Abstract
This dataset is referred to a collection of 41 faba bean (Vicia faba L.) and 15 lentil (Lens culinaris Medik.) accessions from the ex situ repository of the Institute of Biosciences and Bioresources of the Italian National Research Council (CNR-IBBR). All the accessions were grown at the experimental farm “P. Martucci” of the University of Bari “Aldo Moro” (41°01′22.1′′ N 16°54′21.0′′ E) during the growing season 2017–2018, according to a randomized block design with two replicates, each constituted by 10 individual plants. The dataset reports raw and elaborated analytical data determined on the flour produced from individual accessions, concerning proximate composition, bioactive compounds, antioxidant activity, fatty acid composition, and physicochemical and functional properties. Elaborated data might be used to understand the compositional variability within the species and, together with raw data, to highlight peculiar accessions characterized by valuable nutritional and/or technological attitude useful in research institutions and food industries. Furthermore, the data can be used for genetic studies aimed at identifying genomic regions underlying nutritional and technological traits.
Keywords: Pulses, Proximate composition, Fatty acid composition, Technological properties, Bioactive compounds, Faba bean, Lentil, Germplasm biodiversity
Specifications Table
| Subject | Agricultural and Biological Sciences |
| Specific subject area | Food Science |
| Type of data | Data, excel files, images and figures |
| How data were acquired | Proximate composition: Official methods of analysis [1] for moisture, ash and protein content (total nitrogen × 5.7). Solid-liquid extraction with diethyl ether, using a Soxhlet apparatus (SER 148 extractor – Velp Scientifica srl, Usmate Velate, Italy) for the lipid content. Fatty Acid composition: solid-liquid extraction of the lipid fraction; AOCS method for the preparation of fatty acids methyl esters [2]; Gas-chromatographic determination (7890A gas-chromatograph with Flame Ionization Detector and OpenLAB CDS software C.01.07 - Agilent Technologies, Santa Clara, CA, USA) for fatty acids identification. Bioactive compounds and antioxidant activity: preparation of bioactive extracts (solid-liquid extraction) and spectrophotometric analysis (Cary 60 UV–Vis spectrophotometer - Agilent Technologies, Santa Clara, CA, USA). Physicochemical and functional properties: addition of water or oil to the legume flour, mixing and centrifuging [3], [4], [5] (Thermo Fisher Scientific SL16R – Waltham, MA, USA). |
| Data format | Image, raw data, analysed data |
| Parameters for data collection | Forty-one faba bean accessions and fifteen lentil accessions were grown in the experimental farm “P. Martucci” of the University of Bari “Aldo Moro” (41°01′22.1′′ N 16°54′21.0′′ E) during the season 2017–2018, according to a randomized block design with two replicates. Each replicate was constituted by 10 individual plants. The seeds were harvested at physiological maturity, when they were dried. Then the seeds were cleaned and milled to obtain a whole meal flour. All the analytical determinations were carried out in triplicate. |
| Description of data collection | Proximate composition was determined on the flour derived from 41 faba bean and 15 lentil accessions. The protein, lipid, ash, carbohydrate contents, the fatty acid composition, and the bioactive compounds (total phenolic compounds, total anthocyanins, and total carotenoids) were analysed. The total phytate contents and the antioxidant activities (the latter by means of the DPPH assay) were also determined. The physicochemical (Bulk Density; Water Absorption Index; Water Solubility Index) and functional (Water Absorption Capacity; Oil Absorption Capacity) properties of the flour were analysed. |
| Data source location | Department of Soil, Plant and Food Science (DISSPA), University of Bari “Aldo Moro”, Italy. |
| Data accessibility | Data are provided with this article |
Value of the Data
-
•
Data provided in the article describe the nutritional composition as well as the physicochemical and functional properties of faba bean and lentil accessions grown in the same agro-ecological condition, thus highlighting genotypic differences.
-
•
Peculiar accessions characterised by valuable nutritional and/or functional properties were identified, which can be exploited by the food industry to develop legume-based products.
-
•
Data are available for researchers and geneticists for studies aiming to identify genomic regions controlling nutritional and technological traits.
-
•
Data provided in this article provide important information on the description of faba bean and lentil biodiversity.
1. Data Description
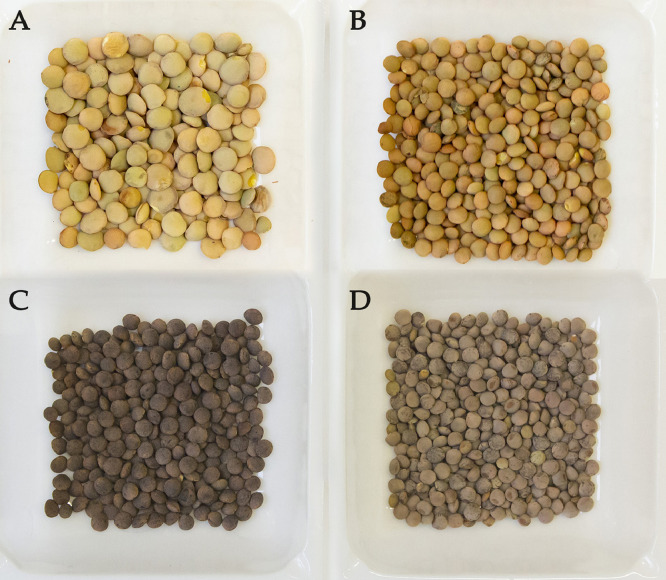
This data article is composed of three tables and seven figures. The data concern 41 faba bean and 15 lentil accessions from different geographical origin, as listed in Table 1. The accessions were characterized by high phenotypic variation (i.e. for color and size), as displayed by the images of some accessions shown in Figs. 1 and 2.
Table 1.
Internal code, CNR-IBBR code, geographical origin, color, weight of 10 seeds (faba beans) and seed diameter (lentils) of the accessions examined.
| Faba bean (Vicia faba L.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | ID Sample | Geographical origin | Color | 10 seed weight (g) | Code | ID Sample | Geographical origin | Color | 10 seed weight (g) |
| FB1 | 110175 | Italy | Green | 13.90 | FB22 | 107547 | Algeria | Green | 14.42 |
| FB2 | 110195 | Italy | Green | 16.65 | FB23 | 112081 | Morocco | Green | 12.65 |
| FB3 | 110201 | Italy | Green | 11.21 | FB24 | 112082 | Morocco | Green | 21.11 |
| FB4 | 110184 | Italy | Purple | 10.59 | FB25 | 112091 | Morocco | Green | 8.59 |
| FB5 | 110360 | Italy | Brown | 6.23 | FB26 | 111894 | Libya | Green | 12.79 |
| FB6 | 110356 | Italy | Green | 10.72 | FB27 | 111900 | Libya | Brown | 6.61 |
| FB7 | 103235 | Italy | Green | 14.11 | FB28 | 111894 | Libya | Green | 17.85 |
| FB8 | 106555 | Italy | Brown | 8.03 | FB29 | 106478 | Ethiopia | Brown | 4.58 |
| FB9 | 106560 | Italy | Purple | 19.00 | FB30 | 106458 | Ethiopia | Green | 8.24 |
| FB10 | 106951 | Italy | Green | 15.67 | FB31 | 108385 | Iran | Brown | 11.93 |
| FB11 | 107640 | Egypt | Green | 10.47 | FB32 | 109167 | Iran | Green | 10.25 |
| FB12 | 108425 | Egypt | Green | 11.41 | FB33 | 109225 | Iran | Brown | 5.91 |
| FB13 | 108428 | Egypt | Green | 6.74 | FB34 | 108947 | Afghanistan | Brown | 8.98 |
| FB14 | 108430 | Egypt | Green | 13.96 | FB35 | 108969 | Afghanistan | Brown | 5.47 |
| FB15 | 108437 | Egypt | Green | 11.96 | FB36 | 109270 | Afghanistan | Brown | 6.96 |
| FB16 | 108438 | Egypt | Brown | 12.65 | FB37 | 109284 | Afghanistan | Brown | 13.90 |
| FB17 | 106406 | Algeria | Green | 10.56 | FB38 | 109282 | Afghanistan | Brown | 5.17 |
| FB18 | 106375 | Algeria | Green | 12.16 | FB39 | 109290 | Afghanistan | Brown | 2.55 |
| FB19 | 106374 | Algeria | Brown | 14.02 | FB40 | 109163 | Pakistan | Brown | 5.55 |
| FB20 | 107537 | Algeria | Brown | 15.62 | FB41 | 109164 | Pakistan | Brown | 8.58 |
| FB21 | 106779 | Algeria | Green | 10.94 | |||||
| Lentil (Lens culinaris Medik.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | ID Sample | Geographical origin | Color | Seed Diameter (mm) | |||||
| LC1 | 106315 | Ethiopia | Dark brown | 4.00 | |||||
| LC2 | 106404 | Algeria | Light green | 5.17 | |||||
| LC3 | 106407 | Algeria | Light green | 5.33 | |||||
| LC4 | 106665 | Italy | Brown | 4.67 | |||||
| LC5 | 106729 | Tunisia | Brown | 4.67 | |||||
| LC6 | 106763 | Tunisia | Light green | 5.00 | |||||
| LC7 | 107400 | Pakistan | Dark brown | 4.17 | |||||
| LC8 | 107414 | Pakistan | Brown | 3.67 | |||||
| LC9 | 107516 | Algeria | Brown | 3.83 | |||||
| LC10 | 107546 | Algeria | Brown | 3.67 | |||||
| LC11 | 111849 | Italy | Light green | 5.17 | |||||
| LC12 | 111910 | Libya | Light green | 5.83 | |||||
| LC13 | 112114 | Morocco | Light green | 4.50 | |||||
| LC14 | Eston-type_1 | Canada | Brown | 4.17 | |||||
| LC15 | Eston-type_2 | Italy | Light green | 4.33 | |||||
Fig. 1.
Phenotypic variation (size and color) of the faba bean accessions. A: small-seeds (accession 108969); B: medium-seeds (accession 109164); C: large-seeds (accession 112082); D: green seeds (accession 112082); E: brown seeds (accession 108385); F: purple seeds (accession 110184).
Fig. 2.
Phenotypic variation (size and color) of lentil accessions. A: large light green seeds (accession 111910); B: small light green seeds (accession Eston_type_2); C: small dark-brown seeds (accession 106315); D: small brown seeds (accession 107516).
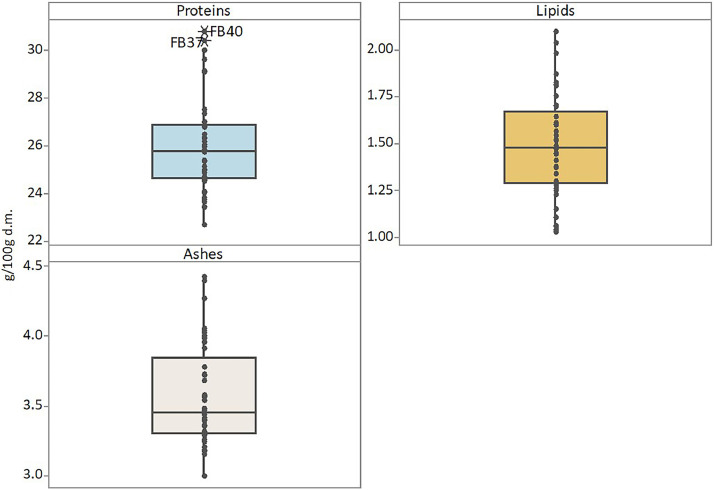
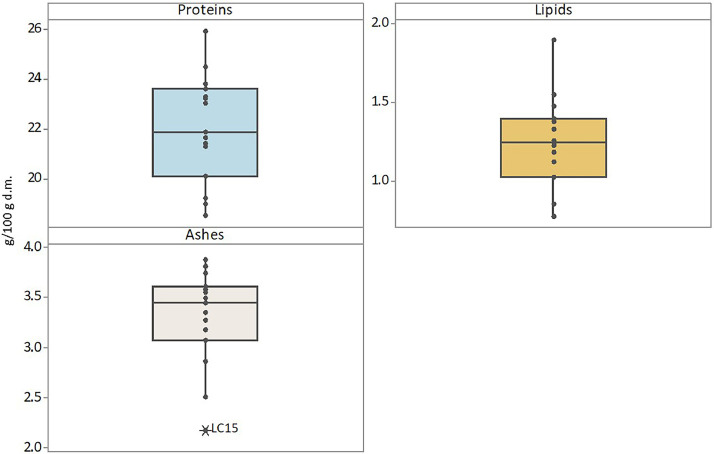
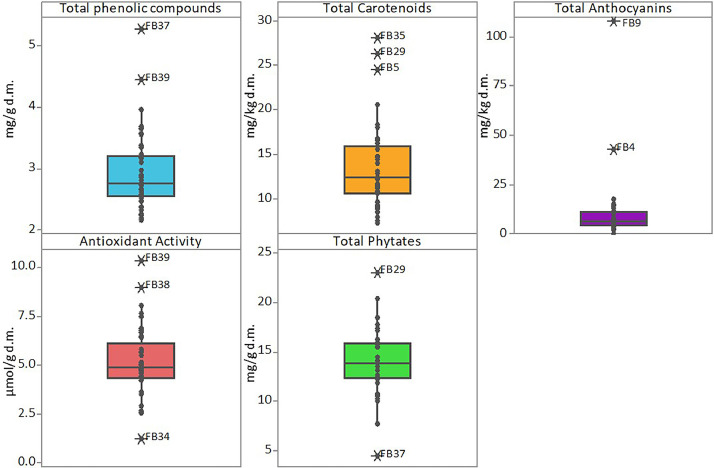
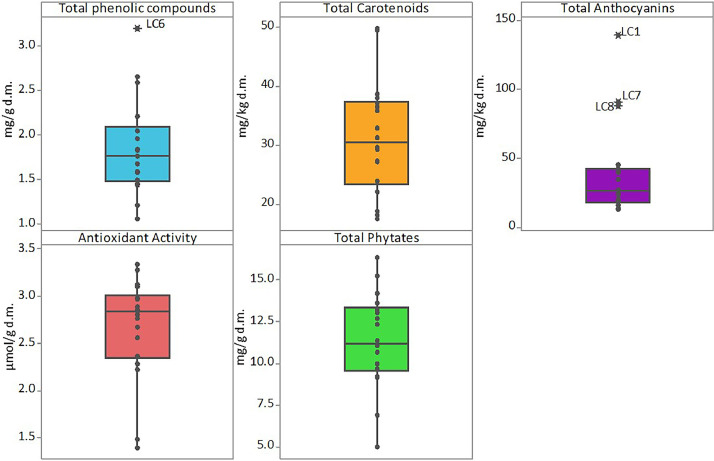
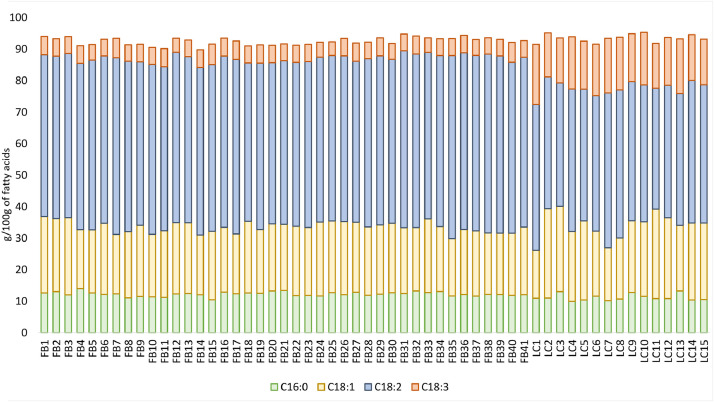
The data article also shows box plots for the protein, lipid and ash contents of faba bean (Fig. 3) and lentil (Fig. 4) accessions, and box plots of the bioactive compounds content of faba bean (Fig. 5) and lentil (Fig. 6) accessions. The outlier accessions, characterised by a peculiar composition, are highlighted in the box plots. The main fatty acids detected in faba bean and lentil accessions are shown in Fig. 7.
Fig. 3.
Box plots of protein, lipid and ash contents (g 100 g−1 of dry matter) of faba bean accessions. Outlier samples, characterised by peculiar composition, are highlighted.
Fig. 4.
Box plots of protein, lipid and ash contents (g 100 g−1 of dry matter) of lentil accessions. Outlier samples, characterised by peculiar composition, are highlighted.
Fig. 5.
Box plot of total phenolic compounds, (mg ferulic acid g−1 on dry matter), total carotenoids, (mg β-carotene kg−1 on dry matter), total anthocyanins (mg cyanidin 3-O-glucoside kg−1 on dry matter), antioxidant activity (μmol trolox eq g−1 on dry matter) and phytate content (mg phytic acid g−1 on dry matter) of faba bean accessions. Names of outlier samples are indicated in the figure.
Fig. 6.
Box plot for total phenolic compounds, (mg ferulic acid g−1 on dry matter), total carotenoids, (mg β-carotene kg−1 on dry matter), total anthocyanins (mg cyanidin 3-O-glucoside kg−1 on dry matter), antioxidant activity (μmol trolox eq g−1 on dry matter) and phytate content (mg phytic acid g−1 on dry matter) of lentil accessions. Names of outlier samples are indicated in the figure.
Fig. 7.
Main fatty acids occurring in faba bean and lentil accessions (g 100g−1 of fatty acids). C16:0 – palmitic acid; C18:1 – oleic acid; C18:2 – linoleic acid; C18:3 – linolenic acid.
Physicochemical and functional properties of the accessions are reported in Tables 2 and 3, respectively. Finally, supplementary materials report all the raw data regarding proximate composition, bioactive compounds and fatty acid composition of faba bean accessions (Supplementary Table S1) and lentil accessions (Supplementary Table S2).
Table 2.
Physicochemical (bulk density; water absorption index; water solubility index) and functional (water absorption capacity; oil absorption capacity) properties of the flour from faba bean accessions.
| Code | ID Sample | Bulk Density (g/mL) | Water Absorbtion Index | Water Solubility Index (%) | Water Absorbtion Capacity (g water/g flour) | Oil Absorbtion Capacity (g oil/g flour) |
|---|---|---|---|---|---|---|
| FB1 | 110175 | 0.78 | 6.23 | 16.01 | 1.45 | 1.07 |
| FB2 | 110195 | 0.77 | 3.91 | 18.70 | 1.37 | 1.20 |
| FB3 | 110201 | 0.78 | 4.54 | 17.20 | 1.29 | 1.11 |
| FB4 | 110184 | 0.80 | 4.14 | 18.27 | 1.15 | 1.00 |
| FB5 | 110360 | 0.82 | 3.93 | 22.87 | 1.25 | 1.09 |
| FB6 | 110356 | 0.79 | 4.38 | 15.67 | 1.32 | 1.03 |
| FB7 | 103235 | 0.77 | 4.22 | 18.45 | 1.18 | 1.08 |
| FB8 | 106555 | 0.76 | 3.90 | 20.88 | 1.24 | 1.13 |
| FB9 | 106560 | 0.77 | 4.08 | 18.07 | 1.10 | 1.00 |
| FB10 | 106951 | 0.78 | 4.04 | 19.42 | 1.18 | 0.98 |
| FB11 | 107640 | 0.78 | 4.02 | 19.08 | 1.10 | 1.10 |
| FB12 | 108425 | 0.79 | 3.93 | 18.79 | 1.19 | 1.06 |
| FB13 | 108428 | 0.76 | 4.48 | 13.58 | 1.30 | 1.07 |
| FB14 | 108430 | 0.79 | 3.94 | 19.42 | 1.29 | 1.08 |
| FB15 | 108437 | 0.80 | 4.68 | 16.08 | 1.32 | 1.06 |
| FB16 | 108438 | 0.78 | 4.07 | 20.04 | 1.12 | 1.07 |
| FB17 | 106406 | 0.84 | 4.13 | 18.43 | 1.31 | 1.07 |
| FB18 | 106375 | 0.75 | 4.02 | 18.44 | 1.30 | 1.04 |
| FB19 | 106374 | 0.77 | 4.27 | 18.05 | 1.33 | 1.04 |
| FB20 | 107537 | 0.78 | 4.11 | 17.26 | 1.23 | 1.07 |
| FB21 | 106779 | 0.78 | 4.20 | 15.44 | 1.36 | 1.05 |
| FB22 | 107547 | 0.76 | 3.94 | 18.07 | 1.30 | 1.03 |
| FB23 | 112081 | 0.77 | 4.58 | 13.32 | 1.31 | 1.02 |
| FB24 | 112082 | 0.77 | 4.16 | 17.27 | 1.25 | 1.06 |
| FB25 | 112091 | 0.76 | 3.87 | 17.85 | 1.21 | 1.08 |
| FB26 | 111894 | 0.77 | 4.36 | 16.68 | 1.17 | 1.04 |
| FB27 | 111900 | 0.81 | 3.49 | 19.87 | 1.12 | 1.00 |
| FB28 | 111894 | 0.79 | 4.25 | 16.80 | 1.28 | 0.51 |
| FB29 | 106478 | 0.81 | 6.40 | 15.10 | 1.11 | 0.98 |
| FB30 | 106458 | 0.76 | 4.36 | 15.21 | 1.29 | 1.00 |
| FB31 | 108385 | 0.77 | 4.93 | 11.93 | 1.22 | 1.05 |
| FB32 | 109167 | 0.76 | 4.16 | 17.46 | 1.12 | 1.04 |
| FB33 | 109225 | 0.76 | 4.22 | 16.64 | 1.17 | 1.06 |
| FB34 | 108947 | 0.77 | 6.71 | 13.24 | 1.47 | 1.02 |
| FB35 | 108969 | 0.80 | 3.73 | 18.50 | 1.21 | 0.99 |
| FB36 | 109270 | 0.79 | 4.23 | 17.72 | 1.07 | 0.99 |
| FB37 | 109284 | 0.79 | 6.32 | 17.55 | 1.08 | 0.95 |
| FB38 | 109282 | 0.79 | 5.86 | 17.19 | 1.18 | 1.00 |
| FB39 | 109290 | 0.83 | 5.99 | 13.10 | 1.09 | 0.97 |
| FB40 | 109163 | 0.81 | 5.91 | 15.27 | 1.08 | 0.98 |
| FB41 | 109164 | 0.80 | 5.70 | 17.75 | 1.08 | 1.01 |
Table 3.
Physicochemical (bulk density; water absorption index; water solubility index) and functional (water absorption capacity; oil absorption capacity) properties of the flour from lentil accessions.
| Code | ID Sample | Bulk Density (g/mL) | Water Absorbtion Index | Water Solubility Index (%) | Water Absorbtion Capacity (g water/g flour) | Oil Absorbtion Capacity (g oil/g flour) |
|---|---|---|---|---|---|---|
| LC1 | 106315 | 0.82 | 4.69 | 9.15 | 0.89 | 0.84 |
| LC2 | 106404 | 0.79 | 5.11 | 12.57 | 0.94 | 0.79 |
| LC3 | 106407 | 0.82 | 4.96 | 9.59 | 1.10 | 0.82 |
| LC4 | 106665 | 0.82 | 5.08 | 8.60 | 0.95 | 0.82 |
| LC5 | 106729 | 0.81 | 4.99 | 9.33 | 1.06 | 0.79 |
| LC6 | 106763 | 0.84 | 5.41 | 2.52 | 1.37 | 0.83 |
| LC7 | 107400 | 0.80 | 5.52 | 11.14 | 1.05 | 0.52 |
| LC8 | 107414 | 0.81 | 5.24 | 13.94 | 1.25 | 0.77 |
| LC9 | 107516 | 0.76 | 5.20 | 14.93 | 0.89 | 0.77 |
| LC10 | 107546 | 1.08 | 5.12 | 14.78 | 0.97 | 0.86 |
| LC11 | 111849 | 0.80 | 5.66 | 9.07 | 1.05 | 0.77 |
| LC12 | 111910 | 0.79 | 5.39 | 10.39 | 1.05 | 0.73 |
| LC13 | 112114 | 0.77 | 5.23 | 12.02 | 1.04 | 0.85 |
| LC14 | Eston-type_1 | 0.77 | 4.17 | 16.64 | 1.10 | 0.64 |
| LC15 | Eston-type_2 | 0.78 | 5.15 | 8.34 | 1.13 | 0.49 |
Supplementary material S1 reports proximate composition, the bioactive compounds content and fatty acid composition of the 41 faba bean accessions examined.
Supplementary material S2 reports the proximate composition, the bioactive compounds and the fatty acid composition of the 15 lentil accessions examined.
2. Experimental Design, Materials and Methods
2.1. Plant material and sample preparation
The germplasm collection consisted of 41 faba bean and 15 lentil accessions from the ex situ repository of the Institute of Biosciences and Bioresources of the Italian National Research Council (CNR-IBBR). The experimental plan was similar to that described in a previous data article [5]. All the plants were grown at the experimental farm “P. Martucci” of the University of Bari “Aldo Moro” (41°01′22.1′′ N 16°54′21.0′′ E) during the growing season 2017–2018, according to a randomized block design with two replicates. Each replicate was constituted by 10 individual plants. Figs. 1 and 2 show the variation of the seed phenotype for some faba bean and lentil accessions, respectively.
After harvesting at crop maturity (dry seeds), samples were carefully cleaned to remove foreign bodies and damaged seeds. Seeds were then classified on the basis of color (determined by visual observation) and for faba bean the weight of 10 seeds was determined using an analytical scale (Mettel Toledo, Columbus, OH, USA), whereas the diameter of the lentil seeds was determined by a caliper. Whole meal flour of each accession was obtained by milling the seeds with a laboratory mill (Model ETA, Vercella Giuseppe, Mercenasco, Italy) equipped with a sieve having holes of 0.6 mm [3].
2.2. Determination of proximate composition
Proximate composition was determined according to the AOAC methods 979.09, 923.03, 925.10, 945.38F, for protein (total nitrogen × 5.7), ash, moisture, and lipid contents, respectively [1]. In particular, protein content was determined by Kjeldahl method, using a DKL8 Digestor and a UDK139 distillation unit (Velp Scientifica, srl, Usmate Velate, Italy), whereas lipid content was determined by means of a Soxhlet apparatus (SER 148 extractor – Velp Scientifica srl, Usmate Velate, Italy) using diethyl ether (Merck KGaA, Darmstadt, Germany) as extracting solvent. Carbohydrate content was determined as difference.
2.3. Determination of fatty acid composition
Fatty acid composition was determined by gas-chromatographic (GC) analysis of fatty acid methyl esters on the lipid fraction extracted using diethyl ether (Merck KGaA, Darmstadt, Germany) with the Soxhlet apparatus. The analytical conditions were similar to those described in a previous data article [5]. The gas-chromatographic system used consisted of a 7890A gas-chromatograph (Agilent Technologies, Salta Clara, CA USA) equipped with a flame ionization detector and an SP2340 fused silica capillary column 60 m × 0.25 mm × 0.2 μm film thickness (Supelco Park, Bellefonte, PA, USA). The temperature of the split injector was 230 °C, with a splitting ratio of 1:50; the detector temperature was 220 °C. The oven temperature was programmed at 160 °C for 1 min, then from 160 to 200 °C, with increments of 1.3 °C min−1, hold 5 min, then from 200 to 240 °C with increments of 10 °C min−1 and final isothermal of 5 min. Helium was utilized as carrier gas at a constant flow rate of 3 mL min−1. The identification of each fatty acid was achieved by comparing the retention time with that of the corresponding methyl ester standard (Merck KGaA, Darmstadt, Germany). The results were expressed as g 100 g-1 of the lipid fraction.
2.4. Determination of bioactive compounds
The determination of bioactive compounds was described in detail in the data article by Summo et al. [5].
2.5. Physicochemical and functional properties of flours
The physicochemical (Bulk density -BD, water absorption index -WAI, and water solubility index -WSI) and functional properties (water absorption capacity -WAC, and oil absorption capacity -OAC) of flours were determined according to Du et al. [4] with the procedures reported in the previous data article by Summo et al. [5].
2.6. Analysis of data
The data were plotted by using the Minitab 17 Software (State College, PA: Minitab, Inc.) and Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA).
Ethics Statement
The analysis did not involve the use of human subjects and animal experiments.
CRediT Author Statement
Davide De Angelis: Writing- Original draft preparation, Data curation, Visualization; Antonella Pasqualone: Writing- Reviewing and Editing, Supervision; Michela Costantini: Data curation, Investigation; Luigi Ricciardi: Writing- Reviewing and Editing, Supervision; Concetta Lotti: Writing- Reviewing and Editing, Methodology; Stefano Pavan: Methodology, Writing- Reviewing and Editing; Carmine Summo: Conceptualization, Writing- Reviewing and Editing, Supervision
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
This research has been performed within the project "LEgume GEnetic REsources as a tool for the development of innovative and sustainable food TEchnological system" supported under the “Thought for Food” Initiative by Agropolis Fondation (through the “Investissements d'avenir” program with reference number ANR-10-LABX-0001-01″), Fondazione Cariplo, and Daniel & Nina Carasso Foundation. We also acknowledge the IBBR-CNR Institute for kindly providing the plant material.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2020.106660.
Appendix. Supplementary materials
References
- 1.AOAC International . 17th edition. Association of Analytical Communities; Gaithersburg, MD: 2006. Official Methods of Analysis. [Google Scholar]
- 2.AOCS . 4th ed. AOCS Press; Champaign, IL: 1993. Official Methods and Recommended Practices of the American Oil Chemists' Society. [Google Scholar]
- 3.Summo C., De Angelis D., Ricciardi L., Caponio F., Lotti C., Pavan S., Pasqualone A. Nutritional, physico-chemical and functional characterization of a global chickpea collection. J. Food Compos. Anal. 2019;84 doi: 10.1016/j.jfca.2019.103306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du S.K., Jiang H., Yu X., Jane J.L. Physicochemical and functional properties of whole legume flour. LWT Food Sci. Technol. 2014;55:308–313. doi: 10.1016/j.lwt.2013.06.001. [DOI] [Google Scholar]
- 5.Summo C., De Angelis D., Ricciardi L., Caponio F., Lotti C., Pavan S., Pasqualone A. Data on the chemical composition, bioactive compounds, fatty acid composition, physico-chemical and functional properties of a global chickpea collection. Data Brief. 2019;27 doi: 10.1016/j.dib.2019.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.