Abstract
SARS-CoV-2, the novel coronavirus strain responsible for the current pandemic of COVID-19, has rendered the entire humanity suffering. Several months have passed since the pandemic has struck. However, the world is still looking for an effective treatment plan to battle the viral infection. The first vaccine just received emergency approval in December 2020 for use in USA and UK. These are excellent news, however, the worldwide distribution of such vaccine, the possibility of virus mutation and the lack of data regarding the long-term effects of such vaccines are a significant concern. In addition, although remdesivir was recently approved by the FDA to be used as a clinical drug against COVID-19, it hasn’t stood out yet as a proven form of therapeutics. Such inability to produce a novel therapy has caused enough inconveniences for the affected people worldwide. Repurposing the already available drugs to fight against the virus seems to be a reasonable option amidst such uncertainty. Given the vast collection of potential treatment candidates to be explored against COVID-19, there is a decent chance that a success in this regard will serve the intermediary purpose of clinically treating the infection until a COVID-19 vaccine is widely distributed worldwide and will be able to treat COVID-19 patients that do not adequately respond to vaccines. Such treatments may prove very useful in future coronavirus outbreaks too. Proper research into these repurposing treatments may yield a certain insight into the field of novel treatment production as well. This review study accumulates a relevant set of information about drugs and vaccines against COVID-19, in terms of their repurposing properties and the specific phases of clinical trials they are undergoing across the world. A potential timeline is also suggested to estimate when an effective result can be expected from the ongoing clinical trials for a better anticipation of the drug landscape. This study will hopefully help accelerate investment of resources into development and discovery of drugs and vaccines against the infection.
Keywords: COVID-19, SARS-CoV-2, phases, clinical trial, vaccine, therapeutics, drug repositioning
Summary
1. Introduction
2. History of COVID-19 and its Treatment
3. Methodology and Sources
4. COVID-19 Drug R&D Landscape
5. Types of Treatments in Clinical Trial
5.1 Biological Treatments
5.2 Chemically Derived Drugs
6. Vaccines
7. Countries and Sponsors Involved in Drug Trials
8. Timeline and Clinical Trial Phases
9. Conclusion
1. Introduction
SARS-CoV-2, a new coronavirus strain detected in Wuhan City, Hubei Province, China is the infectious agent behind the coronavirus disease, 2019 (COVID-19), for which no efficient treatment is available to date1,2. This inability to produce a cure is surprisingly consistent with no approved medication being present for previous two major coronavirus outbreaks (SARS-CoV in 2003 and MERS-CoV in 2015) from the same genus3,4. An effective vaccine is important in order to obtain herd immunity5. The first vaccine just received emergency approval in December 2020 for use in USA and UK. However, it will take months to have it distributed worldwide, its long-term effects are hard to predict, and the possibility of future resistant mutations is a concern. In addition, because of novelty of the SARS-CoV-2 strain and its constant mutative nature, it is hard to synthesize a universal vaccine, so soon enough to be used across the globe right now6,7. In fact, SARS-CoV-2 has affected almost all the countries and provinces in the world and needs immediate attention by extensive approaches to sidestep the symptoms and the disease in a steady manner8,9. Having that in focus, repurposed drugs may serve as an intermediate purpose of treating the disease until a stronger novel drug or an universal vaccine is developed. Repurposed drugs simply work following a manipulated mechanism in already approved drugs for other conditions directed towards weakening the SARS-CoV-2 in a system10. Research into such an area requires a landscape of probable drug candidates to be observed on a regular basis. Now, a good number of drug companies, industries and other institutions around the world are investing into developing aptly repurposed drugs to tackle this global crisis. Here, we have compiled information about 876 clinical trials of various drugs that are currently being researched through tests to be finalized as drug candidates. We have categorized the drugs in terms of their nature to serve a better understanding of the purposes of each drug and the duration the drugs may require completing the clinical trial. By a comparative arrangement, we have presented a dynamic view of drugs to figure out their true potentials.
2. History of COVID-19 and its Treatment
SARS‑CoV‑2 belongs to the subfamily Orthocoronavirinae, family Coronaviridae, order Nidovirales. It comprises of four subtypes with two among them, named α and β, infecting humans, whereas the other two, named γ and δ, more likely to infect animals. It is only the seventh coronavirus known to infect humans until now and only the third to have caused a severe outbreak after SARS-CoV and MERS-CoV. Other coronaviruses, such as HKUI, NL63, OC43 and 229E manifest with milder symptoms compared to the ones in Betacoronavirus genus11,12. Being zoonotic in nature, it is believed that coronaviruses spread to humans from animals. Two cases were previously reported where human infections resulted in drastic disease. The first one was the 2002-2004 SARS outbreak, when humans got infected via the betacoronavirus named SARS-CoV, which is usually found in bats, resulting in 8,422 infections and 916 deaths around the world13. The second one was also produced by a betacoronavirus named MERS-CoV, initiated in Saudi Arabia in 2012, affecting about 3,000 people with concomitant 858 deaths14. The outbreak was well controlled at that time, although the virus came back a couple of times, causing separate outbreaks in South Korea (2015) and in Saudi Arabia (2018)15. MERS and SARS outbreaks never turned into pandemics because of two principal reasons. The first reason is that the reproductive value or R of both SARS-CoV and MERS-CoV were too low (2 or 3 for SARS CoV and below 1 for MERS-CoV), which means that from one infected person two or three more could be infected further. Hence, they were less contagious and avoiding close contact alone could suppress the possibility of being exposed to the diseases16,17. Unfortunately, in the case of SARS CoV-2, the R is around 4, which makes it more intimidating than any other viruses, despite having weaker manifestations18. The second reason is that the affinity of SARS-CoV-2 for angiotensin-converting enzyme 2 (ACE2) receptor is higher than that of SARS-CoV.
As far as drug R&D is concerned, several desperate attempts have been made to treat the COVID-19 since the onset of the emergence of the virus in China. Apparently, the very first approach was made through traditional Chinese medicine therapies (TCM) back in China in December 2019. Although none of those medications were designed exclusively for COVID-19, YinHuQingWen decoction and ShuFengJieDu capsules were suggested in preliminary case treatment with no effective result19. Implementation of systemic corticosteroids following the example of SARS‑CoV to treat pneumonia associated with COVID-19 showed, at least initially, no benefits either12. World Health Organization (WHO) constituted an R&D blueprint to draw suitable therapeutic methods to respond to the virus that included design and development of drugs, vaccines and other clinical techniques. Accompanied by European Medicines Agency, US Food and Drug Administration (FDA) and the Chinese government itself, WHO has already initiated materializing their goals by assembling resources and researchers to delve into establishing efficient vaccines and drugs.
3. Methodology and Sources
We retrieved information from the register of www.clinicaltrial.gov in 4 different categories: “Not yet recruiting”, “Recruiting”, “Enrolling by invitation” and “Active, not recruiting”. We considered going through drugs in all the study phases available including the non-applicable ones with no designation. Since we were studying clinical trials for the landscape, we emphasized on interventional study type of drugs. In addition to this systematic algorithm, we sought assistance from regular and contemporary literature reviews on drugs and vaccines landscape, in order to stay updated with our approach.
4. COVID-19 Drug R&D Landscape
To date, resources are being invested more in the reinforcement of repurposing drugs suiting to COVID-19 cases than in developing novel drugs. That is because of: firstly, synthesizing a novel drug to combat the pandemic at the present time is highly unlikely to serve any purpose considering the unavailability of one perfect design or strategy and also requirement of a huge amount of time. Rather, to make the best use of existing drugs by manipulating them to fight against COVID-19 seems a much smarter approach, if it works. Secondly, in some cases of repurposing drugs, some clinical phases may not be required (phases I and II). Therefore, the drugs may become available on the market faster as compared to the novel ones. Thirdly, both pharmaceutical supply chains and distribution are readily available for such drugs. Besides, applying such drugs in combination with another drug may prove to be much more effective than monotherapy. And the last but not the least, drug repurposing may lead to discovery of new mechanisms of action for old drugs and may pave a way for a new target-based therapeutic in the process as well (Figure 1).
Figure 1. Overview of host pathways and viral replication mechanisms of the repurposed therapeutic drugs undergoing clinical trial against COVID-19.
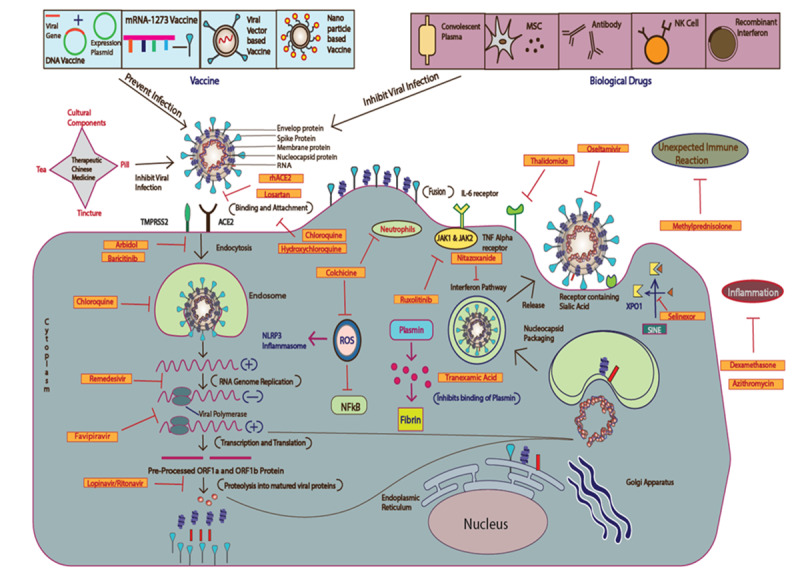
Drugs of both biological and chemical origins are shown along with a number of vaccines involved in repurposed applications in context of their respective pathways and mechanisms.
5. Types of Treatments in Clinical Trial
In current clinical drug research, as of 5th of July, 2020, 62% of trials involve chemical, 33% biological and the remaining 5% include the combination of both chemical and biological drugs in nature (Table 1, Figure 2).
Table 1. Total number of clinical trials for drugs of chemical, biological and combinatorial nature obtained from www.clinicaltrial.gov on 4th of July, 2020.
| Treatment Types | Clinical Trials |
|---|---|
| Chemically Derived Drugs | 542 |
| Biological Treatments | 294 |
| Combined Therapy | 42 |
Figure 2. Percentage of chemically derived drugs, biological treatments and combined therapy in current clinical trials for repurposing against COVID-19.
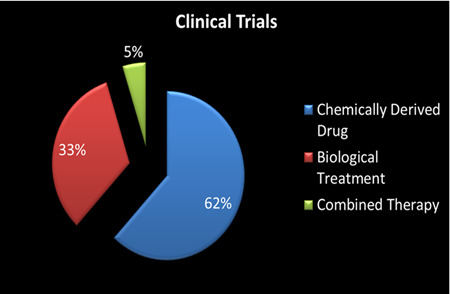
The graph has been generated using the Microsoft Excel application.
5.1 Biological Treatments
Among the biological treatments (Figure 3A), antibody-mediated ones are mostly prevailing, especially tocilizumab and sarilumab being widely used in clinical trials. Convalescent plasma-based treatments are also being evaluated in clinical trials in large facilities, where plasma from cured patients is transferred to the infected patients. Another potential biological target for drug development is the use of stem cell. Several types of mesenchymal stem cells are now being tested as a treatment for COVID-19 patients. Other prospective biological drugs in clinical trials include interferons and NK cells. Even enzymes and peptide-based drugs are under consideration for decisive clinical trials (Table 2).
Figure 3. List of both (A.) biological treatments including vaccines and (B.) chemically derived drugs with their respective clinical trial numbers.
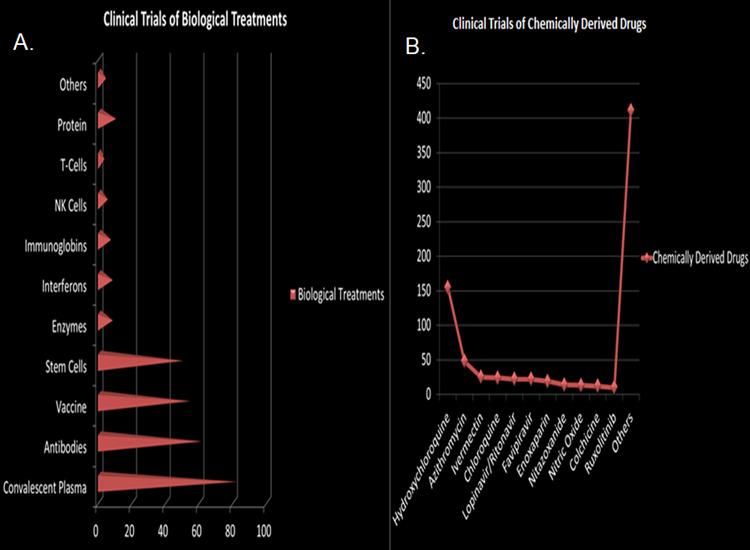
Vaccines mentioned with clinical trial numbers include both SARS-CoV-2 vaccines and different other vaccines which are being tested for repurposing purposes. Drugs of chemical nature with most occurrences in clinical trials are listed in orderly fashion. The graphs have been generated using the Microsoft Excel application.
Table 2. Clinical trials using biological treatments.
| Biological Treatments | Clinical Trials |
|---|---|
| Convalescent Plasma | 82 |
| Antibodies | 61 |
| Vaccines | 54 |
| Stem Cells | 50 |
| Enzymes | 8 |
| Interferon | 8 |
| Immunoglobin | 7 |
| NK Cells | 5 |
| T-Cells | 3 |
| Protein | 9 |
| Others | 4 |
5.1.1 Convalescent Plasma
Convalescent plasma treatment involves transfusion of plasma content enriched with antibodies from a system exposed to a particular pathogen20. For decades now, this technique has served as a successful treatment for a rather short term recovery in individuals21. It works prophylactically for infected patients in preventing severity of the disease22,23. The mode of mechanism of the treatment is to take out the pathogen by cytotoxicity, phagocytosis or neutralization through propelling the antibodies to bind with the pathogens24. Convalescent plasma had already been used against SARS and MERS-CoV before25. South Korea and Taiwan conducted successful studies on convalescent plasma positively impacting severe cases of SARS and MERS-CoV26,27. As for SARS CoV-2, China facilitated a limited study on COVID-19 patients that exhibited a clinical improvement in terms of fever, cough and other symptoms28,29. A recently published article by Shen et al. (2020) reported improved respiratory status, viral loads and pulmonary lesions in patients administered with convalescent plasma leading to a meaningful recovery. However, no conclusive remarks on the efficacy of the therapy were made by the author. The result of another single ongoing clinical trial to determine the efficacy of anti-SARS-CoV-2 inactivated convalescent plasma remains to be seen30. Many clinical trials are currently being registered to observe effects of convalescent plasma in COVID-19 recovery (NCT04432013, NCT04388410, NCT04397757 etc).
5.1.2 Recombinant Interferon
Virus infected cells naturally secrete type-1 interferon31. Combined with other drugs or even alone, interferon can demonstrate a diverse antiviral quality against multiple viruses, such as HCV, respiratory syncytial virus32, and α2β has already been declared to have inhibitory effects on both MERS-CoV and SARS-CoV33. Thus, numerous clinical trials are focusing on the safety and feasibility of the interferon treatment program for COVID-19 management (NCT04293887). Few other clinical trials on peginterferon products are under consideration in United States (NCT04344600, NCT04388709, NCT04331899, and NCT04343976) and in Canada (NCT04354259).
5.1.3 Mesenchymal Stem Cell (MSC)
Stem cell research has been on the table for quite a long time now and mesenchymal stem cell (MSC) is being evaluated extensively for COVID-19. MSCs are basically anti-inflammatory agents that produce paracrine factors to repair damaged tissues and reduce pro-inflammatory cytokines at the same time31. Preclinical trials demonstrated that MSCs coupled with the ability of decreasing inflammatory infiltrate serve to retain endothelial permeability34. Immunomodulating effects of mesenchymal stem cells have already been proven to be effective on avian influenza viruses35. Presently, MSCs extracted from dental pulp and umbilical cord are being experimented for clinical use against COVID-19 (NCT04293692, NCT04269525, NCT04288102, NCT04302519)31. A study by University of Utah on MSCs out of amniotic fluid is in the earliest stage now (NCT04319731).
5.1.4 Antibodies & Immunoglobulin
Pathogen specific antibodies as a part of humoral immune response have always been considered a formidable solution against viral infections. Screening cells and manufacturing specific antibodies for this purpose are rather time-consuming and require incessant labor, yet have been massively successful against the West Nile Virus36. Thus, it is obvious that producing SARS CoV-2 surface specific and epitope-targeting antibodies should be the sustainable and long term solution for COVID-1937. AbCellera from Canada and Eli Lilly and Company from USA are collaborating to develop a functional antibody for neutralizing SARS-CoV-2 from around 500 potential antibody sequences, by screening over 5 million immune cells from one of the early COVID-19 patients in USA31. A significant number of clinical trials focused on antibodies is being conducted, with more emphasis than on other biological drugs (NCT04344782, NCT04348500, NCT04409509, NCT04359901). Intravenous immunoglobulin (IVIG) also exploits a dynamic range of effects on the immune system depending upon doses applied31. As for the low doses of 0.2-0.4g/kg, IVIG works as a replacement therapy in antibody deficiency cases, whereas for higher doses of 2g/kg, IVIG displays its immunomodulating features by inhibiting inflammatory cells proliferation, antibody-dependent cytotoxicity and suppressing phagocytosis38. However, contemporary clinical trials against COVID-19 are focusing only on the low doses of IVIG (NCT04261426)31. Several clinical trials were performed to determine the effectiveness of human immunoglobulin in patients with pneumonia caused by 2019-nCoV33,including one by the Versailles Hospital (NCT04403269) in France and in Spain (NCT04432324).
5.1.5 Natural Killer Cells (NK Cells)
Until today, the highest mortality caused by COVID-19 is seen among elder patients, which to some extent, may be related to the decreasing immune system with age. Therefore, efforts should be made to speed up the innate anti-viral immune response39. Natural killer (NK) cells are important parts of innate immunity system to elicit immediate response against viral infections31. The NK group 2 member A (NKG2A) receptors basically reduce mechanism of action of NK cells to a minimal level40. Overexpression of NKG2A compromises basic innate responses by exhausting both NK cells and CD8+ cells, whereas several other studies on cancers revealed that blocking NKG2A on those cells restores CD8+ and NK cells functions41 and significantly inhibits tumor growth42. Studies have shown that pulmonary migration of NK cells and macrophages exert a special role in eliminating SARS-CoV43. This innate response promotes a stable immunity against the SARS-CoV regardless of assistance from antibodies or CD8+ cells by producing cytokines and chemokines31. A similar approach is in clinical trials in China to unravel whether the same outcome is reproducible in the case of COVID-19 (NCT04280224)29. Many companies attempted to treat COVID-19 via repurposed NK-based products. The Kleo Pharmaceuticals from USA, in association with a South Korean Green Cross LabCell company are working together to develop such products. The placental haematopoetic stem cell-derived NK cells, CYNK-001 is developed by a company named Celularity from USA (NCT04365101)39.
5.2 Chemically Derived Drugs
Chemically derived drugs are comparatively more diverse, as can be determined from this evaluation (Figure 3B). The most talked about among them is hydroxychloroquine, an antimalarial drug reported to have more credibility in eliminating the symptoms than any other, although no peer review article has ever confirmed it44 as of the time we are writing this review. Even a combination of hydroxychloroquine and some other drugs like azithromycin is being tested on subjects to find out whether the supposed inclination towards this particular drug is to be attended to or not. About 542 chemical drugs are under incessant clinical trials around the world and about 150 of them pertaining to hydrochloroquine, chloroquine and their combinations are under rapid clinical trials, making this a statistically significant investment behind one particular type of drug (Table 3). Lopinavir and ritonavir, drug combination used against HIV is also listed as one of the most researched ones. Several antiviral drugs are presented too, with remdesivir, favipiravir being the notable ones. Some of the other chemical drugs which got the most focus are azithromycin, losartan, colchicine, enoxaparin, ivermectin and nitric oxide. Traditional Chinese medicine therapy has also been an initial approach to fight back in China during the onset of the outbreak and is still being researched to this day45.
Table 3. Clinical trials using chemically derived drugs.
| Chemically Derived Drugs | Clinical Trials |
|---|---|
| Hydroxychloroquine | 155 |
| Azithromycin | 48 |
| Ivermectin | 25 |
| Chloroquine | 24 |
| Lopinavir/Ritonavir | 22 |
| Favipiravir | 22 |
| Enoxaparin | 19 |
| Nitazoxanide | 14 |
| Nitric Oxide | 13 |
| Colchicine | 12 |
| Ruxolitinib | 10 |
| Others | 411 |
5.2.1 Remesivir
Remdesivir has been one of the most controversial entries for clinical drug trials against COVID-19. The drug has shown enough credibility and all-encompassing properties against RNA viruses particularly46. It acts as an adenosine analogue which terminates viral replication prematurely30. Initially developed to fight against Ebola virus by Gilead Sciences in USA, remdesivir was not proved to be successful but was considered safe for humans that consequently made it a potential candidate for repurposed drug research against COVID-1947. Consistent with antiviral activities against SARS-CoV and MERS-CoV48,49, the drug was also being considered under clinical trial against SARS-CoV-250. It was first prescribed for the very first reported case in USA on his 7th day in the hospital and he showed a significant improvement the following day with no side-effect51. Remdesivir is being experimented on multiple clinical trials across the world in terms of both single trials (NCT04431453, NCT04280705 etc) and in different combinations (NCT04409262, NCT04315948 etc). A randomized, double-blind, placebo-controlled, multicenter clinical trial at ten hospitals in Hubei, China revealed that remdesivir use was not associated with a difference in time to clinical improvement in patients compared to subjects receiving placebo. The US National Institutes of Health (NIH) conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults hospitalized with COVID-19 with evidence of lower respiratory tract involvement. Study revealed that those who received remdesivir had a median recovery time of 11 days as compared with 15 days in those who received placebo. The Food and Drug Administration (FDA) had made remdesivir available under an emergency-use authorization for the treatment of adults and children with severe COVID-19 disease in early May. In late May, FDA officially issued a conditional approval for remdesivir in Taiwan and eventually other countries like EU and Canada started to adopt the legal approval for a regular use against the disease52.
5.2.2 Favipiravir
The drug favipiravir branded as Avigan was developed by Toyama Chemical (division of Fujifilm, Japan)53. Favipiravir follows the mechanism of competitive inhibition to alter the activity of RNA-dependent RNA-polymerase, by resembling the structural makeup of endogenous guanine54. The drug is already approved for influenza and has also been designated by National Medical Products Administration in China as the first anti-COVID-19 drug of the country31. Repurposing favipiravir for COVID-19 is much of a challenge because of lesser preclinical trials on the drug compared to remdesivir, although both of them are similar in terms of their actions31. Number of favipiravir clinical trials (NCT04445467, NCT04434248, NCT04411433 etc) is marching on with increasing expectations. Stanford Medicine researchers launched a clinical trial to test whether an oral drug can reduce symptoms and viral shedding in people with COVID-19 beginning in July 655. Simultaneously, Glenmark is conducting phase III clinical trials of favipiravir as a COVID-19 monotherapy option with 150 patients, enrolled from 9 leading government and private hospitals across India.
5.2.3 Lopinavir/Ritonavir
A popular drug combination of lopinavir and ritonavir against human immunodeficiency virus (HIV), which is basically a facilitator of protease inhibition in the virus itself, already made its mark as a successful therapeutic agent31. Ritonavir combined with lopinavir leads to increased plasma half-life. Such combination was reported to lead to decreased adverse results in the 2004 SARS outbreak30. Studies showed that they can work as inhibitors to the cysteine protease that coronaviruses encode56-58. Experimental and clinical results proved combination to be effective against both SARS and MERS-CoV50,51,54,59. Hence, the combination of lopinavir and ritonavir was repurposed against the SARS-CoV-2 (NCT04252885) as well. However, only a small improvement was noticed in mild cases49 whereas nothing remarkable could be extracted in the severe ones60. Earlier study in China found faster clearance of SARS-CoV-2 by PCR and faster improvement of chest computed tomography. A randomized, open-label clinical trial comparing LPV/r versus placebo in patients with severe COVID-19 found no statistically significant difference in time to clinical improvement or 28-day mortality. In July, WHO announced to discontinue lopinavir/ritonavir treatment for COVID-19, since clinical trial results showed that hydroxychloroquine and lopinavir/ritonavir produce little or no reduction in the mortality of hospitalized COVID-19 patients when compared to standard of care.
5.2.4 Hydroxychloroquine
Hydroxychloroquine, most famously known as an anti-malarial drug and an anti-immune agent operates by suppressing acidification of cellular endosome during the events of replication and infection61. As for the case of SARS-CoV-2, it has shown its ability to inhibit the replication of this virus as well by interrupting the glycosylation of its receptor protein-ACE262. This claim has been supported by several in-vitro clinical trials of the drug that saw a significant reduction in the copy numbers of SARS-CoV-263. Clinical trials in China concluded that hydroxychloroquine is only effective against COVID-19 associated pneumonia, not against the virus specifically31. A non-randomized clinical trial in France gave out a somewhat optimistic result when hydroxychloroquine was combined with azithromycin (NCT04339816, NCT04335552)64. However, the WHO decided on July 15, 2020 to halt clinical trials of hydroxychloroquine as a potential treatment for hospitalized COVID-19 patients65. When compared with the standard of care in the treatment of hospitalized COVID-19 patients, hydroxy-chloroquine did not result in the reduction of the mortality of the patients.
5.2.5 Arbidol (Umifenovir)
Arbidol acts upon influenza viruses and arboviruses as an entry inhibitor66. It interrupts the endocytosis of influenza virus by preventing the fusion of viral body with endosome through targeting hemagglutinin of viral surface31. Arbidol is already approved in Russia and China for COVID-19 drug trials now as a single agent66. But in a comparative study, favipiravir turned out to be more effective than arbidol67. An in vitro study in China shows that arbidol inhibits SARS-CoV-2 infection whenever applied at a minimal concentration. Nowadays, a dose of (200 mg) arbidol is given three times per day not for more than 10 days to treat COVID-19. A randomized study was also conducted to determine the efficacy of arbidol in combination with recombinant human interferon-α2β to treat mild to moderate pneumonia due to COVID-1968. China awaits two phase IV drug trials of Arbidol (NCT04260594, NCT04286503), to provide a conclusive result by February 2021.
5.2.6 Angiotensin Receptor Inhibitor
For an angiotensin receptor inhibitor, recombinant human angiotensin converting enzyme 2 (rhACE2) is now being recommended because of its ability to block S protein from binding to the host receptor ACE231. In fact, rhACE2 is found to be able to obstruct SARS-CoV-2 replication by a factor of about 5,000 times maximum according to contemporary studies on embryonic cell-mediated organoids69. Applying rhACE2 has been reported to abate serum level of angiotensin 2 that diverts the substrate away from the receptor ACE; eventually intervening with activation of ACE2 as well to prevent ARDS (Acute Respiratory Distress Syndrome)70. In China, a study has been carried out to evaluate the biological and physiological role of rhACE2 NCT04287686) against COVID-19 and so it made to the list of potential drugs against SARS CoV-2 in terms of measurement of plasma level of angiotensin 1-731. A couple of other studies in Austria and Australia (NCT04353596, NCT04394117) are dealing with the final phase of angiotensin inhibitory drugs due to be published in two years.
5.2.7 Thalidomide
Thalidomide was used as a treatment plan against some of the severe inflammatory diseases such as Behcet’s disease and Crohn’s disease and it acts by decreasing synthesis of TNF-α71. Studies also reported the successful use of thalidomide against H1N1-infected mice, as it facilitates production of pro-inflammatory cytokines and reduction of infiltration of inflammatory cells72. Recent emergence of thalidomide as an anti-inflammatory, anti-fibrotic and anti-angiogenic agent has put it in the discussion of being a repurposing drug against certain SARS-CoV-2 strains (NCT04273321, NCT04273581) predicting its immunomodulatory ability of reducing lung defect31.
5.2.8 Methylprednisolone
Pathogenesis of SARS-CoV-2 is supported not only by the direct damage it causes to the host, but also by the excessive immune response that the host cell offers31. Methylprednisolone is one of the potential drugs like thalidomide that can supposedly inactivate such unexpected immune reaction. Nowadays along with antibiotics, oseltamivir, and oxygen therapy methylprednisolone is being used to treat COVID-19 patients33. Several clinical trials are ongoing (NCT04263402, NCT04355247, NCT04343729) to determine whether or not it’s safe and useable31.
5.2.9 Chloroquine
Chloroquine is an anti-malarial drug73. While it has been used as an effective anti-viral drug, as of now it is being considered to be repurposing against COVID-1974. Chloroquine inhibits pH-dependent steps of the replication of many viruses that has already been quite extensively tested both in vitro and in vivo on different virus strains and recently, on SARS-CoV-2. Though treatment with chloroquine showed promising results, it also strongly differed in application between live animals and cell lines. The major finding was that even if chloroquine shows promising results on virus75 and cells, the in vivo application is not so promising75. China, South Korea and Italy allowed for experimental trials of chloroquine against SARS-CoV-276,77. Having a lower therapeutic index, chloroquine may often turn out to be toxic for the user78,79. FDA didn’t approve hydroxychloroquine and chloroquine under emergency use authorization (EUA)80. In short, no conclusive clinical trial has proved efficacy of the drug till now although research is still underway in Mexico (NCT04323527, NCT04342650), Egypt (NCT04353336), USA (NCT04349371), UK (NCT04303507) and many other countries.
5.2.10 Losartan
Losartan is a widely used drug for treating hypertension and left ventricular hypertrophy (heart muscle enlargement) and kidney dysfunction in type-2 diabetes81. It blocks the receptor of angiotensin-2 from entering the cells and raising blood pressure. Losartan is being perceived as an antagonist, since it interferes with the substrate-ACE2 receptor interaction of SARS-CoV-282. However, according to a recent study, such medication for hypertension might end up inducing the host to produce more ACE2 instead and increasing susceptibility of the virus in the process83,84. Various studies are taking place across different phases of development on losartan including a phase IV study by Sharp Healthcare (NCT04340557) and a couple of phase II studies by University of Minnesota (NCT04312009, NCT04311177).
5.2.11 Azithromycin
Azithromycin could reduce the severe lung inflammation caused by SARS-CoV-2 infection, through halting the production of cytokines, along with building immunity against other viruses. Azithromycin along with hydroxychloroquine was able to inhibit SARS-CoV2 replication in a clinical trial in France, however, the result was not reproducible when the antibiotic was administered alone85. Few phase III studies are also being carried out in France solely on azithromycin (NCT04371107, NCT04365582). Gautret et al. reported a 100% viral clearance in nasopharyngeal swabs in their 6 patients whenever they were treated with both the drugs. The findings reported by Molina et al. stood in contrast with those reported by Gautret. They repeated the experiments and found 8 of 11 patients having remarkable comorbidities83.
5.2.12 Ruxolitinib
Ruxolitinib is used to treat intermediate or severe myelofibrosis (bone marrow disorder)83,86 and polycythemia vera (PCV) that occurs when there’s a lack of response to hydroxyurea87. It is a janus kinase inhibitor (JAK inhibitor) particularly involving JAK1 and JAK288. It was reported to cause faster improvement of the COVID-19 patients by Ruxolitinib administration but a phase II clinical trial failed to account this89. Incyte, a biopharmaceutical company, has initiated a phase III clinical trial to assess the safety and efficacy of the drug material (NCT04377620)90. Few other phase II and phase II / phase III clinical trials are focused on ruxolitinib in both America and Europe (NCT04348071, NCT04338958, NCT04359290).
5.2.13 Baricitinib
Baricitinib is another anti-inflammatory drug approved to be used against rheumatoid arthritis (RA), as a janus kinase inhibitor (anti-JAK)91. The anti-inflammatory properties of baricitinib inspired some groups to observe its effect in inflammation92. Oral administration of baricitinib could potentially reduce inflammation in COVID-19 associated ARDS93. National Institute of Allergy and Infectious Diseases (NIAID) launched a study on combination of remdesivir and baricitinib for a better anti-inflammatory outcome than remdesivir alone (NCT04401579)93. As for single studies, baricitinib is currently being researched in various clinical trial phases (NCT04340232, NCT04399798 etc).
5.2.14 Nitric Oxide
Nitric oxide (inhaled nitric oxide (iNO)) produced by Mallinckrodt Pharmaceuticals Inc is known to reduce pulmonary hypertension and hypoxia by lessening ventilation support94. A phase II study is being conducted on iNO in patients afflicted with ARDS resulted from SARS-CoV-2 infection95. A phase III study has also been approved by FDA for ensuring safety and efficacy of the drug in patients with COVID-19 needing supplemental oxygen before advancing to forced ventilation95. On March 20, 2020, FDA granted emergency expanded access allowing its iNO delivery system (INOpulse) to be immediately used for the treatment of COVID-19 (NCT04421508). However, more study results are needed to be evaluated (NCT04212243, NCT04398290) to determine the efficacy of iNO in treating COVID-19 patients.
5.2.15 Vitamin C
Vitamin C also known as ascorbic acid helps boost the immune system through its antioxidant properties and collagen synthesis. It is an effective and simple molecule without any side effects or cross-reaction with available COVID-19 drugs. Some hospitals have been reported to have prescribed high-dose of intravenous vitamin C for the treatment of 50 moderate to severe COVID-19 patients in China. The dose varied between 2 and 10 g per day over a period of 8 to 10 hours of IV (intravenous) infusion. The oxygenation index was improved and all the patients recovered84. A study confirmed the ability of vitamin C to lessen death rate by 20% in patients with lung damage96. Another study in China showed zero death out of 50 intermediate patients treated with high doses of vitamin C. Progenbiome of USA has initiated a couple of phase II clinical trials on Vitamin-C (NCT04334512, NCT04335084) alongside other drugs. A phase III research on only Vitamin-C has also been brought under consideration in Sherbrooke University (NCT04401150, NCT03680274). However, the overall effect and outcome of vitamin C in treating COVID-19 alone or in combination are disputable.
5.2.16 Dexamethasone
Dexamethasone is a corticosteroid used for different inflammation conditions and other diseases like arthritis, lupus, breathing disorders etc. A study in March 2020 concluded that an early dose of dexamethasone would reduce inflammation in COVID-19 associated ARDS and thus control mortality rate to a large extent suppressing ventilation tendency97. Recently, dexamethasone proved to be the first life-saving drug among seriously ill COVID-19 patients. The study comprised of 2,100 candidates receiving dexamethasone compared with 4,300 people given standard care. Dexamethasone reduced death by one third for people on ventilation and reduced risk by one fifth for the patients needing oxygen. But the results have not yet been peer reviewed. Dexamethasone phase III and phase IV clinical trials to conclude in near future will be able to help create an extensive platform for further utilization of the drug (NCT04325061, NCT04395105, NCT04327401 etc).
5.2.17 Oseltamivir
Oseltamivir is an already approved drug against influenza A and B20 that targets the neuraminidase on the surface of influenza virus to inhibit the virus from entering human cells98,99. An initial clinical trial in Wuhan didn't show a success when using the drug for COVID-19 patients. Still, oseltamivir is being studied in a combination with chloroquine and favipiravir around the world. A phase III clinical trial on oseltamivir by Federal Task Force on Science and Technology in Pakistan (NCT04338698) is expected to provide a definitive take on the future of this particular drug.
5.2.18 Colchicine
Colchicine is an anti-inflammatory drug used for gout management along with treating other complications20. It blocks neutrophils from transferring to inflammation sites and inhibits forming inflammasome complex blocking its activation100. Colchicine has been reported to reduce inflammation in the cardiac myocytes of patients with both COVID-19 and myopathies101. Several other studies are being conducted on colchicines, NCT04355143, NCT04322682) for cytokine storm20.
5.2.19 Nitazoxanide
Nitazoxanide is an anti-viral drug inhibiting the expression of viral N protein, shown to have inhibitory effects against MERS-CoV in LLC-MK2 cells, along with several other coronaviruses, mouse hepatitis virus strain A59 (MHV-A59), human enteric corona virus 4408 (HECoV-4408) and bovine coronavirus strain L9 (BCoV-L9)102. Through an unreliable source, it is also reported that the drug suppresses proinflammatory cytokines in peripheral blood mononuclear cells (PBMC) and IL-6 in vivo103. Nitazoxanide 500 mg is in phase IV clinical trials due to be concluded by the end of the current year (NCT04341493, NCT04406246).
5.2.20 Selinexor
Selinexor is an FDA approved drug in high doses, used for cancer conditions, such as relapsed multiple myeloma. Manufactured by Karyopharm Therapeutics Inc, selinexor is an oral selective inhibitor of nuclear export (SINE) in cells104. It has been seen that blocking of nuclear export automatically disrupts the replication of SARS-CoV-2. With that in mind, selinexor is now being explored through phase I and phase II clinical trials, by leading health organizations like Norton Healthcare and Lehigh Valley Health Network. Currently, Karyopharm therapeutics is conducting a phase II clinical trial on the drug (NCT04355676, NCT04349098).
5.2.21 Methothrexate
Methothrexate (MTX) is a drug dedicated to treat different cancer cases, such as breast, skin and lung cancer and to treat psoriasis and rheumatoid arthritis in lower doses. While it is still not known whether SARS-CoV-2 is susceptible to the drug or not, for those COVID-19 patients with rheumatoid arthritis as an additional condition it is recommended to stop their medication on MTX for a while until the complexity is resolved. A Brazilian research team collaborating with an Indian-based contract research organization has initiated a study on methotrexate nano-particles (NCT04352465) to treat COVID-19 by evaluating inflammatory responses in patients105.
5.2.22 Tranexemic Acid
Tranexemic acid or TXA is a derivative of amino acid lysine helping blood coagulate. It carries antifibrinolytic property to reverse excessive blood loss from traumas and surgeries. One of the lesser known symptoms of COVID-19 is coagulopathy. University of Alabama researchers are conducting two phase II clinical trials on TXA (NCT04338074, NCT04338126), to figure out its ability in balancing the coagulation factors in COVID-19 patients106.
5.2.23 Traditional Chinese Medicine
Traditional Chinese Medicine (TCM) which uses phytotherapeutic formulations like teas, pills, powders or tinctures, and cultural components can be traced back 5000 years ago in Chinese medicine. These were already used for SARS-CoV infection as coadjuvant therapy with the enhancement of patient’s symptoms, increased oxyhemoglobin arterial saturation in 200233. TCM synthesized by local Chinese manufacturers claimed to have effect in combating infectious diseases such as COVID-19.
6. Vaccines
With the rising cases of SARS-CoV-2 infection, it is apparent that COVID-19 is going to be prevailing for a long time and the battle against any virus can be ultimately won through an effective and safe vaccine107. The first vaccine (Pfizer-BioNTech mRNA-based vaccine) just received emergency approval in December 2020 for use in USA and UK. Additional vaccines (such as the mRNA-based vaccine from Moderna) will follow. These are excellent news. However, it will take months to have the vaccines distributed worldwide, their long-term effects are hard to predict, and the possibility of future resistant mutations is a concern.
Vaccines can be a reliable therapeutic approach108 by promoting a long term strategy against the virus39. Experts have warned of any shortcut in vaccine development due to public pressure which may elicit antibody-dependent enhancement, resulting in long term consequences109. In spite of these, we really cannot rule out the fact that production of vaccine is the most effective and viable alternative to combat an emerging pandemic, such as COVID-19. Many vaccine clinical trials are currently going across the globe, including a BCG vaccine (NCT04348370, NCT04417335), heat killed Mycobacterium vaccine (NCT04347174, NCT04358809) and MMR vaccine (NCT04357028). ChAdOx1 ncoV-19 (NCT04444674), recombinant nCoV-19 (NCT04313127) and inactivated nCoV, which are in different clinical phases now to develop host immunity (Figure 4A). We are discussing several COVID-19 vaccines in clinical trials below.
Figure 4. A graphical representation of (A) leading countries and sponsors associated with the clinical trials of the COVID-19 drug candidates and (B) phases and predicted timeline of the clinical trials of the COVID-19 drug candidates.
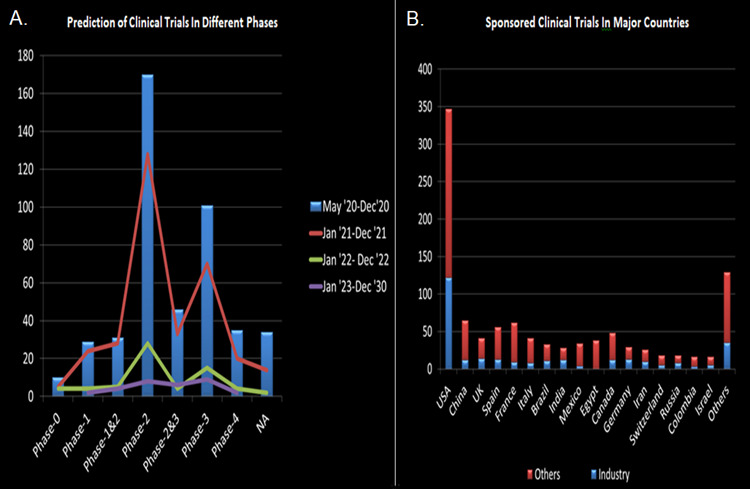
Many countries with at least 10 ongoing clinical trials through different sponsorships are shown by their names. Sponsors are categorized into two distinct groups: industry and others that include universities, organizations, government and government agencies. Different phases of drug trials with respect to specific timelines of potential approval are presented. Some of the drugs are in simultaneous clinical trials of two different phases, whereas the specific phases are not mentioned clearly for some others, hence kept in NA (Not applicable). The graphs have been generated using the Microsoft Excel application.
mRNA-1273 (ClinicalTrials.gov number, NCT04283461), developed by Moderna, an American biotech company in association with National Institute of Allergy and infectious disease (NIAID), has caught the scene for a while now. It is a synthetic mRNA which is surrounded by minute particles composed of lipids. Similar to the Pfizer-BioNTech vaccine (which is also an mRNA-based vaccine), it is supposed to induce antiviral response in the injected cells against spike proteins of SARS CoV-2 (NCT04283461)39,110. No virus was required to formulate the vaccine and platform technology made its production easier and safer for testing (NCT04283461)39,109. Elevated titer of neutralizing antibody was observed in convalescent serum in all the initial 45 candidates whenever 2 dosages were applied of that particular vaccine111. Moderna is very much hopeful that mRNA-1273 will be commercially available on the market soon. The study group published a preliminary report on July 14, 2020 of a phase I, dose-escalation, open-label clinical trial including 45 healthy adults, 18 to 55 years of age, who received two vaccinations, 28 days apart, with mRNA-1273 in a dose of 25 μg, 100 μg, or 250 μg. The mRNA-1273 vaccine induced anti-SARS-CoV-2 immune responses in all participants, and no clinical trial-limiting safety concerns were identified. These findings shed light of hope to the already COVID-19 stricken world.
INO-4800, a DNA vaccine developed by INOVIO Pharmaceuticals (NCT04336410, NCT04447781) is another genetic vaccine that utilizes the process of immune response against specific viruses. Such genetic vaccines do not involve risk of improper protein folding like the protein-based vaccines34,112. The INO-4700 was administered successfully against MERS-S within a DNA vector and INO-4800 is now in clinical trials against SARS-CoV-2-S31. INO-4800 is transdermally applied to healthy candidates through the help of an electroporation device called Cellectra, to determine its immunological features111. Ease in purification and lower expense in production make it potentially preferable over other vaccine types39. In preclinical animal challenge study, INO-4800 provided full protection against SARS-CoV-2 replication in the lungs in mice. 94% of participants demonstrated overall immune responses at week 6 after two doses of INO-4800 in phase I clinical trial with 40 healthy volunteers. The vaccine elicited no serious adverse effects through week 8 with some grade I severity.
ChAd vectors have already been established as safe and 100% efficient with single vaccination113 against viruses like Ebola virus114, Influenza A115 and MERS116. ChAdOx1 vaccine was designed by the University of Oxford in collaboration with AstraZeneca, based upon adenovirus vector against the spike protein S of SARS-CoV-2 (NCT04324606)39. ChAdOx1 vaccination prevented lung damage, indicating promising outcomes in the near future. The vaccine began phase I clinical trial in April. The University later went on to host two more clinical trials on ChAdOx, including one in collaboration with South Africa (NCT04444674, NCT04400838). With all these being said, if successful, ChAdOx is showing immense probability of being one of the most sustainable vaccines against COVID-19, although if the induced immunogenicity in host is propelled towards the vector genome rather than the transgenes, it might cause an unexpected failure in the vaccination process. Over 5,000 healthy volunteers began participating in Brazilian clinical trial of this vaccine. Oxford researchers have begun recruiting for the phase II/phase III clinical trial and planning to apply ChAdOx1 on children.
Nanoparticles-based vaccines are produced through encapsulation by adjoining with antigenic epitopes mimicking viral attacks39. General nanoparticles-based vaccination includes oral and intranasal uptake, which can induce immunity on the mucosal surface, in addition to the systemic immune responses117. This indicates how these vaccines would be effective against respiratory viral infections, including SARS-CoV-239. An investigation was conducted to determine potency between nanoparticle (polyethylenimine) that elicited spike (S) protein of SARS-CoV and naked plasmid vector. Nanoparticle produced elevated level of spike (S) protein specific antibody over naked plasmid vector118. For that purpose, Novavax Inc. has taken the initiative of producing a vaccine based on nanoparticles using spike proteins of SARS-CoV-2 (NCT04368988)39. Another study is under clinical trial as of now evaluating mRNA-lipid nanoparticles-based vaccine previously successful against SARS and MERS-CoV (NCT04283461). Many additional vaccines are now in clinical trials and expected to be approved for use in the near future.
7. Countries and Sponsors Involved in Drug Trials
The United States is dominating the ranking for the number of clinical trials for the highest number of drugs (347). As far as drug research is concerned, in China, they have 65 drug trials ongoing as of now (Figure 4B). France and Spain come next with 62 and 56 ongoing clinical drug trials respectively. Among other most affected nations, Italy has 41 drug trials ongoing, whereas Canada holds about 48 clinical trials attempting to battle COVID-19 (Table 4). It can be derived that North American countries have an upper hand in drug research, whilst other than China, the Asian countries are lagging quite behind. European countries are also providing a decent and above average service in developing drugs. Most of the drug trials are being sponsored by various universities and organizations, while multiple pharmaceutical giants are also investing in a significant number of clinical trials across the globe. There are few other government sponsored organizations, such as NIH and FDA investing in a number of clinical trials.
Table 4. List of countries affiliated with drug R&D with corresponding number of clinical trials through industrial and other (universities and other academic institutions, government funding and other government agencies, e.g. NIH) interventions.
| Country | Industry |
|---|---|
| USA | 122 |
| China | 12 |
| UK | 14 |
| Spain | 13 |
| France | 9 |
| Italy | 8 |
| Brazil | 11 |
| India | 12 |
| Mexico | 4 |
| Egypt | 0 |
| Canada | 12 |
| Germany | 13 |
| Iran | 10 |
| Switzerland | 5 |
| Russia | 8 |
| Colombia | 3 |
| Israel | 5 |
| Others | 35 |
8. Timeline and Clinical Trial Phases
Each of these tested drugs is constantly going through clinical trials and errors to be finally selected for approval. Most of the drug trials are in developmental phase II and III (335 and 195 respectively). 61 drug trials are in the final phase of drug development, while 50 others have no designation at all as in what phase they are in (Figure 4B).
There is no telling as to when a suitable drug may be advertised to cure COVID-19. But as it is estimated, among the 876 clinical drug trials, over half of them may be well predicted by the end of 2020 (Table 5). However, gaining a positive result out of them still asks for absolute confirmation of the feasibility of the drugs from clinical trials on population. That may lead to some of the drugs taking up to 2030 to be finally prescribed as safe. Since the majority of the drugs are currently nearing to the final phase of development, it may be expected that within late 2021, sustainable drug products will be well constructed and readily available or will lay out some standard and similar protocols for an effective drug at the least.
Table 5. Number of ongoing clinical trial phases of drugs in terms of a specific timeline.
| Phases | May '20 – Dec '20 | Jan '21-Dec '21 | Jan '22- Dec '22 |
|---|---|---|---|
| Phase-0 | 10 | 5 | 4 |
| Phase-1 | 29 | 24 | 4 |
| Phase-1&2 | 31 | 28 | 5 |
| Phase-2 | 170 | 128 | 28 |
| Phase-2&3 | 46 | 33 | 4 |
| Phase-3 | 101 | 70 | 15 |
| Phase-4 | 35 | 20 | 4 |
| Not applicable | 34 | 14 | 2 |
9. Conclusion
Worldwide researchers and physicians are doing their best to extract more information about the virus, so that they can figure out effective ways to completely eliminate the lethal pandemic, which has become a threat for human health worldwide. In such emergence of the pandemic, drug repurposing is being considered to be a quicker way to show rapid and effective response. This study tried to systematically address the current scenarios of the clinical trials of the repurposed drugs. Numerous repurposing drug candidates are in clinical trial in many countries simultaneously. Several biological treatments, including convalescent plasma, recombinant interferon, mesenchymal stem cell, antibodies, and the use of the NK cells are undergoing clinical trials and has already exhibited promising results. Among chemically derived drugs, remdesivir was the first treatment for COVID-19 to receive FDA approval. Dexamethasone proved to be the first life-saving drug among seriously ill COVID-19 patients. For hydroxychloroquine, despite showing some promising results at first, clinical trials were later halted at WHO’s recommendation. Although further investigations are needed to be carried out, there is a high probability that within a short period of time, some of these various treatment plans in clinical trials will be approved in terms of safety and efficacy and will be available in the global market at lower cost. Such efforts may facilitate the discovery of new classes of medicines as well. While attempts to synthesize a novel treatment plan continue from the scientific community, the repurposing treatments in clinical trials are bound to provide a reliable ground for withholding the disease until that novelty is achieved. In addition, some of the novel vaccine candidates in clinical trials are already listed here and many more are in the pipeline to undergo clinical trials soon. Such a detailed view of drug and vaccine candidates is expected to assist in and add a new dimension to the ongoing process of developing therapeutic and prevention options against COVID-19, on a huge global platform.
Acknowledgments
M.S.H acknowledges Research Cell, Noakhali Science and Technology University and R and D, Ministry of Science and Technology for research funding. M.S.H also acknowledges the Department of Biotechnology and Genetic Engineering (BGE), Noakhali Science and Technology University for helping with the research facilities. For her contribution in the project through developing the Figure 1 in the manuscript, the authors acknowledge Atqiya Fariha, a student of the Department of BGE. M.S.H., I.H., M.S.S.S., F.R. carried out the studies. M.S.H., I.H., M.S.S.S., F.R. and O.S. participated in drafting the manuscript. N.M.B. and M.M.R. developed the hypothesis, supervised the whole work and helped to prepare and revise the manuscript. All authors read and approved the final manuscript.
Footnotes
Conflict of interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Author have no conflicts of interest to declare.
Severe acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2); The Coronavirus Disease, 2019 (COVID-19); Drug Research and Development (Drug R and D); Angiotensin-Converting Enzyme 2 (ACE2); World Health Organization (WHO); Mesenchymal Stem Cell (MSC); Acute Respiratory Distress Syndrome (ARDS).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.A Novel Coronavirus from Patients with Pneumonia in China, 2019. Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F, Tan Wenjie. The New England journal of medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recommendation of fecal specimen for routine molecular detection of SARS-CoV-2 and for COVID-19 discharge criteria. Ahamed Mim Moonmoon, Naznin Rakhi Nadira, Saha Otun, Rahaman Md Mizanur. Pathogens and global health. 2020;114(4):168–169. doi: 10.1080/20477724.2020.1765651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Dyall Julie, Coleman Christopher M, Hart Brit J, Venkataraman Thiagarajan, Holbrook Michael R, Kindrachuk Jason, Johnson Reed F, Olinger Gene G, Jahrling Peter B, Laidlaw Monique, Johansen Lisa M, Lear-Rooney Calli M, Glass Pamela J, Hensley Lisa E, Frieman Matthew B. Antimicrobial agents and chemotherapy. 2014;58(8):4885–93. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Tan Emily L C, Ooi Eng Eong, Lin Chin-Yo, Tan Hwee Cheng, Ling Ai Ee, Lim Bing, Stanton Lawrence W. Emerging infectious diseases. 2004;10(4):581–6. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harnessing CAR T-cell Insights to Develop Treatments for Hyperinflammatory Responses in Patients with COVID-19. Agarwal Sangya, June Carl H. Cancer Discovery. 2020;10(6):775-778. doi: 10.1158/2159-8290.CD-20-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. Pachetti Maria, Marini Bruna, Benedetti Francesca, Giudici Fabiola, Mauro Elisabetta, Storici Paola, Masciovecchio Claudio, Angeletti Silvia, Ciccozzi Massimo, Gallo Robert C, Zella Davide, Ippodrino Rudy. Journal of translational medicine. 2020;18(1):179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genetic diversity and evolution of SARS-CoV-2. Phan Tung. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID-19 outbreak: Migration, effects on society, global environment and prevention. Chakraborty Indranil, Maity Prasenjit. The Science of the total environment. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genomic exploration light on multiple origin with potential parsimony-informative sites of the severe acute respiratory syndrome coronavirus 2 in Bangladesh. Saha Otun, Hossain Md Shahadat, Rahaman Md Mizanur. Gene reports. 2020;21:100951. doi: 10.1016/j.genrep.2020.100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drug repurposing strategies for COVID-19. Senanayake Suranga L. Future Drug Discovery. 2020;2(2) [Google Scholar]
- 11.Reactivation of Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2): Hoax or hurdle? Saha Otun, Rakhi Nadira Naznin, Towhid Syeda Tasneem, Rahaman Md. Mizanur. International Journal of Healthcare Management. 2020;13(3):265-266. [Google Scholar]
- 12.A new threat from an old enemy: Re‑emergence of coronavirus (Review). Docea Anca Oana, Tsatsakis Aristidis, Albulescu Dana, Cristea Oana, Zlatian Ovidiu, Vinceti Marco, Moschos Sterghios A, Tsoukalas Dimitris, Goumenou Marina, Drakoulis Nikolaos, Dumanov Josef M, Tutelyan Victor A, Onischenko Gennadii G, Aschner Michael, Spandidos Demetrios A, Calina Daniela. International journal of molecular medicine. 2020;45(6):1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SARS: epidemiology. Chan-Yeung Moira, Xu Rui-Heng. Respirology (Carlton, Vic.) 2003;8 Suppl:S9–14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middle East respiratory syndrome coronavirus (MERS-CoV) World Health Organization. 2019. www.who.int. www.who.int.
- 15.Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Fehr Anthony R, Channappanavar Rudragouda, Perlman Stanley. Annual review of medicine. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19 R0: Magic number or conundrum? Viceconte Giulio, Petrosillo Nicola. Infectious disease reports. 2020;12(1):8516. doi: 10.4081/idr.2020.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-19, SARS and MERS: are they closely related? Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Author Correction: A new coronavirus associated with human respiratory disease in China. Wu Fan, Zhao Su, Yu Bin, Chen Yan-Mei, Wang Wen, Song Zhi-Gang, Hu Yi, Tao Zhao-Wu, Tian Jun-Hua, Pei Yuan-Yuan, Yuan Ming-Li, Zhang Yu-Ling, Dai Fa-Hui, Liu Yi, Wang Qi-Min, Zheng Jiao-Jiao, Xu Lin, Holmes Edward C, Zhang Yong-Zhen. Nature. 2020;580(7803):E7. doi: 10.1038/s41586-020-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Yang Yang, Islam Md Sahidul, Wang Jin, Li Yuan, Chen Xin. International journal of biological sciences. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An Update on Current Therapeutic Drugs Treating COVID-19. Wu Renyi, Wang Lujing, Kuo Hsiao-Chen Dina, Shannar Ahmad, Peter Rebecca, Chou Pochung Jordan, Li Shanyi, Hudlikar Rasika, Liu Xia, Liu Zhigang, Poiani George J, Amorosa Louis, Brunetti Luigi, Kong Ah-Ng. Current pharmacology reports. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Luke Thomas C, Kilbane Edward M, Jackson Jeffrey L, Hoffman Stephen L. Annals of internal medicine. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 22.Antibody-mediated regulation of cellular immunity and the inflammatory response. Casadevall Arturo, Pirofski Liise-anne. Trends in immunology. 2003;24(9):474–8. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 23.Serum therapy revisited: animal models of infection and development of passive antibody therapy. Casadevall A, Scharff M D. Antimicrobial agents and chemotherapy. 1994;38(8):1695–702. doi: 10.1128/aac.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A Role for Fc Function in Therapeutic Monoclonal Antibody-Mediated Protection against Ebola Virus. Gunn Bronwyn M., Yu Wen-Han, Karim Marcus M., Brannan Jennifer M., Herbert Andrew S., Wec Anna Z., Halfmann Peter J., Fusco Marnie L., Schendel Sharon L., Gangavarapu Karthik, Krause Tyler, Qiu Xiangguo, He Shinhua, Das Jishnu, Suscovich Todd J., Lai Jonathan, Chandran Kartik, Zeitlin Larry, Crowe James E., Lauffenburger Douglas, Kawaoka Yoshihiro, Kobinger Gary P., Andersen Kristian G., Dye John M., Saphire Erica Ollmann, Alter Galit. Cell Host & Microbe. 2018;24(2):221-233.e5. doi: 10.1016/j.chom.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A serological survey on neutralizing antibody titer of SARS convalescent sera. Zhang Jian-San, Chen Jiang-Ting, Liu Yu-Xuan, Zhang Zhen-Shan, Gao Hong, Liu Yan, Wang Xu, Ning Ye, Liu Yu-Fen, Gao Qiang, Xu Jian-Guo, Qin Chuan, Dong Xiao-Ping, Yin Wei-Dong. Journal of Medical Virology. 2005;77(2):147-150. doi: 10.1002/jmv.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. Yeh Kuo-Ming, Chiueh Tzong-Shi, Siu L. K., Lin Jung-Chung, Chan Paul K. S., Peng Ming-Yieh, Wan Hsiang-Lin, Chen Jenn-Han, Hu Bor-Shen, Perng Cherng-Lih, Lu Jang-Jih, Chang Feng-Yee. Journal of Antimicrobial Chemotherapy. 2005;56(5):919-922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Ko Jae-Hoon, Seok Hyeri, Cho Sun Young, Ha Young Eun, Baek Jin Yang, Kim So Hyun, Kim Yae-Jean, Park Jin Kyeong, Chung Chi Ryang, Kang Eun-Suk, Cho Duck, Müller Marcel A, Drosten Christian, Kang Cheol-In, Chung Doo Ryeon, Song Jae-Hoon, Peck Kyong Ran. Antiviral therapy. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 28.Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. Shen Chenguang, Wang Zhaoqin, Zhao Fang, Yang Yang, Li Jinxiu, Yuan Jing, Wang Fuxiang, Li Delin, Yang Minghui, Xing Li, Wei Jinli, Xiao Haixia, Yang Yan, Qu Jiuxin, Qing Ling, Chen Li, Xu Zhixiang, Peng Ling, Li Yanjie, Zheng Haixia, Chen Feng, Huang Kun, Jiang Yujing, Liu Dongjing, Zhang Zheng, Liu Yingxia, Liu Lei. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Tang Ning, Li Dengju, Wang Xiong, Sun Ziyong. Journal of thrombosis and haemostasis : JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.COVID-19: A review of therapeutics under investigation. Crosby James C, Heimann Matthew A, Burleson Samuel L, Anzalone Brendan C, Swanson Jonathan F, Wallace Douglas W, Greene Christopher J. Journal of the American College of Emergency Physicians open. 2020 doi: 10.1002/emp2.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Tu Yung-Fang, Chien Chian-Shiu, Yarmishyn Aliaksandr A, Lin Yi-Ying, Luo Yung-Hung, Lin Yi-Tsung, Lai Wei-Yi, Yang De-Ming, Chou Shih-Jie, Yang Yi-Ping, Wang Mong-Lien, Chiou Shih-Hwa. International journal of molecular sciences. 2020;21(7) doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treatment of SARS with human interferons. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H W. Lancet (London, England) 2003;362(9380):293–4. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical trials on drug repositioning for COVID-19 treatment. Rosa Sandro G Viveiros, Santos Wilson C. Revista panamericana de salud publica = Pan American journal of public health. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potential application of mesenchymal stem cells in acute lung injury. Lee Jae Woo, Gupta Naveen, Serikov Vladimir, Matthay Michael A. Expert opinion on biological therapy. 2009;9(10):1259–70. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Li Yan, Xu Jun, Shi Weiqing, Chen Cheng, Shao Yan, Zhu Limei, Lu Wei, Han XiaoDong. Stem cell research & therapy. 2016;7(1):159. doi: 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neutralizing antibodies against West Nile virus identified directly from human B cells by single-cell analysis and next generation sequencing. Tsioris Konstantinos, Gupta Namita T., Ogunniyi Adebola O., Zimnisky Ross M., Qian Feng, Yao Yi, Wang Xiaomei, Stern Joel N. H., Chari Raj, Briggs Adrian W., Clouser Christopher R., Vigneault Francois, Church George M., Garcia Melissa N., Murray Kristy O., Montgomery Ruth R., Kleinstein Steven H., Love J. Christopher. Integrative Biology. 2015;7(12):1587-1597. doi: 10.1039/c5ib00169b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Zhou Guangyu, Zhao Qi. International Journal of Biological Sciences. 2020;16(10):1718-1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical uses of intravenous immunoglobulin. Jolles S., Sewell W. A. C., Misbah S. A. Clinical and Experimental Immunology. 2005;142(1):1-11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.COVID-19 therapy and prevention. Elie Claude-Rosny. Discoveries. 2020;8(3):e113. doi: 10.15190/d.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Yaqinuddin Ahmed, Kashir Junaid. Medical Hypotheses. 2020;140:109777. doi: 10.1016/j.mehy.2020.109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. André Pascale, Denis Caroline, Soulas Caroline, Bourbon-Caillet Clarisse, Lopez Julie, Arnoux Thomas, Bléry Mathieu, Bonnafous Cécile, Gauthier Laurent, Morel Ariane, Rossi Benjamin, Remark Romain, Breso Violette, Bonnet Elodie, Habif Guillaume, Guia Sophie, Lalanne Ana Ines, Hoffmann Caroline, Lantz Olivier, Fayette Jérôme, Boyer-Chammard Agnès, Zerbib Robert, Dodion Pierre, Ghadially Hormas, Jure-Kunkel Maria, Morel Yannis, Herbst Ronald, Narni-Mancinelli Emilie, Cohen Roger B., Vivier Eric. Cell. 2018;175(7):1731-1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monalizumab: inhibiting the novel immune checkpoint NKG2A. van Hall Thorbald, André Pascale, Horowitz Amir, Ruan Dan Fu, Borst Linda, Zerbib Robert, Narni-Mancinelli Emilie, van der Burg Sjoerd H, Vivier Eric. Journal for immunotherapy of cancer. 2019;7(1):263. doi: 10.1186/s40425-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. Chen Jun, Lau Yuk Fai, Lamirande Elaine W, Paddock Christopher D, Bartlett Jeanine H, Zaki Sherif R, Subbarao Kanta. Journal of virology. 2010;84(3):1289–301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Two cases report of epidemic stress disorder to novel coronavirus pneumonia. Wang Congjie, Zhou Juan, Zong Chengjuan. Asian journal of psychiatry. 2020;51:102070. doi: 10.1016/j.ajp.2020.102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traditional Chinese Medicine treatment of COVID-19. Xu Jia, Zhang Yunfei. Complementary therapies in clinical practice. 2020;39:101165. doi: 10.1016/j.ctcp.2020.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. Siegel Dustin, Hui Hon C, Doerffler Edward, Clarke Michael O, Chun Kwon, Zhang Lijun, Neville Sean, Carra Ernest, Lew Willard, Ross Bruce, Wang Queenie, Wolfe Lydia, Jordan Robert, Soloveva Veronica, Knox John, Perry Jason, Perron Michel, Stray Kirsten M, Barauskas Ona, Feng Joy Y, Xu Yili, Lee Gary, Rheingold Arnold L, Ray Adrian S, Bannister Roy, Strickley Robert, Swaminathan Swami, Lee William A, Bavari Sina, Cihlar Tomas, Lo Michael K, Warren Travis K, Mackman Richard L. Journal of medicinal chemistry. 2017;60(5):1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 47.A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. Mulangu Sabue, Dodd Lori E, Davey Richard T, Tshiani Mbaya Olivier, Proschan Michael, Mukadi Daniel, Lusakibanza Manzo Mariano, Nzolo Didier, Tshomba Oloma Antoine, Ibanda Augustin, Ali Rosine, Coulibaly Sinaré, Levine Adam C, Grais Rebecca, Diaz Janet, Lane H Clifford, Muyembe-Tamfum Jean-Jacques, Sivahera Billy, Camara Modet, Kojan Richard, Walker Robert, Dighero-Kemp Bonnie, Cao Huyen, Mukumbayi Philippe, Mbala-Kingebeni Placide, Ahuka Steve, Albert Sarah, Bonnett Tyler, Crozier Ian, Duvenhage Michael, Proffitt Calvin, Teitelbaum Marc, Moench Thomas, Aboulhab Jamila, Barrett Kevin, Cahill Kelly, Cone Katherine, Eckes Risa, Hensley Lisa, Herpin Betsey, Higgs Elizabeth, Ledgerwood Julie, Pierson Jerome, Smolskis Mary, Sow Ydrissa, Tierney John, Sivapalasingam Sumathi, Holman Wendy, Gettinger Nikki, Vallée David, Nordwall Jacqueline. The New England journal of medicine. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sheahan Timothy P, Sims Amy C, Graham Rachel L, Menachery Vineet D, Gralinski Lisa E, Case James B, Leist Sarah R, Pyrc Krzysztof, Feng Joy Y, Trantcheva Iva, Bannister Roy, Park Yeojin, Babusis Darius, Clarke Michael O, Mackman Richard L, Spahn Jamie E, Palmiotti Christopher A, Siegel Dustin, Ray Adrian S, Cihlar Tomas, Jordan Robert, Denison Mark R, Baric Ralph S. Science translational medicine. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. Agostini Maria L, Andres Erica L, Sims Amy C, Graham Rachel L, Sheahan Timothy P, Lu Xiaotao, Smith Everett Clinton, Case James Brett, Feng Joy Y, Jordan Robert, Ray Adrian S, Cihlar Tomas, Siegel Dustin, Mackman Richard L, Clarke Michael O, Baric Ralph S, Denison Mark R. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Wang Manli, Cao Ruiyuan, Zhang Leike, Yang Xinglou, Liu Jia, Xu Mingyue, Shi Zhengli, Hu Zhihong, Zhong Wu, Xiao Gengfu. Cell research. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.First Case of 2019 Novel Coronavirus in the United States. Holshue Michelle L, DeBolt Chas, Lindquist Scott, Lofy Kathy H, Wiesman John, Bruce Hollianne, Spitters Christopher, Ericson Keith, Wilkerson Sara, Tural Ahmet, Diaz George, Cohn Amanda, Fox LeAnne, Patel Anita, Gerber Susan I, Kim Lindsay, Tong Suxiang, Lu Xiaoyan, Lindstrom Steve, Pallansch Mark A, Weldon William C, Biggs Holly M, Uyeki Timothy M, Pillai Satish K. The New England journal of medicine. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remdesivir: First Approval. Lamb Yvette N. Drugs. 2020;80(13):1355–1363. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Furuta Yousuke, Komeno Takashi, Nakamura Takaaki. Proceedings of the Japan Academy. Series B, Physical and biological sciences. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Delang Leen, Abdelnabi Rana, Neyts Johan. Antiviral research. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Glenmark Pharmaceuticals Ltd. Glenmark to Commence New Phase 3 Clinical Trial on Combination of Two Anti-viral Drugs Favipiravir and Umifenovir in Hospitalized Patients of Moderate COVID-19 in India. CISION PR News. 2020. https://www.prnewswire.com/in/news-releases/glenmark-to-commence-new-phase-3-clinical-trial-on-combination-of-two-anti-viral-drugs-favipiravir-and-umifenovir-in-hospitalized-patients-of-moderate-covid-19-in-india-836904730.html https://www.prnewswire.com/in/news-releases/glenmark-to-commence-new-phase-3-clinical-trial-on-combination-of-two-anti-viral-drugs-favipiravir-and-umifenovir-in-hospitalized-patients-of-moderate-covid-19-in-india-836904730.html
- 56.Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Chu C M, Cheng V C C, Hung I F N, Wong M M L, Chan K H, Chan K S, Kao R Y T, Poon L L M, Wong C L P, Guan Y, Peiris J S M, Yuen K Y. Thorax. 2004;59(3):252–6. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. Chan Jasper Fuk-Woo, Yao Yanfeng, Yeung Man-Lung, Deng Wei, Bao Linlin, Jia Lilong, Li Fengdi, Xiao Chong, Gao Hong, Yu Pin, Cai Jian-Piao, Chu Hin, Zhou Jie, Chen Honglin, Qin Chuan, Yuen Kwok-Yung. Journal of Infectious Diseases. 2015;212(12):1904-1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Chan K S, Lai S T, Chu C M, Tsui E, Tam C Y, Wong M M L, Tse M W, Que T L, Peiris J S M, Sung J, Wong V C W, Yuen K Y. Hong Kong medical journal = Xianggang yi xue za zhi. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 59.Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Wagstaff Kylie M, Sivakumaran Haran, Heaton Steven M, Harrich David, Jans David A. The Biochemical journal. 2012;443(3):851–6. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. Cao Bin, Wang Yeming, Wen Danning, Liu Wen, Wang Jingli, Fan Guohui, Ruan Lianguo, Song Bin, Cai Yanping, Wei Ming, Li Xingwang, Xia Jiaan, Chen Nanshan, Xiang Jie, Yu Ting, Bai Tao, Xie Xuelei, Zhang Li, Li Caihong, Yuan Ye, Chen Hua, Li Huadong, Huang Hanping, Tu Shengjing, Gong Fengyun, Liu Ying, Wei Yuan, Dong Chongya, Zhou Fei, Gu Xiaoying, Xu Jiuyang, Liu Zhibo, Zhang Yi, Li Hui, Shang Lianhan, Wang Ke, Li Kunxia, Zhou Xia, Dong Xuan, Qu Zhaohui, Lu Sixia, Hu Xujuan, Ruan Shunan, Luo Shanshan, Wu Jing, Peng Lu, Cheng Fang, Pan Lihong, Zou Jun, Jia Chunmin, Wang Juan, Liu Xia, Wang Shuzhen, Wu Xudong, Ge Qin, He Jing, Zhan Haiyan, Qiu Fang, Guo Li, Huang Chaolin, Jaki Thomas, Hayden Frederick G, Horby Peter W, Zhang Dingyu, Wang Chen. The New England journal of medicine. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Effects of chloroquine on viral infections: an old drug against today's diseases? Savarino Andrea, Boelaert Johan R, Cassone Antonio, Majori Giancarlo, Cauda Roberto. The Lancet. Infectious diseases. 2003;3(11):722–7. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Vincent Martin J, Bergeron Eric, Benjannet Suzanne, Erickson Bobbie R, Rollin Pierre E, Ksiazek Thomas G, Seidah Nabil G, Nichol Stuart T. Virology journal. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Positive RT-PCR Test Results in Patients Recovered From COVID-19. Lan Lan, Xu Dan, Ye Guangming, Xia Chen, Wang Shaokang, Li Yirong, Xu Haibo. JAMA. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Gautret Philippe, Lagier Jean-Christophe, Parola Philippe, Hoang Van Thuan, Meddeb Line, Mailhe Morgane, Doudier Barbara, Courjon Johan, Giordanengo Valérie, Vieira Vera Esteves, Tissot Dupont Hervé, Honoré Stéphane, Colson Philippe, Chabrière Eric, La Scola Bernard, Rolain Jean-Marc, Brouqui Philippe, Raoult Didier. International journal of antimicrobial agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. World Health Organization. 2017. https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19
- 66.Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Kadam Rameshwar U, Wilson Ian A. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(2):206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. Chen Chang, Zhang Yi, Huang Jianying, Yin Ping, Cheng Zhenshun, Wu Jianyuan, Chen Song, Zhang Yongxi, Chen Bo, Lu Mengxin, Luo Yongwen, Ju Lingao, Zhang Jingyi, Wang Xinghuan. 2020 doi: 10.3389/fphar.2021.683296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements. Pandey Abhjieet, Nikam Ajinkya Nitin, Shreya Ajjappla Basavaraj, Mutalik Sadhana P, Gopalan Divya, Kulkarni Sanjay, Padya Bharath Singh, Fernandes Gasper, Mutalik Srinivas, Prassl Ruth. Life sciences. 2020;256:117883. doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Monteil Vanessa, Kwon Hyesoo, Prado Patricia, Hagelkrüys Astrid, Wimmer Reiner A, Stahl Martin, Leopoldi Alexandra, Garreta Elena, Hurtado Del Pozo Carmen, Prosper Felipe, Romero Juan Pablo, Wirnsberger Gerald, Zhang Haibo, Slutsky Arthur S, Conder Ryan, Montserrat Nuria, Mirazimi Ali, Penninger Josef M. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Khan Akram, Benthin Cody, Zeno Brian, Albertson Timothy E, Boyd John, Christie Jason D, Hall Richard, Poirier Germain, Ronco Juan J, Tidswell Mark, Hardes Kelly, Powley William M, Wright Tracey J, Siederer Sarah K, Fairman David A, Lipson David A, Bayliffe Andrew I, Lazaar Aili L. Critical care (London, England) 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thalidomide-induced teratogenesis: history and mechanisms. Vargesson Neil. Birth defects research. Part C, Embryo today : reviews. 2015;105(2):140–56. doi: 10.1002/bdrc.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in mice. Zhu Haiyan, Shi Xunlong, Ju Dianwen, Huang Hai, Wei Wei, Dong Xiaoying. Inflammation. 2014;37(6):2091–8. doi: 10.1007/s10753-014-9943-9. [DOI] [PubMed] [Google Scholar]
- 73.Aralen Phosphate. The American Society of Health-System Pharmacists. Archived. 2015.
- 74.A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. Cortegiani Andrea, Ingoglia Giulia, Ippolito Mariachiara, Giarratano Antonino, Einav Sharon. Journal of critical care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.SARS-CoV-2 and the Use of Chloroquine as an Antiviral Treatment. Rebeaud Mathieu E, Zores Florian. Frontiers in medicine. 2020;7:184. doi: 10.3389/fmed.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azioni intraprese per favorire la ricerca e l'accesso ai nuovi farmaci per il trattamento del COVID-19. Agenzia Italiana del Farmaco. 2020. https://www.aifa.gov.it/-/azioni-intraprese-per-favorire-la-ricerca-e-l-accesso-ai-nuovi-farmaci-per-il-trattamento-del-covid-19 https://www.aifa.gov.it/-/azioni-intraprese-per-favorire-la-ricerca-e-l-accesso-ai-nuovi-farmaci-per-il-trattamento-del-covid-19
- 77.Kwak Sung-Sun Physicians work out treatment guidelines for coronavirus. Korea Biomedical Review; Accessed on March 18, 2020. 2020. http://www.koreabiomed.com/news/articleView.html?idxno=7428 http://www.koreabiomed.com/news/articleView.html?idxno=7428
- 78.Severe Illness Associated with Using Non-Pharmaceutical Chloroquine Phosphate to Prevent and Treat Coronavirus Disease 2019 (COVID-19) Georgia Department of Public Health; Accessed on March 31, 2020. 2020. https://www.gachd.org/severe-illness-associated-with-using-non-pharmaceutical-chloroquine-phosphate-to-prevent-and-treat-coronavirus-disease-2019-covid-19/ https://www.gachd.org/severe-illness-associated-with-using-non-pharmaceutical-chloroquine-phosphate-to-prevent-and-treat-coronavirus-disease-2019-covid-19/
- 79.Banner Health experts warn against self-medicating to prevent or treat COVID-19. Banner Health; Accessed on March 25, 2020. 2020. http://bannerhealth.mediaroom.com/chloroquinephosphate http://bannerhealth.mediaroom.com/chloroquinephosphate
- 80.Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020. U.S. Food and Drug Administration; Accessed on March 30, 2020. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-30-2020 https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-30-2020
- 81.Losartan. Drugs.com. 2018. https://www.drugs.com/monograph/losartan-potassium.html. https://www.drugs.com/monograph/losartan-potassium.html.
- 82.Live Science Staff Treatments for COVID-19: Drugs being tested against the coronavirus. Live Science. 2020. https://www.livescience.com/coronavirus-covid-19-treatments.html https://www.livescience.com/coronavirus-covid-19-treatments.html
- 83.Emerging Therapeutic Strategies for COVID-19 Patients. Zhu Shudong, Guo Xialing, Geary Kyla, Zhang Dianzheng. Discoveries. 2020;8(1):e105. doi: 10.15190/d.2020.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruxolitinib. Mesa Ruben A., Yasothan Uma, Kirkpatrick Peter. Nature Reviews Drug Discovery. 2012;11(2):103-104. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- 85.Could azithromycin prevent NHS workers from developing COVID-19? Health Europe. 2020. https://www.healtheuropa.eu/azithromycin-prevent-nhs-workers-developing-covid-19/99271/ https://www.healtheuropa.eu/azithromycin-prevent-nhs-workers-developing-covid-19/99271/
- 86.Practical management of patients with myelofibrosis receiving ruxolitinib. Harrison Claire, Mesa Ruben, Ross David, Mead Adam, Keohane Clodagh, Gotlib Jason, Verstovsek Srdan. Expert Review of Hematology. 2013;6(5):511-523. doi: 10.1586/17474086.2013.827413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruxolitinib versus standard therapy for the treatment of polycythemia vera. Vannucchi Alessandro M, Kiladjian Jean Jacques, Griesshammer Martin, Masszi Tamas, Durrant Simon, Passamonti Francesco, Harrison Claire N, Pane Fabrizio, Zachee Pierre, Mesa Ruben, He Shui, Jones Mark M, Garrett William, Li Jingjin, Pirron Ulrich, Habr Dany, Verstovsek Srdan. The New England journal of medicine. 2015;372(5):426–35. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. Mesa Ruben A. IDrugs : the investigational drugs journal. 2010;13(6):394–403. [PubMed] [Google Scholar]
- 89.Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. Cao Yang, Wei Jia, Zou Liang, Jiang Tiebin, Wang Gaoxiang, Chen Liting, Huang Liang, Meng Fankai, Huang Lifang, Wang Na, Zhou Xiaoxi, Luo Hui, Mao Zekai, Chen Xing, Xie Jungang, Liu Jing, Cheng Hui, Zhao Jianping, Huang Gang, Wang Wei, Zhou Jianfeng. The Journal of allergy and clinical immunology. 2020;146(1):137–146.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruxolitinib Phase III Trial Initiated for Treatment of COVID-19 Cytokine Storm. Ternyila Danielle. https://www.targetedonc.com/view/ruxolitinib-phase-iii-trial-initiated-for-treatment-of-covid-19-symptoms Targeted Oncology. 2020 [Google Scholar]
- 91.A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Bechman Katie, Subesinghe Sujith, Norton Sam, Atzeni Fabiola, Galli Massimo, Cope Andrew P, Winthrop Kevin L, Galloway James B. Rheumatology. 2019;58(10):1755-1766. doi: 10.1093/rheumatology/kez087. [DOI] [PubMed] [Google Scholar]
- 92.Baricitinib Studied as Possible COVID-19 Treatment; Plus Ranitidine Removed from U.S. Market. Kaufman Michele B. https://www.the-rheumatologist.org/article/baricitinib-studied-as-possible-covid-19-treatment-plus-ranitidine-removed-from-u-s-market/ The Rheumatologist. 2020 [Google Scholar]
- 93.Lilly Begins Clinical Testing of Therapies for COVID-19. Eli Lilly and Company. 2020. https://investor.lilly.com/news-releases/news-release-details/lilly-begins-clinical-testing-therapies-covid-19 https://investor.lilly.com/news-releases/news-release-details/lilly-begins-clinical-testing-therapies-covid-19
- 94.Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Chen Luni, Liu Peng, Gao He, Sun Bing, Chao Desheng, Wang Fei, Zhu Yuanjue, Hedenstierna Göran, Wang Chen G. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(10):1531–5. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cennimo David J. What is the role of nitric oxide in the treatment of coronavirus disease 2019 (COVID-19)? Medscape. 2020. https://www.medscape.com/answers/2500114-197460/what-is-the-role-of-nitric-oxide-in-the-treatment-of-coronavirus-disease-2019-covid-19 https://www.medscape.com/answers/2500114-197460/what-is-the-role-of-nitric-oxide-in-the-treatment-of-coronavirus-disease-2019-covid-19
- 96.Senses H. Vitamin C effective against COVID-19: Expert. www.aa.com. 2020.
- 97.Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Villar Jesús, Ferrando Carlos, Martínez Domingo, Ambrós Alfonso, Muñoz Tomás, Soler Juan A, Aguilar Gerardo, Alba Francisco, González-Higueras Elena, Conesa Luís A, Martín-Rodríguez Carmen, Díaz-Domínguez Francisco J, Serna-Grande Pablo, Rivas Rosana, Ferreres José, Belda Javier, Capilla Lucía, Tallet Alec, Añón José M, Fernández Rosa L, González-Martín Jesús M. The Lancet. Respiratory medicine. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 98.Oseltamivir: a review of its use in influenza. McClellan K, Perry C M. Drugs. 2001;61(2):263–83. doi: 10.2165/00003495-200161020-00011. [DOI] [PubMed] [Google Scholar]
- 99.Oral oseltamivir treatment of influenza in children. Whitley R J, Hayden F G, Reisinger K S, Young N, Dutkowski R, Ipe D, Mills R G, Ward P. The Pediatric infectious disease journal. 2001;20(2):127–33. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 100.Colchicine--Update on mechanisms of action and therapeutic uses. Leung Ying Ying, Yao Hui Laura Li, Kraus Virginia B. Seminars in arthritis and rheumatism. 2015;45(3):341–50. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colchicine and the heart: pushing the envelope. Deftereos Spyridon, Giannopoulos Georgios, Papoutsidakis Nikolaos, Panagopoulou Vasiliki, Kossyvakis Charalampos, Raisakis Konstantinos, Cleman Michael W, Stefanadis Christodoulos. Journal of the American College of Cardiology. 2013;62(20):1817–25. doi: 10.1016/j.jacc.2013.08.726. [DOI] [PubMed] [Google Scholar]
- 102.A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Cao Jianzhong, Forrest J Craig, Zhang Xuming. Antiviral research. 2015;114:1–10. doi: 10.1016/j.antiviral.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. Rossignol Jean-François. Journal of infection and public health. 2016;9(3):227–30. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.A clinical trial of low-dose selinexor examines the drug as a potential antiviral and anti-inflammatory treatment for COVID-19. Norton Healthcare. 2020. https://nortonhealthcare.com/news/selinexor-covid-19-trial/ https://nortonhealthcare.com/news/selinexor-covid-19-trial/
- 105.Brazilian Collaboration to Investigate Methotrexate to Treat Severe COVID-19 Cases. TrialSiteNews. 2020. https://trialsitenews.com/brazilian-collaboration-to-investigate-methotrexate-to-treat-severe-covid-19-cases/ https://trialsitenews.com/brazilian-collaboration-to-investigate-methotrexate-to-treat-severe-covid-19-cases/
- 106.Healy M. Drugs for heartburn, gout and depression now being tested as coronavirus treatments, Los Angeles Times. 2020. Los Angeles Times. 2020. https://www.latimes.com/science/story/2020-05-02/researchers-testing-pepcid-as-coronavirus-treatment https://www.latimes.com/science/story/2020-05-02/researchers-testing-pepcid-as-coronavirus-treatment
- 107.COVID-19: Facts and Recommendations from A to Z. Task Force BASE Medicine. Science Insights. 2020;33(1):138-158. [Google Scholar]
- 108.Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Prompetchara Eakachai, Ketloy Chutitorn, Palaga Tanapat. Asian Pacific journal of allergy and immunology. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 109.COVID-19 Vaccines: A Race Against Time in the Middle of Death and Devastation! Khuroo Mohammad S, Khuroo Mohammad, Khuroo Mehnaaz S, Sofi Ahmad A, Khuroo Naira S. Journal of clinical and experimental hepatology. 2020;10(6):610–621. doi: 10.1016/j.jceh.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.[Progress and challenge of vaccine development against 2019-novel coronavirus (2019-nCoV)]. Shi Y, Wang N, Zou Q M. Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine] 2020;54(6):614–619. doi: 10.3760/cma.j.cn112150-20200317-00366. [DOI] [PubMed] [Google Scholar]
- 111.COVID-19: Progress in diagnostics, therapy and vaccination. Liu Xue, Liu Chao, Liu Gang, Luo Wenxin, Xia Ningshao. Theranostics. 2020;10(17):7821–7835. doi: 10.7150/thno.47987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Sheahan Timothy P, Sims Amy C, Leist Sarah R, Schäfer Alexandra, Won John, Brown Ariane J, Montgomery Stephanie A, Hogg Alison, Babusis Darius, Clarke Michael O, Spahn Jamie E, Bauer Laura, Sellers Scott, Porter Danielle, Feng Joy Y, Cihlar Tomas, Jordan Robert, Denison Mark R, Baric Ralph S. Nature communications. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Stanley Daphne A, Honko Anna N, Asiedu Clement, Trefry John C, Lau-Kilby Annie W, Johnson Joshua C, Hensley Lisa, Ammendola Virginia, Abbate Adele, Grazioli Fabiana, Foulds Kathryn E, Cheng Cheng, Wang Lingshu, Donaldson Mitzi M, Colloca Stefano, Folgori Antonella, Roederer Mario, Nabel Gary J, Mascola John, Nicosia Alfredo, Cortese Riccardo, Koup Richard A, Sullivan Nancy J. Nature medicine. 2014;20(10):1126–9. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 114.Safety and Immunogenicity of a Heterologous Prime-Boost Ebola Virus Vaccine Regimen in Healthy Adults in the United Kingdom and Senegal. Venkatraman Navin, Ndiaye Birahim Pierre, Bowyer Georgina, Wade Djibril, Sridhar Saranya, Wright Daniel, Powlson Jonathan, Ndiaye Ibrahima, Dièye Siry, Thompson Craig, Bakhoum Momar, Morter Richard, Capone Stefania, Del Sorbo Mariarosaria, Jamieson Sophie, Rampling Tommy, Datoo Mehreen, Roberts Rachel, Poulton Ian, Griffiths Oliver, Ballou W Ripley, Roman François, Lewis David J M, Lawrie Alison, Imoukhuede Egeruan, Gilbert Sarah C, Dieye Tandakha N, Ewer Katie J, Mboup Souleymane, Hill Adrian V S. The Journal of Infectious Diseases. 2019;219(8):1187-1197. doi: 10.1093/infdis/jiy639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Antrobus Richard D, Coughlan Lynda, Berthoud Tamara K, Dicks Matthew D, Hill Adrian Vs, Lambe Teresa, Gilbert Sarah C. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22(3):668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Folegatti Pedro M, Bittaye Mustapha, Flaxman Amy, Lopez Fernando Ramos, Bellamy Duncan, Kupke Alexandra, Mair Catherine, Makinson Rebecca, Sheridan Jonathan, Rohde Cornelius, Halwe Sandro, Jeong Yuji, Park Young-Shin, Kim Jae-Ouk, Song Manki, Boyd Amy, Tran Nguyen, Silman Daniel, Poulton Ian, Datoo Mehreen, Marshall Julia, Themistocleous Yrene, Lawrie Alison, Roberts Rachel, Berrie Eleanor, Becker Stephan, Lambe Teresa, Hill Adrian, Ewer Katie, Gilbert Sarah. The Lancet. Infectious diseases. 2020;20(7):816–826. doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nanoparticle-Based Vaccines Against Respiratory Viruses. Al-Halifa Soultan, Gauthier Laurie, Arpin Dominic, Bourgault Steve, Archambault Denis. Frontiers in immunology. 2019;10:22. doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coronavirus Pandemic—Therapy and Vaccines. Lundstrom Kenneth. Biomedicines. 2020;8(5):109. doi: 10.3390/biomedicines8050109. [DOI] [PMC free article] [PubMed] [Google Scholar]