Abstract
Uterine niche is one of the emerging complications of caesarean section. With rising caesarean rates, the caesarean-related iatrogenic complications are also on the rise. These include placenta accreta, scar ectopic pregnancy and uterine niche which is a newer entity being described in the recent literature. Uterine niche, also described as uterine isthmocele, caesarean scar defect and diverticulum, is an iatrogenic defect in the myometrium at the site of previous caesarean scar due to defective tissue healing. Patients may have varied symptoms including abnormal uterine bleeding, post-menstrual spotting and infertility, though many women may be asymptomatic and diagnosed incidentally. Diagnosis is made radiologically by transvaginal sonography, saline instillation sonohysterography or magnetic resonance imaging. Occurrence of niche may be prevented by using correct surgical technique during caesarean. Patients may be managed medically; however, subfertility and persistent symptoms may require surgical correction either by hysteroscopic resection or transabdominal or transvaginal repair. This mini-review comprehensively covers the potential risk factors, clinical presentation, diagnosis and management of this increasingly encountered condition due to rising caesarean rates.
Keywords: Uterine niche, Isthmocele, Caesarean scar defect
Introduction
Uterine niche is an iatrogenic pouch-like defect at the site of previous caesarean scar due to defective tissue healing. Other terms used are uterine isthmocele, caesarean scar defect, uterine dehiscence and diverticulum. The niche is defined radiologically as a triangular, hypoechoic or anechoic area at scar site [1, 2]. It has also been described as indentations at least 2 mm deep in the myometrium [1, 3]. There is recent surge in the literature including reviews addressing various aspects of niche [4, 5].
Prevalence
Uterine niche occurs in up to 70% women with previous caesarean of whom 30% are symptomatic [2, 3]. Reported prevalence varies: 24–70% with transvaginal sonography (TVS) and 56–84% with gel/saline instillation sonohysterography (SHG) [1, 3, 6]. This may be an underestimation because many women are asymptomatic and also because clinicians may not recognize niche as a cause of symptoms due to unawareness [7]. Prevalence of 45.6% was reported in a prospective observational study (n = 371) where sonohysterography was done six months post-caesarean [8]. Prevalence increases with increasing number of previous caesareans.
Potential Risk Factors
Niche forms due to poor healing of caesarean scar. Risk factors may be surgery related or patient related [9, 10].
Factors Affecting Lower Uterine Segment
Level Of Uterine Incision
Lower uterine incision towards the cervix results in poor healing, as mucus secreted by cervical glands interferes with myometrial approximation. Mucus gradually increases the niche size also [6, 10].
Caesarean done in advanced labour after cervical effacement and also creation of uterovesical fold of peritoneum influence the level of uterine incision.
Uterine Closure Techniques
Single-layer, decidua sparing closure technique predisposes to incomplete closure, compared to single full-thickness closure. Almost 95% patients with niches had single-layer closure without closing peritoneum. A strong myometrial scar with proper anatomical approximation without tissue strangulation minimizes risk of niches [1, 11].
If muscular edges are thick, they are best approximated by including deeper part in the first layer and the remaining superficial cut edges in the second layer.
Non-perpendicular sutures leading to an irregular myometrium closure, locking sutures or very tight second layer leading to ischemic necrosis result in poorly healed scar predisposing to niche formation.
Thus, double-layer uterine closure using non-locking sutures is the optimal closure technique that results in thicker residual myometrium and hence potentially lower risk of niches.
Suboptimal surgical techniques: Inadequate haemostasis, tissue ischemia, devascularization and excessive tissue manipulation contribute to poor scar healing and adhesions, consequently forming niche.
Adhesions
Adhesion formation with abdominal wall pulls the uterine scar towards abdominal wall, exerting counteracting force opposite to the direction of retracting uterine scar tissue and causing impaired wound healing [10].
Retroflexed Uterus
Patient Factors
Genetic predisposition contributes to impaired healing, poor haemostasis, inflammation, or adhesion formation, post-operative infection [10].
Gestational diabetes (odds ratio, 1.73), previous caesarean (OR, 3.14) and advanced body mass index (OR, 1.06) are independent risk factors. Risk increases by 6% for every additional unit increase in body mass index. Longer active labour prior to emergency caesarean also increases risk (OR, 1.06). However, there is no difference between elective and emergency caesarean [8].
Clinical Presentation
Though most women may remain asymptomatic, post-caesarean niche has been linked to following symptoms:
Post-menstrual Spotting
It is defined as ≥ 2 days of intermenstrual spotting, or ≥ 2 days of brownish discharge after the end of menstruation if bleeding duration exceeds 7 days (discharge is considered normal if bleeding duration is < 7 days) [11, 12]. This is the most predominant symptom seen in 30–55% women at 6–12 months post-caesarean due to collected menstrual blood. The anterior edge of niche obstructs flow of menstrual blood, besides, poor contractility of surrounding fibrosed muscle retains it which is then discharged gradually [1, 3, 9, 10, 12]. When observed prospectively after 1 year of caesarean, post-menstrual spotting was found in 20% women with isthmocele compared to 8.3% women without isthmocele, with 3.34 OR for large defects [13].
Prolonged Bleeding
Impaired menstrual drainage results in prolonged flow. Since not yet specified, it may be described as AUB-N as per FIGO-PALMCOEIN nomenclature of abnormal uterine bleeding (AUB).
Intermittent Spotting
In situ blood formation in the niche, evidenced by free erythrocytes in scar, leads to intermenstrual spotting.
Pain
Women with niche may present with dysmenorrhea (40–50%), chronic pelvic pain (35%), dyspareunia (18%) or suprapubic pain. Pain could be due to abnormal myocontraction to empty niche contents [6, 12].
Midcycle Intrauterine Fluid Accumulation
It may be due to excess mucus formation by retained blood in approximately 45% women.
Caesarean Scar Ectopic Pregnancy
Pregnancy may implant in the niche with risk of rupture.
Secondary Infertility
Probable mechanisms might be chronic inflammation by residual blood or peri-ovulatory fluid accumulation interfering with sperm penetration, fertilization and implantation. A large niche may interfere with conception similar to hydrosalpinx [10, 12].
Problems in IVF
Difficult embryo transfer is encountered in 20% women with niche undergoing IVF, due to a distorted anatomy, specially in a retroflexed uterus. Also, chances of unsuccessful IVF are higher in presence of uterine niche [12].
Bladder Dysfunction
Local accumulation of fluid and scarring were postulated to cause dysfunction due to proximity of niche to the bladder; however, prospective studies did not support this.
Obstetric Complications in Future Pregnancy
There is increased risk of scar ectopic, placenta accreta, scar dehiscence and uterine rupture.
Scar Abscess
Though rare, it has been reported even up to 6 years after caesarean, due to residual blood and mucus that gets infected.
Diagnosis
Niche can be visualized in non-pregnant state using TVS (Fig. 1), SHG, 3-D ultrasound, magnetic resonance imaging or hysteroscopy. Hysterosalpingography may also diagnose a niche. An anechoic space at least 1 mm deep (vertical distance between base and apex), with or without fluid, and at least 2 mm deep in the myometrium at caesarean scar site clinches the diagnosis. There is no consensus yet for diagnostic criteria; however, following features are noted [1, 9, 12, 14, 15]:
Fig. 1.
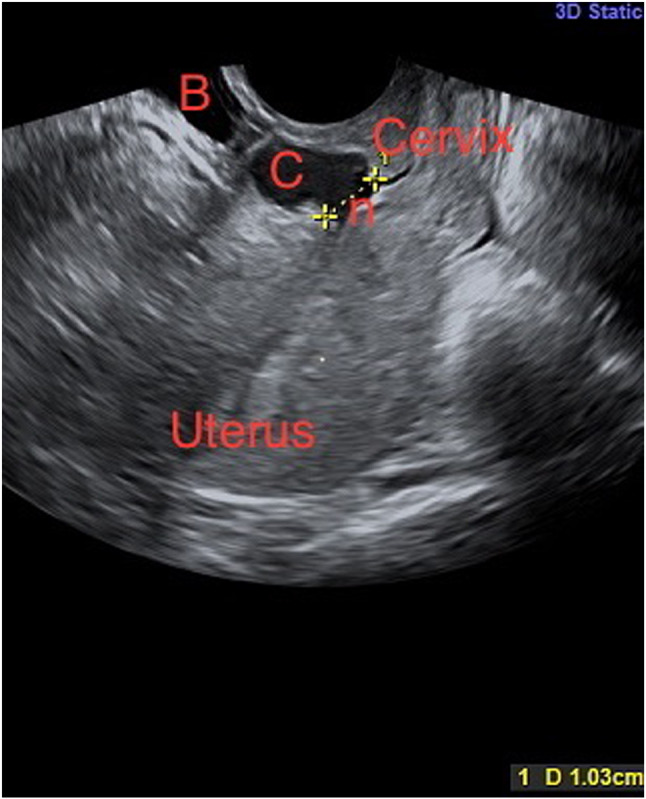
2-D ultrasound showing uterus, cervix, niche (n) measuring 1 cm, collection in the niche (C) and bladder (B) (courtesy Prof. Vatsla Dadhwal)
Niche Size And Residual Myometrium
Residual myometrial thickness (RMT) is the vertical distance between uterine serosa and apex of defect [2]. Large niches are defined when RMT is < 50% of adjacent myometrium or ≤ 2.2 mm on TVS or ≤ 2.5 mm with SHG. Marotta et al. has given cut-off of 3 mm, and ≥ 3 mm RMT is small defect [16]. Absent residual myometrium is termed a total defect.
Niche Shape
Most defects are triangular or semicircular, though round, oval, droplet shape and inclusion cysts are also described. A niche can also be seen as an inward protrusion, i.e. internal scar surface bulging toward uterine cavity, outward protrusion, i.e. external scar surface bulging toward bladder or peritoneal cavity or inward retraction, i.e. external scar surface dimpled toward the myometrium.
Other Niche Features
Other niche features such as concavity, abnormal vascularity, visible serosa, cyst- or polyp-like structure should also be mentioned.
SHG is more accurate for diagnosing niches. Higher prevalence (45% vs. 22%), identification of larger niches and thinner residual myometrium are detected by SHG compared to TVS. Sensitivity and specificity of TVS when compared to SHG are 49% and 100%, respectively. Niches missed with TVS are usually small though they can be clinically relevant. Hence, SHG is the investigation of choice. SHG at 6–12 weeks post-partum when scar is incompletely healed facilitates recognition of scar and small niches, also aided by the thin endometrium during breastfeeding.
Management of Uterine Niche
Indications of treatment: Treatment is indicated only in symptomatic women presenting with secondary infertility, previous scar ectopic, recurrent miscarriage, AUB and bothersome post-menstrual spotting. However, efficacy of treatment is yet to be ascertained. Routine repair of incidentally diagnosed niche with no plans for future childbearing is not recommended.
Treatment options for a uterine niche are as follows:
Medical Treatment
Hormonal therapy symptomatically relieves AUB. Oral contraceptives are suitable if pregnancy is not desired. LNG-IUS was not found to decrease menstrual length [16].
Uterine Sparing Surgical Treatment
Conservative surgical interventions should be considered after eliminating other causes of presenting symptoms. The options include either resection by hysteroscopic route or excision plus repair by transabdominal (laparotomy, laparoscopic, robotic) or vaginal route [11, 17–22]
Hysteroscopic Niche Resection or Isthmoplasty
Technique involves resection of only distal rim, or both distal and proximal edges with resectoscope using bipolar or unipolar current, and coagulation of fragile vessels at the base or even entire niche with ball electrode. Resection facilitates drainage of menstrual blood, though it inevitably increases niche size. Fulgurating base prevents in situ fluid/blood collection. At the end of procedure, flow and pressure of distending medium can be reduced to ensure adequate haemostasis [19, 20]. Complications of hysteroscopic niche resection include uterine perforation, bladder injury especially if overlying RMT < 3 mm, cervical incompetence with proximal rim resection and uterine rupture in subsequent pregnancies [11, 19]. Bladder safety can be ensured by intraoperative ultrasound guidance and filling bladder with methylene blue as shown in HysNiche trial [11].
Niche Repair
Repair is the preferred method when RMT is < 3 mm and essentially involves identification of defect, excision of fibrotic tissue from the edges and re-approximation in 2 layers, either by transabdominal (laparotomy, laparoscopic, robotic) route or by vaginal route. Simultaneous hysteroscopy, Hegar’s dilator, intracervical foley’s catheter, transvaginal or transrectal ultrasound can aid identification. Figure 2 shows the intraoperative findings of transabdominal repair of post-caesarean niche.
Fig. 2.
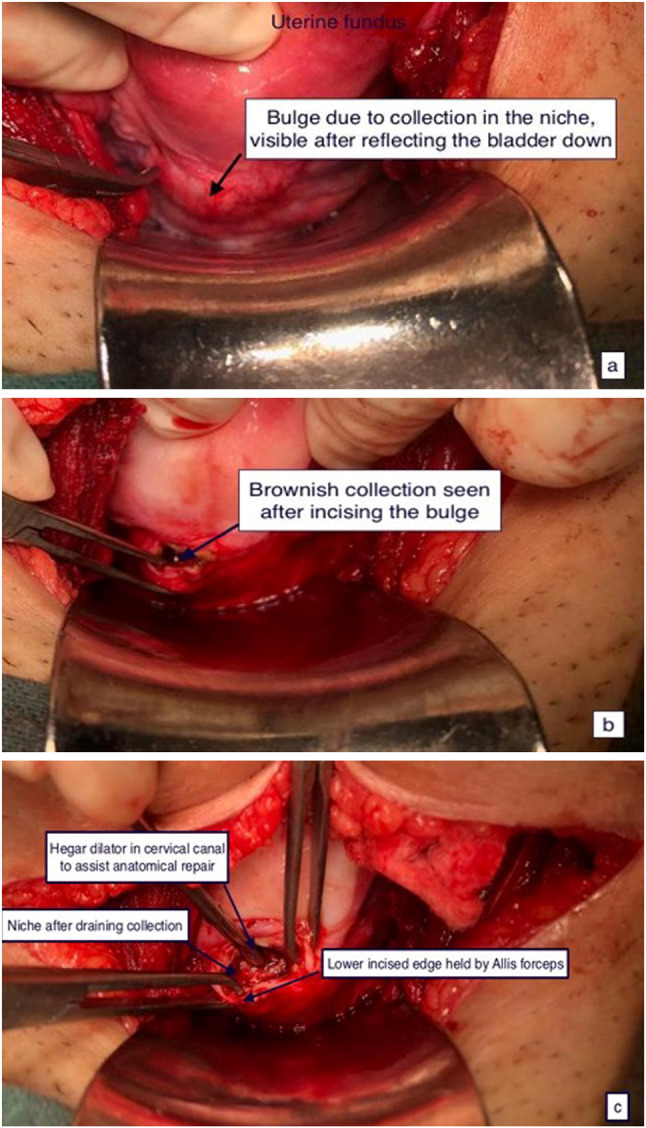
Intraoperative findings of transabdominal repair a bulge prior to incision, b after incision over niche, c after excision of fibrotic tissue from the niche edges (courtesy Prof. Vatsla Dadhwal)
Vervoort’s technique involves laparoscopic repair combined with hysteroscopy with round ligament plication in extremely retroflexed uterus, using hyaluronic acid as adhesion barrier [12]. In Nirgianakis’ ‘Rendezvous technique’, laparoscopy light source is put off with simultaneous hysteroscopy light eliciting the ‘Halloween sign’ or ‘positive diaphanoscopy’ or transillumination where hysteroscopy light shines through the defect [21]. In ‘slip and hook’ technique, Hegar dilator is placed in cervical canal and is blindly slipped anteriorly to bulge out and perforate the defect under laparoscopic vision [22]. In Donnez technique, large isthmoceles are excised laparoscopically using CO2 laser, with round ligaments shortening in retroflexed uterus [18].
Vaginal route can be undertaken by experienced surgeons when niche is not at higher level. After reflecting bladder from cervix, niche is identified, excised and hysterotomy closed in two layers. Transvaginal repair was found to be cost-effective with shorter operation time. Simultaneous hysteroscopy to visualize niche by transillumination and single-port laparoscopy-assisted vaginal repair is also described [16].
Preference of the Route
All surgical techniques appeared effective, and no particular treatment modality was found superior to the other in a recent systematic review of 30 studies [7]. In nutshell, smaller niches of < 2.5–3 mm [16, 25] with RMT > 3 mm can be treated hysteroscopically. Vaginal route is preferred when niche is at the lower level. Transabdominal approach is preferred for large defects especially if the residual myometrium is < 3 mm as bladder can be mobilized out of surgical field offering better niche visualization with lesser bladder injury. It is a better approach for women desiring future pregnancy since uterine wall thickness and strength increase. Among transabdominal routes, laparoscopy and robotic surgery offer advantages of being minimally invasive with lesser morbidity. Incidental endometriosis is reported in 21% women [18]; hence, consent for correction of any associated pathology should also be taken if transabdominal route is planned.
Hysterectomy
Hysterectomy offers definitive treatment for niche-related gynaecological symptoms.
Outcomes of Surgical Management of Post-caesarean Niche
Hysteroscopic niche resection reduces post-menstrual spotting by a median of 3.8 days, with complete resolution of AUB in 72.4% cases [17] and pain improvement in 97% [14]. AUB gets cured in 87.5% patients within first month and 96.8% patients in the second month after surgery [20]. Up to 5% patients may have recurrence [10]. In Vervoort’s study of 101 women with large niche and < 3 mm RMT treated laparoscopically, 79% had symptom relief, post-menstrual spotting reduced by 7 days at 6 months, dysmenorrhea reduced, myometrial thickness increased, and overall, 83.3% women felt very satisfied [12].
After laparoscopic repair, Donnez reported 93% symptom-free patients, increase in mean myometrial thickness from 1.4 to 9.6 mm at 3-month follow-up, 44% pregnancy rate in infertile women, all delivered at full term by elective caesarean. Therefore, effect of increased myometrial thickness on pregnancy outcome during labour could not be estimated [18].
Pregnancy rate varied from 22–71% in various studies [16, 18, 22–24]. Calzolari, in a retrospective study (n = 35), noted isthmocele as the primary cause of infertility in 45.7% (16/35) patients; all 16 women were relieved of AUB and pain, and 9/16(56.3%) conceived after hysteroscopic isthmoplasty. Women who failed to conceive had higher BMI, higher isthmocele grade, higher number of prior caesareans and advanced age [23].
Enderle reported poor obstetric outcomes in his retrospective series of 18 surgically treated women, with approximately 55% miscarriages; hysteroscopy had poorer results as miscarriage occurred in 3/4 patients. One patient who underwent transvaginal repair delivered vaginally; others underwent caesarean [24]. Another study reported pregnancy rate of 71% as 10/14 infertile women conceived including six spontaneous pregnancies after laparotomy, laparoscopy or vaginal approach; eight had caesarean; one had vaginal delivery, and one aborted, with no case of placenta accreta or rupture.
Prevention of Niche Formation
Primary prevention of uterine niche by efforts to minimize caesarean rates and secondary prevention by adopting correct surgical techniques ensuring thicker residual myometrium and strong scar will remain the mainstay in prevention of niches. A recent study in 138 women demonstrated that uterine closure using far-far-near-near double-layer unlocked technique may benefit in reducing isthmocele formation and ensuring sufficient residual myometrium [25].
Conclusion
Post-caesarean uterine niche is a relatively newer clinical entity, and its diagnosis requires high index of suspicion. Emerging evidence has emphasized on careful selection of patients requiring surgical correction, possible learning curve and also yet to be proven efficacy of these treatments. For bleeding symptoms, hormonal treatment is preferable, whereas subfertility may require surgical correction. Hysteroscopic resection is preferred for smaller niches with RMT > 3 mm, niche located lower down can be treated transvaginally, and transabdominal approach is preferred for large defects and in women desiring future pregnancy. Laparoscopy and robotic surgery offer advantages of being minimally invasive with lesser morbidity. Future studies may further delineate factors influencing preference and success of various treatments according to clinical symptoms.
Acknowledgements
We acknowledge contribution of Dr Vatsla Dadhwal, Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, for providing figures for the manuscript.
Dr. Vidushi Kulshrestha
is working as Associate Professor in the Department of Obstetrics and Gynaecology, AIIMS,
New Delhi. She is recipient of prestigious FOGSI-Corion Runner-up Award (2009 and 2013), Dr. C. S. Dawn Prize
(2011) and various best paper/poster awards at national conferences. She has 44 indexed scientific publications and 9 chapters in books. She is one of the founder members of Gynaecological Endocrine Society of India (GESI). She has contributed in framing INDEX TB Guidelines on Extra-pulmonary tuberculosis, FOGSI-GCPR for Abnormal Uterine Bleeding and GOI guidelines for calcium supplementation and Gestational Diabetes Mellitus.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any potential conflict of interest.
Ethical Statement
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Vidushi Kulshrestha is a Associate Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India; Nutan Agarwal is a Consultant Gynaecologist, Head- Fetal Medicine, Department of Obstetrics and Gynaecology, Artemis Hospital, Gurugram, Haryana, India; Ex-Professor, Department of Obstetrics and Gynaecology, AIIMS, New Delhi, India; Garima Kachhawa is a Additional Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bij de Vaate AJ, Brolmann HA, van der Voet LF, van der Slikke JW, Veersema S, Huirne JA, van der Voet LF. Ultrasound evaluation of the cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol. 2011;37:93–99. doi: 10.1002/uog.8864. [DOI] [PubMed] [Google Scholar]
- 2.Naji O, Abdallah Y, Bij De Vaate AJ, Smith A, Pexsters A, Stalder C, McIndoe A, Ghaem-Maghami S, Lees C, Brolmann HA, Huirne JA, Timmerman D, Bourne T. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39:252–259. doi: 10.1002/uog.10077. [DOI] [PubMed] [Google Scholar]
- 3.van der Voet LF, Bij de Vaate AM, Veersema S, Brölmann HA, Huirne JA. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121(2):236–244. doi: 10.1111/1471-0528.12542. [DOI] [PubMed] [Google Scholar]
- 4.Kremer TG, Ghiorzi IB, Dibi RP. Isthmocele: an overview of diagnosis and treatment. Rev Assoc Med Bras. 2019;65(5):714–721. doi: 10.1590/1806-9282.65.5.714. [DOI] [PubMed] [Google Scholar]
- 5.Iannone P, Nencini G, Bonaccorsi G, Martinello R, Pontrelli G, Scioscia M, et al. Isthmocele: from risk factors to management. Rev Bras Ginecol Obstet. 2019;41(1):44–52. doi: 10.1055/s-0038-1676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vikhareva Osser O, Jokubkiene L, Valentin L. Cesarean section scar defects: agreement between transvaginal sonographic findings with and without saline contrast enhancement. Ultrasound Obstet Gynecol. 2010;35:75–83. doi: 10.1002/uog.7496. [DOI] [PubMed] [Google Scholar]
- 7.Setubal A, Alves J, Osório F, Guerra A, Fernandes R, Albornoz J, Sidiroupoulou Z. Treatment for uterine isthmocele, a pouchlike defect at the site of a cesarean section scar. J Minim Invasive Gynecol. 2018;25(1):38–46. doi: 10.1016/j.jmig.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Antila-Långsjö RM, Mäenpää JU, Huhtala HS, Tomás EI, Staff SM. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018;219(5):458.e1–458.e8. doi: 10.1016/j.ajog.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brölmann HA, Bourne T, Huirne JA. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43(4):372–382. doi: 10.1002/uog.13199. [DOI] [PubMed] [Google Scholar]
- 10.Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, Brölmann HA, Mol BW, Huirne JA. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30(12):2695–2702. doi: 10.1093/humrep/dev240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vervoort AJ, Van der Voet LF, Witmer M, Thurkow AL, Radder CM, van Kesteren PJ, et al. The HysNiche trial: hysteroscopic resection of uterine caesarean scar defect (niche) in patients with abnormal bleeding, a randomised controlled trial. BMC Womens Health. 2015;15:103. doi: 10.1186/s12905-015-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vervoort A, Vissers J, Hehenkamp W, Brölmann H, Huirne J. The effect of laparoscopic resection of large niches in the uterine caesarean scar on symptoms, ultrasound findings and quality of life: a prospective cohort study. BJOG. 2018;125(3):317–325. doi: 10.1111/1471-0528.14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antila RM, Mäenpää JU, Huhtala HS, Tomás EI, Staff SM. Association of cesarean scar defect with abnormal uterine bleeding: the results of a prospective study. Eur J Obstet Gynecol Reprod Biol. 2020;244:134–140. doi: 10.1016/j.ejogrb.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 14.van der Voet LF, Vervoort AJ, Veersema S, BijdeVaate AJ, Brölmann HA, Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar: a systematic review. BJOG. 2014;121(2):145–156. doi: 10.1111/1471-0528.12537. [DOI] [PubMed] [Google Scholar]
- 15.Antila-Långsjö R, Mäenpää JU, Huhtala H, Tomás E, Staff S. Comparison of transvaginal ultrasound and saline contrast sonohysterography in evaluation of cesarean scar defect: a prospective cohort study. Acta Obstet Gynecol Scand. 2018;97(9):1130–1136. doi: 10.1111/aogs.13367. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Yang M, Wang Q, Chen J, Ding J, Hua K. Prospective evaluation of five methods used to treat cesarean scar defects. Int J Gynaecol Obstet. 2016;134(3):336–339. doi: 10.1016/j.ijgo.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Abacjew-Chmylko A, Wydra DG, Olszewska H. Hysteroscopy in the treatment of uterine cesarean section scar diverticulum: a systematic review. Adv Med Sci. 2017;62(2):230–239. doi: 10.1016/j.advms.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Donnez O, Donnez J, Orellana R, Dolmans MM. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil Steril. 2017;107(1):289–296. doi: 10.1016/j.fertnstert.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Vervoort A, van der Voet LF, Hehenkamp W, Thurkow AL, van Kesteren P, Quartero H, et al. Hysteroscopic resection of a uterine caesarean scar defect (niche) in women with postmenstrual spotting: a randomised controlled trial. BJOG. 2018;125(3):326–334. doi: 10.1111/1471-0528.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vegas Carrillo de Albornoz A, López Carrasco I, Montero Pastor N, Martín Blanco C, Miró Matos M, Alonso Pacheco L, Moratalla Bartolomé E. Outcomes after hysteroscopic treatment of symptomatic isthmoceles in patients with abnormal uterine bleeding and pelvic pain: a prospective case series. Int J Fertil Steril. 2019;13(2):108–112. doi: 10.22074/ijfs.2019.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nirgianakis K, Oehler R, Mueller M. The Rendez-vous technique for treatment of caesarean scar defects: a novel combined endoscopic approach. Surg Endosc. 2016;30(2):770–771. doi: 10.1007/s00464-015-4226-6. [DOI] [PubMed] [Google Scholar]
- 22.Api M, Boza A, Gorgen H, Api O. Should Cesarean scar defect be treated laparoscopically? A case report and review of the literature. J Minim Invasive Gynecol. 2015;22(7):1145–1152. doi: 10.1016/j.jmig.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Calzolari S, Sisti G, Pavone D, Ciocia E, Bianchini N, Cozzolino M. Prevalence of infertility among patients with isthmocele and fertility outcome after isthmocele surgical treatment: a retrospective study. Ochsner J. 2019;19(3):204–209. doi: 10.31486/toj.18.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enderle I, Dion L, Bauville E, Moquet PY, Leveque J, Lavoue V, Lous ML, Nyangoh-Timoh K. Surgical management of isthmocele symptom relief and fertility. Eur J Obstet Gynecol Reprod Biol. 2020;247:232–237. doi: 10.1016/j.ejogrb.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Kalem Z, Kaya AE, Bakırarar B, Basbug A, Kalem MN. An optimal uterine closure technique for better scar healing and avoiding isthmocele in cesarean section: a randomized controlled study. J Investig Surg. 2019;9:1–9. doi: 10.1080/08941939.2019.1610530. [DOI] [PubMed] [Google Scholar]


