Highlights
-
•
Tangerine, pomegranate and banana processing system.
-
•
Physicochemical and microbiological characteristics of tangerine, pomegranate and banana peels.
-
•
Different uses of tangerine, pomegranate and banana peels.
-
•
Tangerine, pomegranate and banana peels valorisation methods.
Keywords: Banana peel, Environment, Pomegranate peel, Tangerine peel, Valorisation
Abstract
Over the last decade the world has been generating a high quantity of tangerine peel waste (TPW), pomegranate peel waste (PPW) and banana peel waste (BPW). These peels have several economic benefits but there is mismanagement or inappropriate valorisation that could present risks to environment and public health. In the current review, we discussed the use of TPW, PPW and BPW directly for animal feed, soil fertilization, specific compost production and bio-adsorbent. We also discussed the valorisation of these peels for manufacturing the value-added products including enzymes, essential oil and other products that can be used in human food, in medical and cosmetic industry. Additionally, recent studies concerning the valorisation of these peels by biorefinery for bioethanol, biogas and biohydrogen production have been discussed. In the same context some other recent studies about valorisation of microorganisms isolated from these peels for medical, agronomic and industrial interests have been also discussed.
1. Introduction
Pomegranate (Punica granatum) is a fruit from the Punicaceae family, it is derived from the Latin name of the fruit Malum granatum, meaning "granular apple" [1]. The pomegranate is originally from India and Iran, its culture is extending to the whole of the Mediterranean and South-Western American regions [[2], [3], [4]]. The tangerine is considered as the second most important citrus fruit worldwide, its specific species is Citrus reticulata and Rutaceae family [5]. The tangerine is originally from Southeast Asia, and its movement in different areas of the world occurred with the displacement of civilizations and traders [6]. The banana is a tropical fruit harvested throughout the year, its specific species is Musa sapientum, and the Musaceae family, it is native to the Southeast Asian countries [7,8]. All of these fruits are consumed as fresh fruit or as a processed product (juice, jam, wine and vinegar, etc.). Thanks to their physicochemical characteristics, high nutritional content and medicinal benefits, tangerine, pomegranate and banana are three of the most widely consumed fruits in the world. In 2017, the total world production of pomegranate is estimated at 3.8 million metric tons [9]. In 2019, the total world production of tangerine is around 31.7 million metric tons [10]. In 2018, the world production of banana is about 115.74 million metric tons [11]. Besides the very important quantities have produced each year, these fruits generate large amounts of peels.
To minimize management costs and to prevent environmental and human health risks, several uses of residues from processing of tangerine, pomegranate and banana were evaluated. During the last decade, more than 1000 scientific articles have been published, almost 90 % of them have focused on the various TPW, PPW and BPW valorisation pathways worldwide. Every TPW, PPW and BPW valorisation pathway depends on a high number of factors affecting economic viability [[12], [13], [14]]. TPW, PPW and BPW may be used directly or after treatments as animal feed [15,16], soil amendment [[17], [18], [19]], bio-adsorbents [20,21], and the production of the outputs was obtained by the biorefinery process (biogas, bioethanol and biohydrogen) [[22], [23], [24], [25], [26]]. Numerous other studies have focused on the valorisation of TPW, PPW and BPW for the production of value-added products (essential oil, enzymes, food, medical and cosmetic products) [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]].
Environmental constraints must be adequately taken into account with regard to air, soil and water preservation. Thus, the optimal solution must combine the most efficient measures and sustainable valorisation technologies especially in terms of environmental and human health. Over the years, scientists have been focused on the development of various valorisation methods for the full exploitation of TPW, PPW and BPW. However, extensive research is necessary to change traditional perceptions that consider the fruit peels generally and tangerine, pomegranate and banana peels particularly as waste for disposal in public landfills, than as a valuable bioresource reusable in the bio-economy, due to their physicochemical characteristics and their multiple applications in various fields.
The current review provides a global overview of world production, physicochemical and microbiological characteristics of TPW, PPW and BPW and also the various pathways for their valorisation, considering the advantages, disadvantages and factors impacting on each way of valorisation. The purpose is to inform farmers, new scientists, managers of fruit processing industry and the audience about the most suitable solutions for the valorisation of TPW, PPW and BPW. Firstly, to reduce their negative effects in terms of the environment and human health, and secondly, to increase their economic benefits.
2. World production of tangerine, pomegranate and banana fruits and peels
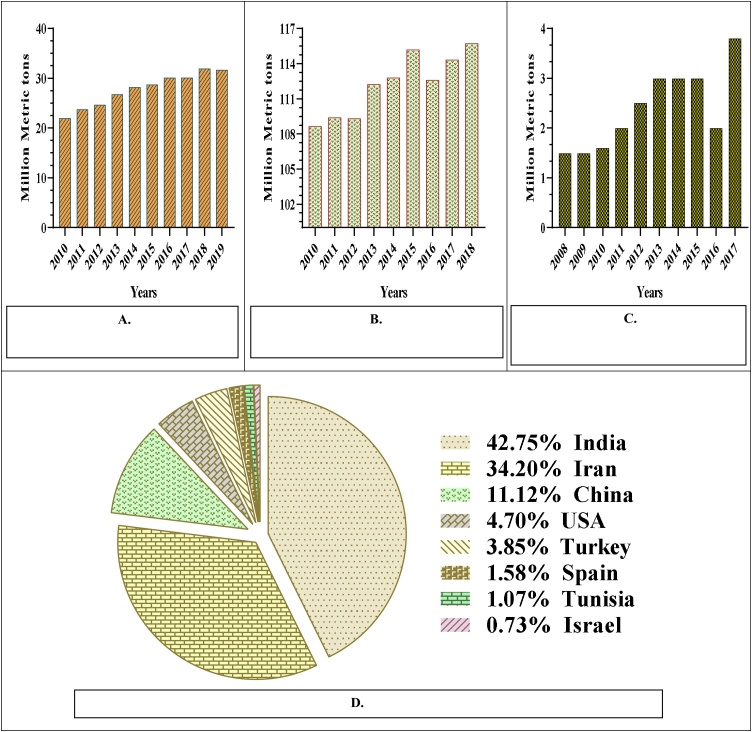
Tangerine is a major fruit crop with a world production of about 31.7 million metric tons in the 2019 [10], its global production quantity is increasing as a result of the increase in its consumption (Fig. 1A). It is cultivated in many countries around the world with tropical and subtropical climate, the most important producers of this fruit in the world are China, Spain, Turkey, Morocco, Egypt, Brazil, United States of America, Japan, Italy and Republic of Korea [11]. The data of the Food and Agriculture Organization of the United Nations have shown that China is the world's largest producer of tangerine with a surface area of 1.34 million ha and a production of 19.21 million metric tons in 2018 [11]. This fruit is largely consumed fresh for its distinctive bittersweet characteristic flavour and its high nutritional contents [38,39]. Furthermore, about 28 % of total tangerine fruit is processed to produce juice and other commercial products [40]. This practice generated a high quantity of the peels because the tangerine consists of 40−50 % of the peel [41], consequently the world generated approximately 12.68–15.85 million metric tons of its peel in 2019.
Fig. 1.
World production of tangerine, banana and pomegranate fruits. A. World production of tangerine. Source. [10]. B. World production of banana. Source. Adapted. [11]. C. World production of pomegranate. Source. Adapted. [9,[304], [305], [306], [307], [308], [309]]. D. Major pomegranate producing countries in 2017. Source. Adapted [310].
Banana is the second most produced fruit in the world, representing 16 % of the total fruit production worldwide [42]. According to the data of the Food and Agriculture Organization of the United Nations, the world banana production was 115.74 million metric tons in 2018 (Fig. 1B), with a surface area of 5.73 million ha [11]. The major banana producers are India, China, Indonesia, Brazil, Ecuador, Philippines, Guatemala, Colombia and Angola, where India is the largest producer with 30.808 million metric tons of production and 884,000 ha of surface area in 2018 [11]. The banana fruit consists of 30−40 % of the peel [43], so the world produced approximately 34.72–46.29 million metric tons of banana peel in 2018.
Unfortunately, there is no reliable data available for the world production of pomegranate, because of the rapidly increasing production, and also it is difficult to calculate the total production. In the current review, we attempted to collect estimates of annual world production, based on the study of published scientific articles. The world production of pomegranate is estimated around 3.8 million metric tons in 2017 [9]. However, the real quantity is higher than this quantity. Additionally, this quantity increased significantly from 2008 to 2017 (Fig. 1C). The most important pomegranate producers in the world (Fig. 1D) are India, Iran, Turkey, China, United States of America, Israel, Egypt, Spain, Afghanistan, Tunisia, Azerbaijan, Morocco, Argentina, Brazil, Chile, Peru, South Africa, Australia and Italy [9], among these producers India is the world's largest producer of pomegranate with a surface area of 234,000 ha and a production of 2.84 million metric tons in 2018 [44]. The pomegranate fruit consists of 50 % of the peel [45], so the world produced an estimated of 1.9 million metric tons of the peel in 2017.
3. Tangerine, pomegranate and banana processing chain
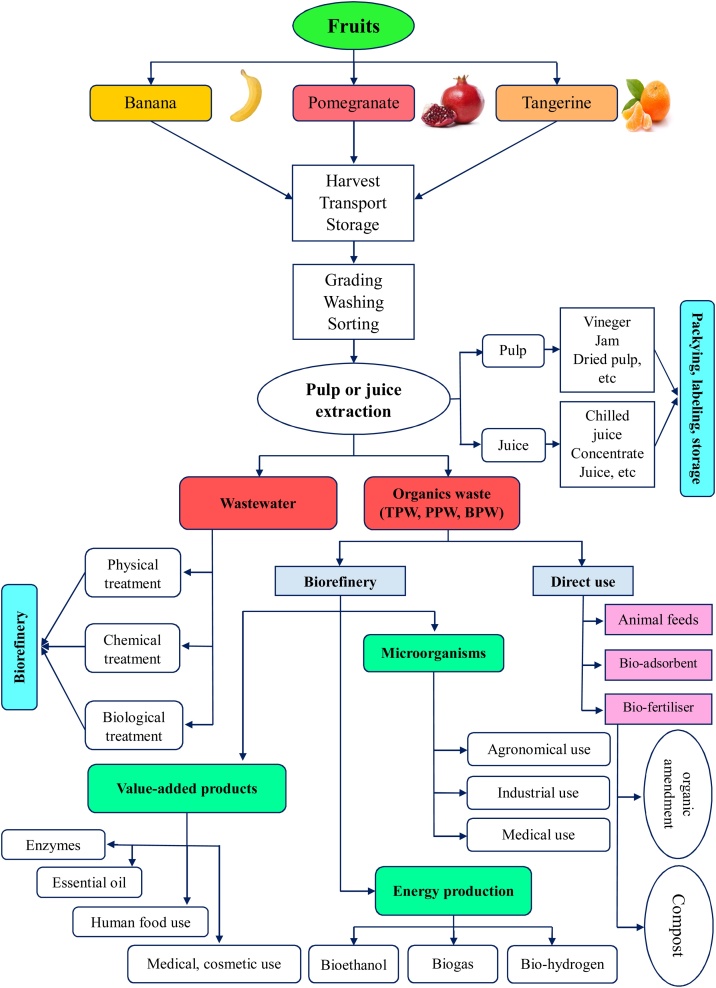
Before tangerine, pomegranate and banana arrive at the processing industry, there are three main stages: harvesting, temporary storage at farms, and transportation. The main harvesting season for tangerine extends from November to January [46]. The pomegranate usually harvested between September and November, where the majority of American pomegranate is generally ready to be harvested from the end of October [47]. Banana is cultivated and harvested throughout the year and it is ready to be harvested 8–10 months after the plantation [48]. Nevertheless, the harvest season depends on the varieties, the climatic and the edaphic factors of each region. At harvest time, it is important to determine the correct maturity stage of the fruit in order to minimize the risk of losses and to ensure a long storage time [41,49]. Numerous studies, such as the study by Coelho de Queiroz et al. [50] which has shown that inappropriate fruit maturity causes physiological disorders during storage and affects storage time. The transport of these fruits is carried out from the production farms towards the supermarkets to be distributed for consumption in fresh form, and towards the processing companies for the manufacture of the processing by-products. Transport cost is variable according to several factors including the distance between the production farms and the processing company or supermarket, the road quality, the transport period, the transported quantity and the fuel cost, etc. Tangerine, pomegranate and banana are very sensitive fruits because of their nature and short storage time at room temperature and their quick deterioration creates a problem for post-harvest management of the fruits causing significant losses to the farmers and the processing industry [9,51,52]. However, maintaining the adequate temperature during the post-harvest period is an important environmental factor to preserve the quality and increase the shelf-life of the fresh fruits [53]. In most countries, fruit is handled, marketed and stored under ambient conditions, when ambient temperatures are still low, with much less commercial storage under refrigerated conditions [54]. But currently, refrigerated storage is the most commercially used storage system for prolonging the storage time of fruits. There are several other storage systems for fruits, such as controlled atmosphere storage, hypobaric storage, cold temperature storage with intermittent heating [54,55]. Recently, numerous researches have been carried out on the adequate storage conditions for tangerines, bananas and pomegranates. For pomegranates, the results of these precious studies suggest a temperature of 5–7 °C and a humidity of over 90 % is necessary to preserve the quality of the fresh fruit [56,57]. For tangerine storage, recommended storage conditions are a temperature of 2–7 °C and a humidity of 80–90 %, to ensure storage time of 2–18 weeks depends on the variety [54]. For the storage of bananas, storage conditions are a minimum temperature of 13.3 °C and a humidity of 85–95 % [55]. The fruit processing industries get the bananas, tangerines and pomegranates directly from the farms. Tangerines are received mixed with a large quantity of leaves. After reception, the fruit is temporarily stored (see condition above), washed, graded, and followed by the juice extraction stage and the production of the other products (Fig. 2). The washing is carried out manually or automatically, depending on each industry. In order to get the maximum benefit from the fruit after processing, experts choose the best destination for each type of fruit. The main product of the tangerine, pomegranate and banana fruit processing industry is the juice, used as a food, thanks to its important nutritional values [58]. In addition, there are other products obtained from the processing of these fruits, such as vinegar, jam, dry peels, flavours for drinks and other food quality products (Fig. 2). The processing of fruits to juice is a process consisting of extracting the juice by several methods, the quality of the juice obtained depends on many factors such as, the variety of fruits, the fruits texture, the maturity stage, the agri-climatic conditions, the fruit pre-treatment, the extraction technique used, the sanitary approaches and the waste disposal [59]. Pre-treatment methods also vary according to peel texture, form and nature of the fruit [58]. Additionally, each type of fruit requires a specific treatment for the separation of pulp, peel and seeds. The processing of fruit into juice is carried out by machines and according to the following steps: Juice extraction (washing, fruit preparation, crushing or milling, fruit-grinding mills and finger cup extractors), juice separation (pressers, rack and cloth press, hydraulic presses, screw-type presses, belt presses, hydrodiffusion extractor and spiral filter press), clarification and stabilization (centrifugation, membrane-based filtration technologies and enzymes for fruit juice clarification), drying/evaporation and concentration, pasteurization and storage [58]. The juice obtained is pasteurized by heating at a higher temperature (60 °C–100 °C) to eliminate pathogenic microorganisms and enzymes for long storage of the juice [60]. The juice produced is packaged and stocked (Fig. 2). But before reaching the final product destination to consumers, there is an essential step, it is the microbiological analysis of the juice, especially for non-pasteurized juice, in order to show that it is safe for human consumption [61].
Fig. 2.
Tangerine, banana and pomegranate processing chain.
The consumption of these fruits in the fresh state and the industry of their processing generated a significant quantity of peels whose quantity is variable from one fruit to another, the processing or consumption of banana fruit generated about 30−40 % of peel [43], the tangerine fruit generated approximately 40−50 % of the peel [41] and the pomegranate fruit generated approximately 50 % of the peel [45]. In view of the world production of these fruits, and the amount of waste generated by each fruit, we are discussing the millions of metric tons of solid organic waste produced each year (see Section 2), and also, we are thinking of a significant amount of wastewater resulting from their processing. In addition, these wastes present risks to the environment and human health (see Section 5). However, these wastes can be used directly as animal feed (see sub-section 7.1.1) or soil biofertilizers (see sub-section 7.1.2), and they may also be recovered in several other ways (see section 7.2).
4. TPW, PPW and BPW characterization
The composition of the fruits generally, tangerine, pomegranate and banana fruits particularly depends on the climatic conditions, the region, the culture, the species, the harvesting period, the maturity and the storage environment [62].
The pomegranate fruit has been known since antiquity by these nutritional properties, composed of the peel, arils and seeds (Table 1), approximately 50 % of the pomegranate's weight is contained in the peel [45,63]. The pomegranate is characterized by a pH lower than 4, and by the presence of hydrolysable tannins, condensed tannins, flavonols, anthocyanins, phenolic and organic acids, and a high sugar content (mainly glucose and fructose), minerals, such as nitrogen (N), phosphorus (P), calcium (Ca), sodium (Na), magnesium (Mg) and potassium (K) [64], heavy metals and vitamins [65,66]. Pomegranate has a high antioxidant activity thanks to different polyphenol compounds mainly ellagitannins, gallotannins, ellagic acid and flavonoids, including anthocyanins, quercetin, kaempferol and luteolin glycosides [67].
Table 1.
Main constituents of tangerine, pomegranate and banana fruits.
| Pulp (%) | Peel (%) | Seeds (%) | |
|---|---|---|---|
| Tangerine fruit* | 48−58 | 40−50 | 2 |
| Pomegranate fruit** | 40 | 50 | 10 |
| Banana fruit*** | 60−70 | 30−40 | 0 |
The banana is one of the most consumed fruits in the world thanks to its high nutritional content, this fruit is composed of the peel and the arils (Table 1). Banana fruit is a source of nutrients containing various minerals, vitamins (vitamins A and C), lipids, proteins, pectin, polysaccharides, flavonoids, alkaloids, steroids and glycosides [68,69]. Banana is widely known for its high content of various antioxidant compounds, such as gallocatechin [70] and dopamine [71].
The tangerine is one of the most popular and most accepted fruits worldwide, this fruit is composed of the peel, arils and seeds (Table 1). Tangerine is very rich in nutritional content, especially fibres, carbohydrates, lipids, proteins, oligo-elements such as calcium (Ca), iron (Fe), manganese (Mn), phosphorus (P), potassium (K), copper (Cu), sodium (Na) and zinc (Zn), fatty acids [6,39,72] and also contains bioactive compounds, such as antioxidants, flavonoids, vitamins (Vitamins C, A, E, K, B1, B2, B3, B5) and other phenolic acids [73].
4.1. Physicochemical characterization
The physicochemical composition of fruit peels in general and of TPW, PPW and BPW in particular depends on the cultivation method of the fruit, the harvest time, the ripeness stage and the different methods used for juice extraction [74].
TPW is rich with bioactive compounds, including ascorbic acid, carotenoids, phenolic compounds (phenolic acid) and flavonoids (hesperidin, naringin, and neohesperidin), lipids (oleic, linoleic, linolenic, palmitic and stearic acids), vitamins (especially vitamin C), insoluble carbohydrates such as cellulose, pectin, other sugar, including glucose and fructose, acids (mainly citric and malic acids), minerals (nitrogen (N), calcium(N) and potassium(k)), essential oil, volatile compounds (alcohol, aldehydes, ketones, esters, hydrocarbons), enzymes (mainly pectinesterase), pigments and carotenoids (carotene, xanthophylls, lutein) and other constituents [41,[74], [75], [76], [77], [78]]. Table 2(a) reports the physicochemical characteristics of TPW.
Table 2.
Physicochemical composition of TPW, PPW et BPW. a. Physicochemical characteristics of TPW; b. Physicochemical characteristics of PPW; c. Physicochemical characteristics of BPW.
| aTPW dry matter | bPPW dry matter | cBPW dry matter | |
|---|---|---|---|
| Chemical composition | |||
| pH | 74.27 | 14.83 | 86.23 |
| Moisture (g water/gdry weight) (%) | 32.50 ± 0.10 g | 47.06 ± 0.07 | 29.80 |
| Proteins (%) | 58.55 | 13.26 ± 0.14 | 15.13 ± 0.14 |
| Lipids (%) | 52.97 | – | 51.70 ± 0.100 |
| Crude fibres (%) | 312.8 | 834.0 | 531.70 ± 0.250 |
| Total dietary fibre (g/g) | − | – | 40.5025 ± 0.2 |
| Ash (%) | 32.881 | 13.31 ± 0.05 | 25.01 |
| Carbohydrates (%) | 518.03 | 486.52 ± 0.18 | 559.00 ± 1.360 |
| Fat (%) | – | 13.31 ± 0.05 | 413.1 ± 0.2 |
| Minerals (mg/g) | 530.96 | – | – |
| Total phenolic (mg GAE/g) | 29.31 ± 12.43 | 6268 | 69-30 |
| Phenolic acids (μg/g) | |||
| Gallic | – | 56208.168 | – |
| Chlorogenic | 20.415 ± 6.3 | 524659.06 | – |
| Caffeic | – | 5228.586 | – |
| Catechol | – | 52423.92 | – |
| Total Flavonoid (mg quercetin/g) | 24.27 ± 1.51 | – | 7196.1 ± 6.70 |
| Total carotenoids (mg/g) | 62.0 | – | – |
| Titratable acid (%) | 30.875 | – | – |
| Hydrolysable tannins (g/kg) | – | 627-172 | – |
| Pectin (%) | 412,82 | 38.1 ± 3.5 | 33.5 |
| Hydrogen cyanide (mg/g) | – | – | 51.33 ± 0.100 |
| Oxalate (mg/g) | – | – | 50.51 ± 0.140 |
| Phytate (mg/g) | – | – | 50.28 ± 0.06 |
| Saponins (mg/g) | – | – | 524.00 ± 0.270 |
| Vitamin C (mg/g) | 37.0 | – | – |
| Essential oils (% w/w) | 14.6 | 20.20 | 30.106 |
| Glucose (% Dry matter) | 839.4 ± 1.1 | 927.94 | 930.1 ± 0.8 |
| Fructose (% Dry matter) | 810.3 ± 0.8 | 932.29 | 915.2 ± 0.7 |
| Physical properties | |||
| Bulk density (g/cc) | 90.849 | 70.62 | 100.39 |
| Particle density (g/cc) | 100.894 | 70.89 | 100.89 |
| Porosity (%) | 924 | 730.88 | 1056.41 |
| Water absorption capacity (ml/g) | – | 76 | 105.1 |
Notes: GAE: Gallic acid equivalents. Source: adapted data; a =1 [135], 2 [292], 3 [39], 4 [32], 6 [293], 5 [72], 7 [18], 8 [176], 9 [294], 10 [295]. b =1 [45], 2 [80], 3 [296], 4 [297], 5 [298], 6 [79], 7 [8], 8 [299], 9 [300]. c =1 [301], 2 [42], 3 [14], 4 [302], 5 [303], 6 [84], 7 [85], 8 [18], 9 [176], 10 [42].
PPW contains several chemical compounds such as hydroxybenzoic acids, gallic acid and ellagic acid, hydroxycinnamic acids, flavone derivatives, yellow colouring molecules and anthocyanidins which give the red colour of the fruit [67,79]. It also contains essential oil [34,80], ellagitanins, such as punicalagine, punicalagine, granatine A and granatine B [3,81]. The pelletierine is also present in the PPW [3]. Table 2(b) reports the physicochemical characteristics of PPW.
BPW contains cellulose, hemicellulose, lignin, proteins, polysaccharides, chlorophyll, pectin and other compounds of low molecular weight [69,82,83]. It also contains beneficial bioactive compounds including phenolics, dopamine, anthocyanins and catecholamines [84]. BPW has a high content of gallocatechins, which makes it a useful dietary source against heart disease and cancer [70], of flavonoids and certain other bioactive compounds, including pro-vitamin A compounds, trans-β-carotene, trans-α-carotene, cryptoxanthin, sterols, cycloartan-type triterpenes, polyunsaturated fatty acids and linolenic acid [85,86]. Also, BPW contains essential oil, phenols, lipids and tannins that have been shown to have good antimicrobial activity [13,87]. Table 2(c) reports the physicochemical characteristics of BPW.
4.2. Microbiological characterization
The microorganisms associated with TPW, PPW and BPW are varied according to varieties, study period, study conditions, and study methods. In literature there are several studies that have focused on the microbiological study of banana, tangerine and pomegranate wastes in the case of compost and vermicompost production [88,89]. However, the study of TPW, PPW and BPW microorganisms is very poorly researched until now. Only a few scientists have focused on the study of these microorganisms, such as El Barnossi et al. [90] that have studied microorganisms associated with the decomposition of tangerine peel in water and soil, in their studies they have shown that the microbial density of tangerine peel has been variable (Table 3). Also, El Barnossi et al. [91] that have focused on the quantitative study of bacteria, yeasts, actinomycetes and moulds associated with decomposing banana and pomegranate waste in water and soil. The results of these researchers are summarised in Table (3). El Barnossi et al. [92] have studied the fungal microorganisms associated with banana and pomegranate wastes, and they have shown different fungal densities associated with banana and pomegranate wastes, in banana wastes microbial biodiversity has been more diverse and the species identified included Aspergillus flavus, Aspergillus niger, Aspergillus ochraceus, Penicillium cyclopium, P. islandicum and P. simplicissium from banana waste and Fusarium oxysporum, Penicillium sp, P. glabrum, P. islandicum from pomegranate waste.
Table 3.
Microbiological characteristics of TPW, PPW and BPW.
| * BPW (CFU/gDw) | *PPW (CFU/gDw) | **TPW (CFU/gDw) | |
|---|---|---|---|
| Total Bacteria | 5.24 107 | 3.039 108 | 2.261 107 |
| Yeasts | 5.86 108 | 7.7318 108 | 5.59 108 |
| Moulds | 1.139 108 | 6.257 108 | 1.017 108 |
| Actinomycetes | 0.00 | 0.00 | 0.00 |
5. Impact of inappropriate valorisation of TPW, PPW and BPW on environment and human health
The processing industry and the consumption of tangerine, pomegranate and banana fruits produce a significant amount of waste every year (See Section 2). A part of these wastes used directly as animal feed, soil biofertilizers or bio-adsorbents (see subsection 7.1.2), another part is distinguished for the biorefinery (see Section 7.2). However, landfills are the most widely used method of waste disposal in the world, especially in developing countries such as Morocco. These landfills represent serious threats to the environment [[93], [94], [95]]. Landfill sites can cause environmental pollution such as pollution of surface water, groundwater, air and soil [[96], [97], [98], [99]]. Agricultural wastes in general contain a very important quantity of organic matter, after their disposal in landfills, the decomposition of agricultural wastes due to landfill microorganisms causes a change in their chemical composition resulting the production of many pollutants through leachates and landfill gases composed of CO2, CH4, CO, H2S and others [95,100]. Landfill leachate, especially from uncontrolled landfills, interacts with soil, surface water and groundwater and degrading their quality [101] and negatively affecting the populated biodiversity [102]. Landfill leachate is a highly polluted liquid mixture containing a wide range of pollutants, such as inorganic compounds including metals (Cd2+, Cr3+, Cu2+, Pb2+, Ni2+, Zn2+), chloride (Cl), sulphates (SO₄²-), organic matter (volatile fatty acids), xenobiotics (pesticides and herbicides), microorganisms, aromatic hydrocarbons, phenols, oil, hydraulic fluids, and other chemicals [93,100,101,103]. In addition, several studies have indicated that the main groundwater pollutants from landfills included chloride (Cl), sodium (Na), phosphorus (P), ammonium (NH₄+), total hardness, total dissolved solids, organic matter, chemical oxygen demand and heavy metals [97,100,[104], [105], [106]]. There are also a pollution risks from these wastes during their treatment and valorisation, such as by the side effects of organic amendments on soil organisms [107,108]. Also, by the excessive applications of low-quality compost can lead to an accumulation of pollutants in the soil, which affects the metabolism of living microbes [109], the continued unwise and large-scale use of compost in soils can lead to the accumulation of heavy metals in the soil [110]. The inappropriate combustion of waste in open air is particularly discouraged because of its serious air pollution [99,110], there are also other pollutants in the water obtained during fruit processing can present risks to the environment and human health.
The most frequent problems with waste disposal in landfills include fires and explosions, vegetation damage, bad landfill odours, air pollution, climate warming and groundwater pollution [111,112]. Additionally groundwater contaminants can be absorbed by plants growing in nearby agricultural fields, potentially creating human and animal health problems [113].
Landfills can cause many negative effects on human health by direct or indirect contact with soil, air and surface or groundwater contaminated with pollutants [95,114]. Direct health hazards mainly concern workers in this field who must be protected, to the extent that contact with the waste and also people living near a landfill site may lead to the inhalation of toxic substances emitted by the site and contact with soil and water polluted, either directly or indirectly through the consumption of contaminated products from the surrounding area [100]. For the general public, the main health hazards are indirectly related to the rearing of disease vectors mainly from flies and rats [110]. Several authors have studied landfills and have concluded that these landfills have negative impact on human health, such as inhalation chemical poisoning, low birth weight, birth defects, neurological diseases, nausea and vomiting, mercury toxicity from consumption of high levels of mercury in fish [115], the increase of cancer cases [116,117] and the respiratory diseases [118,119]. Many other articles have reported an association between exposure to odour control systems, particularly landfills, respiratory and other non-specific symptoms in the public [119,120].
In this section, we have discussed the disposing problem of organic wastes in landfills and also the impact of their inadequate valorisation, clearly these wastes have several adverse impacts on the environment and public health. However, studies are required regarding the impact of these wastes, especially on the transmission of pathogens due to atmospheric pollution in order to protect the environment and public health.
6. Wastewater treatment of TPW, PPW and BPW processing
Currently, the processing industry and the consumption of fresh tangerine, banana and pomegranate fruits generate a significant volume of wastewater because of the increased production and consumption of those fruits. The composition of the wastewater caused by those wastes depends on different water uses. While those fruits are distinguished for consumption in their fresh state, we mainly discuss wastewater polluted by pesticides, biocides, and chemical fertilizers, etc. However, the great volume of wastewater is generated by the processing industry due to treatment, cleaning and cooling. In this case, the composition of wastewater depending on the product processed, such as drink, jam, juice, etc., and during biorefinery process, including biogas, biohydrogen, bioethanol production and other products. Processing industry for fruit produces a large volume of wastewater polluted mainly by highly concentration of organic matter [121]. Organic matter and other types of water contaminants create a favourable environment for the growth of most pathogenic and phytopathogenic organisms, such as bacteria, fungi, protozoa, nematodes and viruses, all of which represent serious problems for humans, animals and environment [122,123]. In this context, a constant effort should be made to protect water resources and also to valorise pollutants in wastewater.
Each type of wastewater requires a specific treatment process according to its composition and the interest of its treatment. It is difficult to establish a universal method for removing all wastewater pollutants at the same time [123]. Wastewater treatment methods require a combination of physical, chemical and biological processes, as well as operations to eliminate insoluble particles, soluble effluent contaminants and microorganisms, and it is also important to respect the factors influencing treatment efficiency, feasibility, environmental impact and sustainability [123]. Since many years, several physical, chemical and biological methods have been used for the treatment of wastewater, such as flotation, sedimentation, evaporation, adsorption, chemical oxidation [122], coagulation, flocculation [124], chemical precipitation, ion exchange, membrane filtration, electrochemistry [125], biodegradation, microbiological treatments, enzymatic decomposition [126], phytoremediation [127], incineration and the liquid-liquid (solvent) extraction [123]. Every method among the methods listed above has its disadvantages and advantages. In their review article, Crini and Lichtfouse [123] have discussed the disadvantages and advantages of those methods, e.g. biological methods have several advantages, such as, simplicity of using microorganisms for biodegradation of organic pollutants, the microorganisms used produce value-added products like extracellular enzymes with high biodegradability, effective removal of biodegradable organic matter, ammoniac (NH3), ammonium (NH4+), high removal of biochemical oxygen demand and suspended solids, etc. Nevertheless, those methods also have disadvantages, such as the necessity of an optimal environment, require management and maintenance of microorganisms as well as physicochemical pre-treatment, require a long process time, low biodegradability of some molecules like colorants, poor discoloration, biological sludge production, and complexity of microbiological mechanisms, etc.
The recovery of wastewater resources can provide environmental and economic benefits; particularly the recuperation of value-added substances from wastewater [122,128]. In their review, Li et al. [129] have discussed wastewater treatment based on microalgae for nutrient recovery and they have indicated that this process has many advantages that can respond to the new demand for improved wastewater treatment. In their review, Liu et al. [130] have discussed and criticized recent research about wastewater treatment using filamentous algae and they have shown that filamentous algae present some advantages over microalgae because of their size, which allows easier harvesting. Cao et al. [128] have discussed the use of photosynthetic bacteria for wastewater treatment with production of value-added products, such as proteins, coenzymes, carotenoids, bacteriochlorines and polyhydroxyalkanoates, etc. Lu et al. [131] have also shown that photosynthetic bacteria are non-toxic and contain many value-added products that can be used in several fields, especially in the agri-food industries. However, wastewater biorefinery presents some disadvantages, especially those related to the complexity of wastewater characteristics and species adaptability. In addition, there are considerable researches deficiency in the fields of wastewater treatment. Therefore, further researches are necessary concerning the selection of species, the effect of nutrient loads, the microorganism interactions with the wastewater compositions, the safety assessment for the use of each microorganism, the separation and purification methods of microorganisms after treatment, and also more fundamental researches about microorganisms applied in the treatment in order to demonstrate the benefits of each method.
7. TPW, PPW and BPW valorisation methods
There are several traditional methods of fruit peel disposal in general and TPW, PPW and BPW in particular, such as landfill and incineration. Because of the high quantity of peels that have generated every day, traditional methods are not sufficient. Furthermore, these methods have a negative impact on the environment and human health (see Section 5), and also the global economy has lost billions of dollars annually through their application. Consequently, the valorisation of any type of wastes requires, before anything else, a good determination of their physicochemical and microbiological characteristics for an adequate valorisation, which preserves our environment and offers an important economic benefit. In this section we will discuss the use of TPW, PPW and BPW directly without differentiation of their compositions, and then we will discuss the valorisation of these peels with differentiation of their chemical, microbiological compositions as well as the products derived from their decomposition under aerobic or anaerobic conditions.
7.1. Direct use
Thanks to these physicochemical and nutritional characteristics (see Section 4), TPW, PPW and BPW could be used directly or after pre-treatments for animal feed and for fertilization and restoration of degraded soil. Moreover, these peels are suitable for the depollution of contaminated environments, especially wastewater, due to their high bio-adsorbent capacity.
7.1.1. Animal feed
Current animal nutrition and health approaches use fruit peels as animal feed, such as TPW, PPW and BPW, which can be used as fresh or dry animal feed. In addition, the flavonoid content of these peels can be considered as antioxidants and phenolic compounds that have the potential to eliminate pathogenic bacteria [132]. According to Monagas et al. [133], the antioxidants that can enter blood vessels of the organism are selectively permeable and prevent hazardous substances to enter the organism. These peels also contain a significant quantity of nutrients and minerals necessary for animals' healthy growth, they are rich in crude fibre, carbohydrates, crude protein and ash [134]. Thus, suggesting that TPW, PPW and BPW (fresh or dried) can be used to partially replace feeds in the conventional animal diet. However, the presence of anti-nutritional content may adversely affect the use of these peels as animal feeds, such as the presence of limonin [12], tannins [13,87] and essential oil [14,80,135], therefore, they must be treated before using them as animal feed. Currently, many studies have focused on the use of PPW as animal feed, such as Shabtay et al. [136], Oliveira et al. [137], Jami et al. [138], Weyl-Feinstein et al. [139] and Varma et al. [15] who have studied the effects of PPW in terms of ruminant health and production. Shabtay et al. [136] have shown that cows feed with concentrated pomegranate extract have produced more milk than control cows. Weyl-Feinstein et al. [139] have demonstrated that milk supplementation with 3.75 % of the concentrated PPW extract for neonatal Holstein calves leads to a reduction in the number of faecal oocysts of intestinal parasite Criptosporidium parvu and diarrhoea intensity. Jami et al. [138] have reported that supplementation of cow's milk with 4 % of concentrated PPW extract significantly increased their dry matter digestibility, fibre protein and milk yield, furthermore, they have also shown that concentrated PPW extract affects significantly the rumen bacterial communities. Varma et al. [15] have concluded that feed supplementation with concentrated PPW extract is feasible to improve calves' health and production. Oliveira et al. [137] have found that pomegranate extract polyphenols have improved mitogen-induced cytokine production and response to vaccination, potentially enhancing calves' immune competence and possibly their health. Concerning TPW, there are several studies that have focused on its use as animal feed, such as Zema et al. [78] which have shown in their review that citrus peels are more suitable for use as feed higher ruminants for meat and milk production. Also, Choi and Lee [140] have studied the physicochemical quality characteristics of the pork patty added with different quantities of TPW, the addition of 0.3 % to 1 % TPW in the pork patty has shown no significant difference in sensory properties compared to the pork patty without TPW, and they have concluded that the addition of TPW could be used as an additive in the pork patty to promote the function of tangerine by-products. Over the last decade, the potential for the use of BPW as a feed additive for livestock [141], poultry [7], aquatic animals, especially African catfish [142], freshwater giant prawn [143], Rohu fish [144], red tilapia performance [145] and rabbits [16] have established. Adeniji et al. [146] have shown that BPW is a good feed source for livestock thanks to its high cellulose and starch content. Nuriyasa et al. [16] have documented that the use of 9 % BPW feed level induces higher consumption, feed digestibility, growth and carcass weight of rabbits compared to those observed without BPW. FAS et al. [7] have concluded that BPW meal can be added up to 10 % in the poultry diet of broilers without any negative effect, which enriches the poultry production industry. Finally, Yangthong et al. [147] have indicated that the inclusion of up to 15 % BPW in the diet does not appear to have a negative effect on fish growth efficiency.
7.1.2. Bio- fertilizer
In the light of the TPW, PPW and BPW physicochemical characteristics (see Section 4), these peels are highly rich in micronutrients, which suggests their use to improve soil quality and therefore crop yields, whether through its use directly as organic soil conditioner or through its use to produce a specific compost to meet the specific requirements of plants.
7.1.2.1. Organic soil amendment
Many farms have used TPW, PPW, and BPW directly or after drying in the soil to improve its physicochemical and microbiological characteristics, as well as plant productivity. The incorporation of these peels into the soil helps the construction of a clay-humic complex, as well as increasing the water retention capacity and decreasing soil permeability. Documentary review of scientific articles over the last decade has revealed that TPW, PPW and BPW have successfully used as fertilisers for improving soil fertility and enriching soil microbiota thanks to their content of minerals required for the plant's growth. TPW, PPW and BPW have a high content of the most nutritious minerals, mainly phosphorus (P), potassium (K), sodium (Na), iron (Fe), zinc (Zn), manganese (Mn) and copper (Cu) [19,148]. BPW is an organic waste rich in high potassium (K) content compared to TPW and BPW [149], also other authors have shown that BPW biochar was found to be high in potassium (K) which could be used as potassium (K) fertilizer, therefore, an alternative source of potassium (K) for plants productivity [19]. Consequently, BPW biochar is not recommended for soil acidity remediation, but it would certainly reduce calcareous requirements when applied as a potassium (K) fertilizer [150]. The incorporation of BPW increases the alkalinity of the soil, as well as the exchange capacity of its anions, which is beneficial for the plants' growth. When BPW decomposed in the soil, the soil's phosphorus (P) content increases, thus, suggesting its incorporation could enrich the soil with phosphorus (P) [151]. Numerous studies have shown that the use of TPW, PPW and BPW as an organic amendment improves soil fertility with other benefits, such as the study by Cao et al. [17] which has indicated that biochar derived from PPW has great potential as an adsorbent for the removal of Cu (II) from the soil, and the study by Sial et al. [152] which has demonstrated that BPW and its biochar have reduced greenhouse gas emissions. Anastopoulos et al. [18] have also demonstrated that the incorporation of TPW and BPW in soil has a significant effect on the bacterial community structure and the bacterial guild abundance associated with nitrous oxide emissions. The usage of TPW, PPW and BPW or their biochar have also helped plants to resist diseases [19,151,153]. Furthermore, the spread of unstable TPW, PPW and BPW to soil could result the depletion of soil oxygen and the emission of harmful odours in treated crop fields. Additionally, the input of these untreated peels in the soil can induce soil permeation with possible pollution of groundwater, soil and air (see Section 5). For these reasons it is necessary to pre-treat these wastes before their utilisation as an organic amendment. In order to integrate their use in a nutrient management strategy for a culture, more studies are required to assess the response of the culture, although long-term effects to several soil physicochemical and microbiological properties will also be helpful, especially the effect on biodiversity and activity of microbial communities which is not available until now.
7.1.2.2. Compost
The composting is the controlled biological transformation of solid organic waste under aerobic conditions by indigenous micro-organisms (bacteria, actinomycetes, yeasts and moulds). The degradation of organic matter results in the release of CO2 from H2O and heat, as well as the production of nutrients. Compost contains a high level of plant nutrients with beneficial effects on the physicochemical and biological soil properties [154]. The production of composts from vegetable residues has known for years. Thanks to these physicochemical and nutritional characteristics TPW, PPW and BPW have succeeded to produce specific composts with very good qualities. However, the excessive moisture content of wet TPW, PPW and BPW makes it difficult to compost by the methods commonly used for solid organic waste, because if the moisture content exceeds 70 %, the aeration of the organic waste becomes very difficult which can prevail anaerobic conditions [155], therefore, this type of waste requires a preliminary mixing with a lignocellulosic support or drying in order to reduce the moisture content at the optimal interval for composting, which is between 45 % and 55 % [155]. TPW, PPW and BPW have high C/N ratios (Table 4), however, at higher C/N ratios (>40), the microbial population will be deficient in nitrogen (N) that can cause the slow-down of the composting process [155], thus, it is interesting to add wastes with a low C/N ratio, such as sewage sludge with a C/N ratio about 8 to reach the optimal C/N ratio between 25 and 35 [156]. In addition, TPW and PPW have a low pH of 4.27 and 4.83 respectively [18,45], which requires the addition of calcium hydroxide (Ca(OH)2) so as to obtain the optimal pH which is between 6 and 7.5 [78]. Several studies have demonstrated that the co-composting of TPW, PPW and BPW with other wastes is an appropriate practice such as Kalemelawa et al. [150] that have conducted researches to improve the composting of BPW with animal manure, poultry litter or earthworms and they have found that inoculation of cow dung or poultry litter greatly facilitated mineralization and the final composts were high potassium (K) and high nitrogen (N) content (>100 g/kg and >2 %), respectively. Another study has indicated that the potassium (K) content of BPW compost is higher compared to other composted wastes, such as municipal solid waste compost [157]. In addition, the highly alkaline pH of BPW compost suggests that it could be useful for remediating acidity in acidic soils [150]. Kucbel et al. [158] have reported that compost from BPW and citrus (tangerine) are very rich in potassium (K). The high potassium (K) and nitrogen (N) content of composts has a high potential for their use to provide a potentially important source of potassium (K) and nitrogen (N) soil nutrients [159]. Besides, Bernal-Vicente et al. [160] and Zema et al. [78] have been shown that citrus compost is characterized by a high content of lignocellulosic compounds, a high C/N ratio and a high pH. For composting PPW, Abou El Nour et al. [161] have evaluated formulations of aerated compost tea in combination with PPW powder to determine their suitability for use as environmentally safe fungicides and they have concluded that it is a promising combination for controlling phytopathogenic fungi and can be used as a natural fungicide effective against hazardous soil-borne fungi.
Table 4.
Carbon and Nitrogen characteristics of TPW, PPW et BPW.
| aTPW | bPPW | cBPW | |
|---|---|---|---|
| C (g/kg) | 560 | 400.9 | 470 |
| N (g/kg) | 13 | 11 | 12.5 |
| C/N | 43 | 39.1 | 38 |
The composting of TPW, PPW and BPW to produce a stable fertiliser resource should be studied as an alternative waste management option to landfill, for a potential benefit to farmers, especially in poor and developing countries, which requires advanced studies about the valorisation of TPW, PPW and BPW by composting, especially the impacts of these composts in terms of soil quality and durability.
7.1.3. Bio-adsorbent
The removal of heavy metal ions from contaminated environments by using fruit peels is an innovative and promising technology. Thanks, to these physicochemical characteristics (see Section 4) TPW, PPW and BPW could be a very good quality bio-adsorbent. The use of TPW, PPW and BPW as bio-adsorbent to eliminate contaminants has been the object of numerous studies. For BPW, most of the studies have focused of the cation adsorption of colorants [162], heavy metals which has indicated by the study of Motaghi and Ziarati [163] which has shown that BPW modified with 1 % citric acid has a large potential capacity to bio-adsorb toxic heavy metal ions (cadmium and lead) from rice, and by the study of Amin et al. [20] which indicated an effective biochar absorption potential prepared using BPW for the removal of copper and lead, also have focused of the acid adsorption which has shown by the study of Pathak et al. [164] which has demonstrated that the use of BPW is extremely effective for the removal of benzoic and salicylic acid from aqueous solutions, and by the study of Pathak and Mandavgane [165] which has shown that microwave-assisted charcoal synthesized from raw BPW has a good potential for removing citric acid from aqueous solution. Also, Alaa El-Din et al. [166] have noted that the adsorbent capacity of BPW performs well as a new bio-adsorbent for oil spill disposal. For PPW, El-Ashtoukhy et al. [167] have documented that PPW is an effective adsorbent for the removal of lead and copper ions from aqueous solutions. Amin [168] has shown that activated carbon prepared from chemically treated PPW is an effective adsorbent for the removal of the blue-106 dye directly from the aqueous solution. For TPW, the study of Abdić et al. [21] has confirmed that TPW is a very interesting material for the removal of Cr, Cu, Mn, Co, Ni, Pb, Cd and Zn from polluted water by these heavy metals, also it has shown that the adsorbent of these heavy metals is a mechanism of ion exchange between the negatively charged groups present in the wall of TPW and the cationic metal ions. Furthermore, Sobhani and Ziarati [169] have revealed that TPW has a high potential for the removal of heavy metals (Pb, Cd and Ni) from plant coriander. Also, Torab-Mostaedi [170] have shown that TPW could be used as an alternative bio-adsorbent for treatment wastewater containing lanthanum (III) and cerium (III).
The use of TPW, PPW and BPW as bio-adsorbent for the treatment of contaminated environments, especially wastewater that is encouraging, thanks to the advantages of being a naturally biomass, low-cost biomass with a high bio-adsorbent capacity. However, they should take into account the suitable pre-treatment and adaptations to improve its performance as bio-adsorbent and study its environmental impacts of their use, especially to living organisms and also to demonstrate their safety to the human population.
7.2. Biorefinery
Biorefinery is an installation integrating biomass conversion processes and related equipment to produce energy and biochemicals from organic biomass waste [171]. Currently, the biorefinery is a new and promising approach to convert organic waste into value-added products. Fig. (2) illustrates the overall biorefinery concept schema for the conversion of TPW, PPW and BPW into energy and value-added products.
7.2.1. Energy production
7.2.1.1. Bioethanol production
Bioethanol is the most widely used liquid biofuel as a fuel or gasoline additive. In addition, it could be used in the production of pharmaceuticals, drinks as well as to replace traditional lead as anti-knock agent [78]. Bioethanol production provides many advantages, such as reducing fossil fuel consumption, increasing energy security and preserving environment, and human health.
TPW, PPW and BPW have suggested as potential feedstocks for bioethanol production due to their high content of fermentable sugar such as glucose, fructose and sucrose, as well as insoluble polysaccharides, such as cellulose and hemicellulose (see Section 4). However, d-limonene of 0.3−0.4 (% v/v) contained in TPW inhibits fermentation by microorganisms [172], because d-limonene causes disruption of the yeast cell wall and alteration of the H+ and K+ transfer chain in glycolysis [173,174], thus necessitating that d-limonene content should be lower than the minimum inhibitory concentration (0.05 % v/v) which could affect fermentation kinetics [174].
Briefly, the bioethanol production process consists of a preliminary pre-treatment to decrease the d-limonene content and subsequent hydrolysis of complex sugar contained in TPW, PPW and BPW by thermal or enzymatic methods into simple sugar, followed by alcoholic fermentation using microorganisms, especially saccharomycetes, which convert the sugar into ethanol [23,175] and produce carbon dioxide by glycolysis, piruvate-decarbossilasys and alcohol-dehydrogenasys [78].
Several pre-treatment methods, such as ammonia fibre explosion, liquid hot water, alkaline hydrolysis, oxidative delignification, steam explosion, acid hydrolysis and biological methods could be used for different lignocellulosic biomasses [78,175] to promote hemicellulose solubilization and lignin modification. Most of these methods are based on thermochemical or thermophysical processes [176]. The pre-treatment of the steam explosion reduces the content of d-limonene to 0.02−0.05 (% v/v) [172], and the popping pre-treatment by Choi et al. [177] reduces the content of d-limonene in tangerine peel waste from 0.21 to 0.01 (% v/v).
The quantitative production of bioethanol depends on several factors including temperature, pH, fermentation time, microorganism density and fungal strains used [178]. Recently, several articles have focused specifically on the production of bioethanol from TPW, PPW and BPW. For TPW, Boluda-Aguilar et al. [172] have indicated that pre-treatment of TPW and fermentation by Saccharomyces cerevisiae produced an ethanol production of 50−60 L/1000 kg of raw TPW. Choi et al. [177] have reported that the fermentation of the pre-treated TPW hydrolysate has been almost completed after 12 h with an ethanol concentration of 46.2 g/L and a yield of 90.6 %. For PPW, bioethanol production using Saccharomyces cerevisiae was 5.58 g/L, using Pichia stipitis was 2.95 g/L [179] and using Kluyveromyces marxianus was 7.20 g/L [22]. For BPW, bioethanol production by Saccharomyces cerevisiae produces an bioethanol content of 4.24 g/L [23].
7.2.1.2. Biogas production
Biogas is a fuel composed of CH4 (37–65 %), CO2 (24–40 %) and H2S (24−427 ppm) [180]. This biofuel is an excellent alternative to fossil fuels and also a clean and renewable energy source. Additionally, it could be used for heating, electricity and vehicle fuel production [181].
Biogas production by anaerobic digestion of organic matter is carried out in three main biochemical steps: hydrolysis/fermentation, acidogenesis and methanogenesis [182]. Currently, researchers are attempting to pre-treat TPW, PPW and BPW before anaerobic digestion or co-digestion to increase the biodegradation efficiency and biogas yield. These biomasses have pre-treated by physical, chemical and biological methods. The composition and yield of the biogas depending on the type of feedstock used, the volatile solid fraction, the C/N ratio, the organic loading level, the hydraulic retention time, the temperature and the pH [183,184]. A number of studies have highlighted that the production of biogas requires an optimal C/N ratio between 20 and 30 and a pH between 6.5 and 7.5 which support the growth and activity of methanogen [185,186]. Achinas et al. [187] have studied the anaerobic co-digestion of BPW with different concentrations of cow manure, in their study, the maximum biogas production rate of BPW with 10 %, 20 % and 30 % cow manure content at 10 gvs.L−1 was 112.18, 89.56 and 94.01 mL.(gvs.d)−1 respectively. In various scientific publications, a methane yield of 55–71.5 % has been documented from BPW's anaerobic digestion [188,189]. Kirtane et al. [190] have shown that the biogas generated by the anaerobic digestion of PPW contains 61–74 % of methane. By using the same biomass, Jain et al. [25] have found that a reasonable quantity of biogas (0.71 m3/kg VS fed) and methane (55.7 %) could be produced by the anaerobic digestion of PPW. Ruiz and Flotats [191] and Gunaseelan [192] have shown that the biochemical methane potential obtained for TPW was 371 and 486 LCH4 kgVS−1, respectively. The use of TPW, PPW and BPW for biogas production serves as a useful bioresource for energy production and also contributes to environmental, economic and communal sustainability.
7.2.1.3. Bio-hydrogen production
Bio-hydrogen (bio-H2) is a clean, renewable biofuel and a promised alternative to fossil fuels because it is clean, does not contribute to greenhouse gases, its combustion generates only water and a high energy content (142.35 kJ/g) [193,194]. Recently, the production of bio-H2 from bioresources, such as food wastes is the main challenge thanks to its high availability and high carbohydrate content. Yet, the high content of cellulose, hemicellulose, fats and proteins in food wastes, especially in fruit peels, is a major problem, which necessitates an adequate and environmentally friendly pre-treatment by physical and chemical methods to facilitate the biodegradation process into simple chemical compounds that could be a good substrate for fermentation [194]. In the last decade, numerous electrochemical, thermochemical and biological processes have been used to generate hydrogen. However, thermochemical and electrochemical processes present environmental risks, because they are non-renewable processes based on their dependence to fossil fuels [195].
To summarize, the production of bio-H2 by anaerobic fermentation in the dark has been studied in the majority of research concerning the production of bio-H2 from food wastes [196]. The production of bio-H2 requires the degradation of the carbohydrate fraction of the waste by initial enzymatic hydrolysis for the production of reducing sugar [197]. This step is followed by thermophilic heterotrophic fermentation of sugar to produce organic acid [198]. Then, the organic acid is converted into hydrogen by dark fermentation processes [194]. The production of bio-H2 is influenced by several physicochemical factors of the wastes used and also by environmental factors such as pH, temperature, and the type of pre-treatment used [196]. Many studies have focused specifically on the production of bio-H2 from food wastes, whereas there is only some studies investigating the production of bio-H2 from citrus peels and BPW, such as the study by Camargo et al. [26] which has demonstrated a high bio-H2 production (13.9 mmol.L−1) in the bio-enhancement trial using Enterococcus casseliflavus and citrus peel waste as a carbon source, and also the study by Nathoa et al. [199] which has indicated the bio-H2 production from BPW by two-phase anaerobic fermentation and produced a yield and hydrogen content of 209.9 mL.g−1 VS and 35 % respectively. Although there are a lot of researches concerning the conversion of agri-industrial wastes into Bio-H2 by dark fermentation processes. To the best of our knowledge, no study is available about the behaviour of TPW and PPW even though they are suitable bioresources for the production of Bio-H2. In addition, new researches are needed to increase the yield of Bio-H2 in order to respond to the increasing global energy demands and to preserve the environment.
7.2.2. Value-added products
Thanks to these physicochemical characteristics (see Section 4), several beneficial compounds could be recovered from TPW, PPW and BPW, these compounds might be used in biological control (essential oil), in food as food additives and also used for cosmetic and medicinal interests.
7.2.2.1. Essential oil extraction and use
Antibiotics discovery was initially considered the miracle that would fight against microbial infections. But nowadays we talk about the end of the miracle, following the appearance of new microorganisms resisting to antibiotic therapies. During the last decade, a large number of essential oil and their compounds have been tested for their antimicrobial activity against bacteria, moulds, yeasts and other pathogenic and phytopathogenic microorganisms in order to minimize the resistance of microorganisms to antibiotics available in the medical market. In this section, the different techniques used for the extraction of essential oil, the chemical composition and the valorisation of essential oil extracted from TPW, PPW and BPW will be discussed.
There are several traditional extraction techniques that have been used to extract essential oil from vegetable matter, such as hydrodistillation, steam distillation, hydro-diffusion, cold pressing, cohobation, enfleurage and solvent extractions [34,[200], [201], [202], [203]]. Hydodistillation is among the first methods that have been applied for the essential oil extraction [203], and it has been applied as a reference method in laboratory-scale studies [204]. Steam distillation is the most common extraction technique in industry thanks to its high efficiency and its versatility for industrial large-scale production [205]. Nevertheless, several scientists, such as Gavahian et al. [203], Gavahian et al. [206] and Cook and Lanaras [201] have shown that traditional extraction and purification methods of essential oil have certain disadvantages, including long extraction time, necessity of large volumes of solvent, low efficiency, low yield, and environmental risks. In the last decade, several scientists have invented new methods for the essential oil extraction to minimise the inconveniences of the traditional methods, among these methods we can mention, the microwave assisted hydrodistillation [202], the microwave extraction without solvents [207], the liquid extraction under pressure [208], the ultrasound-assisted extraction [202], the assisted ohmic hydrodistillation [209], the ohmic-accelerated steam distillation [210], the super and subcritical fluid extraction method [211] and the supercritical CO2 extraction method that is a green method for volatile components extraction (essential oil compounds), this method is commonly used for the extraction of volatile components from the biomass peel [212]. These new methods have several advantages, such as the reduction of extraction time [202,206], the reduction of energy consumption [206], the reduction of environmental impacts [213], and also maintains better control of the extraction process [214]. However, these methods can also have disadvantages like the negative effect on extraction efficiency [213], the production of the same quality of essential oil as traditional methods [210], and their equipment that are too expensive [215].
Essential oil yields extracted from TPW, PPW and BPW have been influenced by species, origin, season, environmental factors, extraction technique and analytical method [135]. There are several scientists who have focused on the extraction of essential oil from these peels, such as Hosni et al. [135], Ferhat et al. [216] and Lota et al. [217] who have demonstrated a yield of 4.62 %, 0.25 %, 0.05−0.6 % of essential oil extracted from TPW, respectively. Hadrich et al. [80] have shown a yield of 0.20 % essential oil extracted from PPW and also Ara and Raofie [34] have indicated a high yield of 1.18 % essential oil which was obtained at a pressure of 350 atm, and a temperature of 55 °C. Kamal et al. [14] have shown that the yield of essential oil in BPW was 0.106 %. Table (5 ) reports the chemical composition of TPW, PPW and BPW essential oil.
Table 5.
Chemical composition of TPW, PPW and BPW essential oil.
| Component | *PPW (%) | Component | **TPW (%) | Component | ***BPW (%) |
|---|---|---|---|---|---|
| α-Pinene | 1.38 | α-Pinene | 0.1 | Isopentyl acetate | 3.84 |
| Camphene | 1.45 | 6-Methyl-5 hepten-2 one | 0.7 | Butyl butanoate | 0.80 |
| β-Pinene | 1.1 | Sabinene | 0.1 | Hexyl butanoate | 3.31 |
| β-caryophyllene | 0.67 | Myrcene | 0.3 | Hexyl isovalerate | 5.43 |
| Limonene | 0.63 | 1,8-Cineole | Tr* | Isoamyl hexanoate | 9.74 |
| Linalool | 0.34 | Limonene | 46.7 | 2-Undecanone | 0.59 |
| Trans-Pinocarveol | 0.28 | (Z)-β-ocimene | Tr* | p-Vinyl guaiacol | 3.72 |
| Borneol | 9.5 | (E)-β-ocimene | 0.4 | Eugenol | 27.60 |
| Terpinen-4-ol | 0.2 | Nonanal | 0.4 | Methyl eugenol | 2.36 |
| β-Cymene | 0.58 | Linalool | 0.7 | Butylated hydroxy toluene | 10.63 |
| p-Cymene | 0.8 | Cis-limonene oxide | Tr* | Trans-Isoelemicin | 8.06 |
| Cis-a-Bergamotene | 1.7 | Trans-limonene oxide | Tr** | Tetradecanal | 0.65 |
| 1,8-cineole | 2.86 | Citronellal | 1.3 | E-Isoamyl cinnamate | 0.35 |
| Butylbenzene | 3.64 | Rose furan epoxide | Tr* | Isoamyl dodecanoate | 1.18 |
| Hexylbenzene | 0.54 | α-Terpineol | Tr* | Methyl palmitate | 0.32 |
| Anethole | 0.1 | Decanol | 0.6 | Ethyl palmitate | 0.68 |
| Phenyloctane | 0.23 | Nerol | 2.3 | n-Heneicosane | 0.30 |
| Pentadecane | 0.25 | Carvone | Tr* | n-Docosane | 0.38 |
| Cadinene | 0.56 | Neral | 14.5 | Tetracosane | 0.89 |
| o-Guaiacol | 0.24 | Geraniol | 3.5 | Pentacosane | 0.94 |
| Camphor | 5.8 | Geranial | 19 | Hexacosane | 1.33 |
| Trans-(+)-Carveol | 0.1 | Neryl formate | Tr** | Octacosane | 3.01 |
| Bornyl acetate | 1.1 | Geranyl formate | Tr* | Nonacosane | 3.34 |
| α-bornyl acetate | 0.42 | Undecanal | Tr** | Triacontane | 2.51 |
| Tetradecane | 0.83 | δ-Elemene | Tr** | Dotriacontane | 0.74 |
| α-Terpineol | 1.65 | Citronellyl acetate | Tr* | Other compound | 7.3 |
| Furancarboxaldehyde | 0.39 | Neryl acetate | 1.1 | ||
| 5-methyl furfural | 0.02 | Geranyl acetate | 3.9 | ||
| β-Caryophyllene oxide | 1.73 | Dodecanal | Tr* | ||
| Lauric acid | 1.69 | β-Caryophyllene | 2.6 | ||
| Hexadecane | 0.76 | Trans-α-bergamotene | 0.1 | ||
| α-Cadinol | 1.8 | α-Humulene | 0.3 | ||
| Heptadecane | 1.3 | β-Bisabolene | 0.2 | ||
| Myristic acid | 1.58 | Germacrene B | Tr* | ||
| Octadecane | 1.25 | (E)-nerolidol | Tr** | ||
| Nonadecane | 1.54 | Caryophyllene oxide | 0.2 | ||
| Dibutyl phthalate | 4.43 | Trans-phytol | Tr* | ||
| Palmitic acid | 11.65 | Other compound | 0.6 | ||
| Eicosane | 1.4 | ||||
| Heneicosane | 1.73 | ||||
| Oleic acid | 12.49 | ||||
| Steric acid | 1.43 | ||||
| Docosane | 3.5 | ||||
| Octacosane | 2.53 | ||||
| Triacontane | 1.72 | ||||
| Other compound | 4.72 |
Notes: Tr*: trace amount (<0.1 %), Tr**: trace amount (<0.01 %). (%): Relative area percent (peak area relative to the total peak area).
Oleic acid, palmitic acid and borneol are the major compounds in PPW essential oil [13]. Numerous studies have proved that borneol is an anti-inflammatory and antioxidant for cortical neurons against oxygen and glucose deprivation [218]. Additionally, Givi et al. [28] have found that PPW essential oil has a significant antifungal activity against Penicillium italicum and Penicillium digitatum, causing post-harvest decay of tangerine, with an inhibition of 14.29 % of P. italicum and 17 % of P. digitatum. Also, the PPW essential oil containing camphor which provides anti-inflammatory, neurotoxic and mucolytic properties [219].
The BPW essential oil has proven a significant antibacterial and antifungal activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Aspergillus fumigatus, Candida albicans, Geotricum candidum and Trichophyton mentagrophytes [14]. The antibacterial and antifungal activities of BPW essential oil may be due to the high content of eugenol, α-cadinol and hydrocinnamyl acetate [220,221]. Also, Bennis et al. [222] have shown that eugenol has an antiseptic and anti-inflammatory activity permitting its use by dentists.
Citrus essential oil has been classified as generally recognized safely, because of its broad spectrum of biological activities, such as antimicrobial activity [223], antifungals [224], antioxidants [225], anti-inflammatory and anxiolytic agents [226]. TPW essential oil is traditionally applied as an antiseptic, antispasmodic, stomachic and sedative, diuretic and also to improve blood circulation [227]. Currently, several TPW essential oil compounds have also been reported for their antimicrobial and enzymatic activity [228,229] and also anti-tumoral due to its limonene and pinene content [230]. In addition, TPW essential oil has been shown to have antibacterial and antifungal activity, inhibiting the growth of several bacteria including Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa and Staphylococcus aureus [27], and several fungi including Penicillium italicum, P. digitatum, P. chrysogenum, Aspergillus niger, A. flavus, Alternaria alternata, Rhizoctonia solani, Curvularia lunata, Fusarium oxysporum and Helminthosporium oryzae [224,[231], [232], [233]]. TPW essential oil has also demonstrated an antiproliferative effect against human embryonic pulmonary fibroblasts [1]. They have been suitable for use as a glazing layer material to enhance the freshness preservation effect in the storage of frozen fish [13].
In this sub-section we have discussed the important usage of TPW, PPW and BPW essential oil, but their uses remain limited. For the previous reason, we urge the scientists to try the use of this essential oil in the various fields, especially in the agri-food and cosmetic field and also to try the combination of this essential oil with them and with antibiotics in order to increase its spectrum of uses, especially against pathogenic microorganisms that develop resistance every now and then. In addition, advanced research is needed concerning the toxicity and the impact of using each essential oil or each of its compound on the environment and human health, specifically to the equilibrium of the normal microflora.
7.2.2.2. Enzymes production
The enzymes are catalytic proteins synthesized by living organisms using substrates under appropriate conditions. Recently, the production of enzymes has increased considerably thanks to their extensive use in a variety of industrial applications, in animal feed, human food (candies, chocolates, cheese, yoghurt, beer and wine), chemical, pharmaceutical and other applications [30,171].
Many scientific research articles have focused on the search for new processes and substrates for the enzyme production. Recently, the use of bioresources, such as agri-food wastes for enzyme production has become a major challenge. Thanks to these physicochemical characteristics TPW, PPW and BPW that have been used as substrates for the production of various enzymes, such as the production of α-amylase by Aspergillus flavus AUMC 11685 from TPW [30], the production of lignocellulolytic enzymes (cellulases, xylanase, laccase, and manganese peroxidase) by Pleurotus dryinus through submerged fermentation of TPW [234], the production of invertase using Cladosporium cladosporioides [235] and using the fungal strain Aspergillus flavus [236] by solid-state fermentation with PPW as substrate, production of tannase from PPW as substrate by Aspergillus niger ITCC [237] and by Bacillus velezensis TA3 under solid-state fermentation [31] and also production of amylase from BPW by Aspergillus niger and Bacillus subtilis [238], and the production of lignocellulose-degrading enzymes (endoglucanase and xylanase) by the submerged fermentation of BPW by Pycnoporus coccineus [239].
7.2.2.3. Human food use
We have discussed that TPW, PPW and BPW are very rich with nutritional elements (see Section 4). Currently, there are a wide range of potential applications as food preservatives, stabilizers, supplements, prebiotics and quality enhancers of the components of TPW, PPW, BPW and their extracts. The pectin extracted from TPW, PPW and BPW is the main product used in human food, this product has various uses as an additive in jam, jellies, marmalades, and in wine as a thickener, stabilizer, texturizer, emulsifier and gelling agent [32,[240], [241], [242]]. Essential oil produced from TPW, BPW and PPW have several advantages in human nutrition, this oil can be used to improve the physicochemical properties of food in order to maintain the aroma, colour, flavour and also to preserve food against microbial contamination [33]. PPW could be used as a valuable ingredient in food products to easily provide nutritional elements, such as minerals, vitamins, β-carotene, and polysaccharides [243]. PPW could be used as a good source of dietary antioxidants with several human health benefits [244]. Pereira and Maraschin [245] have indicated that BPW could be used as a natural source of antioxidants and provitamin. Ventura et al. [246] and Wasila et al. [247] have shown that the addition of PPW extract to jam, juice and wine increased their concentration of phenols, flavonoids and thiols with a significant improvement of their characteristics and stability. Akhtar et al. [81] have found that PPW extracts could be used as prebiotics to inhibit pathogens and promote the growth of beneficial microbiota in the human intestines. Dried TPW has been used as a nutraceutical ingredient in dietary supplements, functional and conventional food [148]. TPW can be used as a culinary seasoning, food supplement and food additive for wine, candies and other food [248,249]. TPW carotenoids could be used as natural food colours as an alternative to artificial colours [74]. Besides, TPW tea contains salvestrols which promote cell death of breast, lung, ovarian and prostate cancer cells and have other human health benefits [148].
Over the past eight years, many studies have focused mainly on the use TPW, PPW and BPW for food storage and preservation. Saleh et al. [250] have shown that the addition of PPW powder is an effective tool for lowering the pH, total volatile nitrogen, and bacterial density in sausage in order to maintain the hygienic quality of beef sausage. Due to the presence of phenols, PPW could improve the quality of stored meat products, i.e. preservation of instrumental colour, limitation of microflora growth, retarding the oxidation of lipids and proteins [251]. Khalid et al. [252] have developed a polycaprolactone/starch/PPW film for antimicrobial packaging applications. Paari et al. [253] have demonstrated that treatment with PPW extract increased the stability of preserved goatfish against lipid oxidation. Khojah [254] has invented a biological coating based on fish gelatine, carrageenan and PPW extract to preserve the overall qualities of the fish fillet. BPW extracts have been shown to prevent oxidation of fish oil [85]. One can see that TPW, PPW and BPW are very polyvalent substrates that could produce a series of valuable compounds with various applications in the improvement and/or preservation of human food.
7.2.2.4. Medical and cosmetic use
In recent years, we have observed an increased interest in the use of TPW, PPW and BPW to improve the immune system and cure diseases, and also used in the cosmetics industry. Essential oil of TPW, PPW and BPW are the main compounds used in the medical and cosmetic field. Essential oil is flavouring agents used to reduce the unpleasant taste of medicines. They have significant antibacterial and antifungal activities against several pathogenic microbial strains [14,28,255] (See sub-section 7.2.2). In therapy, they could be incorporated into cosmetic formulations [34]. In the cosmetics industry, they are used as a fragrant ingredient in many preparations, such as toilet soap, detergent, cream, perfume and other household cleaning products [78,256,257].
Many current studies have indicated that BPW has several benefits in the medical field (Fig. 3). The BPW extract can be used for the treatment of enlarged prostate [258]. BPW has antibacterial activities against Proteus vulgaris and Klebsiella pneumoniae [87]. BPW extracts have used to inhibit the growth of Gloeophyllum trabeum, Rhodonia placenta and Trametes versicolor [35]. The study by Prakash et al. [259] has demonstrated that BPW extracts are an effective inhibitor of A. niger. BPW may be a natural alternative for killing a wart and reducing swelling and irritation after a mosquito bite [260]. Antioxidant compounds contain in BPW are reported to have biological and pharmacological effects that may reduce the negative effects of free radicals [261]. Silver nanoparticles prepared from BPW extract have shown an effective antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans [262]. BPW is a functional food source for cancer and heart disease [70]. Lipids, phenols, and tannins extracted from BPW have proven a good antimicrobial activity against Proteus vulgaris and Klebsiella pneumoniae [87]. PPW would have strong antibacterial, fungal, antiprotozoal and antioxidant properties (Fig. 3) [244,263,264], anti-healing properties [265], anti-inflammatory, anti-infectious, anti-mutagenic and hepatoprotective properties [[266], [267], [268]]. PPW extract has beneficial effects on plasma lipid profile, fatty acid levels and blood pressure for patients with type-2 diabetes [36]. PPW enriched films have shown greater antioxidant activity and antibacterial capacity than the control film based on bean protein [269]. The aqueous extract of PPW could be used to control brown rot caused by the two fungal pathogens Monilinia laxa and Monilinia fructigena, which have been considered as one of the main pathogens of pre and post-harvest tree fruits [37]. The study by Elsherbiny et al. [270] has demonstrated that methanol extract from PPW has a significant effect in inhibiting the germination of Fusarium sambucinum spores. Devatkal et al. [271] have shown a potential application of PPW extract as an antibacterial agent against Pseudomonas stutzeri isolated from poultry meat. Alexandre et al. [272] have found that PPW extracts have antimicrobial activity against eight different strains of pathogenic and contaminating bacteria and against five beneficial bacteria, including Lactobacillus and Bifidobacterium strains. Ismail et al. [273] also have reported an inhibitory effect of PPW extracts against B. subtilis, S. aureus, P. aeruginosa, E. coli, S. typhimurium and A. niger. Kharchoufi et al. [274] have reported the impact of methanolic extract of PPW and aqueous extracts of PPW against Pseudomonas putida. TPW has numerous activities (Fig. 3), especially antioxidant activities of polysaccharides. Polysaccharides have a wide range of pharmacological activities, such as antitumor, antioxidant, immunomodulatory and antiproliferative activities [40,[275], [276], [277]]. Dried TPW is used to treat cough, indigestion, asthma and other diseases [278]. TPW contains flavonoids, which have been known to have anti-inflammatory, anti-cancer and neuroprotective properties [[279], [280], [281]]. Phenolic extract of TPW inhibits proliferation of colorectal cancer cells [29]. Citric acid compounds from TPW extract used as potential tooth whitening materials [282]. Moreover, some bioactive compounds from TPW have been reported to form protective enzymes in the liver and block the deterioration of genetic material in cells [74]. TPW extracts have a capacity to inhibit the growth of Gloeophyllum trabeum, Rhodonia placenta and Trametes versicolor [35].
Fig. 3.
Different medical uses of TPW, PPW and BPW.
7.2.3. Microorganisms valorisation
TPW, PPW and BPW have a relatively high microbial biodiversity [90,283]. Over the last few years, numerous research studies have focused on the recovery of microorganisms isolated from these wastes in the medical, agronomic and industrial fields. For BPW, there are several authors who have been valorising their microorganisms, such as Vaidya and Prasad [284] who have isolated a Bacillus sp able to produce a considerable amount of heat-stable levansucrase with a molecular size of 52 kDa. Grevitara et al. [285] who have isolated and identified a cellulose-degrading bacteria from BPW compost. Mazareli et al. [286] who have isolated a Bacillus sp from banana waste for the production of hydrogen and acids from complex organic substrates in the bioaugmentation process. In addition, Amilia et al. [287] have isolated and screened pectinolytic fungi from BPW for the production of pectinases. For TPW, we found only a few studies that showed the value of its microorganisms, such as the study of Ling et al. [288] that has focused to identify new endophytic yeast strains able to produce Indole-3-acetic acid (IAA) from TPW. Also, Maeno et al. [289] have isolated Pseudofructophilic Leuconostoc citreum Strain F192-5 from TPW able to produce mainly lactate and acetate, with trace amounts of ethanol, from d-glucose metabolism. For PPW, El Barnossi et al. [283] have isolated and identified a Bacillus sp. strain Gn-A11-18 from pomegranate waste that has shown a significant antifungal activity against Candida albicans and A. niger. Almost all PPW studies have focused only about the valorisation of PPW as a substrate, while the valorisation of microorganisms associated with the decomposition of PPW is poorly documented. It is very clear that the microorganisms associated with TPW, PPW and BPW are poorly valorised, which leaves the field of research open in this section in order to better valorise the microorganisms and to benefit as much as possible from this type of bioresources.
8. Conclusion
The physicochemical, microbiological characteristics and nutritional capacity of TPW, PPW and BPW determine the various ways of their valorisation. In this study, we have reviewed various papers published in various journals about the different valorisation pathways of these peels and the factors influencing each one. We have also reviewed articles concerning the impact of these wastes both on the environment and on human health. Studies have shown the use of TPW, PPW and BPW for animal feed, improvement of soil organic matter and nutrient content by using these peels as an organic amendment or for the production of specific composts, in this case preferably in agricultural lands near the processing industries of these fruits in order to minimise transport costs. The possibility of recuperating the energy from TPW, PPW and BPW has been explored by several researchers, who have measured the yield of biogas, bioethanol and bio-hydrogen under different conditions. Yet, the energy recovery systems of these peels should be further improved by appropriate and sustainable techniques in order to minimize the toxic effects of essential oil on microbial load and biodiversity and to optimize the physicochemical characteristics of these peels before their bioconversion.
The current trend of scientific research indicates an increase in articles talking about of the benefits of many compounds recovered from TPW, PPW and BPW. It suggests their use in human food, cosmetic and pharmaceutical industries, such as pectin, flavonoids, proteins, polyphenols, flavouring agents and citric acids, etc. This use could be considered very promising, but it is currently limited by the high processing costs.
As discussed in the present study, many microorganisms have isolated from TPW, PPW and BPW are economically interesting in the medical, agronomic and industrial fields. Therefore, further studies need to be conducted to investigate the microorganisms associated with these peels and their effects both the soil in the case of their use as organic soil conditioner and also on the water in the case of their use as bio-adsorbent.
The study of TPW, PPW and BPW valorisation and their applications in the various fields are still a hotspot for scientific research, which requires a detailed exploration of the mechanisms and long-term risks of their valorisation. Moreover, we still have a long road before the perfect valorisation of TPW, PPW and BPW and the best preservation of the environment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge scientific support received from Laboratory of Biotechnology, Environment, Agri-Food and Health, Faculty of Sciences Dhar El Mahraz, Sidi Mohammed Ben Abdellah University, Fez, Morocco. Special thanks to professor Jawad Garmah and to professor Bounouar Mohammed for their careful oversight, and also to the industrious reviewer and the Editor for their support.
Contributor Information
Azeddin El Barnossi, Email: azeddin.elbarnossi@usmba.ac.ma.
Fatimazhrae Moussaid, Email: mssdfati@gmail.com.
Abdelilah Iraqi Housseini, Email: iraqi4bdel@gmail.com.
References
- 1.Mahesar S.A., Kori A.H., Sherazi S.T.H., Kandhro A.A., Laghari Z.H. Pomegranate (punica granatum) seed oil. In: Ramadan M.F., editor. Fruit Oils Chem. Funct. Springer; Cham: 2019. pp. 691–709. [DOI] [Google Scholar]
- 2.Celik I., Temur A., Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem. Toxicol. 2009;47:145–149. doi: 10.1016/j.fct.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Lansky E.P., Newman R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Kahramanoğlu İ., Usanmaz S. CRC Press; 2016. Pomegranate Production and Marketing. [Google Scholar]
- 5.García-Salas P., Gómez-Caravaca A.M., Arráez-Román D., Segura-Carretero A., Guerra-Hernández E., García-Villanova B., Fernández-Gutiérrez A. Influence of technological processes on phenolic compounds, organic acids, furanic derivatives, and antioxidant activity of whole-lemon powder. Food Chem. 2013;141:869–878. doi: 10.1016/j.foodchem.2013.02.124. [DOI] [PubMed] [Google Scholar]
- 6.El-Otmani M., Ait-Oubahou A., Zacarías L. Postharvest Biol. Technol. Trop. Subtrop. Fruits. Elsevier BV; 2011. Citrus spp: orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime; pp. 437–516. [DOI] [Google Scholar]
- 7.FAS A., Adeyemi O., Oluwole O., Oladunmoye O., Ayo-Ajasa O., Anuoluwatelemi J. Effects of treated banana peel meal on the feed efficiency, digestibility and cost effectiveness of broiler chickens diet. J. Vet. Sci. Anim. Husb. 2015;3 doi: 10.15744/2348-9790.1.603. [DOI] [Google Scholar]
- 8.Pathak P.D., Mandavgane S.A., Kulkarni B.D. Characterizing fruit and vegetable peels as bioadsorbents. Curr. Sci. 2016;110:2114–2123. doi: 10.18520/cs/v110/i11/2114-2123. [DOI] [Google Scholar]
- 9.Kahramanoglu I. Trends in pomegranate sector : production. Postharvest Handling and Marketing. 2019 [Google Scholar]
- 10.2020. USDA (United States Department of Agriculture, Citrus: World Markets and Trade, Washington.https://www.fas.usda.gov/data/citrus-world-markets-and-trade%0Ahttp://www.fas.usda.gov/data/citrus-world-markets-and-trade [Google Scholar]
- 11.2020. FAOSTAT.www.fao.org [Google Scholar]
- 12.Mahato N., Sharma K., Sinha M., Cho M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: current trends and future perspectives. J. Funct. Foods. 2018;40:307–316. doi: 10.1016/j.jff.2017.11.015. [DOI] [Google Scholar]
- 13.He Q., Xiao K. The effects of tangerine peel (Citri reticulatae pericarpium) essential oils as glazing layer on freshness preservation of bream (Megalobrama amblycephala) during superchilling storage. Food Control. 2016;69:339–345. doi: 10.1016/j.foodcont.2016.05.019. [DOI] [Google Scholar]
- 14.Kamal A.M., El-tantawy M.E., Haggag E.G., Shukr M.H., El-garhy A.M.G., Lithy R.M. Vol. 8. 2019. pp. 1808–1816. (Chemical and Biological Analysis of Essential Oils and Pectins Of Banana, Cantaloupe Peels, Guava Pulp and Formulation of Banana Pectin Gel). [Google Scholar]
- 15.Varma V.S., Shabtay A., Yishay M., Mizrahi I., Shterzer N., Freilich S., Medina S., Agmon R., Laor Y. Diet supplementation with pomegranate peel extract altered odorants emission from fresh and incubated calves’ feces. Front. Sustain. Food Syst. 2018 doi: 10.3389/fsufs.2018.00033. [DOI] [Google Scholar]
- 16.Nuriyasa I.M., Puspani E., Bidura I.G.N.G. Growth, feed digestion and carcass characteristics of rabbits fed with banana peel (Acuminata balbisiana) supplementation. Pakistan J. Nutr. 2019;19:19–24. doi: 10.3923/pjn.2020.19.24. [DOI] [Google Scholar]
- 17.Cao Q., Huang Z., Liu S., Wu Y. 2019. Potential of Punica granatum Biochar to Adsorb Cu(II) in Soil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anastopoulos I., Omirou M., Stephanou C., Oulas A. Valorization of agricultural wastes could improve soil fertility and mitigate soil direct N2O emissions. J. Environ. Manage. 2019;250:109389. doi: 10.1016/j.jenvman.2019.109389. [DOI] [PubMed] [Google Scholar]
- 19.Islam M., Halder M., Siddique M.A.B., Razir S.A.A., Sikder S., Joardar J.C. Banana peel biochar as alternative source of potassium for plant productivity and sustainable agriculture. Int. J. Recycl. Org. Waste Agric. 2019;8:407–413. doi: 10.1007/s40093-019-00313-8. [DOI] [Google Scholar]
- 20.Amin M.T., Alazba A.A., Shafiq M. Removal of copper and lead using banana biochar in batch adsorption systems: isotherms and kinetic studies. Arab. J. Sci. Eng. 2018;43:5711–5722. doi: 10.1007/s13369-017-2934-z. [DOI] [Google Scholar]
- 21.Abdić Š., Memić M., Šabanović E., Sulejmanović J., Begić S. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste : tangerine peel. Int. J. Environ. Sci. Technol. 2018;15:2511–2518. doi: 10.1007/s13762-018-1645-7. [DOI] [Google Scholar]
- 22.Demiray E., Ertuğrul Karatay S., Dönmez G. Efficient bioethanol production from pomegranate peels by newly isolated Kluyveromyces marxianus, energy sources, part a recover. Util. Environ. Eff. 2020;42:709–718. doi: 10.1080/15567036.2019.1600621. [DOI] [Google Scholar]
- 23.John I., Yaragarla P., Appusamy A. Production of bioethanol from Banana Peel using isolated cellulase from aspergillus Niger. In: Davim P., Paulo J., Department of Mechanical Engineering, University of Aveiro Aveiro(Ed.), Sivasubramanian V., Subramanian S., editors. Glob. Challenges Energy Environ. Lect. Notes Multidiscip. Ind. Eng. Springer; Singapore, Aveiro: 2020. pp. 9–18. [DOI] [Google Scholar]
- 24.Martínez-Ruano J.A., Caballero-Galván A.S., Restrepo-Serna D.L., Cardona C.A. Techno-economic and environmental assessment of biogas production from banana peel (Musa paradisiaca) in a biorefinery concept. Environ. Sci. Pollut. Res. 2018;25:35971–35980. doi: 10.1007/s11356-018-1848-y. [DOI] [PubMed] [Google Scholar]
- 25.Jain K., Suryawanshi P., Chaudhari A. Recovery of acerbic anaerobic digester for biogas production from pomegranate shells using organic loading approach. Indian J. Biochem. Biophys. 2020;57:86–94. [Google Scholar]
- 26.Camargo F.P., Sakamoto I.K., Silva E.L., Duarte I.C.S., Varesche M.B.A. Bioaugmentation with Enterococcus casseliflavus: a hydrogen-producing strain isolated from Citrus peel waste. Waste Biomass Valorization. 2020 doi: 10.1007/s12649-020-01049-7. [DOI] [Google Scholar]
- 27.Yi F., Jin R., Sun J., Ma B., Bao X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT. 2018;95:346–353. doi: 10.1016/j.lwt.2018.05.011. [DOI] [Google Scholar]
- 28.Givi F., Gholami M., Massah A. Application of pomegranate peel extract and essential oil as a safe botanical preservative for the control of postharvest decay caused by Penicillium italicum and Penicillium digitatum on “Satsuma” mandarin. J. Food Saf. 2019;39:1–11. doi: 10.1111/jfs.12639. [DOI] [Google Scholar]
- 29.Esparza-Martínez J.F., Solis P.G., Miranda-López R., Peña-Caballero V. Inhibition of proliferation of colorectal Cancer cells by phenolic extracts of mandarin (Citrus reticulate) and lime (Citrus aurantifolia) fruit waste. J. Food Nutr. Res. 2019;7:560–567. doi: 10.12691/jfnr-7-8-2. [DOI] [Google Scholar]
- 30.Ali E.H., El-Nagdy M.A., Al-Garni S.M., Ahmed M.S., Rawaa A.M. Enhancement of alpha amylase production by Aspergillus flavus AUMC 11685 on mandarin (Citrus reticulata) peel using submerged fermentation. Eur. J. Biol. Res. 2017;7:154–164. doi: 10.5281/zenodo.818271. [DOI] [Google Scholar]
- 31.Lekshmi R., Arif Nisha S., Kaleeswaran B., Alfarhan A.H. Pomegranate peel is a low-cost substrate for the production of tannase by Bacillus velezensis TA3 under solid state fermentation. J. King Saud Univ. - Sci. 2020;32:1831–1837. doi: 10.1016/j.jksus.2020.01.022. [DOI] [Google Scholar]
- 32.Twinomuhwezi H., Godswill A.C., Kahunde D. Extraction and Characterization of Pectin from Orange (Citrus sinensis), Lemon (Citrus limon) and Tangerine (Citrus tangerina) Am. J. Phys. Sci. 2020;1:17–30. [Google Scholar]
- 33.Tomar O., Akaeca G. Effects of ice cream produced with Lemon, mandarin, and orange peel essential oils on some physicochemical, microbiological and sensorial properties. Kocatepe Vet. J. 2019;12:62–70. doi: 10.30607/kvj.499415. [DOI] [Google Scholar]
- 34.Ara K.M., Raofie F. Application of response surface methodology for the optimization of supercritical fluid extraction of essential oil from pomegranate (Punica granatum L.) peel. J. Food Sci. Technol. 2016;53:3113–3121. doi: 10.1007/s13197-016-2284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbero-López A. Antifungal activity of several vegetable origin household waste extracts against wood-decaying fungi in vitro. Waste Biomass Valor. 2020 doi: 10.1007/s12649-020-01069-3. [DOI] [Google Scholar]
- 36.Grabež M., Škrbić R., Stojiljković M.P., Rudić-Grujić V., Paunović M., Arsić A., Petrović S., Vučić V., Mirjanić-Azarić B., Šavikin K., Menković N., Janković T., Vasiljević N. Beneficial effects of pomegranate peel extract on plasma lipid profile, fatty acids levels and blood pressure in patients with diabetes mellitus type-2: a randomized, double-blind, placebo-controlled study. J. Funct. Foods. 2020;64:102692. doi: 10.1016/j.jff.2019.103692. [DOI] [Google Scholar]
- 37.El Khetabi A., Lahlali R., Askarne L., Ezrari S., El Ghadaroui L., Tahiri A., Hrustić J., Amiri S. Efficacy assessment of pomegranate peel aqueous extract for brown rot (Monilinia spp.) disease control. Physiol. Mol. Plant Pathol. 2020;110 doi: 10.1016/j.pmpp.2020.101482. [DOI] [Google Scholar]
- 38.Azimi F., Fatemi M.H. Multivariate curve resolution-correlation optimized warping applied to the complex GC-MS signals; toward comparative study of peel chemical variability of Citrus aurantium L. varieties. Microchem. J. 2018;143:99–109. doi: 10.1016/j.microc.2018.07.041. [DOI] [Google Scholar]
- 39.Xu M., Tian G., Zhao C., Ahmad A., Zhang H., Bi J., Xiao H., Zheng J. Infrared drying as a quick preparation method for dried tangerine peel. Int. J. Anal. Chem. 2017;2017 doi: 10.1155/2017/6254793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G.T., Ma X.M., Liu S.T., Liao Y.L., Zhao G.Q. Isolation, purification and antioxidant activities of polysaccharides from Grifola frondosa. Carbohydr. Polym. 2012;89:61–66. doi: 10.1016/j.carbpol.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 41.Kashyap K., Kashyap D., Nitin M., Ramchiary N., Banu S. Characterizing the nutrient composition, physiological maturity, and effect of cold storage in khasi mandarin (Citrus reticulata blanco) Int. J. Fruit Sci. 2019:1–20. doi: 10.1080/15538362.2019.1666334. [DOI] [Google Scholar]
- 42.Pathak P.D., Mandavgane S.A., Kulkarni B.D. Fruit peel waste: characterization and its potential uses. Curr. Sci. 2017;113:444–454. doi: 10.18520/cs/v113/i03/444-454. [DOI] [Google Scholar]
- 43.Albarelli J.Q., Rabelo R.B., Santos D.T., Beppu M.M., Meireles M.A.A. Effects of supercritical carbon dioxide on waste banana peels for heavy metal removal. J. Supercrit. Fluids. 2011;58:343–351. doi: 10.1016/j.supflu.2011.07.014. [DOI] [Google Scholar]
- 44.Anonymous . Hortic. Stat. Div. Dep. Agric. Coop. Farmers’ Welf. Minist. Agric. Farmers’ Welfare, Gov. India; 2018. Horticultural Statistics at a Glance 2018; pp. 1–490.http://nhb.gov.in/PDFViwer.aspx?enc=3ZOO8K5CzcdC/Yq6HcdIxC0U1kZZenFuNVXacDLxz28= [Google Scholar]
- 45.Jalal H., Pal M.A., Ahmad S.R., Rather M., Andrabi M., Hamdani S. Physico-chemical and functional properties of pomegranate peel and seed powder. Pharma Innov. J. 2018;7:1127–1131. www.thepharmajournal.com [Google Scholar]
- 46.Chanasut U., Rattanpanone N., Boonyakiat D., Kampoun W. Chilling injury susceptibility of early-season “Sai Nam Peung” tangerine fruit and alteration of α-farnesene and conjugated trienols during low temperature storage. Chiang Mai J. Sci. 2018;45:147–153. [Google Scholar]
- 47.Chater J.M., Merhaut D.J., Jia Z., Mauk P.A., Preece J.E. Fruit quality traits of ten California-grown pomegranate cultivars harvested over three months. Sci. Hortic. (Amsterdam) 2018;237:11–19. doi: 10.1016/j.scienta.2018.03.048. [DOI] [Google Scholar]
- 48.FAO, http://www.fao.org/zhc/detail-events/fr/c/447844/., (2016).
- 49.Hossain M. An overview on post harvest handling and commercial processing of horticultural crops in NEH region of India. Int. J. Sci. Res. 2015;4:2304–2308. doi: 10.21275/v4i11.nov151690. [DOI] [Google Scholar]
- 50.Coelho de Queiroz L.G., Oliveira de Jesus M., Aguiar F.S., Paraizo E.A., Soares M.D.C., Pinheiro J.D.S.M., Mizobutsi G.P., Rodrigues M.L.M., Santos T.C. Influence of different ‘Prata-Anã’ banana bunch ages on post-harvest quality. Am. J. Exp. Agric. 2019;36:1–14. doi: 10.9734/jeai/2019/v36i130227. [DOI] [Google Scholar]
- 51.Rokaya P.R., Baral D.R., Gautam D.M., Shrestha A.K., Paudyal K.P. Effect of altitude and maturity stages on quality attributes of mandarin (Citrus reticulata blanco) Am. J. Plant Sci. 2016;7:958–966. doi: 10.4236/ajps.2016.76091. [DOI] [Google Scholar]
- 52.Murmu S.B., Mishra H.N. Post-harvest shelf-life of banana and guava: mechanisms of common degradation problems and emerging counteracting strategies. Innov. Food Sci. Emerg. Technol. 2018;49:20–30. doi: 10.1016/j.ifset.2018.07.011. [DOI] [Google Scholar]
- 53.Tietel Z., Lewinsohn E., Fallik E., Porat R. Importance of storage temperatures in maintaining flavor and quality of mandarins. Postharvest Biol. Technol. 2012;64:175–182. doi: 10.1016/j.postharvbio.2011.07.009. [DOI] [Google Scholar]
- 54.Ladaniya M. Storage systems and response of citrus fruits. In: Inc E., editor. Citrus Fruit Biol. Technol. Eval. Citus fruit; Atlanta: 2008. pp. 333–373. [DOI] [Google Scholar]
- 55.Kerbel E., Madrid M. Control. Modif. Atmos. Fresh Fresh-Cut Prod. Elsevier Inc.; 2020. Tropical fruits: bananas; pp. 353–362. [DOI] [Google Scholar]
- 56.Kazemi F., Jafararpoor M., Golparvar A. Effects of sodium and calcium treatments on the shelf life and quality of pomegranate. Int. J. Farming Allied Sci. 2013;2:1375–1378. [Google Scholar]
- 57.Okatan V., Çolak A.M., Güçlü S.F., Gündoğdu M. The comparison of antioxidant compounds and mineral content in some pomegranate (Punica granatum L.) genotypes grown in the east of Turkey. Acta Sci. Pol. Hortorum Cultus. 2018;17:201–211. doi: 10.24326/asphc.2018.4.18. [DOI] [Google Scholar]
- 58.Mushtaq M. Fruit Juices Extr. Compos. Qual. Anal. Elsevier BV; 2018. Extraction of fruit juice: an overview; pp. 131–159. [DOI] [Google Scholar]
- 59.Álvarez R., Carvalho C.P., Sierra J., Lara O., Cardona D., Londoño-Londoño J. Citrus juice extraction systems: effect on chemical composition and antioxidant activity of clementine juice. J. Agric. Food Chem. 2012;60:774–781. doi: 10.1021/jf203353h. [DOI] [PubMed] [Google Scholar]
- 60.Ağçam E., Akyildiz A., Dündar B. Fruit Juices Extr. Compos. Qual. Anal. Elsevier BV; 2018. Thermal pasteurization and microbial inactivation of fruit juices; pp. 309–339. [DOI] [Google Scholar]
- 61.Jackson-Davis A., Mendonca A., Hale S., Jackson J., King A., Jackson J. Food Feed Saf. Syst. Anal. Elsevier Inc.; 2018. Microbiological safety of unpasteurized fruit and vegetable juices sold in juice bars and small retail outlets; pp. 213–225. [DOI] [Google Scholar]
- 62.Fadavi A., Barzegar M., Azizi M.H., Bayat M. Note. Physicochemical composition of ten pomegranate cultivars (punica granatum L.) grown in Iran. Food Sci. Technol. Int. 2005;11:113–119. doi: 10.1177/1082013205052765. [DOI] [Google Scholar]
- 63.Sreekumar S., Sithul H., Muraleedharan P., Azeez J.M., Sreeharshan S. Pomegranate fruit as a rich source of biologically active compounds. Biomed Res. Int. 2014:12. doi: 10.1155/2014/686921. ID 686921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mirdehghan S.H., Rahemi M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007;111:120–127. doi: 10.1016/j.scienta.2006.10.001. [DOI] [Google Scholar]
- 65.Tehranifar A., Zarei M., Nemati Z., Esfandiyari B., Vazifeshenas M.R. Investigation of physico-chemical properties and antioxidant activity of twenty Iranian pomegranate (Punica granatum L.) cultivars. Sci. Hortic. (Amsterdam) 2010;126:180–185. doi: 10.1016/j.scienta.2010.07.001. [DOI] [Google Scholar]
- 66.Kandylis P., Kokkinomagoulos E. Food applications and potential health benefits of pomegranate and its derivatives. Foods. 2020;9 doi: 10.3390/foods9020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tabaraki R., Heidarizadi E., Benvidi A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012;98:16–23. doi: 10.1016/j.seppur.2012.06.038. [DOI] [Google Scholar]
- 68.Sansone A.C.M.B., Sansone M., dos Santos Dias C.T., Oliveira do Nascimento J.R. Oral administration of banana lectin modulates cytokine profile and abundance of T-cell populations in mice. Int. J. Biol. Macromol. 2016;89:19–24. doi: 10.1016/j.ijbiomac.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 69.Bediako J.K., Sarkar A.K., Lin S., Zhao Y., Song M.H., Choi J.W., Cho C.W., Yun Y.S. Characterization of the residual biochemical components of sequentially extracted banana peel biomasses and their environmental remediation applications. Waste Manag. 2019;89:141–153. doi: 10.1016/j.wasman.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Someya S., Yoshiki Y., Okubo K. Antioxidant compounds from bananas (Musa Cavendish) Food Chem. 2002;79:351–354. doi: 10.1016/S0308-8146(02)00186-3. [DOI] [Google Scholar]
- 71.Kanazawa K., Sakakibara H. High content of dopamine, a strong antioxidant, in cavendish banana. J. Agric. Food Chem. 2000;48:844–848. doi: 10.1021/jf9909860. [DOI] [PubMed] [Google Scholar]
- 72.Ghanem N., Mihoubi D., Kechaou N., Mihoubi N.B. Microwave dehydration of three citrus peel cultivars: effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind. Crops Prod. 2012;40:167–177. doi: 10.1016/j.indcrop.2012.03.009. [DOI] [Google Scholar]
- 73.Won M.Y., Lee S.J., Min S.C. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2017;39:25–32. doi: 10.1016/j.ifset.2016.10.021. [DOI] [Google Scholar]
- 74.Sharma K., Mahato N., Cho M.H., Lee Y.R. Converting citrus wastes into value-added products: economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Boukroufa M., Boutekedjiret C., Petigny L., Rakotomanomana N., Chemat F. Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 2015;24:72–79. doi: 10.1016/j.ultsonch.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 76.Satari B., Karimi K. Citrus processing wastes: environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018;129:153–167. doi: 10.1016/j.resconrec.2017.10.032. [DOI] [Google Scholar]
- 77.Sharma K., Mahato N., Lee Y.R. 2018. Extraction, Characterization and Biological Activity of Citrus Flavonoids. [Google Scholar]
- 78.Zema D.A., Calabrò P.S., Folino A., Tamburino V., Zappia G., Zimbone S.M. Valorisation of citrus processing waste: a review. Waste Manag. 2018;80:252–273. doi: 10.1016/j.wasman.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Fischer U.A., Jaksch A.V., Carle R., Kammerer D.R. Influence of origin source, different fruit tissue and juice extraction methods on anthocyanin, phenolic acid, hydrolysable tannin and isolariciresinol contents of pomegranate (Punica granatum L.) fruits and juices. Eur. Food Res. Technol. 2013;237:209–221. doi: 10.1007/s00217-013-1981-2. [DOI] [Google Scholar]
- 80.Hadrich F., Cherif S., Gargouri Y.T., Adel S. Antioxidant and lipase inhibitory activities and essential oil composition of pomegranate peel extracts. J. Oleo Sci. 2014;63:515–525. doi: 10.5650/jos.ess13163. [DOI] [PubMed] [Google Scholar]
- 81.Akhtar S., Ismail T., Fraternale D., Sestili P. Pomegranate peel and peel extracts: chemistry and food features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 82.Anwar J., Shafique U., Waheed-uz-Zaman, Salman M., Dar A., Anwar S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010;101:1752–1755. doi: 10.1016/j.biortech.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Silva C.R., Gomes T.F., Andrade G.C.R.M., Monteiro S.H., Dias A.C.R., Zagatto E.A.G., Tornisielo V.L. 2013. Banana Peel As an Adsorbent for Removing Atrazine and Ametryne From Waters; pp. 6–11. [DOI] [PubMed] [Google Scholar]
- 84.González-Montelongo R., Gloria Lobo M., González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010;119:1030–1039. doi: 10.1016/j.foodchem.2009.08.012. [DOI] [Google Scholar]
- 85.Anal A.K., Jaisanti S., Noomhorm A. Enhanced yield of phenolic extracts from banana peels (Musa acuminata Colla AAA) and cinnamon barks (Cinnamomum varum) and their antioxidative potentials in fish oil. J. Food Sci. Technol. 2014;51:2632–2639. doi: 10.1007/s13197-012-0793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Comim S.R.R., Madella K., Oliveira J.V., Ferreira S.R.S. Supercritical fluid extraction from dried banana peel (Musa spp., genomic group AAB): extraction yield, mathematical modeling, economical analysis and phase equilibria. J. Supercrit. Fluids. 2010;54:30–37. doi: 10.1016/j.supflu.2010.03.010. [DOI] [Google Scholar]
- 87.Fapohunda S., Mmom J.U., Fakeye F. Proximate analyses, phytochemical screening and antibacterial potentials of bitter cola, cinnamon, ginger and banana peel. Acad. Arena. 2012;4:8–15. [Google Scholar]
- 88.Wang C., Zuo J., Chen X., Xing W., Xing L., Li P., Lu X., Li C. Microbial community structures in an integrated two-phase anaerobic bioreactor fed by fruit vegetable wastes and wheat straw. J. Environ. Sci. (China) 2014;26:2484–2492. doi: 10.1016/j.jes.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 89.Cerda A., Artola A., Font X., Barrena R., Gea T., Sánchez A. Composting of food wastes: status and challenges. Bioresour. Technol. 2018;248:57–67. doi: 10.1016/j.biortech.2017.06.133. [DOI] [PubMed] [Google Scholar]
- 90.El Barnossi A., Moussaid F., Iraqi H.A. Microbial sequences associated with pomegranate and tangerine peels in water and soil digestion. Proc. BIOSUNE. 2018;1:21–25. [Google Scholar]
- 91.El Barnossi A., Moussaid F., Iraqi H.A. Quantitative research of systematic and functional microbial groups associated with decaying solid green household waste in water and soil. Polish J. Environ. Stud. 2020;29:2631–2639. doi: 10.15244/pjoes/112365. [DOI] [Google Scholar]
- 92.El Barnossi A., Moussaid F., Iraqi H.A. Study of mycoflora associated with the decomposition of solid green household waste in the natural environment. Asian Jr. Microbiol. Biotech. Env. Sc. 2019;21:46–53. [Google Scholar]
- 93.Krčmar D., Tenodi S., Grba N., Kerkez D., Watson M., Rončević S., Dalmacija B. Preremedial assessment of the municipal landfill pollution impact on soil and shallow groundwater in Subotica, Serbia. Sci. Total Environ. 2018;615:1341–1354. doi: 10.1016/j.scitotenv.2017.09.283. [DOI] [PubMed] [Google Scholar]
- 94.Ubavin D., Agarski B., Maodus N., Stanisavljevic N., Budak I. A model for prioritizing landfills for remediation and closure: a case study in Serbia. Integr. Environ. Assess. Manag. 2018;14:105–119. doi: 10.1002/ieam.1967. [DOI] [PubMed] [Google Scholar]
- 95.Tenodi S., Krčmar D., Agbaba J., Zrnić K., Radenović M., Ubavin D., Dalmacija B. Assessment of the environmental impact of sanitary and unsanitary parts of a municipal solid waste landfill. J. Environ. Manage. 2020;258 doi: 10.1016/j.jenvman.2019.110019. 110019 Contents. [DOI] [PubMed] [Google Scholar]
- 96.Mari M., Nadal M., Schuhmacher M., Domingo J.L. Exposure to heavy metals and PCDD/Fs by the population living in the vicinity of a hazardous waste landfill in Catalonia, Spain: health risk assessment. Environ. Int. 2009;35:1034–1039. doi: 10.1016/j.envint.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Akinbile C.O. Environmental impact of landfill on groundwater quality and agricultural soils in Nigeria. Soil Water Res. 2012;7:18–26. doi: 10.17221/4/2011-swr. [DOI] [Google Scholar]
- 98.Stefania G.A., Zanotti C., Bonomi T., Fumagalli L., Rotiroti M. Determination of trigger levels for groundwater quality in landfills located in historically human-impacted areas. Waste Manag. 2018;75:400–406. doi: 10.1016/j.wasman.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 99.Zamri N.S., Khairul M., Kamarudin A., Armi M., Samah A., Shakir A., Saudi M., Wahab N.A., Hafiz M. The environmental pollution and solid waste management in Malaysia. Int. J. Acad. Res. Bus. Soc. Sci. 2019;9:12–23. doi: 10.6007/IJARBSS/v9-i12/6662. [DOI] [Google Scholar]
- 100.Han Z., Ma H., Shi G., He L., Wei L., Shi Q. A review of groundwater contamination near municipal solid waste landfill sites in China. Sci. Total Environ. 2016;569–570:1255–1264. doi: 10.1016/j.scitotenv.2016.06.201. [DOI] [PubMed] [Google Scholar]
- 101.Kjeldsen P., Barlaz M.A., Rooker A.P., Baun A., Ledin A., Christensen T.H. Present and long-term composition of MSW landfill leachate: a review. Crit. Rev. Environ. Sci. Technol. 2002;32:297–336. doi: 10.1080/10643380290813462. [DOI] [Google Scholar]
- 102.Minareci O., Minareci E. Determination of heavy metals in fish, water and, Iran. J. Environ. Heal. Sci. Eng. 2009;6:73–80. [Google Scholar]
- 103.Stefania G.A., Rotiroti M., Buerge I.J., Zanotti C., Nava V., Leoni B., Fumagalli L., Bonomi T. Identification of groundwater pollution sources in a landfill site using artificial sweeteners, multivariate analysis and transport modeling. Waste Manag. 2019;95:116–128. doi: 10.1016/j.wasman.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Azim M., Rahman M.M., Khan R.H., Kamal A. Characteristics of leachate generated At landfill sites and probable risks of surface and groundwater pollution in the surrounding areas : a case study of matuail landfill site, Dhaka. J. Bangladesh Acad. Sci. 2011;35:153–160. [Google Scholar]
- 105.Milosevic N., Thomsen N.I., Juhler R.K., Albrechtsen H.J., Bjerg P.L. Identification of discharge zones and quantification of contaminant mass discharges into a local stream from a landfill in a heterogeneous geologic setting. J. Hydrol. 2012;446–447:13–23. doi: 10.1016/j.jhydrol.2012.04.012. [DOI] [Google Scholar]
- 106.Smahi D., El Hammoumi O., Fekri A. Assessment of the impact of the landfill on groundwater quality: a case study of the mediouna site, Casablanca, Morocco. J. Water Resour. Prot. 2013;5:440–445. doi: 10.4236/jwarp.2013.54043. [DOI] [Google Scholar]
- 107.Bünemann E.K., Schwenke G.D., Van Zwieten L. Impact of agricultural inputs on soil organisms - a review. Aust. J. Soil Res. 2006;44:379–406. doi: 10.1071/SR05125. [DOI] [Google Scholar]
- 108.Domene X., Ramírez W., Mattana S., Alcañiz J.M., Andrés P. Ecological risk assessment of organic waste amendments using the species sensitivity distribution from a soil organisms test battery. Environ. Pollut. 2008;155:227–236. doi: 10.1016/j.envpol.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Lin R., Wang X., Luo Y., Du W., Guo H., Yin D. Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.) Chemosphere. 2007;69:89–98. doi: 10.1016/j.chemosphere.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 110.Meena M.D., Yadav R.K., Narjary B., Yadav G., Jat H.S., Sheoran P., Meena M.K., Antil R.S., Meena B.L., Singh H.V., Singh Meena V., Rai P.K., Ghosh A., Moharana P.C. Municipal solid waste (MSW): strategies to improve salt affected soil sustainability: a review. Waste Manag. 2019;84:38–53. doi: 10.1016/j.wasman.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 111.El-Fadel M., Findikakis A.N., Leckie J.O. Environmental impacts of solid waste landfilling. J. Environ. Manage. 1997;50:1–25. doi: 10.1006/jema.1995.0131. [DOI] [Google Scholar]
- 112.Gworek B., Dmuchowski W., Koda E., Marecka M., Sieczka A., Osi P. Impact of the municipal solid waste łubna landfill on environmental pollution by heavy metals. Water. 2016;8:470. doi: 10.3390/w8100470. [DOI] [Google Scholar]
- 113.Eriksen G.N., Coale F.J., Bollero G.A. Soil nitrogen dynamics and maize production in municipal solid waste amended soil. Agron. J. 1999;91:1009–1016. doi: 10.2134/agronj1999.9161009x. [DOI] [Google Scholar]
- 114.Tiembre I., Koné B.A., Dongo K., Tanner M., Zinsstag J., Cissé G. Epidemiological and clinical aspects of toxic waste intoxication in the district of Abidjan. Heal. Books. 2009;19:189–194. doi: 10.1684/san.2009.0163. [DOI] [PubMed] [Google Scholar]
- 115.Liu A., Ren F., Lin W.Y., Wang J.Y. A review of municipal solid waste environmental standards with a focus on incinerator residues. Int. J. Sustain. Built Environ. 2015;4:165–188. doi: 10.1016/j.ijsbe.2015.11.002. [DOI] [Google Scholar]
- 116.Porta D., Milani S., Lazzarino A.I., Perucci C.A., Forastiere F. Systematic review of epidemiological studies on health effects associated with management of solid waste: a review. Environ. Heal. 2009;8:60. doi: 10.1186/1476-069X-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mattiello A., Chiodini P., Bianco E., Forgione N., Flammia I., Gallo C., Pizzuti R., Panico S. Health effects associated with the disposal of solid waste in landfills and incinerators in populations living in surrounding areas: a systematic review. Int. J. Public Health. 2013;58:725–735. doi: 10.1007/s00038-013-0496-8. [DOI] [PubMed] [Google Scholar]
- 118.Heaney C.D., Wing S., Campbell R.L., Caldwell D., Hopkins B., Richardson D., Yeatts K. Relation between malodor, ambient hydrogen sulfide, and health in a community bordering a landfill. Environ. Res. 2011;111:847–852. doi: 10.1016/j.envres.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Feo G., De Gisi S., Williams I.D. Public perception of odour and environmental pollution attributed to MSW treatment and disposal facilities: a case study. Waste Manag. 2013;33:974–987. doi: 10.1016/j.wasman.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 120.Aatamila M., Verkasalo P.K., Korhonen M.J., Suominen A.L., Hirvonen M.R., Viluksela M.K., Nevalainen A. Odour annoyance and physical symptoms among residents living near waste treatment centres. Environ. Res. 2011;111:164–170. doi: 10.1016/j.envres.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Suwan D., Chitapornpan S., Honda R., Chiemchaisri C. Conversion of organic carbon in food processing wastewater to photosynthetic biomass in photo-bioreactors using different light sources. Environ. Eng. Res. 2014;19:293–298. doi: 10.4491/eer.2014.S1.009. [DOI] [Google Scholar]
- 122.Pariente M.I., Segura Y., Molina R., Martínez F. Wastewater treatment as a process and a resource. Wastewater Treat. Residues Resour. Biorefinery Prod. Biofuels. 2020:19–45. doi: 10.1016/b978-0-12-816204-0.00002-3. [DOI] [Google Scholar]
- 123.Crini G., Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019;17:145–155. doi: 10.1007/s10311-018-0785-9. [DOI] [Google Scholar]
- 124.Lee C.S., Robinson J., Chong M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014;92:489–508. doi: 10.1016/j.psep.2014.04.010. [DOI] [Google Scholar]
- 125.Barakat M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011;4:361–377. doi: 10.1016/j.arabjc.2010.07.019. [DOI] [Google Scholar]
- 126.Basak B., Dey A. Toxic. Waste Manag. Using Bioremediation. IGI Global; Hershey: 2016. Bioremediation approaches for recalcitrant pollutants: potentiality, successes and limitation; pp. 178–197. [DOI] [Google Scholar]
- 127.Morin-Crini N., Crini G., Roy L. Eaux industrielles contaminées. J PUFC, Besançon. 2017;513 [Google Scholar]
- 128.Cao K., Zhi R., Zhang G. Photosynthetic bacteria wastewater treatment with the production of value-added products: a review. Bioresour. Technol. 2020;299:122648. doi: 10.1016/j.biortech.2019.122648. [DOI] [PubMed] [Google Scholar]
- 129.Li K., Liu Q., Fang F., Luo R., Lu Q., Zhou W., Huo S., Cheng P., Liu J., Addy M., Chen P., Chen D., Ruan R. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour. Technol. 2019;291:121934. doi: 10.1016/j.biortech.2019.121934. [DOI] [PubMed] [Google Scholar]
- 130.Liu J., Pemberton B., Lewis J., Scales P.J., Martin G.J.O. Wastewater treatment using filamentous algae – a review. Bioresour. Technol. 2019;298:122556. doi: 10.1016/j.biortech.2019.122556. [DOI] [PubMed] [Google Scholar]
- 131.Lu H., Zhang G., Zheng Z., Meng F., Du T., He S. Bio-conversion of photosynthetic bacteria from non-toxic wastewater to realize wastewater treatment and bioresource recovery: a review. Bioresour. Technol. 2019;278:383–399. doi: 10.1016/j.biortech.2019.01.070. [DOI] [PubMed] [Google Scholar]
- 132.Khalil N. TGF-β: From latent to active. Microbes Infect. 1999;1:1255–1263. doi: 10.1016/S1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 133.Monagas M., Gómez-Cordovés C., Bartolomé B., Laureano O., Ricardo Da Silva J.M. Monomeric, oligomeric, and polymeric Flavan-3-ol composition of wines and grapes from vitis vinifera l. cv. graciano, tempranillo, and cabernet sauvignon. J. Agric. Food Chem. 2003;51:6475–6481. doi: 10.1021/jf030325+. [DOI] [PubMed] [Google Scholar]
- 134.Hassan H.F., Hassan U.F., Usher O.A., Ibrahim A.B., Tabe N.N. Exploring the potentials of banana (Musa Sapietum) peels in feed formulation. Int. J. Adv. Res. Chem. Sci. 2018;5:10–14. doi: 10.20431/2349-0403.0505003. [DOI] [Google Scholar]
- 135.Hosni K., Zahed N., Chrif R., Abid I., Medfei W., Kallel M., Ben Brahim N., Sebei H. Composition of peel essential oils from four selected Tunisian Citrus species: evidence for the genotypic influence. Food Chem. 2010;123:1098–1104. doi: 10.1016/j.foodchem.2010.05.068. [DOI] [Google Scholar]
- 136.Shabtay A., Nikbachat M., Zenou A., Yosef E., Arkin O., Sneer O., Shwimmer A., Yaari A., Budman E., Agmon G., Miron J. Effects of adding a concentrated pomegranate extract to the ration of lactating cows on performance and udder health parameters. Anim. Feed Sci. Technol. 2012;175:24–32. doi: 10.1016/j.anifeedsci.2012.04.004. [DOI] [Google Scholar]
- 137.Oliveira R.A., Narciso C.D., Bisinotto R.S., Perdomo M.C., Ballou M.A., Dreher M., Santos J.E.P. Effects of feeding polyphenols from pomegranate extract on health, growth, nutrient digestion, and immunocompetence of calves. J. Dairy Sci. 2010;93:4280–4291. doi: 10.3168/jds.2010-3314. [DOI] [PubMed] [Google Scholar]
- 138.Jami E., Shabtay A., Nikbachat M., Yosef E., Miron J., Mizrahi I. Effects of adding a concentrated pomegranate-residue extract to the ration of lactating cows on in vivo digestibility and profile of rumen bacterial population. J. Dairy Sci. 2012;95:5996–6005. doi: 10.3168/jds.2012-5537. [DOI] [PubMed] [Google Scholar]
- 139.Weyl-Feinstein S., Markovics A., Eitam H., Orlov A., Yishay M., Agmon R., Miron J., Izhaki I., Shabtay A. Short communication: effect of pomegranate-residue supplement on Cryptosporidium parvum oocyst shedding in neonatal calves. J. Dairy Sci. 2014;97:5800–5805. doi: 10.3168/jds.2013-7136. [DOI] [PubMed] [Google Scholar]
- 140.Choi G.-W., Lee J.-W. Physicochemical quality characteristics of pork patty with tangerine (Citrus unshiu) peel. J. Life Sci. 2017;27:123–130. doi: 10.5352/jls.2017.27.2.123. [DOI] [Google Scholar]
- 141.Nambi-Kasozi J., Sabiiti E.N., Bareeba F.B., Sporndly E. Effect of feeding varying levels of banana peelings supplemented with maize bran, cotton seed cake and Gliricidia sepium on the performance of lactating dairy cows. African J. Agric. Res. 2014;9:720–727. [Google Scholar]
- 142.Lawal M.O. Dietary effects of ripe and unripe banana peels on the growth and economy of production of juvenile catfish (Clarias gariepinus burchell, 1822) J. Fish. 2014;8:220–227. doi: 10.3153/jfscom.201428. [DOI] [Google Scholar]
- 143.Rattanavichai W., Cheng W. Dietary supplement of banana (Musa acuminata) peels hot-water extract to enhance the growth, anti-hypothermal stress, immunity and disease resistance of the giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 2015;43:415–426. doi: 10.1016/j.fsi.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 144.Giri S.S., Jun J.W., Sukumaran V., Park S.C. Dietary administration of banana (Musa acuminata) peel flour affects the growth, antioxidant status, cytokine responses, and disease susceptibility of rohu, labeo rohita. J. Immunol. Res. 2016:1–11. doi: 10.1155/2016/4086591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mones G.H., Jr I.P.A. Performance of red Tilapia (Oreochromis sp.) fed diet with fermented banana (Musa Acuminata × balbisiana) peel at different stages of ripeness following Aeromonas Hydrophila infection. Int. J. Agric. Technol. 2017;13:1041–1064. [Google Scholar]
- 146.Adeniji T.A., Barimalaa I.S., Tenkouano A., Sanni L.O., Hart D.A. Antinutrients and heavy metals in new Nigerian Musa hybrid peels with emphasis on utilization in livestock production. Fruits. 2008;60:65–73. doi: 10.1051/fruits:2007048. [DOI] [Google Scholar]
- 147.Yangthong M., Hutadilok-Towatana N., Ruensirikul J. Use of “Kluai Leb Mu Nang” banana peel as a dietary ingredient in feed for spotted scat (Scatophagus argus linnaeus, 1766) Maejo Int. J. Sci. Technol. 2018;12:221–231. [Google Scholar]
- 148.Lobo M.G., Dorta E. Postharvest Technol. Perish. Hortic. Commod. Elsevier Inc.; 2019. Utilization and management of horticultural waste; pp. 639–666. [DOI] [Google Scholar]
- 149.Essien J.P., Akpan E.J., Essien E.P. Studies on mould growth and biomass production using waste banana peel. Bioresour. Technol. 2005;96:1451–1456. doi: 10.1016/j.biortech.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 150.Kalemelawa F., Nishihara E., Endo T., Ahmad Z., Yeasmin R., Tenywa M.M., Yamamoto S. An evaluation of aerobic and anaerobic composting of banana peels treated with different inoculums for soil nutrient replenishment. Bioresour. Technol. 2012;126:375–382. doi: 10.1016/j.biortech.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 151.Panwar N. Vol. 3. 2015. pp. 121–125. (Studies on Physicochemical Characteristics and Fertility of Soil by Addition of Banana Peels – Waste Management). [Google Scholar]
- 152.Sial T.A., Khan M.N., Lan Z., Kumbhar F., Ying Z., Zhang J., Sun D., Li X. Contrasting effects of banana peels waste and its biochar on greenhouse gas emissions and soil biochemical properties. Process Saf. Environ. Prot. 2019;122:366–377. doi: 10.1016/j.psep.2018.10.030. [DOI] [Google Scholar]
- 153.Siddiqui M.T.H., Nizamuddin S., Mubarak N.M., Shirin K., Aijaz M., Hussain M., Baloch H.A. Characterization and process optimization of biochar produced using novel biomass, waste pomegranate peel: a response surface methodology approach. Waste Biomass Valor. 2019;10:521–532. doi: 10.1007/s12649-017-0091-y. [DOI] [Google Scholar]
- 154.Pane C., Villecco D., Zaccardelli M. Short-time response of microbial communities to waste compost amendment of an intensive cultivated soil in Southern Italy. Commun. Soil Sci. Plant Anal. 2013;44:2344–2352. doi: 10.1080/00103624.2013.803566. [DOI] [Google Scholar]
- 155.Mortier N., Velghe F., Verstichel S. Biotransformation Agric. Waste By-Products Food, Feed. Fibre, Fuel Econ. Elsevier Inc.; 2016. Organic recycling of agricultural waste today: composting and anaerobic digestion; pp. 69–124. [DOI] [Google Scholar]
- 156.Sanchez-Arias V., Fernandez F.J., Villasenor J., Rodriguez L. Enhancing the co-composting of olive mill wastes and sewage sludge by the addition of an industrial waste. Bioresour. Technol. 2008;99:6346–6353. doi: 10.1016/j.biortech.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 157.Mbarki S., Labidi N., Mahmoudi H., Jedidi N., Abdelly C. Contrasting effects of municipal compost on alfalfa growth in clay and in sandy soils: N, P, K, content and heavy metal toxicity. Bioresour. Technol. 2008;99:6745–6750. doi: 10.1016/j.biortech.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 158.Kucbel M., Raclavská H., Růžičková J., Švédová B., Sassmanová V., Drozdová J., Raclavský K., Juchelková D. Properties of composts from household food waste produced in automatic composters. J. Environ. Manage. 2019;236:657–666. doi: 10.1016/j.jenvman.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 159.Kadir A.A., Rahman N.A., Azhari N.W. The utilization of banana peel in the fermentation liquid in food waste composting. IOP Conf. Ser. Mater. Sci. Eng. 2016;136 doi: 10.1088/1757-899X/136/1/012055. [DOI] [Google Scholar]
- 160.Bernal-Vicente A., Ros M., Tittarelli F., Intrigliolo F., Pascual J.A. Citrus compost and its water extract for cultivation of melon plants in greenhouse nurseries. Evaluation of nutriactive and biocontrol effects. Bioresour. Technol. 2008;99:8722–8728. doi: 10.1016/j.biortech.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 161.Abou El Nour M.M., Mona E.A.D.S., Wadi J.M. Suppressive effect of compost/pomegranate peel tea combination against Fusarium oxysporum f. sp. lupini, and Rhizoctonia solani as an alternative synthetic fungicide, Egypt. J. Exp. Biol. 2020;16:13–25. doi: 10.5455/egyjebb.20191208124236. [DOI] [Google Scholar]
- 162.Amel K., Hassen M.A., Kerroum D. Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia. 2012;19:286–295. doi: 10.1016/j.egypro.2012.05.208. [DOI] [Google Scholar]
- 163.Motaghi M., Ziarati P. Adsorptive removal of cadmium and lead from Oryza sativa rice by banana peel as bio-sorbent. Biomed. Pharmacol. J. 2016;9:739–749. doi: 10.13005/bpj/998. [DOI] [Google Scholar]
- 164.Pathak P.D., Mandavgane S.A., Kulkarni B.D. Utilization of banana peel for the removal of benzoic and salicylic acid from aqueous solutions and its potential reuse. Desalin. Water Treat. 2016;57:12717–12729. doi: 10.1080/19443994.2015.1051589. [DOI] [Google Scholar]
- 165.Pathak P.D., Mandavgane S.A. Preparation and characterization of raw and carbon from banana peel by microwave activation: application in citric acid adsorption. J. Environ. Chem. Eng. 2015;3:2435–2447. doi: 10.1016/j.jece.2015.08.023. [DOI] [Google Scholar]
- 166.Alaa El-Din G., Amer A.A., Malsh G., Hussein M. Study on the use of banana peels for oil spill removal. Alexandria Eng. J. 2018;57:2061–2068. doi: 10.1016/j.aej.2017.05.020. [DOI] [Google Scholar]
- 167.El-Ashtoukhy E.S.Z., Amin N.K., Abdelwahab O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination. 2008;223:162–173. doi: 10.1016/j.desal.2007.01.206. [DOI] [Google Scholar]
- 168.Amin N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: adsorption equilibrium and kinetics. J. Hazard. Mater. 2009;165:52–62. doi: 10.1016/j.jhazmat.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 169.Sobhani L., Ziarati P. Study on potential bio-adsorption of Tangerine peel in removal of heavy metals: Pb, Cd and Ni of vegetable coriander. J. Sci. Discov. 2017;1:jsd17020. doi: 10.24262/jsd.1.2.17020. [DOI] [Google Scholar]
- 170.Torab-Mostaedi M. Biosorpcija lantana i cerijuma iz vodenih rastvora pomoću kore mandarine (Citrus reticulata): Ravnotežna, kinetička i termodinamička ispitivanja. Chem. Ind. Chem. Eng. Q. 2013;19:79–88. doi: 10.2298/CICEQ120128043T. [DOI] [Google Scholar]
- 171.Pathak P.D., Mandavgane S.A., Kulkarni B.D. Valorization of pomegranate peels: a biorefinery approach. Waste Biomass Valorization. 2017;8:1127–1137. doi: 10.1007/s12649-016-9668-0. [DOI] [Google Scholar]
- 172.Boluda-Aguilar M., García-Vidal L., González-Castañeda Fdel P., López-Gómez A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010;101:3506–3513. doi: 10.1016/j.biortech.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 173.Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008;19:1130–1138. doi: 10.1016/j.foodcont.2007.12.003. [DOI] [Google Scholar]
- 174.Wilkins M.R. Effect of orange peel oil on ethanol production by Zymomonas mobilis. Biomass Bioenergy. 2009;33:538–541. doi: 10.1016/j.biombioe.2008.08.010. [DOI] [Google Scholar]
- 175.John I., Yaragarla P., Muthaiah P., Ponnusamy K., Appusamy A. Statistical optimization of acid catalyzed steam pretreatment of citrus peel waste for bioethanol production. Bioresour. Technol. Rep. 2017;3:429–433. doi: 10.1016/j.reffit.2017.04.001. [DOI] [Google Scholar]
- 176.Choi I.S., Lee Y.G., Khanal S.K., Park B.J., Bae H.J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy. 2015;140:65–74. doi: 10.1016/j.apenergy.2014.11.070. [DOI] [Google Scholar]
- 177.Choi I.S., Kim J.H., Wi S.G., Kim K.H., Bae H.J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy. 2013;102:204–210. doi: 10.1016/j.apenergy.2012.03.066. [DOI] [Google Scholar]
- 178.Singh A.K., Rath S., Kumar Y., Masih H., Peter J.K., Benjamin J.C., Singh P.K., Singh Dipuraj P. Bio-ethanol production from banana peel by simultaneous saccharification and fermentation process using cocultures Aspergillus niger and Saccharomyces cerevisiae. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:84–96. http://www.ijcmas.com/Archives-18.php [Google Scholar]
- 179.Demiray E., Karatay S.E., Dönmez G. Evaluation of pomegranate peel in ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Energy. 2018;159:988–994. doi: 10.1016/j.energy.2018.06.200. [DOI] [Google Scholar]
- 180.Rasi S., Veijanen A., Rintala J. Trace compounds of biogas from different biogas production plants. Energy. 2007;32:1375–1380. doi: 10.1016/j.energy.2006.10.018. [DOI] [Google Scholar]
- 181.Achinas S., Achinas V. Biogas combustion: an introductory briefing. In: A.V, Artemio N., editors. Biogas Prod. Appl. Glob. Dev. 1st ed. Nova Science Publishers; USA: 2017. pp. 179–193. (Energy Science, Engineering and Technology) [Google Scholar]
- 182.Chen J.F. Green chemical engineering. Engineering. 2017;3:283–284. doi: 10.1016/J.ENG.2017.03.025. [DOI] [Google Scholar]
- 183.Boonsawang P., Rerngnarong A., Tongurai C., Chaiprapat S. Effect of pH, OLR, and HRT on performance of acidogenic and methanogenic reactors for treatment of biodiesel wastewater. Desalin. Water Treat. 2015;54:3317–3327. doi: 10.1080/19443994.2014.909331. [DOI] [Google Scholar]
- 184.Okonkwo U.C., Onokpite E., Onokwai A.O. Comparative study of the optimal ratio of biogas production from various organic wastes and weeds for digester/restarted digester. J. King Saud Univ. - Eng. Sci. 2018;30:123–129. doi: 10.1016/j.jksues.2016.02.002. [DOI] [Google Scholar]
- 185.Corneli E., Dragoni F., Adessi A., De Philippis R., Bonari E., Ragaglini G. Energy conversion of biomass crops and agroindustrial residues by combined biohydrogen/biomethane system and anaerobic digestion. Bioresour. Technol. 2016;211:509–518. doi: 10.1016/j.biortech.2016.03.134. [DOI] [PubMed] [Google Scholar]
- 186.Pontoni L., Panico A., Salzano E., Frunzo L., Iodice P., Pirozzi F. Innovative parameters to control the efficiency of anaerobic digestion process. Chem. Eng. Trans. 2015;43:2089–2094. doi: 10.3303/CET1543349. [DOI] [Google Scholar]
- 187.Achinas S., Krooneman J., Euverink G.J.W. Enhanced biogas production from the anaerobic batch treatment of banana peels. Engineering. 2019;5:970–978. doi: 10.1016/j.eng.2018.11.036. [DOI] [Google Scholar]
- 188.Housagul S., Sirisukpoka U., Boonyawanich S., Pisutpaisal N. Biomethane production from co-digestion of banana peel and waste glycerol. Energy Procedia. 2014;61:2219–2223. doi: 10.1016/j.egypro.2014.12.113. [DOI] [Google Scholar]
- 189.Pisutpaisal N., Boonyawanich S., Saowaluck H. Feasibility of biomethane production from banana peel. Energy Procedia. 2014;50:782–788. doi: 10.1016/j.egypro.2014.06.096. [DOI] [Google Scholar]
- 190.Kirtane R.D., Suryawanshi P.C., Patil M.R., Chaudhari A.B., Kothari R.M. Optimization of organic loading rate for different fruit wastes during biomethanization. J. Sci. Ind. Res. 2009;68:252–255. [Google Scholar]
- 191.Ruiz B., Flotats X. Effect of limonene on batch anaerobic digestion of citrus peel waste. Biochem. Eng. J. 2016;109:9–18. doi: 10.1016/j.bej.2015.12.011. [DOI] [Google Scholar]
- 192.Gunaseelan V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy. 2004;26:389–399. doi: 10.1016/j.biombioe.2003.08.006. [DOI] [Google Scholar]
- 193.Camargo F.P., Sakamoto I.K., Duarte I.C.S., Varesche M.B.A. Influence of alkaline peroxide assisted and hydrothermal pretreatment on biodegradability and bio-hydrogen formation from citrus peel waste. Int. J. Hydrogen Energy. 2019;44:22888–22903. doi: 10.1016/j.ijhydene.2019.07.011. [DOI] [Google Scholar]
- 194.Nayak A., Bhushan B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manage. 2019;233:352–370. doi: 10.1016/j.jenvman.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 195.Chen W.H., Chen S.Y., Kumar Khanal S., Sung S. Kinetic study of biological hydrogen production by anaerobic fermentation. Int. J. Hydrogen Energy. 2006;31:2170–2178. doi: 10.1016/j.ijhydene.2006.02.020. [DOI] [Google Scholar]
- 196.Yasin N.H.M., Mumtaz T., Hassan M.A., Abd Rahman N. Food waste and food processing waste for biohydrogen production: a review. J. Environ. Manage. 2013;130:375–385. doi: 10.1016/j.jenvman.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 197.Alexandropoulou M., Antonopoulou G., Trably E., Carrere H., Lyberatos G. Continuous biohydrogen production from a food industry waste: influence of operational parameters and microbial community analysis. J. Clean. Prod. 2018;174:1054–1063. doi: 10.1016/j.jclepro.2017.11.078. [DOI] [Google Scholar]
- 198.Nath K., Kumar A., Das D. Effect of some environmental parameters on fermentative hydrogen production by Enterobacter cloacae DM11. Can. J. Microbiol. 2006;52:525–532. doi: 10.1139/W06-005. [DOI] [PubMed] [Google Scholar]
- 199.Nathoa C., Sirisukpoca U., Pisutpaisal N. Production of hydrogen and methane from banana peel by two phase anaerobic fermentation. Energy Procedia. 2014;50:702–710. doi: 10.1016/j.egypro.2014.06.086. [DOI] [Google Scholar]
- 200.Aziz Z.A.A., Ahmad A., Setapar S.H.M., Karakucuk A., Azim M.M., Lokhat D., Rafatullah M., Ganash M., Kamal M.A., Ashraf G.M. Essential oils : extraction techniques, pharmaceutical and therapeutic potential - a review. Curr. Drug Metab. 2018;19:1100–1110. doi: 10.2174/1389200219666180723144850. [DOI] [PubMed] [Google Scholar]
- 201.Cook C.M., Lanaras T. Essential oils: isolation, production and uses. Encycl. Food Heal. 2016:552–557. doi: 10.1016/B978-0-12-384947-2.00261-0. [DOI] [Google Scholar]
- 202.Stratakos A.C., Koidis A. Essent. Oils Food Preserv. Flavor Saf. Elsevier Inc.; 2016. Methods for extracting essential oils; pp. 31–38. [DOI] [Google Scholar]
- 203.Gavahian M., Sastry S., Farhoosh R., Farahnaky A. 1st ed. Elsevier Inc.; 2020. Ohmic Heating As a Promising Technique for Extraction of Herbal Essential Oils: Understanding Mechanisms, Recent Findings, and Associated Challenges. [DOI] [PubMed] [Google Scholar]
- 204.Gavahian M., Farahnaky A., Javidnia K., Majzoobi M. Comparison of ohmic-assisted hydrodistillation with traditional hydrodistillation for the extraction of essential oils from Thymus vulgaris L. Innov. Food Sci. Emerg. Technol. 2012;14:85–91. doi: 10.1016/j.ifset.2012.01.002. [DOI] [Google Scholar]
- 205.Masango P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005;13:833–839. doi: 10.1016/j.jclepro.2004.02.039. [DOI] [Google Scholar]
- 206.Gavahian M., Farhoosh R., Javidnia K., Shahidi F., Farahnaky A. Effect of applied voltage and frequency on extraction parameters and extracted essential oils from Mentha piperita by ohmic assisted hydrodistillation. Innov. Food Sci. Emerg. Technol. 2015;29:161–169. doi: 10.1016/j.ifset.2015.02.003. [DOI] [Google Scholar]
- 207.Aboudaou M., Ferhat M.A., Hazzit M., Ariño A., Djenane D. Solvent free-microwave green extraction of essential oil from orange peel (Citrus sinensis L.): effects on shelf life of flavored liquid whole eggs during storage under commercial retail conditions. J. Food Meas. Charact. 2019;13:3162–3172. doi: 10.1007/s11694-019-00239-9. [DOI] [Google Scholar]
- 208.Wianowska D. The influence of purge times on the yields of essential oil components extracted from plants by pressurized liquid extraction. J. AOAC Int. 2014;97:1310–1316. doi: 10.5740/jaoacint.13-318. [DOI] [PubMed] [Google Scholar]
- 209.Gavahian M., Farahnaky A., Majzoobi M., Javidnia K., Saharkhiz M.J., Mesbahi G. Ohmic-assisted hydrodistillation of essential oils from Zataria multiflora Boiss (Shirazi thyme) Int. J. Food Sci. Technol. 2011;46:2619–2627. doi: 10.1111/j.1365-2621.2011.02792.x. [DOI] [Google Scholar]
- 210.Gavahian M., Lee Y.T., Chu Y.H. Ohmic-assisted hydrodistillation of citronella oil from Taiwanese citronella grass: impacts on the essential oil and extraction medium. Innov. Food Sci. Emerg. Technol. 2018;48:33–41. doi: 10.1016/j.ifset.2018.05.015. [DOI] [Google Scholar]
- 211.Ara K.M., Jowkarderis M., Raofie F. Optimization of supercritical fluid extraction of essential oils and fatty acids from flixweed (Descurainia Sophia L.) seed using response surface methodology and central composite design. J. Food Sci. Technol. 2015;52:4450–4458. doi: 10.1007/s13197-014-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bubalo M.C., Vidović S., Redovniković I.R., Jokić S. Food and Bioproducts Processing New perspective in extraction of plant biologically active compounds by green solvents i. Food Bioprod. Process. 2018;109:52–73. doi: 10.1016/j.fbp.2018.03.001. [DOI] [Google Scholar]
- 213.Damyeh M.S., Niakousari M. Ohmic hydrodistillation, an accelerated energy-saver green process in the extraction of Pulicaria undulata essential oil. Ind. Crops Prod. 2017;98:100–107. doi: 10.1016/j.indcrop.2017.01.029. [DOI] [Google Scholar]
- 214.Assami K., Pingret D., Chemat S., Meklati B.Y., Chemat F. Ultrasound induced intensification and selective extraction of essential oil from Carum carvi L. seeds. Chem. Eng. Process. Process Intensif. 2012;62:99–105. doi: 10.1016/j.cep.2012.09.003. [DOI] [Google Scholar]
- 215.Negro V., Mancini G., Ruggeri B., Fino D. Citrus waste as feedstock for bio-based products recovery: review on limonene case study and energy valorization. Bioresour. Technol. 2016;214:806–815. doi: 10.1016/j.biortech.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 216.Ferhat M.A., Meklati B.Y., Smadja J., Chemat F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A. 2006;1112:121–126. doi: 10.1016/j.chroma.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 217.Lota M.L., De Rocca Serra D., Tomi F., Casanova J. Chemical variability of peel and leaf essential oils of 15 species of mandarins. Biochem. Syst. Ecol. 2001;29:77–104. doi: 10.1016/S0305-1978(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 218.Liu R., Zhang L., Lan X., Li L., Zhang T.T., Sun J.H., Du G.H. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience. 2011;176:408–419. doi: 10.1016/j.neuroscience.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 219.Ehrnhöfer-Ressler M.M., Fricke K., Pignitter M., Walker J.M., Walker J., Rychlik M., Somoza V. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a salvia officinalis L. Infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013;61:3451–3459. doi: 10.1021/jf305472t. [DOI] [PubMed] [Google Scholar]
- 220.Ho C.H., Piotrowski J., Dixon S.J., Baryshnikova A., Costanzo M., Boone C. Combining functional genomics and chemical biology to identify targets of bioactive compounds. Curr. Opin. Chem. Biol. 2011;15:66–78. doi: 10.1016/j.cbpa.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 221.Aziz A.N., Ibrahim H., Rosmy Syamsir D., Mohtar M., Vejayan J., Awang K. Antimicrobial compounds from Alpinia conchigera. J. Ethnopharmacol. 2013;145:798–802. doi: 10.1016/j.jep.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 222.Bennis S., Chami F., Chami N., Rhayour K., Tantaoui-Elaraki A., Remmal A. Eugenol induces damage of bacterial and fungal envelope. Moroccan J. Biol. 2004;1:33–39. [Google Scholar]
- 223.Fisher K., Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci. Technol. 2008;19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- 224.Chutia M., Deka Bhuyan P., Pathak M.G., Sarma T.C., Boruah P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT - Food Sci. Technol. 2009;42:777–780. doi: 10.1016/j.lwt.2008.09.015. [DOI] [Google Scholar]
- 225.Rehman Z. Citrus peel extract – a natural source of antioxidant. Food Chem. 2006;99:450–454. doi: 10.1016/j.foodchem.2005.07.054. [DOI] [Google Scholar]
- 226.Pultrini A.D.M., Galindo L.A., Costa M. Effects of the essential oil from Citrus aurantium L. In experimental anxiety models in mice. Life Sci. 2006;78:1720–1725. doi: 10.1016/j.lfs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 227.Odugbemi T. University of Lagos Press; Lagos: 2008. Outlines and Pictures of Medicinal Plants from Nigeria. [Google Scholar]
- 228.Kamal G.M., Anwar F., Hussain A.I., Sarri N., Ashraf M.Y. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J. 2011;18:1275–1282. [Google Scholar]
- 229.Ho S.C., Kuo C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium) Food Chem. Toxicol. 2014;71:176–182. doi: 10.1016/j.fct.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 230.Sun J. D-limonene: safety and clinical applications. Altern. Med. Rev. 2007;12:259–264. [PubMed] [Google Scholar]
- 231.Matan N., Matanb N. Antifungal activities of anise oil, lime oil, and tangerine oil against molds on rubberwood (Hevea brasiliensis) Int. Biodeterior. Biodegrad. 2008;62:75–78. doi: 10.1016/j.ibiod.2007.07.014. [DOI] [Google Scholar]
- 232.Tao N., Jia L., Zhou H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014;153:265–271. doi: 10.1016/j.foodchem.2013.12.070. [DOI] [PubMed] [Google Scholar]
- 233.Wu T., Cheng D., He M., Pan S., Yao X., Xu X. Antifungal action and inhibitory mechanism of polymethoxylated flavones from Citrus reticulata Blanco peel against Aspergillus niger. Food Control. 2014;35:354–359. doi: 10.1016/j.foodcont.2013.07.027. [DOI] [Google Scholar]
- 234.Elisashvili V., Penninckx M., Kachlishvili E., Asatiani M., Kvesitadze G. Use of Pleurotus dryinus for lignocellulolytic enzymes production in submerged fermentation of mandarin peels and tree leaves. Enzyme Microb. Technol. 2006;38:998–1004. doi: 10.1016/j.enzmictec.2005.08.033. [DOI] [Google Scholar]
- 235.Uma C., Gomathi D., Muthulakshmi C., Gopalakrishnan V.K. Optimization and characterization of invertase by Aspergillus fumigatus using fruit peel waste as substrate. Res. J. Pharm. Biol. Chem. Sci. 2010;1:93–101. [Google Scholar]
- 236.Uma C., Gomathi D., Ravikumar G., Kalaiselvi M., Palaniswamy M. Production and properties of invertase from a Cladosporium cladosporioides in SmF using pomegranate peel waste as substrate. Asian Pac. J. Trop. Biomed. 2012;2:605–S611. doi: 10.1016/S2221-1691(12)60282-2. [DOI] [Google Scholar]
- 237.Srivastava A., Kar R. Characterization and application of tannase produced by Aspergillus niger ITCC 6514.07 on pomegranate rind. Braz. J. Microbiol. 2009;40:782–789. doi: 10.1590/S1517-83822009000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jadhav S.A., Kataria P.K., Bhise K.K., Chougule S.A. Original research article amylase production from potato and banana peel waste. Int. J. Curr. Microbiol. App. Sci. 2013;2:410–414. http://www.ijcmas.com [Google Scholar]
- 239.Elisashvili V., Kachlishvili E., Penninckx M. Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J. Ind. Microbiol. Biotechnol. 2008;35:1531–1538. doi: 10.1007/s10295-008-0454-2. [DOI] [PubMed] [Google Scholar]
- 240.Kermani Z.J., Shpigelman A., Pham H.T.T., Van Loey A.M., Hendrickx M.E. Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 2015;44:424–434. doi: 10.1016/j.foodhyd.2014.10.018. [DOI] [Google Scholar]
- 241.Yuliarti O., Matia-Merino L., Goh K.K.T., Mawson J., Williams M.A.K., Brennan C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015;166:479–485. doi: 10.1016/j.foodchem.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 242.Swamy G.J., Muthukumarappan K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017;220:108–114. doi: 10.1016/j.foodchem.2016.09.197. [DOI] [PubMed] [Google Scholar]
- 243.Viuda-Martos M., Fernández-Lóaez J., Pérez-álvarez J.A. Pomegranate and its many functional components as related to human health: a review. Compr. Rev. Food Sci. Food Saf. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 244.Zhu C., Liu X. Optimization of extraction process of crude polysaccharides from Pomegranate peel by response surface methodology. Carbohydr. Polym. 2013;92:1197–1202. doi: 10.1016/j.carbpol.2012.10.073. [DOI] [PubMed] [Google Scholar]
- 245.Pereira A., Maraschin M. Banana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015;160:149–163. doi: 10.1016/j.jep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 246.Ventura J., Alarcón-Aguilar F., Roman-Ramos R., Campos-Sepulveda E., Reyes-Vega M.L., Daniel Boone-Villa V., Jasso-Villagómez E.I., Aguilar C.N. Quality and antioxidant properties of a reduced-sugar pomegranate juice jelly with an aqueous extract of pomegranate peels. Food Chem. 2013;136:109–115. doi: 10.1016/j.foodchem.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 247.Wasila H., Li X., Liu L., Ahmad I., Ahmad S. Vol. 78. 2013. pp. 1166–1172. (Peel Effects on Phenolic Composition, Antioxidant Activity, and Making of Pomegranate Juice and Wine). [DOI] [PubMed] [Google Scholar]
- 248.Ademosun A.O., Oboh G., Olasehinde T.A., Adeoyo O.O. From folk medicine to functional food: a review on the bioactive components and pharmacological properties of citrus peels. Orient. Pharm. Exp. Med. 2018;18:9–20. doi: 10.1007/s13596-017-0292-8. [DOI] [Google Scholar]
- 249.Tian C., Xu H., Li J., Han Z. Characteristics and intestinal immunomodulating activities of water-soluble pectic polysaccharides from Chenpi with different storage periods. J. Sci. Food Agric. 2018;98:3752–3757. doi: 10.1002/jsfa.8888. [DOI] [PubMed] [Google Scholar]
- 250.Saleh E.A., Morshdy A.E.M., Hafez A.E.S.E., Hussein M.A., Elewa E.S., Mahmoud A.F.A. Effect of pomegranate peel powder on the hygienic quality of beef sausage. J. Microbiol. Biotechnol. Food Sci. 2017;6:1300–1304. doi: 10.15414/jmbfs.2017.6.6.1300-1304. [DOI] [Google Scholar]
- 251.Smaoui S., Ben H., Chakchouk A., Fourati M., Sellem I., Elhadef K., Ennouri K., Mellouli L. Pomegranate peel as phenolic compounds source : advanced analytical strategies and practical use in meat products. Meat Sci. 2019;158:107914. doi: 10.1016/j.meatsci.2019.107914. [DOI] [PubMed] [Google Scholar]
- 252.Khalid S., Yu L., Feng M., Meng L., Bai Y., Ali A., Liu H., Chen L. Development and characterization of biodegradable antimicrobial packaging films based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life. 2018;18:71–79. doi: 10.1016/j.fpsl.2018.08.008. [DOI] [Google Scholar]
- 253.Paari A., Naidu H.K., Kanmani P., Satishkumar R., Yuvaraj N., Pattukumar V., Arul V. Evaluation of irradiation and heat treatment on antioxidant properties of fruit peel extracts and its potential application during preservation of goat fish parupenaeus indicus. Food Bioprocess Technol. 2012;5:1860–1870. doi: 10.1007/s11947-011-0552-4. [DOI] [Google Scholar]
- 254.Khojah S.M. Bio-based coating from fish gelatin, K-Carrageenan and extract of pomegranate peels for maintaining the overall qualities of fish fillet. J. Aquat. Food Prod. Technol. 2020:1–13. doi: 10.1080/10498850.2020.1718261. [DOI] [Google Scholar]
- 255.Sultana H.S., Ali M., Panda B.P. Influence of volatile constituents of fruit peels of Citrus reticulata Blanco on clinically isolated pathogenic microorganisms under in-vitro. Asian Pac. J. Trop. Biomed. 2012;2:1299–S1302. doi: 10.1016/S2221-1691(12)60404-3. [DOI] [Google Scholar]
- 256.Sahraoui N., Abert M., El M., Boutekedjiret C., Chemat F. Valorization of citrus by-products using Microwave Steam Distillation (MSD) Innov. Food Sci. Emerg. Technol. 2011;12:163–170. doi: 10.1016/j.ifset.2011.02.002. [DOI] [Google Scholar]
- 257.Bertouche S., Tomao V., Ruiz K., Hellal A., Boutekedjiret C., Chemat F. First approach on moisture determination in food products using alpha-pinene as an alternative solvent for Dean-Stark distillation. Food Chem. 2012;134:602–605. doi: 10.1016/j.foodchem.2012.02.158. [DOI] [Google Scholar]
- 258.Fagbemi J.F., Ugoji E., Adenipekun T., Adelowotan O. Evaluation of the antimicrobial properties of unripe banana(Musa sapientum L) lemon grass (Cymbopogoncitratus S) and turmeric (Curcuma longa) on pathogens. Afr. J. Biotechnol. 2009;8:1176–1182. [Google Scholar]
- 259.Prakash B., Sumangala C.H., Melappa G., Gavimath C. Evaluation of antifungal activity of banana peel against scalp Fungi. Mater. Today Proc. 2017;4:11977–11983. doi: 10.1016/j.matpr.2017.09.119. [DOI] [Google Scholar]
- 260.Kumar K.P.S., Bhowmik D., Duraivel S., Umadevi M. Traditional and medicinal uses of banana. J. Pharmacogn. Phytochem. Tradit. 2012;1:51–63. [Google Scholar]
- 261.Rebello L.P.G., Ramos A.M., Pertuzatti P.B., Barcia M.T., Castillo-Muñoz N., Hermosín-Gutiérrez I. Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Food Res. Int. 2014;55:397–403. doi: 10.1016/j.foodres.2013.11.039. [DOI] [Google Scholar]
- 262.Ibrahim H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015;8:265–275. doi: 10.1016/j.jrras.2015.01.007. [DOI] [Google Scholar]
- 263.Shafiq S.A., Mahdi L.H., Ajja H.A., Muslim S.N. In vitro antimicrobial agents of fruit peel extracts of Punica granatum L. and Citrus sinensis against many bacterial and fungal genera. Int. J. Curr. Sci. 2013;9:101–104. [Google Scholar]
- 264.Ben Hamouda A., Chaieb I., Daami-remadi M., Laarif A. Vol. 4. 2015. pp. 11–15. (In Vitro Evaluation of Insecticidal and Antifungal Potencies of Fruit Peel Extracts of Pomegranate (Punica granatum)). [Google Scholar]
- 265.Murthy K.N.C., K V.R., Veigas J.M., Murthy U.D. Study on wound healing activity of. J. Med. Food. 2004;7:256–259. doi: 10.1089/1096620041224111. [DOI] [PubMed] [Google Scholar]
- 266.Ismail T., Sestili P., Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012;143:397–405. doi: 10.1016/j.jep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 267.Shaban N.Z., El-Kersh M.A.L., El-Rashidy F.H., Habashy N.H. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013;141:1587–1596. doi: 10.1016/j.foodchem.2013.04.134. [DOI] [PubMed] [Google Scholar]
- 268.Zhu C.P., Zhai X.C., Li L.Q., Wu X.X., Li B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015;177:139–146. doi: 10.1016/j.foodchem.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 269.Moghadam M., Salami M., Mohammadian M., Khodadadi M., Emam-Djomeh Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020;104:105735. doi: 10.1016/j.foodhyd.2020.105735. [DOI] [Google Scholar]
- 270.Elsherbiny E.A., Amin B.H., Baka Z.A. Efficiency of pomegranate (Punica granatum L.) peels extract as a high potential natural tool towards Fusarium dry rot on potato tubers. Postharvest Biol. Technol. 2016;111:256–263. doi: 10.1016/j.postharvbio.2015.09.019. [DOI] [Google Scholar]
- 271.Devatkal S.K., Jaiswal P., Jha S.N., Bharadwaj R., Viswas K.N. Antibacterial activity of aqueous extract of pomegranate peel against Pseudomonas stutzeri isolated from poultry meat. J. Food Sci. Technol. 2013;50:555–560. doi: 10.1007/s13197-011-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Alexandre E.M.C., Silva S., Santos S.A.O., Silvestre A.J.D., Duarte M.F., Saraiva J.A., Pintado M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019;115:167–176. doi: 10.1016/j.foodres.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 273.Ismail T., Akhtar S., Sestili P., Riaz M., Ismail A., Labbe R.G. Antioxidant, antimicrobial and urease inhibitory activities of phenolics-rich pomegranate peel hydro-alcoholic extracts. J. Food Biochem. 2016;40:550–558. doi: 10.1111/jfbc.12250. [DOI] [Google Scholar]
- 274.Kharchoufi S., Licciardello F., Siracusa L., Muratore G., Hamdi M., Restuccia C. Antimicrobial and antioxidant features of ‘Gabsiʼ pomegranate peel extracts. Ind. Crops Prod. 2018;111:345–352. doi: 10.1016/j.indcrop.2017.10.037. [DOI] [Google Scholar]
- 275.Zheng J.Q., Mao X.J., Geng L.J., Yang G.M., Xu C.P. Production optimization, preliminary characterization and bioactivity of exopolysaccharides from Incutis tamaricis (Pat.) Fiasson & Niemela. J. Taiwan Inst. Chem. Eng. 2014;45:725–733. doi: 10.1016/j.jtice.2013.08.006. [DOI] [Google Scholar]
- 276.Xia L., Liu X., Guo H., Zhang H., Zhu J., Ren F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods. 2012;4:294–301. doi: 10.1016/j.jff.2011.12.006. [DOI] [Google Scholar]
- 277.wah Ma C., Feng M., Zhai X., Hu M., You L., Luo W., Zhao M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. Eng. 2013;44:886–894. doi: 10.1016/j.jtice.2013.01.032. [DOI] [Google Scholar]
- 278.Shi Q., Liu Z., Yang Y., Geng P., Zhu Y.Y., Zhang Q., Bai F., Bai G. Identification of anti-asthmatic compounds in Pericarpium citri reticulatae and evaluation of their synergistic effects. Acta Pharmacol. Sin. 2009;30:567–575. doi: 10.1038/aps.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chang C.Y., Lin T.Y., Lu C.W., Huang S.K., Wang Y.C., Chou S.S.P., Wang S.J. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology. 2015;50:157–169. doi: 10.1016/j.neuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 280.Cincin Z.B., Kiran B., Baran Y., Cakmakoglu B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed. Pharmacother. 2018;103:336–345. doi: 10.1016/j.biopha.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 281.Justin-Thenmozhi A., Bharathi M.D., Kiruthika R., Manivasagam T., Borah A., Essa M.M. Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT/GSK-3β pathway by Hesperidin in wistar rats. Neurotox. Res. 2018;34:463–476. doi: 10.1007/s12640-018-9904-4. [DOI] [PubMed] [Google Scholar]
- 282.Pratiwi F., Tinata J.K., Prakasa A., Istiqomah, Hartini E., Isworo S. Citric acid compounds of tangerines peel extract (Citrus reticulata) as potential materials teeth whitening. J. Phys. Conf. Ser. 2017:824. doi: 10.1088/1742-6596/824/1/012071. 012071. [DOI] [Google Scholar]
- 283.El Barnossi A., Moussaid F., Iraqi H.A. Antifungal activity of Bacillussp. Gn-A11-18isolated from decomposing solid green household waste in water and soil against Candida albicans and Aspergillus niger. E3S Web Conf. 2020;150 doi: 10.1051/e3sconf/202015002003. [DOI] [Google Scholar]
- 284.Vaidya V., Prasad D.T. Thermostable levansucrase from Bacillus subtilis BB04, an isolate of Banana peel. J. Biochem. Technol. 2012;3:322–327. [Google Scholar]
- 285.Grevitara P.Y., F B.R., P H.S., Dahlia Y., Suarsini E. Isolation and identification of cellulose degrading Bacteria from banana peel compost. El-Hayah. 2018;7:6–11. doi: 10.18860/elha.v7i1.7241. [DOI] [Google Scholar]
- 286.Mazareli R.Cda S., Sakamoto I.K., Silva E.L., Varesche M.B.A. Bacillus sp. isolated from banana waste and analysis of metabolic pathways in acidogenic systems in hydrogen production. J. Environ. Manage. 2019;247:178–186. doi: 10.1016/j.jenvman.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 287.Amilia K.R., Sari S.L.A., Setyaningsih R. Isolation and screening of pectinolytic Fungi from orange (Citrus nobilis tan.) and banana (Musa acuminata L.) fruit peel. IOP Conf. Ser. Mater. Sci. Eng. 2017;193 doi: 10.1088/1757-899X/193/1/012015. [DOI] [Google Scholar]
- 288.Ling L., Li Z., Jiao Z., Zhang X., Ma W., Feng J., Zhang J., Lu L. Identification of novel endophytic yeast strains from tangerine peel. Curr. Microbiol. 2019;76:1066–1072. doi: 10.1007/s00284-019-01721-9. [DOI] [PubMed] [Google Scholar]
- 289.Maeno S., Tanizawa Y., Kajikawa A., Kanesaki Y., Kubota E., Arita M., Dicks L., Endoa A. Pseudofructophilic Leuconostoc citreum Strain F192-5, isolated from Satsuma Mandarin Peel. Appl Env. Microbiol. 2019;85 doi: 10.1128/AEM.01077-19. e01077–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Anwar F., Naseer R., Bhanger M.I., Ashraf S., Talpur F.N., Aladedunye F.A. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J. Am. Oil Chem. Soc. 2008;85:321–330. doi: 10.1007/s11746-008-1204-3. [DOI] [Google Scholar]
- 291.Kaderides K., Papaoikonomou L., Serafim M., Goula A.M. Microwave-assisted extraction of phenolics from pomegranate peels: optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process Intensif. 2019;137:1–11. doi: 10.1016/j.cep.2019.01.006. [DOI] [Google Scholar]
- 292.Irkin R., Dogan S., Degirmenioglu N., Diken M.E., Guldas M. Phenolic content, antioxidant activities and stimulatory roles of citrus fruits on some lactic acid bacteria. Arch. Biol. Sci. 2015;67:1313–1321. doi: 10.2298/ABS140909108I. [DOI] [Google Scholar]
- 293.Wang Y.C., Chuang Y.C., Hsu H.W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–284. doi: 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- 294.Cháfer M., González-Martínez C., Ortolá M.D., Chiralt A., Fito P. Kinetics of osmotic dehydration in orange and mandarin peels. J. Food Process Eng. 2001;24:273–289. doi: 10.1111/j.1745-4530.2001.tb00544.x. [DOI] [Google Scholar]
- 295.Cháfer M., González-Martínez C., Chiralt A., Fito P. Microstructure and vacuum impregnation response of citrus peels. Food Res. Int. 2003;36:35–41. doi: 10.1016/S0963-9969(02)00105-9. [DOI] [Google Scholar]
- 296.Pereira P.H.F., Oliveira T.Í.S., Rosa M.F., Cavalcante F.L., Moates G.K., Wellner N., Waldron K.W., Azeredo H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016;88:373–379. doi: 10.1016/j.ijbiomac.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 297.Ramadan A., El-badrawey S., El-ghany M.A., Nagib R. Recent Trends Dev. Institutional Acad. Perform. High. Specif. Educ. Institutions Egypt Arab World; 2010. Utilization of hydro-alcoholic extracts of peel and rind and juice of pomegranate as natural antioxidants in cotton seed oil; pp. 2443–2464. [Google Scholar]
- 298.Poyrazoğlu E., Gökmen V., Artιk N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Anal. 2002;15:567–575. doi: 10.1006/jfca.2002.1071. [DOI] [Google Scholar]
- 299.Al-Rawahi A.S., Rahman M.S., Guizani N., Essa M.M. Chemical composition, water sorption isotherm, and phenolic contents in fresh and dried pomegranate peels. Dry. Technol. 2013;31:257–263. doi: 10.1080/07373937.2012.710695. [DOI] [Google Scholar]
- 300.Spilmont M., Léotoing L., Davicco M.J., Lebecque P., Miot-Noirault E., Pilet P., Rios L., Wittrant Y., Coxam V. Pomegranate peel extract prevents bone loss in a preclinical model of osteoporosis and stimulates osteoblastic differentiation in vitro. Nutrients. 2015;7:9265–9284. doi: 10.3390/nu7115465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Oberoi H.S., Sandhu S.K., Vadlani P.V. Statistical optimization of hydrolysis process for banana peels using cellulolytic and pectinolytic enzymes. Food Bioprod. Process. 2012;90:257–265. doi: 10.1016/j.fbp.2011.05.002. [DOI] [Google Scholar]
- 302.Wachirasiri P., Julakarangka S., Wanlapa S. The effects of banana peel preparations on the properties of banana peel dietary fibre concentrate, Songklanakarin. J. Sci. Technol. 2009;31:605–611. [Google Scholar]
- 303.Anhwange B.A. Chemical composition of Musa sapientum (Banana) peels. J. Food Technol. 2008;6:263–266. [Google Scholar]
- 304.Holland D., I B. The pomegranate ; new interst in an ancient fruit. Chron. Horticult. 2008;48:12–15. [Google Scholar]
- 305.Bchir B. Liege University; 2011. Contribution to the Study of the Conservation of Pomegranate Seeds by Osmotic Dehydration. [Google Scholar]
- 306.Turfan Ö., Türkyilmaz M., Yemi O., Özkan M. Anthocyanin and colour changes during processing of pomegranate (Punica granatum L.; Cv. Hicaznar) juice from sacs and whole fruit. Food Chem. 2011;129:1644–1651. doi: 10.1016/j.foodchem.2011.06.024. [DOI] [Google Scholar]
- 307.Hernández F., Legua P., Melgarejo-Sánchez P., Martinez Font R. 2012. The Pomegranate Tree in the World: Its Problems and Uses. [Google Scholar]
- 308.Melgarejo-Sánchez P., Martínez J.J., Hernández F., Legua P., Martínez R., Melgarejo P. The pomegranate tree in the world: new cultivars and uses. Acta Hortic. 2015;1089:327–332. doi: 10.17660/ActaHortic.2015.1089.43. [DOI] [Google Scholar]
- 309.Sinha S., Thakur D.S., Mishra P.K., Sinha R. D2-Analysis suggests wider genetic divergence in analysis pomegranate. The Bioscan. 2016;11:1011–1015. [Google Scholar]
- 310.Moghaddam E.H., Shaaban M., Sepahvand A. Medicinal properties of pomegranate. Herb. Med. J. 2019;4:1–13. [Google Scholar]