ABSTRACT
The causal genetic underpinnings of congenital heart diseases, which are often complex and multigenic, are still far from understood. Moreover, there are also predominantly monogenic heart defects, such as cardiomyopathies, with known disease genes for the majority of cases. In this study, we identified mutations in myomesin 2 (MYOM2) in patients with Tetralogy of Fallot (TOF), the most common cyanotic heart malformation, as well as in patients with hypertrophic cardiomyopathy (HCM), who do not exhibit any mutations in the known disease genes. MYOM2 is a major component of the myofibrillar M-band of the sarcomere, and a hub gene within interactions of sarcomere genes. We show that patient-derived cardiomyocytes exhibit myofibrillar disarray and reduced passive force with increasing sarcomere lengths. Moreover, our comprehensive functional analyses in the Drosophila animal model reveal that the so far uncharacterized fly gene CG14964 [herein referred to as Drosophila myomesin and myosin binding protein (dMnM)] may be an ortholog of MYOM2, as well as other myosin binding proteins. Its partial loss of function or moderate cardiac knockdown results in cardiac dilation, whereas more severely reduced function causes a constricted phenotype and an increase in sarcomere myosin protein. Moreover, compound heterozygous combinations of CG14964 and the sarcomere gene Mhc (MYH6/7) exhibited synergistic genetic interactions. In summary, our results suggest that MYOM2 not only plays a critical role in maintaining robust heart function but may also be a candidate gene for heart diseases such as HCM and TOF, as it is clearly involved in the development of the heart.
KEY WORDS: Congenital heart disease, Cardiomyopathy, CG14964, Myomesin, Candidate gene
Editor's choice: MYOM2 plays a critical role in establishing or maintaining robust heart function, and is a candidate gene for heart diseases, such as hypertrophic cardiomyopathy and Tetralogy of Fallot.
INTRODUCTION
Malformations of the heart and their associated diseases, present at birth up to high adult age, are one of the leading causes of death worldwide. A heterogeneous disease with different anatomical variants, physiologic manifestations and genetic underpinnings is hypertrophic cardiomyopathy (HCM), which predominantly causes left ventricular (LV) hypertrophy and outflow tract obstruction (Jones et al., 2017; Maron et al., 2006). The hypertrophy of LV and interventricular septum also causes problems in the electrical system of the heart, which result in life-threatening arrhythmias and an increased risk of sudden death (Driscoll, 2016). HCM can affect individuals of any age, although early manifestations are rare. It is typically caused by monogenic mutations mostly located in sarcomere genes, such as myosin-binding protein C3 (MYBPC3; 30-40% of cases) and β-myosin heavy chain 7 (MYH7; 30-50% of cases) (Vermeer et al., 2016).
Rare deleterious mutations in genes essential for the assembly of the sarcomere have been found in patients with Tetralogy of Fallot (TOF) (Grunert et al., 2014). Congenital heart disease (CHD) is the most common birth defect, with an incidence of about 1% of all newborns worldwide (van der Linde et al., 2011), with TOF being the most common cyanotic form. Although HCM mainly refers to left heart structures, TOF is a cardiac anomaly of a combination of four cardiac features, including ventricular septum defect, overriding aorta, right ventricular (RV) outflow tract obstruction and RV hypertrophy. The burden of a corrected TOF heart is often well tolerated during childhood but there is a particularly significant incidence of symptomatic arrhythmias during the third postoperative decade and thereafter (Cuypers et al., 2014). In general, CHDs like TOF are complex disorders of multifactorial origin, comprising genetic and epigenetic causes, as well as environmental factors that lead to structural defects and heart dysfunction (Rickert-Sperling et al., 2016). Recently, we identified a multigenic background of rare deleterious mutations in several genes, which discriminate TOF cases from controls (Grunert et al., 2014). Here, mutations in sarcomere genes such as MYH7 and MYBPC3 (single TOF cases), as well as titin (TTN) and the M-band protein myomesin 2 (MYOM2) (multiple TOF cases), have been identified.
Myomesin proteins comprise three family members encoded by MYOM1, MYOM2 and MYOM3, and localize to the M-band of the sarcomere. These three myomesin isoforms correlate with the contractile properties in different fiber types (Agarkova and Perriard, 2005; Gautel and Djinović-Carugo, 2016). MYOM1 is expressed in all striated muscles, whereas MYOM2 is expressed in the adult heart and fast fibers, and MYOM3 is expressed only in skeletal muscle intermediate fiber types (Agarkova et al., 2003; Gautel and Djinović-Carugo, 2016; Schoenauer et al., 2005). Moreover, the expression of MYOM2 is weaker in the embryonic human heart but similar to the skeletal muscle, with no expression in the smooth muscle (Schoenauer et al., 2011). In addition, cardiomyocytes (CMs) derived from induced pluripotent stem cells of healthy individuals and TOF patients reveal that MYOM1 and MYOM2 are expressed during cardiac differentiation (Grunert et al., 2020). Furthermore, Myom2 is also expressed in embryonic and adult mouse hearts (Grunert et al., 2014). Composed of tandems of fibronectin type III (FN3) and immunoglobulin type II (Ig) domains, they act as crosslinker for the neighboring thick filaments of myosin in the M-band, and also interact with TTN as its C-terminal part converges to the M-band (Agarkova et al., 2003; Hu et al., 2015). Moreover, MYOM1 and MYOM2 also interact with other sarcomere proteins, such as MYH7 (Obermann et al., 1997, 1998), which is known to be involved in HCM (Maron and Maron, 2013) and other CHDs (Basu et al., 2014; Bettinelli et al., 2013; Postma et al., 2011). Besides sequence alterations, RNA splicing of sarcomere genes, such as troponin T (TNNT1 and TNNT2), troponin I (TNNI1 and TNNI3) and MYH7, were also found to be significantly altered in patients with ischemic cardiomyopathy (Kong et al., 2010) and TOF (Grunert et al., 2016). The connection between these two distinct phenotypes, a monogenetic disease of LV heart structures (HCM) and a multigenic disease of RV heart structures (TOF), seems to be primarily based on genomic but also on transcriptomic alterations in sarcomere genes. These alterations probably contribute to an impaired RV/LV function in both diseases, and cause in short- or long-term clinical outcome arrhythmias and other disorders.
In this study, we identified mutations in the sarcomere gene MYOM2 in two independent cohorts of unrelated TOF and HCM patients. Interestingly, the HCM patients harbored no mutations in the 12 most common HCM disease genes. As the proportion of TOF patients with MYOM2 mutations was quite high and MYOM2 was not described for HCM so far, we further investigated MYOM2 as a candidate gene for TOF and HCM using patient-derived CMs and the Drosophila genetic model system. Drosophila has a high degree of cardiac gene conservation (e.g. NKX2-5, TBX20, GATA4/6 and HAND1/2) (Bodmer, 1993; Han and Olson, 2005; Qian and Bodmer, 2009; Qian et al., 2008) and similar cardiac developmental pathways (e.g. NOTCH signaling which is involved in many CHDs including TOF) (Zhou and Liu, 2014). Moreover, the functional conservation between the vertebrate and fly heart (e.g. autonomous contraction of CMs) (Klassen et al., 2017; Ocorr et al., 2014; Taghli-Lamallem et al., 2016; Wolf et al., 2006) enables the study of the cardiac function of candidate genes for congenital and other heart diseases. Here, we show for the first time that patient-derived CMs exhibit myofibrillar disarray and reduced passive force with increasing sarcomere lengths. Our Drosophila studies show that a fly counterpart of MYOM2, CG14964, controls fly heart size in a gene dosage-dependent manner, regulates myosin levels and also genetically interacts with sarcomere myosin heavy chain, pointing towards an intricate mechanism between MYOM2 and the sarcomere in the context of HCM and TOF.
RESULTS
Identification of MYOM2 as a novel candidate gene in HCM and TOF
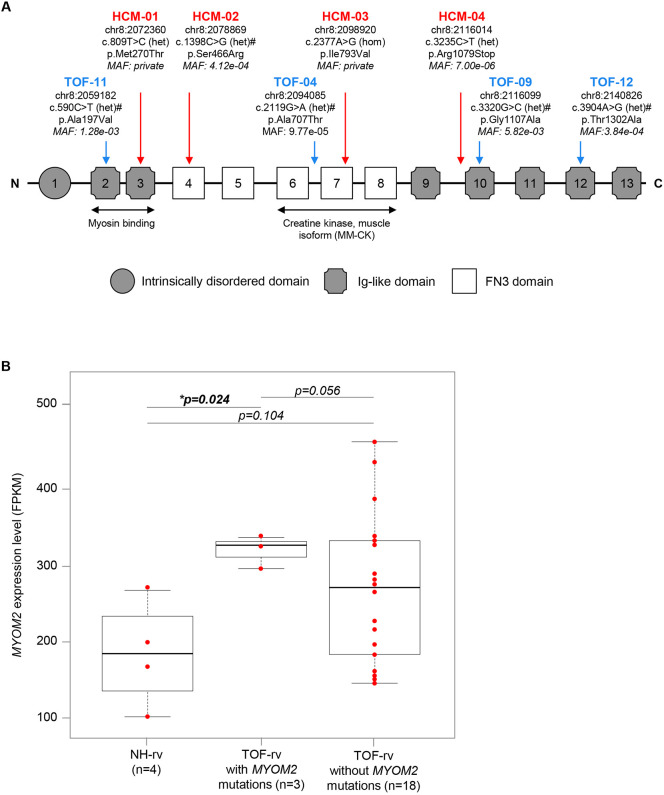
As already mentioned, we have previously shown a multigenic background for TOF by characterizing a cohort of 13 clinically well-defined isolated TOF patients who carried combinations of rare deleterious mutations in genes essential for, among others, apoptosis and cell growth, as well as the structure and function of the sarcomere (Grunert et al., 2014). The cohort comprised mutations in MYH7 and MYBPC3 with single affected cases, as well as TTN and MYOM2 with multiple affected cases (seven and four cases, respectively). For the four cases with MYOM2 mutations (Fig. 1A), we showed that these genetically similar cases shared similar network disturbances in gene expression (Grunert et al., 2014), which should also hold true for other affected genes. Interestingly, the expression level of MYOM2-mRNA is significantly upregulated in TOF patients with mutations compared to normal heart controls, whereas it appears not to be the case for patients without mutations (Fig. 1B). Two of the TOF patients with MYOM2 mutations also harbored rare deleterious variations in TTN (TOF-04 and TOF-11).
Fig. 1.
MYOM2 mutations and expression in patients. (A) Schematic of MYOM2, with its domain structure and mutations found in TOF (blue) and HCM (red) patients. Mutation positions are based on the human reference genome hg38. Nucleotide changes are based on transcript ENST00000262113. Amino acid changes are based on protein ENSP00000262113. Damage prediction by PolyPhen2, SIFT or MutationTaster are indicated by ‘#’. The minor allele frequency (MAF) is based on 71,702 genomes from unrelated individuals of the gnomAD (v3). The binding sites of two interaction partners, MYH7 and creatine kinase (muscle isoform), are indicated below. (B) RNA-seq expression level of MYOM2 in RV tissue of TOF patients and normal hearts (NH). P-value is derived from an unpaired two-sided Student's t-test. Boxplots depict the median and interquartile range, and whiskers show minimum and maximum values. FPKM, fragments per kilobase million; het, heterozygous variation; hom, homozygous variation Schematic based on Agarkova et al. (2005).
We further analyzed the MYOM2 gene for putative mutations in a cohort of 66 unrelated HCM patients who had negative screening results in the known HCM disease genes (MYH7, MYBPC3, TNNT2, TNNI3, TPM1, MYL2, MYL3, ACTC1, TCAP, TNNC1, MYOZ2 and CSRP3). We found four HCM patients with rare probable disease-causing mutations in MYOM2 (Fig. 1A, Fig. S1, Table S1). Interestingly, the mutation p.M270T is located in an Ig-like domain interacting with the light meromyosin part of the β-myosin heavy chain, whereas the mutation p.I793V is located in a FN3-like domain interacting with the muscle isoform of creatine kinase (Fig. 1A). Three of the four single nucleotide variations (SNVs) are missense mutations leading to amino acid changes, and one is a truncating mutation leading to a premature stop (p.R1079X). The p.I793V mutation was the only homozygous variation. Furthermore, we validated the initial genetic screening results in the four affected HCM patients by using a DNA resequencing array composed of known HCM disease genes. This confirmed that none of the four patients carried disease-causing mutations in the 12 most common sarcomere HCM genes. There are no MYOM2 mutations described for HCM so far.
In general, the eight mutations found in MYOM2 in four HCM and four TOF patients were distributed over different domains of the protein (Fig. 1A). Moreover, all variations were rare or even private as for two HCM patients (Fig. 1A), meaning they all have a minor allele frequency of less than 0.01 or zero, based on 71,702 genomes from unrelated individuals of the Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org/) (Karczewski et al., 2020). Of note, almost all candidate mutations found in HCM patients in other studies occur at a very low frequency and ∼50% are found in a single proband or family (Alfares et al., 2015). In addition to their very low frequency, the majority of the variations are predicted to be damaging based on PolyPhen2 (Adzhubei et al., 2013, http://genetics.bwh.harvard.edu/pph2/), SIFT (Vaser et al., 2016, https://sift.bii.a-star.edu.sg/) and MutationTaster (Schwarz et al., 2010, www.mutationtaster.org/) (Fig. 1A).
HCM-derived CMs with MYOM2 mutation show myofibrillar disarray and reduction in passive force
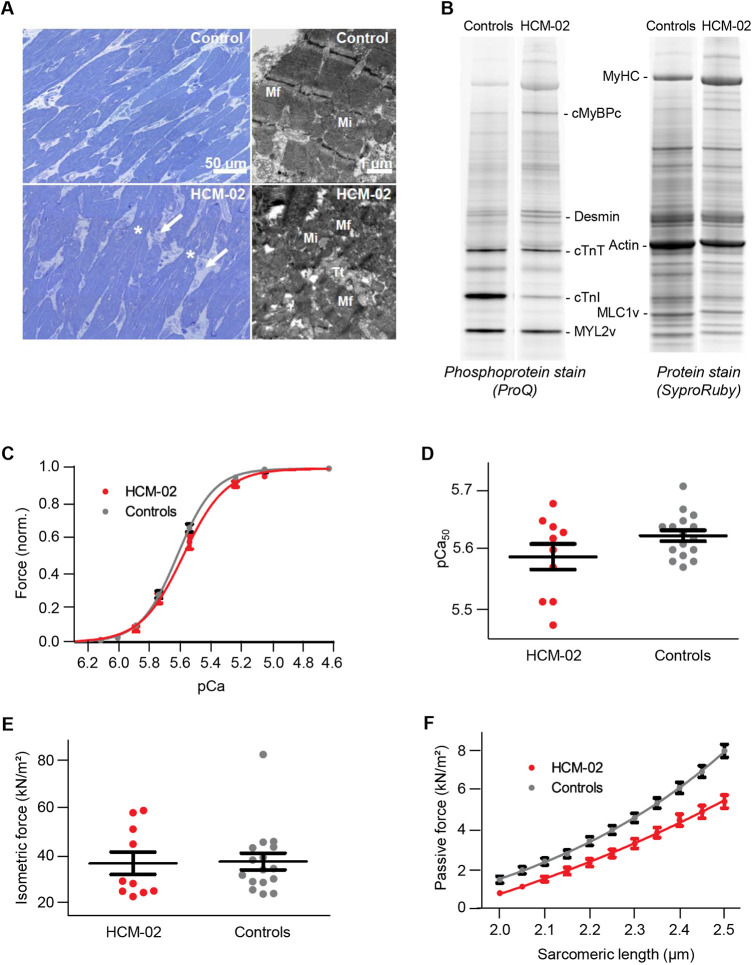
Owing to the severity of the LV hypertrophy, the HCM patient carrying the S466R MYOM2 mutation (HCM-02, Fig. 1A) underwent septal myectomy (see Materials and Methods for clinical courses of HCM patients). We performed morphological analysis of tissue sections, and force measurements on CMs isolated from the interventricular septum of the HCM patient, as well as five unaffected age-matched donors as controls.
Histological analysis showed cellular disarray and mild widening of interstitial spaces, indicative of replacement fibrosis, in the patient but not in control tissue (Fig. 2A). At the ultrastructural level, disarray of myofibrils was a frequent finding in CMs of the patient sample, whereas the myofibrils of the control were regularly oriented and mostly parallel. Thus, the morphological analysis showed an HCM-dependent remodeling of the hypertrophic heart. In addition, gel electrophoretic analyses of the phosphorylation status of HCM-02 and controls showed that cardiac troponin I (cTnI) was particularly strongly phosphorylated in the controls compared to the patient (Fig. 2B, Fig. S2A). The differences might result from the usually strong protein kinase A (PKA)-dependent phosphorylation in control hearts, which especially affects cTnI (Kraft et al., 2013; van der Velden et al., 2003). Yet, it cannot be excluded that the MYOM2 mutation results in additionally reduced cTnI phosphorylation in HCM-02 myocardium. Analysis of the protein quantities of HCM-02 and controls suggested reduced levels of cTnI and MLC2v (herein referring to MYL2) in HCM-02, and an increase in cMyBPC (herein referring to MYBPC3) (Fig. S2B). Presumably the stoichiometry of sarcomere proteins in HCM-02 is maintained due to possible secondary effects of the MYOM2 mutation on other sarcomere proteins.
Fig. 2.
Reduced passive force in CMs derived from a HCM-patient with a MYOM2 mutation. (A) Light and electron micrographs of control (upper panel) and patient HCM-02 (lower panel) myocardial samples. In comparison to the control myocardium, the patient sample showed disoriented CMs (disarray) with great variations in cellular diameter. Irregularly formed connections between CMs (asterisks) and widened interstitial spaces (white arrows) were present. Within CMs, myofibrils (Mf) of the patient frequently showed disarray, with sarcomeres running in various directions, whereas the sarcomeres of control tissue were mostly parallel. Mi, mitochondria; Tt, T-tubule. (B) Example of gel electrophoretic analysis of the phosphorylation status of native myocardial tissue from HCM-02 and controls. For detailed analysis see Fig. S2A. (C) Isometric force generation at increasing calcium concentrations (pCa) normalized to maximum force. Lines were fitted according to a modified Hill equation (Kraft et al., 2013). (D) Calcium concentration at 50% of maximum force generation (pCa50) derived from fitted curves in C. (E) Isometric force at maximum calcium activation (pCa 4.63) appeared not to be altered in HCM-02 CMs carrying the mutation. (F) Passive force at increasing sarcomere length showed a significant reduction for HCM-02 CMs carrying a MYOM2 mutation, compared to controls (P<0.01 for all sarcomere lengths). All functional analyses of CMs from HCM-02 and the control in C-F occurred after adjustment of PKA-dependent phosphorylation (n=16 control CMs; n=10 HCM-02 CMs). Data are mean±s.e.m.
To account for differences in phosphorylation, particularly of PKA-dependent sites in cTnI (Fig. 2B), phosphorylation was adjusted by incubation of all CMs with PKA before force measurements. We also measured force development of CMs at different calcium concentrations and found that mutant CMs have reduced calcium sensitivity. The force-pCa curve was shifted to the right, suggesting more calcium is needed to reach 50% of maximum force development (Fig. 2C). However, the effect was not statistically significant (Fig. 2D). Force generation at maximum calcium activation was not altered (Fig. 2E).
Furthermore, we also determined passive force at increasing sarcomere lengths of CMs from patient HCM-02 and control CMs (Fig. 2F). Interestingly, passive force was significantly lower for patient CMs compared to controls at all sarcomere lengths, indicating that MYOM2 influences passive tension, in addition to TTN. Altogether, these results suggest that the mutation in MYOM2 has an effect on the passive tension of CMs, which may result in altered diastolic function.
Identification of CG14964 as a putative MYOM2 Drosophila ortholog
The occurrence of MYOM2 mutations in both TOF and HCM patients, and its altered RNA expression levels within our TOF cohort, as well as changes in the physiology of HCM-derived mutant CMs, suggest an important function for the development and function of the heart. We hypothesized that it is likely to interact with critical components of the sarcomere, such as MYH7, and therefore investigated any such interaction using the Drosophila model. Drosophila shares similar cardiac functions and pathways, but with lower genetic redundancy and, thus, complexity compared to human or vertebrates in general (Bier and Bodmer, 2004; Bodmer, 1995; Vogler et al., 2009).
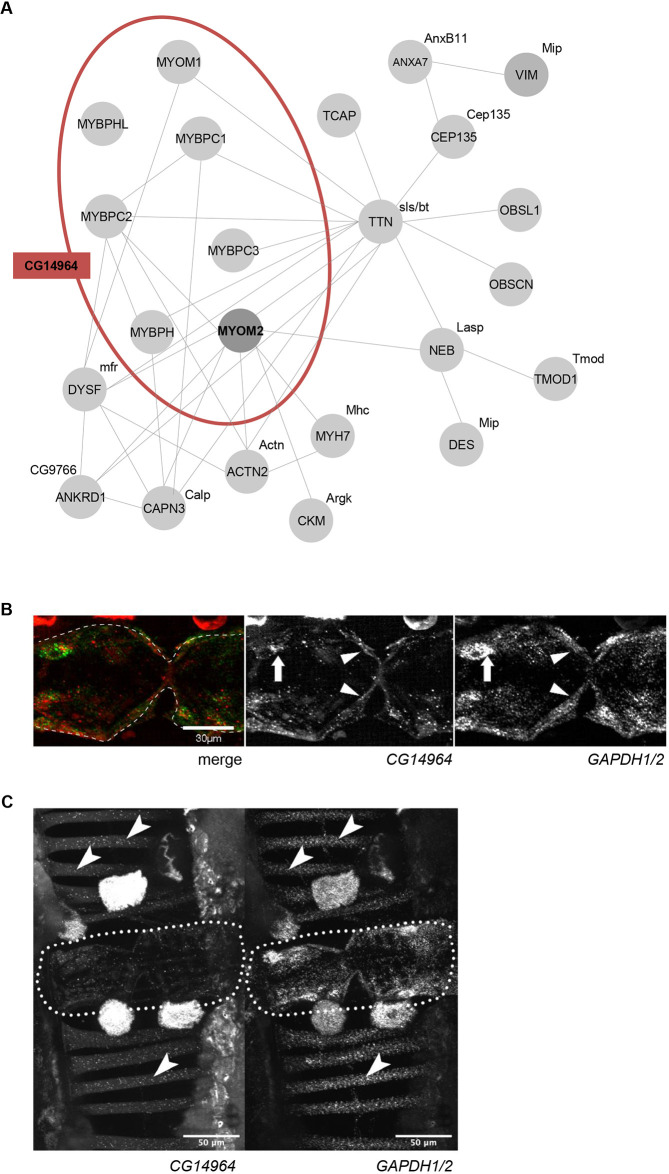
Many sarcomere proteins are conserved from human to Drosophila, like MYH7 (Myosin heavy chain, Mhc, in flies). However, some of these only share a common domain architecture, with orthology being less evident, such as TTN with a large number of FN3 and Ig-like domains similar to the fly gene sallimus (sls), but also to bent (bt). In the case of the myomesin family, several genes with similar domain structure comprising FN3 and Ig-like domains have been reported [DIOPT database (www.flyrnai.org/cgi-bin/DRSC_orthologs.pl), Hu et al., 2011], but only a single fly gene, CG14964, showed a number and an arrangement of domains close to MYOM1, MYOM2 and MYOM3 (Fig. S3A). Of note, CG14964 is also similar to human myosin binding proteins H (MYBPH and MYBPHL), as well as myosin binding proteins C (MYBPC1, MYBPC2 and MYBPC3; Fig. S4). We suggest that in Drosophila, CG14964 inhabits the functional space that is occupied by several human sarcomere genes, including MYOM2 (Fig. 3A, Fig. S3B-D). Similarly, amino acid alignments of bt and sls to the human TTN showed a conserved structure of both proteins in length and regarding the tandem organization of FN3 and Ig domains (Fig. S3C,D), suggesting that both sls and bt adopt the function of TTN. To confirm the potential role of CG14964 in muscle formation, we performed mRNA in situ hybridization (RNAscope, ACDBio) in adult fly abdomens and found that CG14964 was expressed in the heart, specifically in contractile CMs but also in somatic muscles (Fig. 3B,C, Fig. S5). Taken together, the structural conservation of the gene and cardiac expression pattern suggests that CG14964 is the appropriate gene ortholog for studying MYOM2, as well as the cardiac function of other myosin binding proteins in the fly.
Fig. 3.
Interaction network and expression of CG14964 – a putative ortholog of MYOM2. (A) Interaction network of MYOM2, TTN and MYH7 with Drosophila orthologs CG14964, bt and sls. Physical interactions are based on GeneMania v.3.6.0 (Warde-Farley et al., 2010) and other studies (Blandin et al., 2013; Brulé et al., 2010; Hornemann et al., 2003; Obermann et al., 1998). Drosophila orthologs are based on the DIOPT database (Hu et al., 2011). (B) Expression of CG14964 in adult fly hearts (arrows/arrowheads mark perinuclear space with CG14964 (red) and GAPDH1/2 (green) transcript localization). Gapdh1/2 is used as a reference. (C) Expression of CG14964 and GAPDH1/2 in the heart (encircled), and in body wall muscles (arrows).
Heart- and muscle-specific knockdown of CG14964 leads to heart and muscle defects
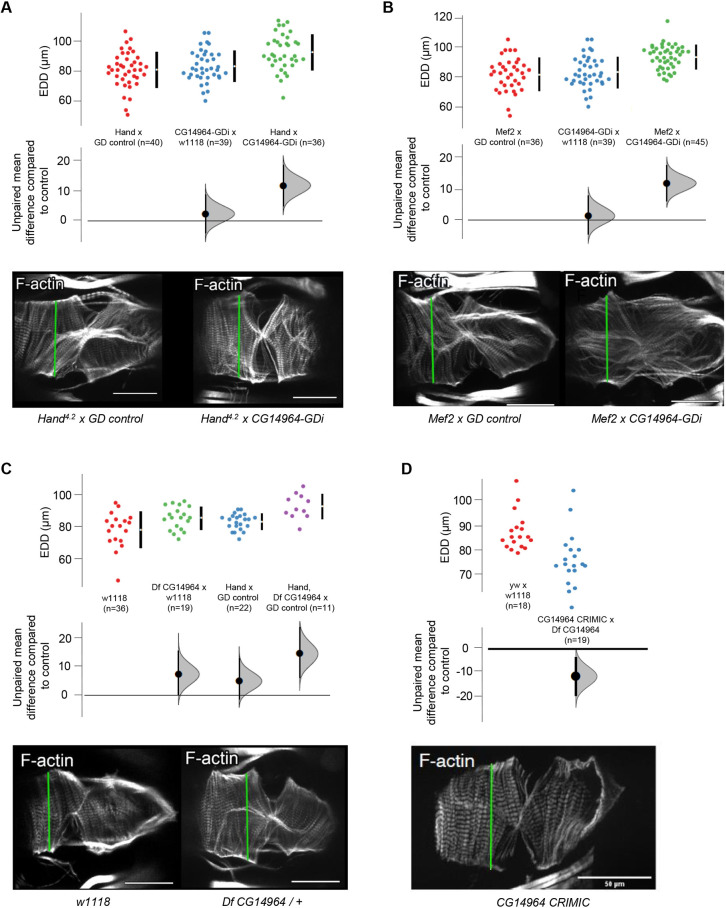
To characterize the function of CG14964, its expression level was reduced by RNAi-mediated knockdown (KD) specifically in the heart or in both somatic muscles and heart. For this purpose, the inducible Gal4-UAS system (Hand4.2-Gal4 and Mef2-Gal4, respectively) was used, as well as two independent RNAi lines [CG14964i-GD and CG14964i-T3 (TRiP, Perkins et al., 2015); see Materials and Methods for further details], which both reduce CG14964 expression in the heart and muscle, as measured by quantitative reverse transcription PCR (RT-qPCR) (in the heart only) and in situ hybridization (Fig. S6A-D). Moreover, a chromosomal deletion covering the CG14964 locus was used alone (i.e. heterozygous deficiency that includes CG14964; CG14964Df; Fig. S6E) or with a loss-of-function allele [i.e. a homozygous CRISPR MiMIC (CRIMIC) insertion; CG14964CRIMIC; Fig. S6F]. For both systemic mutations, CG14964Df and CG14964CRIMIC, we characterized the KD efficiency by RT-qPCR with RNA extracted from whole flies (Fig. S6G-I).
To analyze the cardiac function of CG14964, we applied a semi-automated method to assess the contractility and rhythmicity parameters of mutant adult fly hearts using the semi-automatic optical heartbeat analysis (SOHA) method (Ocorr et al., 2014, 2009; Vogler et al., 2009). Adult flies were dissected to expose the beating heart, filmed and analyzed. In flies with a moderate (∼50%) reduction of CG14964 by RNAi, or heterozygous deficient flies, we observed cardiac dilation of the beating, as well as of fixed heart samples following immunostaining (Fig. 4A-C). Further reduction of CG14964 using a transheterozygous combination of a CRIMIC variant with a deficiency line resulted in cardiac constriction (Fig. 4D). A similar but not significant trend was observed with a strong KD using the TRiP line (Figs S6B,D, S7). These data suggest that the amount of CG14964 reduction dictates the specific heart phenotype, meaning that a moderate reduction causes dilation, whereas a complete loss or strong reduction leads to restriction. Interestingly, we found that constricted hearts of CG14964CRIMIC mutants show increased expression of Mhc (Fig. S9), indicating that reduction of CG14964 might cause myofibrillar hypertrophy in a dosage-dependent manner.
Fig. 4.
Cardiac-specific knockdown of CD14964 leads to dosage-dependent heart defects in the adult fly. (A-D). End-diastolic diameter (EDD) of 3-week-old flies harboring a knockdown of CG14964 by using (A) CG14964-GDi crossed with Hand4.2-Gal4, (B) CG14964-GDi crossed with Mef2-Gal4, (C) CG14964 deficiency or (D) transheterozygous CG14964CRIMIC/deficiency. We observed heart dilation (A-C, mild knockdown) or constriction (D, strong knockdown). All raw data are shown (mean±s.d.), as well as effect size and 95% c.i. below the data. In addition, Phalloidin-stained cardiac myofibrils show altered heart diameters in representative examples (measurements taken at the green lines). Scale bars: 50 μm.
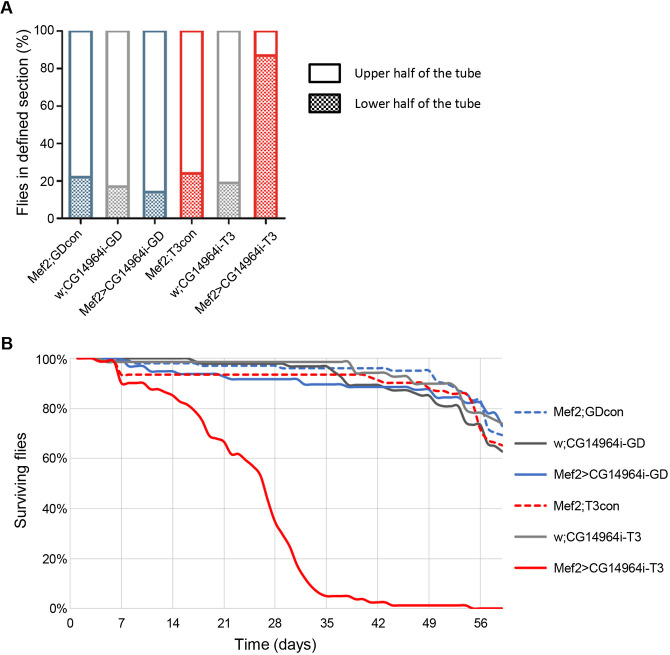
As CG14964 is expressed in somatic muscle, we further examined the role of CG14964 for muscle function in general. Using the rapid iterative negative geotaxis (RING) assay (Gargano et al., 2005) on flies with Mef2-Gal4-mediated KD, we found a significant locomotion (i.e. climbing) defect in 3-week-old CG14964i-T3 flies compared to CG14964i-GD and control flies (Fig. 5A). Similarly, muscle- and heart-specific KD of CG14964 using CG14964i-T3 also resulted in a reduced lifespan of adult flies: the half-survival rate of CG14964i-T3 flies was severely decreased compared to CG14964i-GD and control flies, with fewer than half of the flies surviving beyond week 4 (Fig. 5B). The reduced climbing ability, together with a decreased lifespan of muscle-specific KD of CG14964 in adult flies, indicates an impairment of the overall muscle function, which implies that CG14964 seems to be required in all somatic muscles. This concurs with our observation that CG14964CRIMIC mutants, either homozygous or in trans to the CG14964 deficiency, also showed severely compromised climbing abilities and reduced viability, in addition to cardiac defects. Last, we also tried to overexpress human MYOM2 in Drosophila but, although we could detect the human transcript when driving the hMYOM2 cDNA with Mef2-Gal4 (Fig. S10), we were not successful in obtaining evidence for hMYOM2 by antibody staining or mScarlet localization, indicating that protein translation and/or maturation of hMYOM2 in fly tissue is not trivial.
Fig. 5.
Muscle-specific knockdown of CG14964 causes locomotion defects and reduced lifespan in adult flies. (A) Locomotion test performed using the RING assay on adult flies expressing CG14964i-GD (left) and CG14964i-T3 (right) in muscles (Mef2-Gal4) showed reduced locomotion ability at 3 weeks in Mef2>CG14964i-T3 flies only. Graph shows percentage of fly population in a defined section of the vial after 20 s. (B) A survival assay performed on Mef2>CG14964i-GD and Mef2>CG14964i-T3 with appropriate controls revealed a decreased survival for Mef2>CG14964i-T3, with fewer than half of the flies surviving 28 days versus more than 90% in the other tested lines.
Interaction with Mhc (MYH6/MYH7)
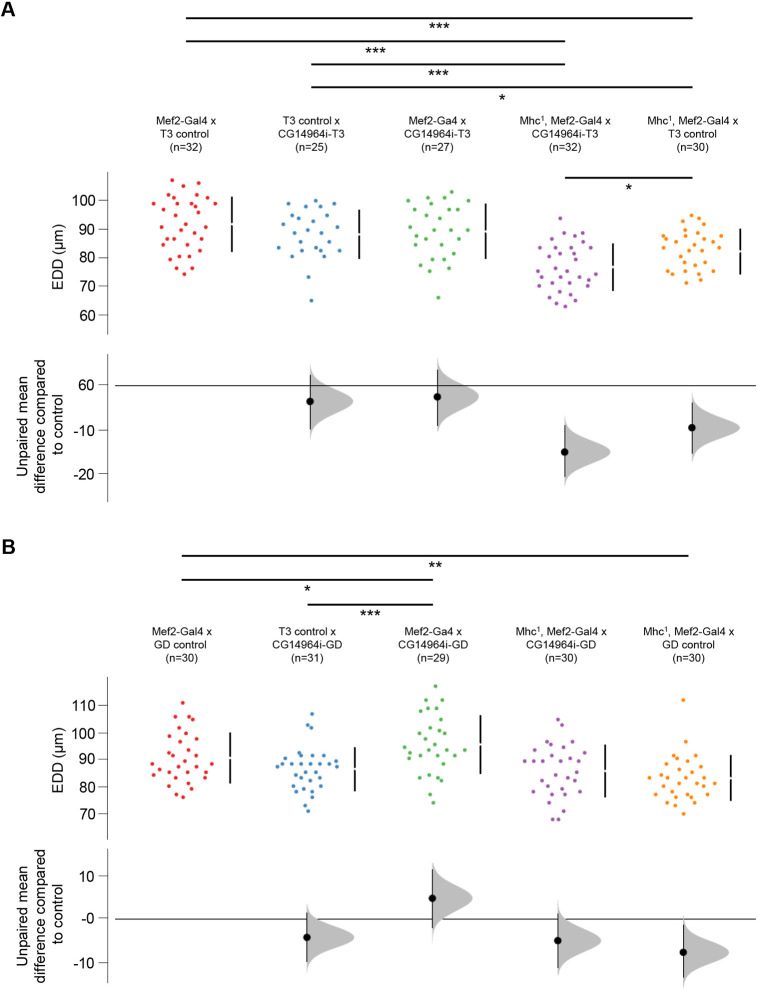
As it was shown that MYOM2 physically interacts with MYH7 in vitro (Obermann et al., 1998), we wanted to test whether CG14964 and Mhc, the fly ortholog for MYH6 and MYH7, could interact at the genetic level in vivo. We used a heterozygous null mutant for Mhc (Mhc1) (O'Donnell and Bernstein, 1988; Wells et al., 1996) combined with different KD lines for CG14964 (both GD and TRiP). Heterozygosity for Mhc1 alone caused a modest but significant cardiac constriction (Fig. 6; far right orange data points). A reduction in CG14964 levels using the strong TRiP line (Fig. 6A; middle green data points), or using the moderate GD line (Fig. 6B; middle green data points), caused a decrease or an increase in heart diameter, respectively, compared to controls (Fig. 6; left data points). Strikingly, the combination of Mhc1/+ with muscle-specific CG14964 KD resulted in a different interaction depending on the RNAi alone, meaning Mhc1/+ combined with CG14964i-T3 resulted in a further constriction, compared to Mhc1/+ alone (Fig. 6A; purple data points), indicating a synergistic enhancement of constriction. Similarly, heterozygosity for CG14964CRIMIC also reduced heart diameter in an Mhc1/+ background (Fig. S8). In contrast, Mhc1/+ combined with CG14964i-GD resulted in intermediate cardiac diameters, compared to either one alone (Fig. 6B; purple data points), indicating a normalization to wild type. This means that the dilation phenotype of CG14964i-GD was reversed by the reduction of Mhc, which was consistent with our observation of significantly increased Mhc levels in a CG14964 mutant background. Taken together, our data suggest that CG14964 and Mhc functionally interact, consistent with the postulated equivalent functions of MYOM2-MYH6/7 in mammals and CG14964-Mhc in the fly heart.
Fig. 6.
Interaction between CG14964 and Mhc. (A) End-diastolic diameters (EDD) are decreased in Mhc1 heterozygous flies (orange) and become further constricted upon strong CG14964 knockdown (purple). (B) Mild CG14964 knockdown causes enlarged hearts (green), which is restricted by Mhc1 heterozygosity (purple). All raw data are shown (mean±s.d.), as well as effect size and 95% c.i. below the data. For Mhc-CG14964 interaction, significance was tested using an unpaired two-sided Student's t-test (*P<0.05; **P<0.01; ***P<0.001).
DISCUSSION
Myofibrils mediate skeletal and cardiac muscle contraction in vertebrates and invertebrates. Their basic unit is the sarcomere with two transverse structures, the Z-disk and the M-band, which anchor thin (actin) and thick (myosin) filaments to an elastic system (Lange et al., 2020). Therefore, alterations of sarcomere proteins influence the contractile performance of the heart and skeletal muscle (Hamdani et al., 2007). In this study, we found rare deleterious genetic variations in the MYOM2 gene of HCM and TOF patients, a well-characterized protein of the sarcomere and a major structural component of its myofibrillar M-band. In humans, there are, besides MYOM2, which is specifically expressed in the heart and skeletal muscle, two other myomesin genes, namely MYOM1 and MYOM3. However, this apparent redundancy in myomesins does not result in a buffering effect per se, which can be seen, for example, in the genomics of arthrogryposis (Pehlivan et al., 2019). Here, loss of function of MYOM2 can result in the termination of gestation of the affected fetus, with cardiac and arthrogryposis findings, without variation in MYOM1 or MYOM3 (Pehlivan et al., 2019).
We observed four affected cases with MYOM2 mutations in our small but phenotypically very homogeneous cohort of 13 isolated TOF patients (∼31%). Moreover, these cases share differential expression profiles in other mutated genes, including MYOM2, and exhibit an aberrant histological phenotype of the RV tissue (Grunert et al., 2014). In a recent study comprising more than 2800 CHD patients [Pediatric Cardiac Genetics Consortium (PCGC)], only 14 cases with rare inherited or de novo mutations in MYOM2 were found, of which two (14%) were TOF patients (Jin et al., 2017). As with many CHD cohorts in general, the variety of CHDs with MYOM2 mutations in the PCGC study was very wide, comprising complex heterotaxias, such as double outlet RV, as well as isolated simple atrial and ventricular septal defects (Jin et al., 2017). However, there are subgroups of TOF patients, which in the case of the PCGC study comprised a relatively small number of cases, and for our small but homogeneous group of patients, in terms of their clinical parameters and features, comprised a relatively high number of cases with MYOM2 mutations. These mutations might contribute to the phenotype during heart development (Grunert et al., 2014), as well as in the long term. The latter is reflected by an arrhythmic burden in adult TOF patients (Khairy et al., 2010; Wu et al., 2014) and that the majority of mutated genes show continuous expression during adulthood (Grunert et al., 2014).
It has been shown that TOF is genetically heterogeneous, and a subgroup is characterized by genetic alterations in other sarcomere genes known to be causative of cardiomyopathy, such as MYH7 and MYBPC3 (Grunert et al., 2014; Vermeer et al., 2016). Thus, we screened for MYOM2 mutations in a cohort of 66 HCM patients that had no mutations in the known disease genes. Our screening revealed a subgroup of four HCM patients with rare MYOM2 mutations, half of which were predicted to be damaging. In general, the genetic testing in HCM was limited by contemporary technologies. However, we have validated the screening approach using an Affymetrix resequencing array as an up-to-date and sensitive method. Approximately 40% of HCM cases have a non-familial subtype (Ingles et al., 2017); however, as data to estimate co-segregation within families were not available we cannot exclude this possibility. Furthermore, it may be possible that referral bias may have led to an overestimate of the frequency of MYOM2 mutation carriers in HCM because of the retrospective nature of this study. However, very few data exist concerning a pathogenic role for mutations in M-band proteins, especially in the myomesin protein family, in cardiomyopathies so far. The first study suggesting a link described the missense mutation p.V1490I in MYOM1, which co-segregated with the phenotype in a family with HCM (Siegert et al., 2011). In a later study, a panel of 62 sarcomere and non-sarcomere genes (including MYOM1 but not MYOM2) in 41 HCM patients was investigated by high-throughput sequencing. In total, they found three rare MYOM1 SNVs, two with and one without deleterious prediction (Bottillo et al., 2016). Furthermore, a panel of 108 cardiomyopathy and arrhythmia-associated genes, including only MYOM1, was screened in 24 patients with restrictive cardiomyopathy (Kostareva et al., 2016). Here, two variants of unknown significance (VUS) were identified in MYOM1. Two further MYOM1 VUS were found in one young sudden death victim with cardiac dilatation by whole-exome sequencing-based molecular autopsy (Shanks et al., 2017). Two further studies screened patients with dilated cardiomyopathy (Akinrinade et al., 2015; Marston et al., 2015). Akinrinade et al. (2015) used a targeted resequencing panel of 101 genes (including MYOM1 and excluding MYOM2) for the screening of 145 Finnish patients and identified one SNV in MYOM1 (designated as VUS) in two individuals. Of note, the study by Marston et al. (2015) investigated a cohort of 30 patients with familial dilated cardiomyopathy in 58 cardiomyopathy-related genes. Indeed, it is the only study including MYOM2 besides MYOM1. However, they found an SNV in MYOM1, but no rare SNVs in MYOM2 were detected. Other larger studies screening different defined gene panels (from 31 to up to 126 genes related to heart disease), by targeted resequencing in cardiomyopathy patients, did not include MYOM1 and MYOM2 in their panels (Cuenca et al., 2016; Haas et al., 2015; Lopes et al., 2013; Walsh et al., 2017). In summary, although one probably disease-associated mutation, as well as seven VUS, have been identified in MYOM1, no, or no rare, MYOM2 variations in cardiomyopathy patients have been described so far.
Functional insight into the role of MYOM2 came from morphological analysis of tissue sections and force measurements on CMs isolated from the interventricular septum of a patient that underwent myectomy (HCM-02). Interestingly, the HCM-derived CMs showed significantly reduced passive force, indicating that the MYOM2 mutation influences the passive properties of the patient-derived CMs. This is an interesting effect that could indicate that, in addition to titin, myomesin 2 also has an influence on the diastolic properties of the CMs and the ventricle. This observation would be in line with the role of myomesin as part of the shock absorber function of the striated muscle M-band that, together with titin, stabilizes the myosin filament lattice longitudinally (Lange et al., 2020; Schoenauer et al., 2005).
In addition, we studied the potential cardiac and muscle function of MYOM2, and the interaction partner MYH7, by assessing the effect of downregulation in Drosophila. Drosophila is an excellent animal model for studying the genetics of human disease mechanisms, which is typically masked due to genetic redundancy present in mammals (Pickett and Meeks-Wagner, 1995). There is no current mammalian animal model for MYOM2, and, in zebrafish, four redundant paralogous myomesin genes exist, of which only MYOM3 has been studied in detail (Xu et al., 2012). Given the advantages of the fly model, we probed the function and genetics of MYOM2 by analyzing the closest functional ortholog in Drosophila, CG14964 (dMnM), a gene that is similar to myomesins, as well as other myosin binding proteins. Cardiac-specific KD or deletion of CG14964 led to dosage-dependent heart defects (i.e. cardiac dilation or constriction), and the muscle-specific KD caused locomotion defects and a reduced lifespan of adult flies. The larger end-diastolic diameter in Drosophila suggests either mild (eccentric) hypertrophy or reduced stiffness of the myofibrils/sarcomeres upon CG14964 KD. This is consistent with reduced passive force at different sarcomere length and, thus, reduced stiffness observed in human CMs with mutation Ser466Arg (HCM-02) in MYOM2. In addition, it supports the hypothesis that CG14964 is a myomesin ortholog in Drosophila. The differences in the KD results may be explained by their efficiencies, given that the strongest KD phenotype was comparable to the combination of two strong alleles in trans. Of note, the cardiac phenotype of CG14964 RNAi appeared to be dose dependent, with mild a reduction causing cardiac dilation that was reversible by a reduction in Mhc dosage, whereas a strong reduction led to cardiac constriction, potentially as a result of an excessive increase in myosin. This is reminiscent of specific mutations in MYH7 (encoding β-myosin heavy chain) in humans that can give rise to, for example, dilation or restriction (Hershberger et al., 2013). In addition to classic HCM, MYH7 mutations may cause cardiomyopathies with different heart morphology and function, such as dilated cardiomyopathy (DCM) and HCM with features of restrictive cardiomyopathy (RCM) (Bondue et al., 2018; Kubo et al., 2007; Møller et al., 2009). Indeed, the cardiac phenotype caused by MYH7 mutations shows a great variety, ranging from late onset DCM, with mild to moderate dilation (Villard et al., 2005), to severe pediatric RCM (Ware et al., 2008). Similarly, in Drosophila, different types of Mhc mutations with inhibited or increased motor activity show cardiac dilation or constriction, respectively (Cammarato et al., 2007; Kronert et al., 2018). However, in contrast to MnM loss of function in which we do not see sarcomere defects, strong KD of Mhc causes breakdown of the sarcomere. Therefore, we hypothesize that the level of MnM is important to fine-tune sarcomere function, potentially by regulating Mhc levels, but not for the overall structure of the sarcomere.
MnM (CG14964) seems to be a hub gene within the interaction network of sarcomere genes, as shown in Fig. 3A. Four interaction partners of MYOM2 are known to carry mutations causing cardiomyopathies (MYH7, ANKRD1, ACTN2 and TTN), whereas two are known to cause muscular dystrophy (DYSF and CAPN3). As 30 to 50% of HCM cases harbor mutations in the sarcomere gene MYH7 (Vermeer et al., 2016), we tested and showed synergistic interaction of CG14964 with Mhc (MYH6/MYH7) in the adult fly.
In summary, this study showed novel rare, and probably disease-relevant, mutations in the sarcomere gene MYOM2 in patients with TOF and HCM, in particular for cardiomyopathy patients. Moreover, the functional characterization of affected patient-derived CMs, as well as functional analyses of the up-to-now unknown fly gene CG14964, as the likely ortholog of MYOM2 as well as other myosin binding proteins, suggest that MYOM2 is involved in the development of the heart and plays critical roles in establishing or maintaining robust heart function. Thus, MYOM2 is a disease candidate gene for HCM and TOF, both of which exhibit an impaired LV and RV function, respectively.
MATERIALS AND METHODS
Phenotyping of HCM patients
The local institutional review board of the Charité – Universitätsmedizin Berlin approved the study and written informed consent was obtained from all participants. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
The study cohort comprised 66 unrelated patients of German origin with HCM (29% female and 71% male). All patients had negative screening results in known HCM disease genes (see below). Although 20 patients showed a non-obstructive form of the disease (about 30%), 46 patients (70%) had hypertrophic obstructive cardiomyopathy (HOCM), presenting with LV outflow tract obstruction at rest and/or with exercise (often accompanied with systolic anterior motion of the anterior mitral leaflet). This distribution is in accordance with the general distribution in HCM (Maron et al., 2006). The cohort was examined on the basis of medical history, physical examination, 12-lead electrocardiogram (ECG), and two-dimensional and M-mode echocardiography. As clinically indicated, cardiac magnetic resonance imaging, heart catheterization, angiography and Holter ECG were performed in some patients. The diagnosis of HCM was made according to the established criteria (Elliott et al., 2007; Gersh et al., 2011). The pathological hallmark of HCM is unexplained LV hypertrophy. Briefly, the major inclusion criterion was the presence of an interventricular septal thickness (IVS) of 13 mm or more (15 mm if sporadic and 13 mm if familial) in the absence of loading conditions (hypertension and valve disease) sufficient to cause the observed abnormality, and in the absence of systemic disease such as amyloidosis.
Genetic analysis in HCM patients
The frequent HCM disease genes MYH7 and MYBPC3, as well as rare disease genes (TNNT2, TNNI3, TPM1, MYL2, MYL3, ACTC1, TCAP, TNNC1, MYOZ2 and CSRP3) were screened as published previously by us and colleagues (Geier et al., 2003; Kabaeva et al., 2002; Mogensen et al., 2004; Perrot et al., 2006, 2005; Posch et al., 2008). Disease-causing mutations in all of these genes were excluded in the 66 HCM patients comprising the study cohort (see above). For validation purposes, 13 randomly selected patients (20% of the cohort) were additionally analyzed by a custom DNA resequencing array composed of 11 HCM disease genes (Affymetrix) as described previously (Fokstuen et al., 2008, 2011). In addition, the four HCM patients who carried a MYOM2 mutation (Table S1) were also analyzed using this technique. Rare probable disease-causing mutations in HCM genes were identified neither in the 13 patients nor in the MYOM2 mutation carriers. Furthermore, mutations in MYOM1 were also excluded in these four patients.
MYOM2 screening was performed using Sanger sequencing. Briefly, the 36 coding exons of MYOM2 were PCR-amplified using flanking intronic primers and directly sequenced using ABI Big Dye Terminator chemistry. The variants that were found were annotated according to the cDNA and protein reference sequence (Genbank ID NM_003970.3; Ensembl ID, ENST00000262113.8; and UniProtKB/Swiss-Prot P54296). The significance of the variants was further analyzed in a first step by considering the nature and location of the change, and its frequency found in large population-based datasets [such as from the Exome Aggregation Consortium (Lek et al., 2016) and the Genome Aggregation Database (Karczewski et al., 2020)] and our own controls [minor allele frequency below 0.02% as recommended by Burke et al. for cardiomyopathies (Burke et al., 2016)]. In a second step, the conservation of the affected amino acid and the possible functional impact of the variants [using mutation prediction tools, such as PolyPhen2 (Adzhubei et al., 2013), SIFT (Vaser et al., 2016) and MutationTaster (Schwarz et al., 2010)] were assessed.
Clinical courses of HCM patients with MYOM2 mutations and control heart tissue
All four unrelated non-familiar cases were characterized by LV hypertrophy and electrocardiogram abnormalities (see detailed clinical data in Table S1). They showed a symptomatic form of HOCM which led to an invasive septal reduction intervention in two of them. All patients were of European (German) ancestry.
The female patient HCM-01 (carrier of mutation p.Met270Thr) presented with symptoms such as angina pectoris, dyspnea on exertion, dizziness, and palpitations at the age of 50 years [New York Heart Association (NYHA) functional class II]. Onset of disease was at the age of 31 years. She was found to have hypertrophic cardiomyopathy with outflow tract (OFT) obstruction (interventricular pressure gradient at rest of 20 mmHg, measured during cardiac catheterization). Echocardiography revealed an IVS thickness of 16 mm and a posterior wall (PW) thickness of 12 mm; ejection fraction (EF) was 35%. The patient was treated with calcium antagonists that improved symptoms.
The male patient HCM-02 (carrier of mutation p.Ser466Arg) had an early onset of disease at the age of 19 years. Later on, he developed a symptomatic disease form with angina, dyspnea on exertion, and syncopes (NYHA class III). He showed a moderate LV hypertrophy with an IVS of 15 mm and with a PW of 14 mm, as well as normal cardiac dimensions at the age of 52 years. Heart catheterization confirmed the diagnosis of HOCM with an OFT gradient of 100 mmHg at rest. Because of this OFT obstruction [including systolic anterior motion (SAM) of the mitral valve and mitral insufficiency], the 53-year-old patient underwent surgical septal myectomy (Morrow procedure), which released the OFT obstruction. Three years later, an automated implantable cardioverter/defibrillator was implanted because of ventricular tachycardia accompanied with recurrent syncopes. Because of atrial flutter, a cavotricuspid isthmus ablation was performed at the age of 68 years.
The male patient HCM-03 (carrier of mutation p.Ile793Val) had disease onset at the age of 50 years. He complained about dyspnea and dizziness (NYHA class II), and the diagnosis of HOCM was made at the age of 56 years. He had a resting OFT gradient of 40 mmHg (measured during catheterization), including SAM. Echocardiography revealed an LV hypertrophy with an IVS of 17 mm and a PW of 14 mm, as well as normal cardiac dimensions; his fractional shortening (FS) was also normal (40-42%). Under high dose calcium antagonist treatment, his OFT gradient was below 10 mmHg and his symptoms improved.
The female patient HCM-04 (carrier of mutation p.Arg1079X) had a late onset of disease at 60 years of age. Hypertrophic cardiomyopathy was diagnosed at the age of 69 years. At this age, she showed symptoms such as palpitations, syncope, angina, and dyspnea (NYHA class III). Echocardiography showed severe LV hypertrophy with an IVS of 23 mm and a PW thickness of 16 mm, as well as normal FS of 42%. Because of an OFT gradient of 95 mmHg at rest, the patient underwent two subsequent transluminal septal ablations (an embolization of the first and second septal branches) at the age of 72 years. These led to a reduction of the gradient and improvement of symptoms, but LV hypertrophy was just slightly reduced (as shown by subsequent echocardiography measurements). Approximately 2 years after septal ablation, symptoms worsened again and the pressure gradient increased to 50 mmHg. Furthermore, the patient developed intermittent atrial fibrillation.
As control tissue, we used flash-frozen tissue from the IVS of five unaffected age-matched non-transplanted donor hearts (three males and two females of age 23 to 56 years) from the Sydney Heart Bank (SHB; for SHB heart codes and details see Table S2).
Morphological analyses
Flash-frozen samples (HCM-02 and control) were slowly thawed on melting fixative (1.5% paraformaldehyde and 1.5% glutaraldehyde in 0.15 M HEPES buffer). After aldehyde fixation, samples were subsequently postfixed with osmium tetroxide, en bloc-stained with uranyl acetate, dehydrated in acetone and embedded in epoxy resin. Semi- and ultrathin sections were cut and stained with Toluidine Blue and lead-uranyl actetate, respectively.
Force measurements, and phosphorylation and protein analyses of CMs from HCM-02 and control myocardium
Protein analysis and force measurements on isolated CMs from heart tissue (IVS) were performed for one HCM patient (HCM-02; male, 53 years), as well as five donor hearts serving as controls (on average 38 years). Written informed consent for use of the tissue and approval of the local ethics committee of Hannover Medical School for the study on human tissue was obtained (No. 507/09). All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. For analysis of phosphorylation of cMyBPC, cTnT, cTnI, and MLC2v in native myocardial tissue from HCM-02 and controls (Fig. S2A), gradient gels were used, and phosphorylation was analyzed by calculating the ratio of Pro-Q Diamond staining (phosphorylated protein) versus SYPRO Ruby staining (total amount of protein) for each band, respectively, as described previously (Kraft et al., 2013). To study relative protein quantities of cMyBPC, cTnT, cTnI, and MLC2v in HCM-02 and control tissue (Fig. S2B), the bands of the respective proteins on the SYPRO Ruby stained gels (Fig. 2B) were analyzed densitometrically and normalized to the alpha-actinin signal in the same lane.
For functional studies, CMs were isolated and force was measured as described in detail previously (Montag et al., 2018). Briefly, CMs were mechanically isolated from myocardial tissue and chemically permeabilized with Triton X-100. Single CMs were mounted between the force transducer and lever arm of a custom-made setup for biomechanical characterization. Active force was measured by incubating CMs in physiological solutions with different calcium concentrations. Passive force was determined in relaxing solution (pCa 8.0) by applying step release and re-stretch of the CMs (Montag et al., 2018) at increasing sarcomere lengths, starting from 1.95 µm up to 2.4 µm. All measurements occurred after incubating the CMs with PKA to adjust PKA-dependent phosphorylation of sarcomeric proteins (Kraft et al., 2013). Sixteen and ten CMs were measured for controls and HCM-02, respectively.
Fly husbandry
All fly stocks were maintained at 25°C on standard fly food medium. The following fly stocks were used: w1118 [3605, Bloomington Drosophila Stock Center (BDSC)], GD control [60000, Vienna Drosophila Resource Center (VDRC)] and TRIP control (36303, BDSC) as control flies; and DMef2-Gal4 (Ranganayakulu et al., 1996) and Hand4.2-Gal4 (Han and Olson, 2005) as driver lines. For CG14964, Df(3L)BSC672 (26524, BDSC) and CRIMIC line CG14964CR01157-TG4.1 (#81199, BDSC) were used; and for CG14964 RNAi GD (43603, VDRC) and TRIP3 (65245, BDSC) were used. For Mhc, we used Mhc1 mutant (O'Donnell and Bernstein, 1988) and MhcYD0783 (50881, BDSC).
Heart functional analysis
To assess heart function in adult flies, we used the Semi-automated Optical Heartbeat Analysis (SOHA) method (Cammarato et al., 2015; Fink et al., 2009; Ocorr et al., 2009). Briefly, adult flies were anesthetized using FlyNap (173025, Carolina) and dissected in artificial hemolymph to expose the dorsal heart (Vogler and Ocorr, 2009). Before filming, the hearts were allowed to equilibrate with oxygenation for 15-20 min. Movies (30 s in length) were recorded with a Hamamatsu Orca Flash 4.0 camera (at 140 frames/sec) using a Zeiss A1 Axioscope (10× magnification). Movie analysis was carried out using SOHA software (Oaktree Technologies, www.sohasoftware.com) (Cammarato et al., 2015).
Overexpression of hMYOM2 in Drosophila
A full-length cDNA of hMYOM2 was obtained [Mammalian Gene Collection Human MYOM2 Sequence-Verified cDNA (CloneId: 6205359, Dharmacon)] and cloned into pUASTattB (Bischof, 2007). For N- or C-terminal tagging of hMYOM2 using Gibson assembly, mScarlet was amplified from a plasmid template using overlapping primers to facilitate in-frame assembly into hMYOM2-pUASattB. Constructs were inserted into attP2 using a commercial injection service (BestGene Inc).
Climbing assay
Climbing defects were quantified using the RING assay which was performed as described previously (Gargano et al., 2005), with the following changes: adult flies were transferred to an empty fly tube and left to adapt for 10 min. The tubes were tapped three times to trigger the negative geotaxis response, and 30 s intervals were recorded to document the distance the flies could climb up. This experiment was performed in triplicate for each biological replicate and the mean of these three replicates was calculated for each experiment.
Survival assay
The measurement of adult lifespan in Drosophila was performed as described previously (Linford et al., 2013). Briefly, control and experimental female flies were collected by CO2 anesthesia and transferred to tubes with fresh standard food on Day 0. Ten vials (containing 10-15 flies) were collected for each genotype (100-150 flies in total). Flies were transferred to a new tube with fresh food every 2 days, and the number of dead flies was counted. This was repeated until the last fly died.
RNA isolation and RT-qPCR
Total RNA was isolated from ∼15-20 adult fly hearts, using TRIzol reagent (Invitrogen) combined with the Quick-RNA MicroPrep Kit (Zymo Research), including a step of DNAse-on-column treatment, following the manufacturer's instructions. RNA quality and quantity were respectively assessed using an Agilent RNA 6000 Pico kit on an Agilent 2100 Bioanalyzer (Agilent Technologies) and Qubit RNA HS assay kit on a Qubit 3.0 Fluorometer (Thermo Fisher Scientific). Total RNA was reverse transcribed using the PrimeScript RT Master Mix (Takara). RNA from three to five adult female whole flies was isolated using TRIzol reagent combined with chloroform/ethanol extraction. RNA quality and quantity were assessed using a Nanodrop spectrometer. cDNA was generated using Superscript IV Reverse Transcriptase (Invitrogen), with additional DNase I treatment or using a QuantiTect Reverse Transcription Kit (Qiagen). SYBR Green-based real-time qPCR (Sybr Green I Master, Roche) was performed on a LightCycler 480 (Roche) and a LightCycler 96 (Roche). Gene expression quantification was determined using the 2−ΔΔCT method (Pfaffl, 2001), with Rp49 as a reference gene. Values were derived from three to five biological replicates.
Immunostainings and fly heart measurements
The immunostaining of fly adult hearts was performed as described previously (Alayari et al., 2009). Fly hearts were dissected as described for the SOHA method (see above), and myofibrils were relaxed using 10 mM EGTA followed by fixation in 4% formaldehyde for 15 min. The sarcomeric structure of the adult heart was visualized using Alexa Fluor 568 Phalloidin (Thermo Fisher Scientific).
Mhc protein level quantification
For Mhc protein level quantification in tissues, 1-week-old female mutant and control flies were stained under identical conditions as described previously (Alayari et al., 2009) using anti-Mhc (1:50, DSHB, 3E8-3D3) and anti-mouse-Alexa Fluor 488 (Jackson Labs, 1:500, 115-545-003). Fly hearts were imaged using a Zeiss Imager M.1 microscope equipped with a 25× dipping lens, with identical imaging settings for each specimen (n=6-9 flies). Mean gray value (intensity) of 5 regions of interest (ROI) per tissue type (cardiomyocytes and ventral longitudinal muscle) were measured for each fly using ImageJ, and the average of mean fluorescent intensity for each fly was calculated for each ROI.
RNAscope
mRNA in situ hybridization for CG14964, to count the number of transcripts for each CM and body wall muscle, and outlining of cells, was carried out as described previously Blice-Baum et al. (2018). The number of transcripts was counted in abdominal body wall muscles and CMs and normalized to the total area of each cell. Gapdh1/2 was used as a reference transcript (control). Similarly, we used a custom probe set to detect hMYOM2 (and pericardin as a control probe) to show ectopic transcript expression of Mef2-Gal4-driven UAS-hMYOM2 in adult fly hearts.
Sequence alignments and ortholog analysis
To compare Drosophila and human protein sequences between CG14964 or bt with MYOM2, as well as bt or sls with TTN, we used the online LAST tool, which is based on a modified standard seed-and-extend approach, and allows plotting of an amino acid alignment in a dot plot manner (Kiełbasa et al., 2011).
Statistical analysis
General statistical analyses were conducted using R or GraphPad Prism. Fly heart data, presented as Cumming estimation plots, were calculated using the R package dabestr, which estimated the unpaired mean differences to the control mean.
Supplementary Material
Acknowledgements
We thank Sean Lal and Amy Li (Anatomy and Histology, School of Medical Sciences, Bosch Institute, University of Sydney, Australia) for providing control heart tissue; and Ante Radocaj and Birgit Piep (Medical School of Hannover, Institute of Molecular and Cell Physiology, Hannover, Germany), as well as Sandra Schochardt-Schuster and Kerstin Mika (Cardiovascular Genetics, Charité – Universitätsmedizin Berlin, Berlin, Germany), for excellent technical assistance. We are deeply grateful to the patients and families for their cooperation.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.A.-P., M.G., A.P., T.K., R.B., G.V., S.R.S.; Methodology: E.A.-P., T.N., T.K., R.B., G.V., S.R.S.; Software: M.G., G.V.; Validation: A.P., G.V.; Formal analysis: M.G., D.H., G.V.; Investigation: E.A.-P., T.N., M.G., O.O., A.P., D.H., F.M., C.M., G.V.; Resources: C.Ö., C.D.R., C.M., T.K., R.B., S.R.S.; Data curation: M.G., G.V.; Writing - original draft: E.A.-P., T.N., M.G., R.B., G.V.; Writing - review & editing: E.A., T.N., M.G., A.P., T.K., R.B., G.V., S.R.S.; Visualization: M.G., O.O., A.P., D.H., F.M., C.M., G.V.; Supervision: T.K., R.B., G.V., S.R.S.; Project administration: T.K., R.B., G.V., S.R.S.; Funding acquisition: T.K., R.B., S.R.S.
Funding
This work was supported by the Einstein BIH Visiting Fellowship of the Stiftung Charité; the Einstein Stiftung Berlin (to S.R.S.); and by the Deutsche Forschungsgemeinschaft (276/3-1 to C.Ö.; 1187/19-1 to T.K.).
Supplementary information
Supplementary information available online at https://dmm.biologists.org/lookup/doi/10.1242/dmm.045377.supplemental
References
- Adzhubei I., Jordan D. M. and Sunyaev S. R. (2013). Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 76, 7.20.1.-. 10.1002/0471142905.hg0720s76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarkova I. and Perriard J.-C. (2005). The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 15, 477-485. 10.1016/j.tcb.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Agarkova I., Ehler E., Lange S., Schoenauer R. and Perriard J.-C. (2003). M-band: a safeguard for sarcomere stability? J. Muscle Res. Cell Motil. 24, 191-203. 10.1023/A:1026094924677 [DOI] [PubMed] [Google Scholar]
- Akinrinade O., Ollila L., Vattulainen S., Tallila J., Gentile M., Salmenperä P., Koillinen H., Kaartinen M., Nieminen M. S., Myllykangas S. et al. (2015). Genetics and genotype-phenotype correlations in Finnish patients with dilated cardiomyopathy. Eur. Heart J. 36, 2327-2337. 10.1093/eurheartj/ehv253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alayari N. N., Vogler G., Taghli-Lamallem O., Ocorr K., Bodmer R. and Cammarato A. (2009). Fluorescent labeling of Drosophila heart structures. J. Vis. Exp. 32, 1423 10.3791/1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfares A. A., Kelly M. A., McDermott G., Funke B. H., Lebo M. S., Baxter S. B., Shen J., McLaughlin H. M., Clark E. H., Babb L. J. et al. (2015). Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet. Med. 17, 880-888. 10.1038/gim.2014.205 [DOI] [PubMed] [Google Scholar]
- Basu R., Hazra S., Shanks M., Paterson D. I. and Oudit G. Y. (2014). Novel mutation in exon 14 of the sarcomere gene MYH7 in familial left ventricular noncompaction with bicuspid aortic valve. Circ. Heart. Fail. 7, 1059-1062. 10.1161/CIRCHEARTFAILURE.114.001666 [DOI] [PubMed] [Google Scholar]
- Bettinelli A. L., Mulder T. J., Funke B. H., Lafferty K. A., Longo S. A. and Niyazov D. M. (2013). Familial ebstein anomaly, left ventricular hypertrabeculation, and ventricular septal defect associated with a MYH7 mutation. Am. J. Med. Genet. A 161A, 3187-3190. 10.1002/ajmg.a.36182 [DOI] [PubMed] [Google Scholar]
- Bier E. and Bodmer R. (2004). Drosophila, an emerging model for cardiac disease. Gene 342, 1-11. 10.1016/j.gene.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F. and Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA.104, 3312-3317. 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed]
- Blandin G., Marchand S., Charton K., Danièle N., Gicquel E., Boucheteil J.-B., Bentaib A., Barrault L., Stockholm D., Bartoli M. et al. (2013). A human skeletal muscle interactome centered on proteins involved in muscular dystrophies: LGMD interactome. Skelet. Muscle 3, 3 10.1186/2044-5040-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blice-Baum A. C., Vogler G., Viswanathan M. C., Trinh B., Limpitikul W. B. and Cammarato A. (2018). Quantifying tissue-specific overexpression of FOXO in Drosophila via mRNA fluorescence in situ hybridization using branched DNA probe technology. Methods Mol. Biol. 1890, 171-190. 10.1007/978-1-4939-8900-3_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R. (1993). The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 118, 719-729. [DOI] [PubMed] [Google Scholar]
- Bodmer R. (1995). Heart development in Drosophila and its relationship to vertebrates. Trends Cardiovasc. Med. 5, 21-28. 10.1016/1050-1738(94)00032-Q [DOI] [PubMed] [Google Scholar]
- Bondue A., Arbustini E., Bianco A., Ciccarelli M., Dawson D., De Rosa M., Hamdani N., Hilfiker-Kleiner D., Meder B., Leite-Moreira A. F. et al. (2018). Complex roads from genotype to phenotype in dilated cardiomyopathy: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc. Res. 114, 1287-1303. 10.1093/cvr/cvy122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottillo I., D'Angelantonio D., Caputo V., Paiardini A., Lipari M., De Bernardo C., Giannarelli D., Pizzuti A., Majore S., Castori M. et al. (2016). Molecular analysis of sarcomeric and non-sarcomeric genes in patients with hypertrophic cardiomyopathy. Gene 577, 227-235. 10.1016/j.gene.2015.11.048 [DOI] [PubMed] [Google Scholar]
- Brulé C., Dargelos E., Diallo R., Listrat A., Béchet D., Cottin P. and Poussard S. (2010). Proteomic study of calpain interacting proteins during skeletal muscle aging. Biochimie 92, 1923-1933. 10.1016/j.biochi.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Burke M. A., Cook S. A., Seidman J. G. and Seidman C. E. (2016). Clinical and Mechanistic Insights Into the Genetics of Cardiomyopathy. J. Am. Coll. Cardiol. 68, 2871-2886. 10.1016/j.jacc.2016.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarato A., Dambacher C. M., Knowles A. F., Kronert W. A., Bodmer R., Ocorr K. and Bernstein S. I. (2007). Myosin transducer mutations differentially affect motor function, myofibril structure, and the performance of skeletal and cardiac muscles. Mol. Biol. Cell 19, 553-562. 10.1091/mbc.e07-09-0890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarato A., Ocorr S. and Ocorr K. (2015). Enhanced assessment of contractile dynamics in Drosophila hearts. BioTechniques 58, 77-80. 10.2144/000114255 [DOI] [PubMed] [Google Scholar]
- Cuenca S., Ruiz-Cano M. J., Gimeno-Blanes J. R., Jurado A., Salas C., Gomez-Diaz I., Padron-Barthe L., Grillo J. J., Vilches C., Segovia J. et al. (2016). Genetic basis of familial dilated cardiomyopathy patients undergoing heart transplantation. J. Heart Lung. Transplant. 35, 625-635. 10.1016/j.healun.2015.12.014 [DOI] [PubMed] [Google Scholar]
- Cuypers J. A. A. E., Menting M. E., Konings E. E. M., Opić P., Utens E. M. W. J., Helbing W. A., Witsenburg M., van den Bosch A. E., Ouhlous M., van Domburg R. T. et al. (2014). Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation 130, 1944-1953. 10.1161/CIRCULATIONAHA.114.009454 [DOI] [PubMed] [Google Scholar]
- Driscoll D. J. (2016). Clinical presentation and therapy of cardiomyopathies. In Congenital Heart Diseases: The Broken Heart. Clinical Features, Human Genetics and Molecular Pathways (ed. Rickert-Sperling S., Kelly R. and Driscoll D.), pp. 667-673. Springer. [Google Scholar]
- Elliott P., Andersson B., Arbustini E., Bilinska Z., Cecchi F., Charron P., Dubourg O., Kühl U., Maisch B., McKenna W. J. et al. (2007). Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 29, 270-276. 10.1093/eurheartj/ehm342 [DOI] [PubMed] [Google Scholar]
- Fink M., Callol-Massot C., Chu A., Ruiz-Lozano P., Belmonte J. C. I., Giles W., Bodmer R. and Ocorr K. (2009). A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. BioTechniques 46, 101-113. 10.2144/000113078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokstuen S., Lyle R., Munoz A., Gehrig C., Lerch R., Perrot A., Osterziel K. J., Geier C., Beghetti M., Mach F. et al. (2008). A DNA resequencing array for pathogenic mutation detection in hypertrophic cardiomyopathy. Hum. Mutat. 29, 879-885. 10.1002/humu.20749 [DOI] [PubMed] [Google Scholar]
- Fokstuen S., Munoz A., Melacini P., Iliceto S., Perrot A., Ozcelik C., Jeanrenaud X., Rieubland C., Farr M., Faber L. et al. (2011). Rapid detection of genetic variants in hypertrophic cardiomyopathy by custom DNA resequencing array in clinical practice. J. Med. Genet. 48, 572-576. 10.1136/jmg.2010.083345 [DOI] [PubMed] [Google Scholar]
- Gargano J. W., Martin I., Bhandari P. and Grotewiel M. S. (2005). Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 40, 386-395. 10.1016/j.exger.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Gautel M. and Djinović-Carugo K. (2016). The sarcomeric cytoskeleton: from molecules to motion. J. Exp. Biol. 219, 135-145. 10.1242/jeb.124941 [DOI] [PubMed] [Google Scholar]
- Geier C., Perrot A., Özcelik C., Binner P., Counsell D., Hoffmann K., Pilz B., Martiniak Y., Gehmlich K., van der Ven P. F. M. et al. (2003). Mutations in the human muscle LIM protein gene in families with hypertrophic cardiomyopathy. Circulation 107, 1390-1395. 10.1161/01.CIR.0000056522.82563.5F [DOI] [PubMed] [Google Scholar]
- Gersh B. J., Maron B. J., Bonow R. O., Dearani J. A., Fifer M. A., Link M. S., Naidu S. S., Nishimura R. A., Ommen S. R., Rakowski H. et al. (2011). 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 58, e212-e260. 10.1161/CIR.0b013e318223e2bd [DOI] [PubMed] [Google Scholar]
- Grunert M., Dorn C., Schueler M., Dunkel I., Schlesinger J., Mebus S., Alexi-Meskishvili V., Perrot A., Wassilew K., Timmermann B. et al. (2014). Rare and private variations in neural crest, apoptosis and sarcomere genes define the polygenic background of isolated Tetralogy of Fallot. Hum. Mol. Genet. 23, 3115-3128. 10.1093/hmg/ddu021 [DOI] [PubMed] [Google Scholar]
- Grunert M., Dorn C., Cui H. and Dunkel I. (2016). Comparative DNA methylation and gene expression analysis identifies novel genes for structural congenital heart diseases. Cardiovasc. Res. 112, 464-477. 10.1093/cvr/cvw195 [DOI] [PubMed] [Google Scholar]
- Grunert M., Appelt S., Schönhals S., Mika K., Cui H., Cooper A., Cyganek L., Guan K. and Sperling S. R. (2020). Induced pluripotent stem cells of patients with Tetralogy of Fallot reveal transcriptional alterations in cardiomyocyte differentiation. Sci. Rep. 10, 10921 10.1038/s41598-020-67872-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J., Frese K. S., Peil B., Kloos W., Keller A., Nietsch R., Feng Z., Müller S., Kayvanpour E., Vogel B. et al. (2015). Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 36, 1123-135a. 10.1093/eurheartj/ehu301 [DOI] [PubMed] [Google Scholar]
- Hamdani N., Kooij V., van Dijk S., Merkus D., Paulus W. J., dos Remedios C., Duncker D. J., Stienen G. J. M. and van der Velden J. (2007). Sarcomeric dysfunction in heart failure. Cardiovasc. Res. 77, 649-658. 10.1093/cvr/cvm079 [DOI] [PubMed] [Google Scholar]
- Han Z. and Olson E. N. (2005). Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132, 3525-3536. 10.1242/dev.01899 [DOI] [PubMed] [Google Scholar]
- Hershberger R. E., Hedges D. J. and Morales A. (2013). Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 10, 531-547. 10.1038/nrcardio.2013.105 [DOI] [PubMed] [Google Scholar]
- Hornemann T., Kempa S., Himmel M., Hayess K., Fürst D. O. and Wallimann T. (2003). Muscle-type creatine kinase interacts with central domains of the M-band proteins myomesin and M-protein. J. Mol. Biol. 332, 877-887. 10.1016/S0022-2836(03)00921-5 [DOI] [PubMed] [Google Scholar]
- Hu Y., Flockhart I., Vinayagam A., Bergwitz C., Berger B., Perrimon N. and Mohr S. E. (2011). An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12, 357 10.1186/1471-2105-12-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.-Y. R., Ackermann M. A. and Kontrogianni-Konstantopoulos A. (2015). The sarcomeric M-region: a molecular command center for diverse cellular processes. Biomed. Res. Int. 2015, 714197 10.1155/2015/714197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles J., Burns C., Bagnall R. D., Lam L., Yeates L., Sarina T., Puranik R., Briffa T., Atherton J. J., Driscoll T. et al. (2017). Nonfamilial hypertrophic cardiomyopathy: prevalence, natural history, and clinical implications. Circ. Cardiovasc. Genet. 2, e001620. 10.1161/CIRCGENETICS.116.001620 [DOI] [PubMed] [Google Scholar]
- Jin S. C., Homsy J., Zaidi S., Lu Q., Morton S., DePalma S. R., Zeng X., Qi H., Chang W., Sierant M. C. et al. (2017). Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat. Genet. 49, 1593-1601. 10.1038/ng.3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. M., Krishnaswamy A., Smedira N. G., Desai M. Y., Tuzcu E. M. and Kapadia S. R. (2017). How symptomatic should a hypertrophic obstructive cardiomyopathy patient be to consider alcohol septal ablation? J. Am. Heart Assoc. 6, e006292. 10.1161/JAHA.117.006292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabaeva Z. T., Perrot A., Wolter B., Dietz R., Cardim N., Correia J. M., Schulte H. D., Aldashev A. A., Mirrakhimov M. M. and Osterziel K. J. (2002). Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 10, 741-748. 10.1038/sj.ejhg.5200872 [DOI] [PubMed] [Google Scholar]
- Karcewski K. J., Francioli L. G., Tiao G., Cummings B. B., Aföldi J., Wang Q., Collins R. L., Laricchia K. M., Ganna A., Birnbaum D. P. et al. (2020). The mutational constraint spectrum quantified from variation in 141,456 humans. Nature581, 434-443. 10.1038/s41586-020-2308-7 [DOI]
- Khairy P., Aboulhosn J., Gurvitz M. Z., Opotowsky A. R., Mongeon F.-P., Kay J., Valente A. M., Earing M. G., Lui G., Gersony D. R. et al. (2010). Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation 122, 868-875. 10.1161/CIRCULATIONAHA.109.928481 [DOI] [PubMed] [Google Scholar]
- Kiełbasa S. M., Wan R., Sato K., Horton P. and Frith M. C. (2011). Adaptive seeds tame genomic sequence comparison. Genome Res. 21, 487-493. 10.1101/gr.113985.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen M. P., Peters C. J., Zhou S., Williams H. H., Jan L. Y. and Jan Y. N. (2017). Age-dependent diastolic heart failure in an in vivo Drosophila model. eLife 6, e20851 10.7554/eLife.20851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S. W., Hu Y. W., Ho J. W. K., Ikeda S., Polster S., John R., Hall J. L., Bisping E., Pieske B., dos Remedios C. G.. et al. (2010). Heart failure–associated changes in RNA splicing of sarcomere genes. Circ. Cardiovasc. Genet. 3, 138-146. 10.1161/CIRCGENETICS.109.904698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostareva A., Kiselev A., Gudkova A., Frishman G., Ruepp A., Frishman D., Smolina N., Tarnovskaya S., Nilsson D., Zlotina A. et al. (2016). Genetic spectrum of idiopathic restrictive cardiomyopathy uncovered by next-generation sequencing. PLoS ONE 11, e0163362 10.1371/journal.pone.0163362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft T., Witjas-Paalberends E. R., Boontje N. M., Tripathi S., Brandis A., Montag J., Hodgkinson J. L., Francino A., Navarro-Lopez F., Brenner B. et al. (2013). Familial hypertrophic cardiomyopathy: functional effects of myosin mutation R723G in cardiomyocytes. J. Mol. Cell. Cardiol. 57, 13-22. 10.1016/j.yjmcc.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Kronert W. A., Bell K. M., Viswanathan M. C., Melkani G. C., Trujillo A. S., Huang A., Melkani A., Cammarato A., Swank D. M. and Bernstein S. I. (2018). Prolonged cross-bridge binding triggers muscle dysfunction in a Drosophila model of myosin-based hypertrophic cardiomyopathy. eLife 7, e38064 10.7554/eLife.38064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Gimeno J. R., Bahl A., Steffensen U., Steffensen M., Osman E., Thaman R., Mogensen J., Elliott P. M., Doi Y. et al. (2007). Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J. Am. Coll. Cardiol. 49, 2419-2426. 10.1016/j.jacc.2007.02.061 [DOI] [PubMed] [Google Scholar]
- Lange S., Pinotsis N., Agarkova I. and Ehler E. (2020). The M-band: the underestimated part of the sarcomere. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118440 10.1016/j.bbamcr.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M., Karczewski K. J., Minikel E. V., Samocha K. E., Banks E., Fennell T., O'Donnell-Luria A. H., Ware J. S., Hill A. J., Cummings B. B. et al. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285-291. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford N. J., Bilgir C., Ro J. and Pletcher S. D. (2013). Measurement of lifespan in Drosophila melanogaster. J. Vis. Exp. 71, 50068. 10.3791/50068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes L. R., Zekavati A., Syrris P., Hubank M., Giambartolomei C., Dalageorgou C., Jenkins S., McKenna W., Plagnol V. and Elliott P. M. (2013). Genetic complexity in hypertrophic cardiomyopathy revealed by high-throughput sequencing. J. Med. Genet. 50, 228-239. 10.1136/jmedgenet-2012-101270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron B. J. and Maron M. S. (2013). Hypertrophic cardiomyopathy. Lancet 381, 242-255. 10.1016/S0140-6736(12)60397-3 [DOI] [PubMed] [Google Scholar]
- Maron M. S., Olivotto I., Zenovich A. G., Link M. S., Pandian N. G., Kuvin J. T., Nistri S., Cecchi F., Udelson J. E. and Maron B. J. (2006). Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 114, 2232-2239. 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- Marston S., Montgiraud C., Munster A. B., Copeland O., Choi O., dos Remedios C., Messer A. E., Ehler E. and Knöll R. (2015). OBSCN mutations associated with dilated cardiomyopathy and haploinsufficiency. PLoS ONE 10, e0138568 10.1371/journal.pone.0138568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J., Perrot A., Andersen P. S., Havndrup O., Klausen I. C., Christiansen M., Bross P., Egeblad H., Bundgaard H., Osterziel K. J. et al. (2004). Clinical and genetic characteristics of alpha cardiac actin gene mutations in hypertrophic cardiomyopathy. J. Med. Genet. 41, e10 10.1136/jmg.2003.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller D. V., Andersen P. S., Hedley P., Ersbøll M. K., Bundgaard H., Moolman-Smook J., Christiansen M. and Køber L. (2009). The role of sarcomere gene mutations in patients with idiopathic dilated cardiomyopathy. Eur. J. Hum. Genet. 17, 1241-1249. 10.1038/ejhg.2009.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag J., Kowalski K., Makul M., Ernstberger P., Radocaj A., Beck J., Becker E., Tripathi S., Keyser B., Mühlfeld C. et al. (2018). Burst-like transcription of mutant and wildtype MYH7-alleles as possible origin of cell-to-cell contractile imbalance in hypertrophic cardiomyopathy. Front. Physiol. 9, 359 10.3389/fphys.2018.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann W. M. J., Gautel M., Weber K. and Fürst D. O. (1997). Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 16, 211-220. 10.1093/emboj/16.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann W. M., van der Ven P. F., Steiner F., Weber K. and Fürst D. O. (1998). Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Mol. Biol. Cell 9, 829-840. 10.1091/mbc.9.4.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K., Fink M., Cammarato A., Bernstein S. and Bodmer R. (2009). Semi-automated Optical Heartbeat Analysis of small hearts. J. Vis. Exp. 31, 1435. 10.3791/1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K., Vogler G. and Bodmer R. (2014). Methods to assess Drosophila heart development, function and aging. Methods 68, 265-272. 10.1016/j.ymeth.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. T. and Bernstein S. I. (1988). Molecular and ultrastructural defects in a Drosophila myosin heavy chain mutant: differential effects on muscle function produced by similar thick filament abnormalities. J. Cell Biol. 107, 2601-2612. 10.1083/jcb.107.6.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlivan D., Bayram Y., Gunes N., Akdemir Z. C., Shukla A., Bierhals T., Tabakci B., Sahin Y., Gezdirici A., Fatih J. M. et al. (2019). The genomics of arthrogryposis, a complex trait: candidate genes and further evidence for oligogenic inheritance. Am. J. Hum. Genet. 105, 132-150. 10.1016/j.ajhg.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., McCall K., Yang-Zhou D., Flockhart I., Binari R., Shim H.-S. et al. (2015). The transgenic RNAi Project at Harvard Medical School: resources and validation. Genetics 201, 843-852. 10.1534/genetics.115.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot A., Schmidt-Traub H., Hoffmann B., Prager M., Bit-Avragim N., Rudenko R. I., Usupbaeva D. A., Kabaeva Z., Imanov B., Mirrakhimov M. M. et al. (2005). Prevalence of cardiac beta-myosin heavy chain gene mutations in patients with hypertrophic cardiomyopathy. J. Mol. Med. (Berl.) 83, 468-477. 10.1007/s00109-005-0635-7 [DOI] [PubMed] [Google Scholar]
- Perrot A., Posch M. G. and Osterziel K. J. (2006). Deletion of Glu at codon 13 in the TCAP gene encoding the Z-disc protein titin-cap/telethonin is a rare non-synonymous polymorphism. Mol. Genet. Metab. 88, 199-200. 10.1016/j.ymgme.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett F. B. and Meeks-Wagner D. R. (1995). Seeing double: appreciating genetic redundancy. Plant Cell 7, 1347-1356. 10.1105/tpc.7.9.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch M. G., Thiemann L., Tomasov P., Veselka J., Cardim N., Garcia-Castro M., Coto E., Perrot A., Geier C., Dietz R. et al. (2008). Sequence analysis of myozenin 2 in 438 European patients with familial hypertrophic cardiomyopathy. Med. Sci. Monit. 14, CR372-CR374. [PubMed] [Google Scholar]
- Postma A. V., van Engelen K., van de Meerakker J., Rahman T., Probst S., Baars M. J. H., Bauer U., Pickardt T., Sperling S. R., Berger F. et al. (2011). Mutations in the sarcomere gene MYH7 in Ebstein anomaly. Circ. Cardiovasc. Genet. 4, 43-50. 10.1161/CIRCGENETICS.110.957985 [DOI] [PubMed] [Google Scholar]
- Qian L. and Bodmer R. (2009). Partial loss of GATA factor Pannier impairs adult heart function in Drosophila. Hum. Mol. Genet. 18, 3153-3163. 10.1093/hmg/ddp254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Mohapatra B., Akasaka T., Liu J., Ocorr K., Towbin J. A. and Bodmer R. (2008). Transcription factor neuromancer/TBX20 is required for cardiac function in Drosophila with implications for human heart disease. Proc. Natl. Acad. Sci. USA 105, 19833-19838. 10.1073/pnas.0808705105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganayakulu G., Schulz R. A. and Olson E. N. (1996). Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev. Biol. 176, 143-148. 10.1006/dbio.1996.9987 [DOI] [PubMed] [Google Scholar]
- Rickert-Sperling S., Kelly R. and Driscoll D. (2016). Congenital Heart Diseases: The Broken Heart. Clinical Features, Human Genetics and Molecular Pathways. Springer. [Google Scholar]
- Schoenauer R., Bertoncini P., Machaidze G., Aebi U., Perriard J.-C., Hegner M. and Agarkova I. (2005). Myomesin is a molecular spring with adaptable elasticity. J. Mol. Biol. 349, 367-379. 10.1016/j.jmb.2005.03.055 [DOI] [PubMed] [Google Scholar]
- Schoenauer R., Emmert M. Y., Felley A., Ehler E., Brokopp C., Weber B., Nemir M., Faggian G. G., Pedrazzini T., Falk V. et al. (2011). EH-myomesin splice isoform is a novel marker for dilated cardiomyopathy. Basic Res. Cardiol. 106, 233-247. 10.1007/s00395-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. M., Rödelsperger C., Schuelke M. and Seelow D. (2010). MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575-576. 10.1038/nmeth0810-575 [DOI] [PubMed] [Google Scholar]
- Shanks G. W., Tester D. J., Nishtala S., Evans J. M. and Ackerman M. J. (2017). Genomic triangulation and coverage analysis in whole-exome sequencing-based molecular autopsies. Circ. Cardiovasc. Genet. 10, e001828. 10.1161/CIRCGENETICS.117.001828 [DOI] [PubMed] [Google Scholar]
- Siegert R., Perrot A., Keller S., Behlke J., Michalewska-Włudarczyk A., Wycisk A., Tendera M., Morano I. and Özcelik C. (2011). A myomesin mutation associated with hypertrophic cardiomyopathy deteriorates dimerisation properties. Biochem. Biophys. Res. Commun. 405, 473-479. 10.1016/j.bbrc.2011.01.056 [DOI] [PubMed] [Google Scholar]
- Taghli-Lamallem O., Plantié E. and Jagla K. (2016). Drosophila in the heart of understanding cardiac diseases: modeling channelopathies and cardiomyopathies in the fruitfly. J. Cardiovasc. Dev. Dis. 3, 7 10.3390/jcdd3010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde D., Konings E. E. M., Slager M. A., Witsenburg M., Helbing W. A., Takkenberg J. J. M. and Roos-Hesselink J. W. (2011). Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 58, 2241-2247. 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- van der Velden J., Papp Z., Zaremba R., Boontje N. M., de Jong J. W., Owen V. J., Burton P. B. J., Goldmann P., Jaquet K. and Stienen G. J. M. (2003). Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc. Res. 57, 37-47. 10.1016/S0008-6363(02)00606-5 [DOI] [PubMed] [Google Scholar]
- Vaser R., Adusumalli S., Leng S. N., Sikic M. and Ng P. C. (2016). SIFT missense predictions for genomes. Nature 11, 1-9. 10.1038/nprot.2015.123 [DOI] [PubMed] [Google Scholar]
- Vermeer A., Wilde A. and Christiaans I. (2016). Human genetics of cardiomyopathies. In Congenital Heart Diseases: The Broken Heart (ed. Rickert-Sperling S., Kelly R. and Driscoll D.), pp. 675-686. Springer. [Google Scholar]
- Villard E., Duboscq-Bidot L., Charron P., Benaiche A., Conraads V., Sylvius N. and Komajda M. (2005). Mutation screening in dilated cardiomyopathy: prominent role of the beta myosin heavy chain gene. Eur. Heart J. 26, 794-803. 10.1093/eurheartj/ehi193 [DOI] [PubMed] [Google Scholar]
- Vogler G. and Ocorr K. (2009). Visualizing the beating heart in Drosophila. J. Vis. Exp. 65, 1425 10.3791/1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler G., Bodmer R. and Akasaka T. (2009). A Drosophila Model for Congenital Heart Disease. Elsevier. [Google Scholar]
- Walsh R., Buchan R., Wilk A., John S., Felkin L. E., Thomson K. L., Chiaw T. H., Loong C. C. W., Pua C. J., Raphael C. et al. (2017). Defining the genetic architecture of hypertrophic cardiomyopathy: re-evaluating the role of non-sarcomeric genes. Eur. Heart J. 38, 3461-3468. 10.1093/eurheartj/ehw603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warde-Farley D., Donaldson S. L., Comes O., Zuberi K., Badrawi R., Chao P., Franz M., Grouios C., Kazi F., Lopes C. T. et al. (2010). The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38, W214-W220. 10.1093/nar/gkq537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware S. M., Quinn M. E., Ballard E. T., Miller E., Uzark K. and Spicer R. L. (2008). Pediatric restrictive cardiomyopathy associated with a mutation in beta-myosin heavy chain. Clin. Genet. 73, 165-170. 10.1111/j.1399-0004.2007.00939.x [DOI] [PubMed] [Google Scholar]
- Wells L., Edwards K. A. and Bernstein S. I. (1996). Myosin heavy chain isoforms regulate muscle function but not myofibril assembly. EMBO J. 15, 4454-4459. 10.1002/j.1460-2075.1996.tb00822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. J., Amrein H., Izatt J. A., Choma M. A., Reedy M. C. and Rockman H. A. (2006). Drosophila as a model for the identification of genes causing adult human heart disease. Proc. Natl. Acad. Sci. USA 103, 1394-1399. 10.1073/pnas.0507359103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.-H., Lu C.-W., Chen H.-C., Chiu S.-N., Kao F.-Y. and Huang S.-K. (2014). Arrhythmic burdens in patients with tetralogy of Fallot: a national database study. Heart Rhythm 12, 604-609. 10.1016/j.hrthm.2014.11.026 [DOI] [PubMed] [Google Scholar]
- Xu J., Gao J., Li J., Xue L., Clark K. J., Ekker S. C. and Du S. J. (2012). Functional analysis of slow myosin heavy chain 1 and myomesin-3 in sarcomere organization in zebrafish embryonic slow muscles. J. Genet. Genomics 39, 69-80. 10.1016/j.jgg.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. L. and Liu J. C. (2014). Role of Notch signaling in the mammalian heart. Braz. J. Med. Biol. Res. 47, 1-10. 10.1590/1414-431X20133177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.