Abstract
Data on the precise mechanisms of the complex interactions of factors related to clinical impact of knee osteoarthritis (KOA) in the elderly population remain limited. To find predictors that explain pain intensity, physical function, and quality of life in elderly KOA subjects, we performed a cross-sectional analysis of the baseline data from a randomized trial. The trial included 104 subjects (aged ≥60) with KOA pain and dysfunctional endogenous pain-inhibitory system activity assessed by conditioned pain modulation (CPM). Three multiple linear regression models were performed to understand the independent predictors of Brief Pain Inventory (BPI), WOMAC function subscale (WOMACFunc), and SF-12 physical subscale (SF12-PCS). Model 1 showed that BPI pain score was predicted by low CPM response, high von-Frey light touch threshold, worse radiological severity as indexed by Kellgren-Lawrence grade (KL), high von-Frey punctate pain intensity and high levels of anxiety (adjusted R2 = 27.1%, F (6,95) = 7.27, P < 0.0001). In model 2, von-Frey light touch threshold, KL, depressive symptoms indexed by Beck Depression Inventory (BDI), level of sleepiness and pain pressure threshold were risk factors for SF12-PCS (adjusted R2 = 31.9%, F (5,96) = 10.5, P < 0.0001). Finally, model 3 showed that WOMACFunc was predicted by BDI, KL and BPI (adjusted R2 = 41%, F (3,98) = 24.42, P < 0.0001). Our data provides an interesting framework to understand the predictors of KOA pain in the elderly and highlights how its related outcomes are affected by disease-specific factors, somatosensory dysfunction and emotional factors.
Keywords: Rehabilitation, Neuroscience, Systems neuroscience, Aging, Musculoskeletal system, Nervous system, Diagnostics, Aging and life course, Elderly, Chronic pain, Knee osteoarthritis, Conditioned pain modulation, Multiple regression analysis, Central sensitization
Rehabilitation; Neuroscience; Systems neuroscience; Aging; Musculoskeletal system; Nervous system; Diagnostics; Aging and life course; Elderly; Chronic pain; Knee osteoarthritis; Conditioned pain modulation; Multiple regression analysis; Central sensitization.
1. Introduction
Knee osteoarthritis (KOA) is the most prevalent form of osteoarthritis (OA) worldwide. It is defined as a complex and multifactorial disease characterized by progressive degeneration of articular cartilage and sclerosis of subchondral bone and an important cause of pain and disability, especially in the elderly population [1, 2]. Approximately 25% of people aged 50 years and over report chronic knee pain, among those 6% have severe knee pain and functional disability [3, 4]. Age is the strongest factor associated with OA development, thus with global aging process, the burden of KOA to the health care systems worldwide is expected to increase [5, 6]. Besides the pain, older individuals with KOA may have a poor quality of life due to stiffness, deformity, limitation in range of motion, dependence to perform activities of daily life; consequently leading to increases in economic and social costs globally [7, 8].
Chronic pain is a multidimensional condition that includes, besides the nociceptive component, an affective-motivational and cognitive-evaluative components [9]. Neuroimaging studies have shown that several brain regions are involved with pain processing, including the dorsolateral prefrontal cortex, sensorimotor cortex and thalamus [10, 11]. Therefore, KOA pain is not purely a result of the structural damage in the knee, but an interaction between local and structural changes at different levels of the neuroaxis [12]. The role of radiological findings on predicting clinical outcomes in KOA remains controversial, and evidence suggests that the degree of pain and disability are only weakly explained by the radiographic severity itself [13, 14]. Several studies have shown that peripheral and central sensitization play an important role in KOA pain persistence [15, 16]. However, it remains poorly understood to what degree central sensitization contributes to self-reported pain severity [17, 18]. Recent findings have suggested a close association between clinical pain and experimental pain measures, such as the descending conditioned pain modulation (CPM), pain pressure threshold (PPT) and temporal summation; however, there are few studies with small sample sizes [19, 20]. Foucher et al also demonstrated that the degree of central pain sensitization might also be affected by the duration of symptoms in KOA patients [21].
Besides altered peripheral and central nociceptive processes, the relationship between mood disorders and KOA pain has been extensively studied and suggests that high levels of psychological distress, such as depression, fear, and anxiety, contribute to pain maintenance and physical limitation [22]. Sharma et al conducted a systematic review and concluded that anxiety and depression could negatively impact health-related quality of life (HRQol) by impairing pain perception and physical functioning among individuals with OA [23]. Since KOA pain can itself generate or exacerbate psychological conditions [24], this relationship seems to be bidirectional, therefore one chronic disease negatively impacting the course of the other disease [25]. Additionally, pain-related cognitions, such as pain catastrophizing and self-efficacy, are consistently related to pain intensity and functional decline in KOA patients [26].
Although it is clear that emotional, somatosensory, and structural knee changes are important independent predictors of pain in OA, there is limited data testing all these predictors together to assess which ones drive pain intensity, physical function and HRQol in KOA. Therefore, the aim of this study is to investigate the risk factors for self-reported pain intensity, physical function and HRQol in an elderly population with KOA using multivariate linear regression models.
2. Methods
2.1. Study design
The present study assesses the baseline data of a randomized clinical trial evaluating the effects of transcranial direct current stimulation on pain intensity due to KOA in an elderly population (NCT03117231) [27]. The study was approved by the Research Ethics Committee at São Paulo Hospital (HSP) (1685/2016). The volunteers were recruited from the HSP outpatient clinics and from general population by advertisements posted in the city of São Paulo, Brazil. All participants provided a written informed consent form before study procedures.
2.2. Participants
A detailed description of the eligibility criteria was reported previously [27]. Briefly, the trial recruited 104 participants aged 60 years and above with a diagnosis of KOA, according to the American College of Rheumatology classification [28]. Eligible subjects met the following criteria: ongoing knee chronic pain (duration ≥6 months); average pain score ≥4 on a 0–10 numeric rating scale (NRS); dysfunctional endogenous pain-inhibitory system (reduction <10% in the pain intensity during the CPM-P test); not diagnosed with any severe acute or chronic uncompensated disease, cognitive impairment or severe depression; no history of epilepsy, syncope, alcohol abuse or traumatic brain injury with residual neurological deficits.
2.3. Assessments
Subjects were queried about demographic, socioeconomic, and clinical information. The following data were collected: age, gender, race, years of education, employment status, marital status, chronic disease conditions, cognitive evaluation (Mini-Mental State Examination), the knee and its region (medial or lateral) most painful. Furthermore, X-rays of the most affected knee were made, and the radiographic severity determined according to Kellgren-Lawrence (KL) 0–4 grading scale [29].
Moreover, the following assessments were performed:
Pain intensity – Brief Pain Inventory (BPI), a short and self-reported questionnaire, was used to assess pain intensity during the last 24 h [30]. The mean of the 4 items (pain right now, pain on average in last 24 h, worst pain in last 24 h, and least pain in last 24 h) that measure pain using an NRS from 0 (“no pain”) to 10 (“the worst pain you can imagine”) was calculated. In the case of bilateral knee pain, the patients were asked to rate their most painful knee.
Health-related quality of life – HRQol was assessed using the 12-Item Short-Form Health Survey scale (SF-12) which includes 8 domains: physical functioning, physical role functioning, bodily pain, general health perceptions, vitality, social functioning, emotional role functioning, and mental health [31]. Two subscales called physical component score (SF12-PCS) and mental component score were calculated. Higher scores are associated with a better quality of life. The SF12-PCS subscale was used in the analysis.
Physical Functioning – Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was collected to evaluate the disease-specific disability [32]. It includes three subscales to measure pain, physical function, and joint stiffness. The function sub-scale (WOMACFunc) that ranges from 0 to 68 (with higher values meaning worse physical performance) was used in the analysis.
Psychological Evaluation – Beck Depression Inventory (BDI) was collected to self-rate depressive symptoms. The questionnaire contains 21 items, yielding total scores ranging from 0 to 63, with higher values meaning greater severity [33].
The mood was also assessed using a 0–100mm visual analogic mood scale (VAMS) by asking the subjects to rate their anxiety, depression, stress, and sleepiness in the moment of the assessment. A higher score indicates worse mood [34].
Quantitative sensory testing – Mechanical detection threshold (MDT) and Mechanical pain threshold (MPT) were evaluated using 20 successive weighted Von Frey fibers (from 0.008 g to 300 g) applied perpendicular to the skin until the filament starts bending, with the eyes closed. The stimulus was kept for approximately 1.5 s and then removed. The test was performed on the thenar region ipsilateral to the most painful knee (hand), and on the most painful knee (knee).
For the MDT, we recorded the smallest weighted fiber that produces light touch and pinprick sensation. Thereafter to detect the MPT, the smallest weighted filament that produces pain was recorded. Following the MPT detection, subjects were asked to rate their pain on a 0–10 NRS, applying the same filament 3 times on the same regions with an interval to avoid sensitization; the mean score was recorded. If the subject did not have a positive response in a trial, the highest weighted fiber (300 g) was considered [35, 36].
Pain Pressure Threshold – PPT was assessed using a 1-cm2 rubber probe algometer (JTECH Medical Industries Inc). The probe was applied perpendicular to the same regions used for MDT and MPT until the patient reported that the pressure sensation became a painful sensation. The same test was performed three times, and the mean score of the recorded values (pressure in Kg at the first “painful sensation” moment) was calculated.
Conditioned Pain Modulation – CPM was performed using pressure as the test stimulus and cold water as the conditioning stimulus. The pressure was applied using the same device, technique and location used for PPT assessments. Following the PPT procedure, participants were asked to submerge their contralateral hand to the most painful knee into a water bath maintained at 10 °C for approximately 1 min. After the 30s of the cold water immersion, the pressure stimulus was reapplied with the same technique (post-CPM trial) [18].
The subjects were asked to report the exact moment when the pressure sensation became a painful sensation before (pre-CPM trial) and after (post-CPM trial) the conditioning stimulus. Participants were also asked to rate this pain sensation, using a 0–10 NRS. A percentage change in pain scores and PPT after CPM procedure were calculated, called CPM-P and CPM-PPT, respectively. The following formula was used: (post-CPM trial score − pre-CPM trial score/pre-CPM trial score) ∗ 100. We expect, after the conditioning stimulus, a negative value for CPM-P - since pain post-CPM should be lower that pre-CPM trial; and a positive valuer for CPM-PPT - since the amount of in Kg should be higher in the post-CPM trial.
2.4. Statistical analysis
Sample characteristics were described using descriptive statistics. Categorical variables were presented through relative and absolute frequencies. While continuous variables were reported by mean and standard deviation, and ordinal variables by median and interquartile range. The normality of data was assessed using visual inspection of the histogram, associated with skewness and kurtosis evaluations. After that, and also considering the central limit theorem, all variables were considered normally distributed.
The association of possible explanatory variables with the outcome variables (pain intensity, physical function, and HRQol) was evaluated with exploratory multiple linear regression models, using the forward stepwise selection method, starting by variable with the highest correlation with the dependent variable. Separate models were performed for each dependent variable. BPI pain score was used as the dependent variable in the “pain intensity” model, WOMACFunc in the “physical function” model and SF12-PCS subscale in the “HRQol” model.
Potential predictors were selected based on the mechanisms related to chronic pain physiology. As independent variables, we included age, gender, KL grade, BDI, VAMS for anxiety, depression, stress and sleepiness, von-Frey MDT, von-Frey MPT, von-Frey NRS (pain intensity following MPT detection), PPT, CPM-PPT, and CPM-P. For the “physical function” model, BPI level was also considered as an independent variable.
In the first step, simple linear regressions were created with each independent variable and dependent variable and sorted according to its variable regression coefficient and coefficient of determination (R-squared). Following this, the variables were entered one by one in the models and maintained depending on the alpha level of significance of the variable coefficient, associated with the R-squared. Variables were selected to be in the final model if provoked a relevant change in the R-squared and/or if they had a significant regression coefficient. The significance level was set at 0.05.
All variables were also assessed for effect modification, and if present (leads to change in more than 10% to the regression coefficient of the variables previously included in the model), the respective variable would be included in the final model. In the 3 models, the assumption of collinearity between each independent variable was evaluated by the variance inflation factor, linearity between dependent variable and independent variables was assessed by visual inspection of individual scatterplots and the homoscedasticity of the model was checked by visual inspection of the histogram of residuals, and the Shapiro-Wilk normality test of the standardized residuals [37].
3. Results
Of 104 subjects, the mean age was 73.9 (±8.01), 88 (84.6%) were women, and the mean educational level was 7 years (±5.52). The sample descriptive statistics are summarized in Tables 1 and 2.
Table 1.
Categorical variables description.
| All subjects | Female, n (%) |
Male, n (%) |
|
|---|---|---|---|
| 88 (84.6) | 16 (15.4) | ||
| Race, n (%) | |||
| White | 43 (41.4) | 42 (47.73) | 1 (6.25) |
| Brown | 41 (39.4) | 29 (32.95) | 12 (75) |
| Black | 13 (12.5) | 12 (13.64) | 1 (6.25) |
| Asian |
7 (6.7) |
5 (5.68) |
2 (12.50) |
| Working status, n (%) | |||
| Unemployed | 15 (14.4) | 13 (14.77) | 2 (12.50) |
| Full-time employed | 4 (3.9) | 3 (3.41) | 1 (6.25) |
| Part-time employed | 1 (0.9) | 1 (1.14) | 0 |
| Retired |
84 (80.8) |
71 (80.68) |
13 (81.25) |
| Marital status, n (%) | |||
| Married | 35 (33.7) | 25 (28.41) | 10 (62.5) |
| Single | 12 (11.5) | 12 (13.64) | 0 |
| Divorced | 13 (12.5) | 10 (11.36) | 3 (18.75) |
| Widowed |
44 (42.3) |
41 (46.59) |
3 (18.75) |
| Comorbidities, n (%) | |||
| Hypertension | 73 (70.2) | 63 (71.59) | 10 (62.50) |
| Diabetes Mellitus | 26 (25) | 20 (22.73) | 6 (37.5) |
| Hypothyroidism | 19 (18.3) | 18 (20.45) | 1 (6.25) |
| Osteoporosis | 26 (25) | 24 (27.27) | 2 (12.50) |
| Stable Coronary Disease |
10 (9.6) |
8 (9.09) |
2 (12.50) |
| Radiological grade, KL, n (%) | |||
| KL I | 26 (25.5) | 19 (21.59) | 7 (43.75) |
| KL II | 32 (31.4) | 27 (30.68) | 5 (31.25) |
| KL III | 30 (29.4) | 26 (29.55) | 4 (25) |
| KL IV | 14 (13.7) | 14 (15.91) | 0 |
| Unknown∗∗ |
2 (0.02) |
2 (2.27) |
0 |
| Knee most affected, n (%) | |||
| Right | 57 (54.8) | 49 (55.68) | 8 (50) |
| Left |
47 (45.2) |
39 (44.32) |
8 (50) |
| Region of knee most affected, n (%) | |||
| Medial | 78 (75) | 66 (75) | 12 (75) |
| Lateral | 26 (25) | 22 (25) | 4 (25) |
Abbreviations: KL: Kellgren-Lawrence.
2 subjects with total knee arthroplasty.
Table 2.
Continuous/ordinal variables description.
| All subjects | Female, n (%) |
Male, n (%) |
|
|---|---|---|---|
| 88 (84.6) | 16 (15.4) | ||
| Age, years, mean (SD) | 73.94 (8.01) | 74.02 (8.10) | 73.5 (7.72) |
| Education, years, mean (SD) | 7.02 (5.52) | 6.85 (5.36) | 7.94 (6.44) |
| Mini-Mental State Examination, mean (SD) | 24.98 (3.98) | 24.60 (4.11) | 27.06 (2.29) |
| BDI, mean (SD) | 13.00 (6.59) | 13.92 (6.45) | 7.94 (4.99) |
| BPI, mean (SD) |
4.59 (1.45) |
4.69 (1.48) |
4.05 (1.15) |
| SF -12, mean (SD) | |||
| Physical Component Summary (PCS) | 32.82 (8.98) | 31.71 (8.54) | 38.95 (9.11) |
| Mental Component Summary (MCS) |
48.52 (11.06) |
47.63 (10.89) |
53.44 (11) |
| WOMAC, mean (SD) | |||
| Pain subscale (0–20) | 9.82 (3.53) | 10.10 (3.56) | 8.25 (2.96) |
| Rigidity subscale (0–8) | 3.53 (2.04) | 3.64 (2.10) | 2.94 (1.57) |
| Function subscale (0–68) | 34.84 (12.15) | 36.16 (11.83) | 27.56 (11.67) |
| Total score (0–96) |
48.09 (15.55) |
49.78 (15.36) |
38.75 (13.52) |
| VAMS, mean (SD) | |||
| Anxiety | 4.16 (2.83) | 4.41 (2.81) | 2.77 (2.59) |
| Depression | 2.49 (2.93) | 2.80 (3.00) | 0.81 (1.81) |
| Stress | 3.41 (2.97) | 3.71 (3.00) | 1.76 (2.20) |
| Sleepiness |
2.90 (2.92) |
3.04 (2.98) |
2.14 (2.52) |
| Von-frey MDT, light touch, median (IQR) | |||
| Knee | 0.60 (1.00) | 0.60 (1.00) | 1.00 (0.98) |
| Hand |
0.40 (0.84) |
0.40 (0.64) |
0.80 (1.12) |
| Von-frey MDT, pinprick, median (IQR) | |||
| Knee | 4.00 (6.00) | 4.00 (6.00) | 4.00 (13.60) |
| Hand |
8.00 (24.00) |
8.00 (24.00) |
9.00 (21.00) |
| Von-frey MPT, median (IQR) | |||
| Knee | 26 (172.00) | 26.00 (91.00) | 43.00 (293.00) |
| Hand |
300.00 (274.00) |
300.00 (274.00) |
300.00 (274.00) |
| Von-frey NRS, mean (SD) | |||
| Knee | 3.08 (1.94) | 3.23 (1.95) | 2.31 (1.75) |
| Hand |
1.76 (1.94) |
1.86 (2.03) |
1.20 (1.26) |
| PPT, mean (SD) | |||
| Knee | 2.06 (1.21) | 1.94 (1.13) | 2.68 (1.47) |
| Hand |
2.72 (1.14) |
2.69 (1.17) |
2.90 (0.98) |
| CPM PPT, mean (SD) | |||
| Knee | 18.51 (29.32) | 19.75 (28.30) | 11.72 (34.62) |
| Hand |
1.87 (26.03) |
0.98 (25.80) |
6.77 (27.56) |
| CPM P, mean (SD) | |||
| Knee | 9.94 (15.06) | 10.83 (15.68) | 5.07 (10.02) |
| Hand | 3.38 (24.61) | 2.75 (25.31) | 6.83 (20.67) |
Abbreviations: BDI: Beck Depression Inventory; BPI: Brief Pain Inventory; SF -12: 12-Item Short Form Health Survey scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; VAMS: Visual Analogic Mood Scale; MDT: Mechanical Detection Threshold; MPT: Mechanical Pain Threshold; NRS: Numeric Rating Scale; PPT: Pain Pressure Threshold; CPM: Conditioned Pain Modulation; CPM-P: Conditioned Pain Modulation - pain; IQR: Interquartile Range; SD: Standard Deviation.
Three different models were created, one for BPI pain score as the dependent variable (model 1), one for SF12-PCS as the dependent variable (model 2), and another one considering WOMACFunc as the dependent variable (model 3). The four assumptions (collinearity, linearity, normality of the standardized residuals, homoscedasticity) were respected.
KL grade was transformed into a dichotomous variable, before the multiple regression analysis. The cut off used to split the variable was determined after analyzing the association between the original variable (KL grade) and the dependent variables in the univariate linear regressions (Table 3). For model 1, the KL IV showed a significant association with BPI, therefore KL grade was dichotomized into group 1 = KL I, II, and III + group 2 = IV. For models 2 and 3, the KL III and IV showed a significant association with the dependent variable, therefore it was dichotomized into group 1 = KL I and II + group 2 = KL III and IV. Two subjects were not classified according to KL grade due to the presence of bilateral knee prosthesis. Therefore, all the models included 102 patients, excluding two patients with previous total knee arthroplasty (TKA).
Table 3.
Simple Linear Regression between KL grade and pain intensity, physical function and quality of life.
| Dependent Variable | Adjusted R2 | KL grade∗∗ | B | SE B | p-value |
|---|---|---|---|---|---|
| BPI pain score | 0.081 | Intercept | 4.269 | 0.273 | <0.001 |
| II | 0.301 | 0.368 | 0.415 | ||
| III | 0.047 | 0.373 | 0.899 | ||
| IV | 1.463 | 0.462 | 0.002∗ | ||
| SF12-PCS | 0.129 | Intercept | 37.616 | 1.629 | <0.001 |
| II | -3.310 | 2.193 | 0.134 | ||
| III | -8.337 | 2.225 | <0.001∗ | ||
| IV | -8.596 | 2.753 | 0.002∗ | ||
| WOMAC function subscale | 0.116 | Intercept | 30.077 | 2.186 | <0.001 |
| II | 2.829 | 2.943 | 0.339 | ||
| III | 6.623 | 2.987 | 0.029∗ | ||
| IV | 14.066 | 3.695 | <0.001∗ |
Abbreviations: KL: Kellgren-Lawrence; BPI: Brief Pain Inventory; SF12-PCS: Physical Component Summary of 12-Item Short Form Health Survey scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Note: KL I was used as the reference variable in the regression.
3.1. Predictors of BPI
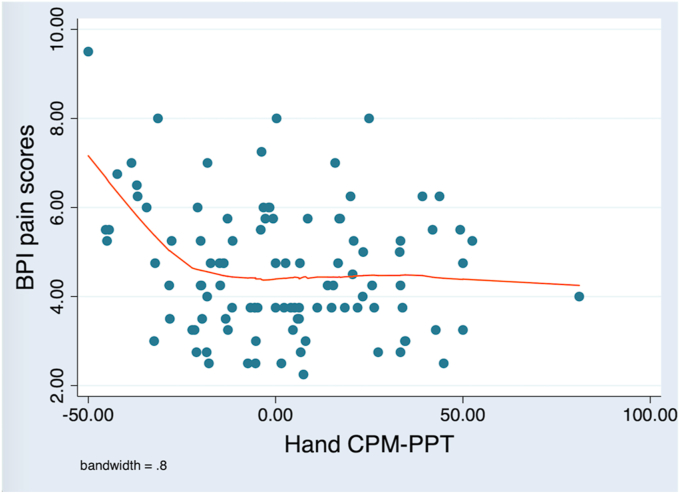
Model 1 resulted in the following final regression equation: BPI pain score = 3.163 + (-0.012 ∗ hand CPM-PPT) + (0.0003 ∗ hand CPM-PPT2) + (0.196 ∗ von-Frey NRS/hand) + (0.771 ∗ KL) + (0.129 ∗ anxiety VAMS) + (0.195 ∗ von-Frey MDT/light touch/knee) (Table 4). The relationship between hand CPM-PPT and BPI pain score was not completely linear, as showed by Figure 1. A linear association was found between BPI pain scores and the negative CPM-PPT values (defective pain modulation), marked by higher BPI scores (worse pain) associated with lower hand CPM-PPT scores (worse pain modulation). A quadratic term (hand CPM-PPT2) was included in the model to correct this not completely linear relationship.
Table 4.
Multiple regression analysis.
| Dependent Variable | Adjusted R2 | F-value | p-value∗ | Predictors | B | SE B | p-value |
|---|---|---|---|---|---|---|---|
| BPI pain score | 0.271 | 7.27 | <0.0001 | Intercept | 3.163 | 0.287 | <0.001 |
| CPM-PPT hand | -0.012 | 0.005 | 0.031 | ||||
| CPM-PPT hand2 | 0.0003 | 0.0001 | 0.031 | ||||
| Von-frey NRS hand | 0.196 | 0.065 | 0.003 | ||||
| KL grade∗∗ | 0.771 | 0.382 | 0.047 | ||||
| VAMS anxiety | 0.129 | 0.044 | 0.004 | ||||
| Von-frey MDT, light touch, knee | 0.195 | 0.078 | 0.014 | ||||
| SF12-PCS | 0.319 | 10.50 | <0.0001 | Intercept | 40.221 | 2.296 | <0.001 |
| BDI | -0.259 | 0.117 | 0.030 | ||||
| KL grade∗∗∗ | -6.202 | 1.548 | <0.001 | ||||
| Von-frey MDT, light touch, knee | -1.266 | 0.446 | 0.006 | ||||
| VAMS sleepiness | -0.791 | 0.266 | 0.004 | ||||
| PPT knee | 1.292 | 0.612 | 0.037 | ||||
| WOMACFunc | 0.410 | 24.42 | <0.0001 | Intercept | 13.382 | 3.208 | <0.001 |
| BDI | 0.890 | 0.142 | <0.001 | ||||
| KL grade∗∗∗ | 4.718 | 1.856 | 0.013 | ||||
| BPI pain score | 1.725 | 0.644 | 0.009 |
Abbreviations: BPI: Brief Pain Inventory; PPT: Pain Pressure Threshold; CPM: Conditioned Pain Modulation; NRS: Numeric Rating Scale; KL: Kellgren-Lawrence; VAMS: Visual Analogic Mood Scale; MDT: Mechanical Detection Threshold; SF12-PCS: Physical Component Summary of 12-Item Short Form Health Survey scale; BDI: Beck Depression Inventory; WOMACFunc: Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
KL grade dichotomized into group 1 = KL I, II and III + group 2 = IV.
KL grade dichotomized group 1 = KL I and II + group 2 = KL III and IV.
Figure 1.
Scatterplot of BPI pain scores X CPM PPT in the hand. There is a significant negative correlation between the two variables (Pearson ρ = -0.20; P = 0.04). However, the linear correlation can be noted for the negative CPM-PPT values (defective pain modulation), that are correlated with higher BPI scores (worse pain).
Model 1 statistically significantly predicted BPI pain scores, F (6, 95) = 7.27, P < 0.0001. This model was able to explain 27.1% of the variance in pain scores. Conditioned pain modulation assessed by CPM-PPT in the hand, von-Frey pain intensity after MPT detection in the hand, radiological evaluation assessed by KL grading method, perception of anxiety at the moment of evaluation detected by the anxiety VAMS and von-Frey light touch MDT detected in the knee were significantly associated with BPI pain scores. Model coefficients suggested that lower CPM-PPT scores in hand (worse pain modulation) are associated with higher scores in BPI (greater pain intensity), higher scores in NRS after MPT detection (higher pain) are related to higher scores in BPI, KL IV is associated with higher scores in BPI, higher values in anxiety VAMS (greater anxiety) are related to higher scores in BPI, light touch MDT detected with larger fibers is associated with higher scores in BPI.
Due to the lack of a completely linear association between hand CPM-PPT and BPI pain score, we also conducted a piecewise linear model dividing the hand CPM-PPT data into negative values and positive values, and found the final regression equation: BPI pain score = 2.983 + (-0.030 ∗ negative hand CPM-PPT) + (0.009 ∗ positive hand CPM-PPT) + (0.192 ∗ von-Frey NRS/hand) + (0.794 ∗ KL) + (0.127 ∗ anxiety VAMS) + (0.203 ∗ von-Frey MDT/light touch/knee). The spline regression statistically significantly predicted BPI pain scores, F (6, 95) = 7.34, P < 0.0001, and similarly to model 1 accounted for 27.4% of the variance in pain scores. This analysis confirmed that there was a linear relationship between hand CPM-PPT and BPI pain scores only for the negative CPM-PPT values, which indicates a defective pain inhibition (Table 5).
Table 5.
Spline regression analysis.
| Dependent Variable | Adjusted R2 | F-value | p-value∗ | Predictors | B | SE B | p-value |
|---|---|---|---|---|---|---|---|
| BPI pain score | 0.274 | 7.34 | <0.0001 | Intercept | 2.983 | 0.319 | <0.001∗ |
| Negative CPM-PPT hand | -0.030 | 0.011 | 0.008∗ | ||||
| Positive CPM-PPT hand | 0.009 | 0.009 | 0.322 | ||||
| Von-frey NRS hand | 0.192 | 0.065 | 0.004∗ | ||||
| KL grade∗∗ | 0.794 | 0.380 | 0.039∗ | ||||
| VAMS anxiety | 0.127 | 0.044 | 0.004∗ | ||||
| Von-frey MDT, light touch, knee | 0.203 | 0.077 | 0.010∗ |
Abbreviations: BPI: Brief Pain Inventory; PPT: Pain Pressure Threshold; CPM: Conditioned Pain Modulation; NRS: Numeric Rating Scale; KL: Kellgren-Lawrence; VAMS: Visual Analogic Mood Scale; MDT: Mechanical Detection Threshold.
KL grade dichotomized into group 1 = KL I, II and III + group 2 = IV.
3.2. Predictors of SF12-PCS
Model 2 resulted in the following final regression equation: SF12-PCS = 40.221 + (-0.259 ∗ BDI) + (-6.202 ∗ KL) + (-1.266 ∗ von-Frey MDT/light touch/knee) + (-0.791 ∗ sleepiness VAMS) + (1.292 + Knee PPT) (Table 4). Model 2 statistically significantly predicted SF12-PCS, F (5, 96) = 10.5, P < 0.0001. This model accounted for 31.9% of the variance in HRQol measured by SF12-PCS subscale. Depressive symptoms assessed by BDI, the radiological evaluation assessed by KL grade, von-Frey light touch MDT detected in the knee, perception of sleepiness at the moment of evaluation detected by the sleepiness VAMS and the PPT measured in the knee were significantly associated with HRQol. The direction of the model coefficients suggested that higher scores in BDI (worse depression) are associated with lower scores in SF12-PCS (worse quality of life), KL III e IV are related to lower scores in SF12-PCS, light touch MDT detected with larger fibers is associated with lower scores in SF12-PCS, higher values in sleepiness VAMS (greater sleepiness) are related to lower scores in SF12-PCS and, also lower PPT in the knee are associated with lower scores in SF12-PCS.
3.3. Predictors of WOMACFunc
Model 3 resulted in the following final regression equation: WOMACFunc = 13.382 + (0.89 ∗ BDI) + (4.718 ∗ KL) + (1.725 ∗ BPI) (Table 4). Model 3 statistically significantly predicted WOMACFunc, F (3, 98) = 24.42, P < 0.0001. This model accounted for 41% of the variability in physical function score around its mean. Depressive symptoms assessed by BDI, radiological evaluation assessed by KL grade, and pain intensity assessed by BPI were significantly associated with physical function. The direction of the regression coefficients indicated that higher scores in BDI (worse depression) are associated with higher scores in WOMACFunc (worse physical function), also KL III e IV are associated with higher scores in WOMACFunc, and higher scores in BPI (greater pain intensity) are related to higher scores in WOMACFunc.
4. Discussion
The present exploratory analysis showed in the same model that higher peripheral structural damage (indexed by KL), increased peripheral sensitization (indexed by light touch Von-Frey in the knee), increased central sensitization (indexed by dysfunctional CPM and hand hyperalgesia) and increased anxiety together lead to higher pain. Additionally, we found that the combination of higher peripheral structural damage (indexed by KL), increased peripheral sensitization (indexed by light touch Von-Frey in the knee), increased central sensitization (indexed by lower PPT in the knee) and worse psychosocial factors (indexed by increased depressive symptoms and sleepiness) also drove to a worse HRQol in such elderly KOA patients. Moreover, a third model demonstrated that worse radiological findings, associated with increased depressive symptoms and higher pain perception lead to worse physical function. To our knowledge, this is the first study to combine clinical, radiological, and neuropsychological variables associated with assessments for peripheral and central sensitization, specifically in elderly patients.
4.1. Risk factors of pain (model 1)
Model 1 suggested that CPM responses affect the reporting of pain perception, notably in those individuals with worse endogenous pain modulation determined by negative scores in CPM-PPT in the hand. The piecewise regression analysis confirmed our results since it showed a significantly linear association only between the negative values of hand CPM-PPT and BPI pain score. This association was not found for CPM-PPT performed in the knee that can be explained by the presence of peripheral sensitization in the knee, highlighting the importance of assessing CPM also in control sites outside the affected joint. It has been shown that impaired central pain modulation could be detected in distant areas from the painful knee due to spreading sensitization [16]. Moreover, CPM-P was not able to predict pain intensity in those individuals, which may be attributed to the subjectiveness of the NRS used to rate their pain during the CPM test, being such more objective the CPM-PPT data. This association between clinical and experimental pain is not yet completely understood, however, it has been theorized that the degree of central and peripheral sensitization may partially explain the severity of individual pain perception, marked mainly by widespread mechanical hyperalgesia and allodynia [19, 38, 39, 40, 41]. In line with these previous findings, our results also showed that higher punctate pain intensity measured in the hand (hyperalgesia) and greater hypoesthesia to light touch measured in the knee are associated with higher pain reports. The degree of hyperalgesia measured in the knee was not associated with pain intensity reporting, which could be related to the high levels of knee hypoesthesia among the study patients.
Regarding the association between structural damage of knee and pain perception, previous literature resulted in conflicting reports [13, 14]. Our results are consistent with the theory that pain severity in individuals with KOA is not fully explained by the degree of radiological involvement. In the present study, only KL IV contributed to the prediction of pain intensity (according to model 1). This finding suggests, as previously shown in prior studies that this discrepancy between radiological measures and subjective pain reports may be explained by other factors, such as psychological factors and central sensitization mechanisms [42].
In addition, higher anxiety perception was associated with higher pain reports. The role of psychological variables, such as depressive and anxiety symptoms, in explaining KOA pain, has been well demonstrated in several studies [26]. Smith et al showed in a study among women with KOA that anxiety and depressive symptoms significantly predict current and next week's pain ratings; however, anxiety had almost twice the effect of depression [43]. Our results failed to demonstrate a predictive value of depressive symptoms for self-reported pain, which may be attributed to the exclusion criteria of severe depression.
These factors together could explain about a third of pain variability scores, thus providing two important insights: (1) treatments targeting only a single target (such as central sensitization) may have limited effect and (2) there is still 2/3 of variability that could not be explained which may indicate lack of precision in the pain outcome used or other factors not investigated or likely a combination of both.
4.2. Risk factors of health-related quality of life (model 2)
Research has been conducted to investigate factors that may improve HRQol following TKA and suggests that a decrease in pain and improvement in function are predictors of improvement in HRQol [44]. However, other studies indicate that the improvement in HRQol following TKA is not fully explained by condition-specific symptoms, such as pain and function [45]. Yakobov et al highlighted the importance of psychological variables in explaining HRQol reports [46]. The present study aimed to improve knowledge about the role of psychological, radiological, and central/peripheral sensitization mechanisms and not pain and function variables in predicting HRQol in KOA subjects. In agreement with previous research, the results of model 2 suggested that worse depressive symptoms and poor quality of sleep negatively influence the HRQol [47, 48]. Also, showed that greater hypoesthesia to light touch and lower PPT, both measured in the knee, are associated with worse HRQol, suggesting that peripheral and central sensitization may influence not only pain scores but also the HRQol in KOA patients, as previously demonstrated by Hansen et al [49]. Also consistent with previous studies, we found that the radiological severity of KOA is associated with HRQol, being worse in KL 3 and 4 [50]. Therefore, a multidimensional approach when treating KOA patients may be beneficial not only to decrease pain perception, but also to improve quality of life. These variables also account for around a third of HRQol variability scores, which may be explained by the absence of pain and function in the model, as potential predictors.
4.3. Risk factors of physical function (model 3)
In agreement with previous studies, model 3 demonstrated that mood disorders are associated with physical disability in KOA patients [43, 51]. Our results suggested that worse depressive symptoms predict worse physical function. To date, the relationship between KOA radiographic severity and physical function is not fully understood, with controversial previous reports [16, 52]. In the present study, consistent with Alkan et al, there was a correlation between severe radiological findings, corresponding to KL 3 and 4, and worse WOMACFunc [52]. We also found that pain intensity correlated positively with function, as also showed by Tambascia et al [53]. These factors explain 41% of function variability scores, which may be explained by the subjectiveness of the physical function outcome selected and/or other variables not explored. In addition, according to our findings, it is reasonable to suppose that in the early stage of KOA severity (KL ≤ 2), interventions guided to treat pain perception and mood disorders should be more cost-effective to improve physical function, compared to TKA procedure.
4.4. Limitations
The present study has some limitations, and its findings must be interpreted with caution. First of all, it was a baseline analysis of a randomized clinical trial. In addition, the analysis was based on a cross-sectional design. Therefore, causation deduction is not suitable. Furthermore, we did not explore all the factors that may influence the outcomes selected, as physical activity, occupation, body mass index, and pain-related cognition variables, such as pain catastrophizing and self-efficacy, that have been shown to play an essential role in chronic pain conditions [46]. Also, some outcomes were collected using subjective scales, such as visual analog scales that have their limitations well established [54]. Finally, our sample had a great majority of females, which is commonly observed in studies with the elderly population, however it may have influenced “gender” no to be in the final models.
5. Conclusion
In conclusion, our study supports evidence that central and peripheral sensitization associated with psychological factors influence the experience of clinical pain in elderly subjects with chronic pain due to KOA. The current study further supports the need for a multidimensional approach to KOA pain treatment, including treatments targeting multiple neural targets.
Declarations
Author contribution statement
D. Tavares: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
V. Trevisani and F. Santos: Conceived and designed the experiments; Wrote the paper.
F. Fregni: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
J. Okazaki, M. Santana, A. Pinto, K. Tutiya and F. Gazoni: Performed the experiments.
C. Pinto: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo / FAPESP, São Paulo, Brazil (Grant ID: 2017/09740-8).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at ClinicalTrials.gov under the registration number NCT03117231.
Acknowledgements
We would like to thank all the physicians and collaborators of the Geriatrics and Gerontology outpatient service in the Federal University of São Paulo.
References
- 1.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Kraan P.M. Osteoarthritis year 2012 in review: biology. Osteoarthr. Cartil. 2012;20:1447–1450. doi: 10.1016/j.joca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Jinks C., Jordan K., Ong B.N., Croft P. A brief screening tool for knee pain in primary care (KNEST). 2. Results from a survey in the general population aged 50 and over. Rheumatology. 2004;43:55–61. doi: 10.1093/rheumatology/keg438. [DOI] [PubMed] [Google Scholar]
- 4.Blagojevic M., Jinks C., Jeffery A., Jordan K.P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr. Cartil. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Du H., Chen S.-L., Bao C.-D., Wang X.-D., Lu Y., Gu Y.-Y., Xu J.-R., Chai W.-M., Chen J., Nakamura H., Nishioka K. Prevalence and risk factors of knee osteoarthritis in Huang-Pu District, Shanghai, China. Rheumatol. Int. 2005;25:585–590. doi: 10.1007/s00296-004-0492-7. [DOI] [PubMed] [Google Scholar]
- 6.Martel-Pelletier J., Maheu E., Pelletier J.-P., Alekseeva L., Mkinsi O., Branco J., Monod P., Planta F., Reginster J.-Y., Rannou F. A new decision tree for diagnosis of osteoarthritis in primary care: international consensus of experts. Aging Clin. Exp. Res. 2019;31:19–30. doi: 10.1007/s40520-018-1077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira D., Ramos E., Branco J. Osteoarthritis. Acta Med. Port. 2015;28:99–106. doi: 10.20344/amp.5477. [DOI] [PubMed] [Google Scholar]
- 8.Suchting R., Colpo G.D., Rocha N.P., Ahn H. The effect of transcranial direct current stimulation on inflammation in older adults with knee osteoarthritis: a Bayesian residual change analysis. Biol. Res. Nurs. 2019 doi: 10.1177/1099800419869845. 1099800419869845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeoka H., Matsuo A., Hiyamizu M., Morioka S., Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci. Lett. 2012;512:12–16. doi: 10.1016/j.neulet.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz J., Minoshima S., Casey K.L. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 11.Boggio P.S., Zaghi S., Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS) Neuropsychologia. 2009;47:212–217. doi: 10.1016/j.neuropsychologia.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Fingleton C., Smart K., Moloney N., Fullen B.M., Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil. 2015;23:1043–1056. doi: 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 13.Serban O., Porojan M., Deac M., Cozma F., Solomon C., Lenghel M., Micu M., Fodor D. Pain in bilateral knee osteoarthritis - correlations between clinical examination, radiological, and ultrasonographical findings. Med. Ultrason. 2016;18:318–325. doi: 10.11152/mu.2013.2066.183.pin. [DOI] [PubMed] [Google Scholar]
- 14.Odding E., Valkenburg H.A., Algra D., Vandenouweland F.A., Grobbee D.E., Hofman A. Associations of radiological osteoarthritis of the hip and knee with locomotor disability in the Rotterdam Study. Ann. Rheum. Dis. 1998;57:203–208. doi: 10.1136/ard.57.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graven-Nielsen T., Wodehouse T., Langford R.M., Arendt-Nielsen L., Kidd B.L. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–2916. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj P., Bajaj P., Graven-Nielsen T., Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93:107–114. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 17.Lluch E., Torres R., Nijs J., Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur. J. Pain. 2014;18:1367–1375. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- 18.Arendt-Nielsen L., Nie H., Laursen M.B., Laursen B.S., Madeleine P., Simonsen O.H., Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Edwards R.R., Ness T.J., Weigent D.A., Fillingim R.B. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003;106:427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Skou S.T., Graven-Nielsen T., Lengsoe L., Simonsen O., Laursen M.B., Arendt-Nielsen L. Relating clinical measures of pain with experimentally assessed pain mechanisms in patients with knee osteoarthritis. Scand. J. Pain. 2013;4:111–117. doi: 10.1016/j.sjpain.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Foucher K.C., Chmell S.J., Courtney C.A. Duration of symptoms is associated with conditioned pain modulation and somatosensory measures in knee osteoarthritis. J. Orthop. Res. 2019;37:136–142. doi: 10.1002/jor.24159. [DOI] [PubMed] [Google Scholar]
- 22.Scopaz K.A., Piva S.R., Wisniewski S., Fitzgerald G.K. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2009;90:1866–1873. doi: 10.1016/j.apmr.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A., Kudesia P., Shi Q., Gandhi R. Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol. Res. Rev. 2016;8:103–113. doi: 10.2147/OARRR.S93516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen G.R., Streltzer J. The psychology of pain. Emerg. Med. Clin. 2005;23:339–348. doi: 10.1016/j.emc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Somers T.J., Keefe F.J., Godiwala N., Hoyler G.H. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr. Opin. Rheumatol. 2009;21:501–506. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- 26.Helminen E.-E., Sinikallio S.H., Valjakka A.L., Vaisanen-Rouvali R.H., Arokoski J.P. Determinants of pain and functioning in knee osteoarthritis: a one-year prospective study. Clin. Rehabil. 2016;30:890–900. doi: 10.1177/0269215515619660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavares D.R.B., Okazaki J.E.F., Rocha A.P., Santana M.V.D.A., Pinto A.C.P.N., Civile V.T., Santos F.C., Fregni F., Trevisani V.F.M. Effects of transcranial direct current stimulation on knee osteoarthritis pain in elderly subjects with defective endogenous pain-inhibitory systems: protocol for a randomized controlled trial. JMIR Res. Protoc. 2018;7 doi: 10.2196/11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T.D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 29.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapstad H., Hanestad B.R., Langeland N., Rustoen T., Stavem K. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Muscoskel. Disord. 2008;9:55. doi: 10.1186/1471-2474-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hungerer S., Kiechle M., von Ruden C., Militz M., Beitzel K., Morgenstern M. Knee arthrodesis versus above-the-knee amputation after septic failure of revision total knee arthroplasty: comparison of functional outcome and complication rates. BMC Muscoskel. Disord. 2017;18:443. doi: 10.1186/s12891-017-1806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 33.Warmenhoven F., van Rijswijk E., Engels Y., Kan C., Prins J., van Weel C., Vissers K. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients., Support. Care Cancer off. J. Multinatl. Assoc. Support. Care Cancer. 2012;20:319–324. doi: 10.1007/s00520-010-1082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahearn E.P. The use of visual analog scales in mood disorders: a critical review. J. Psychiatr. Res. 1997;31:569–579. doi: 10.1016/s0022-3956(97)00029-0. [DOI] [PubMed] [Google Scholar]
- 35.Keizer D., van Wijhe M., Post W.J., Wierda J.M.K.H. Quantifying allodynia in patients suffering from unilateral neuropathic pain using von frey monofilaments. Clin. J. Pain. 2007;23:85–90. doi: 10.1097/01.ajp.0000210950.01503.72. [DOI] [PubMed] [Google Scholar]
- 36.Walk D., Sehgal N., Moeller-Bertram T., Edwards R.R., Wasan A., Wallace M., Irving G., Argoff C., Backonja M.-M. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin. J. Pain. 2009;25:632–640. doi: 10.1097/AJP.0b013e3181a68c64. [DOI] [PubMed] [Google Scholar]
- 37.Osbourne J.W., Waters E. Four assumptions of multiple regression that researchers should always test. Practical Assess. Res. Eval. 2002;8 http://search.ebscohost.com/login.aspx?direct=true&db=eric&AN=EJ670704&site=ehost-live [Google Scholar]
- 38.Meiselles D., Aviram J., Suzan E., Pud D., Eisenberg E. Does self-perception of sensitivity to pain correlate with actual sensitivity to experimental pain? J. Pain Res. 2017;10:2657–2663. doi: 10.2147/JPR.S149663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bevilaqua-Grossi D., Zanin M., Benedetti C., Florencio L., Oliveira A. Thermal and mechanical pain sensitization in patients with osteoarthritis of the knee. Physiother. Theory Pract. 2019;35:139–147. doi: 10.1080/09593985.2018.1441930. [DOI] [PubMed] [Google Scholar]
- 40.Rakel B., Vance C., Zimmerman M.B., Petsas-Blodgett N., Amendola A., Sluka K.A. Mechanical hyperalgesia and reduced quality of life occur in people with mild knee osteoarthritis pain. Clin. J. Pain. 2015;31:315–322. doi: 10.1097/AJP.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 41.Wylde V., Palmer S., Learmonth I.D., Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology. 2012;51:535–543. doi: 10.1093/rheumatology/ker343. [DOI] [PubMed] [Google Scholar]
- 42.Finan P.H., Buenaver L.F., Bounds S.C., Hussain S., Park R.J., Haque U.J., Campbell C.M., Haythornthwaite J.A., Edwards R.R., Smith M.T. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith B.W., Zautra A.J. The effects of anxiety and depression on weekly pain in women with arthritis. Pain. 2008;138:354–361. doi: 10.1016/j.pain.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Silva R.R., Santos A.A.M., de Sampaio Carvalho Junior J., Matos M.A. Quality of life after total knee arthroplasty: systematic review. Rev. Bras. Ortop. 2014;49:520–527. doi: 10.1016/j.rboe.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franklin P.D., Li W., Ayers D.C. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. Clin. Orthop. Relat. Res. 2008;466:2597–2604. doi: 10.1007/s11999-008-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yakobov E., Stanish W., Tanzer M., Dunbar M., Richardson G., Sullivan M.J.L. The prognostic value of pain catastrophizing in health-related quality of life judgments after Total knee arthroplasty. Health Qual. Life Outcome. 2018;16:126. doi: 10.1186/s12955-018-0955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldwin C.M., Griffith K.A., Nieto F.J., O’Connor G.T., Walsleben J.A., Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 48.Rapaport M.H., Clary C., Fayyad R., Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am. J. Psychiatr. 2005;162:1171–1178. doi: 10.1176/appi.ajp.162.6.1171. [DOI] [PubMed] [Google Scholar]
- 49.Hansen S., Vaegter H.B., Petersen K.K. Pre-treatment exercise-induced hypoalgesia is associated with change in pain and function after standardized exercise therapy in painful knee osteoarthritis. Clin. J. Pain. 2019 doi: 10.1097/AJP.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 50.Bernad-Pineda M., de Las Heras-Sotos J., Garces-Puentes M.V. [Quality of life in patients with knee and hip osteoarthritis] Rev. Esp. Cir. Ortop. Traumatol. 2014;58:283–289. doi: 10.1016/j.recot.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 51.He Y., Zhang M., Lin E.H.B., Bruffaerts R., Posada-Villa J., Angermeyer M.C., Levinson D., de Girolamo G., Uda H., Mneimneh Z., Benjet C., de Graaf R., Scott K.M., Gureje O., Seedat S., Haro J.M., Bromet E.J., Alonso J., von Korff M., Kessler R. Mental disorders among persons with arthritis: results from the World Mental Health Surveys. Psychol. Med. 2008;38:1639–1650. doi: 10.1017/S0033291707002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alkan B.M., Fidan F., Tosun A., Ardicoglu O. Quality of life and self-reported disability in patients with knee osteoarthritis. Mod. Rheumatol. 2014;24:166–171. doi: 10.3109/14397595.2013.854046. [DOI] [PubMed] [Google Scholar]
- 53.Tambascia R.A., Vasconcelos R.A., Mello W., Teixeira P.P., Grossi D.B. Pre-operative functional parameters of patients undergoing total knee arthroplasty. Physiother. Res. Int. 2016;21:77–83. doi: 10.1002/pri.1622. [DOI] [PubMed] [Google Scholar]
- 54.Torrance G.W., Feeny D., Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med. Decis. Making. 2001;21:329–334. doi: 10.1177/0272989X0102100408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.