Abstract
Resistant starch (RS), a current health trend, can be obtained from various natural sources. Musa sapientum Linn., ABB group, cv. Kluai Namwa Luang is a good source of RS. This is the first study to investigate the physicochemical properties, RS contents, and prebiotic properties of unpeeled raw banana powder (URB), peeled raw banana powder (PRB), and banana starch (BS) from Kluai Namwa Luang. Their physicochemical properties were characterized by scanning electron microscope, differential scanning calorimeter, and X-ray diffractometer. The RS contents were determined using the Megazyme Resistant Starch Assay Kit. The prebiotic properties are reported as a prebiotic index (PI). The particle morphology of URB, PRB, and BS granules showed a smooth surface with irregular size and shape. Their gelatinization temperatures were 74–78 °C. All samples exhibited typical B-type diffraction patterns. URB contained the highest dietary fiber (9.7 ± 0.2 g per 100 g of dried sample), whereas BS contained the highest RS content (74.1 ± 0.1 g per 100 g of dried sample). Both URB and BS possessed excellent probiotic growth promotion, prebiotic properties with PI values comparable to the commercial inulin, and were highly resistant to digestive enzymes. Therefore, BS from Kluai Namwa Luang is suggested as functional nutrient in health promotion products.
Keywords: Musa sapientum, Banana starch, Raw banana, Resistant starch, Prebiotic
Musa sapientum; Banana starch; Raw banana; Resistant starch; Prebiotic
1. Introduction
Kluai Namwa or Musa sapientum Linn. (ABB genome type), a herbaceous and perennial plant belonging to the Family Musaceae, is an ancient Thai banana. It is a hybrid strain of two wild cultivars of Musa acuminate (AA genome) and Musa balbisiana (BB genome) [1, 2]. In Thailand, the most common cultivars of Kluai Namwa are Kluai Namwa Daeng (white flesh with red core), Kluai Namwa Khao (white flesh with white core), and Kluai Namwa Luang (white flesh with yellow core) [2, 3, 4]. Kluai Namwa Luang is the most popular cultivar cultivated throughout Thailand, because it is most commonly consumed due to the fact that it is easily obtained, cheap, and simple to cultivate in Thailand. The ripe fruit of Kluai Namwa are commonly used as the first solid food fed to infants, as they offer a more balanced diet than any other fruits and vegetables nearby. Its flesh is free of sodium but contains various vitamins which possesses therapeutic value and health benefits [5].
Raw fruit of banana contains high amount of starch that make it an excellent resistant starch (RS) [6, 7, 8]. In addition to starch from bananas, starches with high-resistant starch content also include grain starches that also show smoother grain surfaces and ordered molecular structures, for example, the high content of RS found in jackfruit seeds [9, 10, 11]. When comparing banana starch with cereal and grains starches, it was found that banana starch showed several unique properties including high amylose content (38.6–43.8%), gelatinization temperature (72.1–77.1 °C), relative crystallinity (31.3%), and high RS content (70.1–79.2 %) [12]. Additionally, banana fruit is widely grown and cheap in tropical countries. RS consumption is now a current health trend and used as a functional fiber and nutraceutical for colonic functions. Among five different types of RS (i.e., RS1, RS2, RS3, RS4, and RS5) [13, 14, 15], RS1 represents resistant starch which is physically unable to be digested by enzymes owing to its enclosure in food structures, for example, whole grains [12, 16, 17]. RS2 is the most utilized since it is resistant to enzyme digestion in the gastrointestinal tract. The resistant nature of RS2 is due to the fact of its granular form and its tight packing in a radial pattern that results in relative dehydration. This compact structure limits the accessibility of digestive enzymes, including various types of amylases. Generally, RS2 is found in ungelatinized starch including raw bananas and potatoes. The majority of RS2 granules are B-type structures that are digested more slowly than the others via α-amylase [12, 15, 16, 17]. RS3 represents retrograded amylose occurring in a crystalline non-granular starch or processed foods found in cooled and cooked raw potatoes, potato starch, corn starch, corn flakes, and other grain starches [13, 15, 17, 18, 19]. RS4 corresponds to highly chemically modified starch with the cross-linking. Modified starches obtained by different chemical treatments are contained in this type including chemically modified and thermally modified (repolymerized) starches [12, 14, 15]. RS5 refers to amylose–lipid complexes. The linear molecules of amylose naturally form a single-helical complex with lipids, for example, free fatty acids, phospholipids, and other molecules with hydrophobic moieties [14, 16]. Therefore, RS should be defined as non-digestible starch over a period of 120 min and which has the ability to promote the growth of colonic microorganisms. Additionally, prebiotics selectively stimulate the growth and/or activity of one or a limited number of bacteria in the colon, thereby improving the host's health and providing many health benefits [12, 20, 21]. The breakdown of prebiotic molecules by bacterial fermentation into short-chain fatty acids (SCFAs), for example, acetate, propionate, and butyrate, is crucial for intestinal integrity and function. These SCFAs can modulate the immune system and absorption of minerals such as calcium and iron [21]. On the other hand, they can also maintain normal serum lipid–cholesterol levels [20, 21, 22, 23]. The end products of bacterial fermentation, especially butyrate, are consumed by both bacteria and intestinal epithelium as an energy source. Therefore, prebiotics can help to reduce pathogenic bacteria as well as prevent gastrointestinal diseases and colorectal cancer [20, 21, 22]. Furthermore, prebiotics promote the body's complete absorption of food, resulting in fast growth and health [7, 24]. Moreover, the consumption of RS alters the gut microbial balance (microbiota) and promotes SCFA production by microbial fermentation, and RS consumption can help to reduce gastrointestinal inflammation and reduce pathology in inflammatory bowel disease [25].
Currently, RS2 has been reported to be found in significantly highly amounts in raw banana fruits. However, there has been no studies yet on the RS contents and prebiotic properties of Kluai Namwa Luang. Therefore, this study aimed to focus on the physicochemical properties, RS contents, and prebiotic properties of dry powders from unpeeled raw banana fruit (UPB), peeled raw banana fruit (PRB), and banana starch (BS) from Kluai Namwa Luang. This was expected to further support the research on banana's health attributes as a product.
2. Materials and methods
2.1. Plant materials
Raw Kluai Namwa Luang fruits aged 90–120 days were collected from the Mae Wang District, Chiang Mai Province, Thailand, from January 2017 to March 2017. The voucher specimen of Kluai Namwa Luang was recorded as KNL-001 and kept in the herbarium at the Faculty of Pharmacy, Chiang Mai University, Thailand. Thereafter, the fruit samples were washed thoroughly and wiped until they were dry.
2.2. Chemical reagents
Sodium hydroxide and hydrochloric acid were purchased from RCI Labscan Limited, Bangkok, Thailand. Escherichia coli ATCC 25922, Bacteroides vulgatus ATCC 8482, Bifidobacterium longum ATCC BAA-999 and Total Lactobacillus (sp.) from Lactobacillus casei subsp. rhamnosus TISTR 047 were purchased from ATCC, Manassas, VA, USA. Lactobacillus acidophilus TISTR 450 was purchased from the Thailand Institute of Scientific and Technological Research (TISTR), Thailand. Megazyme Resistant Starch Assay Kit (Lot. K-RSTAR 08/16) was purchased from Megazyme International Ireland Ltd. Bray, Ireland. All other utilized chemicals were of analytical grade.
2.3. Preparation of URB
Raw Kluai Namwa Luang fruits were thinly sliced, with a thickness of approximately 1–2 mm. The sliced banana was then placed on a tray and dried in an oven at 50 °C for 24 h. The dried banana was milled into a fine powder and passed through an 80 mesh sieve. The URB powder was obtained and kept in a sealed container at room temperature until used.
2.4. Preparation of PRB
The peels of raw Kluai Namwa Luang fruits were manually removed. The banana flesh was then thinly sliced, with a thickness of approximately 1–2 mm. Thereafter, they were placed on a tray and dried in an oven at 50 °C for 24 h. The dried banana was milled into a fine powder and passed through an 80 mesh sieve. The PRB powder was obtained and kept in a sealed container at room temperature until used.
2.5. Preparation of BS
BS was extracted from PRB according to a method previously described by Nimsung et al. [26] with some modifications. First, the dried PRB powder was added into a solution of 0.05 N sodium hydroxide at a ratio of 1:5 by volume and stirred constantly for 20 h. The resulting suspension was then centrifuged at 3000 rpm at 10 °C for 15 min. Next, the liquid (supernatant) was decanted and the brown part at the top of the sediment was scraped. The sediment was then added into a new solution of 0.05 N sodium hydroxide with a ratio of 1:5 by volume and stirred for a further 3 h. After that, the suspension was again centrifuged at 3000 rpm at 10 °C for 15 min. The liquid (supernatant) was decanted and the brown part was scraped off from the top of the sediment. The precipitation was resuspended in distilled water with a ratio of 1:2 by volume. The suspension was then filtered through an 80 mesh sieve and set for precipitation for 45 min. The process was repeated 2–3 times. Subsequently, the pH was adjusted to a range of 6.5–7.0 with 0.05 N hydrochloric acid and centrifuged at 3000 rpm at 10 °C for 15 min. The liquid (supernatant) was decanted, and the brown part was scraped off the top of the sediment. Following that, the sediment was dried in a hot-air oven with a temperature of 50 °C for 24 h. Finally, the dried sediment was milled into a fine powder and passed through an 80 mesh sieve. The sieved product (BS) was obtained and stored in sealed containers at room temperature until used.
2.6. Physicochemical properties characterization of URB, PRB, and BS
2.6.1. Morphology
The morphology of URB, PRB, and BS samples were observed by a scanning electron microscope (SEM) (JSM-5910LV, JEOL, Tokyo, Japan). Each sample was arranged on a metal stub using carbon adhesive tape and coated with gold under vacuum conditions. The average diameter and standard deviation were calculated using Image J software version 1.51 K (National Institutes of Health, Bethesda, MD, USA).
2.6.2. Moisture content
The moisture content of the URB, PRB, and BS samples were determined via the weight loss after drying the samples using a digital Electronic Moisture Analyzer (MA 50, Sartorius, Goettingen, Germany). Approximately 2–5 g of the dried powder of each sample was accurately weighed and transferred to an aluminum pan and held at 60 °C until the samples' weight were constant. The moisture content was recorded, and all experiments were done in triplicate.
2.6.3. Quantification of amylose, dietary fiber, soluble dietary fiber, fat, and protein
The samples of URB, PRB, and BS were mechanically crushed before testing. The amylose, dietary fiber, soluble dietary fiber, fat, and protein contents of URB, PRB, and BS were analyzed according to the standard method of the Association of Official Agricultural Chemists (AOAC) [27].
2.6.4. Thermal properties
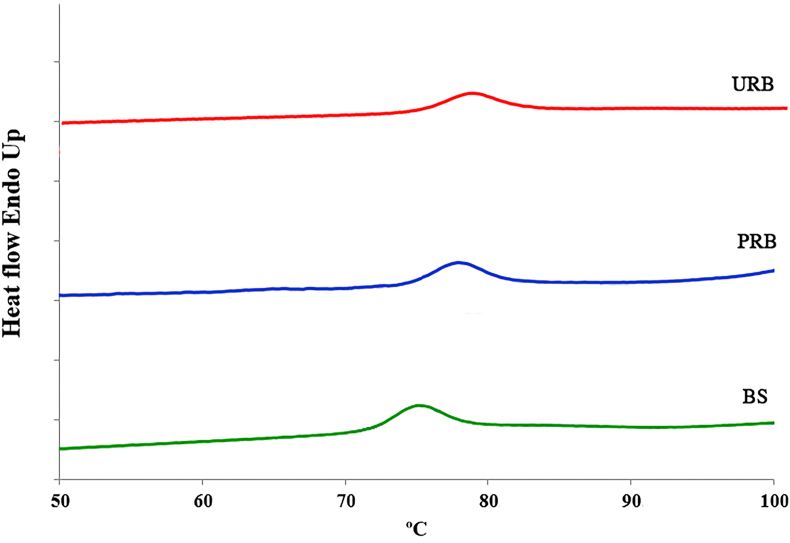
The thermal properties of the URB, PRB, and BS samples were determined according to the methods previously described by Jaiturong et al. [28], using a differential scanning calorimeter (DSC8000, Perkin Elmer, Billerica, MA, USA). Approximately, 2.0 mg of samples were accurately weighed in an aluminum pan with 6 μl of distilled water. The pan was hermetically sealed and equilibrated at room temperature for 24 h. After that, it was heated at a rate of 10 °C/min from 30 to 120 °C to compare it with the reference pan. Onset (T0), peak (Tp), and enthalpy (ΔH) of gelatinization were recorded.
2.6.5. X-ray diffraction
X-ray diffraction patterns of the URB, PRB, and BS samples were examined using an X-ray diffractometer (X'Pert Pro MPD, PANalytical, Almelo, Netherlands). Diffractograms were obtained from 4° to 40° (2θ) at a scanning speed of 4°/min according to the methods previously described by Jane et al. [29].
2.6.6. Fourier transform infrared spectroscopy (FTIR)
The functional groups of the URB, PRB, and BS samples were characterized using FTIR. The FTIR measurements were carried out in attenuated total reflectance (ATR) mode [30]. FTIR was conducted using a Vertex 70 FTIR spectrometer (Bruker Optics Ltd., Banner Lane, Coventry, UK), equipped with a MIRacle™ single reflection ATR accessory (Pike Technologies, Fitchburg, WI, USA) fitted with a diamond internal reflection element. The ATR-FTIR spectra were obtained from a transmittance mode based on 32 scans from 500 to 4000 cm−1 and the resolution was 2 cm−1. The spectral analysis was conducted using the OPUS program version 7.8 (Bruker Optics Ltd., Banner Lane, Coventry, UK).
2.6.7. Nuclear magnetic resonance spectroscopy (NMR)
The 1H-NMR spectra of the URB, PRB, and BS samples were characterized using a Bruker Neo™ 500 NMR spectrometer (Bruker Ltd., Billerica, Massachusetts, US) [31]. The chemical shifts were reported in parts per million (ppm) using H2O impurity in D2O resonated at 7.79 ppm as the internal reference. Coupling constants (J) were reported in hertz (Hz), and coupling patterns were reported as follows: d (doublet). The 1H-NMR spectra were conducted using MestReNova software (Mestrelab Research, Santiago de Compostela, Spain).
2.7. Resistant starch determination
The RS contents of the URB, PRB, and BS powders were analyzed according the AOAC's official method (i.e., 2002.02) with the Megazyme Resistant Starch Assay Kit [32].
2.8. Determination of in vitro starch digestion using α-amylase and trypsin hydrolysis
A solution of 1 unit/ml of α-amylase and 90 units/ml of trypsin in 20 mM sodium phosphate buffer was prepared. Three banana resistant starch samples—URB, PRB, and BS—were prepared as 1% w/v solutions in 15 ml of 20 mM sodium phosphate buffer. Then, 15 ml of enzyme solution was added to a sample solution, and the reaction was then incubated in water bath at 37 °C for 6 h as a control. Each 1 ml of sample was taken at 0, 30, 60, 90, 120, 180, and 240 min to determine the reduction in sugar content, using a 3,5-dinitrosalicyclic acid (DNS) assay [33], and total sugar using the phenol–sulfuric acid method [34]. The sugar concentration in the reaction mixture was determined by a UV visible method based on the measurement of a formed glucose standard. Spectrophotometric measurements were performed with a UV/Vis Spectramax M3. The percentage of hydrolysis of the banana resistant starches were calculated based on reduced sugar released and total sugar content of the sample using Eq. (1):
| % Hydrolysis = 100A/(B – C) | (1) |
where A is the final reducing sugar content, B is the total sugar content, and C is the initial reducing sugar content. All experiments were performed in a triplicate.
2.9. Prebiotic properties of URB, PRB, and BS
The prebiotic properties of URB, PRB, and BS were reported as a prebiotic index (PI) according to a method previously described by of Palframan et al. [35] with some modifications. Briefly, the samples were tested on the growth promotion of representative bacteria in the digestive tract. Lactobacillus paracasei (Lac) and Bifidobacterium longum (Bif) were used as the representative probiotic cultures, while Escherichia coli (Ec) and Clostridium perfringens (Clos) were used as the representative enteric species. All representative strains were obtained from the culture collection units of the Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand. Inulin, a commercial prebiotic, was used as the reference standard for comparison. Each assay was performed in triplicate, measuring the number of viable Log10 colony forming units (Log10 CFU) per ml at T0 for 0 h and at T48 incubation for 48 h, respectively, on 1 % w/v dextrose (standard carbon source in common medium as control), 1 % w/v test banana samples and 1 % w/v inulin as the commercial prebiotic. The prebiotic index (PI) was then calculated using Eq. (2):
| PI = (Bif T48 - Bif T0/Total T48 – Total T0) + (Lac T48 – Lac T0/Total T48 – Total T0) - (Ec T48 - Ec T0/Total T48 - Total T0) - (Clos T48 - Clos T0/Total T48 - Total T0) | (2) |
where Bif T48 is the number of Bifidobacterium longum at sampling time; Bif T0 is the number of Bifidobacterium longum at the beginning; Lac T48 is the numbers of Lactobacillus paracasei at sampling time; Lac T0 is the number of Lactobacillus paracasei at beginning; Ec T48 is the number of Escherichia coli at sampling time; Ec T0 is the number of Escherichia coli at beginning; Clos T48 is the number of Clostridium perfringens at the sampling time; Clos T0 is the number of Clostridium perfringens at the beginning; Total T48 is the numbers of total bacteria at the sampling time; Total T0 is the number of total bacteria at the beginning. All experiments were performed in a triplicate.
2.10. The study of prebiotic properties in promoting probiotic growth
The promotion of probiotic growth of URB, PRB, and BS were recorded as the average number of probiotic bacteria (i.e., Lactobacillus sp.) according to a method previously described by Palframan et al. [35] with some modification. Each sample was tested for the growth promotion of Lactobacillus paracasei, which was the representative bacteria from the digestive tract. The number of viable Log10 CFU per ml at T0 for 0 h and at T48 after incubation for 48 h was measured on 1 %w/v glucose (standard carbon source in common medium as control; MRS) and 1 %w/v sample test. The experiment was performed in triplicate.
2.11. Statistical analysis
The results were determined in triplicates to confirm reproducibility. The data are given as the mean ± S.D. Analysis of variance (ANOVA) was performed using statistical SPSS software version 17 (SPSS Inc, Chicago, Illinois, USA). Duncan's multiple range tests were performed to analyze the significant differences in physicochemical and prebiotic properties, and p < 0.05 was considered as significant.
3. Results and discussion
3.1. Physicochemical properties of URB, PRB, and BS
3.1.1. Morphology
The appearance of URB, PRB, and BS granules, observed by scanning electron microscope (500×), are shown in Figure 1. Various irregular shapes were observed in each sample. Most of them were rounded or slightly elongated and had smooth surfaces. However, fibrous elements were observed in the URB and PRB (fewer) samples but were not detected in the BS samples. A likely explanation may be due to the granule washing procedure during the extraction process of BS. However, the BS granules were not damaged, and the appearance still remained the same, suggesting that the BS preparation technique used in the present study was suitable. The results were well in accordance with a previous study that reported the appearance of banana powder and starch were somewhat rounded and long [36].
Figure 1.
SEM images of (a) URB, (b) PRB, and (c) BS.
The estimated sizes of URB, PRB, and BS were similar, which were 23.5 ± 8.2, 25.7 ± 4.6, and 25.7 ± 4.9 μm, respectively. This is the first study to report the appearance and size of starch granules from Kluai Namwa Luang fruits. It was noted that the granules from Kluai Namwa Luang fruits were smaller than that from other banana varieties, such as Kluai Khai, Kluai Hom Tong, Kluai Namwa, White Manzano, Dwarf Cavendish, green banana (Musa cavendishii) [26, 37, 38]. Additionally, the appearances of banana starch were different depending on banana varieties. Some granules of White Manzano banana starch and Dwarf Cavendish banana starch were oval with ridges or spherical [37], whereas, some granules of green banana (Musa cavendishii) were flat and elongated [38]. The differences in sizes and shapes of the starch granules affected the digestion-resistant properties of the starch [39]. Since larger surface-area-to-volume ratios are commonly found in smaller-sized particles, Kluai Namwa Luang fruits, which yield smaller starch granule sizes, could also yield starch with larger surface-area-to-volume ratios. Therefore, this point could be an outstanding feature of the starch from Kluai Namwa Luang fruits.
3.1.2. Moisture content
To measure the moisture content of URB, PRB, and BS, a convenient and time-efficient automatic moisture detector was used. BS contained the highest moisture content (6.8% ± 0.3% w/w), followed by URB and PRB, of which both had comparable moisture content as shown in Table 1. The results for BS were comparable to that of a previous study by Nimsung et al., who reported moisture contents of dry banana powder and banana starch of 6.9 and 7.2, respectively [26]. Interestingly, URB and PRB had significantly lower moisture content (p < 0.05), which would be another superior feature of the starch from Kluai Namwa Luang fruits. Although starch is considered to be chemically and microbiologically inert under normal storage conditions, excessively dry starches with a humidity lower than the equilibrium humidity are suggested as favorable [40]. Therefore, lower moisture content leads to higher stability, resulting in the longer shelf-life of the starch. Additionally, it is good for storage because the chances of fungal and other microflora growth in the samples during storage time will be reduced. However, protection from high humidity during storage time is also suggested to maintain the stability of the starch [40].
Table 1.
Amylose, Dietary fiber, Soluble dietary fiber, Fat, Protein and RS.
| Sample | Moisture content | Amylose | Dietary fiber | Soluble dietary fiber | Fat | Protein (%Nx6.25) | RS |
|---|---|---|---|---|---|---|---|
| URB | 5.5 ± 0.1b | 25.8 ± 0.1b | 9.7 ± 0.2a | 2.6 ± 0.0a | 0.8 ± 0.0a | 3.7 ± 0.0b | 34.1 ± 0.2a |
| PRB | 5.3 ± 0.2b | 24.2 ± 0.0b | 7.6 ± 0.2a | 2.5 ± 0.0a | 0.6 ± 0.0b | 3.8 ± 0.0a | 42.0 ± 0.1a |
| BS | 6.8 ± 0.3a | 35.6 ± 0.1a | 3.8 ± 0.1b | 1.2 ± 0.2b | 0.3 ± 0.1c | 3.2 ± 0.0c | 74.1 ± 0.1b |
URB = unpeeled raw banana; PRB = peeled raw banana; BS = banana starch; RS = Resistant starch. The results were reported as g of analyzed parameter per 100 g of dried samples.
Values are means of triplicate analysis (n = 3).
In a column, means not sharing a common letter are significantly different at P < 0.05.
3.1.3. Amylose, dietary fiber, soluble dietary fiber, fat, protein, and resistant starch contents
The compositions of amylose, dietary fiber, soluble dietary fiber, fat, protein, and resistant starch (RS) in the URB, PRB, and BS samples are shown in Table 1. It is noted that the amounts of amylose, dietary fiber, and soluble dietary fiber in the URB and PRB samples were significantly different than those of the BS (p < 0.05). The results were well in accordance with a previous study that reported the amylose contents of powder and starch obtained from Kluai Namwa Luang at 25.8 and 43.8 g/100 g (dry weight), respectively [12]. In addition, another previous study reported that the amount of amylose in starch obtained from Kluai Namwa Luang was 28.0 g/100 g (dry weight) [26]. Moreover, research has suggested a significant linear relationship between the apparent amylose and the RS content of both Kluai Namwa Luang powder and starch [12]. This identical relationship was also observed in the present study, since BS, which contains the highest amylose content (35.6 ± 0.1 g/100 g of dried samples), also contained the highest RS content (74.1 ± 0.1 g/00 g of dried samples). Therefore, it could be suggested that amylose plays an important role in resistance to enzymatic digestion of URB, PRB, and BS.
On the other hand, this study remarks that the apparent dietary fiber and soluble dietary fiber content of URB and PRB were significantly higher than that of BS (p < 0.05). The results were well in accordance with the SEM images (Figure 1). Dietary fibers are known as non-starch polysaccharides that are principal components of the plant cell wall such as cellulose, pectin, hemicellulose, etc. [41]. However, these dietary fibers can be removed by granule washing procedures during the extraction process and, hence, resulting in the lower dietary fiber content detected in BS. Therefore, the RS content of BS was significantly enhanced because the dietary fibers had already been removed.
Similar to the dietary fiber content, the contents of fat and protein tended to decrease in BS (p < 0.05). The explanation could be the same, since the granule washing process could not only remove the dietary fibers but also remove the fat and protein from the starch granules. Therefore, the washing process could purify the RS, resulting in the significantly increased RS content of the BS sample (p < 0.05). The results are well in accordance with a previous study that reported the fat and protein contents of peeled green Musa cavendishii and unpeeled green Musa cavendishii tended to decrease with increasing RS content [38].
In brief, starch preparation methods used in the present study could purify and enhance the RS content from Kluai Namwa Luang by removing other components, including dietary fiber, fat, and protein, during the washing process. Therefore, Kluai Namwa Luang powder, especially BS which contained the significantly highest RS content (p < 0.05), is suggested for using as a dietary supplement for enhancing digestive function. Interestingly, the RS content from BS was much higher than that of previous studies. Only 14.3–57.2 g of RS per 100 g of dried samples were detected in the powder and starch from French green bananas, Musa cavendishii, Kluai Namwa, Kluai Hom Tong, and Kluai Khai [6, 38, 42]. Although various factors have been reported to affect the RS content in banana, including differences in banana cultivars, agricultural practices, growing seasons, and methods of starch preparation, the present study highlighted BS from Kluai Namwa Luang as a good source of RS content.
3.1.4. Thermal properties
The thermal properties of starch are important parameters for further utilization, since they are related to the gelatinization temperature and tendency to retrograde properties [43]. The gelatinization characteristics and the thermal properties of the URB, PRB, and BS samples are shown in Figure 2. A broad endothermic peak for each sample was defined at the onset temperature of gelatinization (To), peak gelatinization temperature (Tp), and conclusion temperature (Tc) as shown in Table 2. The results, interestingly, show that URB exhibited a significantly higher gelatinization temperature (p < 0.05). The thermal behaviors of Kluai Namwa Luang in the present study related well with those in previous studies [12, 26]. It is evident that a higher amylose content results in a higher gelatinization temperature. The reason for this is because of the parallel linear chains of amylose which are close together and, hence, require higher temperatures for complete dissociation [43, 44]. On the other hand, granular shape and size, distribution of starch granules, and structural characteristics of crystallinity have been reported to affect the gelatinization temperatures [26, 45, 46]. Since gelatinization significantly increases the susceptibility of starch to digestive enzymes because of the breakage and dissolution of the tight crystalline granular structure, high gelatinization temperatures hence lead to more stable starch granules and higher digestive resistance [47, 48]. However, the degree of disassembly of the starch structure during thermal processing was not a major determinant of the digestibility of gelatinized starch.
Figure 2.
Gelatinization characteristics of URB, PRB and BS from Kluai Namwa Luang.
Table 2.
Thermal properties of URB, PRB and BS.
| Sample | Gelatinization temperature (°C) |
ΔH (J/g) | ||
|---|---|---|---|---|
| To | Tp | Tc | ||
| URB | 75.0 ± 0.8a | 78.1 ± 0.5a | 81.8 ± 0.8a | 10.3 ± 3.1a |
| PRB | 74.3 ± 0.5a | 77.7 ± 0.8a | 81.3 ± 0.6a | 13.4 ± 2.5a |
| BS | 71.3 ± 0.7b | 74.9 ± 0.6b | 78.9 ± 1.0b | 19.8 ± 4.3b |
URB = unpeeled raw banana; PRB = peeled raw banana; BS = banana starch; To = onset temperature; Tp = peak temperature; Tc = conclusion temperature; ΔH = melting enthalpy.
Values are means of trilicate analysis (n = 3).
In a similar way to the gelatinization temperature, BS had a significantly higher endothermic gelatinization enthalpy (ΔH) (p < 0.05). Since enthalpy is the total energy used to break down the crystalline structure of starch, higher ΔH demonstrated the higher bond strength within the molecules of the samples. Various factors have been reported to play a role in bond strength, including the content of amylose, type and arrangement of the crystalline structure, and interactions with other elements in the starch powder [26]. The present study showed that the significantly highest ΔH was detected in BS, which contained the significantly highest amylose content. This was due to the fact that the higher content of amylose led to a very organized arrangement within the molecules of BS, resulting in a wide range of gelatinization temperatures, and a significant amount of energy was needed to break down these bonds [49]. The results of BS related well with the previous study by Nimsung et al. (2007), who reported that the enthalpy values of starch obtained from Kluai Namwa (19.6 J/g) was much higher than that of the powder obtained from Kluai Namwa (15.5 J/g) [26].
3.1.5. X-ray diffraction
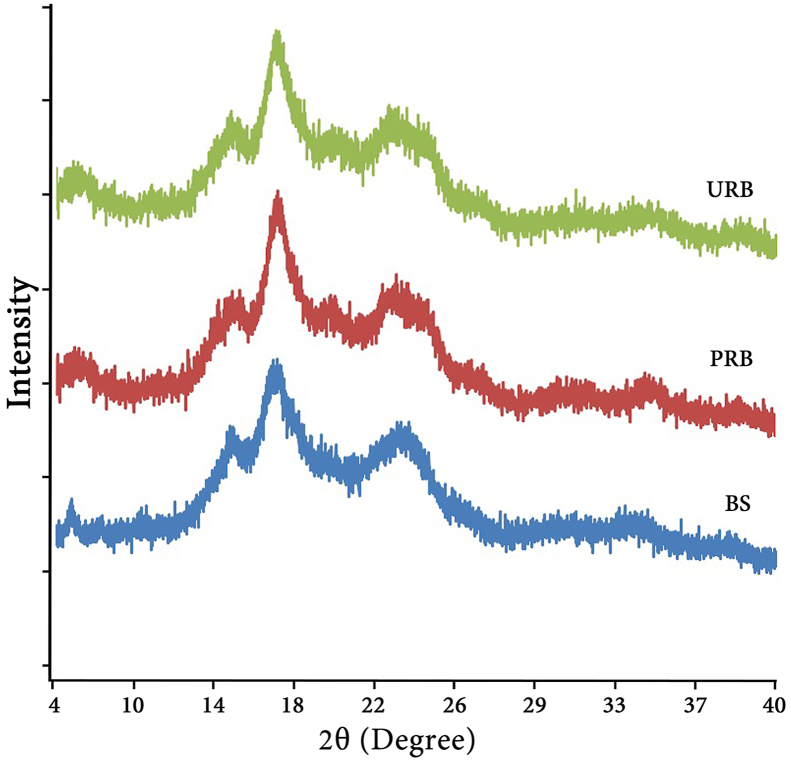
An X-ray diffraction pattern is related to starch digestibility, as an A-type pattern represents a high level of slowly digestible starch, whereas a B-type pattern presents high levels of resistant starch content [50]. Furthermore, a C-type crystalline structure, which is a combination of an A- and B-type, can also be found. However, C-type starch exhibits a greater tendency toward a B-type, i.e., the B-type is predominant over the A-type. Therefore, both B- and C-type crystalline structures are highly resistant to enzyme hydrolysis [36, 37, 51, 52].
The URB, PRB, and BS samples exhibited the same pattern of X-ray diffractograms as shown in Figure 3. Typical B-Type diffraction patterns, with peaks detected around 5.5°, 15°, 17°, and 23° at angle 2θ, were detected in all samples when the highest intensity was at 17°. The highest intensity of X-ray diffraction peaks have been detected differently in different banana starch. The highest peaks were reported to be detected at approximately 5.6°, 15°, 17°, 18°, 20°, 23°, 24°, and 26° at 2θ in various banana starch [36, 52, 53, 54]. The peaks detected around 5.5° and 17° at angle 2θ have been previously defined as fingerprints of B-type crystals from various banana starch [6, 29, 53]. A distinctive peak at 5.5° was in accordance with the B-type crystalline structure of banana starch granules of Kluai Namwa reported by Vatanasuchart et al. (2012) [12]. In addition, the peak at 15° was in accordance with the characteristic peak of banana starch reported by Pineda-Gomez et al. (2014) [38]. Banana powder might be either A-, B-, or C-type crystals, depending on the species, conditions of growth, etc. The present study revealed that starch powder from Kluai Namwa Luang was a B-type crystalline structure that is the most often found in banana powder [6, 51]. Since the resistance of starch granules from some plants to amylase activities is determined by the structure of the starch granules, particularly the presence of B-type crystals [55], URB, PRB, and BS, which exhibited the B-type crystalline pattern, will be resistant to digestive enzymes as well.
Figure 3.
X-ray diffraction patterns of URB, PRB and BS from Kluai Namwa Luang.
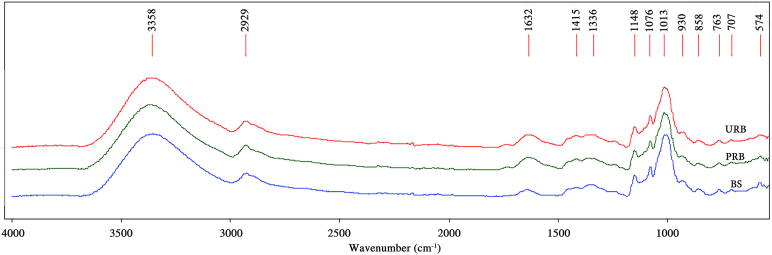
3.1.6. Fourier transform infrared spectroscopy
The FTIR spectra of URB, PRB, and BS are shown in Figure 4. All samples exhibited similar pattern of FTIR spectra. The ordered structures of starch can be seen as sharp peaks with maximum absorbance at 1148 and 1076 cm−1. The molecule type was of a short-range molecular order. This could be supported by the crystallization structure of starch at 1047 cm−1. On the other hand, the molecules also had an amorphous form illustrated by a board band detected at 1013 cm−1. Additionally, the band detected at 3385 cm−1 can be attributed to stretching vibration of the O–H bond and COOH groups. The ratio of 1022/995 cm−1 is widely used to measure the proportion of amorphous to ordered carbohydrate structures in starch [56, 57]. The ratio of 1022/995 cm−1 for URB, PRB, and BS were similar at 0.90, 0.88, and 0.88 μm, respectively. The results of all the sample agreed well with a previous study by Li et al. (2018), who reported that for the ratio 1022/995 cm−1, it also had no significant differences between the flesh starch (0.90) and peel starch (0.88) in other green banana varieties [55].
Figure 4.
Fourier transform infrared spectroscopy patterns of URB, PRB and BS from Kluai Namwa Luang.
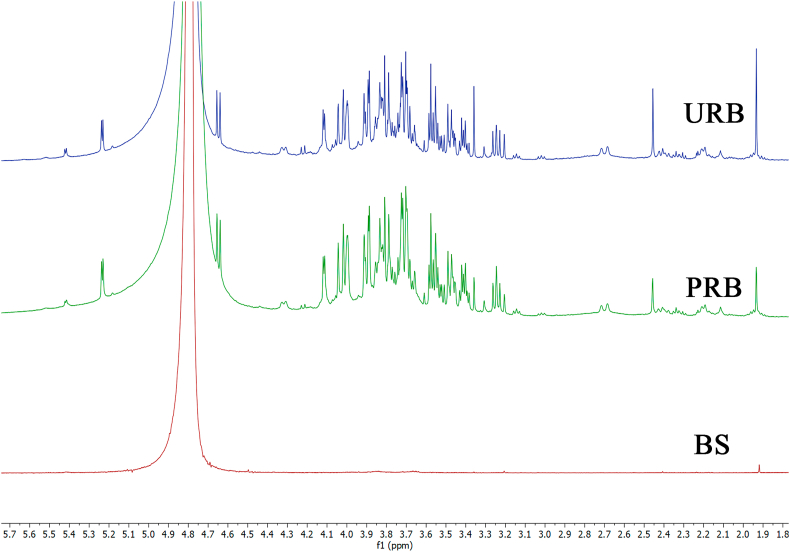
3.1.7. Nuclear magnetic resonance spectroscopy
The NMR spectra of URB, PRB, and BS are shown in Figure 5. The 1H-NMR spectra of polysaccharide URB and PRB displayed overlapping signals ranging from 3 to 5 ppm belonging to the signals of polysaccharide, which were confirmed by the presence of many sugar residue signals. Anomeric protons were used to classify the type of sugar: α-D-sugar and β-D-sugar, which resonated at 4.9–5.8 ppm and 4.4–4.9 ppm, respectively; proton signals resonated at 5.42 ppm (d, J = 3.8 Hz) and were assigned as anomeric protons of sucrose portion, 5.23 ppm (d, J = 3.7 Hz) belonging to α-D-glucose portion, and 4.65 ppm (d, J = 7.9 Hz) assigned as β-D-glucose moiety. Another anomeric signal resonated at 4.22 ppm (d, J = 8.9 Hz) and was assigned as a fructose moiety of sucrose. The results of the URB and PRB samples were in good agreement with a previous study by Suvakanta et al. (2014), who reported that 1H spectrum of from Musa sapientum Linn. is crowded in a narrow region between 3 to 5 ppm typical of polysaccharides [31]. Also, the previous study reported that the anomeric protons were assigned to α-sugar and β-sugar residues due to the presence of signals between 5.08–5.14 ppm and 4.49–4.60 ppm, respectively [31, 58]. In addition, the 1H-NMR spectra of polysaccharide BS had the lowest signals of polysaccharides, because the rapidly digested starch was removed during the extraction of banana starch.
Figure 5.
Nuclear magnetic resonance spectroscopy patterns of URB, PRB and BS from Kluai Namwa Luang.
3.2. In vitro starch digestion kinetics of URB, PRB, and BS
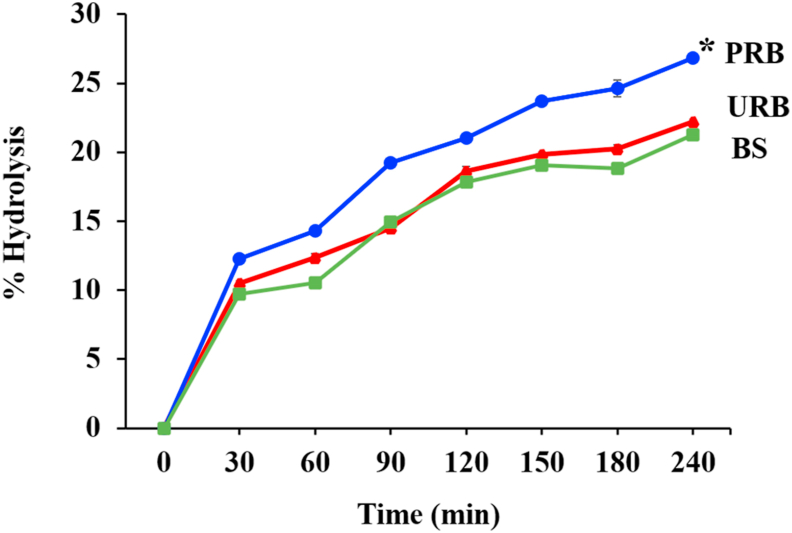
The hydrolysis kinetic of banana starch by α-amylase and trypsin calculated based on reducing sugar released and total sugar content of the samples are shown in Figure 6. Both α-amylase and trypsin are powerful digestive enzymes commonly found in salivary glands, the midgut, the biliary tract, and pancreas mix in the duodenum. The hydrolysis of PRB by α-amylase and trypsin enzymes was significantly higher than URB and BS (p < 0.05), whereas no significant difference between URB and BS was detected. After 24 h, PRB was hydrolyzed by 26.8% ± 0.3%, whereas only 22.2% ± 0.2% and 21.3% ± 0.1% of URB and BS were hydrolyzed. The results show that only a small portion—approximately one-fourth or one-fifth—of banana starch can be hydrolyzed by digestive enzymes in the upper gastrointestinal track. Therefore, banana starch can reach the colon to promote the growth and function of probiotics in the intestine because of their high resistance to α-amylase and trypsin.
Figure 6.
Percentage of banana starch hydrolysis after digest with α-amylase and trypsin enzyme at various times. The values are expressed as mean ± standard deviation (n = 3). The asterisk (∗) indicated significantly different at p < 0.05.
3.3. Prebiotic properties of URB, PRB, and BS
To be beneficial to human health by enhancing digestive function, prebiotic nutrients should selectively stimulate beneficial intestinal bacteria and be able to reduce the growth of non-beneficial or pathogenic bacteria at the same time [19, 20, 59]. Prebiotic properties are normally evaluated and reported as a prebiotic index (PI) that represents their ability engender better probiotic growth than non-beneficial bacteria species [32]. The PI of URB, PRB, and BS are listed in Table 3. A non-prebiotic nutrient (dextrose) and a commercial prebiotic (inulin) were also investigated for their PI value. The results showed that PRB had no prebiotic properties since it possessed a comparable PI value to dextrose (p > 0.05). Interestingly, URB and BS possessed prebiotic properties with comparable PI values to inulin and that of commercial prebiotics (p > 0.05). Therefore, they are suggested for use as dietary supplements to promote the growth of beneficial bacteria. Since both RS and dietary fiber have been known to selectively support the growth of probiotic strains, they were mainly responsible for the prebiotic properties of BS and URB, respectively [59, 60].
Table 3.
Prebiotic index of Dextrose (Control), URB, PRB and BS.
| Sample | Prebiotic index ± SD |
|---|---|
| Dextrose (Control) | 0.17 ± 0.0a |
| URB | 0.40 ± 0.0b |
| PRB | 0.21 ± 0.1a |
| BS | 0.46 ± 0.1b |
| Inulin | 0.47 ± 0.0b |
URB = unpeeled raw banana; PRB = peeled raw banana; BS = banana starch.
Values are means of triplicate analysis (n = 3).
In a column, means not sharing a common letter are significantly different at P < 0.05.
3.4. Promoting growth effect of probiotics of URB, PRB and BS
The abilities of URB, PRB, and BS in promoting the growth of Lactobacillus sp., which was used as representative of probiotics in the present study, are shown in Table 4. Dextrose was used as standard carbon source of culture media. The initial number of Lactobacillus sp. were approximately 6 Log10 CFU, which was similar in all samples. During the first 12 h, the significantly highest Lactobacillus sp. level was detected in the dextrose-treated group. In contrast, BS and URB later increased their levels of probiotics. BS and URB achieve significantly the highest Lactobacillus sp. growth stimulation after 9 h and 18 h, respectively. Although all samples could promote the growth of probiotics, BS and URB possessed the significantly highest growth promotion ability over 18–48 h. Hence, URB and BS from Kluai Namwa Luang not only exhibited the prebiotic properties but also promoted the growth of probiotics. This is consistent with previous studies reporting probiotics bacterial growth and count effects of banana powder from other banana varieties [61, 62, 63].
Table 4.
The average number of probiotic bacteria (Lactobacillus sp.).
| Sample | Total probiotic Lactobacillus sp. (log cfu/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Duration of culture (h) | ||||||||
| 0 h | 3 h | 6 h | 9 h | 12 h | 18 h | 24 h | 48 h | |
| DEX | 6.2 ± 0.1 | 7.3 ± 0.1a | 8.4 ± 0.1a | 8.7 ± 0.1a | 8.8 ± 0.1a | 9.6 ± 0.2b | 9.7 ± 0.1c | 8.2 ± 0.1c |
| URB | 6.1 ± 0.1 | 7.0 ± 0.1c | 7.7 ± 0.1c | 8.5 ± 0.1c | 8.6 ± 0.1c | 9.8 ± 0.1a | 10.0 ± 0.1a | 9.2 ± 0.1a |
| PRB | 6.1 ± 0.1 | 7.1 ± 0.0bc | 7.8 ± 0.2bc | 8.6 ± 0.1b | 8.7 ± 0.1b | 9.6 ± 0.1b | 9.0 ± 0.2d | 8.9 ± 0.1b |
| BS | 6.1 ± 0.1 | 7.1 ± 0.1b | 7.9 ± 0.1b | 8.7 ± 0.1a | 8.8 ± 0.1ab | 9.8 ± 0.2a | 9.9 ± 0.0b | 9.3 ± 0.2a |
DEX = dextrose; URB = unpeeled raw banana; PRB = peeled raw banana; BS = banana starch; Values are means of triplicate analysis (n = 3).
In a column, means not sharing a common letter are significantly different at P < 0.05.
4. Conclusions
URB and BS from Kluai Namwa Luang are essential and potential sources of nutrients which were beneficial for digestive functions. URB is a rich source of dietary fiber (9.7 ± 0.2 g per 100 g of dried sample), whereas BS is as a rich source of RS (74.1 ± 0.1 g per 100 g of dried sample). The B-type crystalline structure of powder and starch from Kluai Namwa Luang resulted in the limitations of various digestive enzymes' accessibility and led to their digestive resistant properties. Both URB and BS exhibited prebiotic properties and had the ability to promote the growth of probiotics which were highly resistance to the hydrolyzation by α-amylase and trypsin. Since URB and BS possessed excellent prebiotic properties, which were similar to the commercial inulin, they are suggested to be developed as a health promotion product. However, chain-length distribution and the molecular weight of banana starch from Kluai Namwa Luang are suggested for further study.
Declarations
Author contribution statement
Patthanakorn Jaiturong, Nachtharinee Laosirisathian: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Busaban Sirithunyalug, Sukum Eitssayeam, Sasithorn Sirilun, Wantida Chaiyana, Jakkapan Sirithunyalug: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Research Council of Thailand, Bangkok, Thailand and Chiang Mai University, Thailand.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was carried out while the authors were studying at Chiang Mai University.
Contributor Information
Wantida Chaiyana, Email: wantida.chaiyana@gmail.com.
Jakkapan Sirithunyalug, Email: jakkapan.s@cmu.ac.th.
References
- 1.Zozimo R.O.B., Ratanasut K., Boonsrangsom T., Sujipuli K. Assessment of genetic diversity among Thai banana cultivars (Musa spp.) based on RAPD and SRAP markers. Int. J. Biosci. 2018;12(4):172–180. [Google Scholar]
- 2.Silayoi B., Babpraserth C., Wanichakul K., Suvitavat K. Banana Genetic Resources in Thailand. Research Institute for Languages Cultures of Asia and Africa; 2005. Multiply useful plants uses and usefulness; pp. 100–112. Thailand. [Google Scholar]
- 3.Silayoi B. Saranukrom Thai. 1st. vol. 30. THAI JUNIOR ENCYCLOPEDIA FOUNDATION by Hismajesty king Bhumibol Adulyadej The Great; 2005. Banana. (Saranukromthai, Thai Junior Encyclopedia Project by Royal Command of H.M. The King). Bangkok, Thailand. [Google Scholar]
- 4.Banana cultivars in Thailand. http://saranukromthai.or.th/sub/book/book.php?book=30&chap=6&page=t30-6infodetail05.html IOP Publishing PhysicsWeb.
- 5.Siriboon N., Banlusilp P. A study on the ripening process of ‘Namwa’ banana. AU J. Tech. 2007;7(4):159–164. http://repository.au.edu/handle/6623004553/13321 [Google Scholar]
- 6.Faisant N., Buleon A., Colonna P., Molis C., Lartigue S., Galmiche J.P. Digestion of raw banana starch in the small intestine of healthy humans: structural features of resistant starch. Br. J. Nutr. 1995;73(1):111–123. [PubMed] [Google Scholar]
- 7.Fuentes-Zaragoza E., Riquelme-Navarrete M.J., Sanchez-Zapata E., Perez- Alvarez J.A. Resistant starch as functional ingredient: a review. Food Res. Int. 2010;43:931–942. [Google Scholar]
- 8.Campuzano A., Rosell C.M., Cornejo F. Physicochemical and nutritional characteristics of banana flour during ripening. Food Chem. 2008;256:11–17. doi: 10.1016/j.foodchem.2018.02.113. [DOI] [PubMed] [Google Scholar]
- 9.Li B., Zhang Y., Xu F., Khan M.R., Zhang Y., Huang C. Supramolecular structure of Artocarpus heterophyllus Lam seed starch prepared by improved extrusion cooking technology and its relationship with in vitro digestibility. Food Chem. 2021;336:127716. doi: 10.1016/j.foodchem.2020.127716. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Zuo H., Xu F., Zhu K., Tan L., Dong W. The digestion mechanism of jackfruit seed starch using improved extrusion cooking technology. Food Hydrocolloids. 2021;110:106154. [Google Scholar]
- 11.Zhang Y., Zhang Y., Li B., Wang X., Xu F., Zhu K. In vitro hydrolysis and estimated glycemic index of jackfruit seed starch prepared by improved extrusion cooking technology. Int. J. Biol. Macromol. 2019;121:1109–1117. doi: 10.1016/j.ijbiomac.2018.10.075. [DOI] [PubMed] [Google Scholar]
- 12.Vatanasuchart N., Niyomwit B., Wongkrajang W. Resistant starch content, in vitro digestibility and physic-chemical properties of flour and starch from Thai bananas. Maejo Int J. Sci. Tech. 2012;6(2):259–271. [Google Scholar]
- 13.Asp N.G., Van Amelsvoort J.M.M., Hautvast J.G.A.J. Nutritional implications of resistant starch. Nutr. Res. Rev. 1996;9(1):1–31. doi: 10.1079/NRR19960004. [DOI] [PubMed] [Google Scholar]
- 14.Hasjim J. Enzyme digestibility of starch and methods to produce enzyme-resistant starch to improve human health. Food Sci. Human Nutr. 2009 Ph.D. Thesis. Iowa State University. [Google Scholar]
- 15.Sajilata M.G., Singhal R.S., Kulkarni P.R. Resistant starch-A review. Compr. Rev. Food Sci. F. 2006;5(1):1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 16.Ai Y. Structures, properties, and digestibility of resistant starch. Food Sci. Human Nutr. 2013 Ph.D. Thesis. Iowa State University. [Google Scholar]
- 17.Gao H., Huang S., Dong T., Yang Q., Yi G. Analysis of resistant starch degradation in postharvest ripening of two banana cultivars: focus on starch structure and amylases. Postharvest Biol. Technol. 2016;119:1–8. [Google Scholar]
- 18.Zhang Y., Zuo H., Xu F., Zhu K., Tan L., Dong W. The digestion mechanism of jackfruit seed starch using improved extrusion cooking technology. Food Hydrocolloids. 2021;110:106154. [Google Scholar]
- 19.Gibson R.G., Roberfroid B.M. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 20.Pandey K.R., Naik S.R., Vakil B.V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015;52(12):7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cashman K. Prebiotics and calcium bioavailability. Curr. Issues Intest. Microbiol. 2003;4(1):21–32. [PubMed] [Google Scholar]
- 22.Dronamraju S.S., Coxhead J.M., Kelly S.B., Burn J., Mathers J.C. Cell kinetics and gene expression changes in colorectal cancer patients given resistant starch – a randomized controlled trial. Gut. 2009;58(3):413–420. doi: 10.1136/gut.2008.162933. [DOI] [PubMed] [Google Scholar]
- 23.Tsen J.H., Lin Y.P., King V. Fermentation of banana media by using κ-carrageenan immobilized Lactobacillus acidophilus. Int. J. Food Microbiol. 2004;91(2):215–220. doi: 10.1016/S0168-1605(03)00376-3. [DOI] [PubMed] [Google Scholar]
- 24.Roberfroid M. Prebiotics: the concept revised. J. Nutr. 2007;137(3):355–363. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 25.Ordiz M.I., May D.T., Mihindukulasuriya K., Martin J., Crowley J., Tarr I.P. The effect of dietary resistant starch type 2 on the microbiota and markers of gut inflammation in rural Malawi children. Microbiome. 2015;3(37) doi: 10.1186/s40168-015-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimsung P., Thongngam M., Naivikul O. Compositions, morphological and thermal properties of green banana flour starch. Kasetsart J. (Nat. Sci.) 2007;41:324–330. [Google Scholar]
- 27.Association of Official . eighteenth ed. AOAC International; Washington, DC, USA: 2005. Analytical Chemist Official Methods of Analysis. [Google Scholar]
- 28.Jaiturong P., Sutjarittangtham K., Eitsayeam S., Sirithunyalug J. Preparation of glutinous rice starch nanofibers by electrospinning. Adv. Mater. Res. 2012;506:230–233. [Google Scholar]
- 29.Jane J.L., Wong K.S., McPherson A.E. Branch-structure difference in starches of A- and B-type x-ray patterns revealed by their Naegeli dextrins. Carbohydr. Res. 1999;300(3):219–227. [Google Scholar]
- 30.Moffat J.G., Qi S., Craig D.Q.M. Spatial characterization of hot melt extruded dispersion systems using thermal atomic force microscopy methods: the effects of processing parameters on phase separation. Pharm. Res. 2014;31(7):1744–1752. doi: 10.1007/s11095-013-1279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suvakanta D., Narsimha M.P., Pulak D., Joshabir C., Biswajit D. Optimization and characterization of purified polysaccharide from Musa sapientum L. as a pharmaceutical excipient. Food Chem. 2014;149:76–83. doi: 10.1016/j.foodchem.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 32.Association of Official Analytical Chemist Official methods of analysis . nineteenth ed. AOAC International; Rockville, MD, USA: 2012. Methods 2002.02. [Google Scholar]
- 33.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. [Google Scholar]
- 34.Dubois M., Gilles K.A., Hhamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 35.Palframan R., Gibson G.R., Rastall R.A. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett. Appl. Microbiol. 2003;37(4):281–284. doi: 10.1046/j.1472-765x.2003.01398.x. [DOI] [PubMed] [Google Scholar]
- 36.Pelissari F.M., Andrade-Mahechal M.M., Sobral P.J.A., Menegalli F.C. Isolation and characterization of the flour and starch of plantain bananas (Musa paradisiaca) Starch/Stärke. 2012;64(5):382–391. [Google Scholar]
- 37.Hung P.V., Cham N.T.M., Truc P.T.T. Characterization of Vietnamese banana starch and its resistant starch improvement. Int. Food Res. J. 2013;20(1):205–211. [Google Scholar]
- 38.Bezerra C.V., Amante E.R., de Oliveira D.C., Rodrigues A.M.C., da Silva L.H.M. Green banana (Musa cavendishii) flour obtained in spouted bed – effect of drying on physic-chemical, functional and morphological characteristics of the starch. Ind. Crops. Prod. 2013;41:241–249. [Google Scholar]
- 39.Brown I.L., McNaught K.J., Ganly R.N., Conway P.L. 1996. Probiotic Compositions. International Patent WO 96/08261/A1. [Google Scholar]
- 40.Hausler O. Starch. In: Rowe C.R., Sheskey J.P., Quinn E.M., editors. Handbook of Pharmaceutical Excipients. sixth ed. Pharmaceutical press; London: 2009. pp. 685–691. [Google Scholar]
- 41.Englyst H.N., Kingman S.M. Dietary Fiber. Springer; Boston, MA: 1990. Dietary fiber and resistant starch; pp. 49–65. [Google Scholar]
- 42.Nimsung P. Kasetsart University; Bangkok, Thailand: 2007. Properties of Raw Banana Flour and Starch Using for Food industry. Master’s Thesis. [Google Scholar]
- 43.Kumar R., Khatkar B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017;54(8):2403–2410. doi: 10.1007/s13197-017-2681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung H.J., Liu Q., Lee L., Wei D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011;25(5):968–975. [Google Scholar]
- 45.Gallant D.J., Bouchet B., Baldwin P.M. Microscopy of starch: evidence of a new level of granule organization. Carbohydr. Polym. 1997;32:177–191. [Google Scholar]
- 46.Shamai K., Bianco-Peled H., Shimoni E. Polymorphism of resistant starch type III. Carbohydr. Polym. 2003;54:363–369. [Google Scholar]
- 47.Park J.E., Bae I.Y., Lee H.G. Effects of amylose contents and degree of gelatinization of rice flour on in vitro starch digestibility, physical characteristics, and morphological properties. Food Eng. Prog. 2017;21(4):341–350. [Google Scholar]
- 48.Wang S., Sun Y., Wang J., Wang S., Copeland L. Molecular disassembly of rice and lotus starches during thermal processing and its effect on starch digestibility. Food Funct. 2016;7(2):1188–1195. doi: 10.1039/c6fo00067c. [DOI] [PubMed] [Google Scholar]
- 49.Hoover R.F., Senanayake S.P.J.N. Composition and physicochemical properties of oat starches. Food Res. Int. 1996;29:15–26. [Google Scholar]
- 50.Magallanes-Cruz P.A., Flores-Silva P.C., Bello-Perez L.A. Starch structure influences its digestibility: a review. J. Food Sci. 2017;82(9):2016–2023. doi: 10.1111/1750-3841.13809. [DOI] [PubMed] [Google Scholar]
- 51.Zhang P., Whilstler R.L., BeMiller J.N., Hamaker B.R. Banana starch: production, physicochemical properties and digestibility-A review. Carbohydr. Polym. 2005;59:443–458. [Google Scholar]
- 52.Campuzano A., Rosell C.M., Cornejo F. Physicochemical and nutritional characteristics of banana flour during ripening. Food Chem. 2018;256:11–17. doi: 10.1016/j.foodchem.2018.02.113. [DOI] [PubMed] [Google Scholar]
- 53.Gallant D.J., Bouchet B., Buleon A., Perez S. Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur. J. Clin. Nutr. 1992;46(Suppl 2):3–16. [PubMed] [Google Scholar]
- 54.Wang J., Huang H.H., Chen P.S. Structural and physicochemical properties of banana resistant starch from four cultivars. Int. J. Food Prop. 2017;20(6):1338–1347. [Google Scholar]
- 55.Jiang G., Liu Q. Characterization of residue from partially hydrolyzed potato and high amylose corn starches by pancreatic α-amylase. Starch/Stärke. 2002;54:527–533. [Google Scholar]
- 56.Li Z., Guo K., Lin L., He W., Zhang L., Wei C. Comparison of physicochemical properties of starches from flesh and peel of green banana fruit. Molecules. 2018;23(9):2312. doi: 10.3390/molecules23092312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sevenou O., Hill S.E., Farhat I.A., Mitchell J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002;31:79–85. doi: 10.1016/s0141-8130(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 58.Zhu H., Yang J., Jiang Y., Zeng J., Zhou X., Yanglin H., Yang B. Morin as a preservative for delaying senescence of banana. Biomolecules. 2018;8:52. doi: 10.3390/biom8030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodrıguez-Ambriz S.L., Islas-Hernández J.J., Agama-Acevedo E., Tovar J., Bello-Pérez L.A. Characterization of a fibre-rich powder prepared by liquefaction of unripe banana flour. Food Chem. 2008;107:1515–1521. [Google Scholar]
- 60.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powthong P., Jantrapanukorn B., Suntornthiticharoen P., Laohaphatanalert K. Study of prebiotic properties of selected banana species in Thailand. J. Food Sci. Technol. 2020;57(7):2490–2500. doi: 10.1007/s13197-020-04284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahore J.G., Shirolkar S.V. Investigation of effect of ripening and processing on prebiotic potential of banana. J. Young Pharm. 2018;10(4):409–413. [Google Scholar]
- 63.Alvarado-Jasso G.M., Camacho-Díaz B.H., Ocampo M.L.A., Jiménez-Ferrer J.E., Mora-Escobedo R., Osorio-Díaz P. Prebiotic effects of a mixture of agavins and green banana flour in a mouse model of obesity. J. Funct. Foods. 2020;64:103685. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.