Abstract
Purpose
We evaluate outcomes of our single center using vertical rectus abdominis myocutaneous (VRAM) flaps for reconstruction after abdominoperineal resection (APR). Our goal was to analyze factors that may affect perineal wound healing, a problematic complication with APR reconstructions due to location and high frequency of neoadjuvant chemoradiation.
Methods
This single-center, retrospective study analyzed all VRAM flap perineal reconstruction patients after APR defect over a 10-year period (from July 2008 to June 2018). Outcome measures focused on factors that may affect perineal wound healing complication rates: cancer stage (I/II vs III/IV), neoadjuvant chemoradiation, surgeon's years in practice (<5 years vs >5 years), and pelvic closed suction drain use.
Results
Twenty-eight patients met inclusion criteria. The overall major perineal wound complication rate was 14.3% (4 patients). Lack of perioperative closed suction pelvic drain use was associated with a significantly higher rate of major perineal wound complications (28.6% vs 0% and p = 0.031). All four major wound complications occurred in patients who did not have a pelvic drain. The major perineal wound complication rate for patients who underwent neoadjuvant chemoradiation was 22% vs 0% with no neoadjuvant chemoradiation (p = 0.107).
Conclusion
While our cohort represents a relatively small single-center study, our 14.3% rate of major perineal wound complications is consistent with previous studies in the literature. Our findings show that perioperative pelvic closed suction drain use is associated with a lower rate of perineal wound complications. While neoadjuvant chemoradiation trended toward a higher incidence of perineal wound complications, it did not reach statistical significance.
Keywords: Vertical rectus abdominus myocutaneous flap, Abdominoperineal resection, Microsurgery, Perineal reconstruction, Pelvic drain, Flap complications
Introduction
Perineal reconstruction has a unique set of challenges. The location gives rise to a high incidence of wound healing problems, particularly in the context of rectal cancer and frequent use of radiation. The use of a vertical rectus abdominis myocutaneous (VRAM) flap is a well-established method for reconstruction, using well-vascularized and nonirradiated tissue to fill the defect.
The VRAM flap is a Mathes and Nahai Type III muscle flap with dual dominant pedicles from the superior and inferior epigastric systems. Its versatility and robustness has earned it a position as one of the workhorse flaps for plastic surgery reconstruction. Its reliable vascular anatomy, sufficient bulk, and large skin paddle make it an excellent option for perineal reconstruction.1 While variations of this flap exist, such as a superiorly pedicled VRAM flap, transverse rectus abdominus myocutaneous flap, muscle only flap, and free flap, our focus is on an inferiorly pedicled VRAM flap as it relates to perineal reconstruction.
One of the most common indications for VRAM flap reconstruction of the perineum is an abdominoperineal resection (APR) in the setting of radiation, either due to low rectal cancer or anal cancer.1,2 According to the American Cancer Society, it is estimated that more than 40,000 new cases of rectal cancer will be diagnosed in the year of 2019, and most of these cases are adenocarcinoma.3 The definitive treatment for locally advanced, nonmetastatic rectal cancer is surgical. The National Comprehensive Cancer Network (NCCN) recommends treatment with neoadjuvant chemoradiotherapy and surgical resection, followed by adjuvant chemotherapy for this stage of disease.4
Anal cancer is a relatively rare condition, comprising of only 0.5% of all cancers, with an estimated 8580 cases in the United States in 2017.3 The majority of anal cancers are epidermoid or squamous cell. However, despite the infrequent occurrence, its incidence is increasing at an alarming rate and it can be a debilitating disease for survivors.5 As Dr. Nigro described the use of chemoradiation rather than surgery in 1974, the treatment algorithm for anal cancer has shifted away from surgical resection as a first-line option. While not all patients may qualify for chemoradiation, the advantages of a sphincter-preserving treatment and the potential to avoid surgical resection are well studied.6 However, even in patients who do qualify for initial chemoradiation, failure rates are high, ranging from 20% to 40%.6, 7, 8 In these cases, persistent disease or locoregional recurrence are treated with salvage APR.
The two primary surgical choices for low rectal cancer and anal cancer are APR or lower anterior resection (LAR). The APR involves en bloc resection of the sigmoid colon, rectum, anus, and perineum, followed by a permanent colostomy. This technique has traditionally been the gold standard surgical treatment for low-lying rectal cancer and recurrent anal cancer. However, with the emergence of newer surgical technique as well as neoadjuvant therapy, APR has been increasingly replaced by sphincter-sparing procedures such as LAR. The LAR includes the resection of sigmoid colon and rectum, and preservation of the anal sphincter, followed by primary colorectal anastomosis or coloanal anastomosis. The tumor location in the relationship with the anal sphincter complex is the primary determinant as to which operation is to be performed. While the sphincter-preserving nature of the LAR is preferable, this surgery cannot be performed if the cancer is too “low” or close to the anal sphincter. The importance of this as it relates to a reconstructive algorithm is that LAR defects typically do not require reconstruction by plastic surgery, while APR defects are more likely to require reconstruction.
While the etiologies and oncological treatment algorithms for low rectal cancer and anal cancer differ, once an APR has been performed in the setting of irradiation, the reconstructive goals remain essentially the same: eliminate dead space created by tumor resection, provide structural support for the pelvic organs, maintain adequate wound closure, and yield an esthetically acceptable final appearance.9 The current standard of care is reconstruction with local autologous tissue. One of the most commonly used pedicle flaps following pelvic oncological resections is the VRAM flap. We aim to provide an overview of the use of a VRAM flap for APR perineal reconstruction and analyze our outcomes over a ten-year period. Additionally, we include a discussion about surgical technique and offer several surgical pearls for this operation.
Materials and methods
This is a single institution retrospective study from the University of California, Irvine Medical Center that includes all patients (n = 28) over the age of 18 who underwent a VRAM flap for perineal reconstruction of an APR defect in the past ten years, from July 2008 to June 2018. Patients less than 18 years of age and those who underwent a VRAM flap reconstruction for something other than an APR defect were excluded from the study. The study was reviewed and exempt by the University of California, Irvine Institutional Board Review on November 5, 2018.
Data were collected from Epic™ and Quest™. Patient and surgical characteristics included sex, reason for APR, tumor stage, and timing of chemotherapy and/or radiation, reconstruction for APR only versus total pelvic exenteration versus APR with some combination of vaginectomy/vulvectomy/bilateral salpingo-oophorectomy, and the use of a pelvic closed suction drain. The plastic surgeon's years of experience either less than or greater than five years was also recorded (Table 1).
Table 1.
Patient and Surgical Characteristics.
| Gender (male:female) | 8:20 |
|---|---|
| Age at surgery (years) | 60.0 ± 11.3 |
| Reason for APR | |
| Anal cancer | 21.4% (6/28) |
| Low rectal cancer | 71.4% (20/28) |
| Other | 7.1% (2/28) |
| Tumor stage | |
| I | 14.3% (4/28) |
| II | 35.7% (10/28) |
| III | 35.7% (10/28) |
| IV | 10.7% (3/28) |
| Recurrence | 42.9% (12/28) |
| Neoadjuvant treatment | |
| Chemoradiation | 75% (21/28) |
| Radiation alone | 3.6% (1/28) |
| Adjuvant treatment | |
| Chemoradiation | 7.1% (2/28) |
| Chemotherapy alone | 10.7% (3/28) |
| Prior unrelated perineal radiation | 7.1% (2/28) |
| Additional resections with APR | |
| Vaginectomy | 65% (13/20) |
| BSO | 15% (3/20) |
| Vulvectomy | 5% (1/20) |
| Sacrectomy | 7.1% (2/28) |
| Use of pelvic closed suction drain | |
| No | 50% (14/28) |
| Yes | 50% (14/28) |
| Plastic surgeon's years of practice | |
| < 5 years | 39.3% (11/28) |
| > 5 years | 60.7% (17/28) |
Complications documented by the operating surgeon(s) in the medical record were analyzed (Table 2). Minor complications were defined as not requiring additional surgical intervention, including superficial perineal wound not requiring additional operative intervention, superficial skin infection, and minor parastomal hernia. Major complications were defined as perineal wound or partial flap necrosis requiring an additional operation, pelvic hernia, abdominal wall hernia, pelvic infection/intra-abdominal abscess, complete flap loss, persistent fistula, deep vein thrombosis (DVT)/pulmonary embolus, and death.
Table 2.
Post Operative Complications.
| Occurrence | Intervention | |
|---|---|---|
| Minor | ||
| Superficial perineal wound | 3.6% (1/28) | Negative pressure wound therapy |
| Superficial skin infection* | 3.6% (1/28) | Extended antibiotics, debridement for concurrent flap necrosis |
| Parastomal hernia | 3.6% (1/28) | None |
| Major | ||
| Perineal wound/partial flap necrosis* | 14.3% (4/28) | Debridement and closure |
| Pelvic hernia | 3.6% (1/28) | Repair with mesh |
| Abdominal wall hernia | 0% (0/28) | |
| Pelvic infection/abscess | 0% (0/28) | |
| Complete flap loss | 0% (0/28) | |
| Persistent fistula | 0% (0/28) | |
| DVT/PE | 0% (0/28) |
one of patient had both a concurrent superficial infection with perineal wound/partial flap necrosis.
Descriptive statistics are presented as frequencies from our sample patient population, n = 28. Variables that may affect major perineal wound complication, such as stage of cancer, neoadjuvant chemoradiation, timing during study, surgeon's years in practice, and the use of a closed suction drain were analyzed (Table 3). Chi-square test (Fisher's exact test) was used to examine the relation between these qualitative variables. A p-value < 0.05 was considered significant.
Table 3.
Wound Complication that Required Additional Operative intervention.
| Stage of Cancer | Low Stage (I/II) | High Stage (III/IV) | p-Value |
|---|---|---|---|
| 7.1% (1/14) | 23.1% (3/13) | 0.456 | |
| Neoadjuvant Chemoradiation | Yes | No | p-Value |
| 22.2% (4/18) | 0% (0/10) | 0.107 | |
| Timing During Study | First 14 Cases | Last 14 Cases | p-Value |
| 21.4% (3/14) | 7.1% (1/14) | 0.280 | |
| Surgeon's Years in Practice | <5 Years | >5 Years | p-Value |
| 27.3% (3/11) | 5.9% (1/17) | 0.114 | |
| Closed Suction Drain | No Drain Used | Drain Used | p-Value |
| 28.6% (4/14) | 0% (0/14) | 0.031 |
All plastic surgeons included in the study performed the reconstructive VRAM using a similar technique. The APR portion of the operation was performed by the colorectal surgeon(s) at our institution.
In the preoperative area, a 5–10 cm wide skin paddle was designed in a vertical ellipse over the right rectus abdominis muscle and a handheld doppler was used to mark perforators that enter the skin paddle. A right VRAM was preferentially chosen for the ease of colostomy placement through the left rectus abdominis muscle when possible. If an ostomy was already present or previous scarring made the right side unusable, the flap was designed using the left side.
The initial midline incision was made from just superior to the umbilicus to the pubis, curving around the right side of the patient's umbilicus. Midline dissection was carried down to linea alba, and the peritoneal cavity was opened in the midline. At this point the APR portion of the operation was performed by a colorectal surgery.
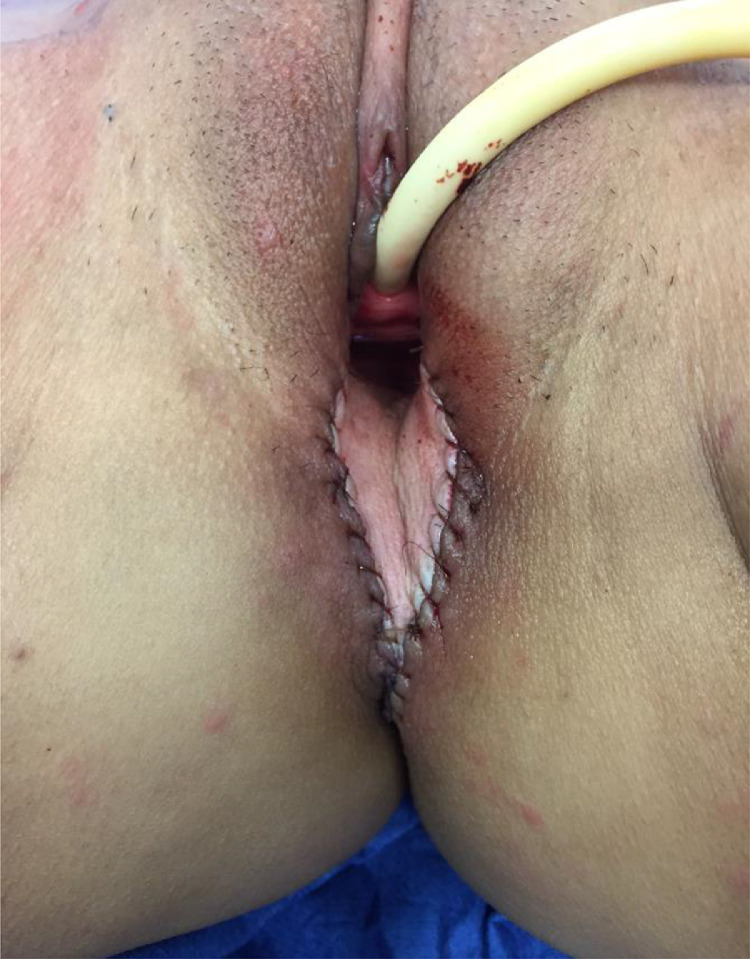
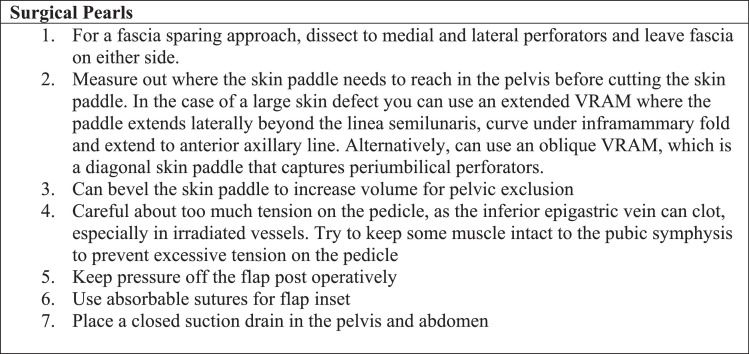
The VRAM skin paddle was then created and the rectus abdominis muscle dissected circumferentially from the rectus sheath. The muscle was then disinserted from the ribs superiorly and the inferior epigastric pedicle was mobilized to prevent kinking or compression. The muscle insertion on the pelvis was usually maintained to prevent tension on the pedicle. However, in several cases the muscle had to be disinserted for the skin paddle to reach the distal aspect of the perineal skin defect. The flap was then rotated medially, so that the most superior aspect of the skin paddle was inserted into the most inferior aspect of the perineal skin defect. The muscle was positioned to obliterate dead space and the pedicle was checked for kinking. Two 15 -French closed bulb suction drains were placed, one deep in the pelvis and the other superficially over the fascia. The skin paddle was inset, which started at the most superior or deep point in the pelvis (Fig. 1). The abdominal donor site was closed after colostomy placement (see summary in Fig. 2).
Fig. 1.
Inset of VRAM flap after APR defect.
Fig. 2.
VRAM flap after APR defect surgical pearls.
Patients remained in bed rest for the first 3–10 days postoperatively on an air mattress, lying on their side and turning every 2 h. Abduction pillows and pillow under sacrum were also often utilized. Mobilization out of bed started by postoperative days 3–5. Patients were advised to sit on a donut for a period of 1–2 h or advised to continue side-to-side lying for additional 2 weeks until cleared by the surgeon. Sitting with direct perineal pressure began once the perineum was healed, usually after 4 to 6 weeks. No heavy lifting/strenuous activity was advised for the first 6 weeks.
Drains were removed once the output was less than 30 mL daily. Antibiotics (cefalexin) were generally continued while drains were in place. Extended duration or change in coverage was implemented if there was concern for soft tissue infection or allergy. Routine patient follow-ups were scheduled at 1–2 weeks, 3–4 weeks then every 3 months for the first year. This included physical exam and wound or flap intervention as needed.
Results
A total of 28 patients had a VRAM flap for an APR defect from July 2008 to June 2018 (Table 1). At the time of surgery, the majority of patients had already undergone neoadjuvant chemoradiation (75%, 21 patients). Of the seven patients who did not undergo neoadjuvant chemoradiation, one had neoadjuvant radiation only, one patient elected to forego neoadjuvant therapy despite it being recommended, one patient had a sarcoma that did not require neoadjuvant therapy, two had a history of past cervical carcinoma for which they had both received radiation > 20 years prior to their new diagnosis and VRAM, and two did not require any neoadjuvant or adjuvant chemoradiation.
Postoperative complications are recorded in Table 2. There were seven patients who had complications for a total complication rate of 25%. Five of the seven complications were considered major complications. Of the five major complications, four were perineal wound/partial flap necrosis that required an additional operation, and one was a pelvic hernia that required repair with mesh. Of the three minor complications, one was a case of superficial perineal wound dehiscence that resolved with negative pressure wound therapy, one was a parastomal hernia that did not require surgical intervention and one was a superficial infection that occurred in a patient with a perineal wound/partial flap necrosis. The overall major perineal wound complication rate was 14.3% (4/28 patients).
Table 3 summarizes our findings for variables that may affect perineal wound healing rates. Not using a pelvic closed suction drain was associated with statistically significant higher rates of major perineal wound complications (28.6% and p-value 0.031), while neoadjuvant chemoradiation trended toward significance. No patients with a pelvic closed suction drain had a major perineal wound complication (0/14 patients).
Discussion
This study represents our ten-year experience with the VRAM flap for APR perineal defects. We demonstrate that good outcomes can be achieved in difficult APR resections, even in the setting of chemotherapy and radiation.
Radiation therapy has been shown to decrease local recurrence and improve survival in the context of anal and rectal cancer; however, it can also be extremely detrimental to wound healing and collapse of dead space.3,5, 6, 7, 8 Radiation-induced free radical damage to the target and surrounding tissues results in the activation of inflammatory pathways, vascular injury, thrombosis, and cell death. Longer term, vessel leak leads to increased collage deposition and stiff fibrotic tissue, while the vascular injury and abnormal inflammatory pathways result in poor vascularity and poor wound healing.10 The affected tissues effectively become noncollapsible, poorly vascularized with poor healing capacity.
Previous studies have compared surgical outcomes for primary closure versus VRAM flap reconstruction in patients undergoing APR with neoadjuvant radiation. These studies have demonstrated a higher rate of perineal wound complications, dehiscence, pelvic abscess, and fluid collections in patients who had primary closure rather than a VRAM flap in the setting of radiation.2,11, 12, 13 The primary advantage of flap reconstruction is to bring in healthy, well-vascularized, and nonirradiated tissue into an irradiated field, to help fill the dead space, and to assist with wound healing and fighting infection.
In our study, 89% of patients had some form of pre or postoperative radiation, and all complications occurred in this patient population. This is consistent with existing literature, as previous studies have shown a high rate of complications in irradiated APR wounds, up to 66% in some cases.10 This includes perineal abscess rates of up to 37%, wound dehiscence/delayed healing rates of up to 31%, and wound infection rates of up to 30%.2,10
Despite the high rate of radiation and/or chemotherapy, our 14% rate of perineal wound complications requiring additional surgical intervention is also consistent with previous studies, which have shown a reoperation rate of 16%−30%.2,11,14 This may be attributable to our low rate of infection, use of pelvic closed suction drains, and/or surgical technique.
There was one superficial infection, which occurred in a patient who had perineal wound/partial flap necrosis requiring surgical intervention. Published rates of infections in APR reconstruction range from 0% to 37%.2,10,11 Our low rate of infections may be due to postoperative antibiotics and pelvic drains. Although there is no consensus on the use of prophylactic antibiotics for closed suction drains,15, 16, 17, 18, 19 it is routine at our institution to continue at least Ancef while in the hospital and transition to Keflex on discharge while these drains are still in. In this study, 89% of patients continued antibiotics beyond 24 h postoperatively. Ancef was not given if the patient was on ceftriaxone or another antibiotic that had gram positive coverage.
We found a statistically significant association between the use of a pelvic closed suction drain and a lower perineal wound complication rate. No patients with a pelvic drain developed a perineal wound complication, while all four major perineal wound complications occurred in patients who did not have a pelvic drain. While several previous studies have evaluated perineal wound healing and the use of a pelvic drain, none have analyzed this in the setting of a VRAM flap for APR reconstruction.
In 1975, Irvin and Goligher published a randomized prospective study analyzing three different methods for perineal wound closure after the excision of the rectum.20 They found that the primary closure of the perineum and use of pelvic drains (whether or not the pelvic peritoneum was closed) was associated with better outcomes than healing by secondary intention. In 1985, Ronald et al. studied perineal wound healing in patients who underwent an APR.21 They closed the levator muscles and perineal tissues but did not approximate the pelvic peritoneum. The small bowel was allowed to fill the pelvic space and drains were placed in the pelvis. The majority had primary closure of the defect, whereas 7 of 57 patients healed by secondary intention. They reported impressive results with nearly all patients healing within two months. The authors hypothesized that their results were “attributable to the elimination of the closed pelvic space.”
Our institution now routinely uses closed suction pelvic drains for all cases of VRAM flap reconstruction of the perineum. The rational is that the pelvic drain assists with the egress of excess pelvic fluid and ultimately helps with eliminating dead space, which is particularly important in irradiated APR defects. Previous studies have shown that drains decrease the rate of seromas and fistulas.22,23
While this study highlights the importance of a pelvic closed suction drain in APR surgery, there are added costs for the drains and additional clinic visits, increased patient discomfort and anxiety, and the drains act as a nidus for bacterial colonization.15,22 What is less clear, however, is whether this bacterial colonization results in an increased rate of infections.15, 16, 17, 18 It is routine at this institution and for many practitioners to prescribe prophylactic antibiotics against gram-positive bacteria while drains are in, but the literature is unclear on this practice.15, 16, 17, 18
While the VRAM flap is the most common flap used at our institution for the reconstruction of perineal defects, several other flaps have been described. These include omental flaps, posterior thigh flaps, gracilis flaps, and gluteus maximus flaps. The advantage of the VRAM flap is that it provides adequate bulk to obliterate pelvic dead as compared to omental and gracilis flaps, has a reliable skin paddle for the perineal skin defect, and readily available with less donor morbidity than the gluteus maximus flap.24, 25, 26, 27, 28, 29, 30, 31, 32
The primary limitation of this study is our small sample size. While not using a pelvic closed suction drain was associated with a statistically significant increase in perineal wound healing complications, neoadjuvant chemoradiation only tended toward statistical significance in this study. Our small sample size could mask the significance of effects of chemoradiation. Existing evidence has shown that the history of radiation should increase wound healing complications in perineal reconstruction.2,10,12 All four patients in our study with major perineal wound healing complications had both a history of radiation and no pelvic closed suction drain. A larger sample size along with randomization could establish statistical significance to and clarify the extent to which radiation versus drain placement contributed to the incidence of wound healing complications in perineal reconstruction with a VRAM flap.
Lastly, there was a wide range in postoperative protocol: timing of bedrest, donut sitting, and ambulation. This varied between surgeons and was ultimately determined by surgical gestalt. A standardized postoperative protocol would have reduced the number of possibly confounding variables.
Conclusion
Our study analyzed the demographics, the disease characteristic, and the outcomes of the patients who underwent the pelvic reconstruction with a VRAM flap after APR at our institution for past 10 years. Our 14.3% rate of perineal wound complications requiring additional surgical intervention is consistent with previous studies that have shown reoperation rates of 16%−30% [1,2,3]. We found that the use of a pelvic closed suction drain was associated with a lower rate of perineal wound complications, while neoadjuvant chemoradiation trended toward having a higher rate of perineal wound complications. Additional, larger multi-institutional or database studies are needed to further clarify the effects of a pelvic closed suction drain and history of radiation in pelvic reconstructions after APR using a VRAM flap.
Declaration of Competing Interest
None.
Funding
None.
References
- 1.Coombs D.M., et al. The vertical rectus abdominis musculocutaneous flap as a versatile and viable option for perineal reconstruction. www.ePlasty.com, Interesting Case, January 16, 2017. [PMC free article] [PubMed]
- 2.Butler C.E., Gundeslioglu A.O., Rodriquez-Bigas M.A. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206(4):694–703. doi: 10.1016/j.jamcollsurg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society . American Cancer Society; Atlanta: 2018. [Cancer Facts & Figures, 2018]https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf Available at: [Google Scholar]
- 4.Benson A.B., 3rd Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018 Jul;16(7):874–901. doi: 10.6004/jnccn.2018.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson R.A., Levine A.M., Bernstein L. Changing patterns of anal canal carcinoma in the United States. J Clin Oncol. 2013 Apr;31(12):1569–1575. doi: 10.1200/JCO.2012.45.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousseau D.L., Jr, Thomas C.R., Jr, Petrelli N.J., Kahlenberg M.S. Squamous cell carcinoma of the anal canal. Surg Oncol. 2005;14:121–132. doi: 10.1016/j.suronc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Bartelink H., Roelofsen F., Eschwege F. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone on the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 8.Pocard M., Tiret E., Nugent K., Dehni N., Parc R. Results of salvage abdominoperineal resection for anal cancer after radiotherapy. Dis Colon Rectum. 1998;41:1488–1493. doi: 10.1007/BF02237294. [DOI] [PubMed] [Google Scholar]
- 9.Sagebiel T.L., Faria S.C., Balachandran A. Pelvic reconstruction with omental and VRAM flaps: anatomy, surgical technique, normal post-operative findings, and complications. Radiographics RSNA. 2011;31(7):2005–2019. doi: 10.1148/rg.317115112. [DOI] [PubMed] [Google Scholar]
- 10.Chessin D.B., Hartley J., Cohen A.M. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: a cohort study. Ann Surg Oncol. 2005;12:104–110. doi: 10.1245/ASO.2005.03.100. [DOI] [PubMed] [Google Scholar]
- 11.Touny A., Haitham O., Maamoon S. Perineal reconstruction using pedicled vertical rectus abdominis myocutaneous flap (VRAM) J Surg Oncol. 2014;110:752–757. doi: 10.1002/jso.23692. [DOI] [PubMed] [Google Scholar]
- 12.Weichman K.E., Matros E., Disa J.J. Reconstruction of peripelvic oncologic defects. Plast Reconstr Surg. Oct 2017;140(4):601–612. doi: 10.1097/PRS.0000000000003703. [DOI] [PubMed] [Google Scholar]
- 13.Spasojevic M., Mariathasan A.B., Goscinski M. Vertical rectus abdominis musculocutaneous flap repair improves perineal wound healing after abdominoperineal resection for irradiated locally advanced rectal cancer. Ann Surg Oncol. 2018 May;25(5):1357–1365. doi: 10.1245/s10434-018-6363-3. [DOI] [PubMed] [Google Scholar]
- 14.Sunsen K.G., Buntzen T.T., Lindegaard J.C. Perineal healing and survival after anal cancer salvage surgery 10 year experience VRAM. Ann Surg Oncol. 2009;16:68–77. doi: 10.1245/s10434-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 15.Reiffel A.J., Pharmer L.A., Weinstein A.L., Spector J.A. A prospective analysis of the association between indwelling surgical drains and surgical site infection in plastic surgery. Ann Plast Surg. 2013 Nov;71(5):561–565. doi: 10.1097/SAP.0b013e31824c905b. [DOI] [PubMed] [Google Scholar]
- 16.Reiffel A.J., Barie P.S., Spector J.A. A multi-disciplinary review of the potential association between closed-suction drains and surgical site infection. Surg Infect (Larchmt) 2013 Jun;14(3):244–269. doi: 10.1089/sur.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadad E., Wiser I., Rosenthal A. Suction drains in esthetic breast implant exchange are associated with surgical site infections: a retrospective cohort study. J Plast, Recon Aesth Surg. 2017 Nov;70(11):1635–1640. doi: 10.1016/j.bjps.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Kaya E., Paksoy E., Ozturk E. Subcutaneous closed suction drainage does not affect surgical site infection rate following elective abdominal operations a prospective randomized clinical trial. Acta Chir Belgica. 2010;110(4):457–462. doi: 10.1080/00015458.2010.11680655. [DOI] [PubMed] [Google Scholar]
- 19.Edwards B.L., Stukenborg G.J., Brenin D.R., Schroen A.T. Use of prophylactic postoperative antibiotics during surgical drain presence following mastectomy. Ann Surg Oncol. 2014 Oct;21(10):3249–3255. doi: 10.1245/s10434-014-3960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irvin T.T., Goligher J.C. A controlled clinical trial of three different methods of perineal wound management following excision of the rectum. Br J Surg. 1975;62:287–291. doi: 10.1002/bjs.1800620409. [DOI] [PubMed] [Google Scholar]
- 21.Tompkins R.G., Warshaw A.L. Improved management of the perineal wound after proctectomy. Ann Surg. 1985 Dec;202(6):760–765. doi: 10.1097/00000658-198512000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao J.W., Raveendran J.A., Maly C. Closed suction drains after subcutaneous mastectomy for gynecomastia do they reduce complications? Aesthetic Plastic Surg. 2017 Dec;41(6):1291–1294. doi: 10.1007/s00266-017-0959-z. [DOI] [PubMed] [Google Scholar]
- 23.Cecka F., Jon B., Lovecek M. The role of drains in pancreatic surgery. Rozhl Chir. 2014;93(9):450–455. [PubMed] [Google Scholar]
- 24.Lefevre J.H., Parc Y., Kerneis S. Abdomino-perineal resection for anal cancer: impact of a vertical rectus abdominis myocutaneous flap on survival, recurrence, morbidity, and wound healing. Ann Surg. 2009;250(5):707–711. doi: 10.1097/SLA.0b013e3181bce334. [DOI] [PubMed] [Google Scholar]
- 25.Mughal Reconstruction of perineal defects. Ann R Coll Surg Engl. 2013;95(8):539–544. doi: 10.1308/003588413X13629960047155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nisar P.J., Scott H.J. Myocutaneous flap reconstruction of the pelvis after abdominoperineal excision. Colorectal Dis. 2009;11:806–816. doi: 10.1111/j.1463-1318.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 27.Peirce C., Martin S. Management of the perineal defect after abdominoperineal excision. Clin Colon Rectal Surg. 2016;29(2):160–167. doi: 10.1055/s-0036-1580627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh Gracilis flap reconstruction of the perineum: an outcome analysis. J Am Coll Surg. 2016 Oct;223(4):602–610. doi: 10.1016/j.jamcollsurg.2016.06.383. [DOI] [PubMed] [Google Scholar]
- 29.Nelson R.A., Butler C.E. Surgical outcomes of VRAM versus thigh flaps for immediate reconstruction of pelvic and perineal cancer resection defects. Plast Reconstr Surg. 2009 Jan;123(1):175–183. doi: 10.1097/PRS.0b013e3181904df7. [DOI] [PubMed] [Google Scholar]
- 30.Chong T.W., Balch G.C., Kehoe S.M.. Reconstruction of large perineal and pelvic wounds using gracilis muscle flaps. Ann Surg Oncol. 2015;22:3738–3744. doi: 10.1245/s10434-015-4435-1. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone M.S. Vertical rectus abdominis myocutaneous versus alternative flaps for perineal repair after abdominperineal excision of the rectum in the era of laparoscopic surgery. Ann Plast Surg. 2017;79(1):101–106. doi: 10.1097/SAP.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 32.Haapamäki M M., Pihlgren V., Lundberg O., Sandzén B., Rutegård J. Physical performance and quality of life after extended abdominoperineal excision of rectum and reconstruction of the pelvic floor with gluteus maximus flap. Dis Colon Rectum. 2011;54(1):101–106. doi: 10.1007/DCR.0b013e3181fce26e. [DOI] [PubMed] [Google Scholar]