Abstract
Objective
To date, treatment options (i.e. psychotherapy, antidepressant medications) for patients with posttraumatic stress disorder (PTSD), are relatively few, and considering their limited efficacy, novel therapies have gained interest among researchers and treatment providers alike. Among patients with chronic pain (CP) about one third experience comorbid PTSD, which further complicates their already challenging pharmacological regimens. Low dose ketamine infusion has shown promise in PTSD, and in treatment of CP, however they have not been studied in comorbid population and under rigorous control conditions.
Methods
We compared the effects of a single dose of either ketamine (0.5 mg/kg) or ketorolac (15 mg) over a 40-minute of IV infusion in CP patients with and without PTSD, in double blind, randomized study. Measures were collected before, during, one day and seven days after the infusion. A planned sample size of 40 patients randomly assigned to treatment order was estimated to provide 80% power to detect a hypothesized treatment difference after the infusion.
Main Outcome and Measures: The primary outcome measures were change in PTSD symptom severity assessed with the Impact of Event Scale–Revised (IES-R) and Visual Analogue Scale (VAS) for pain administered by a study clinician 24 hours post infusion. Secondary outcome measures included Impact of Event Scale–Revised (IES-R), VAS and Brief Pain Inventory (Short Form) for pain 1 week after the infusion.
Results
Both treatments offered comparable improvement of PTSD and CP symptoms that persisted for 7 days after the infusion. Patients with comorbid PTSD and CP experienced less dissociative side effects compared to the CP group. Surprisingly, ketorolac infusion resulted in dissociative symptoms in CP patients only.
Conclusions
This first prospective study comparing effects of subanesthetic ketamine versus ketorolac infusions for comorbid PTSD and CP, suggests that both ketamine and ketorolac might offer meaningful and durable response for both PTSD and CP symptoms.
Keywords: chronic pain, IV infusion, ketamine, posttraumatic stress disorder
Introduction
Posttraumatic stress disorder (PTSD) is a prevalent and debilitating disease characterized by severe and persistent psychological symptoms following exposure to traumatic events. Following a traumatic event, symptoms of one-month duration include four distinct clusters: intrusive recollections; avoidance of trauma reminders; negative alterations in cognitions and mood; and hyperarousal. Moreover, PTSD patients frequently exhibit comorbidity with other internalizing disorders (e.g. anxiety disorders, major depressive disorder). Prevalence rates for PTSD in the general population are estimated at approximately 8%, with higher rates associated with exposure to multiple traumatic events. Prevalence rates for PTSD are significantly higher among military veterans, with current estimates of up to 23%.1 Furthermore, prevalence rates of PTSD in patients with CP have been estimated at 35%.2
To-date, treatment options for United States veterans with PTSD have predominantly included psychotherapies such as prolonged exposure and cognitive processing therapy, and medications including selective serotonin reuptake inhibitors (SSRIs).3 However, novel treatments, such as ketamine infusion, that may offer better efficacy and is less subject to patient dropout and/or side effects have continued to gain interest among researchers and treating providers alike.4–6 Feder et al. reported that one dose of intravenous (IV) ketamine infusion was associated with rapid reduction in PTSD symptom severity and comorbid depressive symptoms compared with midazolam (placebo) with symptom relief persisting for at least seven days.7 Furthermore, repeated IV ketamine infusions on 3 days per week schedule over 12-day period were associated with PTSD and depression symptom relief for up to 41 and 20 days, respectively.8 These initial reports suggest that the effects of low dose ketamine infusions may have a more extended impact on PTSD symptomatology beyond its immediate plasma concentration.
In addition to examining the effects of ketamine on symptoms of depression and PTSD, clinical studies have also examined its effects on mitigating CP symptoms. In a double-blind, randomized, placebo-controlled parallel-group trial of patients with chronic regional pain syndrome (CRPS) type I treated with low dose IV ketamine infusion, Sigtermans et al. reported spontaneous pain relief that lasted for 12 weeks, without functional improvement however.9 Noppers et al. studied 24 fibromyalgia patients using IV infusion of ketamine (0.5 mg/kg over 30 minutes, n = 12) or active placebo (midazolam 5 mg, n = 12) in a randomized double blind trial, concluding that short-term ketamine infusion is insufficient to induce long-term analgesic effects in these patients.10 The effects of ketamine on migraine headache and chronic low-back pain have not been widely studied.11 Thus, few studies involving patients with CP suggest that single doses of ketamine produce short term pain relief probably related to its plasma concentration.
It has been suggested that PTSD and CP might share overlapping brain circuitry,12 and given high rate of comorbidity13 of these conditions, evaluating the potential immediate and extended effect of ketamine infusions in these patients is clearly warranted. Our study sought to examine the effects of a single low dose ketamine infusion as compared to the IV infusion of active placebo (nonsteroidal anti-inflammatory agent – ketorolac) on CP and PTSD symptoms in a double-blind randomized trial of US military veterans with and without PTSD. The choice of potent analgesic – ketorolac, as active comparator, had allowed for effective blinding of treatment assignment in patients with CP symptoms. Based on prior literature, we hypothesized that PTSD symptoms in veterans with both CP and PTSD would decrease following ketamine infusion compared to ketorolac, and that these effects would persist beyond its presence in the brain. We hypothesized that some of the CP symptoms in PTSD patients would also be reduced for a longer period. We further hypothesized that ketorolac would produce pain relief only related to its short-term pharmacokinetics in contrast to ketamine for both groups.
Methods
Subjects aged 18 to 65 were enrolled at the Outpatient Pain Management Clinic of the Ann Arbor Veteran Affairs (VA) Hospital between January 2018 and June 2019 and randomized into four treatment groups: CP subjects treated with ketorolac, CP subjects treated with ketamine, CP+PTSD subjects treated with ketorolac, and CP+PTSD subjects treated with ketamine. CP diagnosis was defined as having symptoms of CP of at least 6 months duration as defined by the International Association for the Study of Pain (IASP).14 PTSD diagnosis was established based on DSM-V using the Clinician-Administered PTSD Scale-5 (CAPS-5)15,16 administered by master’s level clinician; with symptoms persisting for at minimum 3 months prior to assessment. All participants taking psychotropic and/or pain medications were required to be on stable doses for at least 6 weeks prior to study enrollment for the duration of the study. Participants engaged in psychotherapy for PTSD symptoms were required to have sessions that were stable in frequency and duration for at least 6 weeks prior to beginning of the study. Exclusion criteria included inability to speak English, inability or unwillingness to provide written informed consent; moderate-to-severe cognitive impairment (Mini-Mental State Examination scores < 20 administered by a trained clinician); current or lifetime history of psychotic or bipolar disorder; current bulimia or anorexia nervosa, alcohol abuse or dependence in the previous 3 months; serious unstable medical illness or sleep apnea; HTN, prolonged QT interval, peptic ulcer disease or recent history of GI-bleed, renal insufficiency, active substance use disorder, active suicidal or homicidal ideation on presentation; pregnancy (confirmed by baseline lab test), the initiation of female hormonal treatments within 3 months of screening, or inability or unwillingness to use a medically accepted contraceptive method for the duration of the study. The institutional review board at Ann Arbor VA approved the study; it was registered at ClinicalTrials.gov (identifier: NCT04322968). Written informed consent was obtained from all study participants. Participants were not compensated for their time.
Procedures
The entire study was conducted in an outpatient setting. Patients were asked to fast overnight and be accompanied by a driver on the day of the infusion. They were admitted to the Post-Anesthesia Care Unit (PACU) where an indwelling catheter was placed in the antecubital vein of the nondominant arm. Monitoring of pulse and blood pressure, pulse oximetry, and electrocardiographic monitoring was instituted. Participants were required to stay at the clinical site for 4 hours after the medication was given. Follow-up visits occurred at different time points over the course of one week after the infusion period has been completed.
Patient with and without PTSD were randomized to receive either ketamine or ketorolac (Table 1). Single IV infusion of ketamine hydrochloride (0.5 mg/kg) was administered over 40 minutes. Ketorolac 15 mg was reconstituted in 500 cc of normal saline administered over 40 minutes. To prevent the development of nausea and vomiting, a known side effect associated with ketamine infusion, or potential treatment unblinding, 4 mg IV ondansetron was administered to all patients.
Table 1.
The arms and assigned interventions.
| Cohort | Intervention | |
|---|---|---|
| CP patients with PTSD | IV ketamine infusion | IV ketorolac infusion |
| CP patients without PTSD | IV ketamine infusion | IV ketorolac infusion |
The compounds were prepared by research pharmacy and delivered to PACU in blinded fashion. All study personnel, including rater, patients, and data analysts, were blinded to randomization order. Only the anesthesiologist (ARD) performing the infusion was not blinded in order to prepare for possible side effects of each medication. A trained rater administered questionnaires at baseline (prior to infusion), 15 minutes, 40 minutes, 120 and 240 minutes, 24 hours (day 1), 48 hours (day 2) after infusion, and seven days (day 7) after infusion.
Measures
The primary outcomes, PTSD and CP symptom severity, were scored using Impact of Event Scale–Revised (IES-R17) and Visual Analogue Scale (VAS18) respectively one- and seven-days post-infusion. Scores taken one day post-infusion were selected as primary end points because acute sedating and other side effects were expected to have resolved, while potential symptom improvement was expected to persist beyond 24 hours. Secondary outcome measures included scores from the IES-R, VAS and Brief Pain Inventory (BPI Short Form19) assessed seven days following infusion. The anticipated side effects and dissociative, psychotomimetic, and manic symptoms were measured with the Patient-Rated Inventory of Side Effects (PRISE2020) and Clinician-Administered Dissociative States Scale,21 respectively.
Statistical Analysis
Statistical analyses were performed in R (R Development Core Team, 2013; version 3.5.1).22 Three-way repeated measures ANOVAs were conducted in order to assess differences in side effects (i.e. PRISE 20), pain symptoms (i.e. VAS, BPI-P, BPI-I), and dissociative symptoms (i.e. CADSS) by group and administered medication. Two-way repeated measures ANOVAs were conducted to assess differences in PTSD symptoms (i.e. IES-R) by medication in both the CP and CP+PTSD groups separately given predetermined differences in PTSD symptoms by group. Two-way ANOVAs were also conducted to assess the a priori primary outcomes of PTSD (IES-R) and pain (VAS) symptom change from baseline to 24 hours post infusion and the secondary outcomes of PTSD and pain symptom change from baseline to 7 days post infusion. Post-hoc analyses were conducted for all significant three-way interactions to clarify directionality of effects between and within-groups, respectively. Bonferroni corrections were used to adjust for multiple tests. Significance values for all analyses utilized a 95% confidence interval.
Results
Study Participants
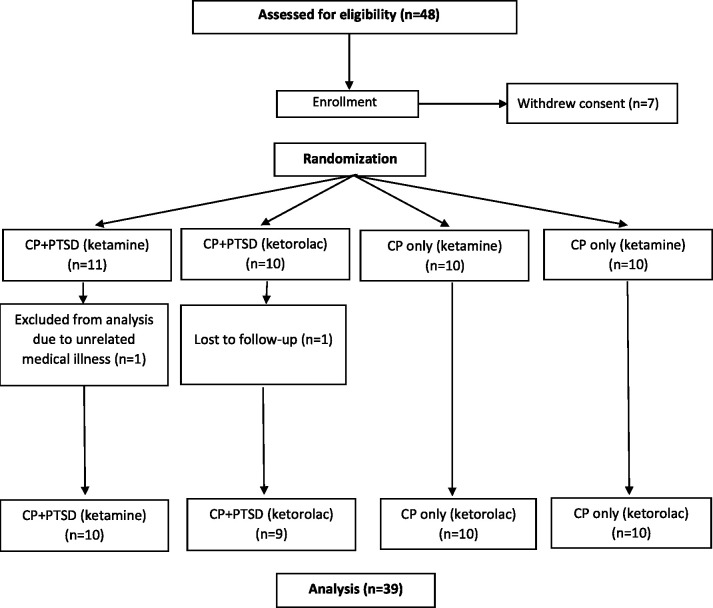
48 potential participants completed informed consent, and out of these, 41 participants (ages 29 to 65) met eligibility criteria and were randomly assigned to receive ketamine or ketorolac infusion (Table 2). Average age did not differ between CP+PTSD (Mean (M) = 42.47, standard deviation (SD) = 9.23) and CP (M = 48.20, SD = 9.23) groups, p > 0.05. There was also no significant difference in age within the CP+PTSD group for those receiving ketamine (M = 45.36, SD = 11.18) or those receiving ketorolac (M = 40.10, SD = 9.73, p > 0.05). Within the CP groups, there was a significant difference in age between those receiving ketamine (M = 43.50, SD = 9.65) and those receiving ketorolac (M = 52.9, SD =8.61), t(17.76) = 2.29, p = 0.03. Gender distribution did not differ between or within CP+PTSD and CP groups, p’s >0.05. All 41 subjects completed all ratings through one-day post-infusion; 1 patient from the CP+PTSD group receiving ketamine dropped out due to an unrelated medical emergency and 1 patient from the CP+PTSD group receiving ketorolac was lost to follow-up (Figure 1).
Table 2.
Demographic and clinical characteristics of study participants.
|
Primary location of pain, N |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant Group | Medication Group | N | Age (M) | Age (SD) | Gender | Ethnicity, Caucasian, N | Weight (M), lbs | Weight (SD) | Low back pain | Neck | Join(s) | Headache | Other chronic pain |
| CP+PTSD | Ketamine | 11 | 45.3 | 11.18 | 7M/4F | 11 | 211.9 | 24.7 | 4 | 2 | 2 | 3 | |
| CP+PTSD | Ketorolac | 10 | 40.1 | 9.73 | 6M/4F | 9 | 204.6 | 51.6 | 8 | 2 | |||
| CP | Ketamine | 10 | 43.5 | 9.65 | 9M/1F | 9 | 214.6 | 63.6 | 8 | 1 | 1 | ||
| CP | Ketorolac | 10 | 52.9 | 8.61 | 9M/1F | 10 | 209.5 | 76.3 | 9 | 1 | |||
Figure 1.
Consolidated standards of reporting trials patient flowchart.
IES-R 1-Day and 7 Days Post Infusion
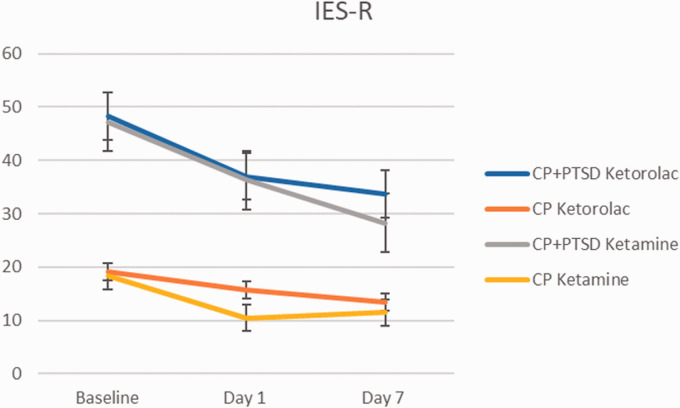
Given preexisting diagnosis of PTSD in the CP+PTSD group, PTSD symptom scores were greater at baseline for the CP+PTSD group than in the CP group and analyses were conducted on groups separately (Figure 2). Results of two-way repeated measures ANOVAs (2(ketorolac, ketamine) X 3(baseline, Day 1, Day 7)) revealed a significant main effect of time on PTSD symptom scores in the CP+PTSD groups such that PTSD symptom decreased significantly from baseline to seven days post-infusion, in both medication groups (F(1, 52) = 9.35, p < 0.01; Figure 2). There was no significant main effect of medication, nor was there an interaction between time and medication type, p’s > 0.05. Follow up t-test analyses revealed significant decreases in PTSD symptom scores from baseline to one day post-infusion (t(32.59)= 2.33, p = 0.03), and from baseline to seven days post-infusion (t(27.53)= 2.93, p < 0.01). There was not a significant symptom change from one day post-infusion through seven days post-infusion (t(31.75)= 0.92, p = 0.37). There were no significant main effects or interactions for analyses on PTSD symptoms in the CP group, p’s > 0.05.
Figure 2.
Change in the Impact of Event Scale-Revised (IES-R) total score over 1 week. Error bars represent standard deviation.
VAS Results
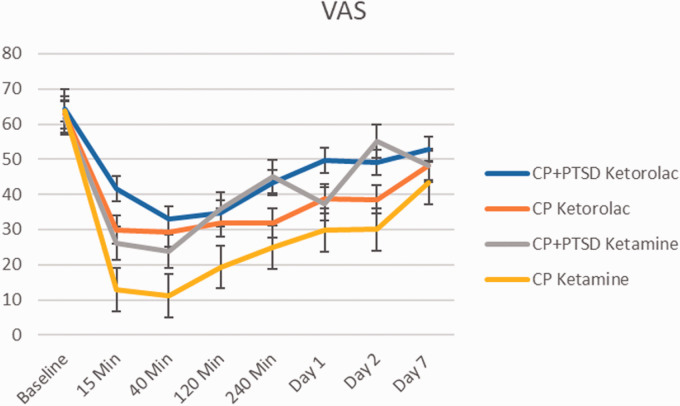
Immediate effects were examined with three-way repeated measures ANOVA (2(Diagnosis: CP, CP+PTSD) X 2(treatment: ketorolac, ketamine) X 4(Time: baseline, 15 min., 40 min., 120 min.). There were significant main effects of medication (F(1, 148) = 14.69, p < 0.001) and time (F(1, 148) = 26.55, p < 0.001) on VAS (Figure 3). There was also significant diagnosis by medication interaction (F(1, 148) = 5.40, p = 0.02), with overall lower pain scores (less effect) (M = 52.2, SD = 25.41) for ketamine as compared to ketorolac in the CP group, (M = 26.78, SD = 28.19; t(74) = 2.18, p < 0.001) but not in CP+PTSD group. There was no significant three-way interaction between group, medication, and time point.
Figure 3.
Change in Visual Analogue Scale (VAS) over 1 week. Error bars represent standard deviation.
Sustained effects were examined using a two three-way ANOVAs 2(Diagnosis: CP, CP+PTSD) X 2(treatment: ketorolac, ketamine) X (Time points). Results revealed no significant effects on VAS scores from baseline to 1 day post-infusion (F(1, 32) = 0.65, p > 0.1). There was a significant main effect of group on VAS symptoms across time points (F(1, 32) = 8.24, p < 0.01). While both groups had improved VAS scores on Day 7, post-hoc t-tests revealed that the CP+PTSD group had significantly greater pain improvement (M= -30.61, SD= 17.71) compared to the CP group (M= -14.28, SD= 15.87); t(33.6)=2.91, p < 0.001.
BPI Pain Scale 7 Days Post Infusion
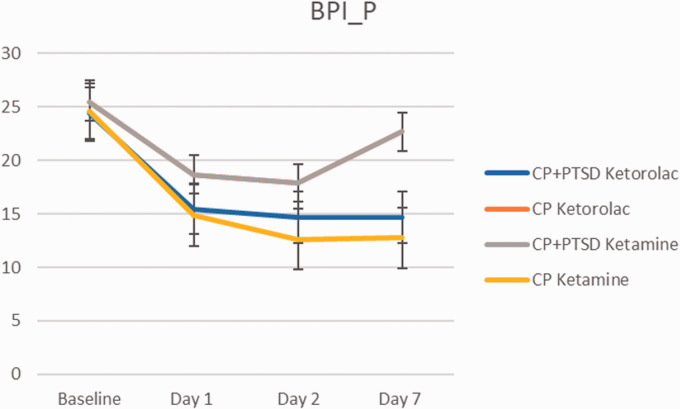
Ketamine led to reduction of BPI-pain scale on Day 1 and 2 after the infusion, with return to higher scores on Day 7 in CP+PTSD group (Figure 4). On three-way repeated measures ANOVA (2(CP, CP+PTSD) X 2(ketorolac, ketamine) X 4(baseline, Day 1, Day 2, Day 7) there was a significant main effect of time (F(3, 33) = 8.24, p < 0.01) and diagnosis by medication interaction (F(1, 137) = 8.76, p < 0.01) with no significant three way interactions, p > 0.05. Within the CP group, ketorolac (M = 21.17, SD = 9.66) was associated with greater pain compared to ketamine (M = 16.61, SD = 8.56; t(69.9) = 2.14, p = 0.035. There were no significant differences in pain score by medication in the CP+PTSD group, p > 0.05.
Figure 4.
Change in brief Pain Inventory Pain Intensity scale over 1 week. Error bars represent standard deviation.
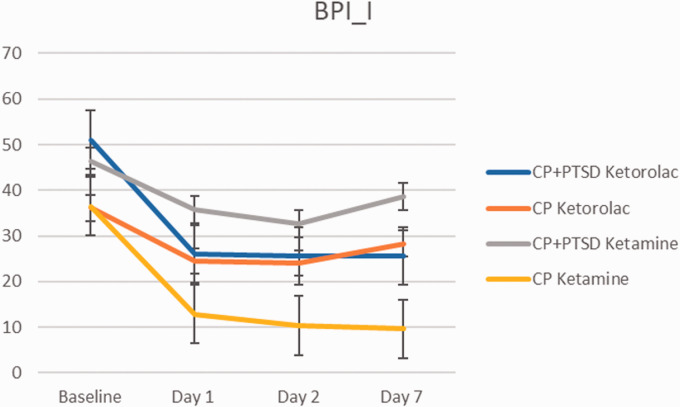
Bpi Pain Interference Scale 7 Days Post Infusion
The BPI-pain interference scale showed the same pattern of time-dependent reduction of symptoms in the CP and CP+PTSD groups (Figure 5). A three-way repeated measures ANOVA (2(CP, CP+PTSD) X 2(ketorolac, ketamine) X 4(baseline, Day 1, Day 2, Day 7)) revealed significant main effect of time point (F(1, 137) = 34.13, p < 0.01) and diagnosis group (F(1, 137) = 19.67, p < 0.01). There was a significant two way interaction between group and medication (F(1, 137) = 8.24, p = 0.004), and a significant three way interaction between group, medication, and time (F(1, 137) = 7.30, p = 0.007). Post-hoc t-tests revealed that Ketamine administration was associated with greater pain scores at baseline (M = 40.00, SD = 12.05) compared to subsequent time points (M = 10.21, SD = 9.47; t(13.2) = 7.08, p < 0.01) only in the CP group. Ketorolac administration was associated with greater scores at baseline (M = 51.1, SD = 13.05) compared to subsequent time points in the CP+PTSD group (M = 25.75, SD = 18.42; t(22.87) = 4.66, p < 0.01). In the CP group alone, ketorolac was associated with greater pain scores (M = 28.25, SD = 18.16) compared to ketamine (M = 18.05, SD = 16.66; t(70.6) = 2.5, p = 0.01).
Figure 5.
Change in brief Pain Inventory Pain Interference scale over 1 week. Error bars represent standard deviation.
Dissociative Symptoms and Side Effects
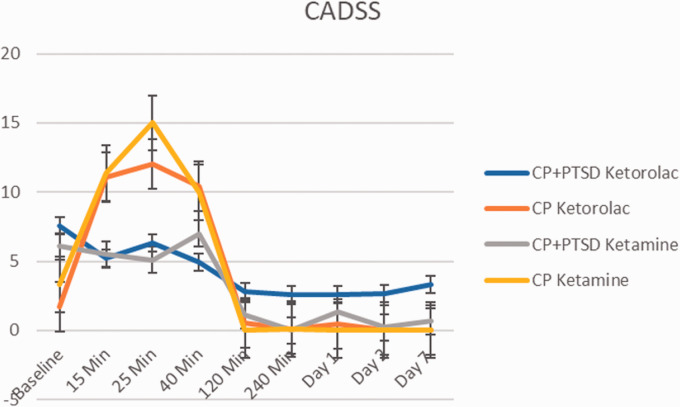
CADSS 7 Days Post Infusion
Both ketamine and ketorolac infusions led to significant increase in dissociative symptoms in CP group, however unexpectedly they had less effect on dissociative symptoms in CP+PTSD group (Figure 6). Results of a three-way repeated measures ANOVA (2(CP, CP+PTSD) X 2(ketorolac, ketamine) X 9(Time Points from baseline to Day 7)) revealed significant main effects of medication (F(1, 327) = 4.20, p = 0.04) and time point (F(1, 327) = 36.42, p < 0.01). There was also a significant medication by time point interaction (F(1, 327) = 10.17, p < 0.001) and a significant three way interaction between group, medication, and time (F(1, 327) = 4.39, p = 0.04). Post-hoc t-test analyses revealed that in the CP group, ketamine administration was associated with greater average dissociative symptom endorsement (M = 4.45, SD = 9.04) throughout the infusion compared to ketorolac (M = 0.53, SD = 1.21; t(88.4) = -3.98, p < 0.001). In the CP+PTSD group, ketamine and ketorolac did not present with significant differences (t(169.98) = 1.228, p > 0.1)).
Figure 6.
Change in Clinician-Administered Dissociative States Scale (CADSS) score over 1 week. Error bars represent standard deviation.
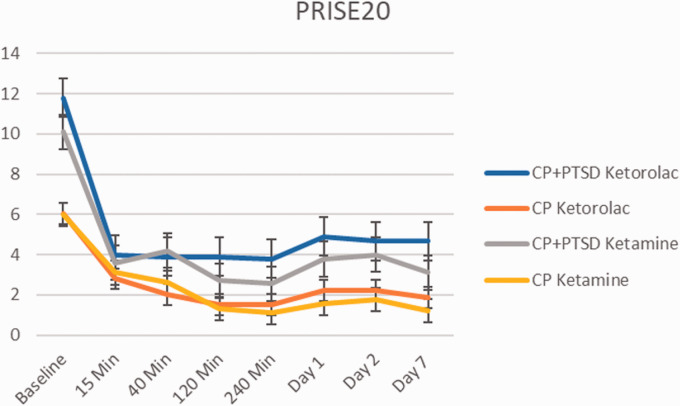
Prise 7 Days Post Infusion
With the Patient-Rated Inventory of Side Effects both ketamine and ketorolac infusion were generally well tolerated by the patients in CP and CP+PTSD groups (Figure 7). No subjects needed acute treatment with β-blockers during ketamine infusion because of blood pressure elevation (systolic blood pressure >180 mm Hg and/or diastolic blood pressure >100 mm Hg). Results of a three-way repeated measures ANOVA (2(CP, CP+PTSD) X 2(ketorolac, ketamine) X 9(Time Points from baseline to Day 7)) revealed significant main effects of group (F(1, 312) = 24.60, p < 0.001) and time point (F(1, 312) = 35.48, p < 0.001). There were no significant interactions between the variables of interest (p’s > 0.05). However, post-hoc analyses revealed baseline side effects scores were significantly lower for the CP (M = 6.16, SD = 4.13) compared to CP+PTSD (M = 10.95, SD = 4.78) group, (t(36.67) = -3.3544, p = 0.002).
Figure 7.
Change in Patient Related Inventory of Side Effects (PRISE) score over 1 week. Error bars represent standard deviation. Both ketamine and ketorolac infusion were generally well tolerated by the patients in chronic pain and PTSD-chronic pain groups.
24-Hours Analysis
To isolate early effects of ketamine and ketorolac infusions, we also compared baseline and first post-infusion day scores on: VAS, dissociative symptoms, and side effects, in all subjects. Two-way repeated measures ANOVAs (2 treatment(ketorolac, ketamine) X 2 time points (Baseline, Day 1)) were used to examine changes in pain, dissociative symptoms, and side effects. There were main effects of time (first day vs. baseline) on all measures, however there was no main effect of medication, nor significant interactions present on any measure (see Supplement for more details).
Discussion
Both single doses of ketamine and ketorolac resulted in comparable improvement of PTSD and CP symptoms over a period of seven days. Our study adds to a growing body of evidence that ketamine infusion may lead to meaningful and durable response for both PTSD and CP symptoms. In contrast to initial hypotheses, these findings suggest that the effects of ketamine were not unique given that ketorolac demonstrated similar effects. We conceived ketorolac as a short-acting active placebo that produces an immediate analgesic effect comparable to ketamine. To our surprise, there was a sustained effect of ketorolac on both PTSD and CP symptoms. While it is possible that these findings reflect placebo effects in both groups, it is also possible that they reflect the unanticipated effects of potent anti-inflammatory agent on PTSD symptoms.
Previous studies examining the effect of ketamine on symptoms of PTSD have not examined comorbidity of PTSD and CP symptoms.7 Thus, the current study focused on CP patients with and without a diagnosis of PTSD. Considering ketamine effects, in a first reported randomized controlled study by Feder et al., a single dose of ketamine resulted in significant reduction of core PTSD symptoms and these benefits remained for up to seven days post infusion. Moreover, depressive symptoms also decreased and persisted up to a week after the infusion.7 Many CP patients cope with depressive symptoms, and depression and CP share common neurobiological pathways.23 Hence the treatment of CP may treat the pain and reduce the depressive symptoms. However, no evidence has been reported on improvement of depressive symptoms following long term ketamine treatment in CRPS patients.9 It is possible that the antidepressant effects of ketamine in CP patients are short-lived.
With respect to the potential effects of ketorolac, several studies demonstrated a relationship between inflammation and PTSD.24,25 Ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), has the potential to attenuate the inflammatory processes relevant to PTSD development. In addition, ketorolac may have central analgesic effects as its subarachnoid administration has been demonstrated to have a direct action by blocking hyperalgesia caused by substance P and stimulation of NMDA receptors.26,27 While the efficacy of NSAID has yet to be examined in treatment of PTSD symptoms, a study using a rat model of PTSD showed that treatment with another NSAID ibuprofen reduced both inflammatory cytokine levels and behavioral symptoms associated with the model.28 The use of ketorolac in the current study was to control for analgesic properties of ketamine; hence ketorolac, a medication with a known short terminal half-life (5.6 hours), was chosen as appropriate positive control as we did not anticipate that the effects of medication would last beyond it known pharmacodynamics and pharmacokinetics. Surprisingly, both ketamine and ketorolac resulted in comparable improvements of CP+PTSD symptoms that persisted for seven days post infusion. Future studies will be able to examine the potential mechanisms involved.
The self-report measures used in our study have been validated for assessment of symptoms of CP and have previously been utilized in ketamine clinical trials.11,29 In our study, ketamine infusion was associated with reduction of BPI-pain and interference scales in CP+PTSD group that persisted for at least 48 hours after the infusion. This is consistent with other reports of ketamine effects persisting beyond the period when ketamine is present in plasma of other tissues,30,31 suggesting that ketamine might be initiating neurobiological processes that have extended effects. This is somewhat surprising since ketamine effects in patients with pain symptoms only had been generally reported as short lived.9,32–35 Similarly, mechanisms of extended effects of ketorolac have not been reported, to the best of our knowledge, to date. Surprisingly, ketorolac infusion, in our study lead to reduction of BPI-pain and interference scales that persisted for a week in CP+PTSD group but had shorter lived effect in CP only group.
Differential responsivity over time to ketamine versus ketorolac in CP+PTSD and CP only subjects, could point toward two distinct brain mechanisms underlying CP and PTSD symptoms.
Additional interesting observation in the current study is that both ketamine and ketorolac infusions were associated with fewer dissociative symptoms in CP+PTSD than in CP group. PTSD had been linked to high level of dissociative symptoms22,36 and ketamine is commonly known to induce dissociative symptoms. Surprisingly, in the current study, subjects with CP+PTSD reported no increase in dissociative symptoms after ketamine infusions while subjects with CP only reported significant increase in dissociative symptoms following both ketamine and ketorolac. This observation can be seen as further evidence of distinct neurobiological processes present in two diagnostic groups. It is also possible, that the lack of prominent dissociative symptoms observed in patients with CP+PTSD who received ketamine, could be due to concomitant use of adjuvant pain medications and/or anticonvulsants prescribed for pain or PTSD. Still presence of dissociative symptoms in CP only group treated with ketorolac is puzzling and requires further investigation. In line with previous studies we did not observe significant emergence of psychedelic symptoms associated with ketamine infusion nor worsening of dissociative symptoms in patients with PTSD.37–39
Our study had several limitations. First, our group sizes are very modest and thus findings have to be replicated in the larger cohort studies. Notably, the lack of inactive placebo control (normal saline infusion) also limits the interpretation of the results. Including a group of patients with PTSD and no diagnosis of CP in the design would also allow for more effective isolation of the medication effects. Our study also does not address depression and suicidal ideation (the latter was an exclusion criteria) in the group of veterans who received ketamine infusion, an important consideration when evaluating therapeutic benefit of the drug. These findings warrant further examination in a larger randomized-controlled clinical trial with the emphasis of identifying those patients who have PTSD and comorbid CP and/or depression symptoms.
In conclusion, this study is the first prospective study of subanesthetic ketamine infusion for comorbid PTSD and CP. The treatment offered comparable improvement of PTSD and CP symptoms that persisted for seven days after the infusion. We found that patients with comorbid PTSD and CP may be less prone to experiencing dissociative side effects of pain treatment compared to CP only group. Surprisingly, ketorolac infusion resulted in dissociative symptoms in CP patients only, raising the question of the neurobiological mechanisms involved. Finally, we did not observe worsening of CP or PTSD symptoms for either treatment. Future studies looking into the common neuroplasticity changes shared by pain and PTSD are warranted to promote the identification of new drug targets and to ameliorate symptoms of PTSD and comorbid CP.
Supplemental Material
Supplemental material, sj-pdf-1-css-10.1177_2470547020981670 for Low Dose Ketamine Infusion for Comorbid Posttraumatic Stress Disorder and Chronic Pain: A Randomized Double-Blind Clinical Trial by Alisher R. Dadabayev, Sonalee A. Joshi, Mariam H. Reda, Tamar Lake, Mark S. Hausman, Edward Domino and Israel Liberzon in Chronic Stress
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alisher R. Dadabayev https://orcid.org/0000-0001-6520-5830
Supplemental material: Supplementary material for this article is available online.
References
- 1.Fulton JJ, Calhoun PS, Wagner HR, et al. The prevalence of posttraumatic stress disorder in operation enduring freedom/operation Iraqi freedom (OEF/OIF) veterans: a meta-analysis. J Anxiety Disord. 2015; 31: 98–107. [DOI] [PubMed] [Google Scholar]
- 2.Asmundson GJ, Bonin MF, Frombach IK.Norton GR. Evidence of a disposition toward fearfulness and vulnerability to posttraumatic stress in dysfunctional pain patients. Behav Res Ther. 2000; 38(8): 801–812. [DOI] [PubMed] [Google Scholar]
- 3.Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019; 76(2): 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Andrea D, Sewell RA. Transient resolution of treatment-resistant posttraumatic stress disorder following ketamine infusion. Biol Psychiatry. 2013; 1 74(9): e13–e14. [DOI] [PubMed] [Google Scholar]
- 5.Peltoniemi MA, Hagelberg NM, Olkkola KT.Saari TI. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016; 55(9): 1059–1077. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018; 43: 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feder A, Parides MK, Murrough JW, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014; 71(6): 681–688. [DOI] [PubMed] [Google Scholar]
- 8.Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions for comorbid posttraumatic stress disorder and treatment-resistant depression. J Clin Psychiatry. 2018; 79: e1–8. [DOI] [PubMed] [Google Scholar]
- 9.Sigtermans MJ, van Hilten JJ, Bauer MC, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009; 145: 304–311. [DOI] [PubMed] [Google Scholar]
- 10.Noppers I, Niesters M, Swartjes M, et al. Absence of long-term analgesic effect from a short-term S-ketamine infusion on fibromyalgia pain: a randomized, prospective, double blind, active placebo-controlled trial. Eur J Pain. 2011; 15(9): 942–949. [DOI] [PubMed] [Google Scholar]
- 11.Patil S, Anitescu M. Efficacy of outpatient ketamine infusions in refractory chronic pain syndromes: a 5-year retrospective analysis. Pain Med. 2012; 13(2): 263–269. [DOI] [PubMed] [Google Scholar]
- 12.Bosco MA, Gallinati JL, Clark ME. Conceptualizing and treating comorbid chronic pain and PTSD. Pain Res Treat. 2013; 2013: 174728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler RC, Chiu WT, Demler O.Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005; 62(6): 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019; 160(1): 19–27. [DOI] [PubMed] [Google Scholar]
- 15.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995; 8(1): 75–90. [DOI] [PubMed] [Google Scholar]
- 16.Gnanavel S, Robert RS. Diagnostic and statistical manual of mental disorders, fifth edition, and the impact of events scale-revised. Chest. 2013; 144(6): 1974–1691. [DOI] [PubMed] [Google Scholar]
- 17.Morina N, Ehring T, Priebe S. Diagnostic utility of the impact of event scale-revised in two samples of survivors of war. PLoS One. 2013; 8(12): e83916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herr KA, Mobily PR. Comparison of selected pain assessment tools for use with the elderly. Appl Nurs Res. 1993; 6(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994; 23(2): 129–138. [PubMed] [Google Scholar]
- 20.Wisniewski SR, Rush AJ, Balasubramani GK.Trivedi MH, Nierenberg AA; STARD Investigators. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006; 12(2): 71–79. [DOI] [PubMed] [Google Scholar]
- 21.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress. 1998; 11(1): 125–136. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R project.org/
- 23.Sheng J, Liu S, Wang Y.Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017; 2017: 9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedert EA, Calhoun PS, Watkins LL, et al. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010; 39(1): 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019; 73(4): 143–153. [DOI] [PubMed] [Google Scholar]
- 26.Ashworth HL, Ong C, Seed PT, et al. The influence of timing and route of administration of intravenous ketorolac on analgesia after hand surgery. Anaesthesia. 2002; 57(6): 535–539. [DOI] [PubMed] [Google Scholar]
- 27.Malmberg AB, Yaksh TL. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993; 79(2): 270–281. [DOI] [PubMed] [Google Scholar]
- 28.Lee B, Sur B, Yeom M.Shim I, Lee H, Hahm DH. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharmacol. 2016; 20(4): 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Herndon C, Anderson S, et al. Intravenous ketamine infusion for complex regional pain syndrome: survey, consensus, and a reference protocol. Pain Med. 2019; 20(2): 323–334. [DOI] [PubMed] [Google Scholar]
- 30.Correll GE, Maleki J, Gracely EJ.Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004; 5(3): 263–275. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg ME, Domsky R, Scaringe D, et al. Multi-day low dose ketamine infusion for the treatment of complex regional pain syndrome. Pain Physician. 2005; 8(2): 175–179. [PubMed] [Google Scholar]
- 32.Bell RF, Dahl JB, Moore RA.Kalso E. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol Scand. 2005; 49(10): 1405–1428. [DOI] [PubMed] [Google Scholar]
- 33.Elia N, Tramer MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain. 2005; 113(1–2): 61–70. [DOI] [PubMed] [Google Scholar]
- 34.Graven-Nielsen T, Aspegren Kendall S, Henriksson KG, et al. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain. 2000; 85: 483–491. [DOI] [PubMed] [Google Scholar]
- 35.Jouguelet-Lacoste J, La Colla L, Schilling D.Chelly JE. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med. 2015; 16(2): 383–403. [DOI] [PubMed] [Google Scholar]
- 36.van Huijstee J, Vermetten E. The dissociative subtype of post-traumatic stress disorder: research update on clinical and neurobiological features. Curr Top Behav Neurosci. 2018; 38: 229–248. [DOI] [PubMed] [Google Scholar]
- 37.Donoghue AC, Roback MG, Cullen KR. Remission from behavioral dysregulation in a child with PTSD after receiving procedural ketamine. Pediatrics. 2015; 136(3): e694–e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartberg J, Garrett-Walcott S, De Gioannis A. Impact of oral ketamine augmentation on hospital admissions in treatment-resistant depression and PTSD: a retrospective study. Psychopharmacology (Berl). 2018; 235(2): 393–398. [DOI] [PubMed] [Google Scholar]
- 39.Womble AL. Effects of ketamine on major depressive disorder in a patient with posttraumatic stress disorder. AANA J. 2013; 81: 118–119. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-css-10.1177_2470547020981670 for Low Dose Ketamine Infusion for Comorbid Posttraumatic Stress Disorder and Chronic Pain: A Randomized Double-Blind Clinical Trial by Alisher R. Dadabayev, Sonalee A. Joshi, Mariam H. Reda, Tamar Lake, Mark S. Hausman, Edward Domino and Israel Liberzon in Chronic Stress