Abstract
Chronic low back pain is a common condition, with high societal costs and often ineffectual treatments. Communication between macrophages/monocytes (MØ) and sensory neurons has been implicated in various preclinical pain models. However, few studies have examined specific MØ subsets, although distinct subtypes may play opposing roles. This study used a model of low back pain/radiculopathy involving direct local inflammation of the dorsal root ganglia (DRG). Reporter mice were employed that had distinct fluorescent labels for two key MØ subsets: CCR2-expressing (infiltrating pro-inflammatory) MØ, and CX3CR1-expressing (resident) macrophages. We observed that local DRG inflammation induced pain behaviors in mice, including guarding behavior and mechanical hypersensitivity, similar to the previously described rat model. The increase in MØ in the inflamed DRG was dominated by increases in CCR2+ MØ, which persisted for at least 14 days. The primary endogenous ligand for CCR2, CCL2, was upregulated in inflamed DRG. Three different experimental manipulations that reduced the CCR2+ MØ influx also reduced pain behaviors: global CCR2 knockout; systemic injection of INCB3344 (specific CCR2 blocker); and intravenous injection of liposomal clodronate. The latter two treatments when applied around the time of DRG inflammation reduced CCR2+ but not CX3CR1+ MØ in the DRG. Together these experiments suggest a key role for the CCR2/CCL2 system in establishing the pain state in this model of inflammatory low back pain and radiculopathy. Intravenous clodronate given after pain was established had the opposite effect on pain behaviors, suggesting the role of macrophages or their susceptibility to clodronate may change with time.
Keywords: CCR2, MCP-1, macrophage, dorsal root ganglion, radicular pain, CX3CR1, INCB3344, clodronate
1. Introduction
Chronic low back pain is a major cause of disability and health care costs with inadequate treatments. (Gaskin and Richard, 2012; Institute of Medicine (US) Committee on Advancing Pain Research, 2011). Chronic low back pain is associated with inflammation.
Monocytes/macrophages (MØ) play critical roles in both inflammation and pain (Chen et al., 2019). Macrophages have often been identified on the basis of their expression of panmacrophage marker IBA1 (ionized calcium-binding adaptor molecule 1), and we have previously shown an increase in IBA1 expression levels in our rat model of chronic low back pain (Xie et al., 2016). However, new studies have revolutionized our understanding of macrophages in health and disease by delineating heterogenous subsets of these cells. Two major subsets are described in the literature based on their expression of either CCR2 or CX3CR1. CCR2 is the primary receptor for CCL2 (also known as monocyte chemoattractant protein 1; MCP-1), an important chemoattractant that mediates migration of pro-inflammatory blood monocytes into sites of tissue inflammation. CX3CR1 is the primary receptor for CX3CL1 (fracktalkine), and high levels of its expression are often used as a marker for resident macrophages within specific tissues and microglia in the spinal cord and brain (although the CCL2-expressing MØ also express low levels of CX3CR1) . (Dominguez and Ardavin, 2010; Saederup et al., 2010; Williams et al., 2017; Wynn and Vannella, 2016; Yamasaki et al., 2012). These two general classes of MØ can be studied in transgenic reporter mice in which the CCR2-expressing cells also express red fluorescent protein, while the CX3CR1-expressing cells express green fluorescent protein (Saederup et al., 2010). A number of immunological studies have used these reporter mice, but relatively few pain studies have made use of these reporter mice, (e.g. a study (Gu et al., 2016) that focused primarily on the spinal cord).
In the present study, we used these reporter mice to examine the role of these two MØ subtypes in a model of low back pain and radiculopathy induced by local inflammation of the lumbar dorsal root ganglia (DRG) with the inflammatory stimulus zymosan. This model, originally developed in rats (Xie et al., 2012; Xie et al., 2006), is intended to mimic the local inflammation at the level of the sensory ganglia that may occur in some forms of low back pain and radiculopathy. An example is back pain caused by ruptured discs, in which the DRG are exposed to the immunogenic nucleus pulposis that is normally sequestered inside the disc away from immune surveillance (Gertzbein et al., 1977). Here, we demonstrate that this DRG inflammation model is readily adapted to mice, and provide data suggesting a key role for CCL2 and the CCR2-expressing MØ in initiating pain behaviors in this model. The CCR2/CCL2 system has been implicated in several different types of pain models, including nerve injury (Abbadie et al., 2003; Gu et al., 2019), chemotherapy (Illias et al., 2018), and peripheral inflammatory (Chun and Kwon, 2019) models. In most of these studies CCR2/CCL2 were found to be pain-initiating or pain-enhancing (for review, see (Chen et al., 2019; Jiang et al., 2020)), but in a few studies a role for pain resolution was suggested. For example, pain-relieving local sympathectomy upregulated CCL2 in the DRG (Tonello et al., 2020), and depletion of CCR2-positive macrophages prolonged recovery from IL-1β-induced hyperalgesia (Willemen et al., 2014). CCL2 and CCR2 can be expressed in sensory neurons especially under pathological conditions; CCL2 can directly excite sensory neurons, and can be released into the spinal cord by sensory neurons (Abbadie et al., 2009; White et al., 2005). A number of studies have examined these mechanisms of action of CCL2, while other studies focus more on the role of macrophages in mediating CCL2 actions (Chen et al., 2019). In the present study, we used the reporter mice to examine the role of MØ subsets in the DRG in this model of inflammatory low back pain and radiculopathy.
2. Methods
2.1. Animals:
Male and female mice (8 - 12 weeks) were used in this study and obtained from Jackson Laboratory, Bar Harbor, ME. To obtain mice with red and green fluorescent labeled macrophage subsets, the strain B6.129(Cg)-Ccr2tm2.1Ifc/J (Ccr2RFP/RFP; strain 017586, (Saederup et al., 2010)), in which CCR2, the receptor for CCL2, has been replaced at both loci by red fluorescent protein (RFP), was crossed with the strain B6.129P2(Cg)-Cx3cr1tm1Litt/J (Cx3cr1GFP/GFP; strain 005582; (Jung et al., 2000)) in which the fractalkine receptor CX3CR1 is replaced at both loci by green fluorescent protein (GFP) (as illustrated in Supplemental Fig. A1). The resulting progeny CCR2+/RFP CX3CR1+/GFP (referred to herein as “reporter” mice) have both RFP-labeled and GFP-labeled subsets of MØ, but have one normal copy of each of the 2 receptors (Saederup et al., 2010). The CCR2RFP/RFP parent strain was used for experiments requiring CCR2 knockout mice. All transgenic mice were viable and showed no obvious developmental defects, confirming previous reports. Experiments with “wildtype” mice used the C57BL/6J mice (strain 000664, Jackson Laboratory), recommended as the control strain for both constructs. Behavioral experiments did not show sex differences. All experiments included animals of both sexes in approximately equal numbers. Data from both sexes is combined in all figures except some supplementary figures, where more details about the analysis of sex as a factor can be found. All efforts were made to minimize animal suffering, reduce the number of animals used, and use alternatives to in vivo techniques, in accordance with the International Association for the Study of Pain guidelines and the National Institutes of Health Office of Laboratory Animal Welfare Guide for the Care and Use of Laboratory Animals, and adhered to animal welfare guidelines established by the University of Cincinnati Institutional Animal Care and Use Committee who approved the experimental protocols used.
2.2. Pain model
The lumbar DRG inflammation model of inflammatory low back pain/radiculopathy was modified from our previously described rat model (Xie et al., 2012). The mice were anesthetized with isoflurane and an incision was made on the back and the overlying paraspinal muscles separated to expose the L4 and L3 transverse processes. No laminectomy was performed. The L4 DRG (analogous to the L5 DRG in rat (Rigaud et al., 2008)) was inflamed by local injection of the immune activator zymosan (Sigma-Aldrich, St. Louis, MO, catalog #Z4250; 2mg/ml, 3 μl in volume which was reduced from 10 μl used in the rat, in incomplete Freund’s adjuvant - IFA) into the L4 intervertebral foramen, above the DRG, via a ½” 30-gauge needle bent at a 90 degree angle inserted into the L4 intervertebral foramen. For sham control animals, the DRG were exposed but no injection was made. For experiments in which DRG tissue was isolated for obtaining mRNA or protein, both L4 and L3 were inflamed. The incision was closed in layers.
2.3. Pain behavior measurements:
Mechanical sensitivity of the hindpaw was tested with von Frey filaments using the up-and-down method (Chaplan et al., 1994), as we have previously reported (Tonello et al., 2020). Experimenters were blinded as to which experimental group the animal was in. Briefly, the mice were first acclimatized (1 hour) in individual clear Plexiglas boxes on an elevated wire mesh platform to facilitate access to the plantar surface of the hind paws. Subsequently, a series of von Frey hairs (0.02, 0.07, 0.16, 0.4, 0.6, 1.0, and 1.4 g; Stoelting Co., Wood Dale, IL) were applied perpendicular to the plantar surface of hindpaw. A test began with the application of the 0.6 g hair. A positive response was defined as a clear paw withdrawal or shaking. Whenever a positive response occurred, the next lower hair was applied, and whenever a negative response occurred, the next higher hair was applied. The testing consisted of 6 stimuli, and the pattern of response was analyzed as the log transforms of the von Frey force, as described in (Mills et al., 2012). Spontaneous guarding behavior was scored (Brennan et al., 1996) as 0 (no guarding, paw flat on floor), 1 (mild shift of weight away from paw), 2 (unequal weight bearing and some part of the foot not touching the floor), or 3 (foot totally raised or not bearing any weight); these scores were recorded just before each application of the von Frey filament (6 observations per paw total) and averaged.
In some experiments, an operant measure of mechanical pain was used as described by Shepherd and Mohapatra (Shepherd and Mohapatra, 2018); briefly, we measured the latency for the mouse to enter a chamber with closely spaced metal probes on the floor; crossing the chamber allowed the mouse to escape from a naturally aversive stimulus (well-lit chamber) and reach a dark chamber but only by encountering the mechanical stimuli to the paws. Measurements were made with the probes at 5, 2, and 0 mm height. The chamber was constructed as described in the original reference, with plastic parts pre-cut and provided by TAP Plastics, San Jose, CA, and push pins from Office Depot (Item # 825-265) that had been slightly blunted with a whetstone.
2.4. Reagents for manipulation of MØ in vivo:
Liposomal clodronate (Van Rooijen and Sanders, 1994) was purchased from Liposoma (cat# 283539, Amsterdam, The Netherlands) and injected intravenously (200 μl per mouse, tail vein injection) at the indicated time point. Control mice received injections of control liposomes as provided by the same manufacturer. The CCR2 antagonist INCB3344 was purchased from APExBIO (cat# A3494, Houston, TX) and administered intravenously (tail vein) on the indicated schedules (100 μl of 0.17 mM solution per mouse per injection, made by diluting 3 μl of a 57.7 mM DMSO stock concentration with 1 ml of normal saline).
2.5. Immunohistochemistry
Mice were anesthetized with isoflurane and lumbar DRGs were removed as fresh tissue, immediately fixed in 4% paraformaldehyde for 2 hours, transferred to 30% sucrose, and cut into 12 μm frozen sections within one day. The lack of cardiac fixation perfusion and the rapid processing of images were found to improve retention of the endogenous fluorescence signals. Images were captured at 10x and 20x using an Olympus BX63F with CellSens Dimensions software, and the intensity of the endogenous red and green fluorescence signal normalized by the area was quantified (intensity/area) in cellular regions of the DRG. In some initial experiments data were also analyzed by counting the number of red and green cells using Image J and normalizing to the area; conclusions were similar with the two methods. The latter method allowed counting of double-labeled cells as well; these were found to be relatively small portion of the total cells and so most subsequent experiments used the intensity method (see Results).
2.6. Enzyme-linked immunosorbent assay (ELISA)
Animals were terminally anesthetized with isoflurane and lumbar DRG (L3 and L4) were rapidly removed and homogenized in RIPA lysis buffer (catalog 20-188, Sigma) containing MS-SAFE protease and phosphate inhibitors (catalog MSSAFE, Sigma), and centrifuged to remove cell debris. Protein concentrations were assessed by the Qubit protein assay kit and samples were analyzed using the CCL2 ELISA assay (catalog MJE00B, R&D System). A standard curve was included in the experiment, and the assay used according to the protocol provided by the manufacturer.
2.7. Statistics and data analysis
Scientific rigor and sample sizes were based on our similar and published experiments (Ibrahim et al., 2018; Tonello et al., 2020)). Graphpad Prism (La Jolla, CA), version 6 software was used for statistical analysis; version 8 was used for 3-way ANOVAs presented in supplementary data. Mice were assigned to experimental groups at random. For analysis of quantified immunohistochemical data, multiple sections per animal were analyzed and the statistics were performed using the average values from each animal. Immunohistochemical experiments were conducted as side-by-side comparisons of experimental and control images, and the data are presented as normalized to the control value to allow for differing absolute intensity values in different experiments. Since ratios were being analyzed, statistics were done on log transformed data although linear data is presented for ease of viewing. Time course data were analyzed using two-way repeated measures ANOVA with Bonferroni posttest to determine on which days experimental groups differed. For these analyses, F values for the comparison between groups are given as F (degrees freedom in numerator, degrees freedom in denominator). Except where indicated, comparisons between groups in other experiments were performed with one-way ANOVA, followed by Bonferroni’s post-hoc analysis, or with unpaired Student’s t-test. Twotailed tests were used throughout. Significance was ascribed for p<0.05. Levels of significance are indicated by the number of symbols, e.g., *, p = 0.01 to <0.05; **, p = 0.001 to 0.01; ***, p < 0.001. Data are presented as mean ± S.E.M.
3. Results
3.1. DRG inflammation leads to mechanical pain behaviors in mice
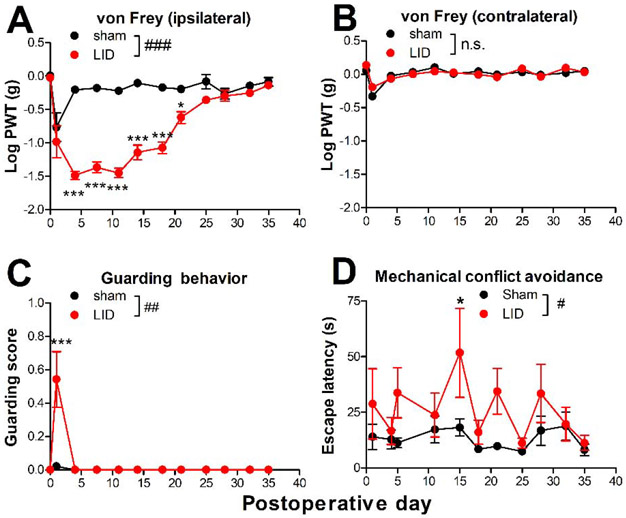
The DRG inflammation model was originally developed in rats and involved inflaming the L5 DRG with 10 μl of 2 mg/ml zymosan in incomplete Freund’s adjuvant (Xie et al., 2012). To adapt the model to mice, the volume injected was reduced to 3 μl and the L4 DRG (analogous to the rat L5 DRG (Rigaud et al., 2008)) was inflamed. As shown in Fig. 1, zymosan-injected animals rapidly developed mechanical allodynia, as indicated by a significant reduction in von Frey threshold in the ipsilateral hindpaw, which lasted for ~3 weeks (Fig. 1A, B). A more modest and short-lasting increase in guarding behavior also developed on the ipsilateral side (Fig. 1C). The results were similar to those in the rat except that the mechanical hypersensitivity resolved faster in mice (3 weeks vs. 4 – 5 weeks) (Xie et al., 2012). To use an operant test in addition to the reflexive von Frey test, we also used the mechanical conflict avoidance test (Shepherd and Mohapatra, 2018), in which the mice must cross a chamber touching a lattice of sharp pins in order to escape from a naturally aversive light stimulus to reach a dark chamber. With this test, the latency to enter the chamber was longer when the pins were set at a 5 mm height instead of a 0 mm height (data not shown), and the latency at the 5 mm height was higher in the LID mice compared to sham controls (Fig. 1D). Results were similar for males and females (see supplemental Fig. A2 for results separated by sex) and data have been combined.
Fig. 1:
Effect of local inflammation (“LID”) of the dorsal root ganglion (DRG) on pain behaviors in mice. Baseline behaviors were measured 1 day prior to surgery; the value is plotted on day 0. On day 0, the L4 DRG was inflamed with 3 μl of zymosan in incomplete Freund’s adjuvant. The control group received sham surgery with no zymosan injection. A, von Frey threshold on the ipsilateral side; B, von Frey threshold on the contralateral side; C, guarding score (maximum score is 3) on the ipsilateral side (no contralateral guarding was observed); D, latency to enter chamber with lattice of pins at 5 mm height; #, p<0.05; ##, p<0.01; ###, p<0.001; significant overall effect for the sham vs. LID factor, or n.s., not significant (2 way repeated measures ANOVA); F(1,14) = 161.7 (A); 0.07, p = 0.80, n.s. (B); 9.7 (C); and 5.48 (D). In panel A and C the interaction effect was also significant. *, p<0.05; **, p<0.01; ***, p<0.001; significant difference between the groups at the indicated time point (Bonferroni's multiple comparisons posttest). N = 8 mice/group, wildtype, 4 of each sex (see also supplemental Fig. A2).
3.2. DRG inflammation predominately increases Ccr2+/RFP MØ in the DRG
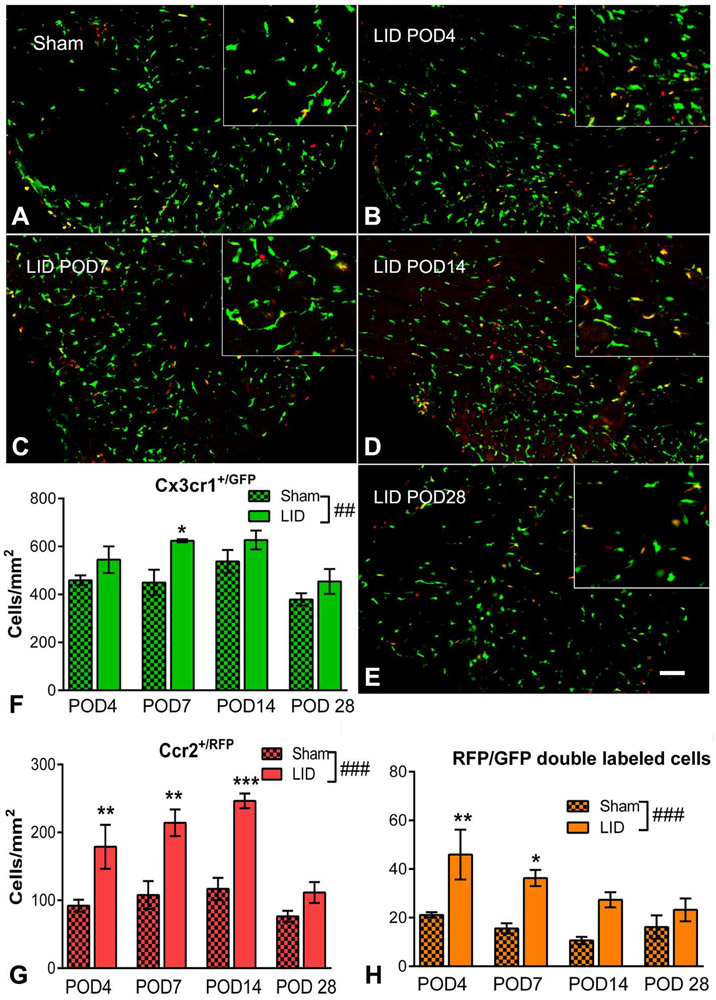
In order to examine the possible roles of different subsets of MØ, we used mice in which MØ expressing the CCR2 receptor also expressed red fluorescent protein, and MØ expressing the CX3CR1 receptor also expressed green fluorescent protein. As shown in Fig. 2 and supplemental Fig. 3A, most labeled cells in the normal or sham operated DRG were resident or Cx3cr1+/GFP MØ; e.g. 80.2% of labeled cells overall in the sham operated animals at the 4 different time points shown in Fig. 2. The relatively high density of resident macrophages in the DRG was similar to that observed in a previous study (Krishnan et al., 2018). However, it was the relative number of Ccr2+/RFP MØ that increased most significantly after DRG inflammation, with significant ~2 fold increases (compared to sham operated animals) on days 4, 7, and 14 after DRG inflammation, decreasing to 1.5 fold (not significant) on day 28 when behavior was beginning to resolve. In contrast, the fold-increases in Cx3cr1+/GFP macrophage density were much smaller, reaching significance only on day 7 (1.4-fold). Qualitatively, the Cx3cr1+/GFP macrophages seemed to undergo a marked shape change after DRG inflammation, with larger cell bodies and forming circular structures putatively around sensory neurons, but this finding was not well captured with our quantitative methods, not reaching significance even if signal intensity rather than cell counts were used (data not shown). Some double labeled MØ were also observed; however, the absolute number was small (generally comprising 1-5% of all labeled MØ; note different y axis scales in Fig. 2); these were not analyzed further and subsequent experiments analyzed the overall red and green intensity/area in DRG sections.
Fig. 2:
Effect of local DRG inflammation (“LID”) on density of Cx3cr1+/GFP, Ccr2+/RFP, and double-labeled MØ in cellular areas of the DRG. DRG sections were obtained from sham operated animals, or at the indicated postoperative days (“POD”) after DRG inflammation, and the RFP and GFP signals measured (sample images shown as RFP/GFP merged). Scale bar, 50 μm. Summary data show the density of GFP, RFP, and double labeled cells. ##, p<0.01; ###, p<0.001; significant overall effect of the LID/sham factor (2 way repeated measures ANOVA); F(1,6) = 22.4 (Cx3cr1+/GFP; F), 117.2 (Ccr2+/RFP; G) and 45.9 (double-labeled cells, H). The interaction effect was not significant in any of the groups. *, p<0.05; **, p<0.01; ***, p<0.001; significant difference between the LID and sham values at the indicated time point (Bonferroni's multiple comparisons posttest). N = 4 reporter mice/group (both sexes) at each time point. Images from inflamed DRG were always compared side-by-side with images from sham operated DRG at the same time point; the example shown of the sham DRG is from the day 4 sham group. Sample images from normal DRG and other sham POD can be seen in supplemental Fig. A3. Insets are 1.75 x magnification.
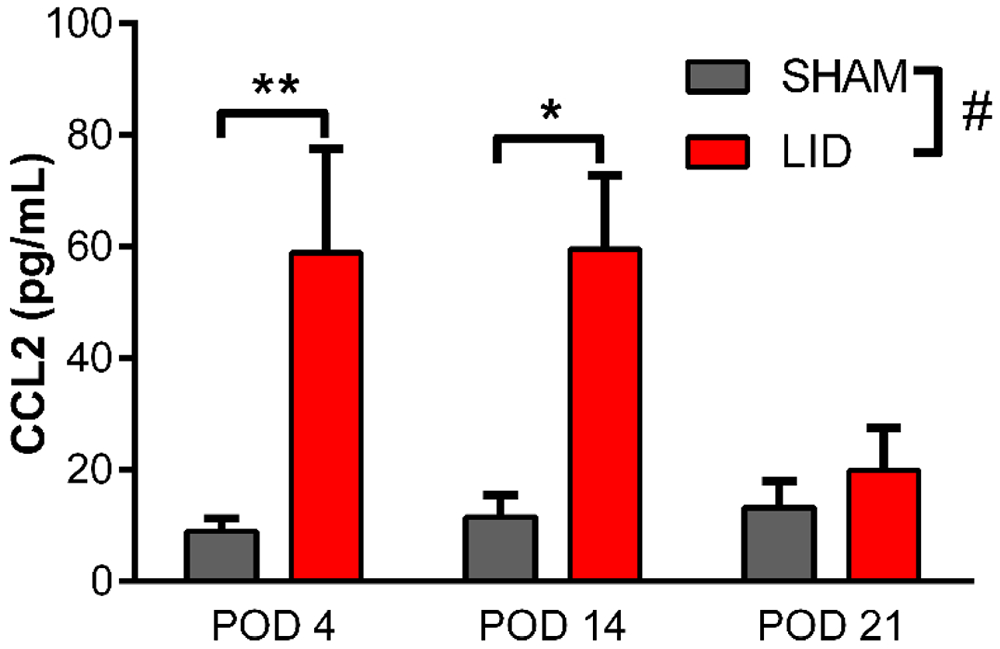
3.3. DRG inflammation increases CCL2 in the DRG
The increase in Ccr2+/RFP MØ after DRG inflammation suggested that CCL2 levels in the DRG might be increased, as we previously reported in the rat model (Xie et al., 2016). This was confirmed by ELISA assay of protein isolated from inflamed and sham DRGs. As shown in Fig. 3, CCL2 increased 5-6 fold on day 4 and day 14 after DRG inflammation, returning to near control values on day 21, a time point at which mechanical hypersensitivity (and numbers of Ccr2+/RFP MØ) was beginning to resolve.
Fig. 3.
Effect of DRG inflammation on CCL2 protein levels in the DRG. Inflamed DRGs (“LID”) or DRGs from sham-operated controls were obtained at the indicated postoperative day (POD), and CCL2 concentration measured with ELISA. #, p<0.05; significant overall effect for the sham vs. LID factor. **, p<0.01; *, p<0.05; significant difference between sham and LID groups at the indicated time points (2 way repeated measures ANOVA with Bonferroni posttest); F(1,6) = 9.63, p = 0.02 for LID vs. sham factor. The interaction factor was also significant (p = 0.03). N = 4 mice per group (wildtype; both sexes).
3.4. Reducing macrophage infiltration reduces pain behaviors:
To further examine the role of infiltrating Ccr2+/RFP MØ, we used three different methods in order to reduce macrophage infiltration:
Global knockout of the CCR2 receptor:
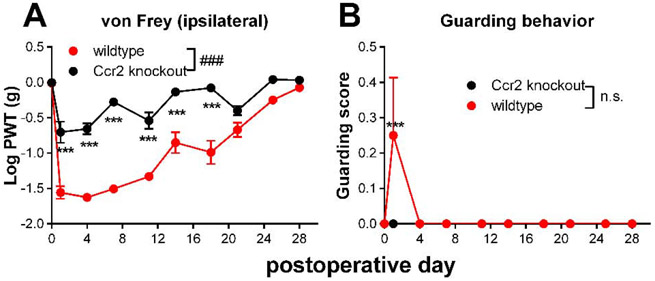
First, we used genetic knockout of the CCR2 receptor, by using the parent strain Ccr2RFP/RFP in which both copies of the Ccr2 gene have been replaced by RFP. As shown in Fig. 4, mice of this genotype had significantly smaller von Frey responses to DRG inflammation. There were no sex differences in the genotype effect (Supplemental Fig. A4) and no effect of genotype on baseline thresholds.
Fig. 4:
Effect of Ccr2 knockout on behaviors induced by local inflammation of the dorsal root ganglion (DRG). Baseline behaviors were measured on 2 days prior to surgery, the average value is plotted on day 0. On day 0, the L4 DRG was inflamed. A, von Frey threshold on the ipsilateral side; B, ipsilateral guarding score (maximum score is 3). ###, p<0.001; significant overall effect for genotype factor (2 way repeated measures ANOVA); F(1,14) = 127.7 (A); 2.33, not significant (n.s.), p = 0.15 (B). In both panels the interaction effect was significant. **, p<0.01; ***,p<0.001; significant difference between the groups at the indicated time point (Bonferroni's multiple comparisons posttest). N = 8 mice/group, 4 of each sex. Data separated by sex can be found in supplemental Fig. A4.
CCR2 inhibitor:
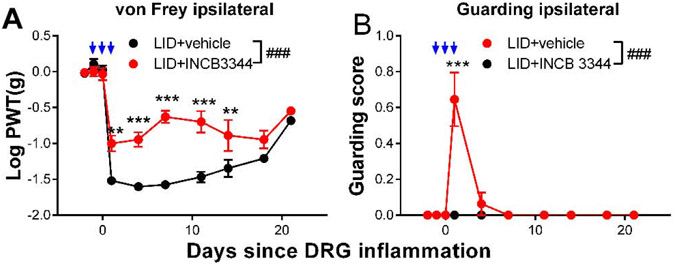
As a second method of investigating the role of the Ccr2+-expressing MØ in the DRG inflammation model, we used the small molecule specific CCR2 inhibitor INCB3344 (Brodmerkel et al., 2005). The drug was injected intravenously on three sequential days, the day before, of, and after DRG inflammation. As shown in Fig. 5, this treatment reduced mechanical hypersensitivity and paw guarding induced by DRG inflammation. This effect was observed in both sexes (Supplemental Fig. A5).
Fig. 5:
Effect of CCR2 inhibitor INCB3334 on behaviors induced by local inflammation of the dorsal root ganglion (“LID”). Baseline behaviors were measured on 2 days prior to and just prior to the DRG inflammation surgery on day 0. INCB3334 or vehicle was injected intravenously on days −1, 0, and 1 (blue arrows; behaviors measured 2 hours later). A, von Frey threshold on the ipsilateral side; B, ipsilateral guarding score (maximum score is 3). ###, p<0.001; significant overall effect for INCB3344/vehicle factor (2 way repeated measures ANOVA); F(1,14) = 49.9 (A); 18.9 (B). In both panels the interaction effect was also significant. **, p<0.01; ***,p<0.001; significant difference between the groups at the indicated time point (Bonferroni's multiple comparisons posttest). N = 8 mice/group, wildtype, both sexes. Data separated by sex can be found in supplemental Fig. A5.
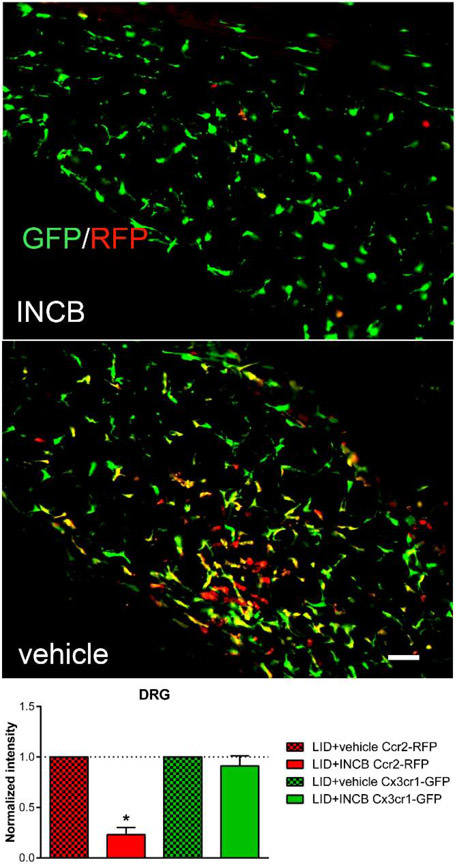
The INCB3344 injections reduced the Ccr2+/RFP MØ in the inflamed DRG, but not the Cx3cr1+/GFP MØ as measured on day 4 after DRG inflammation (Fig. 6). The INCB3344 treatment reduced RFP intensity to 23% of that seen in vehicle treated animals.
Fig. 6:
Effect of CCR2 inhibitor INCB3334 on Cx3cr1+/GFP and Ccr2+/RFP macrophage density. Reporter mice received either INCB3334 or vehicle, intravenously, for 3 sequential days; on the second day, the DRG was inflamed. 4 days after DRG inflammation DRGs were obtained from INCB3344 treated (top) or vehicle treated (middle) the RFP and GFP signals measured (images shown as RFP/GFP merged). Intensity data in sections from INCB3334-treated mice were normalized to the intensity in vehicle treated mice measured in side-by-side experiments (dotted line). Scale bar, 50μm. Summary data: *, p<0.05; significant difference between vehicle and INCB3334 treated groups, ratio t-test. N = 3 reporter mice per group, both sexes.
3.5. Macrophage depletion with liposomal clodronate had time-dependent effects on pain behaviors
Liposomal Clodronate:
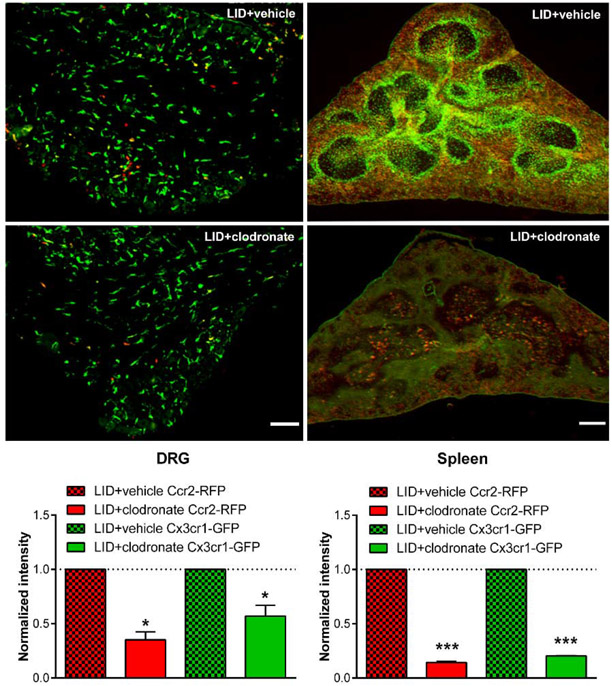
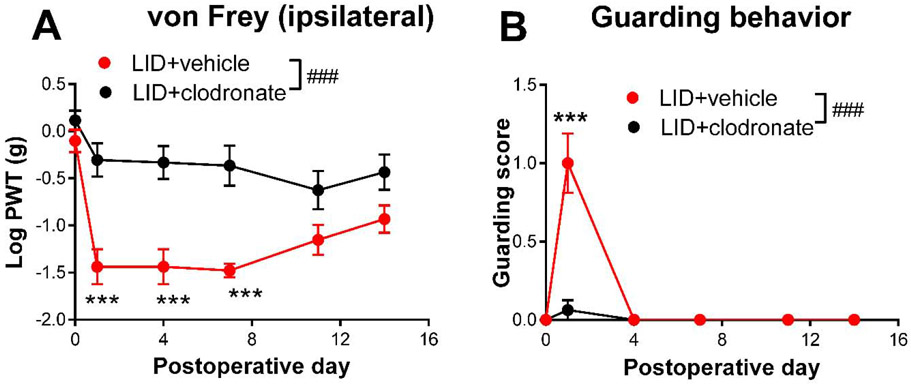
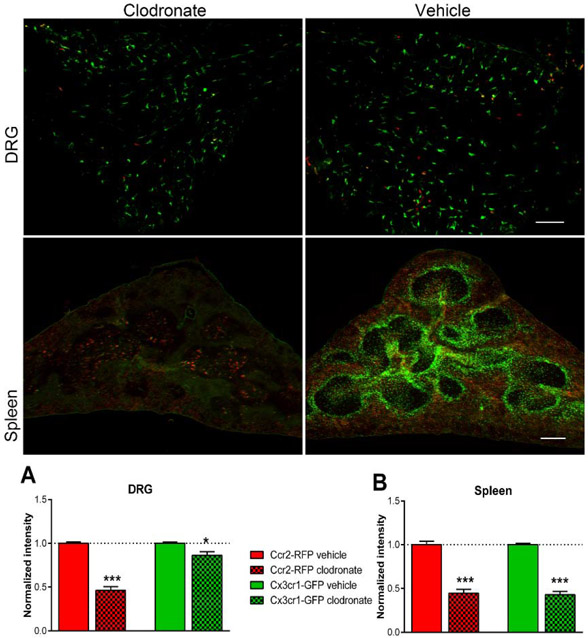
As a third method for manipulating MØ, we used liposomal clodronate, injected intravenously 2 days prior to DRG inflammation. The liposome-encapsulated cytotoxin clodronate is taken up by MØ, a method commonly used to deplete circulating monocytes that has varying effects on resident macrophages (Van Rooijen and Sanders, 1994) but does not deplete spinal cord microglia (Peng et al., 2016). As shown in Fig. 7, this treatment significantly reduced the von Frey and guarding responses in wildtype mice. Effects were similar in males and females (Supplemental Fig. A6). The magnitude of the clodronate effect on measured behaviors was similar to the magnitude of global CCR2 knockout. As we have previously reported (Tonello et al., 2020), macrophages were successfully depleted by systemic clodronate (Supplemental Fig. A7) in wildtype mice. In the reporter mice, the clodronate treatment markedly reduced Ccr2+/RFP MØ in the inflamed DRG to 46% of the control value, but had only a minor but significant effect on Cx3cr1+/GFP MØ (Fig. 8), reducing to 86% of the control value. Both values are close to the values predicted from the data in Fig. 2 by assuming that clodronate causes the loss of LID-induced increases in Ccr2+/RFP and double-labeled MØ (predicted values of RFP intensity to 50% and GFP intensity to 86% of the values observed in vehicle treated animals on POD 4). As confirmation of depletion of circulating monocytes, sections of the spleen also showed marked reduction in both Cx3cr1+/GFP and Ccr2+/RFP monocytes (Fig. 8).
Fig. 7:
Effect of intravenous liposomal clodronate on behaviors induced by local inflammation (“LID”) of the dorsal root ganglion (DRG). Baseline behaviors were measured 1 day prior to surgery, the value is plotted on day 0. Liposomal clodronate was injected on day −2. The control group received vehicle liposome injection. On day 0, the L4 DRG was inflamed. A, von Frey threshold on the ipsilateral side; B, ipsilateral guarding score (maximum score is 3). ###, p<0.001; significant overall effect for the vehicle vs. clodronate factor (2 way repeated measures ANOVA); F(1,14) = 14.1 (A); 22.18(B). In both panels the interaction effect was also significant. ***,p<0.001; significant difference between the groups at the indicated time point (Bonferroni's multiple comparisons posttest. N = 8 mice/group, wildtype, 4 of each sex. Data separated by sex can be found in supplemental Fig. A6.
Fig. 8:
Effect of pretreatment with liposomal clodronate on Cx3cr1+/GFP and Ccr2+/RFP MØ density. Reporter mice received either intravenous liposomal clodronate or vehicle control injections. 2 days later, the DRG were inflamed. DRG (top, scale bar 20 μ ) and spleen sections (middle; scale bar 200 μ) were obtained 4 days after inflammation and the RFP and GFP signals measured (images shown as RFP/GFP merged). Insets are 1.75 x magnification. Intensity data in sections from clodronate-treated mice were normalized to the intensity in vehicle treated mice measured in side-by-side experiments (dotted lines). *, p<0.05; *** p<0.001; significant difference between vehicle and clodronate treated groups, ratio t-test. N = 4 reporter mice per group, both sexes.
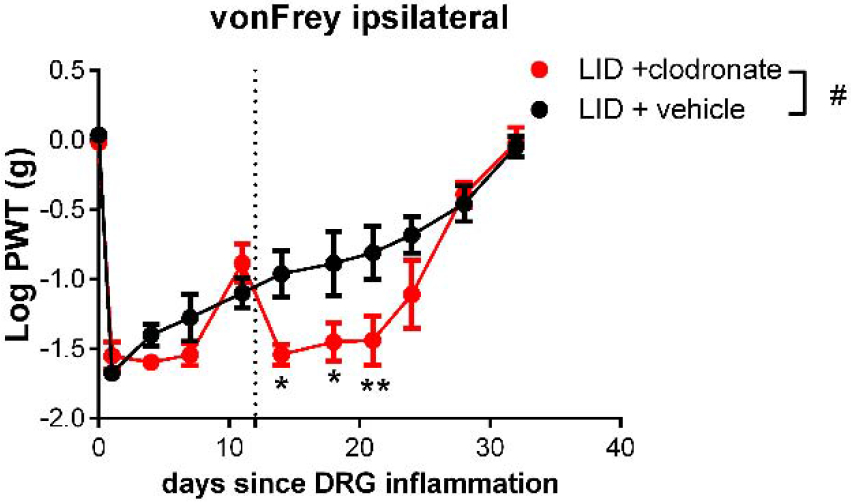
Delayed clodronate injection:
As an initial experiment to determine whether the role of infiltrating MØ changed with time, we injected liposomal clodronate 12 days after DRG inflammation, a time at which mechanical hypersensitivity was already well established. As shown in Fig. 9, this delayed injection had an effect opposite to that of clodronate injected just prior to DRG inflammation, causing a small but significant decrease in the von Frey threshold. The guarding behavior had already disappeared by day 12, and scores remained at 0 after the delayed clodronate injection (data not shown). Also in contrast to the early clodronate injection, delayed clodronate injection significantly depleted not only Ccr2+/RFP MØ (to 35% of the value seen in vehicle treated animals), but also Cx3cr1+/GFP MØ (to 57% of the value seen in vehicle treated animals) in the inflamed DRG of reporter mice, measured 2 days after the injection (Fig. 10).
Fig. 9:
Effect of CCR2 delayed liposomal clodronate injection on mechanical hypersensitivity induced by local inflammation of the dorsal root ganglion (“LID”). Baseline behaviors were measured on 2 days prior to the DRG inflammation surgery on day 0, and the average is plotted on day 0. Liposomal clodronate or vehicle was injected intravenously on day 12 (dotted line). #, p<0.05; significant overall effect for clodronate vs. vehicle factor (2 way repeated measures ANOVA); F(1,14) = 5.8. The interaction effect was also significant. *, p<0.05; **, p<0.01; significant difference between the groups at the indicated time point (Bonferroni's multiple comparisons posttest). N = 8 mice/group, wildtype, both sexes.
Fig. 10:
Effect of delayed treatment with liposomal clodronate on Cx3cr1+/GFP and CCR2+/RFP macrophage density. Reporter mice received DRG inflammation on day 0, followed on day 12 by either intravenous liposomal clodronate or vehicle control injections. 2 days later, the DRG and spleen were isolated. RFP and GFP signals were measured in DRG (left, scale bar 20 μm ) and spleen sections (right; scale bar 200 μ ). Intensity data in sections from clodronate-treated mice were normalized to the intensity in vehicle treated mice measured in side-by-side experiments (dotted line). *, p<0.05; ***, p<0.001; significant difference between vehicle and clodronate treated groups, ratio t-test. N = 4 reporter mice per group, both sexes.
4. Discussion
In this study we demonstrated that the local DRG inflammation model of low back pain and radiculopathy first developed in rats (Xie et al., 2012; Xie et al., 2006) could be readily extended into mice. This allowed us to examine the roles of genetically labeled subsets of MØ in the model. We found that the CCR2-expressing MØ subset played an important role in the initiation of mechanical and spontaneous pain in the model. Two of the manipulations used to affect this subset, CCR2 knockout and systemic application of a CCR2 inhibitor, would be expected to have global effects and may have had multiple sites of action. However, the similarity of these two treatments’ effects on pain behaviors to the effect of liposomal clodronate provides evidence for the importance of effects on macrophages rather than only neuronal effects. Liposomal clodronate, which temporarily depleted macrophages just prior to DRG inflammation and reduced the increase in CCR2-expressing MØ in the inflamed DRG, while having only a minor effect on the numbers of CX3CR1-expressing MØ in the DRG, would not a priori be expected to directly affect neurons. The behavioral effects of clodronate on pain behaviors were maintained for the duration of the experiment (15 days), although recovery of circulating MØ after i.v. clodronate in mice requires less than a week (Sunderkotter et al., 2004), suggesting a key role of clodronate-sensitive MØ in the establishment phase of pain behaviors. Further evidence for the role of the CCR2-expressing MØ was the ability of a specific CCR2 antagonist, INCB3344, to reduce pain behaviors as well as reducing the DRG inflammation-induced increase of CCR2-expressing (but not CX3CR1-expressing) MØ in the DRG, similarly to clodronate.
Our data supporting a key role for this subset of MØ per se are particularly interesting in view of the many studies showing important roles for direct excitatory CCL2/CCR2 effects on sensory neurons and in the spinal cord (where CCL2 can be released by sensory neurons), in a number of different types of pain models including those involving nerve injury, chemotherapy-induced pain, and peripheral inflammation (e.g. (Chun and Kwon, 2019; Illias et al., 2018; Serrano et al., 2010; Van Steenwinckel et al., 2011; White et al., 2005; Zhang et al., 2013; Zhang and Koninck, 2006)). In some of these studies possible roles for macrophages were not examined, and in some of the studies primarily very acute effects of CCR2 inhibition on pain behaviors or neuronal excitability were examined. We would in general not have observed such acute effects in our experimental paradigms. Conversely, studies supporting a key role for macrophages in mediating CCL2/CCR2 effects in pain behaviors also involved a number of different types of pain models (e.g. (Bravo-Caparros et al., 2020; Hackel et al., 2013; Trevisan et al., 2016; Van Steenwinckel et al., 2015)). Peripheral nerve injury models may induce MØ infiltration at the injury site, in the DRG, or even in the spinal cord, and there has been some controversy about which site is most important (e.g. see (Yu et al., 2020) and references therein), as well as demonstrations that MØ at these two sites may have different properties (Komori et al., 2011). However, these considerations may not be important in the model used for our study, since it involves direct local inflammation at the site of the DRG and there is no peripheral nerve injury site. Macrophages in the DRG are uniquely situated to affect sensory neuron function, may differ functionally from those infiltrating peripheral nerve (see above). Our model may be very well suited to exploring the function of these DRG macrophages.
Most of the above cited studies (also see Introduction) imply a pro-nociceptive role for the CCL2/CCR2 system during the pain induction process, regardless of whether the most important direct site of action is sensory neurons or infiltrating MØ or a synergism between the two. Since neuronal effects of CCL2 are also pronociceptive, in our model there may be a mutually reinforcing effect of macrophage and neuronal sites of action of the experimental manipulations we used. However, there is less agreement about the role of CCL2/CCR2 in the maintenance phase of pain. Studies disagree about whether the CCL2 upregulation or sensitivity to CCR2 inhibition is maintained or only seen at the beginning of various pain models (Lee and Zhang, 2012; Noh et al., 2020; Willemen et al., 2014; Zhang and Koninck, 2006; Zhu et al., 2014). Recently, we have demonstrated that local sympathectomy promotes resolution of chemotherapy-induced pain by increasing not only anti-inflammatory cytokines but also CCR2 and CCL2 transcripts (Tonello et al., 2020), suggesting an alternative beneficial role for the CCL2/CCR2 signaling. Here, we found that clodronate injections were anti-nociceptive if done on day 14, after pain was well established, which would be consistent with a change in the role of CCR2+ MØ with time. Another explanation for this result is that the CX3CR1+ macrophages in the DRG play an antinociceptive or pain-resolving role, and were depleted by delayed clodronate because the inflammation of the DRG increased local drug accessibility. Further studies are needed to elucidate the role of different MØ subsets at later times. Our previous study suggested a pain-resolving role for CCL2 in the DRG in the context of mediating antinociceptive effects of local sympathectomy a chemotherapy pain model (Tonello et al., 2020). Understanding the role of CCL2/CCR2 in pain maintenance will be important for developing possible therapeutic applications targeting this system.
This study agrees with preclinical studies using other models of low back pain implicating CCL2/CCR2 as an important pro-nociceptive factor (White et al., 2005; Zhu et al., 2014). Some human studies also support a role of the CCL2/CCR2 system in low back pain of various etiologies (Lippi et al., 2017; Palada et al., 2019; Peng et al., 2017; Romero-Sanchez et al., 2011; Teodorczyk-Injeyan et al., 2018).
Our study cannot rule out roles for the CX3CR1-expressing MØ population. There was little change in the numbers of these cells in the DRG after DRG inflammation, clodronate or INCB treatment, but a change in morphology was noted. The relatively large numbers of the CX3CR1-expressing MØ we observed in the DRG (compared to, for example, peripheral nerve) and our interpretation that these are resident macrophages are consistent with previous studies (Kolter et al., 2020; Krishnan et al., 2018). Study of the role of these cells is complicated by the fact that the green label also marks microglia in the spinal cord. In addition, a small population of patrolling monocytes in the blood are also Cx3Cr1-positive, and have been shown to play a pronociceptive role in a chemotherapy pain model(Montague et al., 2018; Old et al., 2014).
In summary, this study made use of mouse genetic tools to begin to examine the role of 2 key defined subpopulations of MØ, and established a key role for CCL2 and CCR2-expressing MØ in initiating pain behaviors in a model of low back pain induced by local inflammation of the lumbar DRG. Future studies may focus on using the same genetic tools to isolate distinct populations of DRG MØ at different time points, to determine how their molecular properties may contribute to initiation and maintenance of pain.
Supplementary Material
Highlights for Li Zhang et al.
CCR2- and CX3CR1-expressing macrophage subsets may play distinct roles in pain
Local DRG inflammation (a radiculopathy model) increased pain behaviors in mice
DRG inflammation increased CCL2 and CCR2+ macrophages in the DRG
Three methods of reducing CCR2+ macrophage infiltration reduced pain behaviors
Genetically identifying macrophage subsets can elucidate macrophage roles in pain
Acknowledgements:
Funded in part by NIH grants NS045594, NS055860, and AR068989 to J.-M. Zhang. and NS113243 to T. Berta. The authors thank Andrew Shepherd and D.P. Mohapatra for helpful discussions about establishing the mechanical conflict avoidance assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA, 2009. Chemokines and pain mechanisms. Brain Res Rev 60, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ, 2003. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A 100, 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Caparros I, Ruiz-Cantero MC, Perazzoli G, Cronin SJF, Vela JM, Hamed MF, Penninger JM, Baeyens JM, Cobos EJ, Nieto FR, 2020. Sigma-1 receptors control neuropathic pain and macrophage infiltration into the dorsal root ganglion after peripheral nerve injury. FASEB J 34, 5951–5966. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF, 1996. Characterization of a rat model of incisional pain. Pain 64, 493–501. [DOI] [PubMed] [Google Scholar]
- Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue CB, Newton RC, Scherle P, Vaddi K, 2005. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol 175, 5370–5378. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, 1994. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Chen O, Donnelly CR, Ji RR, 2019. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol 62, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S, Kwon YB, 2019. The CCL2 elevation in primary afferent fibers produces zymosan-induced hyperalgesia through microglia-mediated neuronal activation in the spinal dorsal horn. Brain Res Bull 149, 53–59. [DOI] [PubMed] [Google Scholar]
- Dominguez PM, Ardavin C, 2010. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 234, 90–104. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P, 2012. The economic costs of pain in the United States. J Pain 13, 715–724. [DOI] [PubMed] [Google Scholar]
- Gertzbein SD, Tait JH, Devlin SR, 1977. The stimulation of lymphocytes by nucleus pulposus in patients with degenerative disk disease of the lumbar spine. Clinical Orthopaedics & Related Research 123, 149–154. [PubMed] [Google Scholar]
- Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, Ren Y, DiCicco-Bloom E, Young W, Dong H, Wu LJ, 2016. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep 16, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Qiu Z, Cheng N, Chen C, Hei Z, Li X, 2019. Identification of potential mechanism and hub genes for neuropathic pain by expression-based genome-wide association study. J Cell Biochem 120, 4912–4923. [DOI] [PubMed] [Google Scholar]
- Hackel D, Pflucke D, Neumann A, Viebahn J, Mousa S, Wischmeyer E, Roewer N, Brack A, Rittner HL, 2013. The connection of monocytes and reactive oxygen species in pain. PLoS One 8, e63564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SIA, Xie W, Strong JA, Tonello R, Berta T, Zhang JM, 2018. Mineralocorticoid Antagonist Improves Glucocorticoid Receptor Signaling and Dexamethasone Analgesia in an Animal Model of Low Back Pain. Front Cell Neurosci 12, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illias AM, Gist AC, Zhang H, Kosturakis AK, Dougherty PM, 2018. Chemokine CCL2 and its receptor CCR2 in the dorsal root ganglion contribute to oxaliplatin-induced mechanical hypersensitivity. Pain 159, 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Advancing Pain Research, C., and Education., 2011. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press, Washington DC. [PubMed] [Google Scholar]
- Jiang BC, Liu T, Gao YJ, 2020. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther, 107581. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR, 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20, 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter J, Kierdorf K, Henneke P, 2020. Origin and Differentiation of Nerve-Associated Macrophages. J Immunol 204, 271–279. [DOI] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Inada T, Hisaoka T, Senba E, 2011. Site-specific subtypes of macrophages recruited after peripheral nerve injury. Neuroreport 22, 911–917. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Bhavanam S, Zochodne D, 2018. An Intimate Role for Adult Dorsal Root Ganglia Resident Cycling Cells in the Generation of Local Macrophages and Satellite Glial Cells. J Neuropathol Exp Neurol 77, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang J, 2012. Heterogeneity of macrophages in injured trigeminal nerves: cytokine/chemokine expressing vs. phagocytic macrophages. Brain Behav Immun 26, 891–903. [DOI] [PubMed] [Google Scholar]
- Lippi G, Dagostino C, Buonocore R, Aloe R, Bonaguri C, Fanelli G, Allegri M, 2017. The serum concentrations of leptin and MCP-1 independently predict low back pain duration. Clin Chem Lab Med 55, 1368–1374. [DOI] [PubMed] [Google Scholar]
- Mills C, Leblond D, Joshi S, Zhu C, Hsieh G, Jacobson P, Meyer M, Decker M, 2012. Estimating efficacy and drug ED50's using von Frey thresholds: impact of Weber's law and log transformation. J Pain 13, 519–523. [DOI] [PubMed] [Google Scholar]
- Montague K, Simeoli R, Valente J, Malcangio M, 2018. A novel interaction between CX3CR1 and CCR2 signalling in monocytes constitutes an underlying mechanism for persistent vincristine-induced pain. J Neuroinflammation 15, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh MC, Mikler B, Joy T, Smith PA, 2020. Time Course of Inflammation in Dorsal Root Ganglia Correlates with Differential Reversibility of Mechanical Allodynia. Neuroscience 428, 199–216. [DOI] [PubMed] [Google Scholar]
- Old EA, Nadkarni S, Grist J, Gentry C, Bevan S, Kim KW, Mogg AJ, Perretti M, Malcangio M, 2014. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest 124, 2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palada V, Ahmed AS, Finn A, Berg S, Svensson CI, Kosek E, 2019. Characterization of neuroinflammation and periphery-to-CNS inflammatory cross-talk in patients with disc herniation and degenerative disc disease. Brain Behav Immun 75, 60–71. [DOI] [PubMed] [Google Scholar]
- Peng J, Gu N, Zhou L, U, B.E., Murugan M, Gan WB, Wu LJ, 2016. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 7, 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZY, Chen R, Fang ZZ, Chen B, Wang ZH, Wang XY, 2017. Increased local expressions of CX3CL1 and CCL2 are related to clinical severity in lumbar disk herniation patients with sciatic pain. J Pain Res 10, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH, 2008. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain 136, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sanchez C, Tsou HK, Jan MS, Wong RH, Chang IC, Londono J, Valle-Onate R, Howe HS, Yu D, Leung BP, Wei JC, 2011. Serum monocyte chemotactic protein-1 concentrations distinguish patients with ankylosing spondylitis from patients with mechanical low back pain. J Spinal Disord Tech 24, 202–207. [DOI] [PubMed] [Google Scholar]
- Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF, 2010. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One 5, e13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Pare M, McIntosh F, Elmes SJ, Martino G, Jomphe C, Lessard E, Lembo PM, Vaillancourt F, Perkins MN, Cao CQ, 2010. Blocking spinal CCR2 with AZ889 reversed hyperalgesia in a model of neuropathic pain. Mol Pain 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Mohapatra DP, 2018. Pharmacological validation of voluntary gait and mechanical sensitivity assays associated with inflammatory and neuropathic pain in mice. Neuropharmacology 130, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ, 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172, 4410–4417. [DOI] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan JA, McGregor M, Triano JJ, Injeyan SH, 2018. Elevated Production of Nociceptive CC Chemokines and sE-Selectin in Patients With Low Back Pain and the Effects of Spinal Manipulation: A Nonrandomized Clinical Trial. Clin J Pain 34, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonello R, Xie W, Lee SH, Wang M, Liu X, Strong JA, Zhang JM, Berta T, 2020. Local Sympathectomy Promotes Anti-inflammatory Responses and Relief of Paclitaxel-induced Mechanical and Cold Allodynia in Mice. Anesthesiology 132, 1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan G, Benemei S, Materazzi S, De Logu F, De Siena G, Fusi C, Fortes Rossato M, Coppi E, Marone IM, Ferreira J, Geppetti P, Nassini R, 2016. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139, 1361–1377. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A, 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174, 83–93. [DOI] [PubMed] [Google Scholar]
- Van Steenwinckel J, Auvynet C, Sapienza A, Reaux-Le Goazigo A, Combadiere C, Melik Parsadaniantz S, 2015. Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun 45, 198–210. [DOI] [PubMed] [Google Scholar]
- Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, Sarret P, Pohl M, Melik Parsadaniantz S, 2011. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci 31, 5865–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ, 2005. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A 102, 14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A, 2014. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 15, 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Randolph GJ, Zinselmeyer BH, 2017. A Polecat's View of Patrolling Monocytes. Circ Res 120, 1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM, 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Chen S, Strong JA, Li A-L, Lewkowich IP, Zhang J-M, 2016. Localized sympathectomy reduces mechanical hypersensitivity by restoring normal immune homeostasis in rat models of inflammatory pain. J Neurosci 36, 8712–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Kim D, Shahrestani S, Zhang JM, 2012. Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience 206, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang J-M, 2006. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience 142, 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Liu L, Lin J, Ransohoff RM, 2012. Role of CCR2 in immunobiology and neurobiology. Clinical and Experimental Neuroimmunology 3, 16–29. [Google Scholar]
- Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI, 2020. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, Dougherty PM, 2013. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. J Pain 14, 1031–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Koninck Y, 2006. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 97, 772–783. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cao S, Zhu MD, Liu JQ, Chen JJ, Gao YJ, 2014. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. J Pain 15, 516–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.