Abstract
The variation in chemical composition of essential oils (EOs) as affected by plant phenology and environmental factors is a crucial limitation in standardization of EOs and thus their effective implementation into novel organic farming. The cultivation of medicinal and aromatic plants (MAP) using bio-elicitors has been advocated as genuine tool to sustainably assure higher quantity and quality EOs. Herein, a field trial and laboratory bioassays were undergone to decipher the impact of a local arbuscular mycorrhizal fungi (AMF) inoculation on agro-morphological yield, EO output and differential suppression of common bean (Phaseolus vulgaris L.) root rot incited by Fusarium solani. The field experiment was laid out following a completely randomized block design (CRBD) with two treatments; non-inoculated control and AMF-inoculation in triplicates. The effects of the EOs were tested against mycelial growth, conidia and common bean seeds germination. Promising concentrations were thereafter assessed for their suppressive effect against Fusariun solani in planta. A significantly higher collar diameter, hypocotyl branching and subsequent Mycorrhizal Dependency (MD) in mycorrhizal lemongrass [(+)AMF] compared to non-inoculated counterpart [(-)AMF]. The resulting EOs consistently repressed F. solani Fs4 conidia germination and mycelium deployment in a dose-dependent manner, with minimum inhibitory concentration (MIC) of 500 ppm for both the EOs, and 125 ppm for the reference fungicide (Ridomil Plus 44 WP). The EO from mycorrhizal lemongrass protected the common bean plantlets from infection by F. solani Fs4 both in laboratory and greenhouse conditions, leading to healthier and vigorous plantlets. The best protection rate was once more obtained with EO from AMF-primed lemongrass at the concentration of 1000 ppm (65.0%) while the lowest disease severity was obtained with Ridomil Plus 44 WP (63.13%). Overall, AMF inoculation shifted the lemongrass biochemical processes with subsequent impact on growth, and an enhanced suppression in Fusarium root rot under greenhouse conditions.
Keywords: AM fungi, Lemongrass, Essential oil, Fusarium root rot, Protection, Engineering, Chemistry, Agricultural science, Environmental science, Biological sciences
AM fungi; Lemongrass; Essential oil; Fusarium root rot; Protection; Engineering; Chemistry; Agricultural science; Environmental science; Biological sciences.
1. Introduction
Soil-borne diseases are major crops yield-limiting factors threatening food security worldwide (Erper et al., 2008). Fungal plant pathogens like Pythium spp., Rhizoctonia solani, Thielaviopsis basicola, Verticillium spp., Sclerotinia spp., Phytophthora spp. and Fusarium spp., have been cited as causative agents (Abawi and Pastor-Corrales, 1990). They may each infect independently the host causing characteristic symptoms, or in complexes, resulting in more severe cases (Erper et al., 2008). Among soil borne diseases, Fusarium root rot (FRR), caused by the fungus Fusarium solani f. sp. phaseoli (Fsp) W.C. Snyder & H.N. Hansen has a cosmopolitan distribution. The former prevails wherever common bean (Phaseolus vulgaris L) is grown across the world. In fact, F. solani causes hypocotyl rot, seeds and seedlings decay at pre and post-emergences (damping-off) and root rot in common bean, with substantial yield decline culminating at as high as 100% in susceptible cultivars (Mukankusi et al., 2011; Nzungize et al., 2012).
Soil disinfestation, and the usage of disease-free or fungicide coated planting materials may help in dwarfing the destructive effects of the phytopathogens. Chemically diverse fungicides such as thiram (Thiram 70 S), benomyl (Benlate), Dithane M-45 (Mancozed) and Captafol (Difolatan) have been screened out against FRR under greenhouse and open field with controversial outcomes, given the resurgence of the damages on fibrous roots out of the protection area at certain distance of seed placement (Abawi and Pastor-Corrales, 1990; Eke et al., 2019). In addition to the questionable efficiency of chemical fungicides, the worldwide trend toward environmentally-friendly approaches for plant disease control calls for steady mitigation of agrochemicals since they present extensive setbacks such as the development of resistances and non-target effects (Gianinazi et al., 2010; Eke et al., 2019). Thus, attention has been paid to the usage of plant derived fungicidal products such as plant extracts and essential oils (EO) in agriculture (Tak and Isman 2016). Isolated from medicinal and aromatic plants (MAP) plant-based drugs have occupied key position in traditional health care systems since ancient times. Moreover, the suppressive action of various EOs toward many agriculturally impactful phytopathogens has been evidenced (Nana et al., 2015a; Amini et al., 2016).
However, estimates reveal that 95% of MAP currently used for diverse purposes are harvested in wild, without any remediation measure (Karki, 2002), thought a sustainable balance between the rate of harvest and any resource capital is required (McChesney et al., 2007). As such, there is actually strong advocacy in cooperating the cultivation of MAP into traditional agricultural system, to tackle the unsustainable harvest. Besides, it's well established that many component of the environment influence significantly plants growth and metabolic processes with subsequent repercussion on their inherent bioactivity (Karagiannidis et al., 2011; Lakušic et al., 2013). Nonetheless, reports pertaining to the stabilisation and or the optimisation of the performances of cultivated MAP are still fragmentary though the quality assurance across the whole production system would be necessary to sustainably assure the quality of the end products (Amujoyegbe et al., 2012).
Elsewhere, the symbiotic association between the roots of more than 90% of higher plants and rhizospheric arbuscular mycorrhizal fungi (AMF) termed “Mycorrhizae” has been credited with improved fitness and altered host plants metabolic processes (Smith and Read, 2008; Yang et al., 2015; Eke et al., 2015). A straight relationship has indeed been drawn between Mycorrhizae frequency and the quantitative and qualitative changes in the EOs production of numerous aromatic plants including but not limited to Ocimum basilicum (Copetta et al., 2006), Mentha arvensis (Freitas et al., 2004), Mentha piperita (Mucciarelli et al., 2003) and Chrysopogon zizanioides (Adams et al., 2003). Recently, a preliminary field experiment conducted in the framework of this study, aiming to screen out the effects of harvest periods and a local AMF inoculum on C. citratus EO's yield and quality as well as biomass production unveiled an accumulated biomass and a deep shift in the chemical composition (Fokom et al., 2019). We thus anticipated that EO from mycorrhizal lemongrass plants could bear improved antifungal potential towards F. solani, a recently described virulent bean root rot causing fungus in Cameroon.
2. Materials and methods
2.1. Description of the experimental farm
The experiment was conducted at the experimental farm of the Faculty of Sciences of the University of Yaoundé I, Cameroon between longitude 11°31′00″ E and latitude 3°52′00″ N. The site is located between 500 to 900 m height from the sea level within the 5th agro-ecological zone of Cameroon, characterized by a bimodal rainfall (1500 mm) pattern. The cropping was a raining season maize (Zea mays L.) and cassava (Minihot esculenta) rotation system. The on-site soil is a Sandy-clay-loam complex. The other physiochemical characteristics included; Organic matter (3.62%), C/N ratio (20.5), cation exchange capacity (29 meq/100 g) and pH of 5.2 (Fokom et al., 2019). Maize straw and weeds were removed and the field was plowed using the conventional manual hoe-ploughing tillage system to approximately 15–20 cm depth. Two plots with 0.5 m interval for non-inoculated control and AMF-inoculation were prepared with three replications each.
2.2. Symbiotic organism, sowing and growth conditions
An AMF inoculum made up of a mixture of propagule units of Glomus aggregatum, Gigaspora margarita and Glomus intraradices (1/1/1), provided by the Laboratory of Soil Microbiology of the Biotechnology Centre, University of Yaoundé I was utilized in this experiment. Prior to sowing, 200 g of AMF inoculum containing approxymately 5000 spores and mycelium fragments were placed as seed bed in pokets made at 0.5 m interval (Nwaga et al., 2010; Tchameni et al., 2012). Afterward, lemongrass seeds, obtained from the seed bank of the Department of Plant Physiology of the University of Yaoundé I were washed under running tap water and surface disinfected with 2% Chlorox (NaOCl) for 5 min and blotted dried. One seed was then placed in each poket and covered with a loose layer of soil. The control plots without mycorrhizal inoculum [(-)AMF] were instead provided with 200 g of sterilize inoculum [(121 °C; 1h), two runs]. The plots (experimental blocs) were randomly organised with AMF treatment [(+) AMF] and control without AMF [(-) AMF].
2.3. Assessment of total chlorophyll content
Leaves chlorophyll content is an indicator of plants fitness. The impact of AMF on plant vigour could therefore be estimated through the evaluation of leaves photosynthetic pigments content. Briefly, 100 mg leaves of 14 months old plant were excised (fourth abaxial leaves), and extracted upon grinding in a mortar and pestle with 10 ml of ice cold acetone (80%). The mixture was centrifuged at 13 000g (Eppendorf, 5810R) for 2 min and the supernatant collected. The pellets were re-extracted thrice following the same procedure. The supernatants were pooled and the absorbance readout was done at 663 and 645 nm and the total chlorophyll content was calculated using the Eq. (1) as follows (Lichtenthaler, 1987)
| Total Chlorophyll = (20.2 × A 645–8.02 × A 663) × 100 /mg. | (1) |
Where: 20.2 and 8.02 = Arnon's constances; A645, A663 = Absorbance recorded at 645 and 663 nm respectively.
2.4. Assessment of root colonization by AMF
In order to ascertain mycorrhizae formation upon inoculation, fine lemongrass roots from each treatment were carefully collected and washed with tap water to remove farm adhering soil. Further, the roots were acidified (HCl, 5%) and bleached using 10% potassium hydroxide (KOH) and stained (63 ml glycerin, 875 ml lactic acid, 0.1 g acid fuchsine and 63 ml water) for 14 h on laboratory bench. The excess stain was removed with 5% lactic acid. Characteristic mycorrhizal anatomic structures were visualized under a Nikon YS100 microscope (100–400 magnification) and the AMF root colonization percentages was calculated as per the gridline intersect method from Giovannetti and Mosse (1980). Positive counts consisted of arbuscules, vesicles and/or mycelia within the roots.
2.5. Essential oil extraction
Fourteen months after sowing, the aerial parts of lemongrass plants from each treatment and replicates were harvested and pooled together to make a composite sample. The samples were immediately transferred to the Laboratory for Phtytobiochemisty and Medicinal Plants Studies and subjected to extraction by hydro-distillation using a Clevenger-type apparatus. The freshly harvested samples were weighed and placed in a heat-proof container. Tap water, representing two to three folds the total weight of the biological samples was then added and the overall was heated up and maintained to boiling point for around 6 h. The essences were collected by decantation and water traces were removed through an anhydrous sodium sulphate and stored in dark glass bottle at 4 °C until usage.
2.6. Pathogenic fungal pathogen and inoculum preparation
The root rot causing fungal pathogen, Fusarium solani Fs4 (Accession: JN232142.1) was previously isolated from common bean roots exhibiting typical symptoms of root rots as described by Abawi and Pastor Corrales (1990). Isolation material was collected in a commercial farm devastated by the disease, and the fungus was isolated (Al-Askar and Rashad, 2010). Information's pertaining to its identity and virulence toward common bean seedlings are extensively documented (Nana et al., 2015b; Toghueo et al., 2016; Eke et al., 2016; Eke et al., 2016, 2016, 2019).
Prior to the experiments, the pathogen's inoculum, made up of macroconidia, microconidia and chlamidospores was prepared by scraping off the propagules on 10 days old PDA cultures using glass wire and sterile distilled water. The obtained slurry, was filtered and adjusted to 2×105 cell.ml−1 using Mallassez haemocytometer and saline solution (NaCl, 0.09%).
2.7. In vitro antifungal assays
2.7.1. Mycelial growth inhibition assay
The EOs from either mycorrhizal and non- mycorrhizal lemongrass were assessed for their inhibitory action against Fusarium solani Fs4 mycelial development using the food poisoning method described by Grover and Moore (1962). Briefly, EOs and reference fungicide; Ridomil plus 44 WP were foremost dissolved in Tween 80 emulsifier (0.5% v/v) and added aseptically to sterile (121 °C, 15 min) Potato Dextrose Broth (PDB, Himedia, India) medium in 40 ml Eppendorf tubes so as to obtain final concentrations range of 62.5–1000 ppm selected from pre-tests where total inhibition of the pathogen's mycelial growth was obtained at the single dose of 1000 ppm. PDB with tween 80 was considered as negative controls. Thereafter, 50 μL of Fusarium solani Fs4 conidial suspension (2 × 105 conidia.ml−1) were inoculated in each tube. Four replications were prepared for each treatment and the overall experiment was repeated twice. The test and controls sets were incubated at 25 ± 2 °C for 10 days and the mycelial mats were harvested through filtration using pre-weighed filter paper and oven-dried (60 °C) till constant weight. The growth inhibition (GI) was thereafter calculated with reference to the negative control using the formula (2):
| GI (%) = [(Mc - Mt)/Mc] × 100 | (2) |
Where: Mc and Mt are mat mycelia (mg) of Fusarium solani Fs4 grown in inhibitor free PDB and inhibitors (EO and Ridomil Plus 44 WP) amended PDB, respectively.
2.7.2. Assay for inhibition of pathogen's conidial germination
The M38-A2 method (CLSI, 2008) with slight modifications was utilized to test the effects of studied EOs and Ridomil Plus 44 WP on F. solani Fs4 conidial germination. In brief, sterile PDB (Himedia, India) medium (100 μL) were seeded in the wells of a 96-wells microtiter plate. Thereafter, stock solutions (100 μL) of either EOs or Ridomil Plus 44 WP were added in the first line wells so as to obtain the double of the desired inhibitor's concentration. Upon homogenization, a twofold serial dilution was undergone by transferring successively 100 μL of the content of the precedent wells into subsequent ones. Later on, 100 μL F. solani conidia suspension, calibrated at 4 × 105 cells.ml−1 were pipetted in the wells. Wells with no inhibitors served as negative controls. The conidia germination was monitored after 10, 12, 14, 18, 20, 22 and 24 h of incubation by optical density (OD600 nm) readout (Magelan Infinite pro M200). The germination at each EO concentration was assessed as per the following formula (3):
| Germination rate (ΔOD) = ODtx-ODt0 | (3) |
where ODt0: Optical density readout of a given well at time t = 0 (immediately after addition of pathogen's conidia in the mixture) whilst ODtx stands for optical density readout at time t = x (x < 0).
The lowest concentration showing no growth of F. solani upon subculture on fresh PDA medium (without EOs) was defined as the Minimum Inhibitory Concentration (MIC).
2.8. Assessment of common bean seeds germination
2.8.1. Allelopathy/bio-priming effect of EOs towards bean seeds
The procedure described by Toghueuo et al. (2016) was used to evaluate the effects of the tested EOs on common bean seeds germination or germination inhibition (Allelopathy). Bean seeds of the genotype GLP 190c, obtained from the germplasm of the National Institute of Agricultural Research for Development (IRAD) of Foumbot (West-Cameroon) were selected for any visible damage and surface-disinfected with Chlorox (5%, 1 min). The sterilizing solution was then removed by three rounds rinsing with sterile distilled water and blotted dried. The sterilized seeds were thereafter dipped in EOs suspensions for 2 h, and immediately transferred in 9.0cm Petri dishes provided with water-soaked tissue papers. The negative control seeds were soaked in sterile distilled water. Five plates with 10 seeds each were prepared per treatment and the whole experiment was conducted twice. The mean percentage of seeds germination was calculated in function of the total number of seeds per treatment following the formula (4) below.
| [(NGc-NGt)/ NGc]∗100. | (4) |
Where: NGc and NGt are respectively the number of germinated seeds in the negative controls and treated plates.
2.8.2. Effects of EOs on bean seeds germination under F. solani Fs4 assault
To assure whether the EOs can promote bean seed germination while protecting them from F. solani attack, sterilized seeds were soaked in EOs suspensions as described above. The reference fungicide (Ridomil Plus 44 WP), prepared in water at the dose recommended by the manufacturer (3.3 g.l−1) was used to coat the seeds. Both the EOs-treated and fungicide-treated seeds were allow to dry and afterward transferred into 9.0 cm Petri dishes (10 seeds/plate) provided with two layers of tissue paper. Then, 10 ml of F. solani conidia suspension (4 × 105 cells.ml−1) prepared in PDB were seeded in each plate. The control plate was rather provided with equal volume of sterile PDB medium. 5 replications were prepared for each treatments and the overall experiment was performed twice. Germinated bean seeds were counted upon 10 days incubation and the percentage of germination was calculated with reference to the controls (Kumar et al., 2014).
2.9. In planta Fusarium root rot suppression assay
To ascertain the protective effect of the EOs oils under natural conditions, a pot experiment was conducted in the greenhouse of the Biocontrol agent's sub-unit of the Laboratory for Phytobiochemisty and Medicinal Plants Studies of the University of Yaoundé I, Cameroon. The growth substrate (soil) was collected from the A horizon of a common bean farm beneath the University and classified as Rhodic kanduidlut type following the U.S. soil taxonomy. The soil was checked for stones and woods particles from the bush and thereafter air-dried. The dried soil was then sieved with 4-mm mesh sieve and mixed up with river sand (3:1 v/v soil-sand ratio). The thus prepared substrate was autoclaved (121 °C, 1 h) twice, infested with F. solani conidia (3000 conidia/g of soil) and filled in the pots (Abawi and Pastor Corrales, 1990). Sterile seeds (GLP 190C) previously soaked in EOs or Ridomil plus 44 WP as described above were sown in the pots (3 seeds/pot). The pots were arranged in a greenhouse in a randomized block designed with 6 replications of 7 treatments as follow: (1) Control (non-inoculated soil and inhibitor-free seeds), (2) F. solani (infested soil with F. solani Fs4 and inhibitor-free seeds), (3) Fs_(-)AMF500 [(infested soil with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 500 ppm (CMI)], (4) Fs_(-)AMF1000 [(infested with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 1000 ppm (2CMI)], (5) Fs_(+)AMF500 [(infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 500 ppm (CMI)], (6) Fs_(+)AMF1000 [(infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 1000 ppm (2CMI)], (7) Fs_Ridomil plus (infested soil with F. solani Fs4 and seed coated with the reference fungicide; Ridomil plus 44 WP). 28 days after sowing, agro-morphological parameters were recorded, and the plant vigour was calculated (Toghueuo et al., 2016). The FRR incidence and severity were evaluated by visual and microscopic observations of the necrotic lesions on roots and hypocotyl and through the 1 to 9 disease rating scale of the CIAT (Abawi and Pastor Corrales, 1990; Eke et al., 2016). The FRR severity was thereafter expressed in percentage as per the formula (5) of Filion et al. (2003) as.
| FRR severity = ∑ 100∗[(DSi∗ n)/N∗DS] | (5) |
Where: DSi = severity (1–9 scale) of a given plant, n = number of plants similar severity, N = total number of plants of the treatment, and DS = highest severity recorded (on the 1–9 scale).
2.10. Statistical analysis
Numeric data collected from the overall experiments were subjected to the analysis of variance (ANOVA) using Statgraphics (version 5.1) statistical software for windows. Distances between the computerized mean values were measured either by the paired T-test when comparing the responses of the two EOs, or the Duncan's multiple comparison test when the treatments were greater than 2. The differences between means were significant at p < 0.05.
3. Results
3.1. Mycorrhizal inoculation improves lemongrass growth and chlorophyll content
Data reporting the effects of mycorrhizal inoculation on the growth and the chlorophyll synthesis of lemongrass are presented (Table 1). The T-test revealed a significantly higher collar diameter (p = 0.02), branching (p = 0.015) and total chlorophyll synthesis in mycorrhizal lemongrass [(+)AMF] compared to the non-inoculated counterpart [(-)AMF]. Likewise, the Mycorrhizal efficiency (ME), indicating the increase in growth parameters of AMF-inoculated plants over the uninoculated ones showed a substantial accumulation in biomass production upon mycorrhization.
Table 1.
Changes in lemongrass growth, chlorophyll content and root colonization rate.
| Treatment | Parameter |
||||
|---|---|---|---|---|---|
| Collar diameter (cm) | No of Branches | Colonization rate (%) | Total chlorophyll (mg.g−1 Fresh weight) | ME (%)1 | |
| (-) AMF | 0.57 ± 0.05b | 3.75 ± 0.4a | 7.0 ± 4.0b | 13.5 ± 2.6b | 0 |
| (+) AMF | 1.13 ± 0.2a | 2.5 ± 0.5b | 43 ± 6.0a | 15.82 ± 4.8a | 92.9 |
(-) AMF: Essential oil from non-mycorrhizal lemongrass, (+)AMF: Essential oil from mycorrhizal lemongrass
Mycorrhizal Efficiency, standing for the increase rate expressed in percentage following mycorrhizal inoculation. The results are displayed in terms of Mean ± standard deviation (SD). Mean values in each column with different alphabet are significantly different at α = 0.05.
3.2. Inhibitory effects of essential oils and reference fungicide on F. solani Fs4 mycelial growth
The inhibitory profiles of EOs from mycorrhizal and non-mycorrhizal lemongrass as well as Ridomil Plus 44 WP are shown (Table 2). Obviously, and irrespective of EOs, the inhibition percentages increased as the concentration augmented. At the concentration of 500 ppm, for both the EOs, and at 125 ppm for the reference fungicides, no visible growth of the pathogen was recorded, indicating a significant difference between the EOs and the reference fungicide. Taken at concentrations range of 62.5–250 ppm, the mycelial growth inhibition exhibited by the (+)AMF] EO was higher compared to the non-inoculated [(-) AMF] plants though non-significant.
Table 2.
F. solani mycelial growth inhibition by essential oils and Ridomil Plus 44WP at differing concentrations.
| Conc (%) | Growth inhibition (%) |
||
|---|---|---|---|
| (-) AMF | (+) AMF | Ridomil Plus 44 WP | |
| 0 | 0.0 ± 0.0e | 0.0 ± 0.0d | 68.4 ± 3.2c |
| 62.5 | 16.5 ± 2.1d | 23.5 ± 2.1c | 82.7 ± 6.4b |
| 125 | 38 ± 4.2c | 46 ± 12.7b | 100 ± 0.0a |
| 250 | 71 ± 4.2b | 89 ± 15.5a | 100 ± 0.0a |
| 500 | 100 ± 0.0a | 100 ± 0.0a | 100 ± 0.0a |
| 1000 | 100 ± 0.0a | 100 ± 0.0a | 100 ± 0.0a |
(-) AMF: Essential oil from non-mycorrhizal lemongrass, (+)AMF: Essential oil from mycorrhizal lemongrass
The results are displayed in terms of Mean ± standard deviation (SD). Mean values in each column with different alphabet are significantly different at α = 0.05.
3.3. Inhibitory effects of essential oils from mycorrhizal and non- mycorrhizal inoculated lemongrass on F. solani conidia germination
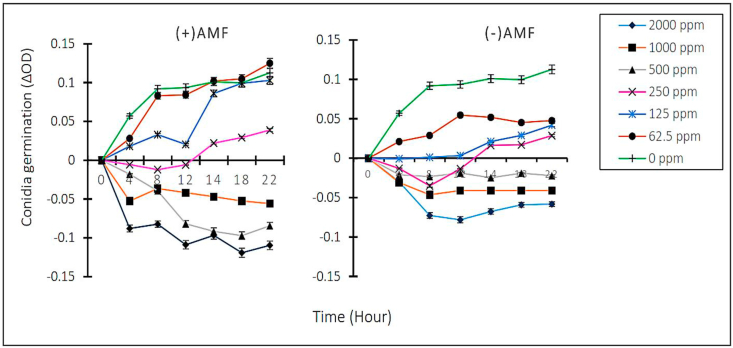
The data reveal that the F. solani conidia germination rate varied as function of concentration within and between EOs (Figure 1). Irrespective of the essential oil involved, a negative correlation was found between the essential oils concentration and conidia germination in the sense that conidia germination speed dropped steadily as the EOs concentration increased. Between EOs, at lowest doses (62.5, 125 and 250 ppm), the overall inhibition of conidia germination of the EO from mycorrhizal lemongrass [(+)AMF] was significantly (p < 0.05) higher compared to the un-inoculated counterpart (-)AMF]. At higher concentrations (500, 1000 and 2000 ppm) reversely, no growth was recorded upon subculture on solid medium with the EO from mycorrhizal lemongrass [(+)AMF] upon 8 h incubation till the end of the experiment. Whilst at 500 ppm, growth re-occurred with the EO from non-mycorrhizal lemongrass (-)AMF indicating a better performance of the EO from mycorrhizal lemongrass (MIC = 500 ppm; 8 h) over the non-mycorrhizal counterpart (MIC = 1000 ppm; 8 h).
Figure 1.
Inhibitory profiles of EOs from non-inoculated and AMF-inoculated lemongrass plantlets towards F. solani spores germination in function of time and concentration. (-) AMF: Essential oil from non-mycorrhizal lemongrass, (+)AMF: Essential oil from mycorrhizal lemongrass. Each curve with a given colour indicates the germination rate of F. solani's conidia at a given concentration.
3.4. Essential oils from mycorrhizal and non-mycorrhizal inoculated lemongrass impact common bean seeds germination in presence and absence of F. solani Fs4
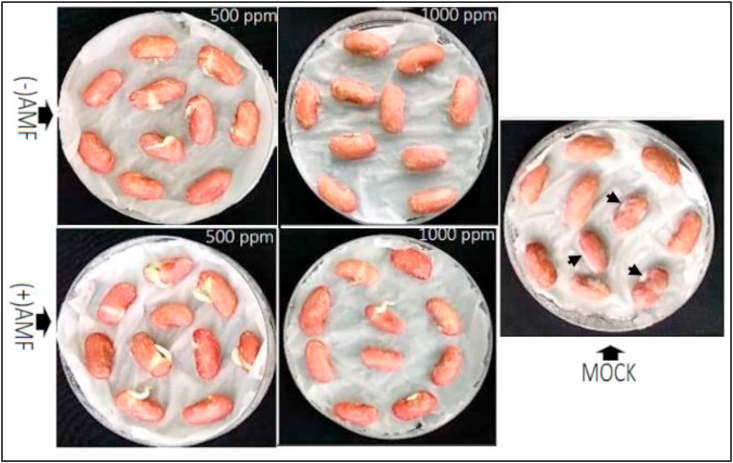
Prior to pot trial, the EOs were screened out at their respective CMI and 2CMI on common bean seeds germination under sterile and F. solani infected environments (Table 3). In the absence of the pathogen, as referred to the mock, both the EOs significantly slowed down the common bean seed germination at the concentration of 1000 ppm, indicating an allelopathic reaction at that concentration. Meanwhile, at 500 ppm the later rather enhanced the germination power by 7% and 19% respectively for EOs from non-inoculated and AMF-primed lemongrass. The ANOVA indicated a significantly higher germination rate at 500 ppm for (+)AMF EO compared to (-)AMF EO. In the presence of F. solani (Figure 2), while no germination was recorded in infected bean seeds, a protective effect was obvious upon coating the seeds with the EOs (p < 0.05). Irrespective of the EO, the concentration 500 ppm was the best in both protecting and enhancing seed germination compared to 1000 ppm. The best germination rate being recorded with the EO from (+)AMF lemongrass (p < 0.05).
Table 3.
Effects of essential oils from mycorrhizal and non- mycorrhizal primed lemongrass, on common bean seeds germination in presence and absence of F. solani Fs4.
| Treatment | Germination percentage |
|||||
|---|---|---|---|---|---|---|
| Without F. solani |
Infected With F. solani |
|||||
| Control | 500 ppm | 1000 ppm | Control | 500 ppm | 1000 ppm | |
| (-) AMF | 81.5 ± 4.9e | 89 ± 2.4e | 64.5 ± 0.7d | 0.0 ± 0.0a | 45 ± 7.07c | 35 ± 7.07b |
| (+) AMF | 81.5 ± 4.9e | 100 ± 0.0f | 69.5 ± 7.7d | 0.0 ± 0.1a | 65.0 ± 2.4d | 34.5 ± 3.5b |
(-) AMF: Essential oil from non-mycorrhizal lemongrass; (+) AMF: Essential oil from mycorrhizal lemongrass.
Mean values in each column with different alphabet are significantly different at α = 0.05.
Figure 2.
Germination of common bean seeds coated with either EO from non-primed [(-) AMF] and AMF-primed [(+) AMF] lemongrass, at different concentrations. Mock, stands for non-treated seeds seeded in F. solani infected Petri plates. Arrow indicate fungal invasion on bean seeds.
3.5. FRR suppressive test
3.5.1. FRR suppression with essential oils from AMF-primed lemongrass or not
The results indicate that, seeds dressing with EOs significantly suppressed both the disease incidence and severity compared to the negative control (inhibitor-free seeds planted in F. solani infested soil (p < 0.05) with protection rate ranging from 0 to 65.0% and 0–63.13% for root rot incidence and severity, respectively. The best protection was recorded with essential oils from AMF-primed lemongrass at the concentration of 1000 ppm (65.0%) as far as the FRR incidence is concerned while the lowest disease severity was obtained with the reference fungicide, Ridomil Plus 44 WP (63.13%) (see Table 4).
Table 4.
Effects of common bean seeds coating with essential oils from mycorrhizal and non- mycorrhizal primed lemongrass and Ridomil on FRR incidence and severity.
| Treatment | DI (%) | Protection rate (%) | [DS (%)∗] | Protection rate (%) |
|---|---|---|---|---|
| Control | 0.0a | 0.0 | 1,0a | 0.0 |
| F. solani (Fs) | 100.0e | 0.0 | 78.2e | 0.0 |
| Fs_(-) AMF500 | 55.0d | 45.0 | 42.2d | 46.03 |
| Fs_(-) AMF1000 | 43.0c | 57.0 | 36.7c | 53.06 |
| Fs_ (+) AMF500 | 46.5c | 54.5 | 40.6d | 48.08 |
| Fs_ (+) AMF1000 | 35.0b | 65.0 | 32.4c | 58.56 |
| Fs_Ridomil Plus | 41.7c | 59.3 | 28.8b | 63.13 |
Control (non-inoculated soil and inhibitor-free seeds), F. solani (infested soil with F. solani Fs4 and inhibitor-free seeds), Fs_(-)AMF500 (infested soil with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 500 ppm), Fs_(-)AMF1000 (infested with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 1000 ppm), Fs_(+)AMF500 (infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 500 ppm), Fs_(+)AMF1000 (infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 1000 ppm), Fs_Ridomil plus (infested soil with F. solani Fs4 and seed coated with the reference fungicide; Ridomil plus 44 WP). DI = Disease Incidence; DS = Disease Severity; Mean values in each column with different alphabet are significantly different at α = 0.05. ∗Disease severity expressed as percentage according to Filion et al. (2003).
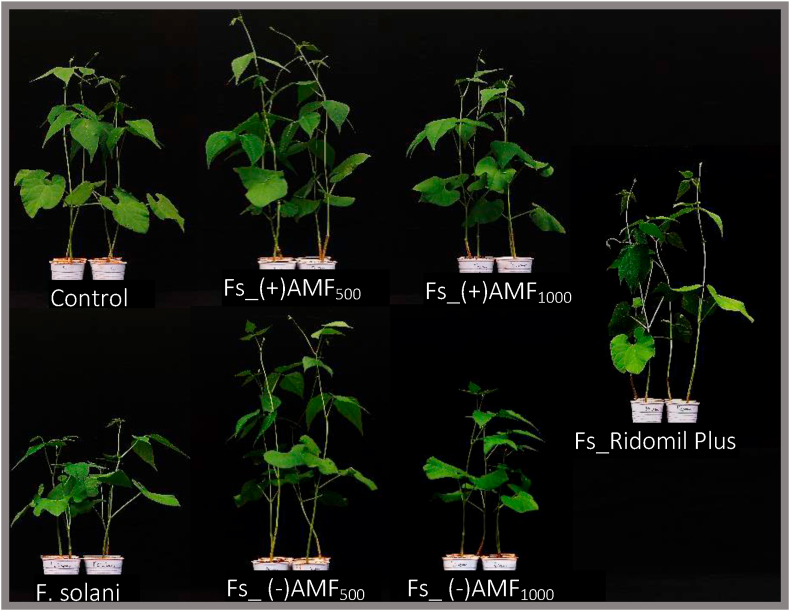
3.5.2. Common bean seeds coating with essential oils from either AMF-primed lemongrass or not impact the growth in the presence of F. solani
The data displayed in Table 5 unveiled the beneficial effects of bean seeds coating with lemongrass EOs and Ridomil compared to F. solani infected and negative controls. While symptoms like leaves chlorosis, stunting and premature defoliation were observed in plantlets emerging from F. solani infested pots only (Figure 3), reiterating the aggressiveness of the fungus. A substantial increase could be observed in plantlets whose seeds were previously dressed with either essential oils or the reference fungicide (p < 0.05) compared to the untreated controls. Globally, the root and shoot length and their respective dry masses were magnified by approximately two-folds upon seeds coating with AMF-primed EO at 1000 ppm compared to other treatments. This clearly indicated that AMF-primed lemongrass oils, protected the common bean plantlets from being infected, leading to more biological matter yield.
Table 5.
Effects of common bean seeds coating with essential oils from mycorrhizal and non- mycorrhizal primed lemongrass and Ridomil on common bean seedlings growth.
| Treatment | Agro-morphological parameter |
||||
|---|---|---|---|---|---|
| Root length (cm) | Shoot length (cm) | Root dry weight (g) | Shoot dry weight (g) | Vigour index | |
| Control | 20.3 ± 2.2b | 48.1 ± 5.4bc | 0.31 ± 0.01b | 3.43 ± 1.21b | 5435 |
| F. solani (Fs) | 12.8 ± 2.4a | 29.4 ± 6.6a | 0.18 ± 0.01a | 1.9 ± 0.6a | 2815 |
| Fs_ (-) AMF500 | 21.3 ± 1.6b | 45.6 ± 3.4b | 0.32 ± 0.1b | 2.8 ± 0.74b | 4654 |
| Fs_ (-) AMF1000 | 25.9 ± 4.2c | 52.8 ± 6.7c | 0.57 ± 0.1c | 4.6 ± 1.30c | 5548 |
| Fs_ (+) AMF500 | 17.5 ± 4.8ab | 42.6 ± 5.5b | 0.33 ± 0.02b | 3.32 ± 1.10b | 4525 |
| Fs_ (+) AMF1000 | 26.6 ± 4.7c | 54.6 ± 2.8d | 0.74 ± 0.2d | 5.01 ± 1.7c | 7814 |
| Fs_Ridomil Plus | 23.9 ± 7.1bc | 50.2 ± 4.7c | 0.49 ± 0.18c | 4.50 ± 1.9c | 5812 |
Control (non-inoculated soil and inhibitor-free seeds), F. solani (infested soil with F. solani Fs4 and inhibitor-free seeds), Fs_(-) AMF500 (infested soil with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 500 ppm), Fs_(-) AMF1000 (infested with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 1000 ppm), Fs_(+) AMF500 (infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 500 ppm), Fs_(+) AMF1000 (infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 1000 ppm), Fs_Ridomil plus (infested soil with F. solani Fs4 and seed coated with the reference fungicide; Ridomil plus 44 WP). Mean values in each column with different alphabet are significantly different at α = 0.05.
Figure 3.
Effects of common bean seeds coating with essential oils from mycorrhizal and non- mycorrhizal primed lemongrass at different concentrations and Ridomil on FRR and plant growth. Control (pots with un-inoculated soil and non-treated seeds); Control (non-inoculated soil and inhibitor-free seeds), F. solani (infested soil with F. solani Fs4 and inhibitor-free seeds), Fs_(-) AMF500 (infested soil with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 500 ppm), Fs_(-) AMF1000 (infested with F. solani Fs4 and seed coated with EOs from non-mycorrhizal lemongrass at the concentration of 1000 ppm), Fs_(+) AMF500 (infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 500 ppm), Fs_(+) AMF1000 (infested soil with F. solani Fs4 and seed coated with EOs from mycorrhizal lemongrass at the concentration of 1000 ppm), Fs_Ridomil plus (infested soil with F. solani Fs4 and seed coated with the reference fungicide; Ridomil plus 44 WP).
4. Discussion
The efforts towards reducing the usage of pesticides in agricultural systems initiated in the second half of the 20th century has led to the investigation of alternative approaches of which botanical pesticides (Nana et al., 2015a). In spite of the broad spectrum activity of EOs against a plethora of agriculturally impactful pests and pathogens, just few manufactured pesticides is commercially availablereason being the instability in EOs composition which runs counter the legislation governing botanical pesticides validation. To address this task earlier findings focus on techniques likegenetic manipulations, the usage of elicitation products and new biotechnological methods to grow MAPs, aiming at both increasing the production and assuring standardized EOs composition (Mahmoud and Croteau, 2002; Iannicelli, 2016). Here, we attempted to assess the effects of mycorrhizal inoculation on the growth, essential oil production and the suppressive effects of the resulting EOs towards a local highly virulent common bean (Phaseolus vulgaris L.) root rot causing pathogen; F. solani Fs4.
Our data unveiled a significantly higher biological yield and mycorrhizal dependency in mycorrhizal lemongrass compared to uninoculated ones. The obtained data are in agreement with our previous data indicating an accumulated biomass in mycorrhizal lemongrass compared to non-inoculated control (Fokom et al., 2019), and further lay emphasis on the impact of AMF in improving the growth of many higher plants on earth (Ngakou et al., 2007; Nana et al., 2015b; Gomoung et al., 2017). Tentative explanation of such trait has pointed out foremost mycorrhiza (up to 43% colonization rate relative to the normal control) with their subsequent established repercussion on plant fitness. In fact, a set of physical and metabolic shifts occur in the host and its surrounding environment including the exploration of more volume of soil by extra-radical mycelia, leading to an enhanced uptake of water and essential micro and macro nutrients (Schnepf and Leitner, 2011; Azcón-Aguilar et al., 2003; Gosling et al., 2006; Eke et al., 2019). The tested EOs displayed significant inhibition in F. solani Fs4 mycelial growth and conidia germination in a dose-dependent manner. The MIC were 500 ppm for the EOs, and 125 ppm for the Ridomil Plus 44 WP as far as the mycelial growth is concerned. The mycelial growth inhibition recorded with EO from AMF-inoculated lemongrass was significantly higher compared non-inoculated counterpart though non-significant. Reversely, the EO from (+)AMF was statistically more performant in inhibiting conidia germination compared to EO from non-mycorrhizal lemongrass. Similar inhibitory effects of EOs has been reported against a vast array of phytopathogens including Alternaria alternata, Ralstonia solanacearum, Penicillium citrinum, Rhyzopius stolonifera, Phytophthora megakarya, Phytophthora nicotians, Phytophthora colocaseae, Phytophthora capsici and Fusarium solani (Bowers and Locke, 2004; Helal et al., 2006; Mahanta et al., 2007; Paret et al., 2010; Bi et al., 2012; Nana et al., 2015a; Sameza, 2016; Amini et al., 2016) threatening world food security. Lemongrass EOs for instance has earlier been credited with hypocholesterolemy, antimutagenic, antiprotozoal, cytoprotection, antioxidation, and antimalarial and anti-inflammatory activities in addition to their antifungal potentials (Vazquez-Briones et al., 2015). The overall activities being more often related to their composition in neral, citral and geranial as major components (Adukwu et al., 2016; Fokom et al., 2019). At the moment, clear mechanism of action undergone by any of these phytochemicals are still to be demonstrated but, it is well-known that EOs oftentimes act by disrupting the cell wall and cytoplasmic membrane of fungi and bacteria, leading to leakage of intracellular compounds and probably cell death by lysis. Otherwise, EOs components may disturb membrane channels and pumps, altering the ionic balance between the cells inner and outer compartments causing cell physical alteration and death without releasing the cellular contents (Tian, 2012). The increasing emphasis on EOs as genuine tool in the fight against disease and pest is not only related to the individual efficacy of their putative major components, but to the multitude of mechanisms driven by their chemically diverse constituents, which makes then important weapon to fight the resistance phenomenon. Therefore, synergistic relationships are likely the mostly indicated mode underlying the efficiency of complex mixtures like EOs (Vidyasagar and Tabassum, 2013). Many scenarios have been postulated accordingly. While Kim and Park (2012) pointed out additivity and/or synergism between components bearing individual antimicrobial potential, optional mode indicates that non-active minors ingredients may act either as adjuvants by facilitating the influx of active principles having their target in the cytoplasm or nucleus or the optimisation of an active ingredient through the formation of more active chemical complex (Shiota et al., 2004; Sibanda and Okoh, 2007). The recorded discrepancy among both the EOs (from AMF-inoculated or not) could be due to the shift in the biochemical changes perpetrated in the composition of the essences of AMF-inoculated plants. Of note, inorganic phosphorus, which increased provision by AMF through mycorrhiza has been proven, are essential reactant in the biosynthesis of terpenoids; the major components of EOs (Ashour et al., 2010). Also it was observed in our previous study that the percentage of myrcene was significantly higher in EO from mycorhizal plant 14 month post planting than control lemongrass plant (Fokom et al., 2019).
Moreover, the EO extracted from mycorrhizal lemongrass protected the common bean plantlets from infection by F. solani Fs4 both in laboratory and under greenhouse conditions, leading to healthier and vigorous plantlets with reference to plants emerging from F. solani infected common bean plantlets alone. The best reduction in root rot incidence protection rate was obtained with EOs from AMF-primed lemongrass at the concentration of 1000 ppm (65.0%) while the lowest disease severity was obtained with the reference fungicide, Ridomil Plus 44 WP (63.13%). Similar reductions have been shown by several EOs towards soil-borne diseases in cucumber, melon, pepper and Cocoa (Nana et al., 2015a; Amini et al., 2016). The recorded data are in accordance with the in vitro assays where a significant lethal effect of the EOs towards the conidia of F. solani was evidenced. It is likely that once in the rhizosphere, the essential oils may have diffused in the soil and killed the infectious propagules providing a sterile environment around the seeds (Abawi and Pastor-Corrales, 1990). As a consequence of the disease reduction, the common bean plants grew faster but at doses 2000 ppm the growth of the plants were significantly reduced. This could be ascribed to the phenomenon of allelopathy which has long been cited as inherent attributes of EOs. Overall, we obtained prominent effects at 500 and 1000 μL.l−1. This promising output end to somehow resolve the issue of standardization of EOs, and provides a clear insight on the cost-effectiveness of the application of essential oils in open field. Indeed, seeds dressing require less amount of EOs and can address the volatility issue often cited as the most limiting factor associated to the use of EOs in crop protection.
5. Conclusion
In this study, we recorded lemongrass biomass accumulation, improved essential oils yield upon mycorrhization under field conditions. Likewise, EO from mycorrhizal lemongrass consistently inhibited F. solani Fs4 development, significantly more than the non-inoculated counterpart. At 1000 ppm, both the essential oils enhanced the common bean seeds germination, and protected plantlets from being infected by F. solani Fs4 both in laboratory and greenhouse conditions, leading to more healthy and vigorous plants. Overall, AMF inoculation had reoriented the metabolome of lemongrass plant. Leading to an enhanced growth and antifungal potential of the resulting EO towards F. solani. But the constance in the chemical composition should be demonstrated overtime in order to standardize the product and move ahead in the search of organic alternative to agrochemicals.
Declarations
Author contribution statement
Pierre Eke, Souleymanou Adamou, Raymond Fokom: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Vanessa Dinango Nya: Performed the experiments.
Tsou Fokou Patrick Valer:Contributed reagents, materials, analysis tools or data; Wrote the paper.
Louise Nana Wakam, Dieudonné Nwaga, Fabrice Fekam Boyom: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Notre Dame Institute for Advanced Study and Pierre Eke was supported by Wallace Laboratories (US).
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abawi G.S., Pastor-Corrales M.A. Centro Internacional de Agricultura Tropical (CIAT) first ed./ Kandrups Bogtrykkeri edition; Cali, Colombia: 1990. Root rots of beans in Latin America and Africa: diagnosis, research methodologies, and management strategies. [Google Scholar]
- Adams R.P., Pandey R.N., Dafforn M.R., James S.A. Vetiver DNA-fingerprinted cultivars: effects of environment on growth, oil yields and composition. J. Essent. Oil Res. 2003;15:363–371. [Google Scholar]
- Adukwu E.C., Bowles M., Edwards-Jones V., Bone H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and and pure. Appl. Microbiol. Biotechnol. 2016;100:9619–9627. doi: 10.1007/s00253-016-7807-y. Constraints. Trends in Plant Science, TRPLSC 1484 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Askar A.A., Rashad Y.M. Arbuscular mycorrhizal fungi: a biocontrol agent against common bean Fusarium root rot disease. Plant Pathol. J. 2010;9:31–38. [Google Scholar]
- Amini J., Farhang V., Javadi T., Nazemi J. Antifungal effect of plant essential oils on controlling Phytophthora species. Plant Pathol. J. 2016;32(1):16–24. doi: 10.5423/PPJ.OA.05.2015.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amujoyegbe B.J., Agbedahunsi J.M., Amujoyegbe O.O. Cultivation of medicinal plants in developing nations: means of conservation and poverty alleviation. Int. J. Med. Aromatic Plants. 2012;2:345–353. [Google Scholar]
- Ashour M., Wink M., Gershenzon J. Biochemistry of terpenoids: monoterpenes, sesquiterpenes and diterpenes. In: Wink M., editor. second ed. Vol. 40. Wiley; New York: 2010. (Annual Plant Reviews: Biochemistry of Plant Secondary Metabolism). [Google Scholar]
- Azcón-Aguilar C., Palenzuela J., Roldan A., Bautista S., Vallejo R., Barea J.M. Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification threatened Mediterranean shrublands. Appl. Soil Ecol. 2003;14:165–175. [Google Scholar]
- Bi Y., Jiang H., Hausbeck M.K., Hao J.J. Inhibitory effects of essential oils for controlling Phytophthora capsici. Plant Dis. 2012;96:797–803. doi: 10.1094/PDIS-11-11-0933. [DOI] [PubMed] [Google Scholar]
- Bowers J.H., Locke J.C. Effect of formulated plant extracts and oils on population density of Phytophthora nicotianae in soil and control of Phytophthora blight in the greenhouse. Plant Dis. 2004;88:11–16. doi: 10.1094/PDIS.2004.88.1.11. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Clinical and Laboratory Standards Institute; suite 1400, Wayne, Pennsylvania 190871898, USA: 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard-second ed. CLSI Document M38-A2. [Google Scholar]
- Copetta A., Lingua G., Berta G. Effects of three AM fungi on growth, distribution of glandular hairs and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza. 2006;16:485–494. doi: 10.1007/s00572-006-0065-6. [DOI] [PubMed] [Google Scholar]
- Eke P., Chatue C.G., Nana W.L., Toghueo K.R.M., Tsouh F.P.V., Jesus-Marie A., Fekam B.F. Mycorrhiza consortia in dual inoculation with Fusarium solani f. sp. Phaseoli control the root rot in common bean (Phaseolus vulgaris L.) Biol. Contr. 2015;103:240–250. [Google Scholar]
- Eke P., Nana W.L., Toghueo K.R.M., Tsouh F.P.V., Jesus-Marie A., Fekam B.F. Integrated assessment of phytostimulation and biocontrol potential of endophytic trichoderma spp. against common bean (Phaseolus vulgaris L.) root rot fungi complex in Centre region, Cameroon. Int. J. Pure Appl. Biosc. 2016;4(4):50–68. [Google Scholar]
- Eke P., Nana W.L., Tsouh F.P.V., Ekounda T.V., Kuleshwar P.S., Kamdem W.T.H., Fekam B.F. Improved nutrient status and Fusarium root rot mitigation with an inoculant of two biocontrol fungi in the common bean (Phaseolus vulgaris L.) Rhizosphere. 2019;12:100172. [Google Scholar]
- Erper I., Karaca H.G., Ozkoc I. Root rot disease incidence and severity on some legume species grown in Samsun and the fungi isolated from roots and soils Arc. Phytopathol. Plant Prot. 2008;41:501–506. [Google Scholar]
- Filion M., St.-Arnaud M., Jabaji-Hare S.H. Quantification of Fusarium solani f. sp. Phaseoli in mycorrhizal bean plants and surrounding mycorrhizosphere soil using realtime polymerase chain reaction and direct isolations on selective media. Phytopathology. 2003;93:229–235. doi: 10.1094/PHYTO.2003.93.2.229. [DOI] [PubMed] [Google Scholar]
- Fokom R., Adamou S., Essono D., Ngwasiri D.P., Eke P., Teugwa M.C., Tchoumbougnang F., Fekam B.F., Amvam Z.P.H., Nwaga D., Sharma A.K. Growth, essential oil content, chemical composition and antioxidant properties of lemongrass as affected by harvest period and arbuscular mycorrhizal fungi in field conditions. Ind. Crop. Prod. 2019;138:111477. [Google Scholar]
- Freitas M.S., Martins M.A., Carvalho A.J., Carneiro R.F. Crescimento e produção de fenóis totais em carqueja [Baccharis trimera (Less.)] napresençae na ausência de adubação mineral. Rev. Bras. Pl. Botuc Med. 2004;6:30–34. [Google Scholar]
- Gianinazzi S., Gollotte A., Binet M.N., van Tuinen D., Redecker D., Wipf D. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20:519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- Giovannetti M., Mosse B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–499. [Google Scholar]
- Gomoung D., Mbailao M., Toukam T.S., Ngakou A. Influence of cross-inoculation on groundnut and Bambara groundnut Rhizobium symbiosis: contribution to plant growth and yield in the field at Sarh (Chad) and Ngaoundere (Cameroon) Am. J. Plant Sci. 2017;8:1953–1966. [Google Scholar]
- Gosling P., Hodge A., Goodlass G., Bending G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006;113:17–35. [Google Scholar]
- Grover R., Moore J. Toximetric studies of fungicides against brown rot organism: S. fructicola et Slaxa. Phytopathology. 1962;52:876–880. [Google Scholar]
- Helal G.A., Sarhan M.M., Abu Shahla A.N.K., Abou Elkhair E.K. Antimicrobial activity of some essential oils against microorganism deteriorating fruit juices. MYCOBIOLOGY. 2006;34:219–229. doi: 10.4489/MYCO.2006.34.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannicelli J. Effect of polyploidization in the production of essential oils in Lippia integrifolia. Ind. Crop. Prod. 2016;81:20–29. [Google Scholar]
- Karagiannidis N., Thomidis T., Lazari D., Panou-Filotheou E., Karagiannidou C. Effect of three Greek arbuscular mycorrhizal fungi in improving the growth, nutrient concentration, and production of essential oils of oregano and mint plants. Sci. Hortic.-Amsterdam. 2011;129:329–334. [Google Scholar]
- Karki M.B. Paper presented at the Regional Workshop at Wise Practices and Experimental Learning in the Conservation and Management of Himalayan Medicinal Plant. December 15–20, 2002. Organic conversion & certification: a strategy for improved value addition and marketing of medicinal plants products in the Himalayas. Kathmandu, Nepal. [Google Scholar]
- Kim E., Park K. Fumigant antifungal activity of myrtaceae essential oils and constituents from Leptospermum petersonii against three Aspergillus species. Mol. 2012;17:10459–10469. doi: 10.3390/molecules170910459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Mohammad S., Mukesh S., Anuradha S., Sonika P., Antima S. Enhancing seed germination and vigor of Chickpea by using potential and effective strains of Trichoderma species. Virol. Mycol. 2014;3:128. [Google Scholar]
- Lakuši´c D., Risti´c M., Slavkovska V., Lakuši´c B. Seasonal variations in the composition of the essential oils of Rosemary (Rosmarinus officinalis, Lamiaceae) Nat. Prod. Commun. 2013;8:131–134. [PubMed] [Google Scholar]
- Lichtenthaler H.K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Mahanta J.J., Chutia M., Bordoloi M., Pathak M.G., Adhikary R.K., Sarma T.C. Cymbopogon citratus L. essential oil as a potential antifungal agent against key weed moulds of Pleurotus spp. spawns. Flavour Fragrance J. 2007;22:525530. [Google Scholar]
- Mahmoud S.S., Croteau R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002;7:366–373. doi: 10.1016/s1360-1385(02)02303-8. [DOI] [PubMed] [Google Scholar]
- McChesney J.D., Venkataraman S.K., Henri J.T. Plant natural products: back to the future or into extinction? Phytochemistry. 2007;68:2015–2022. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Mucciarelli M., Scannerini S., Bertea C., Maffei M. In vitro and in vivo peppermint (Mentha piperita) growth promotion by nonmycorrhizal fungal colonization. New Phytol. 2003;158:591–597. doi: 10.1046/j.1469-8137.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- Mukankusi C.M., Melis R.J., Derera J., Buruchara R.A. A screening technique for resistance to Fusarium root rot of common bean. Afr. J. Plant Sci. 2011;5:15161. [Google Scholar]
- Nana W.L., Eke P., Fokom R., Bakanrga V.I., Begoude B.D., Tchana T., Tchameni N.S., Kuate J., Chantal M., Fekam B.F. Antimicrobial activity of Syzygium aromaticum and Zanthoxylum xanthoxyloides essential oils against Phytophthora megakarya. J. Phytopathol. 2015;163:632–641. [Google Scholar]
- Nana W.L., Eke P., Atogho T.B., Toghueo K.R.M., Chatue C.G., Pokaa N.A., Ekounda T.V., Etoa F.X., Fekam B.F. In-vitro fungitoxic effect of cold and hot water extracts of three Ocimum species on fungi causing common bean (Phaseolus vulgaris L.) root rot in Cameroon. Int. J. Curr. Microbiol. Appl. Sci. 2015;4(5):596–606. [Google Scholar]
- Ngakou A., Nwaga D., Ntonifor N.N., Tamo M., Nebane C.L.N., Parh I.A. Contribution of arbuscular mycorrhizal Fungi (AMF), rhizobia and metarhizium anisopliae to cowpea production in Cameroon. Int. J. Agric. Res. 2007;2:754–764. [Google Scholar]
- Nwaga D., Jansa J., Abossolo A. The potential of soil beneficial microorganisms for slash-and-burn agriculture in the humid forest zone of Sub Saharan Africa. In: Dion P., editor. Soil Biology and Agriculture in the Tropics. Springer-Verlag; Berlin, Heidelberg: 2010. pp. 81–107. [Google Scholar]
- Nzungize J.R., Lyumugabe F., Busogoro J.P., Baudoin J.P. Pythium root rot of common bean: biology and control methods. A review. Biotechnol. Agron. Soc. Environ. 2012;16:40–413. [Google Scholar]
- Paret M.L., Cabos R., Kratky B.A., Alvarez A.M. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010;94:521–527. doi: 10.1094/PDIS-94-5-0521. [DOI] [PubMed] [Google Scholar]
- Sameza M.L. Evaluation of clove essential oil as a mycobiocide against rhizopus stolonifer and fusarium solani, tuber rot causing fungi in yam (Dioscorea rotundata Poir) J. Phytopathol. 2016 [Google Scholar]
- Schnepf A., Leitner D. Tagung Der Österreichischen Gesellschaft Für Wurzelforschung; 2011. Modelling the Effect of Arbuscular Mycorrhizal Fungi on Plant Phosphate Uptake; pp. 61–64. [Google Scholar]
- Shiota S., Shimizu M., Sugiyama J., Morita Y., Mizushima T., Tsuchiya T. Mechanisms of action of corilagin and tellimagrandin I that remarkably potentiate the activity of ß-lactams against methicillin resistant Staphylococcus aureus. Microbiol. Immunol. 2004;48:67–73. doi: 10.1111/j.1348-0421.2004.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Sibanda T., Okoh A. The challenges of overcoming antibiotic resistance: plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr. J. Biotechnol. 2007;6:2886–2896. [Google Scholar]
- Smith S.E., Read J.D. 3 edition. Acad. Press; London, UK: 2008. Mycorrhizal Symbiosis. [Google Scholar]
- Tak J.H., Isman M.B. Metabolism of citral, the major constituent of lemongrass oil, in the cabbage looper, Tricohoplusia ni, and effects of enzyme inhibitors on toxicity and metabolism. Pestic. Biochem. Physiol. 2016;133:20–25. doi: 10.1016/j.pestbp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Tchameni N.S., Nwaga D., Nana W.L., Ngonkeu M.E.L., Fokom R., Etoa F.X. Growth enhancement, amino acid synthesis and reduction in susceptibility towards Phytophthora megakarya by Arbuscular Mycorrhizal Fungi inoculation in cocoa plants. J. Phytopathol. 2012 [Google Scholar]
- Tian J. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PloS One. 2012;7 doi: 10.1371/journal.pone.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghueo R.M.K., Eke P., González Í.Z., Aldana B.R., Nana W.L., Boyom F.F. Biocontrol and growth enhancement potential of two endophytic Trichoderma spp. from Terminalia catappa against the causative agent of Common Bean Root Rot (Fusarium solani) Biol. Contr. 2016;16:1–44. [Google Scholar]
- Vazquez-Briones M.C., Hernandez L.R., Guerrero-Beltran J.A. Physicochemical and antioxidant properties of Cymbopogon citratus essential oil. J. Food Res. 2015;4:36–45. [Google Scholar]
- Vidyasagar G.M., Tabassum N. Antifungal investigations on plant essential oils. a review. Int. J. Pharm. Pharmaceut. Sci. 2013;5(2):19–28. [Google Scholar]
- Yang Y., Han X., Liang Y., Ghosh A., Chen J., Tang M. The combined effects of Arbuscular Mycorrhizal Fungi (AMF) and Lead (Pb) Stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0145726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.