Abstract
Purpose
To report the case of a ten-year old girl with torpedo maculopathy with a complete vitelliform lesion and describe associated optical coherence tomography (OCT), OCT angiography (OCTA), multifocal electroretinogram (ERG) and adaptive optics ophthalmoscopy (AOO) imaging of the lesion.
Observations
An asymptomatic ten-year old girl with visual acuity of 20/15 OU was referred for evaluation of possible Best's disease of her left eye. The unilaterality, location, and shape of the lesion was consistent with torpedo maculopathy. OCT and autofluorescence (AF) revealed that the entire lesion was composed of subretinal hyperreflective material that was hyperautofluorescent, consistent with vitelliform material. Within the boundary of the lesion, OCTA showed reduced choriocapillaris density while adjacent to the lesion, the choriocapillaris density was slightly increased. Microperimetry demonstrated normal sensitivity in both eyes, electrooculograms (EOG) were normal and multifocal ERG showed symmetrical mildly supernormal amplitudes. Additionally, AOO demonstrated that nasal to the lesion there were clusters of hyper-reflective areas, and immediately adjacent to the lesion cones were poorly resolved. However, there was a return to more normal photoreceptor architecture outside of the lesion.
Conclusions and Importance
Torpedo maculopathy lesions typically present with outer retinal attenuation and retinal pigmented epithelium (RPE) atrophy. Vitelliform material was recently observed for the first time in association with Torpedo maculopathy in a case report that described small vitelliform material at the periphery of the lesion. We report the second case of torpedo maculopathy associated with a vitelliform lesion and the first description of a torpedo lesion composed fully of presumed vitelliform material. We also describe findings of OCTA, multifocal ERG and AOO imaging in torpedo maculopathy with vitelliform lesion.
Keywords: Adaptive optics ophthalmoscopy, Multifocal electroretinogram, Multimodal imaging, Optical coherence tomography angiography, Torpedo maculopathy, Vitelliform
1. Introduction
First described by Gass in 1992 as a ‘hypopigmented nevus of the RPE in the macula’, torpedo maculopathy consists of an asymptomatic, unilateral, flat, well-circumscribed, hypopigmented macular lesion located along the horizontal raphe.1 The lesion appears as a torpedo or oval shaped defect in the retinal pigmented epithelium (RPE) with tapered edges directed towards the fovea.2 Torpedo maculopathy lesions typically present with outer retinal attenuation and RPE atrophy. Despite its unique characteristic appearance, its etiology is unknown.
Vitelliform material associated with a torpedo lesion has only been described in one prior report,3 and was noted only at the periphery of the lesion. Our report describes the first report of torpedo maculopathy with a complete vitelliform lesion.
Optical coherence tomography angiography (OCTA) and other multimodal imaging modalities (i.e. multifocal electroretinogram [mfERG] and adaptive optics ophthalmoscopy [AOO]) provide insight into retinal and vascular anatomy and function, and have just recently been used to describe torpedo lesions.4 OCTA, mfERG and AOO have been used to investigate choroidal structure5 retinal function,6 and cone anatomy and density6,7 in torpedo lesions. However, we report the first case of AOO, multifocal ERG, and OCTA in a torpedo lesion with vitelliform material. These unique findings may help elucidate a potential mechanism for the etiology of torpedo lesions.
2. Case report
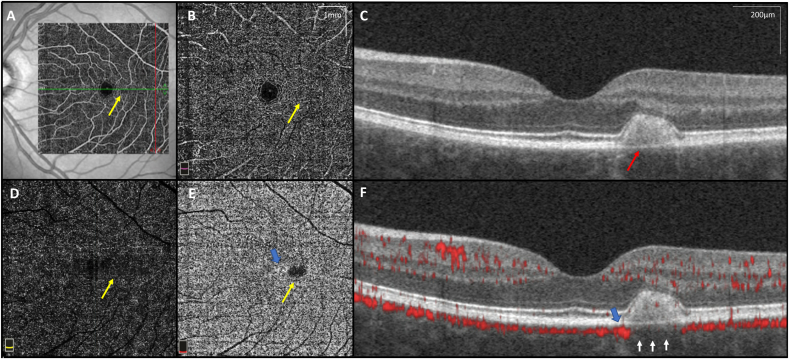
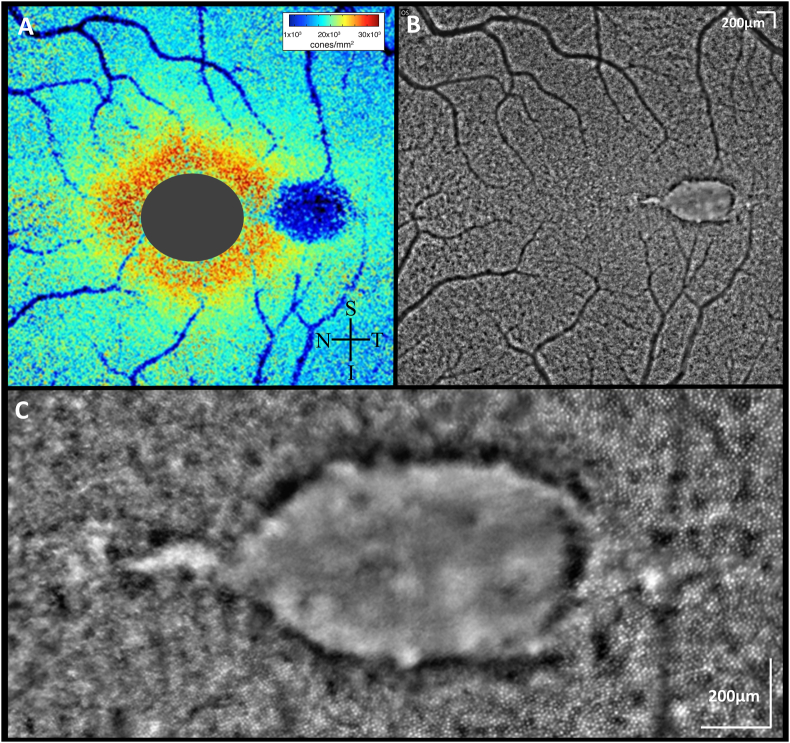
A ten-year old girl was referred for evaluation of possible Best's disease of her left eye. The patient was asymptomatic and Snellen visual acuity measured 20/15 OU. Fundus exam of her right eye was unremarkable. The left eye demonstrated a well-defined, spindle-shaped yellow lesion along the horizontal raphe with a pigmented margin (Fig. 1A). Spectral domain OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) of this lesion showed a large subretinal hyperreflective deposit, consistent with vitelliform material (Fig. 1B). Fundus autofluorescence (FAF) was consistent with this finding, demonstrating strong hyperautofluorescence of the lesion (Fig. 1C). An electo-oculogram (EOG) was normal and microperimetry showed normal sensitivity over the lesion. Multifocal electroretinogram showed symmetric mildly supernormal amplitudes with normal latency. OCTA (RTVue-XR Avanti; Optovue, Inc., Fremont, CA) showed reduced density of the choriocapillaris in the area of the lesion and increased density at the lesion border (Fig. 2). This was quantified using our institution's previously published scan protocol and analysis methods.8,9 Further imaging with AOO, using previously published protocols10 showed that nasal and temporal to the lesion there were clusters of hyper-reflective areas, and immediately adjacent to the lesion cones were poorly resolved. However, there was a return to more normal photoreceptor architecture outside of the lesion (Fig. 3).
Fig. 1.
Fundus photo, Spectral domain optical coherence tomography (SD-OCT), and Fundus autofluorescence. (A) Fundus photo of patient's left eye showing a fusiform hypopigmented lesion in the temporal raphe consistent with torpedo maculopathy. (B) Horizontal SD-OCT of through the lesion showing RPE atrophy, mild attenuation of the outer retina, and a subretinal hyperreflective deposit, consistent with vitelliform material. (C) Fundus autofluorescence of the lesion demonstrating strong hyperautofluorescence.
Fig. 2.
Optical coherence tomography angiography (OCTA). OCTA en face images (6.0 × 6.0mm scan size – yellow scale bar 1 × 1mm) with sectioning through the lesion (yellow arrows) including (A) Superficial and (B) Deep choroidal vessels, (C) OCT B-scan showing vitelliform substance (red arrow), and (D) Outer retina without visible vasculature as expected. (E) En face OCTA image showing choroidal capillaries with adjacent increased capillary density (blue arrow) adjacent to the lesion, and decreased density below the lesion (yellow arrow) (F) OCTA B-scan showing decreased density of choriocapillaris beneath the vitelliform lesion (white arrows) and increased choriocapillaris density adjacent to the torpedo lesion (blue arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Adaptive Optics Ophthalmoscopy (AOO). AOO imaging of the torpedo lesion revealed photoreceptors were not visualized at the same focus level as adjacent to the torpedo lesion. The (A) Voronoi cone density plot thus indicates reduced cone density in the area of the lesion, though cones are most likely axially displaced rather than lost. These images from the rtx1™ flood-illuminated AOO camera from Imagine Eyes (Orsay, France) have been montaged with i2K Retina software (DualAlign, LLC, Clifton Park, NY, USA) using previously published protocols.10 Because Rtx1 is unable to detect individual cones at the fovea where the inter-cone spacing is smaller than the spatial resolution, the cone density map in this study has a gray oval to mask this area; (B) AOO montage showing torpedo lesion with mixture of hypo and hyper-reflective spots; (C) Magnified AOO image showing healthy cones temporal to the lesion with non-discernible cone cells within the lesion.
3. Discussion
The etiology of torpedo maculopathy is unknown and lesions can present as RPE atrophy and attenuation of outer retinal structures with (Type 2) or without (Type 1) an excavated hyporeflective subretinal cleft.11 There is ongoing debate as to whether these findings are an evolution of the same disease process11 versus distinct phenotypic entities.12
Vitelliform material was recently observed for the first time in association with Torpedo maculopathy in a case report that described small vitelliform material at the periphery of the lesion.3 Thomas et al. noted a hyperautofluorescence at the inferior periphery of torpedo lesions, although no vitelliform material was visualized.2
Our case is only the second to report torpedo maculopathy associated with a vitelliform lesion and the first description of a torpedo lesion composed fully of presumed vitelliform material. As vitelliform is rare in torpedo lesions, it is possible that this could be a torpedo-like lesion, distinct from classic torpedo lesions. However, this novel finding of a vitelliform lesion may clarify prior hypotheses regarding the etiology of torpedo lesions. One hypothesis includes a developmental defect in the RPE, which has been supported by embryologic studies.13 Shields et al. noted that this macular bulge of fetal RPE development correlated with the size and location of torpedo maculopathy and speculated that torpedo maculopathy could represent a persistent defect in the development of the RPE in the fetal temporal bulge.13 It is possible that this bulge could be due to overlying vitelliform material that may degrade and regress, eventually leaving behind the typical subretinal cleft, RPE atrophy, and loss of outer retinal layers.
OCTA imaging of our case shows decreased choriocapillaris density in the area of torpedo maculopathy with increased choriocapillaris density adjacent to the lesion. Although possible, the appearance of reduced capillary density could be secondary to signal blockage from the overlying vitelliform material, these vascular findings have also been demonstrated in other cases of torpedo maculopathy without vitelliform lesions5,14 as well as in a clinical-pathological correlation report of a torpedo-like lesion.15 The consistency of these findings, despite the variable clinical presentations, suggests there may be a common pathway between the choriocapillaris abnormalities and the torpedo lesions. The underlying cause of these changes in the choriocapillaris remains unknown but may be due to a localized malformation of choriocapillaris, displacement of the choriocapillaris secondary to bulging RPE, or localized malformation of RPE with subsequent atrophy of the choriocapillaris.
We now report new multimodal imaging findings of vitelliform torpedo maculopathy. Only two previous studies have described multifocal ERG findings in torpedo maculopathy.7,16 Ours is the first report of multifocal ERG findings in a patient with vitelliform torpedo maculopathy, which demonstrated mildly supernormal amplitudes with a mildly prolonged latency, rather than a focal amplitude decrease as previously described.7,16 Additionally, this is only the third report of AOO imaging technology in torpedo maculopathy,6,7 but unique given the vitelliform material. Montaged images using rtx1™ flood-illuminated AOO camera from Imagine Eyes (Orsay, France) and i2k Retina (DualAlign, LLC, Clifton Park, NY, USA), showed loss of the visualization of cone photoreceptors within the lesion. It is unclear whether there was an actual loss of focal photoreceptors, or simply displacement of photoreceptors by the underlying vitelliform lesion. However, sensitivity as measured by microperimetry was normal at a point correlating with the lesion and OCT showed a mostly intact, but displaced ellipsoid zone. Most likely, the loss of cone density as measured by AOO reflects anterior displacement of the cells out of the plane of focus by the subretinal vitelliform resulting in poor visualization, rather than actual loss. This would be consistent with the SD-OCT findings that show vitreal displacement of the outer photoreceptor layers in those areas adjacent to the lesion. Previous reports using adaptive optics scanning laser ophthalmoscopy (AOSLO) of vitelliform lesions have shown similar findings depending on the studied disease.17 Hugo et al. recently described AOO findings in three cases of torpedo maculopathy. They found that cone density was lower and cone spacing was higher in torpedo lesions. As in our case, they also cautioned that interpretation of AOO cone density can be heavily influenced by the focal plane and illumination angle.6
4. Conclusions
This case provides a possible explanation of the etiology behind torpedo lesions. Our findings suggest that torpedo maculopathy could be preceded by a bulge of vitelliform material in the location of the future torpedo lesion. Over time, this material may degrade and regress, eventually leaving behind the typical subretinal cleft, RPE atrophy, and loss of outer retinal layers. It is possible that this process occurs during gestational development, leaving behind only the vitelliform footprints prior to any clinical examination. Additionally, we report multimodal imaging findings (including AOO, OCTA, microperimetry, EOG, and multifocal ERG) in vitelliform torpedo maculopathy. Future studies using these imaging techniques on non-vitelliform torpedo lesions may help to clarify and enforce the significance of these findings.
Patient consent
Written consent from patient's parent was obtained for publication of this report.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
All authors have no financial disclosures.
Acknowledgements
Supported by grant P30 EY010572 from the National Institutes of Health (Bethesda, MD), and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY)
References
- 1.Roseman R.L., Gass J.D. Solitary hypopigmented nevus of the retinal pigment epithelium in the macula. Arch Ophthalmol. 1992;110:1358–1359. doi: 10.1001/archopht.1992.01080220020005. [DOI] [PubMed] [Google Scholar]
- 2.Thomas A.S., Flaxel C.J., Pennesi M.E. Spectral-domain optical coherence tomography and Fundus autofluorescence evaluation of Torpedo maculopathy. J Pediatr Ophthalmol Strabismus. 2015;52 doi: 10.3928/01913913-20150303-01. Online:e8-10. [DOI] [PubMed] [Google Scholar]
- 3.Dolz-Marco R., Saffra N.A., Freund K.B. Torpedo maculopathy presenting with a vitelliform lesion. Retina. 2017;37:e19–e20. doi: 10.1097/IAE.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 4.Grimaldi G., Scupola A., Sammarco M.G., Marullo M., Blasi M.A. Morpho-functional evaluation of torpedo maculopathy with optical coherence tomography angiography and microperimetry. Am J Ophthalmol Case Rep. 2018;10:165–168. doi: 10.1016/j.ajoc.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla R., Pujari A., Rakheja V., Kumar A. Torpedo maculopathy: a primary choroidal capillary abnormality? Indian J Ophthalmol. 2018;66:328–329. doi: 10.4103/ijo.IJO_784_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugo J., Beylerian M., Denion E. Multimodal imaging of torpedo maculopathy including adaptive optics. Eur J Ophthalmol. 2019;30(2):NP27–NP31. doi: 10.1177/112067211982777212. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesh R., Yadav N.K., Sinha S., Mehta R., Akkali M.C. Structural-functional correlation using adaptive optics, visual fields, optical coherence tomography and multifocal electroretinogram in a case of torpedo maculopathy. Indian J Ophthalmol. 2019;67:1502–1505. doi: 10.4103/ijo.IJO_2044_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M., Hwang T.S., Campbell J.P. Projection-resolved optical coherence tomographic angiography. Biomed Optic Express. 2016;7:816–828. doi: 10.1364/BOE.7.000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao S.S., Jia Y., Liu L. Compensation for reflectance variation in vessel density quantification by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:4485–4492. doi: 10.1167/iovs.16-20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S., Gale M.J., Fay J.D. Assessment of different sampling methods for measuring and representing macular cone density using flood-illuminated adaptive optics. Invest Ophthalmol Vis Sci. 2015;56:5751–5763. doi: 10.1167/iovs.15-16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong E.N., Fraser-Bell S., Hunyor A.P., Chen F.K. Novel optical coherence tomography classification of torpedo maculopathy. Clin Exp Ophthalmol. 2015;43:342–348. doi: 10.1111/ceo.12435. [DOI] [PubMed] [Google Scholar]
- 12.Shirley K., O’Neill M., Gamble R., Ramsey A., McLoone E. Torpedo maculopathy: disease spectrum and associated choroidal neovascularisation in a paediatric population. Eye. 2018;32(8):1315–1320. doi: 10.1038/s41433-018-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields C.L., Guzman J.M., Shapiro M.J., Fogel L.E., Shields J.A. Torpedo maculopathy at the site of the fetal "bulge. Arch Ophthalmol. 2010;128:499–501. doi: 10.1001/archophthalmol.2010.29. [DOI] [PubMed] [Google Scholar]
- 14.Ali Z., Shields C.L., Jasani K., Aslam T.M., Balaskas K. Swept-source optical coherence tomography angiography findings in Torpedo maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2017;48:932–935. doi: 10.3928/23258160-20171030-10. [DOI] [PubMed] [Google Scholar]
- 15.Parsons M.A., Rennie I.G., Rundle P.A., Dhingra S., Mudhar H., Singh A.D. Congenital hypertrophy of retinal pigment epithelium: a clinico-pathological case report. Br J Ophthalmol. 2005;89:920–921. doi: 10.1136/bjo.2004.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzzonetti L., Petroni S., Catena G., Iarossi G. Optical coherence tomography and electrophysiological findings in torpedo maculopathy. Doc Ophthalmol. 2015;130:65–70. doi: 10.1007/s10633-014-9472-8. [DOI] [PubMed] [Google Scholar]
- 17.Kay D.B., Land M.E., Cooper R.F. Outer retinal structure in best vitelliform macular dystrophy. JAMA Ophthalmol. 2013;131:1207–1215. doi: 10.1001/jamaophthalmol.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]