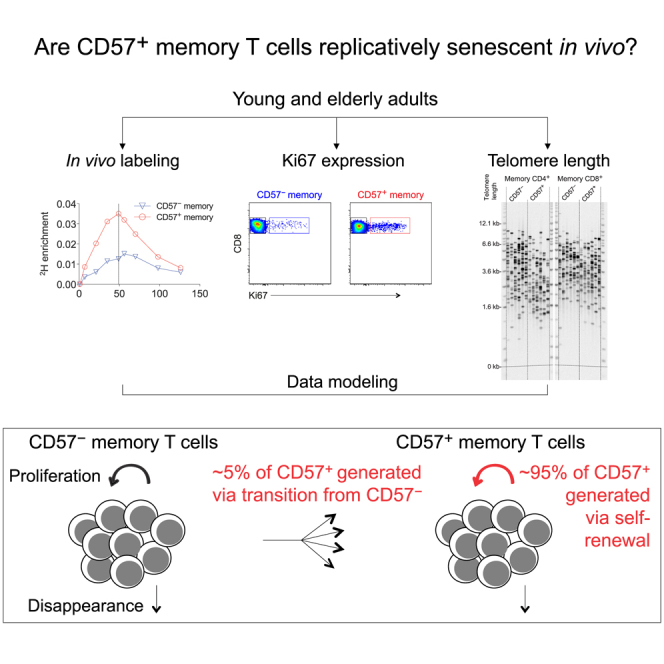
Summary
A central paradigm in the field of lymphocyte biology asserts that replicatively senescent memory T cells express the carbohydrate epitope CD57. These cells nonetheless accumulate with age and expand numerically in response to persistent antigenic stimulation. Here, we use in vivo deuterium labeling and ex vivo analyses of telomere length, telomerase activity, and intracellular expression of the cell-cycle marker Ki67 to distinguish between two non-exclusive scenarios: (1) CD57+ memory T cells do not proliferate and instead arise via phenotypic transition from the CD57− memory T cell pool; and/or (2) CD57+ memory T cells self-renew via intracompartmental proliferation. Our results provide compelling evidence in favor of the latter scenario and further suggest in conjunction with mathematical modeling that self-renewal is by far the most abundant source of newly generated CD57+ memory T cells. Immunological memory therefore appears to be intrinsically sustainable among highly differentiated subsets of T cells that express CD57.
Keywords: Enter keywords here
Graphical Abstract
Highlights
-
•
CD57+ memory T cells are not replicatively senescent in vivo
-
•
CD57+ memory T cells are maintained primarily via self-renewal in vivo
In vitro studies have led to the widely held assumption that replicatively senescent memory T cells express the carbohydrate epitope CD57. Using a variety of experimental approaches and mathematical modeling of composite datasets, Ahmed et al. show that CD57+ memory T cells proliferate and self-renew in vivo.
Introduction
Immune senescence has been linked with the accumulation of terminally differentiated lymphocytes that fail to proliferate in response to antigenic challenge. It has also been suggested that surface expression of CD57, a terminally sulfated glycan carbohydrate epitope (Abo and Balch, 1981), identifies memory T cells that lack the capacity to proliferate (Brenchley et al., 2003). In line with these widely accepted paradigms, highly differentiated effector memory T cells that express CD45RA, known as TEMRA cells, become more prevalent with age (Nociari et al., 1999) and often express CD57 (Ladell et al., 2008).
Contrary to the notion of differentiation-linked senescence, memory T cells that express CD57 can be induced to proliferate in vitro, at least under optimized conditions (Chong et al., 2008; Izquierdo et al., 1990). Parallel strands of evidence have further suggested a key role for these cells as immune effectors. For example, memory CD8+ T cells rarely express CD57 in conjunction with programmed death-1 (PD-1) (Petrovas et al., 2009), a marker associated with exhaustion (Day et al., 2006; Freeman et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006), and functionally replete memory CD4+ and CD8+ T cells with cytotoxic potential typically express high levels of CD57 (Casazza et al., 2006; Chattopadhyay et al., 2009; Chong et al., 2008; Kern et al., 1999; Le Priol et al., 2006; Takata and Takiguchi, 2006; van Leeuwen et al., 2002). Virus-specific memory T cells have also been identified in the TEMRA compartment (Appay et al., 2008). In the CD8+ lineage, these cells have been associated with protective effects, most notably during acute (Northfield et al., 2007) and chronic human immunodeficiency virus type 1 (HIV-1) infection (Addo et al., 2007), and in a study of elite controllers with eventual disease progression, increasing levels of viral replication appeared to drive the formation of CD28−CD57+ memory CD8+ T cells, potentially indicating a reactive escalation in the cytotoxic response to HIV-1 (Benito et al., 2018). Accordingly, CD57+ memory T cells enrich the immune system with important antigen-dependent effector functions and, by extension, do not necessarily represent an irrelevant “cul-de-sac” in the lymphocyte differentiation pathway.
In this study, we used deuterium labeling to quantify the proliferation of CD57− and CD57+ memory T cells in vivo and supplemented these analyses with ex vivo measurements of telomere length, telomerase activity, and intracellular expression of the cell-cycle marker Ki67. We then used mathematical modeling to evaluate two non-exclusive hypothetical scenarios: (1) CD57+ memory T cells arise from the CD57− memory T cell compartment as a consequence of progressive differentiation; and/or (2) CD57+ memory T cells self-renew via intracompartmental proliferation and thereby contribute to long-term immunological memory.
Results
CD57− and CD57+ Memory T Cells Exhibit Similar Rates of Deuterium Incorporation
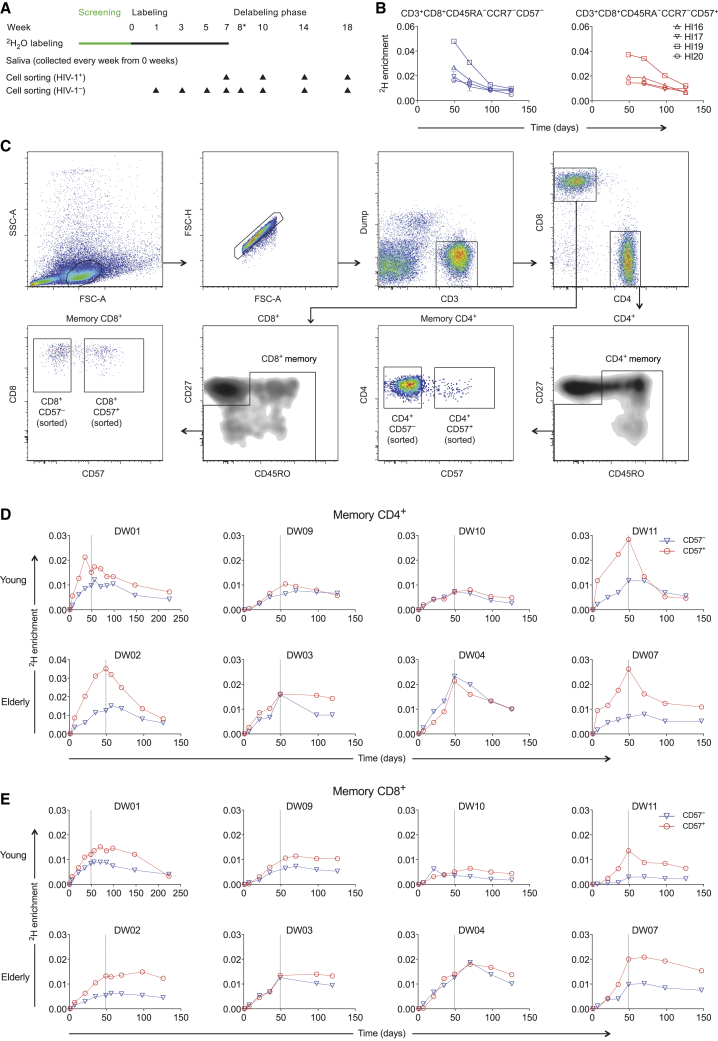
Preliminary in vivo labeling data were derived from studies of volunteers with chronic HIV-1 infection (aged 36–53 years), all of whom were antiretroviral drug-free at the time of experimentation and seropositive for cytomegalovirus (CMV; n = 4; Table S1). The labeling protocol is outlined in Figure 1A. Venous blood was sampled at weeks 7 (end of labeling), 10, 14, and 18, and at each time point, CD57− and CD57+ memory CD8+ T cells were flow-sorted from the CD45RA−CCR7− subset at >98% purity (Figure S1). This gating strategy was designed to exclude TEMRA cells, which were assessed separately in an earlier report (Ladell et al., 2008). Considerable rates of 2H labeling and delabeling were observed among CD45RA−CCR7−CD57− and CD45RA−CCR7−CD57+ memory CD8+ T cells (Figure 1B).
Figure 1.
CD57− and CD57+ Memory T Cells Exhibit Similar Rates of Deuterium Incorporation
(A) Schematic representation of the 2H2O labeling protocol and sampling time points.
(B) Experimental labeling data for CD57− and CD57+ memory CD8+ T cells sampled from the HIV-1-infected volunteers in cohort 1. The corresponding flow cytometric gating strategy is shown in Figure S1.
(C) Successive panels depict the flow cytometric gating strategy used to sort CD57− and CD57+ memory T cells from the CD4+ and CD8+ lineages (cohort 2). Lymphocytes were identified in a forward scatter-area versus side scatter-area plot, and single cells were identified in a forward scatter-area versus forward scatter-height plot. Boolean gates were drawn for analysis only to exclude fluorochrome aggregates. Viable CD3+CD14−CD19− cells were then identified in the CD4+ and CD8+ lineages, and sort gates were fixed on CD57− and CD57+ memory cells after exclusion of potentially naive CD27brightCD45RO− cells.
(D) Experimental labeling data for CD57− and CD57+ memory CD4+ T cells sampled from the healthy volunteers in cohort 2.
(E) Experimental labeling data for CD57− and CD57+ memory CD8+ T cells sampled from the healthy volunteers in cohort 2.
Immune activation enhances the turnover of memory T cells in the setting of chronic HIV-1 or HIV-2 infection (Hegedus et al., 2014; McCune et al., 2000; Vrisekoop et al., 2015; Zhang et al., 2013). We therefore sought to confirm these preliminary findings in a more comprehensive labeling study of healthy volunteers (aged 29–83 years), all of whom were seronegative for HIV-1 and seropositive for CMV. Recruitment was stratified to include equal numbers of young (aged 29–47 years) and elderly individuals (aged 60–83 years), the latter representing a population in which immune senescence was more likely (total n = 8; Table S1). Venous blood was sampled during the labeling phase (weeks 1, 3, and 5), at the end of labeling (week 7), and during the delabeling phase (weeks 8, 10, 14, and 18) (Figure 1A). At each time point, CD57− and CD57+ memory T cells were flow-sorted from the CD4+ and CD8+ lineages at >98% purity after gating out potentially naive CD27brightCD45RO− events (Figures 1C and S1).
In each coreceptor-defined lineage, similar patterns of 2H labeling and delabeling were observed among CD57− and CD57+ memory T cells, and equivalent (n = 3) or greater rates of 2H labeling (n = 5) were observed among CD57+ memory T cells compared with CD57− memory T cells (Figures 1D and 1E). Importantly, the corresponding 2H label enrichments in body water followed an expected rise-and-fall profile (Figure S2), and in the context of age-related immune senescence, no intralineage or intrasubset differences in the kinetics of 2H accumulation or loss were apparent between young and elderly volunteers (Figures 1D and 1E).
Ki67+ Cells Are Readily Detectable in the CD57+ Memory T Cell Pool
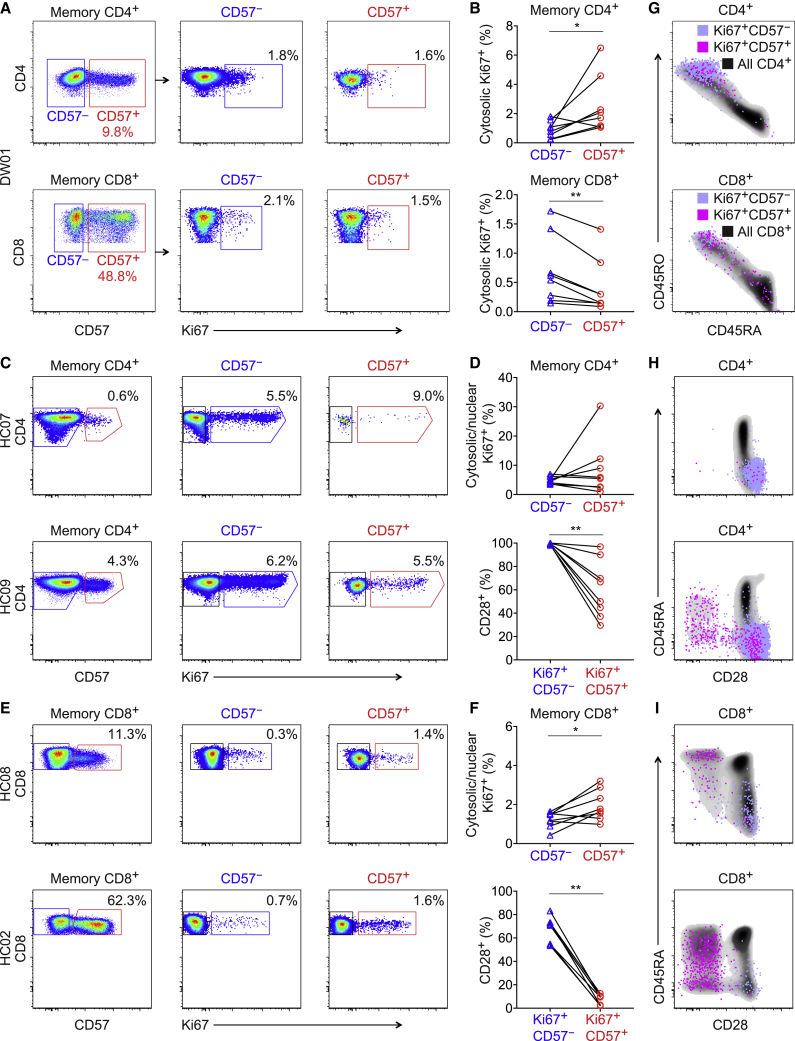
To corroborate these findings, we measured the expression of Ki67, an intracellular marker that accumulates during active phases of the cell cycle (Gerdes et al., 1983; Miller et al., 2018). Cytosolic expression of Ki67 was detected in the CD4+ lineage at mean frequencies of 1% among CD57− memory T cells and 2.9% among CD57+ memory T cells (p = 0.02, paired samples Wilcoxon test; Figures 2A and 2B) and in the CD8+ lineage at mean frequencies of 0.7% among CD57− memory T cells and 0.4% among CD57+ memory T cells (p = 0.008, paired samples Wilcoxon test; Figures 2A and 2B). Higher frequencies were observed using a different approach that simultaneously exposed intranuclear antigens. Cytosolic/nuclear expression of Ki67 was detected in the CD4+ lineage at mean frequencies of 4.9% among CD57− memory T cells and 8.6% among CD57+ memory T cells (p = 0.742, paired samples Wilcoxon test; Figures 2C and 2D) and in the CD8+ lineage at mean frequencies of 1.2% among CD57− memory T cells and 1.9% among CD57+ memory T cells (p = 0.039, paired samples Wilcoxon test; Figures 2E and 2F).
Figure 2.
Ki67+ Cells Are Readily Detectable in the CD57+ Memory T Cell Pool
(A) Representative flow cytometric data from a labeled volunteer (DW01) showing cytosolic expression of Ki67 among memory CD4+ (top) or CD8+ T cells (bottom) gated as CD57− (blue) or CD57+ (red).
(B) Percent cytosolic expression of Ki67 among memory CD4+ (top) or CD8+ T cells (bottom) gated as CD57− (blue triangles) or CD57+ (red circles). ∗p < 0.05, ∗∗p < 0.01. Paired samples Wilcoxon test.
(C) Representative flow cytometric data from unlabeled volunteers (n = 2) showing cytosolic/nuclear expression of Ki67 among memory CD4+ T cells gated as CD57− (blue) or CD57+ (red). HC07 was seronegative for CMV.
(D) Top: percent cytosolic/nuclear expression of Ki67 among memory CD4+ T cells gated as CD57− (blue triangles) or CD57+ (red circles). Bottom: percent expression of CD28 among the corresponding Ki67+CD57− (blue triangles) and Ki67+CD57+ memory CD4+ T cells (red circles). ∗∗p < 0.01. Paired samples Wilcoxon test.
(E) Representative flow cytometric data from unlabeled volunteers (n = 2) showing cytosolic/nuclear expression of Ki67 among memory CD8+ T cells gated as CD57− (blue) or CD57+ (red). HC02 was seropositive for CMV, and HC08 was seronegative for CMV.
(F) Top: percent cytosolic/nuclear expression of Ki67 among memory CD8+ T cells gated as CD57− (blue triangles) or CD57+ (red circles). Bottom: percent expression of CD28 among the corresponding Ki67+CD57− (blue triangles) and Ki67+CD57+ memory CD8+ T cells (red circles). ∗p < 0.05, ∗∗p < 0.01. Paired samples Wilcoxon test.
(G) Phenotypic characteristics of Ki67+CD57− and Ki67+CD57+ memory CD4+ (top) or CD8+ T cells (bottom) shown overlaid on density clouds representing the corresponding total CD4+ (top) or CD8+ T cell populations (bottom). Related to (A).
(H) Phenotypic characteristics of Ki67+CD57− and Ki67+CD57+ memory CD4+ T cells shown overlaid on density clouds representing the corresponding total CD4+ T cell populations. Related to (C). Key as in (G).
(I) Phenotypic characteristics of Ki67+CD57− and Ki67+CD57+ memory CD8+ T cells shown overlaid on density clouds representing the corresponding total CD8+ T cell populations. Related to (E). Key as in (G).
In further analyses, we assessed the phenotypic characteristics of Ki67+CD57− and Ki67+CD57+ memory T cells in the CD4+ and CD8+ lineages. As expected, Ki67+ memory CD4+ T cells predominantly expressed CD45RO, with or without CD57, whereas Ki67+ memory CD8+ T cells were phenotypically more heterogeneous and often expressed CD45RA in conjunction with CD57 (Figure 2G). Moreover, Ki67+CD57− memory T cells expressed CD28 at higher frequencies than Ki67+CD57+ memory T cells, both in the CD4+ lineage (p = 0.008, paired samples Wilcoxon test; Figures 2D and 2H) and in the CD8+ lineage (p = 0.008, paired samples Wilcoxon test; Figures 2F and 2I). Similar patterns of expression were observed for CCR7 (Figure S3).
To link these findings with the labeling data, we compared the phenotypic characteristics of Ki67+CD57− and Ki67+CD57+ memory T cells with the phenotypic characteristics of CD57− and CD57+ memory T cells sampled from the healthy volunteers in cohort 2. In both coreceptor-defined lineages, CD57− memory T cells expressed CD28 and CCR7 at higher frequencies than CD57+ memory T cells, akin to the corresponding Ki67+ memory T cells (Figure S4). Of note, CD57+ memory CD4+ T cells mostly lacked CD27 but commonly expressed CD127 and PD-1, whereas CD57+ memory CD8+ T cells were generally more differentiated and rarely expressed CD27, CD127, or PD-1 (Figures S4 and S5). Age had no apparent influence on these phenotypic characteristics (data not shown).
CD57− and CD57+ Memory T Cells Have Similar Division Histories
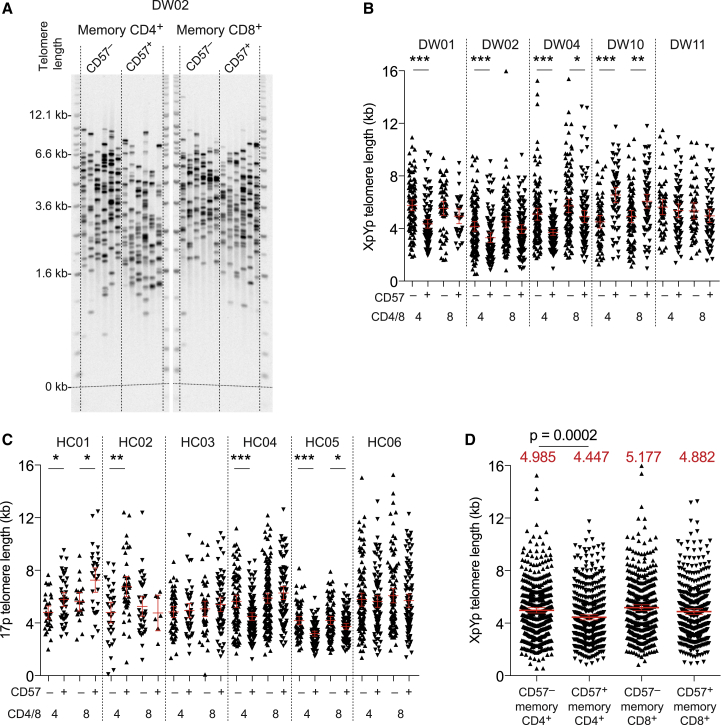
To refine our understanding of these datasets, we measured XpYp or 17p telomere lengths in the CD57− and CD57+ memory T cell pools (Figure 3A). Telomere lengths were distributed in a heterogeneous manner and overlapped considerably across CD57-defined subsets in the CD4+ and CD8+ lineages. In some volunteers, significant differences in mean telomere length were observed between the CD57− and CD57+ memory T cell populations, most commonly in the CD4+ lineage, but no consistent directional change was apparent between CD57-defined subsets in either the CD4+ or the CD8+ lineage (Figures 3B and 3C). However, pooling the XpYp data from labeled volunteers revealed that telomere lengths were maintained to a slightly greater extent in the CD57− memory CD4+ T cell population compared with the CD57+ memory CD4+ T cell population (Figure 3D), and pooling the 17p data from volunteers in cohort 3 yielded a similar result with borderline significance (p = 0.037, Mann-Whitney U test; data not shown). Telomerase activity was generally low, as expected given the infrequent expression of Ki67, but marginally higher levels were detected among CD57− memory T cells compared with CD57+ memory T cells in both coreceptor-defined lineages. These data were reported previously for reference in another labeling study of the volunteers in cohort 2 (Ahmed et al., 2016).
Figure 3.
CD57− and CD57+ Memory T Cells Have Similar Division Histories
(A) Representative single telomere length analysis (STELA) data showing XpYp telomere lengths among CD57− and CD57+ memory CD4+ or CD8+ T cells sampled from a labeled volunteer (DW02).
(B) XpYp telomere lengths among CD57− and CD57+ memory CD4+ or CD8+ T cells sampled from labeled volunteers (cohort 2). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Mann-Whitney U test.
(C) 17p telomere lengths among CD57− and CD57+ memory CD4+ or CD8+ T cells sampled from unlabeled volunteers (cohort 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Mann-Whitney U test.
(D) Pooled XpYp telomere length data for the volunteers shown in (B). Red lines show means with 95% confidence intervals. Mean values are specified above each column. Significance was assessed using the Mann-Whitney U test.
CD57− and CD57+ Memory T Cells Self-Renew In Vivo
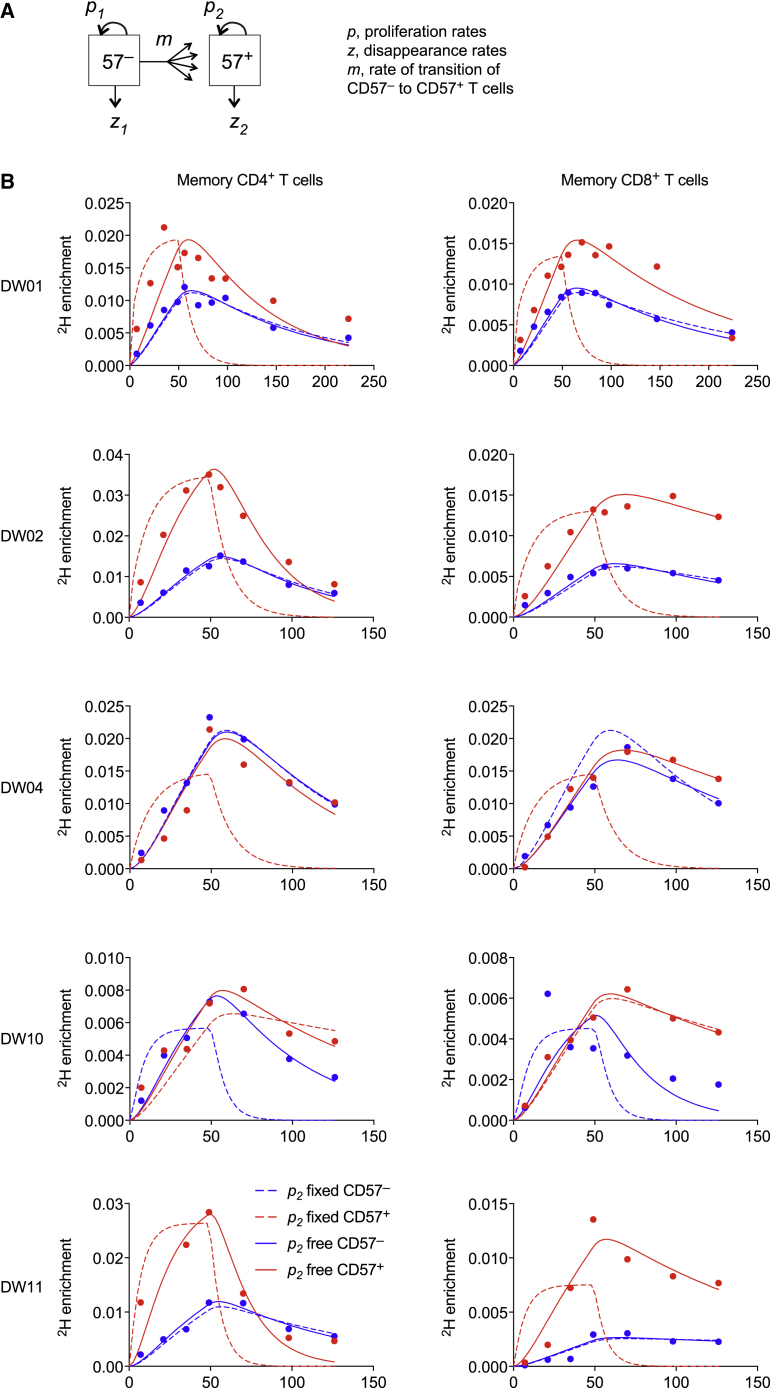
To integrate these findings, we fitted mathematical models simultaneously to the 2H enrichment data and the telomere length data, allowing CD57− memory T cells to become CD57+ memory T cells in the CD4+ and CD8+ lineages (Figure 4A). This approach was designed to capture both possible explanations for the accumulation of label in the corresponding CD57+ compartments, namely that CD57− memory T cells proliferated and acquired expression of CD57 and/or that CD57+ memory T cells proliferated and retained expression of CD57. Proliferation rates were denoted by p1 and p2 for CD57− and CD57+ memory T cells, respectively, such that replicative senescence in the CD57+ subsets was represented by the constraint p2 = 0. Telomeres shorten by an average of 50 bp per cell division (De Boer and Noest, 1998). In some cases, this rate of erosion can be counteracted by the activity of telomerase, a possibility that was included in the model assumptions via an additional parameter, termed K.
Figure 4.
CD57− and CD57+ Memory T Cells Self-Renew In Vivo
(A) Schematic representation of the mathematical model.
(B) Model fits to the measured data (dots) for CD57− and CD57+ memory CD4+ (left) or CD8+ T cells (right) with p2 constrained to zero (dashed lines) or free (solid lines).
Fits were restricted to the volunteers for whom labeling data and telomere length data were available (n = 5). The general model (with p2 free) fitted the data well for CD57− and CD57+ memory T cells in the CD4+ and CD8+ lineages (Figure 4B; Table 1). Importantly, the proliferation rate estimates for the CD57+ subsets were positive, with 95% confidence intervals that did not overlap zero (i.e., p2 > 0). This conclusion was robust to different rates of telomere shortening per cell division and changes in the parameter K (Figure S6). Model performance was considerably worse if proliferation was disallowed in the CD57+ memory T cell populations (i.e., p2 = 0) (Figures 4B and S7). Indeed, the median p value in a comparison of the models was 5 × 10−20 (F test), which provided strong evidence to reject the null hypothesis of the simpler model, namely that CD57+ memory T cells were unable to proliferate (i.e., p2 = 0). Moreover, the median small-sample-corrected Akaike information criterion (AICc) difference was 106, which indicated that the simpler model provided a substantially worse description of the data (i.e., fit after adjustment for model complexity). The best fits were therefore consistent with substantial proliferation in the CD57+ compartments, such that influx from the CD57− compartments typically contributed only ∼5% of all newly generated CD57+ memory T cells (Table 1).
Table 1.
Parameter Estimates from Model Fits to the Experimental Data
| ID | p1 (% per day) | SE (% per day) | z1 (% per day) | SE (% per day) | p2 (% per day) | SE (% per day) | z2 (% per day) | SE (% per day) | mR (% per day) | SE (% per day) | Self-renewal (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ T Cells | |||||||||||
| DW01 | 0.33 | 0.04 | 0.0088 | 0.27 | 0.59 | 0.08 | 1.24 | 0.37 | 0.02 | 0.01 | 97 |
| DW02 | 0.65 | 0.06 | 1.89 | 0.31 | 2.08 | 0.23 | 3.77 | 0.58 | 0.17 | 0.03 | 93 |
| DW04 | 0.53 | N/D | 1.43 | N/D | 0.53 | N/D | 1.65 | N/D | 0.00 | N/D | 100 |
| DW10 | 0.58 | 0.05 | 1.80 | 0.28 | 0.49 | 0.05 | 0.92 | 0.22 | 0.00 | 0.00 | 99 |
| DW11 | 0.44 | 0.06 | 1.24 | 0.33 | 1.64 | 0.02 | 5.24 | 0.66 | 0.45 | 0.02 | 79 |
| Median | 0.53 | 1.43 | 0.59 | 1.65 | 0.02 | 97 | |||||
| CD8+ T Cells | |||||||||||
| DW01 | 0.26 | 0.02 | 0.73 | 0.13 | 0.40 | 0.03 | 0.70 | 0.13 | 0.02 | 0.00 | 96 |
| DW02 | 0.22 | 0.02 | 0.86 | 0.22 | 0.42 | 0.04 | 0.45 | 0.19 | 0.04 | 0.01 | 92 |
| DW04 | 0.37 | N/D | 0.91 | N/D | 0.37 | N/D | 0.62 | N/D | 0.00 | N/D | 100 |
| DW10 | 0.55 | 0.17 | 3.60 | 1.53 | 0.34 | 0.11 | 0.63 | 0.56 | 0.02 | 0.02 | 95 |
| DW11 | 0.07 | 0.01 | 0.24 | 0.34 | 0.32 | 0.03 | 0.81 | 0.04 | 0.07 | 0.01 | 83 |
| Median | 0.26 | 0.86 | 0.37 | 0.63 | 0.02 | 95 | |||||
The best-fit estimates are shown. A limited number of data points were available from one volunteer (DW04). The asymptotic covariance matrix method was used to calculate standard errors (SEs). The percentage of new CD57+ T cells generated via intracompartmental proliferation (right column) was calculated as 100 × p2/(p2 + mR). ID, identification number; N/D, not determined.
Discussion
In this study, we used in vivo deuterium labeling and ex vivo analyses of telomere length, telomerase activity, and intracellular expression of the cell-cycle marker Ki67 to investigate the paradigm that replicatively senescent memory T cells can be identified via the surrogate marker CD57. We detected similar rates of proliferation among CD57− and CD57+ memory T cells in both coreceptor-defined lineages. These results were supported by flow cytometric analyses, which revealed the presence of actively dividing cells in the corresponding CD57+ memory T cell populations. Marginally higher levels of telomerase activity were detected among CD57− memory T cells compared with CD57+ memory T cells in the CD4+ and CD8+ lineages, consistent with a relatively small biological effect, and in line with recent observations (Fali et al., 2018), telomere lengths were maintained to a slightly greater extent among CD57− memory CD4+ T cells compared with CD57+ memory CD4+ T cells. In contrast, telomere lengths were distributed around similar means in the CD57− and CD57+ memory CD8+ T cell populations. Mathematical modeling of the experimental data further suggested that self-renewal via intracompartmental proliferation rather than replenishment via phenotypic conversion was by far the most abundant source of newly generated CD57+ memory CD4+ and CD57+ memory CD8+ T cells.
CD57 was originally recognized as a differentiation antigen on the surface of NK cells (Abo and Balch, 1981) and subsequently associated with other lymphocyte subsets in germinal centers (Ritchie et al., 1983). In peripheral blood, CD57+ memory T cells accumulate throughout life, especially after infection with CMV (Gratama et al., 1989). These associations with age and persistent antigenic drive were mechanistically linked in a seminal in vitro study, which reported that replicatively senescent memory CD8+ T cells expressed CD57 (Brenchley et al., 2003). However, an earlier study had reached a different conclusion (Izquierdo et al., 1990), and later experiments showed that CD57+ memory CD8+ T cells were able to proliferate in vitro in the presence of certain growth factors, potentially mimicking the in vivo microenvironment (Chong et al., 2008). Similar findings were reported in another study, although markedly higher response frequencies on a per-cell basis were noted in the CD57− subset compared with the CD57+ subset (Le Priol et al., 2006). Nonetheless, the proportion of responding cells in the CD57+ subset was more than sufficient to maintain homeostatic turnover, at least according to a deuterium labeling study of bulk memory T cell populations (Zhang et al., 2013).
TEMRA cells are somewhat resistant to apoptosis (Gupta and Gollapudi, 2007) and retain deuterium in the CD8+ lineage with an estimated half-life of approximately 25 years, assuming simple exponential decay without phenotypic conversion (Ladell et al., 2008). In response to extreme stimulation with supraphysiological concentrations of phytohemagglutinin and interleukin-2, CD8+ TEMRA cells that expressed CD57 were recently found to be more susceptible to cell death than CD8+ TEMRA cells that lacked CD57 (Verma et al., 2017). This observation was thought to indicate a functional dichotomy between CD57-defined subsets within the CD8+ TEMRA compartment. However, it does not necessarily follow that a similar dichotomy exists under homeostatic conditions, because terminally differentiated CD57+ memory CD8+ T cells may be protected from excessive stimulation in vivo by a lack of costimulatory receptors, such as CD27 and CD28.
In summary, we have shown that CD57+ memory T cells in the CD4+ and CD8+ lineages self-renew in vivo, enabling the long-term maintenance of functionally replete immunological memory. It remains to be determined how this process is regulated in terms of antigenic drive versus homeostatic signals as a function of differentiation status, but nonetheless, it is clear from the presented data that replicatively senescent memory T cells cannot be defined solely via surface expression of CD57.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-CD3–APC-H7 (clone SK7) | BD Biosciences | Cat#641415; RRID:AB_2870309 |
| Anti-CD4–PE-Cy5.5 (clone S3.5) | Thermo Fisher Scientific | Cat#MHCD0418; RRID:AB_10376013 |
| Anti-CD8–BV711 (clone RPA-T8) | BioLegend | Cat#301044; RRID:AB_2562906 |
| Anti-CD14–V500 (clone M5E2) | BD Biosciences | Cat#561391; RRID:AB_10611856 |
| Anti-CD19–V500 (clone HIB19) | BD Biosciences | Cat#561121; RRID:AB_10562391 |
| Anti-CD27–QD605 (clone CLB-27/1) | Thermo Fisher Scientific | Cat#Q10065; RRID:AB_2556450 |
| Anti-CD28–APC (clone CD28.2) | BD Biosciences | Cat#559770; RRID:AB_398666 |
| Anti-CD28–BV421 (clone CD28.2) | BioLegend | Cat#302930; RRID:AB_2561910 |
| Anti-CD45RA–ECD (clone 2H4LDH11LDB9) | Beckman Coulter | Cat#M2711U; RRID:AB_10640553 |
| Anti-CD45RA–PE (clone HI100) | BD Biosciences | Cat#555489; RRID:AB_395880 |
| Anti-CD45RO–ECD (clone UCHL1) | Beckman Coulter | Cat#IM2712U; RRID:AB_10639537 |
| Anti-CD57–FITC (clone NK-1) | BD Biosciences | Cat#555619; RRID:AB_395986 |
| Anti-CD57–PE-Cy7 (clone NK-1) | BioLegend | Cat#359624; RRID:AB_2632689 |
| Anti-CD127–BV421 (clone A019D5) | BioLegend | Cat#351310; RRID:AB_10960140 |
| Anti-CCR7–BV421 (clone G043H7) | BioLegend | Cat#353208; RRID:AB_11203894 |
| Anti-CCR7–FITC (clone 150503) | BD Biosciences | Cat#561271; RRID:AB_10561679 |
| Anti-CCR7–PE-Cy7 (clone 3D12) | BD Biosciences | Cat#557648; RRID:AB_396765 |
| Anti-CXCR3–BV421 (clone G025H7) | BioLegend | Cat#353716; RRID:AB_2561448 |
| Anti-Ki67–AF647 (clone B56) | BD Biosciences | Cat#558615; RRID:AB_647130 |
| Anti-Ki67–FITC (clone B56) | BD Biosciences | Cat#556026; RRID:AB_396302 |
| Anti-PD-1–BV421 (clone EH12.2H7) | BioLegend | Cat#329920; RRID:AB_10960742 |
| Biological Samples | ||
| Peripheral blood from adults infected with HIV-1 | San Francisco General Hospital, San Francisco, CA, USA | N/A |
| Peripheral blood from healthy adult volunteers | St George’s Hospital, London, UK | N/A |
| Peripheral blood from healthy adult volunteers | Cardiff University School of Medicine, Cardiff, UK | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Heavy water (2H2O) | Cambridge Isotope Laboratories | Cat#DLM-4TPB-PK |
| Pentafluorobenzyl hydroxylamine | Sigma-Aldrich | Cat#194484 |
| Sodium dodecyl sulfate | Thermo Fisher Scientific | Cat#10593355 |
| Tris(hydroxymethyl) methylamine | Thermo Fisher Scientific | Cat#77-86-1 |
| Ethylenediaminetetraacetic acid | Sigma-Aldrich | Cat#E6511-100G |
| Sodium hydroxide | Thermo Fisher Scientific | Cat#J/7620/15 |
| Sodium phosphate dibasic | Sigma-Aldrich | Cat#7558-79-4 |
| Hydrochloric acid | Thermo Fisher Scientific | Cat#7647-01-0 |
| Critical Commercial Assays | ||
| LIVE/DEAD Fixable Aqua Dead Cell Stain Kit | Thermo Fisher Scientific | Cat#L34966 |
| Cytofix/Cytoperm Kit | BD Biosciences | Cat#554715 |
| Foxp3 Transcription Factor Staining Buffer Kit | Thermo Fisher Scientific | Cat#00-5521-00 |
| QIAmp DNA Mini Kit | QIAGEN | Cat#51304 |
| QIAmp DNA Micro Kit | QIAGEN | Cat#56304 |
| Oligonucleotides | ||
| XpYpE2: TTGTCTCAGGGTCCTAGTG | Eurofins Genomics | Custom |
| 17pserev1: GAATCCACGGATTGCTTTGTGTAC | Eurofins Genomics | Custom |
| Telorette2: TGCTCCGTGCATCTGGCATCTAACCCT | Eurofins Genomics | Custom |
| Teltail: TGCTCCGTGCATCTGGCATC | Eurofins Genomics | Custom |
| Software and Algorithms | ||
| DiVa version 8 | BD Biosciences | https://www.bdbiosciences.com/en-us |
| FlowJo software version 9.9.4 | FlowJo LLC | https://www.flowjo.com |
| Phoretix 1D Quantifier | Nonlinear Dynamics | http://www.nonlinear.com/about/totallab |
| Prism version 8 | GraphPad | https://www.graphpad.com |
| Other | ||
| DreamTaq polymerase | Thermo Fisher Scientific | Cat#EP0702 |
| Pwo polymerase | Sigma-Aldrich | Cat#11644955001 |
| dNTPs | Promega | Cat#U1511 |
| α-33P dCTP | PerkinElmer | Cat#BLU013H100UC |
| Megaprime | VWR | Cat#RPN1607 |
| Hybond-XL | VWR | Cat#RPN15205 |
| Agarose MP | Sigma-Aldrich | Cat#11388983001 |
| 1 kb ladder | Agilent | Cat#201115 |
| 2.5 kb ladder | Bio-Rad | Cat#1708205 |
| FACSVantage SE | BD Biosciences | https://www.bdbiosciences.com/en-us |
| FACSAria | BD Biosciences | https://www.bdbiosciences.com/en-us |
| Special Order Research Product FACSAria II | BD Biosciences | https://www.bdbiosciences.com/en-us |
| GC/MS (5873/6980) | Agilent | https://www.agilent.com |
| DB-17 column | Agilent | https://www.agilent.com |
| Tetrad2 Thermal Cycler | Bio-Rad | https://www.bio-rad.com |
| Typhoon FLA 9500 Phosphorimager | GE Healthcare | https://www.cytivalifesciences.com/ |
Resource Availability
Lead Contact
Further information and requests for reagents and resources should be directed to and will be fulfilled by the Lead Contact, Kristin Ladell (ladellk@gmail.com).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The datasets reported in this study are available on request from the Lead Contact, Kristin Ladell (ladellk@gmail.com).
Experimental Model and Subject Details
Three groups of human volunteers participated in this work. Cohort 1: volunteers with chronic HIV-1 infection (aged 36–53 years) were recruited for preliminary in vivo labeling studies (n = 4 males; Table S1). All were antiretroviral drug-free and seropositive for CMV. Cohort 2: healthy volunteers (aged 29–83 years) were recruited for more extensive in vivo labeling studies (n = 3 females; n = 5 males; Table S1). All were seronegative for hepatitis C virus and HIV-1 and seropositive for CMV. Cohort 3: additional healthy volunteers (aged 28–58 years) were recruited for phenotypic studies and measurements of telomere length and telomerase activity (n = 7 females; n = 7 males). Similar experiments were performed using venous blood samples donated by 5 of the 8 volunteers in cohort 2. All studies were conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was granted by the University of California Committee on Human Research (cohort 1), the London-Chelsea Research Ethics Committee (cohort 2), and the Cardiff University School of Medicine Research Ethics Committee (cohort 3).
Method Details
Measurement and Analysis of Deuterium Enrichment in T Cell DNA
T cell proliferation in vivo was measured using deuterium (2H) labeling as described previously (Busch et al., 2007; Hellerstein et al., 1999; Ladell et al., 2008; McCune et al., 2000; Neese et al., 2001; Westera et al., 2013). Briefly, volunteers received heavy water (2H2O) orally for 7 weeks (Figure 1A), and deuterium incorporation into the DNA of flow-sorted T cells was quantified via gas chromatography/mass spectrometry (Agilent 5873/6980) (Ladell et al., 2008). DNA was released by boiling and hydrolyzed according to standard protocols, and deoxyribonucleosides were derivatized using pentafluorobenzyl hydroxylamine (Sigma-Aldrich). Gas chromatography/mass spectrometry was performed in negative chemical ionization mode using a DB-17 column (Agilent). The M+1/M+0 isotopomer ratio was monitored at mass-to-charge (m/z) 436/435. To normalize for body water enrichment, weekly saliva samples were analyzed for 2H2O content via calcium carbide-induced acetylene generation, monitoring at m/z 27/26 (Previs et al., 1996).
Flow Cytometry and Cell Sorting
T cell subsets of interest were flow-sorted from freshly isolated peripheral blood mononuclear cells (PBMCs) at >98% purity using a FACSVantage SE, a FACSAria, or a Special Order Research Product FACSAria II (all from BD Biosciences). Cells were stained with combinations of the following reagents: (1) anti-CD3–APC-H7 (clone SK7), anti-CD14–V500 (clone M5E2), anti-CD19–V500 (clone HIB19), anti-CD28–APC (clone CD28.2), anti-CD45RA–PE (clone HI100), anti-CD57–FITC (clone NK-1), anti-CCR7–FITC (clone 150503), and anti-CCR7–PE-Cy7 (clone 3D12) from BD Biosciences; (2) anti-CD4–PE-Cy5.5 (clone S3.5), anti-CD27–QD605 (clone CLB-27/1), and LIVE/DEAD Fixable Aqua from Thermo Fisher Scientific; (3) anti-CD8–BV711 (clone RPA-T8), anti-CD28–BV421 (clone CD28.2), anti-CD57–PE-Cy7 (clone NK-1), anti-CD127–BV421 (clone A019D5), anti-CCR7–BV421 (clone G043H7), anti-CXCR3–BV421 (clone G025H7), and anti-PD-1–BV421 (clone EH12.2H7) from BioLegend; and (4) anti-CD45RA–ECD (clone 2H4LDH11LDB9) and anti-CD45RO–ECD (clone UCHL1) from Beckman Coulter. Viable CD57− and CD57+ memory T cells were identified in the CD4+ and/or CD8+ lineages after exclusion of CD27brightCD45RO− (Figure 1C) or CD45RA+CCR7+ events (Figure S1). Cytosolic expression of Ki67 was evaluated using anti-Ki67–AF647 (clone B56; BD Biosciences) in conjunction with a Cytofix/Cytoperm Kit (BD Biosciences), and cytosolic/intranuclear expression of Ki67 was evaluated using anti-Ki67–FITC (clone B56; BD Biosciences) in conjunction with a Foxp3 Transcription Factor Staining Buffer Kit (Thermo Fisher Scientific). Data were analyzed with FlowJo software version 9.9.4 (FlowJo LLC).
Single Chromosome Telomere Length Analysis
DNA was extracted from 3,000 flow-sorted T cells using a QIAmp DNA Micro Kit (QIAGEN). Single telomere length analysis (STELA) was carried out at the XpYp or the 17p telomere as described previously (Capper et al., 2007). Briefly, 0.75 μL of the Telorette-2 linker (10 μM) was added to genomic DNA eluted in 35 μL of Tris (10 mM). Multiple PCRs were then performed for each test DNA. Each reaction was set up in a final volume of 10 μL containing 250 pg of DNA and the telomere-adjacent and Teltail primers at a final concentration of 0.5 μM in 75 mM Tris-HCl pH 8.8, 20 mM (NH4)2SO4, 0.01% Tween-20, and 1.5 mM MgCl2, with 0.5 U of a 10:1 mixture of Taq (Thermo Fisher Scientific) and Pwo polymerase (Sigma-Aldrich). The reactions were processed in a Tetrad2 Thermal Cycler (Bio-Rad). DNA fragments were resolved via 0.5% Tris-acetate-EDTA agarose gel electrophoresis and identified via Southern hybridization with a random-primed α-33P-labeled (PerkinElmer) TTAGGG repeat probe, together with probes specific for molecular weight markers at 1 kb (Agilent) and 2.5 kb (Bio-Rad). Hybridized fragments were detected using a Typhoon FLA 9500 Phosphorimager (GE Healthcare). The molecular weights of the DNA fragments were calculated using Phoretix 1D Quantifier (Nonlinear Dynamics).
Measurement of Telomerase Activity
Flow-sorted T cells were lyzed and assayed in two steps using a modified SYBR Green real-time quantitative telomeric repeat amplification protocol (Wege et al., 2003). Standard curves were obtained from serial dilutions of a 293T cell extract with known telomerase activity. Experimental telomerase activity was calculated with reference to 293T cells and expressed as relative telomerase activity (Ct293T/Ctsample).
Quantification and Statistical Analysis
General Statistics
Unmatched groups were compared using the Mann-Whitney U test, and matched groups were compared using the paired samples Wilcoxon test. Significance was assigned at p < 0.05.
Mathematical Modeling
Mechanistic ordinary differential equation-based models were developed to assess the dynamics of CD57− and CD57+ memory T cells (Costa Del Amo et al., 2018; Patel et al., 2017). These subsets were modeled as dependent populations to investigate the possibility that label acquisition in the CD57+ compartment was a consequence of proliferation-linked differentiation in the CD57− compartment. Accordingly, CD57− and CD57+ memory T cells were allowed to proliferate and die or exit the circulation, and CD57− memory T cells were allowed to gain expression of CD57. The phenotypic conversion of CD57− memory T cells into CD57+ memory T cells was considered over n rounds of division, including the possibility that n = 0.
In the applied model, CD57− memory T cells (x1) became CD57+ memory T cells (x2) at a rate m:
where p1 and p2 are the rates of proliferation of CD57− and CD57+ memory T cells, respectively, and z1 and z2 are the rates of disappearance of CD57− and CD57+ memory T cells, respectively. The possibility that surface expression of CD57 could be acquired during clonal expansion was accommodated in the permitted values for m (bounds during fitting [0,40]).
To minimize the number of free parameters, label enrichment among CD57+ memory T cells was assumed to originate either from dividing CD57− memory T cells that differentiated into CD57+ memory T cells or from dividing CD57+ memory T cells. A model in which the acquisition of CD57 was not coincident with clonal expansion resulted in a substantially worse fit to the data and was not pursued further. The fraction of label thus became:
where p1 and p2 are as above, z1∗ and z2∗ are the rates of loss of labeled CD57− and CD57+ memory T cells, respectively, L1 and L2 are the fractions of labeled deoxyadenosine among CD57− and CD57+ memory T cells, respectively, R is the ratio of CD57− to CD57+ memory T cells (x1/x2), and bw is the amplification factor estimated from label acquisition among granulocytes, assuming 100% turnover in 7 weeks. Data were available from 4 volunteers and gave a population average value for bw of 3.5, consistent with previous studies (Ahmed et al., 2015; Lahoz-Beneytez et al., 2016). The value of bw was therefore fixed at 3.5. Finally, U(t) is an empirical function used to describe the availability of label in body water:
as described previously (Vrisekoop et al., 2008), where U(t) represents the fraction of labeled precursor in body water at time t (in days), f is the fraction of labeled precursor in ingested water, τ is the length of the labeling period, δ is the turnover rate of body water per day, and β is the plasma enrichment attained at the end of day 0. Parameters were estimated by fitting the above functions to the deuterium labeling data measured in saliva. The resulting fits of U(t) to saliva measurements are shown in Figure S2.
Inclusion of Telomere Length Data in Model Fits
The impact of cell division on telomere length was modeled as described previously (De Boer and Noest, 1998). Telomere length data were available from 5 of the 8 volunteers in cohort 2. The model was fitted simultaneously to the labeling data and the telomere length data using the free parameters p1 and p2 to describe the rates of proliferation of CD57− and CD57+ memory T cells, respectively, z1∗ and z2∗ to describe the rates of disappearance of labeled CD57− and CD57+ memory T cells, respectively, and mR, the rate of conversion from CD57− to CD57+ memory T cells (m) multiplied by the ratio of the frequency of CD57− memory T cells to the frequency of CD57+ memory T cells (R).
Estimation of Telomere Length Change
The rate of change in telomere loss indices for CD57− memory T cells was defined according to a previous report (De Boer and Noest, 1998) as follows:
where p1, p2, m, and R are as above, K is the length of telomere loss upon clonal expansion in units of division, and μ1 and μ2 are the average number of divisions undergone by CD57− and CD57+ memory T cells, respectively. The difference in telomere length between CD57− and CD57+ memory T cells was estimated as:
where ε is the average number of base pairs (bp) lost per division (taken to be 50 bp [De Boer and Noest, 1998]), giving the following expression for the difference in telomere length:
Fitting Procedure
The function U(t) was fitted to the deuterium labeling data measured in saliva, and the free parameters f, β, and δ were estimated for each individual. The resulting parameterized U(t) functions were then used as fixed inputs during simultaneous fitting of the deuterium labeling and telomere length data from CD57− and CD57+ memory T cells using the equations for L1, L2, and ΔC above. The free parameters were p1, p2, z1, z2, and mR. As telomerase is highly active during clonal expansion, the telomere length loss index (K) was initially set to 0 (Bodnar et al., 1996; Collins, 2006). This assumption was subsequently relaxed to explore the impact of variations in K (Figure S6). The contribution of self-renewal to the production of new CD57+ memory T cells was defined as:
To ensure that the labeling data and the telomere length data contributed equally to the fit, all residuals were normalized by the mean, and the deuterium residuals were divided by the number of labeling data points. Conclusions were analyzed for robustness against changes in the number of telomere base pairs lost per division. Scenarios in which CD57+ memory T cells did not proliferate were also tested by fixing p2 to 0. Model performance was evaluated using the F test and the AICc (Burnham and Anderson, 2002). The model was fitted to the data using non-linear least-squares regression implemented via the algorithm Pseudo in the FME package in R (Soetaert and Petzoldt, 2010).
Acknowledgments
The authors extend their profound thanks to all study participants. This work was funded by Cancer Research UK (grant C17199/A18246), the Medical Research Council (grant G1001052), and the Wellcome Trust (grant 093053/Z/10/Z). J.L.-B. was supported by the European Union Seventh Framework Programme (grant 317040, QuanTI). Pilot studies were funded in part by the National Institutes of Health (NIH) via grant RO1 AI43866 to M.K.H. and grants R37 AI40312 and U01 AI43641 to J.M.M., who also received a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research and was supported by an NIH Director’s Pioneer Award, part of the NIH Roadmap for Medical Research, via grant DPI OD00329. Research conducted at the University of California San Francisco Clinical and Translational Science Institute under the guidance of J.M.M. was supported by the National Center for Research Resources, part of the NIH Roadmap for Medical Research, via grant UL1 RR024131-01. D.A.P. was supported by a Wellcome Trust Senior Investigator Award (100326/Z/12/Z). B.A. was supported by the European Union Seventh Framework Programme (grant 317040, QuanTI), Leukemia and Lymphoma Research (grant 15012), Medical Research Council (grants G1001052 and J007439), and Wellcome Trust via an Investigator Award (103865/Z/14/Z).
Author Contributions
M.K.H., J.M.M., D.A.P., D.C.M., and K.L. designed experiments; R.A., K.L.M., R.E.J., L.R., Y.Z., and K.L. performed experiments; C.B., M.K.H., J.M.M., D.M.B., D.A.P., D.C.M., and K.L. supervised experiments; R.A., K.L.M., R.E.J., L.R., D.M.B., D.C.M., and K.L. analyzed data; J.L.-B. and B.A. modeled data; D.A.P., D.C.M., B.A., and K.L. wrote the manuscript. All authors contributed intellectually and approved the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: December 15, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.108501.
Contributor Information
David A. Price, Email: priced6@cardiff.ac.uk.
Derek C. Macallan, Email: macallan@sgul.ac.uk.
Becca Asquith, Email: b.asquith@imperial.ac.uk.
Kristin Ladell, Email: ladellk@gmail.com.
Supplemental Information
References
- Abo T., Balch C.M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J. Immunol. 1981;127:1024–1029. [PubMed] [Google Scholar]
- Addo M.M., Draenert R., Rathod A., Verrill C.L., Davis B.T., Gandhi R.T., Robbins G.K., Basgoz N.O., Stone D.R., Cohen D.E. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS ONE. 2007;2:e321. doi: 10.1371/journal.pone.0000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Westera L., Drylewicz J., Elemans M., Zhang Y., Kelly E., Reljic R., Tesselaar K., de Boer R.J., Macallan D.C. Reconciling estimates of cell proliferation from stable isotope labeling experiments. PLoS Comput. Biol. 2015;11:e1004355. doi: 10.1371/journal.pcbi.1004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Roger L., Costa Del Amo P., Miners K.L., Jones R.E., Boelen L., Fali T., Elemans M., Zhang Y., Appay V. Human stem cell-like memory T cells are maintained in a state of dynamic flux. Cell Rep. 2016;17:2811–2818. doi: 10.1016/j.celrep.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V., van Lier R.A., Sallusto F., Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- Benito J.M., Ortiz M.C., León A., Sarabia L.A., Ligos J.M., Montoya M., Garcia M., Ruiz-Mateos E., Palacios R., Cabello A., ECRIS integrated in the Spanish AIDS Research Network Class-modeling analysis reveals T-cell homeostasis disturbances involved in loss of immune control in elite controllers. BMC Med. 2018;16:30. doi: 10.1186/s12916-018-1026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar A.G., Kim N.W., Effros R.B., Chiu C.P. Mechanism of telomerase induction during T cell activation. Exp. Cell Res. 1996;228:58–64. doi: 10.1006/excr.1996.0299. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Karandikar N.J., Betts M.R., Ambrozak D.R., Hill B.J., Crotty L.E., Casazza J.P., Kuruppu J., Migueles S.A., Connors M. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. Second Edition. Springer-Verlag; 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. [Google Scholar]
- Busch R., Neese R.A., Awada M., Hayes G.M., Hellerstein M.K. Measurement of cell proliferation by heavy water labeling. Nat. Protoc. 2007;2:3045–3057. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- Capper R., Britt-Compton B., Tankimanova M., Rowson J., Letsolo B., Man S., Haughton M., Baird D.M. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza J.P., Betts M.R., Price D.A., Precopio M.L., Ruff L.E., Brenchley J.M., Hill B.J., Roederer M., Douek D.C., Koup R.A. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P.K., Betts M.R., Price D.A., Gostick E., Horton H., Roederer M., De Rosa S.C. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong L.K., Aicheler R.J., Llewellyn-Lacey S., Tomasec P., Brennan P., Wang E.C. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur. J. Immunol. 2008;38:995–1000. doi: 10.1002/eji.200737687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat. Rev. Mol. Cell Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Del Amo P., Lahoz-Beneytez J., Boelen L., Ahmed R., Miners K.L., Zhang Y., Roger L., Jones R.E., Marraco S.A.F., Speiser D.E. Human TSCM cell dynamics in vivo are compatible with long-lived immunological memory and stemness. PLoS Biol. 2018;16:e2005523. doi: 10.1371/journal.pbio.2005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- De Boer R.J., Noest A.J. T cell renewal rates, telomerase, and telomere length shortening. J. Immunol. 1998;160:5832–5837. [PubMed] [Google Scholar]
- Fali T., Fabre-Mersseman V., Yamamoto T., Bayard C., Papagno L., Fastenackels S., Zoorab R., Koup R.A., Boddaert J., Sauce D., Appay V. Elderly human hematopoietic progenitor cells express cellular senescence markers and are more susceptible to pyroptosis. JCI Insight. 2018;3:e95319. doi: 10.1172/jci.insight.95319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G.J., Wherry E.J., Ahmed R., Sharpe A.H. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gratama J.W., Fridell E., Lenkei R., Oosterveer M.A., Ljungström I., Tanke H.J., Linde A. Correlation between cytomegalovirus and Toxoplasma gondii serology and lymphocyte phenotypes in peripheral blood and cord blood. Scand. J. Infect. Dis. 1989;21:611–616. doi: 10.3109/00365548909021688. [DOI] [PubMed] [Google Scholar]
- Gupta S., Gollapudi S. Effector memory CD8+ T cells are resistant to apoptosis. Ann. N Y Acad. Sci. 2007;1109:145–150. doi: 10.1196/annals.1398.017. [DOI] [PubMed] [Google Scholar]
- Hegedus A., Nyamweya S., Zhang Y., Govind S., Aspinall R., Mashanova A., Jansen V.A., Whittle H., Jaye A., Flanagan K.L., Macallan D.C. Protection versus pathology in aviremic and high viral load HIV-2 infection—the pivotal role of immune activation and T-cell kinetics. J. Infect. Dis. 2014;210:752–761. doi: 10.1093/infdis/jiu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein M., Hanley M.B., Cesar D., Siler S., Papageorgopoulos C., Wieder E., Schmidt D., Hoh R., Neese R., Macallan D. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- Izquierdo M., Balboa M.A., Fernández-Rañada J.M., Figuera A., Torres A., Iriondo A., López-Botet M. Relation between the increase of circulating CD3+ CD57+ lymphocytes and T cell dysfunction in recipients of bone marrow transplantation. Clin. Exp. Immunol. 1990;82:145–150. doi: 10.1111/j.1365-2249.1990.tb05418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F., Khatamzas E., Surel I., Frömmel C., Reinke P., Waldrop S.L., Picker L.J., Volk H.D. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 1999;29:2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ladell K., Hellerstein M.K., Cesar D., Busch R., Boban D., McCune J.M. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J. Immunol. 2008;180:7907–7918. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoz-Beneytez J., Elemans M., Zhang Y., Ahmed R., Salam A., Block M., Niederalt C., Asquith B., Macallan D. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 2016;127:3431–3438. doi: 10.1182/blood-2016-03-700336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Priol Y., Puthier D., Lécureuil C., Combadière C., Debré P., Nguyen C., Combadière B. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J. Immunol. 2006;177:5145–5154. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- McCune J.M., Hanley M.B., Cesar D., Halvorsen R., Hoh R., Schmidt D., Wieder E., Deeks S., Siler S., Neese R., Hellerstein M. Factors influencing T-cell turnover in HIV-1-seropositive patients. J. Clin. Invest. 2000;105:R1–R8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I., Min M., Yang C., Tian C., Gookin S., Carter D., Spencer S.L. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018;24:1105–1112.e5. doi: 10.1016/j.celrep.2018.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese R.A., Siler S.Q., Cesar D., Antelo F., Lee D., Misell L., Patel K., Tehrani S., Shah P., Hellerstein M.K. Advances in the stable isotope-mass spectrometric measurement of DNA synthesis and cell proliferation. Anal. Biochem. 2001;298:189–195. doi: 10.1006/abio.2001.5375. [DOI] [PubMed] [Google Scholar]
- Nociari M.M., Telford W., Russo C. Postthymic development of CD28−CD8+ T cell subset: age-associated expansion and shift from memory to naive phenotype. J. Immunol. 1999;162:3327–3335. [PubMed] [Google Scholar]
- Northfield J.W., Loo C.P., Barbour J.D., Spotts G., Hecht F.M., Klenerman P., Nixon D.F., Michaëlsson J. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ TEMRA cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J. Virol. 2007;81:5759–5765. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.A., Zhang Y., Fullerton J.N., Boelen L., Rongvaux A., Maini A.A., Bigley V., Flavell R.A., Gilroy D.W., Asquith B. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017;214:1913–1923. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C., Casazza J.P., Brenchley J.M., Price D.A., Gostick E., Adams W.C., Precopio M.L., Schacker T., Roederer M., Douek D.C., Koup R.A. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C., Chaon B., Ambrozak D.R., Price D.A., Melenhorst J.J., Hill B.J., Geldmacher C., Casazza J.P., Chattopadhyay P.K., Roederer M. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J. Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs S.F., Hazey J.W., Diraison F., Beylot M., David F., Brunengraber H. Assay of the deuterium enrichment of water via acetylene. J. Mass Spectrom. 1996;31:639–642. doi: 10.1002/(SICI)1096-9888(199606)31:6<639::AID-JMS336>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ritchie A.W., James K., Micklem H.S. The distribution and possible significance of cells identified in human lymphoid tissue by the monoclonal antibody HNK-1. Clin. Exp. Immunol. 1983;51:439–447. [PMC free article] [PubMed] [Google Scholar]
- Soetaert K., Petzoldt T. Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. J. Stat. Softw. 2010;33:1–28. [Google Scholar]
- Takata H., Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J. Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- van Leeuwen E.M., Gamadia L.E., Baars P.A., Remmerswaal E.B., ten Berge I.J., van Lier R.A. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J. Immunol. 2002;169:5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- Verma K., Ogonek J., Varanasi P.R., Luther S., Bünting I., Thomay K., Behrens Y.L., Mischak-Weissinger E., Hambach L. Human CD8+ CD57− TEMRA cells: too young to be called “old”. PLoS ONE. 2017;12:e0177405. doi: 10.1371/journal.pone.0177405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrisekoop N., den Braber I., de Boer A.B., Ruiter A.F., Ackermans M.T., van der Crabben S.N., Schrijver E.H., Spierenburg G., Sauerwein H.P., Hazenberg M.D. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrisekoop N., Drylewicz J., Van Gent R., Mugwagwa T., Van Lelyveld S.F., Veel E., Otto S.A., Ackermans M.T., Vermeulen J.N., Huidekoper H.H. Quantification of naive and memory T-cell turnover during HIV-1 infection. AIDS. 2015;29:2071–2080. doi: 10.1097/QAD.0000000000000822. [DOI] [PubMed] [Google Scholar]
- Wege H., Chui M.S., Le H.T., Tran J.M., Zern M.A. SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res. 2003;31:e3. doi: 10.1093/nar/gng003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westera L., Zhang Y., Tesselaar K., Borghans J.A., Macallan D.C. Quantitating lymphocyte homeostasis in vivo in humans using stable isotope tracers. Methods Mol. Biol. 2013;979:107–131. doi: 10.1007/978-1-62703-290-2_10. [DOI] [PubMed] [Google Scholar]
- Zhang Y., de Lara C., Worth A., Hegedus A., Laamanen K., Beverley P., Macallan D. Accelerated in vivo proliferation of memory phenotype CD4+ T-cells in human HIV-1 infection irrespective of viral chemokine co-receptor tropism. PLoS Pathog. 2013;9:e1003310. doi: 10.1371/journal.ppat.1003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets reported in this study are available on request from the Lead Contact, Kristin Ladell (ladellk@gmail.com).