general adult population. Part Fibre Toxicol 9: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SS, Chen CC and Yang CY (2014). Short-term effect of fine particulate air pollution on daily mortality: a case-crossover study in a tropical city, Kaohsiung, Taiwan. J Toxicol Environ Health A 77: 467–477. [DOI] [PubMed] [Google Scholar]
- Utell MJ, Frampton MW, Zareba W, Devlin RB and Cascio WE (2002). Cardiovascular effects associated with air pollution: Potential mechanisms and methods of testing. Inhal Toxicol 14: 1231–1247. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R and Hogg JC (2001). Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am J Respir Crit Care Med 164: 826–830. [DOI] [PubMed] [Google Scholar]
- Vidale S and Campana C (2018). Ambient air pollution and cardiovascular diseases: From bench to bedside. Eur J Prev Cardiol 25: 818–825. [DOI] [PubMed] [Google Scholar]
- Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Mohlenkamp S, Nonnemacher M, Jakobs H, Erbel R, Jockel KH, Hoffmann B and Heinz G Nixdorf Recall Investigator (2015). Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med 72: 656–663. [DOI] [PubMed] [Google Scholar]
- Vodonos A, Awad YA and Schwartz J (2018). The concentration-response between long-term PM2.5 exposure and mortality; A meta-regression approach. Environ Res 166: 677–689. [DOI] [PubMed] [Google Scholar]
- Wang G, Jiang R, Zhao Z and Song W (2013). Effects of ozone and fine particulate matter (PM2.5) on rat system inflammation and cardiac function. Toxicol Lett 217: 23–33. [DOI] [PubMed] [Google Scholar]
- Wang M, Zheng S, Nie Y, Weng J, Cheng N, Hu X, Ren X, Pei H, and Bai Y (2018). Association between short-term exposure to air pollution and dyslipidemias among type 2 diabetic patients in northwest China: A population-based study. Int J Environ Res Public Health 15: 576–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang L, Moreno-Vinasco L, Lang GD, Siegler JH, Mathew B, Usatyuk PV, Samet JM, Geyh AS, Breysse PN, Natarajan V and Garcia JG (2012). Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol 9: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wellenius GA, Hickson DA, Gjelsvik A, Eaton CB and Wyatt SB (2016). Residential proximity to traffic-related pollution and atherosclerosis in 4 vascular beds among African American adults: Results from the Jackson Heart Study. Am J Epidemiol 184: 732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiong L and Tang M (2017). Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol 37: 644–667. [DOI] [PubMed] [Google Scholar]
- Watkinson WP, Campen MJ, Nolan JP and Costa DL (2001). Cardiovascular and systemic responses to inhaled pollutants in rodents: effects of ozone and particulate matter. Environ Health Perspect 109: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters A, Dreyfuss C, Pochet S, Hendrick P, Berkenboom G, van de Borne P and Argacha JF (2013). Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension 62: 352–358. [DOI] [PubMed] [Google Scholar]
- Wauters A, Vicenzi M, De Becker B, Riga JP, Esmaeilzadeh F, Faoro V, Vachiery JL, van de Borne P and Argacha JF (2015). At high cardiac output, diesel exhaust exposure increases pulmonary vascular resistance and decreases distensibility of pulmonary resistive vessels. Am J Physiol Heart Circ Physiol 309: 2137–2144. [DOI] [PubMed] [Google Scholar]
- Wilker EH, Mittleman MA, Coull BA, Gryparis A, Bots ML, Schwartz J and Sparrow D (2013). Long-term exposure to black carbon and carotid intima-media thickness: The normative aging study. Environ Health Perspect 121: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, Herder C, Roden M, Koenig W, Meisinger C and Peters A (2016). Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes 65: 3314–3326. [DOI] [PubMed] [Google Scholar]
- Yan Z, Jin Y, An Z, Liu Y, Samet JM and Wu W (2016). Inflammatory cell signaling following exposures to particulate matter and ozone. Biochim Biophys Acta 1860: 2826–2834. [DOI] [PubMed] [Google Scholar]
- Yatera K, Hsieh J, Hogg JC, Tranfield E, Suzuki H, Shih CH, Behzad AR, Vincent R and van Eeden SF (2008). Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques. Am J Physiol Heart Circ Physiol 294: 944–953. [DOI] [PubMed] [Google Scholar]
- Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC and Rajagopalan S (2009). Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci 111: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Schneider A, Stolzel M, Ruckerl R, Cyrys J, Pan X, Zareba W, Koenig W, Wichmann HE and Peters A (2007). Ambient source-specific particles are associated with prolonged repolarization and increased levels of inflammation in male coronary artery disease patients. Mutat Res 621: 50–60. [DOI] [PubMed] [Google Scholar]
- Zanoli L, Lentini P, Granata A, Gaudio A, Fatuzzo P, Serafino L, Rastelli S, Fiore V, D’Anca A, Signorelli SS and Castellino P (2017). A systematic review of arterial stiffness, wave reflection and air pollution. Mol Med Rep 15: 3425–3429. [DOI] [PubMed] [Google Scholar]
- Zhang X, Staimer N, Gillen DL, Tjoa T, Schauer JJ, Shafer MM, Hasheminassab S, Pakbin P, Vaziri ND, Sioutas C and Delfino RJ (2016a). Associations of oxidative stress and inflammatory biomarkers with chemically characterized air pollutant exposures in an elderly cohort. Environ Res 150: 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM, Hasheminassab S, Pakbin P, Longhurst J, Sioutas C and Delfino RJ (2016b). Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ Health 15: 3–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Jin C, Su Y, Li J and Zhu B (2016). Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ Pollut 212: 627–635. [DOI] [PubMed] [Google Scholar]