Abstract
Introduction: We aimed to analyze patients with acute and chronic joint involvements in sarcoidosis.
Methods: This is a retrospective multicenter analysis of patients with proven sarcoidosis, as defined by clinical, radiological, and histological criteria, with at least one clinical and/or ultrasonographic synovitis.
Results: Thirty-nine patients with sarcoid arthropathy were included, and among them 19 had acute sarcoidosis (Lofgren's syndrome). Joint involvement and DAS44-CRP were not significantly different in acute and chronic sarcoid arthropathies. Acute forms were more frequent than chronic sarcoid arthropathy in Caucasians, without any difference of sex or age between these 2 forms. Joint involvement was frequently more symmetrical in acute than chronic forms (100 vs. 70%; p < 0.05), with a more frequent involvement in wrists and ankles in acute forms, whereas the tender and swollen joint counts and the DAS44-CRP were similar between the 2 groups. Skin lesions were significantly more frequent in patients with acute forms [17 (89%) vs. 5 (25%); p < 0.05] and were erythema nodosum in all patients with Löfgren's syndrome and sarcoid skin lesions in those with chronic sarcoidosis. Among 20 patients with chronic sarcoidosis, treatment was used in 17 (85%) cases, and consisted in NSAIDs alone (n = 5; 25%), steroids alone (n = 5; 25%), hydroxychloroquine (n = 2; 20%), methotrexate (n = 3; 15%), and TNF inhibitors (n = 2; 10%). A complete/partial joint response was noted in 14 (70%) cases with a DAS44-CRP reduction of 2.07 [1.85–2.44] (from 3.13 [2.76–3.42] to 1.06 [0.9–1.17]; p < 0.05).
Conclusion: Sarcoid arthropathies have different clinical phenotypes in acute and chronic forms and various treatment regimens such as hydroxychloroquine and methotrexate could be used in chronic forms.
Keywords: sarcoid arthropathy, outcome, methotrexate, infliximab, sarcoidosis
Introduction
Sarcoidosis is a heterogeneous systemic granulomatous disease affecting mostly lung and lymphatic nodes. Non-caseating granulomas are the characteristic histopathological feature. Joint involvement, also known as sarcoid arthropathy, is observed in 6–35% of patients, and asymptomatic bone involvement in 3–13% of patients. Acute-onset arthritis is generally characterized by symmetric arthritis, and in Lofgren's syndrome is associated with bilateral hilar adenopathies and erythema nodosum, with remission usually occurring within 6–10 weeks (1–5). Chronic sarcoid arthropathy is characterized by persistent oligo or poly-arthritis in 20% of patients, with 40% of arthralgia (1, 2). Few studies have described the prevalence and features of sarcoid arthropathies, and association with other organ involvements (2, 4, 6, 7). Non-steroidal anti-inflammatory drugs (NSAIDs) are effective in acute sarcoidosis, but little is known about the efficacy of other therapies in steroid-dependent and refractory forms of sarcoid arthropathy. We aimed to describe clinical characteristics of sarcoid arthropathies and describe the treatment.
Patients and Methods
Study Scheme
The study was observational with a retrospective analysis. A call for observation was sent to members of the “French Inflammatory Joint Disease Working Group” (Club Rhumatismes et Inflammation) and the National French Society of Internal Medicine (SNFMI) from July 2017 to November 2018. Physicians were asked to fill in a defined table. The study complied with the recommendations of the Declaration of Helsinki, and being observational and retrospective, ethics committee approval was not necessary according to the French local law. Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Patients
Patients were included if they were aged of 18 years or more, had histologically-proven sarcoidosis (except for typical acute forms of Löfgren syndrome) and at least one episode of arthritis, defined as the presence of clinical and/or ultrasonographic synovitis (8–11). The definition of ultrasonographic synovitis was used as usually done in patients with rheumatoid arthritis or other inflammatory arthritis. Exclusion criteria were: patients with other granulomatous diseases, associated rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, microcrystalline arthritis or infectious arthritis (3). All patients' medical records were reviewed by 2 investigators (Carlotta Cacciatore and Arsène Mekinian).
Data Collection
Clinical data was collected as follows: age, ethnicity, sex, date of diagnosis, disease duration, and presence of skin, lung, heart, ocular, and neurological involvements. Articular assessment, which was collected at diagnosis and during follow-up, included: tender joint count, swollen joint count, localization of joint involvement and DAS44-CRP. Synovitis was diagnosed by a clinical examination and/or joint ultrasonography: among 39 included patients with clinical synovitis, 31 have undergone joint ultrasonography and have all ultrasound-confirmed synovitis. Synovitis was defined as synovial hypertrophy ≥2, or synovial hypertrophy ≥1+PD signal ≥1, according to the OMERACT definitions (12). Joint activity was quantified using DAS28-CRP at diagnosis and during the follow up. The use of non-steroidal anti-inflammatory drugs (NSAIDs), steroids and/or synthetic disease-modifying antirheumatic drugs (DMARDs), hydroxychloroquine and TNF antagonists was collected. Laboratory data included serum hemoglobin, platelet, neutrophil and lymphocyte counts, calcium, creatinine, alanine aminotransferase, aspartate aminotransferase, C-reactive protein (CRP), erythrocyte sedimentation rates (ESR), gamma-globulin, angiotensin converting enzyme (ACE), rheumatoid factor, antinuclear and anti-citrullinated protein antibodies (ACPA). Sarcoidosis was considered as acute if remission was completely reached within 12 weeks. Löfgren's syndrome was defined in patients with acute sarcoidosis in the presence of arthritis, bilateral hilar adenopathy and erythema nodosum, and fever (6, 13). All remaining cases were defined as chronic sarcoid arthropathy. Efficacy of each drug was compared to each other, and all treatment lines were analyzed. Complete articular response to treatment was defined as total disappearance of arthralgia and synovitis, with a DAS44-CRP <1.6 (14, 15). Partial response was defined as an improvement of at least 50% of swollen joint count (i.e., number of synovitis). Non-response was defined as all remaining cases. For each line of treatment, the reasons of interruption has been codified among inefficacy, adverse events and remission.
Statistical Analysis
Data is expressed as medians with ranges and numbers with frequencies. Student t-test or Wilcoxon-Mann Whitney test were used to compare quantitative data and Chi-square or Fisher's exact tests for qualitative variables. All analyses were done using R software (R Foundation, Vienna, Austria version 3.0.2) and a p < 0.05 was considered as significant.
Results
Thirty-nine patients (64% women) were included with a median age of 41 years [25–75] (Table 1). Among 39 patients with clinical arthritis, 31 (82%) had done ultrasound echography and all these 31 cases had ultrasound-confirmed synovitis. The pattern of arthritis was mostly symmetric polyarthritis affecting ankles in 33 (85%) cases, wrists in 18 (46%) cases and metacarpo-phalangeal joints in 12 (31%) cases. None reported spine involvement or dactylitis. Median tender and swollen joint count at diagnosis was 6 [1–28] and 2 [1–6], respectively. C-reactive protein levels were at 22 mg/l [1–271] and ACE at 65 U/L [21.2–300]. Median DAS44-CRP at diagnosis was 3.4 [2.3–5.9]. No patients had positive rheumatoid factor or ACPA, nor radiological structural damage. Acute forms were diagnosed in 19 (49%) patients and among them 17 (89%) have Löfgren syndrome. The remaining 20 (51%) patients have chronic sarcoid arthropathy. Acute forms were more frequent than chronic sarcoid arthropathies in caucasians, without any difference of sex or age between these 2 forms. Joint involvement was frequently more symmetrical in acute than chronic forms (100 vs. 70%; p < 0.05), with a more frequent involvement in wrists and ankles in acute forms, whereas the tender and swollen joint counts and the DAS44-CRP were similar between the 2 groups (Table 1). Skin lesions were significantly more frequent in patients with acute forms [17 (89%) vs. 5 (25%); p < 0.05] and were erythema nodosum in all patients with Löfgren's syndrome and sarcoid skin lesions in those with chronic sarcoidosis. Ocular involvement was present only in patients with chronic sarcoidosis (n = 4, 20%): one chorioretinitis and anterior uveitis (n = 3). There were no significant differences in calcium levels, ACE, gammaglobulin, and lymphocyte levels at diagnosis between patients with acute and chronic sarcoid arthropathies (data not shown).
Table 1.
Characteristics of patients with joint involvement and acute/chronic sarcoidosis.
| Characteristics |
All patients N = 39 |
Acute sarcoidosis N = 19 |
Chronic sarcoidosis N = 20 |
|---|---|---|---|
| Age (years), medians [ranges] | 38 [23–70] | 34.5 [23–70] | 42 [25–65] |
| Female sex (n;%) | 25 (64) | 13 (68) | 12 (60) |
| Caucasians (n;%) | 19 (48.7) | 12 (63) | 7 (35)* |
| Extra-articular involvement | |||
| Skin involvement (n;%) | 22 (56) | 17 (89) | 5 (25)* |
| Ocular involvement (n;%) | 4 (10) | 0 | 4 (20)* |
| Hilar and mediastinal adenopathies (n;%) Lung involvement (n;%) |
19 (49) 32 (82) |
8 (42) 16 (84) |
11 (55)* 16 (80) |
| Heart involvement (n;%) CNS involvement (n;%) |
2 (5) 1 (2.5) |
0 0 |
2 (10) 1 (5) |
| Joint involvement | |||
| Symmetrical (n;%) Wrist (n;%) Ankles (n;%) Metacarpo-phalangeal (n;%) Knees (n;%) |
33 (85) 18 (46) 32 (82) 12 (31) 16 (41) |
19 (100) 5 (26) 17 (89) 4 (21) 6 (32) |
14 (70)* 10 (50)* 13 (65)* 6 (30) 10 (50) |
| Tender joints medians [ranges] | 6 [1–28] | 4 [2–28] | 6 [1–12] |
| Swollen joints medians [ranges] | 2 [1–6] | 2 [1–4] | 2 [1–6] |
| DAS 44-CRP medians [ranges] | 3.4 [2.3–5.9] | 3.7 [2.3–5.9] | 3.4 [2.3–3.5] |
| Laboratory data | |||
| Lymphocytes (G/l) medians [ranges] | 1.410 [0.66–3.35] | 1.8 [0.66–2.50] | 1.11 [0.71–3.35] |
| Gammaglobulins (g/l) medians [ranges] | 11.6 [7.5–24.7] | 11.4 [7.5–13.3] | 12.3 [7.8–24.7] |
| Calcium levels (mg/l) medians [ranges] | 2.34 [2.16–2.57] | 2.3 [2.16–2.53] | 2.36 [2.26–2.57] |
| ACE (UI/l) medians [ranges] | 65 [21.2–300] | 55 [21.20–111] | 76.5 [36–300] |
| First line treatments | 34 (87) | 17 (89) | 17 (85) |
| NSAIDs alone (n;%) | 15 (38) | 10 (53) | 5 (25)* |
| Steroids alone (n;%) | 9 (23) | 4 (21) | 5 (25) |
| Hydroxychloroquine (n;%) With NSAIDs With steroids |
4 (10) 3 (7.5) 0 |
2 (10) 2 (10) 0 |
2 (10) 1 (5) 0 |
| Methotrexate (n;%) With NSAIDs With steroids |
4 (10) 1 (2.5) 2 (5) |
1 (5) 1 (5) 0 |
3 (15) 0 2 (10) |
| TNF inhibitors (n;%) With steroids |
2 (5) | 0 0 |
5 (25) 5 (10) |
| Follow-up (months) medians [ranges] | 18 [3–264] | 20.5 [3–57] | 62.5 [3–264]* |
Values are medians with interquartiles and numbers with frequencies.
p < 0.05.
ACPA, anti Citrullinated Peptide antibodies; ANA, anti-nuclear antibodies; CRP, C-reactive protein; ESR, erythrocyte-sedimentation rate; NSAIDs, non-steroidal anti-inflammatories; RF, rheumatoid factor.
During the median follow-up of 18 [3–264] months, 34 patients (87%) received at least one therapy among NSAIDs, steroids, methotrexate, hydroxychloroquine or TNF inhibitors. Among the 19 patients with acute sarcoidosis, treatment was used in 17 (89%) cases, and consisted of NSAIDs alone (n = 10; 53%), steroids alone (n = 4; 21%), hydroxychloroquine (n = 2; 10%), and methotrexate (n = 1; 5%) (Table 1). A complete/partial joint response was noted in 15 (79%) cases with a DAS44-CRP reduction of 2.45 [1.66–2.66] (from 3.37 [2.62–3.48] to 0.92 [0.89–1.58]; p < 0.05).
Among 20 patients with chronic sarcoidosis, a first-line treatment was used in 17 (85%) cases, and consisted of NSAIDs alone (n = 5; 25%), steroids alone (n = 5; 25%), hydroxychloroquine (n = 2; 20%), methotrexate (n = 3; 15%), and TNF inhibitors (n = 5; 25%) (Table 1). A complete/partial joint response was noted in 14 (70%) cases with a DAS44-CRP reduction of DAS44-CRP reduction of 2.07 [1.85–2.44] (from 3.13 [2.76–3.42] to 1.06 [0.9–1.17]; p < 0.05). A second-line therapy for chronic sarcoidosis was used in 8 cases: methotrexate (n = 6), steroids alone and hydroxychloroquine (n = 1 each) with a joint response in 6 cases. A third-line therapy for sarcoid chronic arthropathy was used in 5 cases and among them TNF inhibitors in 5 cases with joint responses in all cases.
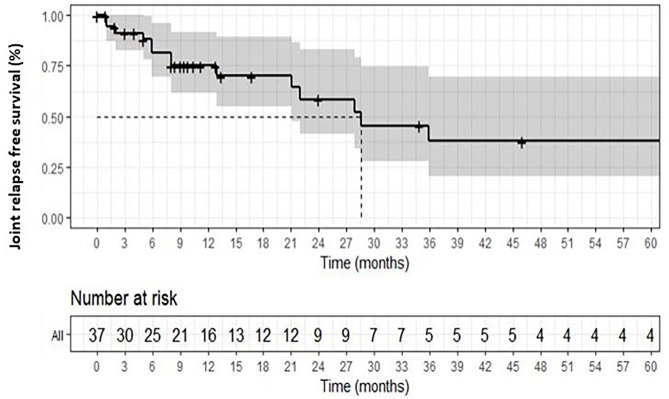
NSAIDs was the most frequent first-line option in acute sarcoidosis [10 (53%) vs. 5 (25%); p < 0.05], but other first-line therapy frequencies were not significantly different between the 2 groups. The median time of treatment was at 2.5 months [0.6–7]. The joint relapse-free survival was at 26.6 months in the overall group (Figure 1).
Figure 1.
Overall relapse-free survival in sarcoid arthropathy.
Discussion
This study reports clinical and laboratory features of acute and chronic sarcoid arthropathies, and shows some significant differences in joint and extra-articular involvements. The acute forms have a more symmetrical joint distribution with predominant skin involvement, whereas chronic sarcoid arthropathies have more frequent ocular involvements (16).
Joint involvement in sarcoidosis ranges from 6 to 35% (2, 11, 13–15, 17–19). Clinical features of acute and chronic sarcoid arthropathies have rarely been analyzed (20, 21). The chronic form is usually associated with parenchymal lung or other organ involvements and is relatively rare (up to 7%) (4, 18, 22, 23). In our study, swollen joint count and DAS44 CRP were comparable in both sarcoid arthropathies, but acute forms were frequently more symmetrical and affected ankles (4). DAS-28 score is not validated in the setup of sarcoidosis, and in particular does not include the ankle involvement which could be frequent in acute forms, but has already previously been used in other inflammatory arthritis and sarcoid arthritis (24, 25). Consistently with previous studies, skin involvement (mostly erythema nodosum) was particularly frequent in acute sarcoid arthropathy, whereas ocular involvement occurred just in chronic subsets (26). The presence and features of hilar and lung involvements were not discriminatory between acute and chronic sarcoid forms.
Management of sarcoid arthropathy is poorly studied (11, 27): most data involves acute forms, with chronic forms only described in small case-series. In this study, we compared the efficacy of various treatment regimens on sarcoid arthropathy with clinical synovitis. Using pooled lines of various therapies, we showed no significant differences in joint responses using hydroxychloroquine, methotrexate and TNF antagonists. Previous studies confirmed the efficacy of NSAIDs and steroids for acute sarcoid arthropathy (13, 18, 28), as we reported in the present study. For chronic sarcoid arthropathy, small case-series showed the benefit of methotrexate (29, 30), whereas in a retrospective study among 10 patients treated with TNF antagonists, despite initial rapid efficacy, no clinical amelioration was noted after 1 year of treatment (24). Judson et al. conducted the only trial evaluating sarcoidosis joint manifestations under infliximab. No satisfactory effect on joint disease was observed at 24 and 48 weeks, even though significant attenuation of involvement for all combined organs was observed at 28 weeks (22). TNF- antagonists could also have a steroid-sparing effect, in particular in chronic sarcoidosis (22, 24, 25).
Our study has several biases that limit definitive conclusions. Firstly, it is a retrospective study with small sample sizes, and the small number of patients treated by TNF antagonists does not allow definitive conclusions about efficacy of this therapy in joint involvement. We assessed disease activity with DAS44-CRP score usually used for rheumatoid arthritis, as some other previous studies (24), because no validated score exists to evaluate joint activity in sarcoidosis.
Conclusion
Sarcoid arthropathies have different clinical phenotypes in acute and chronic forms and various treatment regimens such as hydroxychloroquine and methotrexate could be used in chronic forms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.565420/full#supplementary-material
References
- 1.Nessrine A, Zahra AF, Taoufik H, Nessrine A, Zahra AF, Taoufik H. Musculoskeletal involvement in sarcoidosis. J Bras Pneumol. (2014) 40:175–82. 10.1590/S1806-37132014000200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser H, Vos K, Zanelli E, Verduyn W, Schreuder GM, Speyer I, et al. Sarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factors. Ann Rheum Dis. (2002) 61:499–504. 10.1136/ard.61.6.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glennås A, Kvien TK, Melby K, Refvem OK, Andrup O, Karstensen B, et al. Acute sarcoid arthritis: occurrence, seasonal onset, clinical features and outcome. Br J Rheumatol. (1995) 34:45–50. 10.1093/rheumatology/34.1.45 [DOI] [PubMed] [Google Scholar]
- 4.Kobak S, Sever F, Usluer O, Goksel T, Orman M. The clinical characteristics of sarcoid arthropathy based on a prospective cohort study. Ther Adv Musculoskelet Dis. (2016) 8:220–4. 10.1177/1759720X16670598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Kofahi K, Korsten P, Ascoli C, Virupannavar S, Mirsaeidi M, Chang I, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. (2016) 12:1623–34. 10.2147/TCRM.S74476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungprasert P, Crowson CS, Matteson EL. Clinical characteristics of sarcoid arthropathy: a population-based study. Arthritis Care Res. (2016) 68:695–9. 10.1002/acr.22737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz C, Restrepo-Escobar M, Martínez-Muñoz M, Echeverri A, Márquez J, Pinto LF. Differences between patients with sarcoidosis with and without joint involvement treated for fifteen years in a third level hospital. Reumatol Clin. (2020) 16:45–8. 10.1016/j.reumae.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Statement on Sarcoidosis Am J Respir Crit Care Med. (1999) 160:736–55. 10.1164/ajrccm.160.2.ats4-99 [DOI] [PubMed] [Google Scholar]
- 9.Le Bras E, Ehrenstein B, Fleck M, Hartung W. Evaluation of ankle swelling due to Lofgren's syndrome: a pilot study using B-mode and power Doppler ultrasonography. Arthritis Care Res. (2014) 66:318–22. 10.1002/acr.22099 [DOI] [PubMed] [Google Scholar]
- 10.Kellner H, Späthling S, Herzer P. Ultrasound findings in Löfgren's syndrome: is ankle swelling caused by arthritis, tenosynovitis or periarthritis? J Rheumatol. (1992) 19:38–41. [PubMed] [Google Scholar]
- 11.Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. (2013) 39:277–97. 10.1016/j.rdc.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Agostino MA, Terslev L, Aegerter P, Backhaus M, Balint P, Bruyn GA, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-part 1: definition and development of a standardised, consensus-based scoring system. RMD Open. (2017) 3:e000428. 10.1136/rmdopen-2016-000428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byun CW, Yang SN, Yoon JS, Kim SH. Lofgren's syndrome-acute onset sarcoidosis and polyarthralgia: a case report. Ann Rehabil Med. (2013) 37:295–9. 10.5535/arm.2013.37.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann RM, van der Heijde D, Gardiner PV, Szumski A, Marshall L, Bananis E. DAS28-CRP and DAS28-ESR cut-offs for high disease activity in rheumatoid arthritis are not interchangeable. RMD Open. (2017) 3:e000382 10.1136/rmdopen-2016-000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burmester GR, Ferraccioli G, Flipo R-M, Monteagudo-Sáez I, Unnebrink K, Kary S, et al. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. (2008) 59:32–41. 10.1002/art.23247 [DOI] [PubMed] [Google Scholar]
- 16.Sweiss NJ, Patterson K, Sawaqed R, Jabbar U, Korsten P, Hogarth K, et al. Rheumatologic manifestations of sarcoidosis. Semin Respir Crit Care Med. (2010) 31:463–73. 10.1055/s-0030-1262214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirabayashi Y, Ishii T. The DAS28-ESR cutoff value necessary to achieve remission under the new Boolean-based remission criteria in patients receiving tocilizumab. Clin Rheumatol. (2013) 32:123–7. 10.1007/s10067-012-2103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of sarcoidosis 1946-2013: a population-based study. Mayo Clin Proc. (2016) 91:183–8. 10.1016/j.mayocp.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in Black women in the United States. Chest. (2011) 139:144–50. 10.1378/chest.10-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio-Rivas M, Franco J, Corbella X. Sarcoidosis presenting with and without Löfgren's syndrome: Clinical, radiological and behavioral differences observed in a group of 691patients. Joint Bone Spine. (2020) 87:141–7. 10.1016/j.jbspin.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Bechman K, Christidis D, Walsh S, Birring SS, Galloway J. A review of the musculoskeletal manifestations of sarcoidosis. Rheumatology. (2018) 57:777–83. 10.1093/rheumatology/kex317 [DOI] [PubMed] [Google Scholar]
- 22.Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. (2008) 31:1189–96. 10.1183/09031936.00051907 [DOI] [PubMed] [Google Scholar]
- 23.Eschard JP, Etienne JC. [Osteoarticular manifestations of sarcoidosis]. Rev Med Interne. (1994) 15(Suppl. 3):305–7S. [PubMed] [Google Scholar]
- 24.Banse C, Bisson-Vaivre A, Kozyreff-Meurice M, Vittecoq O, Goëb V. No impact of tumor necrosis-factor antagonists on the joint manifestations of sarcoidosis. Int J Gen Med. (2013) 6:605–11. 10.2147/IJGM.S44542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banse C, Goëb V. Do not forget the joint involvement of sarcoidosis. Immunotherapy. (2015) 7:599–600. 10.2217/imt.15.24 [DOI] [PubMed] [Google Scholar]
- 26.Schupp JC, Freitag-Wolf S, Bargagli E, Mihailović-Vučinić V, Rottoli P, Grubanovic A, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. (2018) 51:1700991. [DOI] [PubMed] [Google Scholar]
- 27.Awada H, Abi-Karam G, Fayad F. Musculoskeletal and other extrapulmonary disorders in sarcoidosis. Best Pract Res Clin Rheumatol. (2003) 17:971–87. 10.1016/j.berh.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Matsui K, Adachi M, Kawasaki Y, Matsuda K, Shinohara K. Sarcoidosis acutely involving the musculoskeletal system. Intern Med. (2007) 46:1471–4. 10.2169/internalmedicine.46.6375 [DOI] [PubMed] [Google Scholar]
- 29.Suda T, Sato A, Toyoshima M, Imokawa S, Yoshitomi A, Tamura R, et al. Weekly low-dose methotrexate therapy for sarcoidosis. Intern Med Tokyo JPN. (1994) 33:437–40. 10.2169/internalmedicine.33.437 [DOI] [PubMed] [Google Scholar]
- 30.Kaye O, Palazzo E, Grossin M, Bourgeois P, Kahn MF, Malaise MG. Low-dose methotrexate: an effective corticosteroid-sparing agent in the musculoskeletal manifestations of sarcoidosis. Rheumatology. (1995) 34:642–4. 10.1093/rheumatology/34.7.642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.