Abstract
Purpose
The aims of this study were to evaluate the 5-year cumulative survival rate (CSR) of implants placed with guided bone regeneration (GBR) compared to implants placed in native bone, and to identify factors contributing to implant failure in regenerated bone.
Methods
This retrospective cohort study included 240 patients who had implant placement either with a GBR procedure (regenerated bone group) or with pristine bone (native bone group). Data on demographic features (age, sex, smoking, and medical history), location of the implant, implant-specific features, and grafting procedures and materials were collected. The 5-year CSRs in both groups were estimated using Kaplan-Meier analysis. Risk factors for implant failure were analyzed with a Cox proportional hazards model.
Results
In total, 264 implants in the native bone group and 133 implants in the regenerated bone group were analyzed. The 5-year CSRs were 96.4% in the regenerated bone group and 97.5% in the native bone group, which was not a significant difference. The multivariable analysis confirmed that bone status was not an independent risk factor for implant failure. However, smoking significantly increased the failure rate (hazard ratio, 10.7; P=0.002).
Conclusions
The 5-year CSR of implants placed in regenerated bone using GBR was comparable to that of implants placed in native bone. Smoking significantly increased the risk of implant failure in both groups.
Keywords: Cumulative survival rate, Risk factors, Dental implants, Bone regeneration
Graphical Abstract
INTRODUCTION
To obtain predictable outcomes of implants, a sufficient quantity and quality of hard tissue is required at the recipient site for successful osseointegration [1]. However, advanced resorption, resulting in a lack of alveolar ridge volume, often occurs due to periodontal disease, trauma, pathology, and infection, and insufficient alveolar ridge volume can hinder primary stability and the positioning of implants for optimal prosthetic plans [2]. Among the surgical approaches to overcome localized bone defects, guided bone regeneration (GBR) has been widely utilized in implant practice based on scientific and clinical evidence [3,4,5].
GBR can be performed either simultaneously with implant placement (the 1-stage approach) or prior to the insertion of implants (the 2-stage approach or staged approach) using barrier membranes and different types of graft materials. The morphology of a peri-implant bone defect reflects the healing potential of bone regeneration and acts as an important determinant in the choice of a surgical approach [4,6]. The simultaneous approach can be considered in cases of dehiscence, fenestration, and intra-alveolar defects [7,8]. A resorbable or non-resorbable membrane combined with particulate bone might be considered for a peri-implant defect surrounded by neighboring bone walls to stabilize the augmented volume. If bone deficiency restricts the primary stability of the implant and causes unfavorable hard and soft tissue support, the staged approach can be used to consolidate the augmented bone first, with an appropriate healing time [9,10].
Several studies have evaluated the clinical outcomes of implants with GBR and reported predictable results [11,12,13,14]. A systematic review on the correction of dehiscence and fenestration defects with GBR showed a survival rate of 95.7% (range, 84.7%–100%) irrespective of the type of membrane or graft material [12]. In lateral bone augmentation, the survival rate of implants was also comparable to that of implants placed in native bone [11,14] and in staged GBR, the survival rate was found to be 99%–100% in a follow-up period of up to 5 years, and adequate ridge dimensions were achieved, with success rates from 87% to 95% [11]. Vertical ridge augmentation using GBR has also been reported in a limited number of studies with successful outcomes, although it cannot be considered as a general approach [15].
Despite the high survival and success rates reported for implants placed with GBR, clear conclusions have not yet been reached regarding the long-term stability of the regenerated bone, and a lack of consensus remains regarding the confounding factors that affect the clinical outcomes of implants with GBR [11,16,17]. In light of the possibility that regenerated bone itself may be a potential risk for implant failure, and the need to identify any other factors that might contribute to adverse outcomes in regenerated bone, this study evaluated the 5-year follow-up data of implants placed with GBR using either the 1- or 2-stage approach. Therefore, the purpose of the present study was to compare the cumulative survival rate (CSR) of implants placed in regenerated bone from GBR to that of implants placed in native bone within a 5-year follow-up and to identify risk factors associated with implant failure.
MATERIALS AND METHODS
Study design and inclusion of subjects
The present retrospective cohort study was conducted in accordance with the World Medical Association Helsinki Declaration and with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study protocol was approved by Institutional Review Board (IRB) at the Kyung Hee University Dental Hospital (approval No. KHD IRB-006-2).
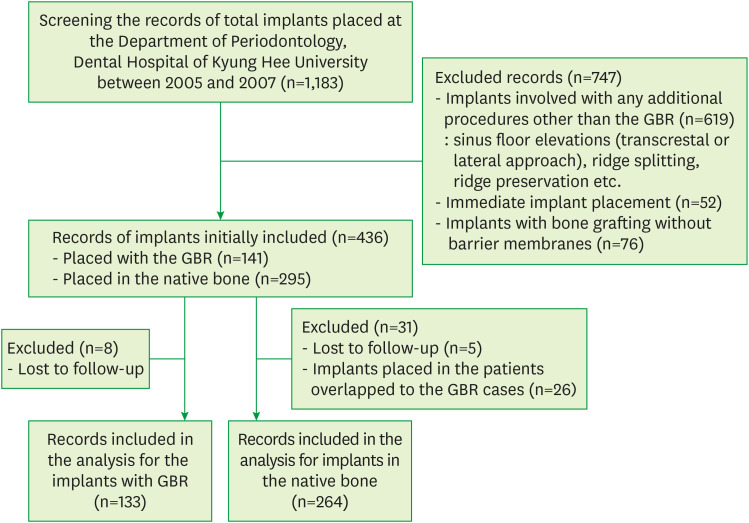
From electronic and paper dental records, all 1,183 that were placed at the Department of Periodontology, Dental Hospital of Kyung Hee University between 2005 and 2007 were screened. Records from this period were chosen to enable both mid-term and long-term follow-up with serial evaluation in further study. Implants that were placed with any additional procedures other than GBR, including sinus floor elevation through the transalveolar or lateral approach, ridge splitting, and ridge preservation, were excluded (619 implants). Implants that were immediately placed (52 implants) were also excluded, as were cases of bone grafting without a barrier membrane (76 implants). The initial abstraction included the records of 436 implants, of which 141 implants were placed with only a GBR procedure (the regenerated bone group) and 295 implants were placed in native bone with no additional surgical procedures (the native bone group). Records of patients who had uncontrolled systemic diseases and any conditions that might affect bone metabolism (e.g., chemotherapy or radiotherapy of the head and neck area, bisphosphonate mediation) or were lost to follow-up after the prosthetic delivery (8 implants in the regenerated bone group and 5 implants in the native bone group) were excluded. Finally, implants placed in patients overlapping with the regenerated bone group (26 implants) were also excluded from the native bone group (Figure 1).
Figure 1.
Flow chart of the retrospective cohort study.
GBR: guided bone regeneration.
Clinical procedures
The regenerated bone group in this study included i) implants that were placed with only a GBR procedure, ii) implants placed in sites that had localized horizontal and/or vertical ridge augmentation, iii) GBR performed with bone graft materials and/or autogenous bone in combination with barrier membranes, and iv) implants placed simultaneously with GBR (1-stage approach) or after a certain period of healing (2-stage approach). The native bone group included implants that were placed in the healed ridge and fully engaged in the natural bone with no need for additional bone grafting.
The 1-stage approach in the regenerated bone group allowed simultaneous implant placement at the recipient site, where primary stability was obtained despite deficient ridge width in Siebert class I defects [18]. The resulting osseous defects, including dehiscence, fenestration, and self-contained spaces, were treated with particulate bone graft materials and/or autogenous bone chips covered with barrier membranes. Seibert class II or class III defects and class I defects [18] showing severe ridge collapse were treated with the 2-stage approach, in which the GBR procedure was performed in advance and the implants were placed after the healing period for bone consolidation. A mean healing period of 7 months (range, 4–9 months) was needed for the 2-stage approach in the present study. The bone graft materials included deproteinized bovine bone minerals (DBBMs; Bio-Oss®, Geistlich Pharma AG, Wolhusen, Switzerland), biphasic calcium phosphate (MBCP®, Biomatlante, Vigneux de Bretagne, France), and irradiated cancellous bone allografts (ICB; Rocky Mountain Tissue Bank, Denver, CO, USA). They were mixed with or without autogenous bone harvested from intraoral donor sites. A resorbable membrane (Bio-Gide, Geistlich Pharma AG) or a nonresorbable titanium-reinforced and expanded polytetrafluoroethylene membrane (ePTFE; Gore-Tex, WL Gore & Associates, Flagstaff, AZ, USA) was used to cover the grafted area.
Four types of implant systems with a sand-blasted, large-grit, and acid-etched (SLA) surface treatment (Implantium™, Dentium, Seoul, Korea; ITI®, Institute Straumann AG, Waldenburg, Switzerland; SPI®, Thommen Medical AG, Waldenburg, Switzerland) or an anodized surface (Replace Select™, Nobel Biocare, Göteborg, Sweden) were installed in the present study.
Prosthetic loading was performed after a mean healing period of 6 months in both groups after implant placement and patients were recalled every 3 months after the final prosthesis for the first year and twice a year thereafter.
Analysis of the CSR
An implant-level analysis of the CSR was conducted using the proportion of implants still present in situ (censored) at the end of the follow-up period [19]. For the patient-level CSR, failure was defined as at least 1 implant being removed from the patient. The incidence of implant loss for any reason (e.g., implant fracture, mobility, or uncontrolled recurrent peri-implantitis) was recorded as implant failure.
Statistical analysis
Data analysis was performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to characterize patients and implants in the regenerated bone group and the native bone group. Differences in the distribution of covariates between groups were analyzed using the Pearson χ2 test (for age, sex, smoking, location of the implant, implant system, and the diameter and length of the implant) or the Fisher exact test (for medical history). The differences in the mean age and follow-up periods after loading between the 2 groups were analyzed using the Wilcoxon rank sum test. The level of statistical significance was set at 5% (P<0.05).
The overall 5-year implant-level and patient-level CSRs in the regenerated bone group and the native bone group, as well as the CSR of the implants according to each covariate (age, sex, smoking, medical history, position, system, surface, and the diameter and length of the implant), were subjected to Kaplan-Meier analysis [20] with the log-rank test. Covariates showing P values <0.05 were included among the confounding factors in the multivariate analysis. A Cox proportional hazard model was used to compare the CSR between the 2 groups after adjusting for confounding factors (age, smoking, location of the implant, implant system, and implant diameter) to identify risk factors associated with implant failure. The results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs) with P values.
RESULTS
Demographic information of patients and implants
In total, 240 patients with 397 implants were analyzed in this retrospective study. The descriptive statistics of these patients are summarized in Table 1. The native bone group included 264 implants placed in 158 patients (92 men and 66 women) with a mean age of 52.2±10.5 years (range, 21–84 years). The regenerated bone group included 133 implants placed in 82 patients (42 men and 40 women) with a mean age of 48.7±10.7 years (range, 19–69 years). There were no significant differences between the 2 groups in the distribution of 10-year age groups, sex, smoking, or medical history. However, the mean age was significantly different (P=0.016) between the 2 groups. A total of 349 (87.9%) implants were placed in nonsmokers and 48 (12.1%) were placed in smokers. The native bone group had 232 (87.9%) implants in nonsmokers and 32 (12.1%) implants in smokers. In the regenerated bone group, there were 117 (88.0%) implants in nonsmokers and 16 (12.0%) implants in smokers. There were no significant differences in the proportion of implants placed in nonsmokers or smokers between the 2 groups.
Table 1. Demographic information of the patients.
| Variables | Groups | P value | ||
|---|---|---|---|---|
| Native bone | Regenerated bone | |||
| Total number of patients | 158 | 82 | ||
| Age (yr) | 52.2±10.5 (21–84) | 48.7±10.7 (19–69) | 0.016a) | |
| ≤30 | 7 (4.4) | 6 (7.3) | 0.185 | |
| 31–60 | 123 (77.8) | 68 (82.9) | ||
| ≥61 | 28 (17.7) | 8 (9.8) | ||
| Sex | 0.338 | |||
| Male | 92 (58.2) | 42 (51.2) | ||
| Female | 66 (41.8) | 40 (48.8) | ||
| Smoking | 0.836 | |||
| Yes | 20 (12.7) | 9 (11.0) | ||
| No | 138 (87.3) | 73 (89.0) | ||
| Medical history | 0.854 | |||
| Diabetes mellitus | 9 (5.7) | 7 (8.5) | ||
| Osteoporosis | 2 (1.3) | 1 (1.2) | ||
| Others | 32 (20.3) | 15 (18.3) | ||
In medical history, the category of “others” included hypertension, cardiovascular disease, a history of tuberculosis, hepatitis B, fatty liver, and hyperthyroidism/hypothyroidism. Values are presented as mean±standard deviation (range) or number (%).
a)Statistically significant difference between the groups using the Wilcoxon rank sum test (P<0.05).
Information on the implants is presented in Table 2. In the native bone group, most implants were located at posterior sites (mandible, 71.2%; maxilla, 24.6%). However, in the regenerated bone group, most implants were placed in the posterior mandible (39.8%), followed by the anterior maxilla (28.6%) and the posterior maxilla (25.6%). In both groups, most implants had SLA surface treatment (66.3% in the native bone group and 64.7% in the regenerated bone group). The location of the implants, implant system, and the diameter of the implants showed significant differences between the 2 groups. The length of the implants placed in both the native bone group and the regenerated bone group ranged from 8 mm to 13 mm, except for one 6-mm implant in the regenerated bone group. In the processing of the data, the implants were subdivided according to the standard length of 10 mm, yielding subgroups with an implant length of ≥10 mm or <10 mm [21,22]. The length of implants showed no significant difference between the native bone group and regenerated bone group. The mean follow-up period after prosthetic loading was 33.78±14.0 months (range, 1–59 months) in the native bone group and 30.63±12.1 months (range, 0–60 months) in the regenerated bone group, which was a significant difference between the 2 groups (P=0.01).
Table 2. Information on dental implants placed in the native and regenerated groups.
| Variables | Group | P value | ||
|---|---|---|---|---|
| Native bone (n=264) | Regenerated bone (n=133) | |||
| Location of implant | 0.000a) | |||
| Anterior maxilla | 5 (1.9) | 38 (28.6) | ||
| Posterior maxilla | 65 (24.6) | 34 (25.6) | ||
| Anterior mandible | 6 (2.3) | 8 (6.0) | ||
| Posterior mandible | 188 (71.2) | 53 (39.8) | ||
| Implant system | 0.000a) | |||
| Implantium™ | 64 (24.2) | 55 (41.4) | ||
| Replace Select™ | 89 (33.7) | 47 (35.3) | ||
| SPI® | 53 (20.1) | 24 (18.0) | ||
| ITI® | 58 (22.0) | 7 (5.3) | ||
| Implant diameter (mm) | 0.000a) | |||
| 3.3–3.5 | 29 (11.0) | 58 (43.6) | ||
| 3.8–4.3 | 209 (79.2) | 73 (54.9) | ||
| ≥4.8 | 26 (9.8) | 2 (1.5) | ||
| Implant length (mm) | 0.325 | |||
| <10 | 49 (18.6) | 19 (14.3) | ||
| ≥10 | 215 (81.4) | 114 (85.7) | ||
Values are presented as number (%).
a)Statistically significant difference between the groups using the Pearson χ2 test (P<0.05).
In the regenerated bone group, most implants (n=109; 82.0%) were placed simultaneously with the GBR procedure (the 1-stage approach), while 24 (18.0%) implants in the regenerated bone group were placed at sites previously augmented with GBR after a consolidation period (the 2-stage approach) (Table 3). In the selection of graft materials, DBBM was mostly utilized in the GBR procedure (75.9%) with a resorbable membrane and the 1-stage approach (66.1%) or with a nonresorbable membrane with the 2-stage approach (91.7%). Membrane exposure was found at 11 sites (10.1%) with the 1-stage approach and at 4 sites (16.7%) with the 2-stage approach.
Table 3. Surgical information of the GBR group.
| Variables | GBR group | ||
|---|---|---|---|
| One-stage (n=109) | Two-stage (n=24) | ||
| Graft materials | |||
| Autogenous bone only | 6 (5.5) | 0 (0) | |
| Autogenous bone and DBBM | 6 (5.5) | 1 (4.2) | |
| Autogenous bone and FDBA | 1 (0.9) | 0 (0) | |
| DBBM only | 85 (78.0) | 9 (37.5) | |
| FDBA only | 1 (0.9) | 2 (8.3) | |
| FDBA and MBCP | 10 (9.2) | 12 (50.0) | |
| Membrane | |||
| Resorbable | 72 (66.1) | 2 (8.3) | |
| Nonresorbable | 37 (33.9) | 22 (91.7) | |
| Membrane exposure | 11 (10.1) | 4 (16.7) | |
| Resorbable | 7 | 0 | |
| Non-resorbable | 4 | 4 | |
Values are presented as number (%).
GBR: guided bone regeneration, DBBM: deproteinized bovine bone mineral, FDBA: mineralized freeze-dried bone allografts, MBCP: micro- and macro-porous biphasic calcium phosphate, One-stage: implant placement simultaneously with GBR, Two-stage: staged approach for implant placement after the GBR site had healed.
CSRs in the native bone group and the regenerated bone group
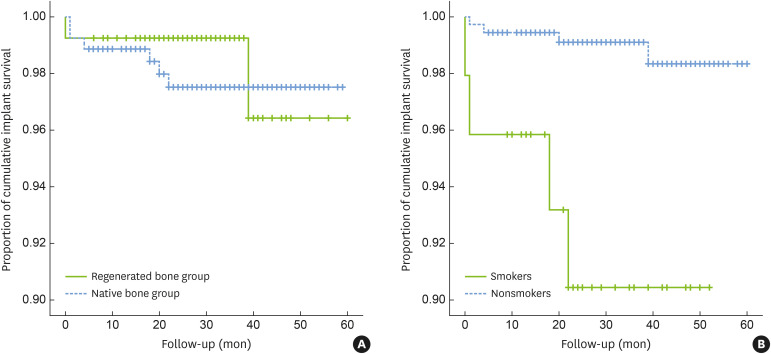
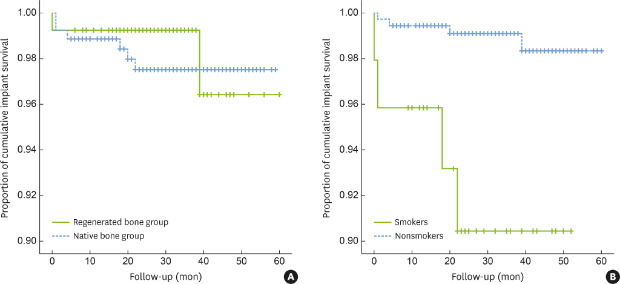
The implant-level 5-year CSR was 97.5% in the native bone group, with a mean follow-up of 33.8±14 months (range, 6–59 months), and 96.4% in the regenerated bone group, with a mean follow-up of 30.6±12 months (range, 6–60 months). This difference was not significant (P=0.66) (Figure 2A). The patient-level 5-year CSR was 96.84% and 97.56% in the native bone group and in the regenerated bone group, respectively, with no significant difference between the groups (P=0.79). There were 6 implant failures in 264 implants in the native bone group and 2 implant failures in 133 implants in the regenerated bone group (Table 4). In the native bone group, 4 implants were removed due to mobility associated with occlusal interference after prosthetic loading, of which 3 implants failed within 4 months and 1 implant failed at 22 months. Two implants were removed due to recurrent peri-implantitis at 18 and 20 months after loading, respectively. In the regenerated bone group, failed implants were found at GBR sites where the 2-stage approach was used. One of the implants failed at the time of abutment connection during prosthetic delivery. Bone dehiscence was detected on the buccal side during explantation. The other failed implant, which was 6 mm in length, showed mobility after 3 years of loading.
Figure 2.
The 5-year CSRs of implants. (A) CSRs showed no significant difference between the regenerated bone group and the native bone group, (B) smokers showed a significantly lower CSR than nonsmokers.
CSR: cumulative survival rate.
Table 4. Descriptions of cases of implant failure.
| Group | Sex/age | Position | Implant system, diameter×length | Time of implant failure | Other information |
|---|---|---|---|---|---|
| Native bone | M/62 | #36 | Replace Select™ | 22 months after loading | Smoking habit, occlusal interference, implant mobility |
| 4.3×10 mm | |||||
| Native bone | F/78 | #46 | SPI® | 20 months after loading | Recurrent peri-implantitis |
| 4.2×9.5 mm | |||||
| Native bone | M/56 | #16 | Replace Select™ | 1 month after loading | Smoking habit, implant mobility |
| 4.3×13 mm | |||||
| Native bone | M/56 | #24 | Replace Select™ | 1 month after loading | Implant mobility |
| 3.5×10 mm | |||||
| Native bone | M/51 | #12 | Replace Select™ | 4 months after loading | Implant mobility |
| 3.5×13 mm | |||||
| Native bone | M/47 | #26 | ITI® | 18 months after loading | Smoking habit, recurrent peri-implantitis |
| 4.1×10 mm | |||||
| Regenerated bone | M/49 | #37 | ITI® | 39 months after loading | Two-stage, GBR using DBBM and non-resorbable membrane |
| 4.1×6 mm | |||||
| Regenerated bone | M/44 | #13 | Replace Select™ | During prosthetic delivery | Smoking habit, 2-stage, GBR using autogenous bone, DBBM and resorbable membrane |
| 3.5×13 mm |
GBR: guided bone regeneration, DBBM: deproteinized bovine bone mineral.
Variables associated with implant failure and risk factor analysis
A univariate analysis of the 5-year CSR of implants according to each study variable was conducted (Table 5). The risk of implant failure was significantly different between smokers and nonsmokers (CSR, 90.4% and 98.3%, respectively; P=0.0004) (Figure 2B). In the multivariate Cox proportional hazards model, confounding factors of biologic relevance (age), status of bone (regenerated or native bone), and variable significantly associated with implant failure (smoking) were selected (Table 6). Significant association between implant failure and smoking was consistently found and implant failure was 10.7 times more likely in smokers than in nonsmokers (HR, 10.653; P=0.002). The risk factor of smoking was then subdivided into the following 4 subgroups: subgroup 1, nonsmokers in the native bone group; subgroup 2, smokers in the native bone group; subgroup 3, nonsmokers in the regenerated bone group; and subgroup 4, smokers in the regenerated bone group. The 5-year CSRs of subgroups 1, 2, 3, and 4 were 98.3%, 88.9%, 99.2%, and 93.8%, respectively. The multivariate Cox proportional hazards model showed that implant failure was 10.9 times more likely in subgroup 2 and 9.1 times more likely in subgroup 4 than in subgroup 1. However, statistical significance was only found for the comparison between subgroup 2 and subgroup 1 (P=0.005) (Table 6).
Table 5. Univariate analysis of the factors associated with implant failure.
| Factors | P value |
|---|---|
| Age | 0.28 |
| Sex | 0.06 |
| Smoking | 0.0004a) |
| Medical history | 0.88 |
| Location of the implant | 0.35 |
| Implant system | 0.21 |
| Implant diameter | 0.46 |
| Implant length | 0.57 |
a)Statistically significant difference in the cumulative survival rate of smokers and nonsmokers using Kaplan-Meier analysis with the log-rank test (P<0.05).
Table 6. Multivariate Cox proportional hazards model for the analysis of potential risk factors associated with implant failure.
| Factors | Hazard ratio | 95% CI | P value | ||
|---|---|---|---|---|---|
| Potential risk factors associated with implant failures | |||||
| Bone status (regenerated bone) | 0.870 | 0.169–4.491 | 0.868 | ||
| Age | 1.061 | 0.981–1.146 | 0.138 | ||
| Smoking | 10.653 | 2.472–45.913 | 0.002a) | ||
| Risk factor of smoking associated with implant failure | |||||
| Comparison with subgroup 1 | |||||
| Subgroup 2 | 10.872 | 2.039–57.962 | 0.005b) | ||
| Subgroup 3 | 0.906 | 0.091–8.990 | 0.933 | ||
| Subgroup 4 | 9.094 | 0.827–100.054 | 0.071 | ||
CI: confidence interval, subgroup 1: nonsmokers in the native bone group, subgroup 2: smokers in the native bone group, subgroup 3: nonsmokers in the regenerated bone group, subgroup 4: smokers in the regenerated bone group.
a)In comparison with nonsmokers, smokers had a 10.7 times higher risk for implant failure, with a statistically significant difference (P<0.05); b)In comparison with subgroup 1, subgroup 2 had a 10.9 times higher risk for implant failure, with a statistically significant difference (P<0.05).
DISCUSSION
Implant placement in an atrophic ridge has been successfully carried out using GBR, with predictable survival rates of dental implants. However, interest has recently emerged in the long-term stability of GBR and factors affecting the clinical outcomes, rather than simply demonstrating implant survival. In the present retrospective study, the 5-year CSR of implants placed in regenerated bone using GBR was compared to that of implants placed in native bone, and risk factors associated with implant failure were evaluated.
The 5-year CSR of the regenerated bone group was 96.4%, which was not significantly different from that of the native bone group (97.5%). This finding is in accordance with previous reports demonstrating a high survival rate of implants in regenerated bone; however, only a few studies have included a direct comparison with a control group [11,23,24]. In a systematic review, the CSR of implants placed in regenerated bone using barrier membrane ranged from 79% to 100% after 5 years of function, and it was similar to that of implants placed in non-regenerated bone based on second to third levels of evidence [25]. The multivariable analysis with a Cox proportional hazards model in the present study also demonstrated that the status of bone (native or regenerated bone) was not an independent risk factor for implant failure. Although the different sample size between the 2 groups is a limitation of this study, our findings support the idea that implant survival in regenerated bone and native bone is comparable.
Despite the high success rates of GBR (over 90% in most studies), some studies have reported success rates ranging from 61.5% to 100% with study designs involving different types of defect morphology, follow-up periods, and surgical procedures [13]. A multicenter retrospective study was conducted on the survival of implants over the course of 11 years, and the risk factors for failure were demonstrated by a logit model [26]. Based on those results, bone quality, bone augmentation, and GBR were suggested to be risk factors despite the high overall survival rate of the implants. However, since descriptive information about the failed implants was not provided, it might be difficult to distinguish the actual factors with significant influences given the possibility of overlapping confounding factors in a single failed implant. Findings of a lower survival rate of implants in grafted bone and a significant association between implant failure and the presence of a bone graft were sometimes based on heterogeneous data that included other types of grafting procedures, such as sinus augmentation and autogenous block graft, or confined types of implant systems [27]. Therefore, it is still necessary to collect extensive data under refined criteria considering potential confounding factors.
Potential risk factors for implant failure include demographic, health status, anatomic, implant- and prosthodontic-specific, and ancillary procedure-related variables [28,29]. Age, smoking, fixture length, implant location, and/or bone quality were suggested to be associated with early failure, generally caused by the loss of osseointegration [29,30,31,32]. Factors contributing to late failure after prosthetic loading included dental plaque and occlusal overload [33]. In the present study, smoking significantly increased the failure rate of implants in both the native bone group and the regenerated group by 10.7-fold (P=0.0004). When the 2 groups were subdivided according to smoking or nonsmoking, smokers in both the native bone group and the regenerated bone group showed a higher risk of implant failure (with HRs of 10.9 and 9.1, respectively) than nonsmokers in the native bone group. Although a statistically significant difference was only found between smokers and nonsmokers in the native bone group (P=0.005), due to the relatively small sample size of smokers in the regenerated bone group, our result confirmed the deleterious effect of smoking on the survival of dental implants, irrespective of bone status. This finding is in accordance with results of previous studies documenting that the use of tobacco is an important risk factor with regard to the loss of dental implants, especially for early healing in osseointegration before functional loading [30,34,35]. However, further data on the severity of smoking habits and its effects on the failure of implants in regenerated bone should be evaluated.
Previous studies have reported that implants placed in the maxilla experienced more failures than those in the mandible, with the posterior maxilla showing the highest risk [29,36]. Despite the contradictory findings [37], the reasons were often described as the poor bone density of the posterior teeth and the higher occlusal force exerted on them. In this study, the location of the implant was not a significant risk factor for implant failure, although implants placed in the maxillary region showed slightly lower CSRs (95.4% for the anterior area and 96.8% for the posterior area) than those placed in the mandible (100% for the anterior area and 98% for the posterior area). However, it should be noted that the distribution of implant locations was significantly different between the groups, as most implants in the native group were placed in the posterior mandible (71.2%) and the sample size of the anterior region in the native group was very limited, as this area generally experiences advanced collapse of buccal bone.
The surgical procedure of GBR was done using either the 1-stage (109 implants) or 2-stage (24 implants) approach according to the severity of ridge atrophy. The proportion of the sample size was slanted towards the 1-stage approach. Although implant failure in the regenerated bone group occurred only in the 2-stage approach, resulting in a significantly lower 5-year CSR (91.7%) than was found for the 1-stage approach (100.0%, P=0.01), this outcome was based on biased data and should be interpreted with caution. In this study, autogenous bone and various graft materials were used to maintain augmented space beneath the barrier membrane. Although autogenous bone is superior in terms of its biologic properties, a systematic review has reported that implant survival seems to be independent of the biomaterial used for augmentation [38].
Membrane exposure is known to be a major postoperative complication in GBR, and the present study had 15 cases (11.3%) out of 133. It is somewhat controversial whether membrane exposure has a significant effect on the outcome of GBR. Recent systematic reviews have suggested that non-exposed sites have greater resolution of the vertical dimension of the defect, with a weighted mean difference of 1.01 mm in 1-stage GBR, and a greater gain of width of 3.1 mm in 2-stage GBR [5]. In addition, the weighted mean change in alveolar bone height has been reported to be 6-fold higher at non-exposed sites than at exposed sites, which was a significant difference [39]. Despite its negative effect on bone formation, membrane exposure was not associated with implant failure in the present study and interestingly, no cases of implant failure occurred in smokers due to an exposed membrane.
The present study has some limitations inherent to its retrospective design, and also has the possibility of a biased interpretation in the statistics for confounding factors. To determine the stability of regenerated bone, further studies should also analyze defect morphology and dimensions at baseline, radiographic findings of peri-implant bone loss, and precise success criteria during the long-term follow-up of implants. In addition, future research should evaluate patient-reported outcomes in GBR and assess complications (both biological and technical in nature) that might affect the peri-implant hard and soft tissues over the course of long-term observations. Within the limitations of this study, it could be concluded that the survival of implants placed with GBR was comparable to that of implants placed in native bone in a 5-year follow-up and that smoking was a significant risk factor for implant failure in both groups.
Footnotes
Funding: This work was supported by a grant from Kyung Hee University in 2017 (KHU-20170845).
- Conceptualization: Ji-Youn Hong, Eun-Young Shin, Seung-Il Shin.
- Formal analysis: Ji-Youn Hong, Eun-Young Shin, Yeek Herr, Jong-Hyuk Chung, Hyun-Chang Lim, Seung-Il Shin.
- Investigation: Ji-Youn Hong, Eun-Young Shin, Hyun-Chang Lim.
- Methodology: Ji-Youn Hong, Eun-Young Shin, Yeek Herr, Jong-Hyuk Chung, Hyun-Chang Lim, Seung-Il Shin.
- Project administration: Yeek Herr, Jong-Hyuk Chung, Seung-Il Shin.
- Writing - original draft: Ji-Youn Hong, Eun-Young Shin, Hyun-Chang Lim.
- Writing - review & editing: Ji-Youn Hong, Eun-Young Shin, Yeek Herr, Jong-Hyuk Chung, Hyun-Chang Lim, Seung-Il Shin.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22 Suppl:49–70. [PubMed] [Google Scholar]
- 2.Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17 Suppl 2:136–159. doi: 10.1111/j.1600-0501.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 3.Nyman S, Lang NP, Buser D, Bragger U. Bone regeneration adjacent to titanium dental implants using guided tissue regeneration: a report of two cases. Int J Oral Maxillofac Implants. 1990;5:9–14. [PubMed] [Google Scholar]
- 4.Benic GI, Hämmerle CH. Horizontal bone augmentation by means of guided bone regeneration. Periodontol 2000. 2014;66:13–40. doi: 10.1111/prd.12039. [DOI] [PubMed] [Google Scholar]
- 5.Sanz-Sánchez I, Ortiz-Vigón A, Sanz-Martín I, Figuero E, Sanz M. Effectiveness of lateral bone augmentation on the alveolar crest dimension: a systematic review and meta-analysis. J Dent Res. 2015;94:128S–142S. doi: 10.1177/0022034515594780. [DOI] [PubMed] [Google Scholar]
- 6.Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994;9:13–29. [PubMed] [Google Scholar]
- 7.Gher ME, Quintero G, Assad D, Monaco E, Richardson AC. Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol. 1994;65:881–891. doi: 10.1902/jop.1994.65.9.881. [DOI] [PubMed] [Google Scholar]
- 8.Dahlin C, Lekholm U, Becker W, Becker B, Higuchi K, Callens A, et al. Treatment of fenestration and dehiscence bone defects around oral implants using the guided tissue regeneration technique: a prospective multicenter study. Int J Oral Maxillofac Implants. 1995;10:312–318. [PubMed] [Google Scholar]
- 9.Buser D, Dula K, Belser UC, Hirt HP, Berthold H. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int J Periodontics Restorative Dent. 1995;15:10–29. [PubMed] [Google Scholar]
- 10.Simion M, Trisi P, Piattelli A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int J Periodontics Restorative Dent. 1994;14:496–511. [PubMed] [Google Scholar]
- 11.Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy) J Clin Periodontol. 2008;35:173–202. doi: 10.1111/j.1600-051X.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009;20 Suppl 4:113–123. doi: 10.1111/j.1600-0501.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- 13.Clementini M, Morlupi A, Canullo L, Agrestini C, Barlattani A. Success rate of dental implants inserted in horizontal and vertical guided bone regenerated areas: a systematic review. Int J Oral Maxillofac Surg. 2012;41:847–852. doi: 10.1016/j.ijom.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Milinkovic I, Cordaro L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int J Oral Maxillofac Surg. 2014;43:606–625. doi: 10.1016/j.ijom.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 2008;35:203–215. doi: 10.1111/j.1600-051X.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 16.Klinge B, Flemmig TF Working Group 3. Tissue augmentation and esthetics (Working Group 3) Clin Oral Implants Res. 2009;20 Suppl 4:166–170. doi: 10.1111/j.1600-0501.2009.01774.x. [DOI] [PubMed] [Google Scholar]
- 17.Beretta M, Cicciù M, Poli PP, Rancitelli D, Bassi G, Grossi GB, et al. A restrospective evaluation of 192 implants placed in augmented bone: long-term follow-up study. J Oral Implantol. 2015;41:669–674. doi: 10.1563/aaid-joi-D-14-00123. [DOI] [PubMed] [Google Scholar]
- 18.Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend Contin Educ Dent. 1983;4:437–453. [PubMed] [Google Scholar]
- 19.van Steenberghe D, Quirynen M, Naert I. In: Lang NP, Karring T, Lindhe J, editors. Survival and success rates with oral endosseous implants; Proceedings of the 3rd European Workshop on Periodontology: Implant Dentistry; Berlin; Quintessence. 1999. pp. 242–254. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Telleman G, Raghoebar GM, Vissink A, den Hartog L, Huddleston Slater JJ, Meijer HJ. A systematic review of the prognosis of short (<10 mm) dental implants placed in the partially edentulous patient. J Clin Periodontol. 2011;38:667–676. doi: 10.1111/j.1600-051X.2011.01736.x. [DOI] [PubMed] [Google Scholar]
- 22.Mezzomo LA, Miller R, Triches D, Alonso F, Shinkai RS. Meta-analysis of single crowns supported by short (<10 mm) implants in the posterior region. J Clin Periodontol. 2014;41:191–213. doi: 10.1111/jcpe.12180. [DOI] [PubMed] [Google Scholar]
- 23.Zitzmann NU, Schärer P, Marinello CP. Long-term results of implants treated with guided bone regeneration: a 5-year prospective study. Int J Oral Maxillofac Implants. 2001;16:355–366. [PubMed] [Google Scholar]
- 24.Benić GI, Jung RE, Siegenthaler DW, Hämmerle CH. Clinical and radiographic comparison of implants in regenerated or native bone: 5-year results. Clin Oral Implants Res. 2009;20:507–513. doi: 10.1111/j.1600-0501.2008.01583.x. [DOI] [PubMed] [Google Scholar]
- 25.Hämmerle CH, Jung RE, Feloutzis A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol. 2002;29 Suppl 3:226–231. doi: 10.1034/j.1600-051x.29.s3.14.x. [DOI] [PubMed] [Google Scholar]
- 26.Mayta-Tovalino F, Mendoza-Martiarena Y, Romero-Tapia P, Álvarez-Paucar M, Gálvez-Calla L, Calderón-Sánchez J, et al. An 11-year retrospective research study of the predictive factors of peri-implantitis and implant failure: analytic-multicentric study of 1279 implants in Peru. Int J Dent. 2019;2019:3527872. doi: 10.1155/2019/3527872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sesma N, Pannuti C, Cardaropoli G. Retrospective clinical study of 988 dual acid-etched implants placed in grafted and native bone for single-tooth replacement. Int J Oral Maxillofac Implants. 2012;27:1243–1248. [PubMed] [Google Scholar]
- 28.Chuang SK, Wei LJ, Douglass CW, Dodson TB. Risk factors for dental implant failure: a strategy for the analysis of clustered failure-time observations. J Dent Res. 2002;81:572–577. doi: 10.1177/154405910208100814. [DOI] [PubMed] [Google Scholar]
- 29.Noda K, Arakawa H, Kimura-Ono A, Yamazaki S, Hara ES, Sonoyama W, et al. A longitudinal retrospective study of the analysis of the risk factors of implant failure by the application of generalized estimating equations. J Prosthodont Res. 2015;59:178–184. doi: 10.1016/j.jpor.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Pérez A, Moya-Villaescusa MJ, Caffesse RG. Tobacco as a risk factor for survival of dental implants. J Periodontol. 2007;78:351–359. doi: 10.1902/jop.2007.060299. [DOI] [PubMed] [Google Scholar]
- 31.Mesa F, Muñoz R, Noguerol B, de Dios Luna J, Galindo P, O’Valle F. Multivariate study of factors influencing primary dental implant stability. Clin Oral Implants Res. 2008;19:196–200. doi: 10.1111/j.1600-0501.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- 32.Kang DY, Kim M, Lee SJ, Cho IW, Shin HS, Caballé-Serrano J, et al. Early implant failure: a retrospective analysis of contributing factors. J Periodontal Implant Sci. 2019;49:287–298. doi: 10.5051/jpis.2019.49.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung DM, Oh TJ, Lee J, Misch CE, Wang HL. Factors affecting late implant bone loss: a retrospective analysis. Int J Oral Maxillofac Implants. 2007;22:117–126. [PubMed] [Google Scholar]
- 34.Strietzel FP, Reichart PA, Kale A, Kulkarni M, Wegner B, Küchler I. Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol. 2007;34:523–544. doi: 10.1111/j.1600-051X.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 35.Tran DT, Gay IC, Diaz-Rodriguez J, Parthasarathy K, Weltman R, Friedman L. Survival of dental Implants placed in grafted and nongrafted bone: a retrospective study in a university setting. Int J Oral Maxillofac Implants. 2016;31:310–317. doi: 10.11607/jomi.4681. [DOI] [PubMed] [Google Scholar]
- 36.Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- 37.Zinser MJ, Randelzhofer P, Kuiper L, Zöller JE, De Lange GL. The predictors of implant failure after maxillary sinus floor augmentation and reconstruction: a retrospective study of 1045 consecutive implants. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:571–582. doi: 10.1016/j.oooo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Al-Nawas B, Schiegnitz E. Augmentation procedures using bone substitute materials or autogenous bone - a systematic review and meta-analysis. Eur J Oral Implantology. 2014;7 Suppl 2:S219–S234. [PubMed] [Google Scholar]
- 39.Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72:512–516. doi: 10.1902/jop.2001.72.4.512. [DOI] [PubMed] [Google Scholar]