Abstract
The disease, cystic fibrosis, is caused by the malfunction of the cystic fibrosis transmembrane conductance regulator. Expression of functional CFTR may normally regulate extracellular pH via control of bicarbonate efflux. Reports also suggest that the CFTR may be a Cl-/HCO3- exchanger. If true, this could be very important for treatment of CF given the airway host defense system is quite sensitive to pH, and acidic pH been found to increase mucus viscosity. We compared evidentiary support of four possible models of CFTR's role in the transport of bicarbonate: 1) CFTR as a Cl-channel that permits bicarbonate conductance, 2) CFTR as an anion Cl-/HCO3- exchanger (AE), 3.) CFTR as both a Cl-channel and an AE, and 4.) CFTR as a Cl-channel that allows for transport of bicarbonate and regulates an independent AE. The effect of stimulators and inhibitors of CFTR and AEs were evaluated via iodide efflux and studies of extracellular pH. This data, as well as that published by others, suggest that while CFTR may support and regulate bicarbonate flux it is unlikely it directly performs Cl-/HCO3- anion exchange.
Keywords: Cystic fibrosis, Bicarbonate transport, CFTR, Microphysiometry, Efflux
Highlights
-
•
Four possible models of CFTR's role in the transport of HC03- were evaluated.
-
•
Cells exposed to modulators of CFTR/AE activity were studied by efflux and pHo.
-
•
Efflux: While CFTR inhibition reduced anion efflux, AE inhibition did not.
-
•
pHo: In Cl-free buffer conditions CFTR activation reduced pHo acidification.
-
•
CFTR may regulate but unlikely it directly performs Cl-/HCO3- exchange.
1. Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) is a protein found within the phospholipid bilayer of epithelial cells along the apical surface [1]. This protein regulates salt transport and epithelial fluid secretion [1]. CFTR is an ABC transporter, meaning it requires ATP in order to regulate gating and conductance of anions, such as chloride (Cl−) and bicarbonate (HCO3-) [[2], [3], [4], [5]]. Cystic fibrosis (CF) is caused by a genetic mutation within the DNA sequence which codes for the CFTR gene [6]. The most common mutation of the CFTR gene is ΔF508, which results in the protein becoming degraded before reaching the apical membrane [1,7]. The protein is targeted by ubiquitin-proteasome pathway which sends it to the proteasome for degradation [1]. The disease pathology is mainly due to a lack of Cl− conductance, however, it is difficult to attribute all symptoms of CF solely to changes in Cl− conductance [8,9].
The aim of this study was to examine bicarbonate conductance through CFTR as well as investigate the “Muallem hypothesis” which proposed that CFTR is an anion exchanger (AE) [10]. Since mucins become viscous in lower pH this might be important given the classic manifestation of cystic fibrosis is thickened mucus that plugs the vas deferens (fertility), the pancreas (digestion), and the lungs (infections). The specific goals of this research were to examine the Cl− channel conductance of CFTR expressed in stable cell lines, as well as examine the conditions necessary for CFTR-dependent HCO3− conductance to take place. The direct characterization of Cl− conductance in these cell lines was accomplished through complete sample replacement iodide efflux experiments [11,12]. Examining the conditions necessary for CFTR dependent HCO3- conductance was achieved by tracking extracellular pH (pHo) through the use of a microphysiometer [[13], [14], [15]], a semiconductor-based instrument which detects the rate at which a cell acidifies its environment. Cells were perfused with physiological buffers containing various CFTR and AE activators and inhibitors and resulting changes in ion flux and pHo were dynamically monitored.
In testing the “Muallem hypothesis” we made the assumption that the channel and exchanger properties of CFTR were two separate entities and focused on three predictions proposed by the Muallem group [10]; 1) bicarbonate transport should be Cl-dependent and stimulated by forskolin; 2) glibenclamide (a known CFTR inhibitor) should stop efflux of chloride through the CFTR channel but should not prevent the action of CFTR as an exchanger; and 3) DIDS (an anion exchanger blocker) should effect and block bicarbonate movement through CFTR's anion exchanger in cells expressing CFTR.
2. Materials and methods
2.1. Cell culture
The cells used in this study were C127 mouse mammary epithelial control cells, cells stably expressing wild-type CFTR, and those expressing ΔF508-CFTR. These cells were obtained from Genzyme Corporation (Dr. Seng Cheng). These are stable cell lines that were either transfected with the BPV vector alone (bovine papilloma-based vector with neomycin resistant genes) or transfected with BPV vector containing either cDNA for wild-type CFTR (2WT2 cells) or ΔF508 CFTR (508–8 cells). C127 cell lines were grown in Dulbecco's Modified Eagles Medium (DMEM) containing 10% fetal bovine serum (FBS), 2 mM glutamine, 100 units/ml penicillin, and 10 μg/ml streptomycin.
2.2. Iodide efflux
I-125 efflux (substituted for Cl−) was measured using complete sample replacement with 30-sec intervals. Efflux buffer was (in mM): 10 HEPES; pH 7.4, 5.4 KCl, 1.8 CaC12, 1.0 NaH2PO4, 0.8 MgSO4, and 1 mg/ml glucose (either with 150.0 of NaCl or NaI). Protocol and efflux rate constants were generate as described by Venglarik et al., 1990 [12].
2.3. Measurement of extracellular pH
C127 cells were prepared per manufacturer's recommendations before transfer into sensor chambers. Cells were passaged into six 12-mm diameter disposable 3.0 μm porous polycarbonate capsules in standard DMEM supplemented with 10% FBS and 5% P/S as described previously [13,16]. The cells and sensor chambers were first equilibrated with the running medium (in mM) 148 NaCl, 5.0 KCl, 1.0 CaCl2, and 2.0 HEPES, 1.8 mg/ml glucose; pH 7.4 for about 10 min. A spacer, which defines the internal size of the cell area used for recording, was then placed on the capsule cell surface. A capsule insert, which traps the cells between microporous membranes and a spacer, was then placed on top. The cell capsules were transferred to the equilibrated sensor chambers with a silicon chip (pH detector) on the bottom and placed on to the recording instrument. A two mm diameter circular region in the center of cell capsule was used for pH recordings by a light emitting diode (LED) located underneath the sensor chamber. To enhance the detection of subtle pH changes, sensor chambers were perfused with a nominally bicarbonate-free running media with a low buffering capacity. The media was also degassed/debubbled and warmed to 37 °C, before perfusing cells, and pHo was continually monitored. Reservoirs of cell media were connected to the flow chamber by tubing, and the flow rate was set to 100 μl/min. Each cycle lasted 2 min and consisted of a perfusion phase and an interruption phase. During the perfusion phase the medium was continuously passed through the chamber for an interval of 80 s. During the interruption phase, the pumps were halted for 40 s, during which the rate of acidification within the chamber was calculated, recorded and plotted by the software (termed rate data). The flow was then resumed, and the next cycle begun, with rates of acidification returning again to baseline.
2.4. Statistical analysis
Except where noted, all data are reported as mean ± standard error (SEM). Statistical significance was assessed by performing two-tailed student's t-tests. When using microphysiometer data, the notation “n = x(y)” was used, where “x” stands for traditional trial number and denotes the number of cell capsules used. The “y” stands for the number of multiple replications performed on a single cell capsule for the duration of a single experiment. For example, n = 2(4) would indicate two different cell capsules, each tested twice for the same condition. Multiple curves of normalized acidification rate obtained through the multiple replications would then be averaged to represent a response from a cell capsule under a certain condition. For all microphysiometer data, each capsule was represented as the mean of all trials, however, only the number of capsules tested was used as a trial number in statistical tests. In iodide efflux studies, data was obtained from 35 mm cell culture dishes and each dish of cells was used as a single trial number. The data were again represented as an average curve of all trials for a single experimental condition.
3. Results
3.1. CFTR activation increased iodide efflux
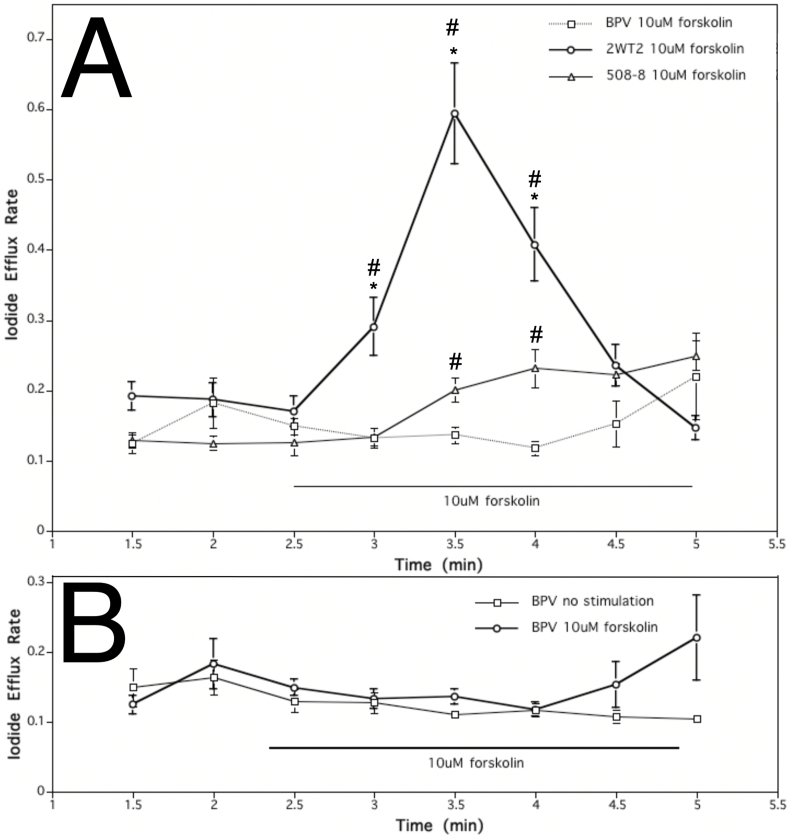
Application of 10 μM forskolin, a cAMP elevating agent, was used to stimulate CFTR and thus elicit a significant increase in iodide efflux in "wild type" cells, expressing wild-type CFTR, when compared to "mutant" cells, expressing ΔF508-CFTR and vector-transfected controls (Fig. 1). Comparing iodide efflux rate, change in concentration over time, of wild type cells before stimulation (at 2.5 min) and at the second time point during forskolin treatment (at 3.5 min), 10 μM forskolin caused about a 3.5-fold increase in efflux rate and yielded an increase in rate constant of 0.42. The increase in iodide efflux observed in forskolin-stimulated cells expressing wild-type CFTR was significantly higher than that from cell lines expressing mutant CFTR or null controls. By contrast, forskolin only caused a small gradual increase in iodide efflux from ΔF508-CFTR mutant cells (0.07 increase in rate constant) and no change in control cells (Fig. 1A). The iodide efflux rate of mutant cells stimulated with 10 μM forskolin was comparable but distinct from the baseline level of found in a vector-transfected null control cell line (Fig. 1B). CFTR activation successfully increased iodide efflux thus establishing the stability of the experimental system and its controls, and enabling the next step, inhibitors added to test the impact of restricted movement of specific ions.
Fig. 1.
Effect of CFTR activator (10μM forskolin) on iodide efflux rate of cell lines stably expressing wild type, mutant ΔF508-CFTR versus null control. (A.) Effect of 10 μM forskolin treatment on iodide efflux rate of control cells transfected with vector alone (BPV), and cells stably expressing wild type CFTR (2WT2) or ΔF508-mutant CFTR (508–8). When compared to mutant and control cells, 10 μM forskolin (cAMP elevating agent) exposure elicited a rapid and significant increase in iodide efflux in wild type cells. Each point represents an average of 20 experiments for wild type and mutant cell lines and 6 experiments for the control cell line (*p < 0.01 compared to 508–8; #p < 0.01 compared to BPV; error bars as SEM). (B.) Effect of 10 μM forskolin treatment versus non-treatment in rate of iodide efflux response of control cell line (vector transfected BPV cells). Each point represents an average of 6 trials (error bars as SEM).
3.2. Anion exchanger (AE) inhibition did not reduce iodide efflux
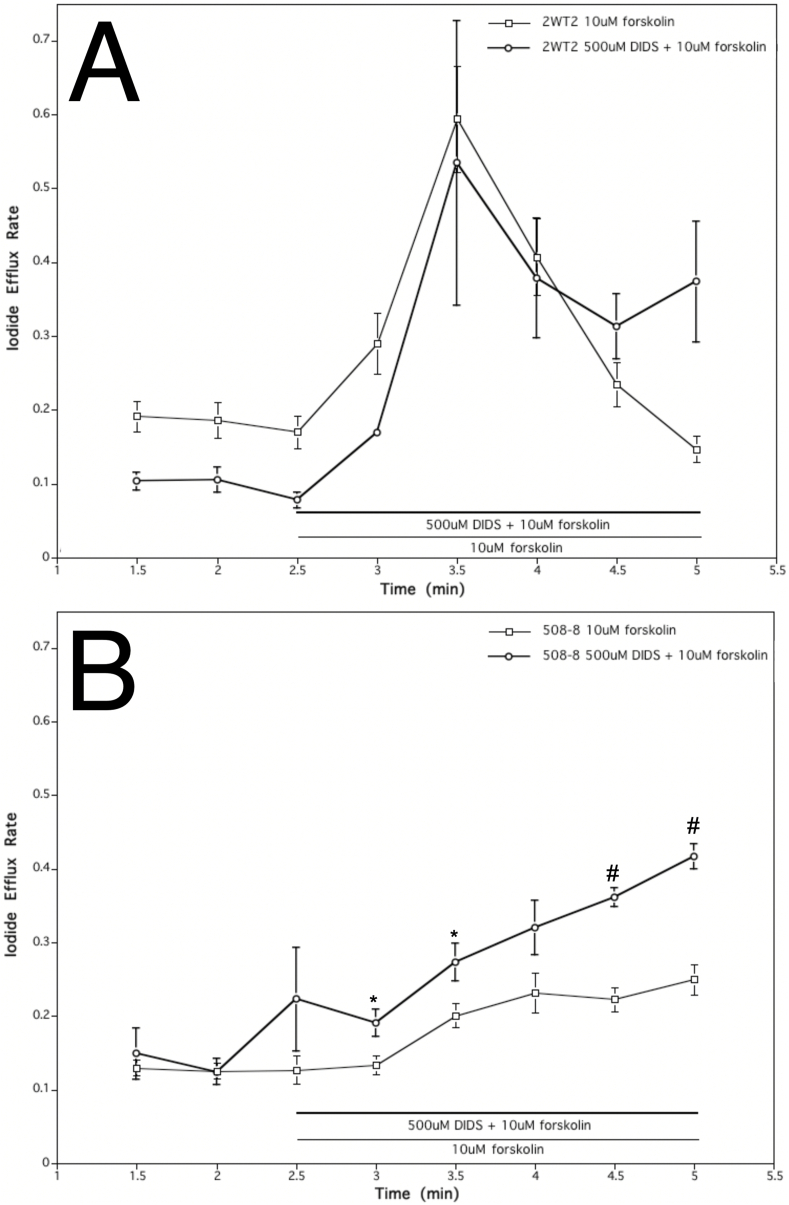
Concurrent treatment of wild-type cells with 500 μM DIDS (AE inhibitor) and 10 μM forskolin elicited a significant increase in iodide efflux similar to that seen in the controls with forskolin alone (Fig. 2A). The efflux rate increased about 5-fold compared to the pretreatment (between 2.5 and 3.5 min). This yielded a change in rate constant of 0.46 (Fig. 2A). The average increase in efflux rate of wild type cells with both DIDS and forskolin was slightly higher than the mean increase from wild type cells treated solely with forskolin (Fig. 2A). Cells expressing ΔF508-CFTR did not change their iodide movement (Fig. 2B). The efflux from ΔF508-CFTR cells compared under two conditions (DIDS + forskolin, forskolin alone) revealed no inhibition. Given anion exchange inhibitors did not reduce efflux in either cell line, expressing wild-type or mutant CFTR, this undermines the possibility that CFTR directly performs Cl-/HCO3- exchange.
Fig. 2.
Effect of AE inhibitor (500 μM DIDS) on iodide efflux rate of cell lines stably expressing wild type and mutant ΔF508-CFTR. A. Effect of 500 μM DIDS and 10 μM forskolin treatment of wild type cells resulted in an almost identical iodide efflux rate as that elicited by treatment with 10 μM forskolin alone. Each point represents an average of 20 experiments for 10 μM forskolin treatment and 6 experiments for studies with 500 μM DIDS and 10 μM forskolin (error bars as SEM). B. Effect of 500 mM DIDS and 10 μM forskolin on mutant cells did not show a similar increase in iodide efflux. Each point represents an average of 20 experiments for 10 μM forskolin treatment and 6 experiments for studies with 500 μM DIDS and 10 mM forskolin (*p < 0.05; #p < 0.01; error bars as SEM).
3.3. CFTR inhibition reduced iodide efflux rates
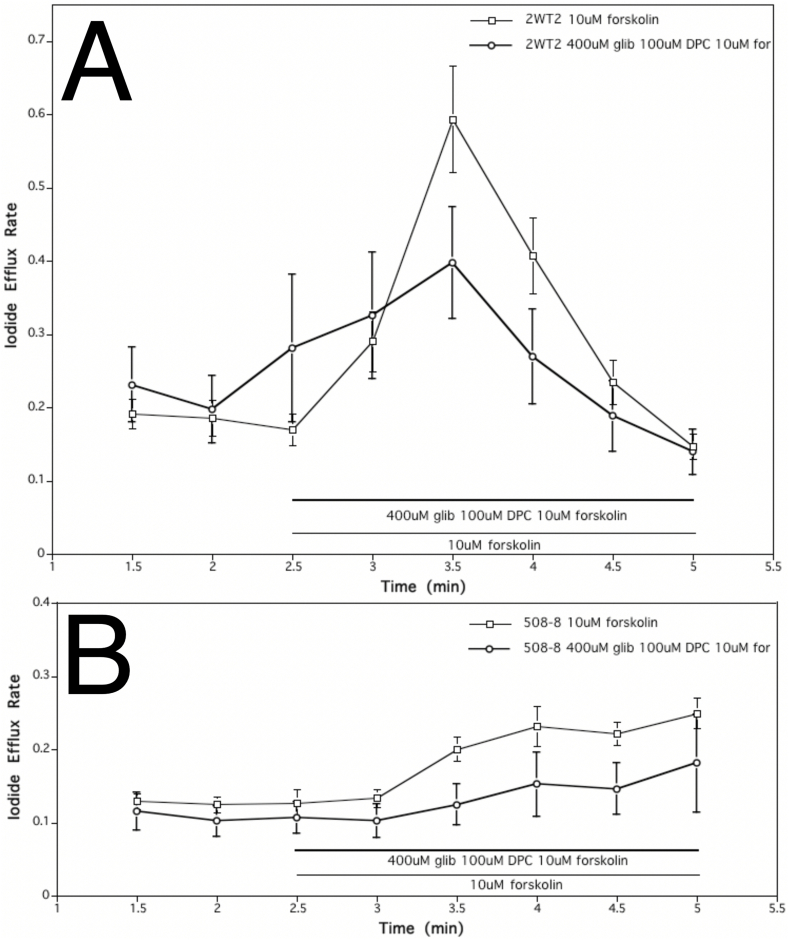
Application of 400 μM glibenclamide and 100 μM DPC (CFTR channel inhibitors) alongside 10 μM forskolin, decreased iodide efflux in wild type cells indicating, at least partial CFTR inhibition (Fig. 3A). Glibenclamide and DPC suppressed the increase in iodide efflux elicited by forskolin to only about 1.5-fold in wild type cells, causing a total change in rate of 0.12 (Fig. 3A). Yet when the difference versus a 3.5-fold increase caused by forskolin alone was not found to be statistically significant (p = 0.06). The same treatment in ΔF508-CFTR mutant cells did not cause any significant change in the overall rate of efflux in either cells treated with forskolin alone, or treated with forskolin and both glibenclamide and DPC (Fig. 3B). Both conditions did, however, cause a gradual steady increase in efflux rate for the duration of the experiment (1.7-fold increase with forskolin alone and 2-fold increase with inhibitors and forskolin) (Fig. 3B). No significant difference between the two treatments was observed.
Fig. 3.
Effect of CFTR inhibitors (400 μM glibenclamide, 100 μM DPC) on iodide efflux rate of cell lines stably expressing wild type and mutant ΔF508-CFTR.A. Application of 400 μM glibenclamide, 100 μM DPC, and 10 μM forskolin decreased iodide efflux in cell line expressing wild-type CFTR (2WT2 cells), indicating partial CFTR inhibition. Each point represents an average of 20 experiments for 10 μM forskolin treatment and 6 experiments for studies with 400 μM glibenclamide, 100 μM DPC and 10 μM forskolin (error bars as SEM). B. Treatment of cell line expressing ΔF508-CFTR (508–8 cells) with 400 μM glibenclamide, 100 μM DPC and 10 μM forskolin did not cause any significant change in the overall rate of efflux whether treated with forskolin alone, or with inhibitor cocktail and forskolin. The differences in efflux rate constants were not statistically significant (p = 0.24 at t = 3; p = 0.02 at t = 3.5; p = 0.13 at t = 4; p = 0.06 for at t = 4.5). Each point represents an average of 20 experiments for 10 μM forskolin treatment and 6 experiments for studies with 400 μM glibenclamide, 100 μM DPC and 10 μM forskolin (error bars as SEM).
If CFTR inhibition occurred, wild type cells treated with CFTR inhibitors (400 μM glibenclamide and 100 μM DPC) and the stimulator (10 μM forskolin) were expected to behave similarly to ΔF508-CFTR mutant cells treated with a stimulator. A significant difference was, however, observed between the two treatments. Wild type cells exhibited a rate of efflux increased by about 1.5-fold, causing a change in rate of efflux by 0.12, while mutant cells showed a change in rate of only 0.07, thus again indicating only partial inhibition of CFTR. In this case, the application of ion channel blockers reduced CFTR-dependent iodide efflux in both cell lines, which is supportive of the role where CFTR serves as an anion channel.
3.4. CFTR activation reduces extracellular acidification (causes alkalinization)
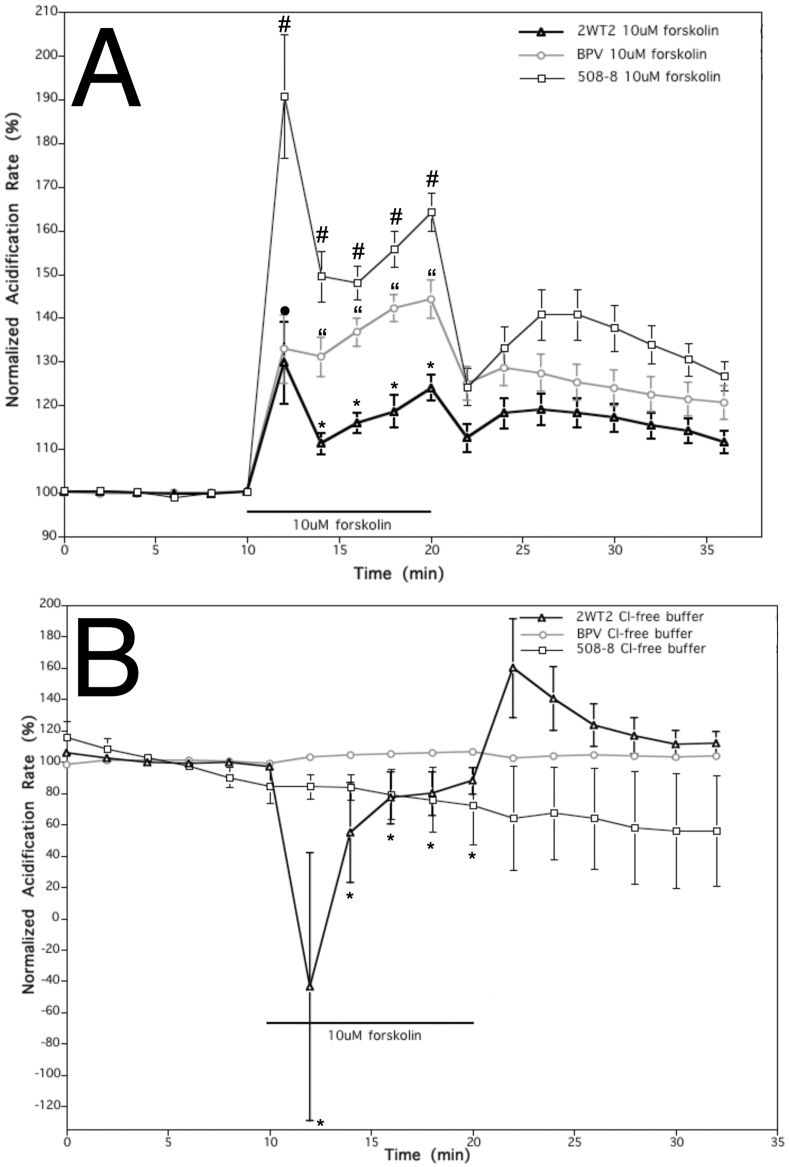
It has previously been shown that activation of CFTR, by cAMP elevating agent forskolin, can lead to decreased extracellular acidification (or increased extracellular alkalinization) of CFTR-expressing cells, and the opposite in CFTR-deficient control cells [10]. During this study, acidification rates were similarly compared between cells stably expressing wild-type CFTR, mutant ΔF508-CFTR and controls. After a 10-min exposure to 10 μM forskolin, a reduced extracellular pH acidification response was observed in cells expressing wild-type CFTR when compared to mutant and control cells (Fig. 4A). Immediately after stimulus acidification rates initially increased in all cell lines: mutant (90%), control cells (35%), and wild-type cells (35%). Four minutes after exposure to forskolin, the initial acidification rate for mutant cells reduced to a 50% (drifting between 45 and 65%) above pretreatment level. On the other hand, cells expressing wild-type CFTR displayed a distinctly reduced acidification rate profile from both the mutant and control cells. In response to forskolin stimulation the wild-type cells only exhibited a peak increase in acidification rate of 25% above that of the pretreatment state. Even after forskolin washout, the acidification rates of cell types remained distinct from one another for some time. Given the application of reagents known to elevate ion conductance in CFTR lead to a concomitant extracellular alkalinization, this is suggestive that wild-type CFTR can conduct bicarbonate ions and impact pH without requiring anion exchange.
Fig. 4.
Impact of forskolin (CFTR stimulator) and Cl-free conditions (AE inhibitor) on cells expressing wild type and ΔF508-CFTR on extracellular pH assayed via Microphysiometry. A. Effect of 10 μM forskolin treatment on normalized acidification rates of control cells (BPV) and cell line stably expressing wild-type CFTR (2WT2 cells) or mutant ΔF508-CFTR (508–8 cells). A 10-min exposure to 10 μM forskolin resulted in a significantly lower increase in acidification rate in wild-type cells when compared to mutant and control cells. Each point represents an average of n = 26(39) for wild type cells, n = 26(38) for mutant cells, and n = 13(20) for control cells (*p < 0.01 comparing 2WT2 to 508–8 and BPV; #p < 0.01 comparing 508–8 to 2WT2 and BPV; “p < 0.01 comparing BPV to 508–8 and 2WT2; • p < 0.01 comparing 2WT2 to 508–8; error bars as SEM). B. In Cl-free media a 10 μM forskolin treatment decreased normalized acidification rates of cells expressing wild type CFTR (2WT2) but had no impact on cells expressing mutant ΔF508-CFTR (508–8) or controls (BPV). Each point represents an average of n = 8 for wild type cells, n = 10 for mutant cells, and n = 4(8) for control cells (*p < 0.01 comparing 2WT2 and 508–8 to BPV cells; error bars as SEM).
3.5. Cl- free buffer reduces extracellular acidification rates
Our final studies monitored acidification rates of cells in Cl-free media with stimulation by 10 μM forskolin. By introducing a Cl-free environment on the extracellular side, thus creating a concentration gradient for Cl-to exit the cells and HCO3- to enter, we could better examine the possibility that CFTR is a Cl-/HCO3- exchanger. If CFTR were an AE, HCO3- would only be able to enter the cell via CFTR upon Cl-exit. Thus, one might predict if CFTR was an AE, in the Cl-free environment, forskolin stimulation might lead to a greater increase in extracellular acidification rate as compared to mutant and control cells. Following the switch to Cl-free medium containing 10 μM forskolin, the acidification rate of wild type cells decreased by about 1.4-fold while there was no change in either mutant or control cells (Fig. 4B). This decrease in acidification rates of wild type cells was rather short lived (Fig. 4B). To compare, exposure to 10 μM forskolin in running media containing Cl-, elicited an increase in acidification rate in wild type cells of about 1.35-fold. Four minutes following the drug exposure acidification rate in wild type cells was reduced to 1.25-fold above baseline. The Cl-free conditions did not reverse, but rather increased alkalinization of extracellular environment of cells expressing wild-type CFTR. If CFTR had anion exchanger qualities, when comparing wild type cells to mutant and control cells, we would not expect to see forskolin-stimulated reduction in extracellular acidification rate (i.e. extracellular alkalinization) in cells overexpressing functional wild-type CFTR.
4. Discussion
The aim of this study was to characterize the regulation of HCO3- transport and extracellular pH by CFTR. Four models of CFTR function were assessed throughout this study: 1) CFTR as a Cl-channel that permits HCO3- conductance, 2) CFTR as an anion Cl-/HCO3- exchanger (AE), 3.) CFTR as both a Cl-channel and an AE, and 4.) CFTR as a Cl-channel that allows for transport of HCO3- and regulates an independent AE. The Muallem hypothesis stated that three conditions were required for CFTR to be considered an anion exchanger: bicarbonate transport should be Cl-dependent and stimulated with forskolin, glibenclamide and DPC should stop efflux of chloride through the channel, but should not prevent action of CFTR as an anion exchanger, and DIDS should block bicarbonate movement through CFTR's anion exchanger in expression cells. However, none of these conditions were met during the experiments.
Glibenclamide and DPC prevented forskolin-stimulated bicarbonate efflux through CFTR (as observed by increased acidification rate following the inhibitor treatment). DIDS treatment did not prevent bicarbonate efflux stimulated with forskolin; rather it was found to increase it in all cell lines used. This was indicative of inhibition of AEs found in all three cell lines, and not an AE specific to the wild-type CFTR expressing line. Additionally, the decrease in acidification rate during the simultaneous DIDS and forskolin treatment was greater than with DIDS alone only in wild type cells. The results imply that upon activation of CFTR, with forskolin, there is an additional base efflux, which compensates for an increased metabolic rate.
The most important piece of evidence collected stemmed from the studies with Cl-free media where treatment with forskolin in wild type cells caused a large decrease in acidification compared to the media containing Cl-. This aided in the conclusion that HCO3- transport through CFTR was not Cl-dependent which opposes the proposed model that it was mediated by an exchange mechanism.
Choi et al.'s [10] findings that suggested CFTR served as a Cl-/HCO3- exchanger. This could be explained by a tight interaction between CFTR and other Cl-/HC03- exchangers, such as members of SLC26 transporter family like DRA and A6.
CFTR has been shown to regulate these transporters and therefore Cl-/HCO3- exchange through interactions with PDZ domains [17]. PDZ mediated interactions of CFTR with other transporters, however are not an isolated event with SLC26 family. CFTR shows multiple interactions with PDZ proteins such as EBP50, Na+/H+ exchanger type 3 kinase A, CAP70, and so on [18]. This is suggested to play a role in the regulation of epithelial fluid and electrolyte transport that not only functions as a Cl-channel but also affects other exchangers and channels [19,20]. CFTR is thought to interact with other cell proteins, such as ENaC, through their respective PDZ domains and scaffolding proteins [12]. There have been suggestions that CFTR may affect Na + absorption through epithelial Na + channels (ENaC), K+ secretion through luminal K+ channels, and HCO3- salvage through Na+/HCO3- exchange [19,20].
These studies support CFTR function as presented by model 4: CFTR as a Cl-channel that allows for transport of HCO3- and regulates an independent AE.
Yet is pH important in CF? Yes, yet more data is needed in vivo. Consider, that the airway host defense system is sensitive to acidic pH [21], which could explain the persistent infections characteristic of CF lungs. And the classic manifestation of cystic fibrosis is mucus that plugs the vas deferens (fertility), the pancreas (digestion), and the lungs (infections). It also has been shown that acidic pH which is present young human airway cells [22] will increase the airway mucus viscosity in pig models [23]. Ropes of mucus form in the human submucosal glands [24] and impede the clearance of mucins out of the lung [25] perhaps due to a loss of bicarbonate [26].
CRediT authorship contribution statement
Marija K. Massey: Methodology, Investigation, Data curation, Formal analysis, Writing - original draft. Michael J. Reiterman: Validation, Visualization, Writing - review & editing. Jad Mourad: Writing - original draft, Visualization, Writing - review & editing. Douglas B. Luckie: Conceptualization, Methodology, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank Drs. John Wilterding, Karl Olson and Seth Hootman as well as students Stephen Cahill, Chi Lim, Vishal Malhorta for assistance and scholarly discussions in the generation of this work. This work was funded by the Cystic Fibrosis Foundation and Pennsylvania Cystic Fibrosis Inc.
References
- 1.Hutt D.M., Olsen C.A., Vickers C.J., Herman D., Chalfant M., Montero A., Leman L.J., Burkle R., Maryanoff B.E., Balch W.E., Ghadiri M.R. Potential agents for treating cystic fibrosis: cyclic tetrapeptides that restore trafficking and activity of ΔF508-CFTR. ACS Med. Chem. Lett. 2011;2:703–707. doi: 10.1021/ml200136e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szollosi A., Muallem D.R., Csanády L., Vergani P. Mutant cycles at CFTR's non-canonical ATP-binding site support little interface separation during gating. J. Gen. Physiol. 2011;137:549–562. doi: 10.1085/jgp.201110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz B.D. Airway epithelial cells: 'Bicarbonate in' ≠ 'Bicarbonate out. J Physiol. 2012;590:5263–5264. doi: 10.1113/jphysiol.2012.242586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D., Steward M.C. The role of CFTR in bicarbonate secretion by pancreatic duct and airway epithelia. J. Med. Invest. 2009;56(Suppl):336–342. doi: 10.2152/jmi.56.336. [DOI] [PubMed] [Google Scholar]
- 5.Kim D., Liao J., Hanrahan J.W. The buffer capacity of airway epithelial secretions. Front. Physiol. 2014;5:188. doi: 10.3389/fphys.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartoszewski R.A., Jablonsky M., Bartoszewska S., Stevenson L., Dai Q., Kappes J., Collawn J.F., Bebok Z. A synonymous single nucleotide polymorphism in DeltaF508 CFTR alters the secondary structure of the mRNA and the expression of the mutant protein. J. Biol. Chem. 2010;285:28741–28748. doi: 10.1074/jbc.M110.154575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukacs G.L., Verkman A.S. CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol. Med. 2012;18:81–91. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller C.M., Benos D.J. CFTR! Am. J. Physiol. 1992;263:C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- 9.Quinton P.M. Physiological basis of cystic fibrosis: a historical perspective. Physiol. Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 10.Choi J.Y., Lee M.G., Ko S., Muallem S. Cl- -dependent HCO3- transport by cystic fibrosis transmembrane conductance regulator. J. Pancreas. 2001;2:243–246. [PubMed] [Google Scholar]
- 11.Marshall J., Fang S., Ostedgaard L.S., O'Riordan C.R., Ferrara D., Amara J.F., Hoppe H., IV. Scheule R.K., Welsh M.J., Smith A.E., Cheng S.H. Stoichiometry of recombinant cystic fibrosis transmembrane conductance regulator in epithelial cells and its functional reconstitution into cells in vitro. J. Biol. Chem. 1994;269:2987–2995. [PubMed] [Google Scholar]
- 12.Venglarik C.J., Bridges R.J., Frizzell R.A. A simple assay for agonist-regulated Cl and K conductances in salt-secreting epithelial cells. Am. J. Physiol. 1990;259:C358–C364. doi: 10.1152/ajpcell.1990.259.2.C358. [DOI] [PubMed] [Google Scholar]
- 13.McConnell H.M., Owicki J.C., Parce J.W., Miller D.L., Baxter G.T., Wada H.G., Pitchford S. The cytosensor microphysiometer: biological applications of silicon technology. Science. 1992;257:1906–1912. doi: 10.1126/science.1329199. [DOI] [PubMed] [Google Scholar]
- 14.Hafner F. Cytosensor Microphysiometer: technology and recent applications. Biosens. Bioelectron. 2000;15:149–158. doi: 10.1016/s0956-5663(00)00069-5. [DOI] [PubMed] [Google Scholar]
- 15.Eklund S.E., Thompson R.G., Snider R.M., Carney C.K., Wright D.W., Wikswo J., Cliffel D.E. Metabolic discrimination of select list agents by monitoring cellular responses in a multianalyte microphysiometer. Sensors. 2009;9:2117–2133. doi: 10.3390/s90302117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luckie D.B., Singh C.N., Wine J.J., Wilterding J.H. CFTR activation raises extracellular pH of NIH/3T3 mouse fibroblasts and C127 epithelial cells. J. Membr. Biol. 2001;179:275–284. doi: 10.1007/s002320010052. [DOI] [PubMed] [Google Scholar]
- 17.Ko S.B., Zeng W., Dorwart M.R., Luo X., Kim K.H., Millen L., Goto H., Naruse S., Soyombo A., Thomas P.J., Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat. Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunzelmann K. CFTR: interacting with everything? News Physiol. Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 19.Lee M.G., Choi J.Y., Luo X., Strickland E., Thomas P.J., Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl-/HCO3- exchange in mouse submandibular and pancreatic ducts. J. Biol. Chem. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- 20.Ahn W., Kim K.H., Lee J.A., Kim J.Y., Choi J.Y., Moe O.W., Milgram S.L., Muallem S., Lee M.G. Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3- salvage mechanisms in model systems and the mouse pancreatic duct. J. Biol. Chem. 2001;276:17236–17243. doi: 10.1074/jbc.M011763200. [DOI] [PubMed] [Google Scholar]
- 21.Pezzulo A.A., Tang X.X., Hoegger M.J., Alaiwa M.H., Ramachandran S., Moninger T.O. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y., Salinas D., Nielson D.W., Verkman A.S. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell Physiol. 2006;290(3):C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 23.Tang X.X., Ostedgaard L.S., Hoegger M.J., Moninger T.O., Karp P.H., McMenimen J.D., Choudhury B., Varki A., Stoltz D.A., Welsh M.J. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Invest. 2016;126(3):879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostedgaard L.S., Moninger T.O., McMenimen J.D., Sawin N.M., Parker C.P., Thornell I.M., Powers L.S., Gansemer N.D., Bouzek D.C., Cook D.P., Meyerholz D.K., Abou Alaiwa M.H., Stoltz D.A., Welsh M.J. Gel-forming mucins form distinct morphologic structures in airways. Proc. Natl. Acad. Sci. U. S. A. 2017;174(26):6842–6847. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoegger M.J., Fischer A.J., McMenimen J.D., Ostedgaard L.S., Tucker A.J., Awadalla M.A., Moninger T.O., Michalski A.S., Hoffman E.A., Zabner J., Stoltz D.A., Welsh M.J. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen E.Y., Yang N., Quinton P.M., Chin W.C. A new role for bicarbonate in mucus formation. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299(4):L542–L549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]