Abstract
Antimicrobial resistance to antibiotics is a serious health problem worldwide, for this reason, the search for natural agents with antimicrobial power against pathogenic microorganisms is of current importance. The objective of this work was to evaluate the antioxidant capacity (ABTS+ and DPPH), antimicrobial activity, and polyphenol compounds of methanolic and aqueous extracts of Jacaranda mimosifolia flowers. The antimicrobial activity against Bacillus cereus ATCC 10876, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC 51299, Escherichia coli ATCC 25922, Listeria monocytogenes ATCC 19115, Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium ATCC 14028, Staphylococcus aureus ATCC 25923, and Streptococcus mutans ATCC 25175, was determined using the Kirby Bauer technique. The results of polyphenolic compounds showed a high amount of total flavonoids in the methanolic and aqueous extracts (503.3 ± 86.5 and 245. 7 ± 27.8 mg Rutin Equivalents/g DW, respectively). Quercetin, gallic acid, caffeic acid, and rutin were identified by the HPLC-DAD technique, while in the GC-MS analysis, esters, fatty acids, organic compounds, as well as monosaccharides were identified. Higher antioxidant capacity was detected by the ABTS technique (94.9% and 62.6%) compared to DPPH values (52.5% and 52.7 %) for methanolic and aqueous extracts, respectively. The methanolic extract showed a greater inhibitory effect on gram-positive bacteria, with a predominant higher inhibition percentage on Listeria monocytogenes and Streptococcus mutans (86% for both). In conclusion, Jacaranda flower extracts could be a natural antimicrobial and antioxidant alternative due to the considerable amount of polyphenolic compounds, and serve as a sustainable alternative for the isolation of active ingredients that could help in agriculture, aquaculture, livestock, pharmaceutics, and other industrial sectors, to remediate problems such as oxidative stress and antimicrobial abuse.
Keywords: Jacaranda mimosifolia, Antioxidant, Antimicrobial, Phenolic compounds
Jacaranda mimosifolia; Antioxidant; Antimicrobial; Phenolic compounds.
1. Introduction
The abuse and inappropriate utilization of antibiotics to combat infectious diseases caused by microorganisms such as S. aureus, E. faecalis, S. mutans, L. monocytogenes, P. aeruginosa, and E. coli has generated an increase in antibiotic-resistant microorganisms and a diversification of resistance genes in bacteria (OMS, 2016). The immoderate use of these substances in humans, animals, and agriculture, combined with the not biodegradable characteristic of several of these compounds facilitate the transfer of resistant genes (Alós, 2015). Natural extracts are traditional components in drug development with a special use as natural preservatives that have raised as an alternative for antibiotic treatments. Some of their health-promoting properties are attributed to the presence of compounds with antimicrobial activities (Wei et al., 2013; Dai et al., 2020). The use of natural plant-derived antimicrobials can be highly effective in reducing the antibiotic dependence, minimizing the possibility of pathogen resistance to these components, and representing an alternative to cope with this global problem.
Diverse investigations have reported the antioxidant, antimicrobial, anticancer, and other biological properties of flowers and their extracts (Voon et al., 2012; Li et al., 2014; Al-Shukaili and Hossain., 2019; Karra et al., 2020). Moreover, flowers are common components in traditional medicine (Li et al., 2014) that can be gathered directly from the wild vegetation, allowing the population to take advantage of this resource (Magaña et al., 2010). Jacaranda is a member of the dicot family Bignoniaceae Juss., endemic plants of South America distributed in tropical areas throughout the world (Sánchez, 2011). Some species of this family are used in traditional medicine for the treatment of wounds, rheumatism, and colds. Additionally, its anti-malaria and antimicrobial activity have also been reported by Khamsan (2012). The objective of this work was to evaluate the antioxidant capacity (ABTS+ and DPPH), antimicrobial activity, and polyphenol compounds of methanolic and aqueous extracts of Jacaranda mimosifolia flowers. The extracts of this flower could be a potential sustainable alternative as an antimicrobial component and solution of oxidative stress problems in other organisms.
2. Materials and methods
2.1. Samples
Fresh flowers of Jacaranda mimosifolia, with no apparent physical, insect or microbial damage were collected in the months of March to May 2017 at the University Center of the Autonomous University of Querétaro, between the geographical coordinates ranging from 20° 35′ 33.7″ North latitude and 100° 24′ 44.5” West longitude. The flower petals were carefully removed (without anther, stamen or sepals) and were freeze-dried (freeze dryer Labconco FreeZone 4.5 L model 230 V, Kansas City, USA) for 48 h at -50 °C. Samples were ground, sieved (mesh size 30), sheltered from light, and stored until analysis at 4 °C.
2.2. Determination of total phenolic compounds
2.2.1. Extracts preparation
Distilled water and methanol were used as solvents for the extraction of bioactive compounds. 50 mg of Jacaranda flower sample was weighed and mixed individually with 150 ml of solvent and kept under stirring in an orbital shaker (Lab Companion, Model SI 600R Bench top shaker) at 160 rpm for 24 h at room temperature and dark conditions (Cardador-Martínez et al., 2002). The methanol extracts were rota-evaporated and aqueous extracts were lyophilized.
2.2.2. Determination of total phenolic content, tannins and total flavonoids
The Folin-Ciocalteu method (Singleton and Rossi, 1965), modified for use in 96-well microplate, was used for total phenolic content determination of flower extracts. The total phenolic content was expressed as mg Gallic Acid Equivalents (GAE)/g flower Dry Weight (DW). Condensed tannins were quantified at 492 nm (MULTISKAN GO, Thermo Fisher Scientific, Finland) and expressed in mg (+) Catechin Equivalents (CE)/g DW as described by Feregrino-Pérez et al. (2008). Total flavonoids determination was carried out using the methodology of Oomah (2005) where samples were measured at 404 nm and expressed as mg of Ruthin Equivalents/g DW.
2.3. Phytochemical profiling
2.3.1. HPLC-DAD phenolic compound analysis
Analyses of phenolic compounds were performed on an Alliance 2695LC system (Waters) equipped with a 2998 diode array detector (DAD) set at 220–540 nm detection range, and a Symmetry C18 reverse phase column (100 × 4.6mm) (Waters). The eluent was a mixture of solvent A (water acidified with 0.1% formic acid) and solvent B (acetonitrile grade HPLC). A 30 μl volume of the phenolic extract was filtered through a 0.4 μm pore diameter syringe filter prior injection. Elution gradient was 5%–40% (B) from 0 to 18 min, 40%–90% (B) from 18 to 20 min and 90% (B) from 20 to 24 min at a flow rate of 0.5 mL/min and 35 °C. Individual compound identification and quantification was obtained by comparison of retention times and using a calibration curve of pure standard.
2.3.2. Gas-chromatography-mass spectrometry (GC-MS) analysis
A GC–MS system (Agilent GC Series 7890A and Agilent single quadrupole MS detector model 5975C), with electron impact model (70 eV), a mass range of 50–700 m/z, HP-5MS capillary column (30 m × 0.25 mm i.d. x 0.25 μm), and splitless injection (at 250 °C) to 2.5 min/splitless time, was used for metabolite identification. The GC–MS control and data processing was performed by comparing their mass spectra using the Chem-Station (Agilent Technologies) software. Metabolite identification was analyzed through the spectral matching (MS) with commercialized libraries (NIST) based on the retention time, m/z, percentage of spectral similarity, and the internal standard.
2.4. Determination of antioxidant capacity
2.4.1. DPPH method
DPPH antioxidant capacity (2,2-Diphenyl-1-picrylhydrazyl) was determined with the methodology described by Williams (1995) and modified by Fukumoto and Mazza (2000). The experiments were performed in triplicate. An absorbance of 520 nm was used at a time of 30 min. The results were expressed as the percentage of antiradical activity (%ARA) based on the following formula: %ARA = [(A.Control)–(A.Sample)/A.Control]∗100 where A.Control represents the absorbance of the DPPH solution and A.Sample represents the absorbance of the sample with DPPH solution.
2.4.2. ABTS method
ABTS (2,2′-azino-bis-(3-ethyl benzothiazolin-6-ammonium sulphonate)) assay was performed according to the method described by Nenadis et al. (2004). It was measured at 734 nm (MULTISKAN GO, Thermo Fisher Scientific, Finland), analyzing the samples in triplicate. The results were expressed as the percentage inhibition of ABTS based on the following formula: %ABTS_inhibition = [(A.Control)-(A.Sample)/A.Control]∗100 where A.Control represents the absorbance of the ABTS solution and A.Sample represents the absorbance of the sample with ABTS solution.
2.5. Antimicrobial activity
2.5.1. Microorganism and growth conditions
Nine types of microorganisms were obtained from the collection of the Microbiology Laboratory, School of Chemistry, of the Autonomous University of Queretaro for the evaluation of the antibacterial activities of the flower extracts (methanol and aqueous). Bacillus cereus ATCC 10876, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC 51299, Listeria monocytogenes ATCC 19115, Staphylococcus aureus ATCC 25923, Streptococcus mutans ATCC 25175 (gram-positive), Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Salmonella typhimurium ATCC 14028 (gram-negative) were standardized to a concentration of 108 CFU/mL for the antibacterial susceptibility test.
2.5.2. Antimicrobial potential
Both methanol and aqueous extracts were used to evaluate the antimicrobial potential by using the modified agar disk diffusion method (Kirby et al., 1957; Washington, 1981) against a culture of pathogenic gram-positive and gram-negative bacterial strains. Four spots were uniformly placed inside the sensidisks: (1) methanol extract, (2) aqueous extract, (3) positive control (160 mg of Gentamicin), and (4) negative control (water or methanol).
Bacterial culture (100 μL) was inoculated into test tubes with molten Müller-Hinton agar at 45 °C. Afterward, the inoculation was placed into the plates according to the previously described arrangement. Six-diameter disks of sterilized Whatman filter paper were impregnated with 25 μL of the aqueous or methanol extracts (which corresponds to 20 and 145 equivalent mg of Gallic acid, for each extract, respectively), dried, and then, gently pressed on the inoculated agar plates. Subsequently, the plates were incubated at 37 °C for 24 h. The experiment was replicated three times. The inhibition halo was calculated by measuring the inhibition diameter and using the following formula of percent inhibition for each bacteria for each treatment: %inhibition = [(Y-Z)/Y]∗100 where Y represents the growth of the bacteria without extract, Z represents the growth of bacteria with extract.
2.6. Statistical analysis
The results were presented as the mean ± standard error. Data were analyzed using a one-way ANOVA to determine significant differences. When differences were found, a mean comparison through a Tukey test was performed for the amount of polyphenolic compounds, antioxidant capacity, and the inhibition halo diameter between microorganisms. A Dunnet test was performed to find statistical difference between the methanol and aqueous extracts with respect to the positive control (Gentamicin).
3. Results and discussion
3.1. Determination of total phenolic content, tannins and total flavonoids
Plants have a secondary metabolism responsible for the biosynthesis of compounds called secondary metabolites which are involved in various non-essential processes, as stress adaptation, plant-to-plant communication, and pollinator attraction. Phenols, flavonoids, and condensed tannins, among other compounds, are examples of secondary metabolites. Phenols are organic compounds that contain at least one phenolic group and an aromatic ring attached to a hydroxyl group. Various biological activities, such as antioxidant, antibacterial, and antifungal, have been attributed to these specialized compounds (Li et al., 2016; Liu et al., 2016; Dai et al., 2020). The antimicrobial activity of phenolic compounds has been related to their ability to denature proteins and modify extracellular pH and hydrophobicity, considering them as surfactants (Büsra Konuk and Ergüden, 2020). The condensed tannin and flavonoids are the most important plant pigments for flower coloration. They are a subclass of polyphenols with an antioxidant, anti-inflammatory, antiplatelet, antiviral, antibacterial, vasodilator, as well as an antiallergic, and hepatoprotective properties, making these components a focus of scientific interest as possible adjuvants in treatments of a wide variety of diseases including those of microbial origin (Álvarez and Orallo, 2003). Additionally, flavonoids have inhibitory activity against diverse pathogens in plants, expanding their possible applications to the agricultural sector (Faggio et al., 2017).
Jacaranda mimosifolia flowers have a higher amount of total phenols, expressed as mg Gallic acid equivalent (GAE)/g DW (Table 1), compared to seed oil (0.02793 mg GAE/g) reported in Van-Nieuwenhove et al. (2019) and compared to the aqueous extracts of flowers reported by Rana et al. (2013) (49.80 mg GAE/g), both of the same species. Similarly, the results reported in the present research for total phenols are higher than those reported by Li et al. (2014) for 51 types of edible and wildflowers, with ranges from 0.13 to 11.48 mg GAE/g DW for the water-soluble and fat-soluble fractions, additionally, the total phenolic contents varied from 0.50 to 24.36 mg GAE/g DW. The same trend was observed for Jambu (Acmella olerarea) flowers, which are used in Amazon food and medicine, for both, in a dry and wet basis (5.65 ± 0.44 and 0.58 ± 0.04 mg GAE/g DW, respectively) (Andrade et al., 2018). Mak et al. (2013) showed lower results for ethanol and aqueous of hibiscus flower extracts (45.98 ± 1.07 and 54.36 ± 1.69 mg GAE/g DW, respectively), while for Cassia flower extracts (262.24 ± 4.50 and 94.68 ± 1.92 mg GAE/g DW, respectively) the results were superior compared to J. mimosifolia flowers (Table 1). The intrinsic (genetic, extracting solvent) and extrinsic (environmental, handling and development stage) factors have a strong influence on the production of phenolic compounds of plants (Fratianni et al., 2007).
Table 1.
Quantification of polyphenolic compounds in the methanol and aqueous extracts of Jacaranda mimosifolia flowers.
| Total Phenols (mg GAE/g DW) | Condensed Tannin (mg CE/g DW) | Total Flavonoids (MG Ruthin/g DW) | |
|---|---|---|---|
| Methanol extract | 64.94 ± 10.45A | 0.360 ± 0.098B | 503.34 ± 86.55A |
| Aqueous extract | 78.50 ± 4.76A | 1.335 ± 0.096A | 245.72 ± 27.88B |
Superscript letters per column are found to be significantly different using ANOVA and the Tukey test at a significant level p < 0.05.
The amount of tannins differed significantly depending on the solvent medium, obtaining minor concentration for the methanol extract in comparison to the aqueous extract (Table 1). The concentration of tannins presented by the Jacaranda mimosifolia flowers is lower than those presented by other types of hibiscus flowers (2849.4 ± 121.1 and 4420.9 ± 110.7 mg CE/100 g DW), and Cassia flowers (1779.8 ± 139.2 and 103.3 ± 6.9 mg CE/100 g DW for ethanol and aqueous extracts, respectively) (Mak et al., 2013). The same trend is observed for Limonium delicatulum flowers for methanol and aqueous extracts (48.38 ± 0.70 and 8.14 ± 0.44 mg CE/g DW, respectively) (Medini et al., 2014), presenting higher values than these of flowers of J. mimosifolia. The presence of tannins can contribute to the elimination of free radicals depending on the number of aromatic rings, molecular weight, and nature of the hydroxyl group substitution (Cai et al., 2006).
The concentration of flavonoids is higher than the concentration of the other evaluated polyphenols (Table 1). The presence of flavonoids has been described in several investigations with extracts of flowers of the same species. Rana et al. (2013) reported 8.90 mg/g in the aqueous fraction of extracts with organic solvents, whereas Medini et al. (2014) reported 5.55 ± 2.95 and 2.15 ± 0.16 mg Ruthin/g DW for methanol and aqueous extracts of L. delicatulum flowers, respectively. Furthermore, it is observed that the amount of flavonoids is greatly influenced by the type of solvent medium used for the extraction. Flavonoids, the major group of phenolic compounds, contain a C6–C3–C6 skeleton and are by far the most diverse and common secondary metabolites in plants. Diverse modifications to the C3, such as hydroxylation, methylation, prenylation, and glycosylation, can occur to the baseline flavonoid molecules, producing a large number of flavonoids with diverse structures and different properties (Zha et al., 2019). Epidemiological studies indicate that flavonoids have health benefits (Gutiérrez-Venegas et al., 2019). The affection of cell membrane synthesis, nucleic acid inhibition, and metabolism of various bacterial strains provides flavonoids with bactericidal and bacteriostatic characteristics (Muhammad et al., 2019). However, the best-known property of flavonoids is the antioxidant effects, which are often much higher than those of vitamin E and vitamin C (Prior and Cao, 2000).
3.2. Phytochemical profiling
Metabolic profiling is a tool for screening the bioactive compounds and for the elucidation of regulative principles and pathways. Phenolic compounds are the base for the formation of different metabolites. Fatty acids and phytosterols are also related to the biosynthesis of metabolic pathways.
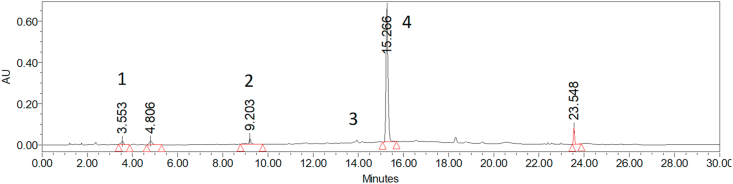
For the HPLC analysis, gallic acid, caffeic acid, rutin, and quercetin (in elution order) were identified by comparison of the standard retention time and the UV spectra, and then quantified in the extract. Quercetin was the most abundant compound as shown in Figure 1 and Table 2. Diverse studies indicate that quercetin may have antioxidant, anti-inflammatory, anticancer, and gastroprotective activities (Bathori et al., 2004; Kanadaswami et al., 2005). Additionally, Li and Xu (2008), demonstrated the ability of some lotus leaf extracts with high concentration of quercetin to inhibit the growth of oral pathogenic bacteria. Rauha et al. (2000) reported antimicrobial effects of Finnish plant extracts containing phenolic compounds and other flavonoids as quercetin, quercetin-glycosides and derives.
Figure 1.
HPLC chromatograms of phenolic compounds identified in Jacaranda mimosifolia Flowers (220–540 nm): 1) gallic acid, 2) caffeic acid, 3) rutin and 4) quercetin.
Table 2.
Compounds identified in Jacaranda flowers by HPLC-DAD.
| Peak | Compound | RT (min) | λ max (nm) | mg/g DW of flower sample |
|---|---|---|---|---|
| 1 | Gallic acid | 3.55 | 219, 27 | 6.35 |
| 2 | Caffeic acid | 9.20 | 240, 32 | 3.00 |
| 3 | Rutin | 13.93 | 254, 35 | Traces |
| 4 | Quercetin | 15.27 | 266, 34 | 83.5 |
For the GC-MS analysis, the screening of the J. mimosifolia extracts resulted in the presence of esters, organic compounds, fatty acids, monosaccharides, carotenoids and phytosterols. The mass spectrum range of the components was compared with the compound registry of the NIST and CAS library where 54 components were identified. The names of the recognized compounds, molecular weight, retention time, and concentration are presented in Table 3.
Table 3.
Identified compounds in Jacaranda flowers by GC-MS.
| No. | Compound | %∗ | m/z | NIST/CAS | M.wt | RT |
|---|---|---|---|---|---|---|
| 1 | Silane, methoxytrimethyl | 56.7 | 39 | CAS: 1825-61-2 | 104 | 1.45 min |
| 2 | Acetamide, 2,2,2-trifluoro-N-(trimethylsilyl)- | 71.5 | 119 | CAS: 21149-38-2 | 257 | 2.40 min |
| 3 | Trisiloxane, octamethyl- | 34.4 | 103 | CAS: 107-51-7 | 236 | 2.60 min |
| 4 | Cystathionine, bis(trimethylsilyl) ester | 29.4 | 215 | CAS: 73090-79-6 | 366 | 3.27 min |
| 5 | L-Asparagine, N, N2-bis(tert-butyldimethylsilyl), tert-butyldimethylsilyl ester | 10.4 | 156 | CAS: 96381-41-8 | 474 | 4.34 min |
| 6 | Phosphoric acid, tris(trimethylsilyl) ester | 97.6 | 76 | CAS: 10497-05-9 | 314 | 5.17 min |
| 7 | Hydroquinone bis(trimethylsilyl) ether | 58.6 | 167 | CAS: 2117-24-0 | 254 | 6.84 min |
| 8 | [1,1′-Bicycloropropyl]-2-octanoic acid, 2′-hexyl-, methyl ester | 52.7 | 155 | CAS: 56687- | 322 | 7.65 min |
| 9 | Butanedioic acid, [(trimethylsilyl)oxy]-, bis(trimethylsilyl)ester | 88.6 | 67 | CAS: 38166-11-9 | 350 | 8.34 min |
| 10 | L-Proline, 5-oxo-1-(trimethylsilyl)-, trimethylsilyl ester | 79.3 | 83 | CAS: 30274-77-2 | 273 | 8.97 min |
| 11 | Mannosamine | 17 | 119 | NIST: 127688 | 179 | 9.11 min |
| 12 | Benzoic acid, 3-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 21.3 | 94 | CAS: 3782-84-1 | 282 | 9.55 min |
| 13 | α-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate | 16.9 | 236 | CAS: 54477-01-9 | 331 | 10.63 min |
| 14 | Benzoic acid, 4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 72.8 | 77 | CAS: 27750-57-8 | 296 | 10.88 min |
| 15 | α-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate | 17.8 | 236 | CAS: 54477-01-9 | 331 | 11.22 min |
| 16 | 4-Trimethylsilyloxy-4-trimethylsilyloxycarbonylmethyl-2,5-cyclohexadiene-1-one | 96.4 | 108 | NIST: 280345 | 312 | 11.57 min |
| 17 | Pregn-5-en-20-one, 3-(acetyloxy)-16, 17-epoxy-6-methyl-, (3β, 16α)- | 20.2 | 270 | CAS: 55349-94-5 | 386 | 13.00 min |
| 18 | α-D-Galacotopyranose, 1,2,3-tris-O-(trimethylsilyl)-, cyclic methylboronate | 12 | 228 | CAS: 56196-95-3 | 420 | 13.31 min |
| 19 | Dihydroxyacetone dimer, tetra (trimethylsilyl)- | 28.2 | 85 | CAS: 28527-65-3 | 468 | 13.44 min |
| 20 | D-Psicofuranose, pentakis (trimethylsilyl) ether (isomer 2) | 14.1 | 202 | NIST: 380128 | 540 | 13.66 min |
| 21 | Glucopyranoside, methyl 2,3,5,6-tetrakis-O-(trimethylsilyl)-, α-D- | 27.2 | 130 | CAS: 6736-96-5 | 482 | 13.90 min |
| 22 | D-(-)-Fructofuranose, pentakis (trimethylsilyl) ether (isomer 1) | 18.4 | 208 | NIST:380166 | 540 | 14.11 min |
| 23 | L-(-)-Sorbofuranose, pentakis (trimethylsilyl) ether | 11.2 | 168 | NIST: 380164 | 540 | 14.25 min |
| 24 | D-(-)Ribofuranose, tetrakis (trimethylsilyl) ether (isomer 1) | 14.3 | 148 | NIST: 380115 | 438 | 14.28 min |
| 25 | D-Xylopyranose, 1,2,3,4-tetrakis-O-(trimethylsilyl)- | 4.74 | 95 | CAS: 55555-45-8 | 438 | 14.63 min |
| 26 | Glucofuranoside, methyl 2,3,5,6-tetrakis-O-(trimethylsilyl)-α-D- | 21.7 | 130 | CAS: 6736-96-5 | 482 | 14.73 min |
| 27 | 1,5-Anhydro-D-sorbitol, tetrakis (triemethylsilyl) ether | 21.7 | 230 | NIST: 380138 | 452 | 15.11 min |
| 28 | 1,2,3-Propanetricarboxylic acid, 2-[(trimethylsilyl)oxy]-,tris (trimethylsilyl) ester | 13.4 | 70 | CAS: 14330-97-3 | 480 | 15.22 min |
| 29 | Ribitol, 1,2,3,4,5-pentakis-O-(trimethylsilyl)- | 15.9 | 185 | CAS: 32381-53-6 | 512 | 15.33 min |
| 30 | β-D-(+)-Talopyranose, pentakis (trimethylsilyl) ether | 6.08 | 151 | NIST: 380171 | 540 | 15.57 min |
| 31 | 14-Methyl-pentadecane1,2-diol, bis(trimethylsilyl) ether | 24.4 | 132 | NIST: 336515 | 402 | 16.12 min |
| 32 | Digitoxin | 9.76 | 258 | CAS: 71-63-6 | 764 | 16.69 min |
| 33 | Galactopyranose, 1,2,3,4,6-pentakis-O-(trimethylsilyl)-, β-d- | 7.07 | 35 | CAS: 1769-00-2 | 540 | 17.03 min |
| 34 | Hexdecanoic acid, trimethylsilyl ester | 96.1 | 157 | CAS: 55520-89-3 | 328 | 17.48 min |
| 35 | Tetradecane, 2,6,10-trimethyl- | 16.9 | 112 | CAS: 14905-56-7 | 240 | 18.22 min |
| 36 | Heptadecanoic acid, 10-methyl-, methyl ester | 35.4 | 169 | CAS: 2490-25-7 | 298 | 18.63 min |
| 37 | Silane, [(3,7,11,15-tetramethyl-2-hexadecenyl)oxy]trimethyl- | 60.3 | 77 | CAS: 57397-39-4 | 368 | 19.32 min |
| 38 | Imidazole, 2-fluoro-1-triacetylribofuranosyl- | 16.4 | 232 | NIST: 1292585 | 344 | 19.35 min |
| 39 | 9,12-Octadecadienoic acid (Z,Z)-, trimethylsilyl ester | 90.4 | 122 | CAS: 56259-07-5 | 352 | 19.74 min |
| 40 | α-Linoleic acid, trimethylsilyl ester | 84.8 | 127 | CAS: 97844-13-8 | 350 | 19.84 min |
| 41 | Octadecanoic acid, trimethylsilyl ester | 94.3 | 211 | CAS: 18748-91-9 | 356 | 20.12 min |
| 42 | E-8-Methyl-9-1-ol-acetate | 22.3 | 169 | NIST: 130814 | 268 | 20.70 min |
| 43 | α-D-Glucopyranosiduronic acid, 3-(5-ethylhexahydro-2,4,6-trioxo-5-pyrimidinyl)-1,1-dimethylpropyl 2,3,4-tris-O-(trimethylsilyl)-, methyl ester | 29.6 | 340 | CAS: 55556-81-5 | 648 | 21.02 min |
| 44 | 9-Octadecenamide, (Z)- | 49.7 | 128 | CAS: 301-02-0 | 281 | 21.62 min |
| 45 | Erucic acid | 8.72 | 188 | CAS: 112-86-7 | 338 | 23.21 min |
| 46 | 9,12,15-Octadecanouc acid, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl]ethyl ester, (Z,Z,Z)- | 44.2 | 189 | CAS: 55521-23-8 | 496 | 23.561min |
| 41 | Estra-1,3,5 (10)trien-17β-ol | 62.1 | 168 | CAS: 2529-64-8 | 256 | 24.05 min |
| 42 | 2-Monopalmitoylglycerol trimethylsilyl ether | 57 | 229 | CAS: 53212-97-8 | 474 | 24.44 min |
| 43 | Hexadecanoic acid, 2,3-bis [(trimethylsilyl)oxy]propyl ester | 97.9 | 164 | CAS: 1188-74-5 | 474 | 25.06 min |
| 44 | Docosanoic acid, trimethylsilyl ester | 53.7 | 140 | CAS: 74367-36-5 | 412 | 25.80min |
| 45 | 1-Monolinoleoylglyccerol trimethylsilyl ether | 58.4 | 230 | CAS: 54284-45-6 | 498 | 27.94 min |
| 46 | 2-Monostearin trimethylsilyl ether | 77 | 161 | CAS: 53336-13-3 | 502 | 28.74 min |
| 47 | 1-Monolinoleoylglyccerol trimethylsilyl ether | 77.6 | 230 | CAS: 54284-45-6 | 498 | 28.92 min |
| 48 | 9-Octadecenamide, (Z)- | 55.3 | 124 | CAS: 301-02-0 | 281 | 29.20 min |
| 49 | Octadecanoic acid, 2,3-bis [(trimethylsilyl)oxy]propyl ester | 95.9 | 129 | CAS: 1188-75-6 | 502 | 29.72 min |
| 50 | Squalene | 61.1 | 96 | CAS: 111-02-4 | 410 | 30.66 min |
| 51 | 2-Monopalmitoylglycerol trimethylsilyl ether | 58.1 | 229 | CAS: 53212-97-8 | 474 | 32.77 min |
| 52 | Eicosane, 7-hexyl | 20.3 | 179 | CAS: 55333-99-8 | 366 | 33.03 min |
| 53 | Octadecanoic acid, 2,3-bis [(trimethylsilyl)oxy]propyl ester | 33.2 | 313 | CAS: 1188-75-6 | 502 | 34.27 min |
| 54 | (+)-α-Tocopherol, O-trimethylsilyl- | 62.7 | 259 | CAS: 2733-26-8 | 502 | 37.97 min |
The reported percentage corresponds to the percentage of similarity produced by the software library of the mass spectrometer.
Mostafa et al. (2014) identified different classes of phytochemicals as fatty acids, sterols and flavonoids in some Jacaranda species. In the present research, the most abundant identified compounds in the extracts of J. mimosifolia were esters, however, there is a considerable portion of fatty acids such as Erucid acid, which has been reported in the Brassicaceae family, specifically in E. sativa. Gulfraz et al. (2011) reported that the Erucic acid present in seeds of E. sativa, in both free and tryglyceride form, are responsible for the antimicrobial activity in Gram-negative and Gram-positive bacteria. In addition, there are also compounds of interest such as Digitoxin, Imidazole, and α-Tocopherol. Digitoxin is a cardiac glycoside with a potent antiviral and cancer cell growth inhibition activities (Cai et al., 2014). Imidazole is a heterocyclic ring and a structural part of some alkaloids that confers antifungal and antioxidant activity (Shalini et al., 2010) and α-Tocopherol is an important natural antioxidant with antiproliferative and protein kinase C-supressing effects (Engin 2009). Due to the diverse compounds found with the GC-MS analysis, the phenolic profile of the Jacaranda extract suggests that flavonoid quercetin in conjunction with esters, fatty acids, and other organic compounds could be responsible for the biological activities found in this study.
3.3. Determination of antioxidant capacity
The antioxidant activity in the methanol and aqueous extracts was evaluated with the most used methods to analyze antioxidant activity in vitro, ABTS and DPPH (Table 4). The methanol extract of J. mimosifolia flower showed a higher inhibition percentage on the ABTS radicals compared to the DPPH method with no significant difference between both extracts. These results are consistent with those reported by Rana et al. (2013) for aqueous extracts of flowers of the same species. The Jacaranda flower extracts presented a greater antioxidant effect, compared to other extracts of plants used within gastronomy and traditional medicine such as chia (Salvia hispanica) (2.4%–66.3% inhibition of ABTS and 4.7%–47.6% inhibition of DPPH) (Poungoué-Kaatou, 2006), saffron (15.7% inhibition of DPPH) (Gallo, 2010), and chamomile flower (14.5% inhibition of DPPH), taking into consideration that the difference in the inhibition percentage is also attributed to the used concentrations, type of extraction, environmental conditions and type of evaluated species (Salazar, 2009). A high concentration of polyphenols in the sample, mainly of the flavonoid type, contributes to greater efficiency in the elimination of free radicals. Mikolajczak et al. (2020) demonstrated a positive correlation (r = 0.75) between flavonoid content and antioxidant activity in various edible flowers. Thus, natural antioxidants can act as free radical scavengers, chain breakers, pro-oxidant metal ion complexes, and singlet-oxygen formation inhibitors (Tosun et al., 2011). On the other hand, recent studies have reported a greater amount of phenolic compounds, and therefore a greater antioxidant activity, in some flowers than other fruits and vegetables (De Morais et al., 2020). Phenolic compounds, in addition to their antioxidant capacity, have been proven a beneficial role as antimicrobial components and gene expression regulators involved in inflammation processes (Gallo, 2010).
Table 4.
Determination of antioxidant capacity by ABTS and DPPH methods in methanol and aqueous extracts of Jacaranda mimosifolia flowers.
| ABTS (% Inhibition) | DPPH (% ARA∗) | |
|---|---|---|
| Methanol extract | 94.91 ± 0.01A | 52.51 ± 0.31A |
| Aqueous extract | 62.63 ± 0.06B | 52.77 ± 0.01A |
ARA = anti-radical activity. Extract concentration: 1 g DW/10 mL of solvent. Superscript letters per column are found to be significantly different using Tukey test at a significant level p < 0.05.
3.4. Antimicrobial activity
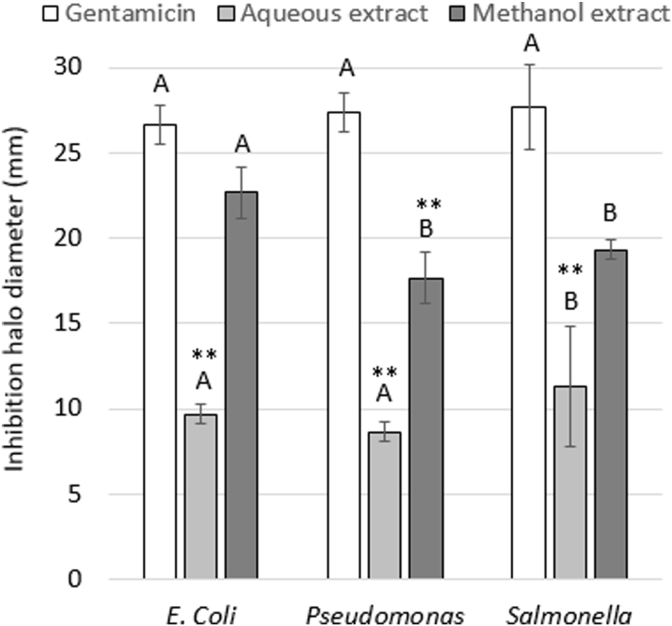
Figure 2 and Figure 3 show the diameter of the inhibition halos generated by the methanol and aqueous extracts of Jacaranda mimosifolia flowers against gram-positive and gram-negative microorganisms, respectively. The positive control (Gentamicin) was taken as a reference to determine the antimicrobial activity, presenting a diameter of the inhibition halo ranging from 26 to 33 mm depending on each of the evaluated microorganisms. Gentamicin is a broad-spectrum aminoglycoside antibiotic which action mechanism is through active transport, crossing the cellular membrane of susceptible bacteria and irreversibly binding to the 30S ribosomatic subunits, preventing the start of protein synthesis, and inducing cell death (Yoshizawa et al., 1998).
Figure 2.
Diameter of the inhibition halo generated by the methanol and aqueous extracts of Jacaranda mimosifolia flowers against gram-positive microorganisms. The results express the average of four independent experiments with three replicates each. Different letters per bar color indicate statistical difference between microorganisms (Tukey Test p > 0.05). ∗∗ Indicates statistical difference with respect to the positive control (Gentamicin) (Dunnet Test p > 0.05).
Figure 3.
Diameter of the inhibition halo generated by the methanol and aqueous extracts of Jacaranda mimosifolia flowers against gram-negative microorganisms. The results express the average of four independent experiments with three replicates each. Different letters per bar color indicate statistical difference between microorganisms (Tukey Test p > 0.05). ∗∗ Indicates statistical difference with respect to the positive control (Gentamicin) (Dunnet Test p > 0.05).
The methanol extract showed greater antimicrobial activity with a range of 17.66–28.33 mm of inhibition halo, compared to the aqueous extract with a range of 8.66–16.33 mm) for all the evaluated microorganisms. On the other hand, the gram-positive microorganisms presented a larger diameter of the inhibition halo compared to the gram-negative for both methanol and aqueous extracts. The most susceptible gram-negative bacterias were E. coli (22.66 ± 1.52 mm) for methanol and S. typhimurium (11.33 ± 3.51 mm) for aqueous extracts. These results show that Jacaranda mimosifolia extracts could be a potential resource to avoid resistance to antibiotics, a recurrent health problem, principally in gastrointestinal diseases caused by toxins of these bacteria. For gram-positive, the most susceptible were B. ceureus (28.33 ± 3.51 mm) for methanol and S. aureus (16.33 ± 3.51 mm) for aqueous extracts. Species of the genus Jacaranda are part of the folklore and ethnobotanical practices in certain areas of South America. The flowers of this tree are used as medicinal treatments and have been attributed with anti-inflammatory, anti-parasitic, anti-fungal, and anti-cancer properties (Hussain et al., 2007), while other species in this genus have been used to treat both viral and bacterial infections (Gachet and Schühly, 2009). Pereira et al. (2015) reported an inhibition halo of 96.9% and 96.6% on Bacillus cereus and S. aureus, respectively, using an ethanol extract of leaves of J. oxyphylla. Zatta et al. (2009) evaluated the antibacterial activity of Jacaranda decurrens leaves against 25 strains of Pseudomonas aeruginosa, an opportunistic pathogen that causes respiratory tract infections, showing significant inhibitory activity. On the other hand, J. mimosifolia is part of the traditional medicine in Ecuador, used to purify the blood and treat venereal diseases (Acosta-Solís, 1992). Hendra et al. (2018) evaluated nine metabolites isolated from J. mimosifolia flowers, including phenylethanoid β-D-glucopyranose, a new compound, which did not presented inhibitory activity against on S. aureus, E. coli, Klebsiella penumoniae, P. aeruginosa, Acinetobacter baumannii, Candida albicans and Cryptococcus neoformans at a low concentration (32 μg/ml). Furthermore, the results of the present work coincide with the results of Rojas et al. (2006), which reported inhibitory activity against B. cereus and E. coli for aqueous extract of J. mimosifolia leaves.
Figure 4 shows the inhibition percentages of bacterial growth. The methanol extracts presented higher percentages compared to the aqueous extracts. The results showed the following susceptibility of the microorganisms against the methanol extract of J. mimosifolia flower; Listeria = S. mutans > B. cereus = E. coli > B. subtilis > E. faecalis > Salmonella > S. aureus > Pseudomonas, presenting inhibition percentages ranging from 86.4 to 64.6%. In contrast, lower ranges were observed for the aqueous extract of J. mimosifolia flower (52.3%–31.7%), with the following trend; B. subtilis > B. cereus > S.aureus > Salmonella = S. mutans > Listeria > E. faecalis > E. coli > Pseudomonas.
Figure 4.
Inhibition percentage of the methanol and aqueous extracts of Jacaranda mimosifolia flowers against microorganisms of interest. White bars indicate percentage inhibition for methanol extract and gray bars correspond to the aqueous extract. Gentamicin was used as a positive control (black bar), presenting a minimum or null bacterial growth. Negative controls (methanol and water) showed no inhibition halos. The results express the average of three independent experiments with three replicates each. Different letters per bar color indicate statistical difference between microorganisms (Tukey Test p > 0.05).
The results of the methanolic extract of J. mimosifolia flower are relevant since the percentage of inhibition on Listeria monocytogenes and Streptoccus mutans (86.4% in both cases) qualifies it as an alternative of natural antimicrobial to prevalent health problems worldwide. Several studies on the antibacterial activity of plant extracts and plant parts on Listeria monocytogenes have been performed; this pathogen is responsible for listeriosis, a disease with a high mortality rate (20%) in risk populations and considered a major food safety problem worldwide (Pouillot et al., 2012; Ceruso et al., 2020). Some of these investigations attribute the inhibitory effect on L. monocytogenes to the presence of phytochemical compounds, such as alkaloids, polyphenolic compounds, gallic acid, glycosides, tannic acid, flavonoids and sterols (Batool et al., 2018). Following this line, in the present work, 86.4% of inhibition for Streptococcus mutans was found with the methanol extract of the Jacaranda flower. This information is significant since S. mutans is present in the multifactorial process that originates dental caries, and this might be the first assay of J. mimosifolia extracts on this microorganism as no previous reports were found. The high concentration of flavonoid-type compounds reported in this work could be responsible for the inhibitory effect on these types of microorganisms. Gutiérrez-Venegas et al. (2019) tested various polyhydroflavones against a group of microorganisms related to the dental caries process where bacteria and fungi were included by measuring the inhibition diameter; in all cases, a bacteriostatic effect was observed on the evaluated bacteria and fungi. In recent years, research related to the formation of anti-cariogenic biofilms has been carried out using polyphenolic compounds, mainly flavonoids, observing an inhibitory effect on the adhesion of hydroapatite pellets treated with proanthocyanidins exposed to Streptococcus, resulting in a decrease of hydrophobicity in bacteria depending on the concentration of various flavonoids, increase of membrane permeability of bacteria, as well as decrease of acids produced by S. mutans in biofilms with the flavonoid apigenin (Koo et al., 2002, 2003).
In particular, part of the antimicrobial activity has been attributed to the phenolic compounds present in the extracts from plants. Studies on the chemical components presented in J. mimosifolia indicate the presence of triterpenes, quinones, fatty acids, flavonoids, and acetosides, a novel phenylethanoid dimmer (Moharram and Marzouk, 2007). Li et al. (2017) indicated that the most common compounds that act as antimicrobials include the members of the flavonoids family which are activated and/or synthesized in the presence of biotic and abiotic stress. On the other hand, Mori et al. (1987) showed that the presence of hydroxyl groups in 3 ′, 4′ and 5′of the B ring and in C3 influences the antimicrobial activity of flavonoids. Data corroborated by Zheng et al. (1996) demonstrated a relationship between the structure and antibacterial activity against gram-positive bacteria using methylated flavones, and that the most active were those with hydroxyl groups at C-5 and C-7 and three substitutions at ring B.
4. Conclusion
Jacaranda mimosifolia flowers represent a source of polyphenolic compounds, showing that methanol and aqueous extracts have high antioxidant activity. Furthermore, both extracts showed antibacterial activity against gram-positive and gram-negative pathogens, with a predominance over gram-positive bacteria. The antioxidant and antibacterial results obtained in this work raise the possibility of using and revaluing Jacaranda flowers as a source of compounds of interest to the pharmaceutical, food, medical, cosmetic, and agricultural sectors. The findings of the present study justify deeper research on the applications of the J. mimosifolia flower as an antimicrobial of natural origin.
Declarations
Author contribution statement
A.A. Feregrino-Perez: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
H. Aguirre-Becerra: Analyzed and interpreted the data; Wrote the paper.
S.A. Pineda-Nieto: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
B.L. Álvarez-Mayorga: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
D.M.R. Pastrana: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
J.F. García-Trejo and R.G. Guevara-González: Contributed reagents, materials, analysis tools or data.
Funding statement
A.A. Feregrino-Perez was supported by FOFI 2018.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the Food Microbiology Laboratory of the Faculty of Chemistry for the support provided in this work.
References
- Acosta Solís M. Fundación Ecuatoriana de Estudios Sociales FESO. Vol. 234. Abya-Yala, Quito.; 1992. Vademecum of medicinal plants from Ecuador; p. 112. [Google Scholar]
- Alós J.I. Resistencia bacteriana a los antibióticos:una crisis global. Enferm. Infecc. Microbiol. Clín. 2015;33(10):692–699. doi: 10.1016/j.eimc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Al-Shukaili N.B.M.D.A., Hossain M.A. Antimicrobial and cytotoxic potential of seeds and flowers crude extracts of sunflower. Grain Oil Sci.Tech. 2019;2:103–108. [Google Scholar]
- Álvarez Castro.E., Orallo Cambeiro.F. Actividad biológica de los flavonoides (I). Acción frente al cancer. Offarm: Farm. Soc. 2003;22(10):130–140. [Google Scholar]
- Andrade Neves.D., Emídio Cunha E.C., Teixeira Duarte M.C., Teixeira Duarte R.M., Braga Ribeiro A., Teixeira Godoy H. Industrial Crops and Products; 2018. Effect of Cooking Process on the Free-Radical Scavenging Capacity and Antimicrobial Activity of Acmella Oleracea. On line. [Google Scholar]
- Bathori M., Zupko I., Hunyadi A. Monitoring the antioxidant activity of extracts originated from various Serraturla species and isolation of flavonoids from Serratula coronate. Fitoterapia. 2004;75:162–167. doi: 10.1016/j.fitote.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Batool R., Kalsoom A., Akbar I., Arshad N., Jamil N. Antilisterial effect of rosa damascena and Nymphaea alba in Mus musculus. BioMed Res. Int. 2018 doi: 10.1155/2018/4543723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büsra Konuk H., Ergüden B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020 doi: 10.1007/s12223-020-00787-4. [DOI] [PubMed] [Google Scholar]
- Cai H., Wang H.-Y.L., Venkatadri R., Fu D.-X., Forman M., Bajaj S.O., Li H., O´Doherty G.A., Arav-Boger R. Digitoxin analogues with improved anticytomegalovirus activity. ACS Med. Chem. Lett. 2014;5:395–399. doi: 10.1021/ml400529q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.Z., Sun M., Xing J., Luo Q., Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Cardador-Martínez A., Castaño-Tostado E., Loarca-Piña G. Antimutagenicactivity of natural phenolic compounds in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food Addit. Contam. 2002;19(1):62–69. doi: 10.1080/02652030110062110. [DOI] [PubMed] [Google Scholar]
- Ceruso M., Clement J.A., Todd M.J., Zhang F., Huang Z., Anastasio A., Pepe T., Liu Y. The inhibitory effect of plant extracts on growth of the foodborne pathogen, Listeria monocytogenes. Antibiotics. 2020;9:319. doi: 10.3390/antibiotics9060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Han R., Xu Y., Li N., Wang J., Dan W. Recent progress of antibacterial natural products: future antibiotics candidates. Bioorg. Chem. 2020 doi: 10.1016/j.bioorg.2020.103922. [DOI] [PubMed] [Google Scholar]
- de Morais J.S., SantÁna A.S., Dantas A.M., Silva B.S., Lima M.S., Borges G.C. Antioxidant activity and bioaccessibiity of phenolic compounds in white, red, blue, purple, yellow and orange edible flowers through a simulated intestinal barrier. Food Res. Int. 2020;131:109046. doi: 10.1016/j.foodres.2020.109046. [DOI] [PubMed] [Google Scholar]
- Engin K.N. Alpha-tocopherol: looking beyond an antioxidant. Mol. Vis. 2009;15:855–860. [PMC free article] [PubMed] [Google Scholar]
- Faggio C., Sureda A., Morabito S., Sanches-Silva A., Mocan A., Nabavi S.F., Nabavi S.M. Flavonoids and platelet aggregation: a brief review. Eur. J. Pharmacol. 2017;807:91–101. doi: 10.1016/j.ejphar.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Feregrino-Pérez A.A., Berumen L.C., García-Alcocer G., Guevara-González R.G., Ramos-Gomez M., Reynoso-Camacho R., Acosta-Gallegos J.A., Loarca-Piña G. Composition and chemopreventive effect of polysaccharides form common beans (Phaseolus vulgaris L.) on azoxymethane-induced colon cancer. J. Agric. Food Chem. 2008;56:8737–8744. doi: 10.1021/jf8007162. [DOI] [PubMed] [Google Scholar]
- Fratianni F., Tucci M., De-Palma M., Pepe R., Nazzaro F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori) Food Chem. 2007;104:1282–1286. [Google Scholar]
- Fukumoto L.R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. Agric. Food Chem. 2000:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Gachet M.S., Schühly W. Jacaranda-An ethnopharmacological and phytochemical review. J. Etnhopharmacol. 2009;121:14–27. doi: 10.1016/j.jep.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Gallo M.F. Microwave assisted extraction of phenolic compounds from four different spices. Molecules. 2010:6365–6374. doi: 10.3390/molecules15096365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulfraz M., Sadiq A., Tariq H., Imran M., Qureshi R., Zeenat A. Phytochemical analysis and antibacterial activity of Eruca sativa seed. Pakistan J. Bot. 2011;43(2):1351–1359. [Google Scholar]
- Gutiérrez-Venegas G., Gómez-Mora J.A., Meraz-Rodríguez M.A., Flores-Sánchez M.A., Ortiz-Mirnada L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendra R., Willis A., Keller P.A. Phytochemical studies on the Australian native plant species Acacia pycnantha and Jacaranda mimosifolia D.Don. Nat. Prod. Res. 2018 doi: 10.1080/14786419.2018.1483922. [DOI] [PubMed] [Google Scholar]
- Hussain H., Krohn K., Ahmad V.U., Miana G.A., Green I.R. 2007. Lapachol: an Overview. Special Issue Reviews And Accounts ARKIVOC; pp. 145–171. [Google Scholar]
- Kanadaswami C., Lee L.T., Lee P.P. The antitumor activities of flavonoids. Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- Karra S., Sebii H., Jardak M., Bouazid M.A., Attia H., Blecker C., Besbes S. Male date palm flowers:Valuable nutritional food ingredients and alternative antioxidant source and antimicrobial agent. South Afr. J. Bot. 2020;131:181–187. [Google Scholar]
- Khamsan N.S.L.S. The isolation of bioactive flavonoids from Jacaranda obtusifolia H. B. K. ssp. rhombifolia (G. F. W. Meijer) Gentry. Acta Pharm. 2012;62:181–190. doi: 10.2478/v10007-012-0014-1. [DOI] [PubMed] [Google Scholar]
- Kirby W.M.M., Yoshihara G.M., Sundsted K.S., Warren J.H. Clinical usefulness of a single disc method for antibiotic sensitivity testing. Antibiot. Annu. 1957;1956–1957:892. [PubMed] [Google Scholar]
- Koo H., Hayacibara M.F., Schobel B.D., Cury J.A., Rosalen P.L., Park Y.K., Vacca-Smith A.M., Bowen W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- Koo H., Pearson S.K., Scott-Anne K., Abranches J., Cury J.A., Rosalen P.L., Park Y.K., Marquis R.E., Bowen W.H. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immunol. 2002;17:337–343. doi: 10.1034/j.1399-302x.2002.170602.x. [DOI] [PubMed] [Google Scholar]
- Li An-Na., Sha Li., Hua-Bin Li., Dong-Ping Xu., Xiang-Rong, Feng Xu., Chen Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funtional Foods. 2014;6:319–330. [Google Scholar]
- Li H., Xue J., Zhou W., Sun Y., Liu W., Lin W., Liu A., Song A., Zhu H. Enhanced production of total flavones from Inonotus baumii by multiple strategies. Prep. Biochem. Biotechnol. 2017;48:103–112. doi: 10.1080/10826068.2017.1365248. [DOI] [PubMed] [Google Scholar]
- Li M., Xu Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch Pharm. Res. (Seoul) 2008;31(5):640–644. doi: 10.1007/s12272-001-1206-5. [DOI] [PubMed] [Google Scholar]
- Li Q., He Y.N., Shi X.W., Kang L.Y., Niu L.Y., Wang X.G., Feng W. Clerodens E–J, antibacterial caffeic acid derivatives from the aerial part of Clerodendranthus spicatus. Fitoterapia. 2016;114:110–114. doi: 10.1016/j.fitote.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Liu H.X., Tan H.B., Qiu S.X. Antimicrobial acylphloroglucinols from the leaves of Rhodomyrtus tomentosa. J. Asian Nat. Prod. Res. 2016;18:535–541. doi: 10.1080/10286020.2015.1121997. [DOI] [PubMed] [Google Scholar]
- Magaña Alejandro.M.A., Mariaca Méndez R., Gama Campillo L.M. 2010. El uso de las plantas medicionales en las comunidades Mayachontales de Nacajuca, Tabasco, México; pp. 213–262. Polibotánica. [Google Scholar]
- Mak Y.W., Chuan L.O., Ahmad R., Bhat R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts. J. King Saud Univ. Sci. 2013;25:275–282. [Google Scholar]
- Medini F., Fellah H., Ksouri R., Abdelly Ch. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014;8:216–224. [Google Scholar]
- Mikolajczak N., Sobiechowska D.A., Tanska M. Edible flowers as a new source of natural antioxidants for oxidative protection of cold-pressed oils rich in omega-3 fatty acids. Food Res. Int. 2020;134:109216. doi: 10.1016/j.foodres.2020.109216. [DOI] [PubMed] [Google Scholar]
- Moharram F.A., Marzouk M.S.A. Anovelphenylethanoid dimer and flavonoids from Jacaranda mimosaefolia. Z. Naturforsch. 2007;62:1213–1220. [Google Scholar]
- Mori A., Nishino C., Enoki N., Tawata S. Antibacterial activity and mode of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry. 1987;26:223–224. [Google Scholar]
- Mostafa N.M., Eldahshan O.A., Singab A.N.B. The genus Jacaranda (Bignoniaceae): a updated review. Pharmacogn. Commun. 2014;4:31–39. [Google Scholar]
- Muhammad K., Saeed-ur-Rahman, Muhammad B., Huang D. Role of flavonoids in plant interactions with the environment and against human pathogens-A review. J. Int. Agric. 2019;18(1):211–230. [Google Scholar]
- Nenadis N., Wang L.-F., Tsimidou M., Zhang H.-Y. Estimation of scavening activity of phenolic compounds using the ABTS assay. Agric. Chem. Food. 2004;52(15):4669–4674. doi: 10.1021/jf0400056. [DOI] [PubMed] [Google Scholar]
- OMS . 2016. Organización Mundial de la Salud. Obtenido de Temas de Salud.http://www.who.int/topics/infectious_diseases/es/ [Google Scholar]
- Oomah B.A.-M.-P. Phenolics and antioxidative activities in common beans(Phaseolus vulgaris L.) J. Sci. Food Agric. 2005:935–942. [Google Scholar]
- Pereira V.V., Silva R.R., Santos M.H., Dias D.F., Moreira M.E.C., Takahashi J.A. Antioedematogenic activity, acetylcholinesterase inhibition and antimicrobial properties of Jacaranda oxyphylla. Nat. Prod. Res. 2015 doi: 10.1080/14786419.2015.1095744. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- Pouillot R., Hoelzer K., Jackson K.A., Henao O.L., Silk B.J. Relative risk of listeriosis in Foodborne Diseases Active Surveillance Network (FoodNet) sites according to age, pregnancy, and ethnicity. Clin. Infect. Dis. 2012;54:S405–S410. doi: 10.1093/cid/cis269. [DOI] [PubMed] [Google Scholar]
- Poungoué-Kaatou G. Antioxidant activity and total phenolic content of Ingenious Salvia species on Investigation of their pharmacological activities. Pitochemistry. 2006:74–90. [Google Scholar]
- Prior R.L., Cao G. Flavonoids: diet and health relationship. Nutr. Clin. Care. 2000;3(5):279–288. [Google Scholar]
- Rana A., Bhangalia S., Singh H.P. A new phenylethanoid glucoside from Jacaranda mimosifolia. Nat. Prod. Res. 2013;27(13):1167–1173. doi: 10.1080/14786419.2012.717290. [DOI] [PubMed] [Google Scholar]
- Rauha J.P., Remes S., Heinonen M. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000;56:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- Rojas J.J., Ochoa V.J., Ocampo S.A., Muñoz J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BioMed Central Comp. Alt. Med. 2006;6:1–6. doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar Aranda R.D.-R.-G.-L. Evaluation of the biological activity of herbal products business. Univ. Med. 2009;(11):156–164. [Google Scholar]
- Sánchez J.L. 2011. Árboles ornamentales. Obtenido de Árboles ornamentales.http://www.arbolesornamentales.es/Jacaranda%20mimosifolia.pdf [Google Scholar]
- Shalini K., Sharma P.K., Kumar N. Imidazole and its biological activities: a review. Chem. Sin. 2010;1(3):36–47. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Tosun M., Celik F., Ercisli S.O., Yilmaz S. International Conference on Environmental and Agriculture Engineering (IPCBEE) Vol. 15. 2011. Bioactive contents of commercial cultivars and local genotypes of walnut(Juglansregia L.) [Google Scholar]
- Van Nieuwenhove C.P., Mayona A., Castro-Gómez P., Fontecha J., Sáez G., Zárate G., Luna Pizarro P. Comparative study of pomegranate and jacaranda seeds as functional components for the conjugated linolenic acid enrichment of yogurt. LWT - Food Sci. Technol. 2019;111:401–407. [Google Scholar]
- Voon H.C., Bhat R., Gulam R. Flower extracts and their essential oils as potential antimicrobial agents. Compr. Rev. Food Sci. Food Saf. 2012;11:34–55. [Google Scholar]
- Washington J.A. Springer Verlag; New York, USA: 1981. Laboratory Procedures in Clinical Microbiology. [Google Scholar]
- Wei Y.M., Chuah L.O., Ahmad R., Bhat R. Antioxidant and antibacterial activities of hibiscus (Hibiscus rosa-sinensis L.) and Cassia (Senna bicapsularis L.) flower extracts. J. King Saud Univ. Sci. 2013;25:275–282. [Google Scholar]
- Williams C.J. Flavonoid profilesin leaves, flowers and stems of forty-nine members of the phaseolinae. Biochem. Systemat. Ecol. 1995:655–667. [Google Scholar]
- Yoshizawa S., Fourmy D., Puglisi J.D. Structural origins of gentamicin. EMBO J. 1998;17:6437–6448. doi: 10.1093/emboj/17.22.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatta D.T., Pimienta F.C., Tres-Venzol L.M.F., Fiuza T.S., Bara M.T.F., Cunha L.C., Pucci L.L., Garrote C.F.D., Oliveira F.N.M., Paula J.R. Estudio da Atividade Antibacteriana contra cepas de Psudomonas aeruginosa e da Toxicidade Aguda das folhas da Jacaranda decurrens. Lat. Am. J. Pharm. 2009;28(4):485–489. [Google Scholar]
- Zha J., Wi X., Gong G., Koffas M.A.G. Pathway enzyme engineering for flavonoid production in recombinant microbes. Metab. Eng. Commun. 2019;9 doi: 10.1016/j.mec.2019.e00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.F., Tan R.X., Liu Z.L. Two flavones from Artemisia giraldii and their antimicrobial activity. Planta Med. 1996;62:160–162. doi: 10.1055/s-2006-957841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.