Abstract
Purpose
Vitamin D deficiency may cause bone loss and increased inflammation, which are well-known symptoms of periodontal disease. This study investigated whether serum 25-hydroxyvitamin D (25(OH)D) levels are associated with periodontal disease status and tooth loss.
Methods
Cross-sectional data from 5,405 individuals aged ≥50 years (2,253 males and 3,152 females) were obtained from the 2008–2010 Dong-gu study, a prospective cohort study of risk factors for chronic diseases. Periodontal examinations were conducted to evaluate the number of remaining teeth, the periodontal probing depth (PPD), the clinical attachment level (CAL), and bleeding on probing. The percentages of sites with PPD ≥4 mm and CAL ≥4 mm were recorded for each participant. The severity of periodontitis was classified using the Centers for Disease Control and Prevention and the American Academy of Periodontology case definitions. Serum 25(OH)D levels were classified as reflecting severe deficiency, deficiency, insufficiency, or sufficiency. Multivariate linear regression analysis was performed to assess the associations of serum 25(OH)D levels with periodontal parameters and the number of remaining teeth after adjusting for confounders including age, smoking status, alcohol consumption status, month of blood collection, and physical activity. Multivariate logistic regression was used to evaluate the association between serum vitamin D levels and severe periodontitis. An overall statistical analysis and a stratified analysis by sex were performed.
Results
Overall, the rates of severe deficiency, deficiency, insufficiency, and sufficiency were 6.5%, 67.9%, 22.4%, and 3.2%, respectively. After adjustment for confounders, vitamin D levels were directly associated with the number of remaining teeth, an association that was significant in males, but not in females. Sufficient serum 25(OH)D was associated with a low frequency of severe periodontitis.
Conclusions
This population-based cross-sectional study indicates that low serum 25(OH)D is significantly associated with tooth loss and severe periodontitis in Koreans aged 50 years and older.
Keywords: Cross-sectional studies, Periodontitis, Tooth loss, Vitamin D
Graphical Abstract
INTRODUCTION
Oral health is a key component of whole-body health that affects quality of life. Poor oral health reduces quality of life by restricting the diet, changing the facial appearance, and impairing communication [1]. Periodontal disease contributes to the deterioration of oral health in elderly people. The morbidity and severity of periodontal diseases tend to become exacerbated as an individual ages [2]. Periodontal disease is the primary cause of tooth loss in older individuals [3,4,5]. A long-term study on tooth loss in those over 65 years old has revealed that tooth loss continues to occur late in life and that a greater level of attachment loss is definitively associated with tooth loss [6].
Several factors contribute to the development of periodontal diseases, which are induced by bacterial infection and characterized by inflammatory and immune reactions. Vitamin D plays an important role in the maintenance of calcium and bone homeostasis, as well as in immune function. It also has anti-inflammatory properties [7,8]. These facts suggest that vitamin D could be associated with periodontal disease. Many studies have been conducted on the relationships between serum 25-hydroxyvitamin D (25(OH)D) levels, periodontal disease, and tooth loss [9,10,11,12,13,14,15,16,17,18,19]. The results from a study carried out by Dietrich et al. [16] with an overall sample of adults at least 20 years old suggest that, among participants over 50 years old, serum 25(OH)D concentration is significantly associated with attachment loss. In another study by Dietrich et al. [17] with participants aged 13 to 90 years, the ratio of bleeding on probing (BOP) sites of the participants in the highest 25(OH)D quintile was 20% less than that of the participants in the lowest 25(OH)D quintile. Thus, optimal ranges of serum vitamin D may reduce susceptibility to gingival inflammation [17]. In contrast, Krall et al. [18] reported that the usage of calcium and vitamin D supplements was not associated with attachment loss. Since conflicting results have been produced regarding the correlations between vitamin D levels, periodontal disease, and periodontal status, the associations among these variables are still a subject of debate. Limited research has been conducted on the relationships of vitamin D levels with severe periodontitis and tooth loss in Koreans. Thus, the purpose of this study was to scrutinize the effect of serum concentrations of 25(OH)D on periodontal status, severe periodontitis, and tooth loss in community-dwelling adults aged ≥50 years residing in the district of Dong-gu in the metropolitan city of Gwangju, Korea.
MATERIALS AND METHODS
Study population
This study was performed based on data from the Dong-gu study, a prospective study designed to analyze the prevalence, incidence, and predictors of chronic diseases among the population of an urban area in the city of Gwangju, Korea. The Dong-gu study was performed at the Dong-gu Public Health Center between 2007 and 2010. In total, the Dong-gu study included 9,260 participants (3,711 males and 5,549 females), all of whom were at least 50 years old, were eligible residents of the area, and agreed to take part in the study [20]. The present study analyzed 5,621 participants from the Dong-gu study who underwent oral examinations between 2008 and 2010. Participants who lacked an assessment of serum vitamin D levels or who had insufficient records of periodontal estimates and other test results were excluded. The final analysis included 5,405 individuals (2,253 males and 3,152 females; Figure 1). All participants in this study were fully informed and consented to participate. This study was approved by the Institutional Review Board (IRB) of Chonnam National University Hospital (IRB No: I-2008-05-056).
Figure 1. Flowchart of the final study sample.
25(OH)D: 25-hydroxyvitamin D.
Questionnaire and body measurements
Trained interviewers obtained information on sociodemographics (age, sex, and education level), medical history, and lifestyle habits using a standardized questionnaire. Education level was divided into middle school or less and high school or more. Participants were classified as current smokers or non-smokers according to their reported smoking status, and they were classified into current drinkers or non-drinkers according to their reported alcohol consumption. Physical activity during leisure time in the past week was rated on a 5-point scale, and based on this rating, participants were classified into a regular exercise group (reported as “often” or “always”) and a non-exercise group (reported as “never,” “occasionally,” or “sometimes”). Height and weight were measured over light clothing with participants standing with bare feet. Body mass index (BMI) was also assessed and was calculated as weight in kilograms divided by the square of height in meters. The usage of medication to treat hypertension and diabetes was self-reported.
Medical assessment: measurement of serum 25(OH)D concentrations
Blood samples from all participants were obtained from the antecubital vein after 10 hours of fasting overnight. The serum was separated from the blood as soon as it was obtained and was cryopreserved at −70°C until analysis. The concentration of serum 25(OH)D was measured by a chemiluminescent microparticle immunoassay, and the minimum observable concentration of serum 25(OH)D was 3.0 ng/mL (ARCHTECT i2000, Abbott Laboratories, Chicago, IL, USA). As serum 25(OH)D levels vary throughout the year, the time of year of blood collection was also recorded. Serum vitamin D levels were classified as severe deficiency (<10.0 ng/mL), deficiency (10.0–19.9 ng/mL), insufficiency (20.0–29.9 ng/mL), or sufficiency (≥30.0 ng/mL). This classification was based on previous studies [21,22].
Periodontal assessment
Periodontal assessments were conducted by 3 expert examiners. For all participants, half of the oral cavity was randomly selected, and each quadrant of the maxilla and mandible was evaluated. Periodontal assessments included the number of remaining teeth, the periodontal probing depth (PPD), the length from the cemento-enamel junction (CEJ) to the gingival margin, and BOP. PPD was examined using a Williams probe (Hu-Friedy, Chicago, IL, USA) at 6 different sites of all teeth except the third molars, and the measured value was rounded to the nearest millimeter. The clinical attachment level (CAL) was calculated by measuring the difference between the CEJ and the PPD. The following parameters were also recorded: the percentage of sites with PPD ≥4 mm (PPD 4%), the percentage of sites with CAL ≥4 mm (CAL 4%), and the percentage of sites exhibiting BOP (BOP%). The severity of periodontitis was defined based on the Centers for Diseases Control and Prevention and the American Academy of Periodontology case definitions [23]. Severe periodontitis was defined as the presence at least 2 teeth with CAL ≥6 mm and at least 1 tooth with PPD ≥5 mm in interproximal sites. In addition, the total number of teeth including the third molars in the oral cavity was evaluated through clinical examinations and radiography at the time of the periodontal assessment.
Statistical analysis
General characteristics of the participants were presented according to sex and serum 25(OH)D status. These general characteristics were expressed as mean±standard deviation or as percentages. Differences between groups were compared using the t-test and analysis of variance for continuous variables, while the chi-square test was used for categorical variables. Multivariable linear regression analysis was performed to assess the relationships between serum vitamin D level and periodontal parameters including number of remaining teeth, BOP%, PPD 4%, and CAL 4%. Sex, age, blood collection time, BMI, smoking status, alcohol consumption status, physical activity, and the usage of medication for hypertension and diabetes were selected as confounding variables and adjusted for in the analysis. Multivariate logistic regression was performed to evaluate the association between serum vitamin D level and severe periodontitis. Statistical analysis was performed, and stratification analysis according to sex was presented. P values less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
The demographic characteristics of the study participants are shown in Table 1. The average age of the participants was 64.9±8.1 years old, and 58.3% of them were females. On average, the males were older than the females (66.1 years vs. 64.1 years, respectively). The mean serum 25(OH)D level was higher for males than females (19.4 ng/mL vs. 15.3 ng/mL, respectively). Males were more likely than females to smoke cigarettes, drink alcohol, exercise regularly, and use antidiabetic medication. Males had significantly higher levels of PPD 4%, CAL 4%, and severe periodontitis than females, but no significant difference was observed in the number of remaining teeth and BOP%.
Table 1. Demographic characteristics of study participants.
| Characteristics | Total | Male | Female | P value | |
|---|---|---|---|---|---|
| No. | 5,405 | 2,253 | 3,152 | ||
| 25(OH)D (ng/mL) | 17.0±5.8 | 19.4±6.0 | 15.3±5.1 | <0.001 | |
| Age (yr) | 64.9±8.1 | 66.1±7.9 | 64.1±8.1 | <0.001 | |
| Education (middle school or less) | 2,031 (37.6) | 1,224 (54.3) | 807 (25.6) | <0.001 | |
| Body mass index (kg/m2) | 24.3±2.9 | 23.9±2.7 | 24.6±2.9 | <0.001 | |
| Current smoking status | 594 (11.0) | 541 (24.0) | 53 (1.7) | <0.001 | |
| Alcohol consumption status | 2,541 (47.0) | 1,525 (67.7) | 1,016 (32.2) | <0.001 | |
| Regular exercise | 1,915 (35.4) | 923 (41.0) | 992 (31.5) | <0.001 | |
| Antihypertensive medication | 1,937 (35.8) | 799 (35.5) | 1,138 (36.1) | 0.628 | |
| Antidiabetic medication | 702 (13.0) | 338 (15.0) | 364 (11.5) | <0.001 | |
| Month of blood collection | 0.282 | ||||
| April | 1,157 (21.4) | 505 (22.4) | 652 (20.7) | ||
| May | 1,899 (35.1) | 783 (34.8) | 1,116 (35.4) | ||
| June | 1,714 (31.7) | 717 (31.8) | 997 (31.6) | ||
| July | 635 (11.7) | 248 (11.0) | 387 (12.3) | ||
| No. of remaining teeth | 22.2±8.0 | 22.4±7.9 | 22.0±8.2 | 0.167 | |
| PPD 4% | 7.6±11.8 | 9.4±13.7 | 6.2±10.1 | <0.001 | |
| CAL 4% | 22.9±19.5 | 29.2±21.6 | 18.4±16.3 | <0.001 | |
| BOP% | 10.8±14.4 | 10.8±15.1 | 10.8±13.9 | 0.987 | |
| Severe periodontitisa) | 1,289 (25.1) | 772 (36.1) | 517 (17.3) | <0.001 | |
Data are presented as mean±standard deviation or number (%). P values were obtained using analysis of variance for continuous variables and the χ2 test for categorical variables.
25(OH)D: 25-hydroxyvitamin D, PPD 4%: the percentage of sites with probing depth ≥4 mm, CAL 4%: the percentage of sites with clinical attachment loss ≥4 mm, BOP%: the percentage of sites which bled after probing.
a)The severity of periodontitis was defined based on Centers for Disease Control/American Academy of Periodontology criteria. Fifty-one participants were not classified because of insufficient records.
The characteristics of the study participants according to serum 25(OH)D levels are shown in Table 2. Females had a higher frequency of vitamin D deficiency than males. In males, participants with severe deficiency were relatively more likely to be older, to be highly educated, to have a lower BMI, and to smoke cigarettes and relatively less likely to drink alcohol and to exercise regularly. Among females, participants with severe deficiency were relatively more likely to be older, to smoke cigarettes, and use hypertension medication and relatively less likely to be highly educated, to drink alcohol, and to exercise regularly.
Table 2. Characteristics of study participants according to vitamin D status and sex.
| Characteristics | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe deficiency | Deficiency | Insufficiency | Sufficiency | P value | Severe deficiency | Deficiency | Insufficiency | Sufficiency | P value | ||
| No. | 51 (2.3) | 1,280 (56.8) | 799 (35.5) | 123 (5.5) | 299 (9.5) | 2,392 (75.9) | 413 (13.1) | 48 (1.5) | |||
| 25(OH)D (ng/mL) | 8.8±0.8 | 15.8±2.6 | 23.6±2.7 | 34.3±4.7 | <0.001 | 8.5±1.1 | 14.3±2.6 | 23.5±2.7 | 34.6±3.8 | <0.001 | |
| Age (yr) | 68.3±10.0 | 66.5±7.9 | 65.2±7.6 | 66.7±8.5 | 0.002 | 66.1±8.6 | 63.8±8.0 | 64.0±8.0 | 65.1±7.9 | <0.001 | |
| Education (middle school or less) | 18 (35.3) | 577 (45.1) | 362 (45.3) | 72 (58.5) | 0.014 | 244 (81.6) | 1,776 (74.2) | 300 (72.6) | 25 (52.1) | <0.001 | |
| Body mass index (kg/m2) | 22.3±2.9 | 23.9±2.8 | 24.0±2.6 | 23.3±2.4 | <0.001 | 24.4±2.9 | 24.6±3.0 | 24.5±2.7 | 24.0±2.8 | 0.216 | |
| Current smoking status | 21 (41.2) | 328 (25.6) | 169 (21.2) | 23 (18.7) | 0.001 | 13 (4.3) | 35 (1.5) | 5 (1.2) | 0 (0.0) | 0.002 | |
| Alcohol consumption status | 28 (54.9) | 822 (64.2) | 588 (73.6) | 87 (70.7) | <0.001 | 64 (21.4) | 795 (33.2) | 145 (35.1) | 12 (25.0) | <0.001 | |
| Regular exercise | 13 (25.5) | 536 (41.9) | 324 (40.6) | 50 (40.7) | 0.136 | 82 (27.4) | 752 (31.4) | 133 (32.2) | 25 (52.1) | 0.008 | |
| Antihypertensive medication | 15 (29.4) | 464 (36.2) | 279 (34.9) | 41 (33.3) | 0.680 | 122 (40.8) | 868 (36.3) | 128 (31.0) | 20 (41.7) | 0.042 | |
| Antidiabetic medication | 11 (21.6) | 203 (15.9) | 111 (13.9) | 13 (10.6) | 0.163 | 39 (13.0) | 287 (12.0) | 36 (8.7) | 2 (4.2) | 0.074 | |
| Month of blood collection | <0.001 | <0.001 | |||||||||
| April | 24 (47.1) | 348 (27.2) | 116 (14.5) | 17 (13.8) | 131 (43.8) | 459 (19.2) | 52 (12.6) | 10 (20.8) | |||
| May | 15 (29.4) | 486 (38.0) | 257 (32.2) | 25 (20.3) | 120 (40.1) | 862 (36.0) | 122 (29.5) | 12 (25.0) | |||
| June | 11 (21.6) | 343 (38.0) | 298 (37.3) | 65 (52.8) | 37 (12.4) | 776 (32.4) | 164 (39.7) | 20 (41.7) | |||
| July | 1 (2.0) | 103 (8.0) | 128 (16.0) | 16 (13.0) | 11 (3.7) | 295 (12.3) | 75 (18.2) | 6 (12.5) | |||
| No. of remaining teeth | 18.5±9.5 | 21.6±8.5 | 22.9±7.5 | 22.1±8.6 | <0.001 | 21.1±8.8 | 22.4±7.8 | 23.0±7.3 | 22.8±7.3 | 0.022 | |
| PPD 4% | 8.2±16.1 | 9.3±13.5 | 9.8±13.9 | 8.1±13.1 | 0.579 | 5.5±10.7 | 6.3±10.1 | 6.4±9.8 | 6.7±9.5 | 0.645 | |
| CAL 4% | 36.4±25.9 | 29.2±21.9 | 29.1±21.1 | 26.7±19.6 | 0.146 | 18.3±17 | 18.4±16.4 | 18.3±16.0 | 19.5±14.1 | 0.956 | |
| BOP% | 10.1±17.2 | 10.2±14.7 | 11.6±15.4 | 12.5±16.5 | 0.152 | 8.0±11.2 | 11.0±13.9 | 11.8±15.2 | 12.1±15.3 | <0.001 | |
| Severe periodontitisa) | 17 (37.0) | 450 (37.2) | 269 (35.1) | 36 (31.3) | 0.544 | 60 (21.7) | 382 (16.8) | 69 (17.4) | 6 (13.3) | 0.207 | |
Data are presented as mean±standard deviation or number (%).
P values were obtained using analysis of variance for continuous variables and the chi-square test for categorical variables.
25(OH)D: 25-hydroxyvitamin D, PPD 4%: the percentage of sites with probing depth ≥4 mm, CAL 4%: the percentage of sites with clinical attachment loss ≥4 mm, BOP%: the percentage of sites which bled after probing.
a)The severity of periodontitis was defined based on Centers for Disease Control/American Academy of Periodontology criteria. Fifty-one participants were not classified because of insufficient records.
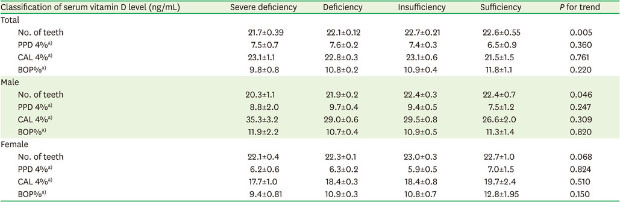
To evaluate the relationship between serum 25(OH)D level and periodontal disease status, multivariable linear regression analysis was performed (Table 3). After adjusting for sex, age, blood collection time, BMI, smoking, alcohol consumption, regular exercise, usage of antihypertensive medication, and usage of antidiabetic medication, an increasing serum 25(OH)D category was associated with an increased number of remaining teeth among all participants. On average, participants in the sufficient group had 0.9 more remaining teeth than those in the severely deficient group. In an analysis stratified according to sex, an increasing serum 25(OH)D category was significantly associated with an increased number of remaining teeth in males, but not in females. Compared to the severely deficient group, the sufficient group had an average of 2.1 additional remaining teeth in males and 0.6 in females. However, the serum 25(OH)D level was not associated with other periodontal parameters such as PPD 4%, CAL 4%, or BOP%. Table 4 shows the association between serum vitamin D levels and severe periodontitis. High vitamin D levels were associated with a low frequency of severe periodontitis. Compared to the group of individuals with vitamin D deficiency, the odds ratios (ORs) for those with severe deficiency, insufficiency, and sufficiency were 1.22 (95% confidence interval [CI], 0.92–1.61), 0.96 (95% CI, 0.82–1.13), and 0.81 (95% CI, 0.55–1.17), respectively. However, in males, the ORs were the highest among the participants with vitamin D deficiency. Compared to the group of participants with vitamin D deficiency, the ORs of those with severe deficiency, insufficiency, and sufficiency were 0.87 (95% CI, 0.46–1.61), 0.90 (95% CI, 0.74–1.10), and 0.77 (95% CI, 0.50–1.16), respectively.
Table 3. Associations of vitamin D levels with number of remaining teeth and periodontal parameters.
| Classification of serum vitamin D level (ng/mL) | Severe deficiency | Deficiency | Insufficiency | Sufficiency | P for trend | |
|---|---|---|---|---|---|---|
| Total | ||||||
| No. of teeth | 21.7±0.39 | 22.1±0.12 | 22.7±0.21 | 22.6±0.55 | 0.005 | |
| PPD 4%a) | 7.5±0.7 | 7.6±0.2 | 7.4±0.3 | 6.5±0.9 | 0.360 | |
| CAL 4%a) | 23.1±1.1 | 22.8±0.3 | 23.1±0.6 | 21.5±1.5 | 0.761 | |
| BOP%a) | 9.8±0.8 | 10.8±0.2 | 10.9±0.4 | 11.8±1.1 | 0.220 | |
| Male | ||||||
| No. of teeth | 20.3±1.1 | 21.9±0.2 | 22.4±0.3 | 22.4±0.7 | 0.046 | |
| PPD 4%a) | 8.8±2.0 | 9.7±0.4 | 9.4±0.5 | 7.5±1.2 | 0.247 | |
| CAL 4%a) | 35.3±3.2 | 29.0±0.6 | 29.5±0.8 | 26.6±2.0 | 0.309 | |
| BOP%a) | 11.9±2.2 | 10.7±0.4 | 10.9±0.5 | 11.3±1.4 | 0.820 | |
| Female | ||||||
| No. of teeth | 22.1±0.4 | 22.3±0.1 | 23.0±0.3 | 22.7±1.0 | 0.068 | |
| PPD 4%a) | 6.2±0.6 | 6.3±0.2 | 5.9±0.5 | 7.0±1.5 | 0.824 | |
| CAL 4%a) | 17.7±1.0 | 18.4±0.3 | 18.4±0.8 | 19.7±2.4 | 0.510 | |
| BOP%a) | 9.4±0.81 | 10.9±0.3 | 10.8±0.7 | 12.8±1.95 | 0.150 | |
Values are presented as adjusted mean±standard error of the mean. Values were estimated via linear regression analysis and adjusted for age, sex, month of blood collection, body mass index, smoking, alcohol consumption, regular exercise, antihypertensive medication, and antidiabetic medication.
PPD 4%: the percentage of sites with probing depth ≥4 mm, CAL 4%: the percentage of sites with clinical attachment loss ≥4 mm, BOP%: the percentage of sites which bled after probing.
a)A total of 228 edentulous participants were excluded from the analysis.
Table 4. Odds ratios and 95% confidence intervals for severe periodontitis.
| Classification of serum vitamin D level (ng/mL) | Severe deficiency | Deficiency | Insufficiency | Sufficiency |
|---|---|---|---|---|
| Total | 1.22 (0.92–1.61) | 1 (reference) | 0.96 (0.82–1.13) | 0.81 (0.55–1.17) |
| Male | 0.87 (0.46–1.61) | 1 (reference) | 0.90 (0.74–1.10) | 0.77 (0.50–1.16) |
| Female | 1.29 (0.93–1.76) | 1 (reference) | 1.04 (0.77–1.37) | 0.77 (0.29–1.71) |
All models were adjusted for age, sex, month of blood collection, body mass index, smoking status, alcohol consumption status, regular exercise, antihypertensive medication, and antidiabetic medication.
In total, 228 edentulous participants and 51 participants without sufficient records were excluded from the analysis.
DISCUSSION
In this study, we assessed the correlation between serum 25(OH)D levels and periodontal status, the prevalence of severe periodontitis, and tooth loss in a large sample of community-dwelling Koreans over 50 years old. We found that after adjusting for confounders, lower serum 25(OH)D levels were associated with a decreased number of remaining teeth (a relationship that was statistically significant in males) and with a high frequency of severe periodontitis.
This study was performed based on data from the Dong-gu study, which investigated the population of a Korean urban area (Dong-gu, Gwangju, Korea). The participants were residents of the regional community, and many individuals enrolled in the study. The examined sites of the periodontium were half of the dental arch and 6 sites on each tooth, which were relatively more specific than the sites investigated in other community periodontal investigations. However, this study had some limitations. One such limitation is that tooth loss is caused both by periodontal diseases and by other factors, such as caries, trauma, and fracture. Therefore, the number of remaining teeth does not solely reflect periodontal conditions. Further inspection of the time and cause of tooth loss would have helped to elucidate any causal relationship between periodontal status and tooth loss. Since this study only included those who agreed to participate in the study, participants’ health and periodontal conditions may show a positive bias compared to the overall population.
Vitamin D consists of a group of fat-soluble prohormones (vitamin D2 and D3), which are obtained from dietary intake and supplements. Ultraviolet B radiation causes 7-dehydrocholesterol to be converted to vitamin D3 in the skin [24]. Vitamin D is well known for its role in calcium homeostasis and bone metabolism. Vitamin D increases the levels of calcium and phosphate in blood by stimulating intestinal absorption, bone resorption, and renal reabsorption [25]. It helps provide optimal conditions for bone mineralization and is important for the development and maintenance of the mineralized skeleton [26]. Vitamin D also activates phagocytosis involving monocytes and enhances monocyte differentiation [27]. Moreover, vitamin D has anti-inflammatory properties, as it inhibits the secretion of proinflammatory cytokines including interferon-gamma, tumor necrosis factor-alpha, and interleukin-12 [17]. Furthermore, a myriad of evidence from both observational and experimental studies has supported the possibility that vitamin D may have non-calcemic effects on the body [28].
Serum 25(OH)D levels were used as an indicator of vitamin D concentration. 25(OH)D is metabolically converted from vitamin D originating from dietary sources or the skin. Although the normal range of serum 25(OH)D concentrations has not yet been consistently defined, a concentration between 20 and 100 ng/mL is often considered normal, and a normal range of 30 to 60 ng/mL is commonly accepted in studies. The definition of vitamin D deficiency is similarly not conclusive and varies from <20 ng/mL to <30 ng/mL among previous studies. According to the classification of serum 25(OH)D level by the American Medical Association, deficiency is defined as a concentration of <30 nmol/L. Hwang et al. [21] examined alterations of parathyroid hormone level and bone mineral density in a study on the optimal serum 25(OH)D level in Korean adults over 49 years old. The results suggested that a serum 25(OH)D level >30 ng/mL is not associated with osteoporosis, while decreased bone density and an increased risk of osteoporosis begin to occur at serum 25(OH)D levels under 20 ng/mL. In Koreans, vitamin D deficiency has been suggested to be indicated by levels below 20 ng/mL. In this study, therefore, the definition of vitamin D deficiency used in the study of Hwang et al. [21] was adopted. In the present study, we defined vitamin D deficiency as a serum 25(OH)D level <20 ng/mL and sufficiency as a serum 25(OH)D level >30 ng/mL. The staging of insufficiency and sufficiency was similarly adopted from previous studies [21,22].
This cross-sectional study showed that 59% of males and 86% of females were in a vitamin D-deficient state according to the definition of deficiency as a serum 25(OH)D level <20 ng/mL. These rates are quite high. As indicated by some previous studies, vitamin D deficiency rates in the American and European elderly have reached 40% to 100%. One study indicated that more than 50% of postmenopausal women taking medication for osteoporosis exhibited below-optimal (<30 ng/mL) 25(OH)D levels [14]. Around the world, 50% of the population has vitamin D deficiency. In particular, this deficiency has been commonly observed in Western Europe, the Middle East, India, China, and Japan. In one study conducted by Lips et al. [29] in 2006, the highest prevalence of vitamin D deficiency—defined as serum 25(OH)D levels lower than 30 ng/mL—was seen in South Korea, followed by Japan and Lebanon. In keeping with these results, the Dong-gu study sample showed a very high frequency of vitamin D deficiency.
Vitamin D can affect periodontal disease in association with bone metabolism [30]. Some studies have reported a correlation between vitamin D levels and periodontal status. Dietrich et al. [17] reported that compared with the ratio of BOP sites among the lowest 25(OH)D quintile of the population, the ratio of BOP sites among the highest 25(OH)D quintile was 8 times greater. Miley et al. [31] assessed the periodontal status of 51 participants receiving periodontal maintenance therapy. Compared with 28 participants who did not take vitamin D and calcium supplements, 23 participants who took supplements for more than 18 months had shallower probing depths, fewer bleeding sites, fewer furcation involvements, and less attachment loss [31]. Millen et al. [32] observed 628 postmenopausal women over 5 years to evaluate the relationship between serum 25(OH)D levels and periodontal disease. The study indicated that changes in alveolar crest height, CAL, PPD, and gingival bleeding were not associated with serum 25(OH)D level [32]. Lee et al. [19] classified serum 25(OH)D levels of ≤20 ng/mL as vitamin D deficiency based on data obtained from 6011 participants of the Korean National Health and Nutrition Examination Survey (2007–2009) and examined whether vitamin D deficiency was associated with a community periodontal index of code 3 or higher. Their results showed that serum vitamin D level was not significantly associated with a community periodontal index of code 3 or higher, but among current smokers, serum vitamin D levels were associated with periodontal status [19]. In this study, no association between serum 25(OH)D levels and CAL or PPD was evident; however, compared to the vitamin D-deficient group, the odds of severe periodontitis among those with vitamin D sufficiency were reduced by 19% (OR, 0.81; 95% CI, 0.55–1.17). In line with this finding, Abreu et al. [12] showed that for every unit (ng/mL) increase in serum 25(OH)D levels, the odds of periodontal disease were significantly reduced by 12% (OR, 0.885; 95% CI, 0.785–0.995) in a pilot study conducted in 38 Puerto Rican adults, and concluded that lower serum vitamin D levels are associated with periodontitis in this population.
In this study, after adjustment for confounding variables, serum 25(OH)D levels were found to be associated with tooth loss. Zhan et al. [9] used the data from 1,904 participants from the Study of Health in Pomerania with a 5-year follow-up period and explored the association between serum 25(OH)D levels and tooth loss, as well as the progression of periodontal diseases. Their results showed that after adjusting for multiple confounders, each 10-µg/L increase in serum 25(OH)D concentration was associated with a 13% decrease in the risk of tooth loss [9]. However, in the OsteoPerio study including postmenopausal women, the data did not show an association between serum 25(OH)D status and tooth loss [11]. Our results showed an association between serum 25(OH)D level and tooth loss, and after stratification by sex, this association was significant in males but not in females. As this study was cross-sectional, it has a limitation in that it cannot be used to elucidate any causal relationship between vitamin D concentration and tooth loss. Hence, further long-term follow-up is needed.
In conclusion, this study indicated that serum vitamin D levels were negatively associated with tooth loss and severe periodontitis in a sample of Korean adults over 50 years old. These results suggested that evaluating serum vitamin D status would be valuable in the screening of periodontitis patients.
Footnotes
Funding: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A5A2027521).
- Conceptualization: Min-Ho Shin, Ok-Su Kim.
- Formal analysis: Min-Ho Shin, Chang-Kyun Choi, Ok-Su Kim.
- Investigation: Hyunju Kim, Min-Ho Shin, Suk-Ja Yoon, Sun-Seog Kweon, Young-Hoon Lee, OkJoon Kim, Young-Joon Kim, HyunJu Chung, Ok-Su Kim.
- Methodology: Min-Ho Shin, Ok-Su Kim, Sun-Seog Kweon, Young-Hoon Lee.
- Project administration: Min-Ho Shin, Sun-Seog Kweon, Young-Hoon Lee.
- Writing - original draft: Hyunju Kim.
- Writing - review & editing: Hyunju Kim, Ok-Su Kim.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kandelman D, Petersen PE, Ueda H. Oral health, general health, and quality of life in older people. Spec Care Dentist. 2008;28:224–236. doi: 10.1111/j.1754-4505.2008.00045.x. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 3.Holm-Pedersen P, Schultz-Larsen K, Christiansen N, Avlund K. Tooth loss and subsequent disability and mortality in old age. J Am Geriatr Soc. 2008;56:429–435. doi: 10.1111/j.1532-5415.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 4.Coll PP, Lindsay A, Meng J, Gopalakrishna A, Raghavendra S, Bysani P, et al. The prevention of infections in older adults: oral health. J Am Geriatr Soc. 2020;68:411–416. doi: 10.1111/jgs.16154. [DOI] [PubMed] [Google Scholar]
- 5.Wu B, Hybels C, Liang J, Landerman L, Plassman B. Social stratification and tooth loss among middle-aged and older Americans from 1988 to 2004. Community Dent Oral Epidemiol. 2014;42:495–502. doi: 10.1111/cdoe.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren JJ, Watkins CA, Cowen HJ, Hand JS, Levy SM, Kuthy RA. Tooth loss in the very old: 13–15-year incidence among elderly Iowans. Community Dent Oral Epidemiol. 2002;30:29–37. doi: 10.1034/j.1600-0528.2002.300105.x. [DOI] [PubMed] [Google Scholar]
- 7.Yao SG, Fine JB. A review of vitamin D as it relates to periodontal disease. Compend Contin Educ Dent. 2012;33:166–171. [PubMed] [Google Scholar]
- 8.Khammissa RAG, Ballyram R, Jadwat Y, Fourie J, Lemmer J, Feller L. Vitamin D deficiency as it relates to oral immunity and chronic periodontitis. Int J Dent. 2018;2018:7315797. doi: 10.1155/2018/7315797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan Y, Samietz S, Holtfreter B, Hannemann A, Meisel P, Nauck M, et al. Prospective study of serum 25-hydroxy vitamin D and tooth loss. J Dent Res. 2014;93:639–644. doi: 10.1177/0022034514534985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimenez M, Giovannucci E, Krall Kaye E, Joshipura KJ, Dietrich T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014;17:844–852. doi: 10.1017/S1368980013000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlesen S, Mai X, Wactawski-Wende J, LaMonte MJ, Hovey KM, Genco RJ, et al. Vitamin D status and tooth loss in postmenopausal females: the Buffalo osteoporosis and periodontal disease (OsteoPerio) study. J Periodontol. 2016;87:852–863. doi: 10.1902/jop.2016.150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abreu OJ, Tatakis DN, Elias-Boneta AR, López Del Valle L, Hernandez R, Pousa MS, et al. Low vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health. 2016;16:89. doi: 10.1186/s12903-016-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ketharanathan V, Torgersen GR, Petrovski BE, Preus HR. Radiographic alveolar bone level and levels of serum 25-OH-vitamin D3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health. 2019;19:83. doi: 10.1186/s12903-019-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuk AM, Quiñonez CR, Saarela O, Demmer RT, Rosella LC. Joint effects of serum vitamin D insufficiency and periodontitis on insulin resistance, pre-diabetes, and type 2 diabetes: results from the National Health and Nutrition Examination Survey (NHANES) 2009–2010. BMJ Open Diabetes Res Care. 2018;6:e000535. doi: 10.1136/bmjdrc-2018-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millen AE, Hovey KM, LaMonte MJ, Swanson M, Andrews CA, Kluczynski MA, et al. Plasma 25-hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. J Periodontol. 2013;84:1243–1256. doi: 10.1902/jop.2012.120445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–580. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 18.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111:452–456. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Je DI, Won SJ, Paik DI, Bae KH. Association between vitamin D deficiency and periodontal status in current smokers. Community Dent Oral Epidemiol. 2015;43:471–478. doi: 10.1111/cdoe.12173. [DOI] [PubMed] [Google Scholar]
- 20.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, et al. Cohort profile: the Namwon study and the Dong-gu study. Int J Epidemiol. 2014;43:558–567. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 21.Hwang YC, Ahn HY, Jeong IK, Ahn KJ, Chung HY. Optimal serum concentration of 25-hydroxyvitamin D for bone health in older Korean adults. Calcif Tissue Int. 2013;92:68–74. doi: 10.1007/s00223-012-9669-3. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 23.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann B, Meurer M. Vitamin D metabolism. Dermatol Ther. 2010;23:2–12. doi: 10.1111/j.1529-8019.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 25.Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci. 2009;51:11–20. doi: 10.2334/josnusd.51.11. [DOI] [PubMed] [Google Scholar]
- 26.Stein SH, Livada R, Tipton DA. Re-evaluating the role of vitamin D in the periodontium. J Periodontal Res. 2014;49:545–553. doi: 10.1111/jre.12149. [DOI] [PubMed] [Google Scholar]
- 27.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Han J, Song Y, Zhang J, Wang Z. Serum levels of 25-hydroxyvitamin D, oral health and chronic obstructive pulmonary disease. J Clin Periodontol. 2012;39:350–356. doi: 10.1111/j.1600-051X.2012.01852.x. [DOI] [PubMed] [Google Scholar]
- 29.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA. Optical serum 25-hydroxyvitamin D levels for multiple health outcomes. In: Reichrath J, editor. Sunlight, vitamin D and skin cancer. New York (NY): Springer; 2008. pp. 55–71. [DOI] [PubMed] [Google Scholar]
- 31.Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, Anderson Spearie CL, et al. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol. 2009;80:1433–1439. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millen AE, Andrews CA, LaMonte MJ, Hovey KM, Swanson M, Genco RJ, et al. Vitamin D status and 5-year changes in periodontal disease measures among postmenopausal women: the Buffalo OsteoPerio study. J Periodontol. 2014;85:1321–1332. doi: 10.1902/jop.2014.130686. [DOI] [PMC free article] [PubMed] [Google Scholar]