Abstract
Aims
Screening for asymptomatic atrial fibrillation (AF) could prevent strokes and save lives, but the AF burden of those detected can impact prognosis. New technologies enable continuous monitoring or intermittent electrocardiogram (ECG) snapshots, however, the relationship between AF detection rates and the burden of AF found with intermittent strategies is unknown. We simulated the likelihood of detecting AF using real-world 2-week continuous ECG recordings and developed a generalizable model for AF detection strategies.
Methods and results
From 1738 asymptomatic screened individuals, ECG data of 69 individuals (mean age 76.3, median burden 1.9%) with new AF found during 14 days continuous monitoring were used to simulate 30 seconds ECG snapshots one to four times daily for 14 days. Based on this simulation, 35–66% of individuals with AF would be detected using intermittent screening. Twice-daily snapshots for 2 weeks missed 48% of those detected by continuous monitoring, but mean burden was 0.68% vs. 4% in those detected (P < 0.001). In a cohort of 6235 patients (mean age 69.2, median burden 4.6%) with paroxysmal AF during clinically indicated monitoring, simulated detection rates were 53–76%. The Markovian model of AF detection using mean episode duration and mean burden simulated actual AF detection with ≤9% error across the range of screening frequencies and durations.
Conclusion
Using twice-daily ECG snapshots over 2 weeks would detect only half of individuals discovered to have AF by continuous recordings, but AF burden of those missed was low. A model predicting AF detection, validated using real-world data, could assist development of optimized AF screening programmes.
Keywords: Atrial fibrillation, Screening, Asymptomatic, ECG
What’s new?
In an asymptomatic screened cohort of individuals found to have at least one episode of atrial fibrillation (AF) during 2 weeks of continuous electrocardiogram (ECG) monitoring, only 52% would have their AF detected by twice-daily 30 s ECG snapshots during the same period based on over 34 000 simulations.
The median AF burden of the individuals detected to have AF with twice-daily screening for 14 days was 4.0%, while for those who would be missed it was only 0.68%.
AF burden in asymptomatic screened populations is lower and its distribution different to AF seen in clinically indicated ECG monitoring.
The Markovian model of AF detection using mean episode duration and mean burden can approximate detection rates for any range of characteristics with ≤9% error across the range of screening frequencies and durations considered.
The Markovian model of AF detection, available online, can help guide future strategies for screening.
Introduction
It has been estimated that ∼13% of individuals with atrial fibrillation (AF) are undiagnosed and among patients with stroke about 20% have a new AF diagnosis.1 The significant clinical consequences of undiagnosed AF, along with the availability of therapies proven effective at preventing severe consequences such as stroke, have supported the potential value of screening for silent AF among at-risk populations.2 However, the evidence of overall benefit for systematic screening relative to opportunistic screening via pulse palpation or ECG has been limited to date, leading to conflicting recommendations.3–5
In recent years, there has been a proliferation of devices which detect AF via ECG recordings—from implantable monitors continuously recording the ECG for months, to consumer-focused devices and wearables providing 30 seconds rhythm strips anytime and anywhere. In addition, advanced algorithms have been introduced to autonomously detect AF in single-lead rhythm strips, and possibly even predict future AF risk from a sinus rhythm 12-lead ECG.6 These new technologies, along with availability of more patient-friendly, effective anticoagulants, have reinvigorated interest in screening for AF.
Three broad systematic screening strategies have been tested in large populations: multi-year continuous ECG monitoring with an implantable cardiac monitor (ICM) as in the LOOP and REVEAL AF studies7,8; long-term with an adhesive ECG sensor patch (2 weeks) as in the mSToPS study9; and intermittent, 30 s ECG screening snapshots with a handheld ECG at various frequencies, intervals and durations, as in the STROKESTOP study.10 Both continuous and intermittent strategies offer advantages and disadvantages.
To better understand the likelihood of correctly identifying an individual who has occasional, asymptomatic AF episodes by intermittent snapshots vs. continuous monitoring, several investigators have attempted to simulate effectiveness of intermittent monitoring using datasets of continuously monitored individuals found to have new AF.11,12 However, prior studies have been limited by the use of data obtained from individuals with ICMs (pacemaker or defibrillator in 93%) for clinical indications, rather than an asymptomatic screening population. A recent study using an implantable loop recorder to screen for asymptomatic AF estimated the fortnightly AF burden likely to be associated with the detection of AF through twice-daily ECG checks in 205 individuals with at least one AF episode over the course of 3 years.7
Using continuous ECG data from asymptomatic individuals found to have AF in the mSToPS trial, we simulated the likelihood of diagnosing AF in this population via different intermittent screening strategies and estimated the AF burden likely to be associated with AF detected, or missed, by intermittent recordings. Using a much larger dataset of over 6200 individuals with paroxysmal AF during clinically indicated ECG patch monitoring we expanded the model to include individuals with higher burdens of paroxysmal AF.13 Finally, to generalize AF detection results to any type of AF burden and measurement protocol we developed a Markovian model to simulate AF events in an individual, and demonstrated that the model accurately approximates AF detection probability in data from our two cohorts.
Methods
Study population
Both datasets included in this analysis used the iRhythm Zio®XT, an FDA approved, single-use, water-resistant, continuous ambulatory single-lead ECG monitoring skin adhesive patch that retains in memory the wearer’s ECG for up to 2 weeks. The first cohort includes 69 individuals who participated in a nationwide trial of AF screening and were found to have newly diagnosed silent AF on patch monitoring.9 The clinically monitored cohort includes 6235 individuals who underwent clinically indicated extended cardiac rhythm evaluation and had episodic AF.13 The clinical indications for testing are unknown.
Available data include the start and stop time of each AF event, and start and stop time of the monitored period.
For each person (designated formulaically as ‘s’), we have the precise timing of the recorded AF intervals (the total number of discrete episodes designated by ‘Q’), from which we derive the duration of each event, ,…,. We calculated the average length of AF events for person ‘s’, namely as, and the fraction of time in AF for the monitored period (bs), which is the AF burden for that person in the monitored period.
The screening cohort comprises data from 69 individuals, with median monitored time of 14.0 days (IQR: 13.1–14.0 days). The clinically monitored cohort contains sensor patch reports from 6235 unique individuals, with median monitored time of 13.8 days (IQR: 12.5–14.0 days).
In both datasets, artefacts determined by the manufacturer’s specification were removed. If an interval labelled as artefact was preceded and followed by an AF event, the two AF events were merged and considered as a unique AF event, including the short interval labelled as artefact in the total episode duration.
Analytic methods
In order to test the efficacy of AF screening with short ECG snapshots of 30 s, we simulated period monitoring for each subject, with 30 s snapshots repeated every δ = 24 h (M = 1 measurement per day), δ = 12 h (M = 2 measurements per day), δ = 8 h (M = 3 measurements per day), or δ = 6 h (M = 4 measurements per day). The initial snapshot starts at a random time, while the next snapshot happens after an interval of δ + ε, where ε is a random variable normally distributed with zero mean and standard deviation equal to 30 min, in order to simulate some variability in the actual measurement time. The measurements are repeated for the different lengths of the screening time (from D = 1 day to D = 14 days).
The simulated, intermittent AF screening was labelled as successful if at least one AF event was detected for the individual (by definition, all individuals analysed had at least one AF event recorded during continuous recording). We assume that an AF event is detected if the person is in AF during the entire 30 s of the screening.
The performance of intermittent, 30 s screening strategies is determined by selecting a specific choice of the parameters (M, the number of measurements per day; and D, the number of days of the screening interval), simulating an AF screening for each person in the dataset, and calculating the fraction in whom at least one AF event has been identified. This procedure is repeated 500 times for each person in the asymptomatic screened cohort and 50 times for each person in the clinically monitored cohort (choosing a different starting time at random) in order to obtain the best approximation of the fraction of people who might be correctly classified with AF in the specific dataset. Therefore, the results are based on a total of ∼350 000 simulated screening strategies in the combined cohorts.
Markovian model of atrial fibrillation
We proposed the use of a two-state continuous-time Markov chain, where the first state (N) represents normal sinus rhythm (or other non-AF rhythms), while the second state (A) represents an AF interval. The time spent in each of the two states is distributed according to an exponential distribution, with parameter λN and λA, for non-AF and AF states, respectively. Exponential distributions have the memoryless property (or Markov property), i.e. at any time in such a system, the distribution of the time in which the process will remain in the current state does not depend on the time already spent in the same state. Consequently, the probability of changing state (e.g. starting an AF event while in normal sinus rhythm) does not depend on the time passed since the last AF event. See Supplementary material for analytical derivations of this model.
Statistical analysis
The distribution of the AF burden for the individuals in each of the two cohorts is calculated by defining the burden intervals from 0 to 100%, with a 2.5% step, and then calculating for each cohort the fraction of individuals in each of these steps.
Accuracy is verified by calculating the 95% confidence interval of the average fraction of individuals with AF detected for the different repetitions (for each choice of the number of measurements per day M and the length of the screening time D). Differences between median burdens were calculated by the Wilcoxon Rank-Sum test.
Results
Asymptomatic screened cohort
The AF burden for individuals in the asymptomatic screening cohort found to have newly diagnosed AF is relatively low, as observed in Figure 1A: median AF burden was 1.9% (IQR: 0.6–4.1%) with a median number of four discrete episodes of AF (IQR: 1–27) over 14 days. Median duration of the longest individual episode of AF was 227 min (IQR: 49–611).
Figure 1.
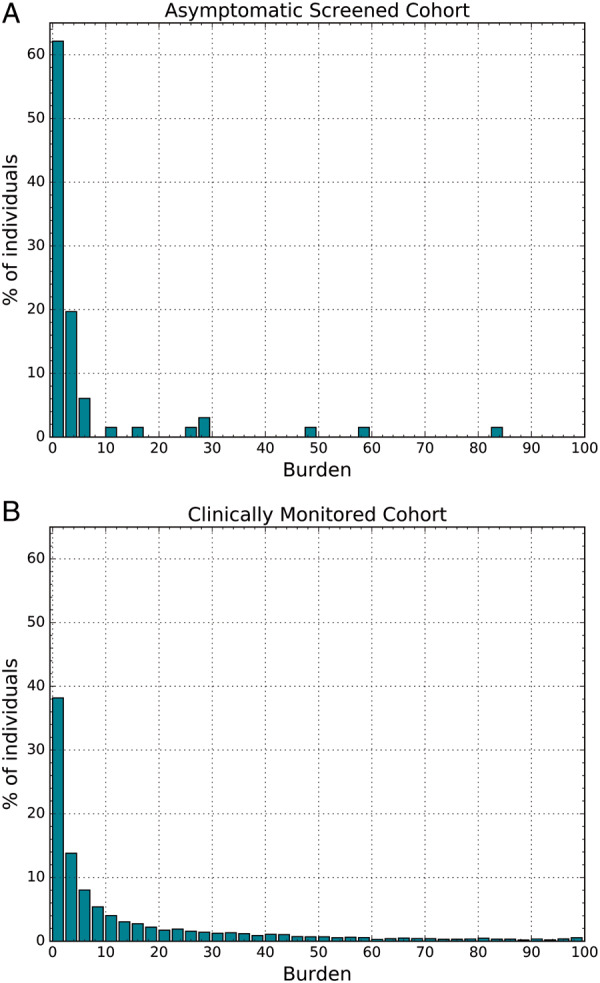
Atrial fibrillation (AF) burden (percentage of monitored time in AF) in (A) the asymptomatic, screened cohort, and (B) the clinically monitored cohort.
As a consequence, the percentage of people diagnosed with AF after simulated screening with short, recurring measurements is relatively low, as shown in Figure 2B. As a key case, we consider the individuals detected by two 30 s ECG snapshots per day over a 14-day period (the screening frequency in STROKESTOP) which at 52% is just over half of individuals. For one measurement per day and 14 days of screening, we would anticipate being able to detect AF in only 35% of individuals, increasing to 61% and 66% with three or four ECG snapshots per day, respectively.
Figure 2.
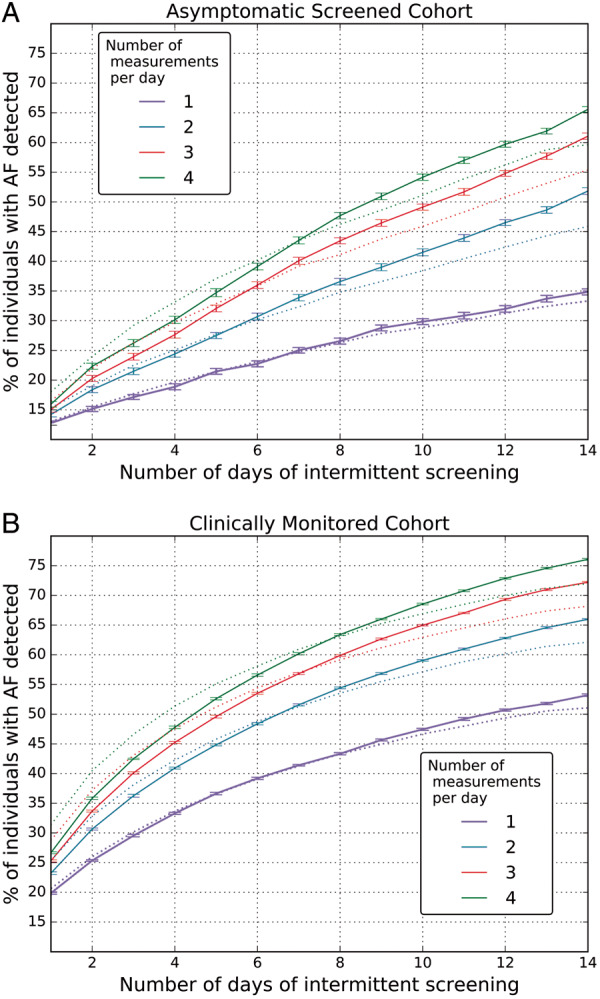
Percentage of individuals with atrial fibrillation (AF) detected in (A) the asymptomatic, screened cohort, and (B) the clinically monitored cohort. Different colours are for the number of daily 30 s ECG measurements performed. The solid lines are the actual data, while the dotted lines are the data obtained with the Markovian model.
The probability of detecting an AF episode while monitoring with intermittent screenings increases as the AF burden for the individual increases. Using two ECG snapshots per day over a 14-day period, for the 52% of individuals detected, the median burden was 4.0%, IQR: 2.49–27.2% (median values over all the screening repetitions), while the median individual average duration of an AF episode was 233 min, IQR: 49–914. For the remaining 48% whose AF was not detected by twice-daily screening, median burden was much lower at 0.68%, IQR: 0.18–1.58% (P < 0.001), while the duration of the average individual episode of AF was 8 min, IQR: 3–70 (P < 0.001).
Performance of the Markovian model in the asymptomatic screened cohort
The Markovian model was used to create an artificial dataset from the original dataset in order to test the accuracy of the AF detection estimation on artificial data with characteristics similar to real data. The performance of the AF detection on the artificial data created with the Markovian model very much mirrors the performance in the actual asymptomatic cohort data (Figure 2A). The estimation error is limited to a maximum of 9% for all considered lengths of measurements (from 1 to 14 days) and for all frequencies of measurement (from 1 to 4) per day.
Clinically monitored cohort
The AF burden for individuals in the clinically monitored cohort with AF episodes is substantially higher than in the asymptomatic cohort and has a different distribution (Figure 1B). The median burden of AF was 4.6% (IQR: 1.2–17.4%) with a median number of 21 discrete episodes of AF (IQR: 4–91) over 14 days and the median duration of the longest individual episode of AF was 324 min (IQR: 87–1082).
The percentage of people in this cohort detected with AF by using 30 s intermittent ECG measurements from one to four times a day for up to 14 days is depicted in Figure 2B. As in the screened cohort, the probability of detecting an AF episode while monitoring with intermittent screenings depends on the individual’s AF burden. As the AF burden in the individuals undergoing clinically indicated monitoring is much higher, the likelihood of AF detection is higher at all frequencies and durations of intermittent screening. As a key case, we again consider the people detected by two 30 s ECG snapshots per day over a 14-day period, which is 66% of all individuals with AF. For this 66% of detected patients, AF median burden was 11.9%, IQR: 4.9–30.5% (overall screening repetitions), median average AF episode was 76 min, IQR: 16–393. For the remaining 34% (non-detected), AF median burden was 0.77%, IQR: 0.17–1.88%, median average AF episode was 4 min, IQR: 1–24—similar to that in the asymptomatic cohort.
Performance of the Markovian model in the clinically monitored cohort
As in the asymptomatic screened cohort, the performance of AF detection on the artificial data created with the Markovian model mirrors the performance in the actual data. The accuracy of this model is relatively consistent. The model slightly overestimates the fraction of individuals detected with AF for short monitoring time (<1 week), while it underestimates the fraction of individuals detected with AF for monitoring time between 7 and 14 days. As for the asymptomatic cohort, the estimation error is not greater than 9%.
Atrial fibrillation burden changes during a day
Minor changes in the probability of an AF event are observed depending on time of the day (Figure 3), see Supplementary Document for further details.
Figure 3.
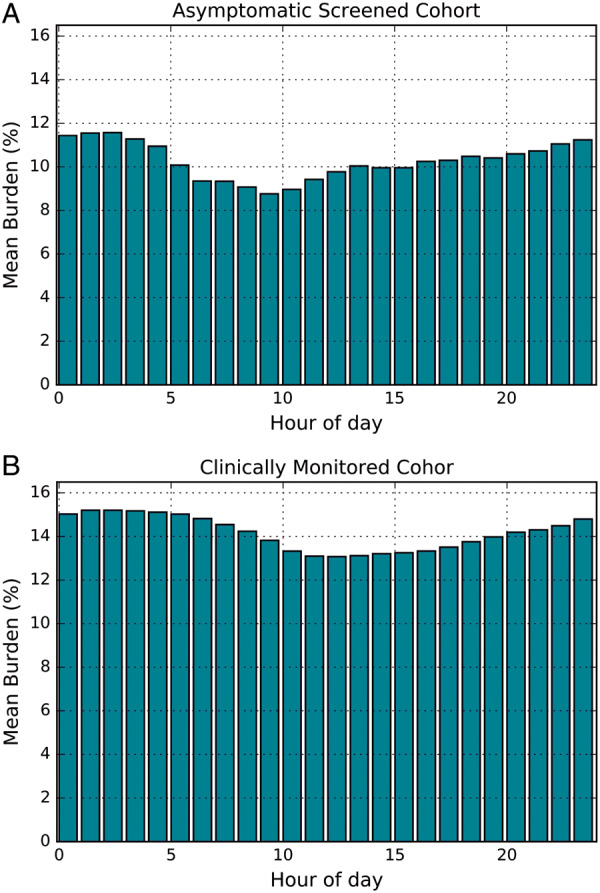
Cohort average burden of atrial fibrillation (AF) per hour in (A) the asymptomatic screened cohort, and (B) the clinically monitored cohort.
Markovian model simulation
In the previous sections, we demonstrated that given AF burden and average length of AF events, the Markovian model can generate realistic data and that the detection accuracy using 30 s ECG snapshots is well approximated by the model data. The model can be used for two different goals. First, given any AF burden and corresponding averages of AF events, it is possible to generate artificial data and estimate the detection percentage for any number of days of monitoring, number of measurements per day, and length of each monitoring interval. In addition, it is possible to use the model to obtain theoretical results of the detection likelihood of at least one AF event.
In Figure 4, we show the case of two measurements per day for 14 days and depict the percentage of individuals with AF detected as a function of their AF burden, for different values of the average length of an AF episode. The difference in detection likelihood for short and frequent AF intervals is minimal for an average length of an AF event between 6 min and 4 h. In this case, with an AF burden of 5% it is possible to detect approximately 77% of people with AF, while with an AF burden of 10% it is possible to detect more than 90%. If a person has infrequent and longer episodes, detection has a lower percentage of success, even for high values of the AF burden.
Figure 4.
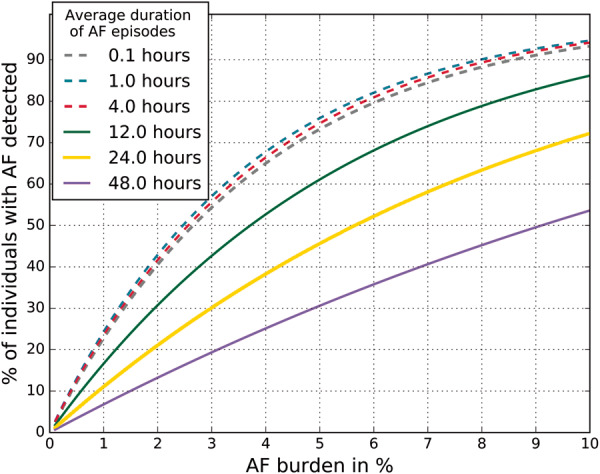
Probability of detecting at least one atrial fibrillation (AF) event when all individuals undergo two 30 s ECG screenings per day for a total of 14 days, as a function of the AF burden and the average length of the AF events. In the Figure, as is the average length for an AF episode, while the actual length of each AF episode for an individual varies substantially, according to the exponential variable model described in the Methods section.
This figure serves as a relevant example of the performance of the model. Investigators, clinicians, or health providers can use the model found at scripps.edu/quer_af_det and enter the variables of their choosing—screening programme characteristics and AF characteristics of the cohort they wish to identify—to generate the corresponding information.
Discussion
Over just the last several years, the technologies that allow for screening for AF outside of routine appointments with a healthcare provider have rapidly expanded and now include watches, smartphones, patches, subcutaneous implantable devices, and more. This has led to an equally large and growing number of studies, some involving well over 100 000 participants, using very different strategies, but all exploring the question as how to best identify an individual with asymptomatic AF (www.safer.phpc.cam.ac.uk/about-screenforaf/aims-and-objectives, www.ukbiobank.ac.uk/heart-monitor).14,15 However, because most existing knowledge of the clinical consequences of AF, and the value of preventative interventions is based on experience with individuals discovered clinically to have AF, there currently exists a large knowledge gap between the increasing ease of screening for AF and the evidence base needed to guide what to do with that information.
It is possible that future research will show that screening only benefits individuals with high AF burdens, making single timepoint screening, or intermittent, infrequent monitoring of greatest value.16,17 Certainly, paroxysmal AF seems to have somewhat lower risk of stroke than persistent AF. On the other hand, it is also conceivable that future research might find that any episode of AF, irrespective of duration or burden, is a marker of actionable risk to trigger aggressive preventive interventions with or without initiating anticoagulant therapy,18 though decisions on anticoagulant initiation remain an important management conundrum. Therefore, there is value in better understanding the likelihood of various screening methods to identify individuals with asymptomatic paroxysms of AF of varying lengths, frequencies, and overall AF burdens.
In the current work, using a unique data set derived from a cohort of 69 asymptomatic individuals participating in an AF screening trial, and found to have at least one episode of AF lasting >30 s during 14 days of continuous ECG monitoring, we were able to model the probability of identifying an individual with asymptomatic AF as having AF using different intermittent 30 s ECG screening strategies. Using this model, we would estimate that a screening strategy with twice daily, 30 s ECGs for 14 days, would have identified just over half of the individuals who were found to have asymptomatic AF in the mSToPS trial.9,10 It is important to note from our simulation that the median burden of the 48% of individuals who would be missed by this intermittent strategy was only 0.68% compared to a median burden of 4.0% for those detected. Therefore, if an investigator’s goal is to identify individuals with AF who would benefit from the initiation of anticoagulation, it will be crucial to identify the AF burden in a 2 week monitoring period, or other individual characteristics, at which the risk of stroke is sufficient to justify anticoagulant prophylaxis. This trade-off between increased detection of low-burden, possibly low-risk AF by continuous monitoring strategies, could be minimized by defining an intermittent monitoring strategy that would diminish the potential for missing individuals with a high burden.19
In order to expand the applicability of the simulation to a much larger population with a greater variety of patterns of AF paroxysms and higher burdens we supplemented the results of the asymptomatic, screened cohort with data from 6235 individuals who underwent the same duration of continuous ECG monitoring with the same device, but for a variety of clinical indications, and were found to have paroxysms of AF. A novel though not completely unexpected finding is the difference in distribution of AF burden between the clinically indicated and screening cohorts, with much higher burden in those monitored for a clinical indication, most likely symptomatic paroxysmal AF. The median AF burden of those detected by a twice-daily ECG checks for 14 days was 11.9%, compared to only 4.0% of those in the screening cohort. In the KP-RHYTHM study16 of clinically indicated monitoring, only the highest tertile of burden (11.4%) had an increased risk of stroke. The median AF burden in that study was 4.4% (IQR 1.1–17.2%), which is almost identical to the AF burden in our clinically indicated cohort (4.2%, IQR 1.2–17.4%). An important unknown is whether the relationship between stroke risk and AF burden might differ in those with a clinical indication for monitoring compared to asymptomatic people undergoing screening for AF.
The Markovian model, developed using data from both cohorts, and therefore a wide range of AF burdens, can enable researchers to develop an intermittent screening strategy that is based on the predicted likelihood of identifying individuals with the AF characteristics of greatest interest to them. It is the size and variability of the AF characteristics of our large population that we believe is its greatest value relative to other recent simulation work of AF screening strategies.9,10 While much work remains to precisely identify those who benefit from the initiation of anticoagulation therapy, other interventions such as implementing a formal weight loss programme or aggressive treatment of sleep apnoea might be actioned by the detection of any AF.
Limitations of the study
One limitation of our analyses is based on the assumption that personal-use, wireless ECG devices will always correctly detect an AF event when present, similar to prior studies.7,8 Indeed, automatic AF detection from short and noisy signals sensed by portable ECG devices may have inaccuracies: if algorithms are manipulated to enhance sensitivity and reduce false negatives, there will be a concomitant reduction in specificity.20
Our results are less applicable to AF screening strategies based on near-continuous photoplethysmography tracking from a wearable device in very large, lower-risk populations. Early work has suggested this is quite feasible,16,17 and prospective studies, supported by watch-obtained ECGs, are currently underway (www.heartline.com).
Additionally, in our simulation, ECG snapshot intervals were taken at random in the 24 h, while in a clinical study the measurements are likely to be performed only during the day. While there was only a small difference in the AF burden between day and night, the average AF burden for the people in both datasets was higher during night, which could result in a small overestimation of the probability of detection of AF for ECG snapshots taken only during the day.
Conclusion
Using data from an AF screening study in asymptomatic people with newly detected AF on continuous ECG recordings of ∼14 days duration, and real-world data in over 6300 people with paroxysmal AF using the same device, we were able to carry out over 350 000 simulations to estimate the sensitivity of screening strategies involving any range of daily ECG snapshot frequencies and duration up to 14 days. We found that an intermittent screening protocol of twice-daily ECG snapshots for 14 days will detect about two-thirds of individuals with AF in a population with a median burden of about 5%. However, those detected will have a much greater AF burden than those with AF but in whom AF is missed by the ECG snapshots. A Markovian model that performed well in this real-world data set will help inform future AF research and design of AF screening programmes.
Funding
This work was supported by the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences grant (UL1TR002550). Conflict of interest: Dr G.Q. reports grants from Janssen, during the conduct of the study. Dr B.F. reports grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from BMS-PFizer, personal fees and non-financial support from Daiichi-Sankyo, non-financial support from Alivecor, personal fees and non-financial support from Omron, outside the submitted work. Dr S.R.S. reports grants from Janssen, during the conduct of the study; grants from Qualcomm Foundation, personal fees from Novartis, other from Livongo, outside the submitted work.
Data availability
No new data were generated or analysed in support of this research.
Supplementary Material
References
- 1. Jaakkola J, Mustonen P, Kiviniemi T, Hartikainen JE, Palomaki A, Hartikainen P. et al. Stroke as the first manifestation of atrial fibrillation. PLoS One 2016;11:e0168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J. et al. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation 2017;135:1851–67. [DOI] [PubMed] [Google Scholar]
- 3. Mairesse GH, Moran P, Van Gelder IC, Elsner C, Rosenqvist M, Mant J, ESC Scientific Document Group et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE). Europace 2017;19:1589–623. [DOI] [PubMed] [Google Scholar]
- 4. Jonas DE, Kahwati LC, Yun JDY, Middleton JC, Coker-Schwimmer M, Asher GN.. Screening for atrial fibrillation with electrocardiography: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;320:485–98. [DOI] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 6. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ. et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 7. Diederichsen SZ, Haugan KJ, Kronborg C, Graff C, Hojberg S, Kober L. et al. A comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk long-term monitored with implantable loop recorder. Circulation 2020;141:1510–22. [DOI] [PubMed] [Google Scholar]
- 8. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Elkind MSV. et al. Rhythm monitoring strategies in patients at high risk for atrial fibrillation and stroke: a comparative analysis from the REVEAL AF study. Am Heart J 2020;219:128–36. [DOI] [PubMed] [Google Scholar]
- 9. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS. et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018;320:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M.. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- 11. Charitos EI, Stierle U, Ziegler PD, Baldewig M, Robinson DR, Sievers HH. et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 2012;126:806–14. [DOI] [PubMed] [Google Scholar]
- 12. Yano Y, Greenland P, Lloyd-Jones DM, Daoud EG, Koehler JL, Ziegler PD.. Simulation of daily snapshot rhythm monitoring to identify atrial fibrillation in continuously monitored patients with stroke risk factors. PLoS One 2016;11:e0148914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wineinger NE, Barrett PM, Zhang Y, Irfanullah I, Muse ED, Steinhubl SR. et al. Identification of paroxysmal atrial fibrillation subtypes in over 13,000 individuals. Heart Rhythm 2019;16:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y. et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 16. Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH. et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol 2018;3:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR. et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 18. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R. et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 19. Freedman B, Schnabel R, Calkins H.. Opportunistic electrocardiogram screening for atrial fibrillation to prevent stroke. JAMA Cardiol 2019;4:91–2. [DOI] [PubMed] [Google Scholar]
- 20. Gadaleta M, Rossi M, Topol EJ, Steinhubl SR, Quer G.. On the effectiveness of deep representation learning: the atrial fibrillation case. Computer (IEEE) 2019;52:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.


