Abstract
Introduction:
Although the literature supports the importance of physical activity in the oncological context, in Italy a large number of patients are not sufficiently active.
Methods:
The present study aimed to explore factors influencing an active lifestyle in cancer patients during oncological treatments. Semi-structured focus groups, including 18 patients with different cancer types, were conducted at the Oncology Unit in the University Hospital Trust of Verona (Italy). The interviews were audio-recorded, transcribed verbatim, and analyzed with content analysis.
Results:
According to the Health Belief Model, transcripts were categorized into the following themes: benefits, barriers, and cues to action. Patients reported a series of physical, physiological, and psychological benefits deriving from an active lifestyle. The main barriers hampering the physical activity participation were represented by treatment-related side effects, advanced disease, and some medical procedures, for example, ileostomy. Several strategies that can trigger patients to exercise were identified. Medical advice, social support from family and friends, features such as enjoyment, setting goals, and owning an animal can motivate patients to perform physical activity. At the same time, an individualized program based on patients’ characteristics, an available physical activity specialist to consult, more detailed information regarding physical activity in the oncological setting, and having accessible structures were found important facilitators to implementing active behavior.
Conclusions:
Overall, patients have a positive view regarding physical activity, and a variety of obstacles and cues to action were recognized. Considering this information may help to improve adherence to a physical activity program over time, consequently increasing the expected benefits.
Keywords: physical activity, cancer, clinical setting, barriers, motivations, facilitators, attitude, behavior
Introduction
Cancer is the second leading cause of mortality in Italy,1 and it is expected an increase in cases and deaths between 2018 and 2040, by 22% and 35%, respectively.2 Oncological patients usually receive several integrated and multimodal therapies that may damage patients’ bodily integrity, harming their quality of life.3 The cancer treatment-related side effects are usually cumulative and consequently may entail a progressive impairment of both the physical and psychological status in patients.4
Physical activity has emerged as an important complementary supportive care for cancer patients.3 Evidence suggests a positive outcome in terms of reduction of recurrence and mortality risks5 and amelioration of several treatment-related side effects, such as nausea, vomiting, peripheral neuropathy, fatigue, arthralgia, or myalgia.6 From a physical and psychological point of view, physical activity is associated with an increase in cardiorespiratory fitness,7 muscular strength,8 and an improvement in body composition,8 quality of life,9 anxiety,3 and depression levels.3
Despite the benefits, in Italy, only 7% of cancer patients appears to be sufficiently active.10
Numerous aspects can interfere or contrarywise facilitate patients to adopt an active lifestyle during cancer treatments. Some of these are in common with the general population (eg, weather, lack of time), whereas others are strictly related to the disease history.11 Also the attitude, that is, the perception of a behavior as positive or negative, can affect the intention and the maintenance of physical activity performance, also in the oncological context.12 Developing a physical activity program that considers these features may increase the compliance and the maintenance over time of an active lifestyle by the patients. Unfortunately, in Italy, the literature on physical activity and cancer is still limited, with no clear data regarding the adherence to a physical activity program.13 Although several investigations have explored attitude, barriers, motivators, and facilitators able to influence the adoption of physical activity in oncological settings,14-24 no data on Italian patients are available. In order to fill this gap and to successfully design a future interventional study, we have qualitatively explored those factors influencing the adoption of an active lifestyle in cancer patients visiting the outpatients’ facilities at the Oncology Unit in Verona Hospital.
Methods
Design
This study applied a qualitative approach, through a series of focus groups, to assess the factors influencing physical activity behavior in the cancer setting. This study applied a qualitative approach, through a series of focus groups, to assess the factors influencing physical activity behavior in the cancer setting. The theoretical framework driving the investigation was the Health Belief Model, postulating that health-related practice, especially related to disease conditions, is influenced by several factors, including susceptibility and seriousness of the disease, perceived benefits, and barriers towards a behavior, cues to action, and self-efficacy.25 Because this study was related to health promoting factors, rather than to perceptions of severity or vulnerability to peculiar conditions, it focused on patients’ perceptions of the benefits of physical activity, barriers to engagement, and cues to action that would promote physical activity.
All the study procedures were conducted in compliance with Helsinki and Oviedo declaration, and the protocol adhered to Good Clinical Practice principles and Italian legislation. The project was reviewed and approved by the local Ethics Committee for Clinical Trials (Prot. N. 67002), University of Verona. It was carried out following the Standards for Reporting Qualitative Research (SRQR) guidelines for qualitative research.26
Participants and Recruitment
We recruited participants that met the following criteria: (i) were ≥18 years of age, (ii) had a confirmed cancer diagnosis, (iii) were currently patients at the outpatient Department of Oncology in the University Hospital Trust of Verona (Italy), (iv) spoke fluently Italian, and (v) signed the informed consent. No exclusion criteria were applied. A purposive sample strategy was used to recruit patients. The participants were identified by the dedicated psycho-oncologist, DT, working at the Oncology Department of University Hospital Trust of Verona. With a face to face approach, the psycho-oncologist introduced the study to the patients, and if they agreed to participate, the interviews were organized. Written informed consent was obtained from included patients the day of the interviews before starting the focus group. Pseudonyms were used to report the data to protect the participants’ identities.
Data Collection
To explore the factors that influence the physical activity behavior in cancer setting, a flexible semi-structured interview guide, based on the Health Belief Model,25 was developed by AA, DT, and ML. Between September 2019 and February 2020, 5 focus groups (n = 4, 4, 4, 3, and 3) were held in a meeting room at the Oncology Department in University Hospital Trust of Verona. Each discussion lasted up approximately 70 minutes, was audio-recorded and transcribed verbatim. The last author (ML) moderated the discussions; meanwhile, AA and LR observed and assisted. ML is an Associate Professor in Sports Science and Methodology at Verona University with expertise in physical activity and health promotion. AA is a PhD student involved in exercise in oncological patients, with previous interview experiences, and LR is a master’s degree student in preventive and adapted physical activity. The sample size was established using the data saturation principle, that is, data collection continued until no new information seemed to emerge from the discussions. At the end of each focus group, participants completed a questionnaire to provide information about their socioeconomic and demographic data (eg, birth date, education level, perceived economic insecurity, marital status, and occupational status). Patients’ medical history was obtained from medical charts.
Analysis
The data were analyzed with the Atlas.tiTM software, using the content analysis.27 This process comprised some essential steps and involved AA, LR, and ML, who independently examined the transcripts. The investigators read and re-read the entire text several times to get a general idea of the discussion and identify the salient concepts. Subsequently, the analysis process consisted of inductively coding the features relevant to the research questions. Then, the codes were grouped into categories and organized in themes.27
Finally, all 3 authors reviewed the analysis in a process called triangulation, which consisted in comparing the codes, the categories, arriving to a consensus on final themes.27
Results
Participants and Demographics
A total of 36 patients were screened for eligibility. During the recruitment 18 patients declined to participate, due to: unavailability in the proposed days (n = 11), worsening in health conditions (n = 5), and lack of interest (n = 2). Five focus groups with a total of 18 participants, after the discussion among researchers, permitted us to achieve data saturation.
Demographic and medical variables are reported in Table 1. The mean age of the participants was 55.2 ± 10.3 years, 16 were married, 13 had at least higher education, up to age 18-19. All participants were on active treatment; the most frequent cancer sites were upper gastro-intestinal (n = 6) and breast (n = 6), with a mean time from diagnosis of 25.7 months. The data were analyzed according to the Health Belief Model (Figure 1), and the following common themes were categorized: (1) benefits, (2) barriers, and (3) cues to action.
Table 1.
Participants’ Characteristics.
| Variable | |
|---|---|
| Age,a mean (SD) | 55.2 (10.3) |
| Body mass index,b mean (SD) | 23.7 (3.0) |
| Education, N | |
| Secondary (upto 14 years) | 5 |
| Secondary (upto 18-19 years) | 8 |
| College/University | 4 |
| Postgraduate | 1 |
| Marital status, N | |
| Single | 1 |
| Married | 16 |
| Divorced | 1 |
| Employment, N | |
| Part-time employed | 4 |
| Full time employed | 9 |
| Retired | 4 |
| Unemployed | 1 |
| Family income,c N | |
| Barely adequate | 8 |
| Adequate | 9 |
| More than adequate | 1 |
| Met physical activity guidelines (90+ min/week), N | |
| Yes | 10 |
| No | 8 |
| Tumor site, N | |
| Colorectal | 1 |
| Pancreas | 6 |
| Ovary | 1 |
| Lung | 1 |
| Breast | 6 |
| Head and neck | 1 |
| Melanoma | 1 |
| Thymus | 1 |
| Stage, N | |
| I | 1 |
| II | 3 |
| III | 4 |
| IV | 10 |
| Months from diagnosis, mean (SD) | 25.7 (16.5) |
| Treatment, N | |
| Surgery | 10 |
| Chemotherapy | 17 |
| Radiation therapy | 8 |
| Hormone therapy | 5 |
| Immunotherapy | 3 |
| Target therapy | 3 |
| Current treatment status, N | |
| Incoming | 0 |
| Ongoing | 18 |
| Ended | 0 |
| Comorbidity, N | |
| Yes | 10 |
| No | 8 |
Abbreviations: SD, standard deviation; N, number.
Expressed in years.
Expressed in units of kg/m2.
Perceived economic insecurity assessed by the question: Does your monthly income cover your monthly expenditure?
Figure 1.
Health Belief Model theoretical framework applied to physical activity in cancer patients.
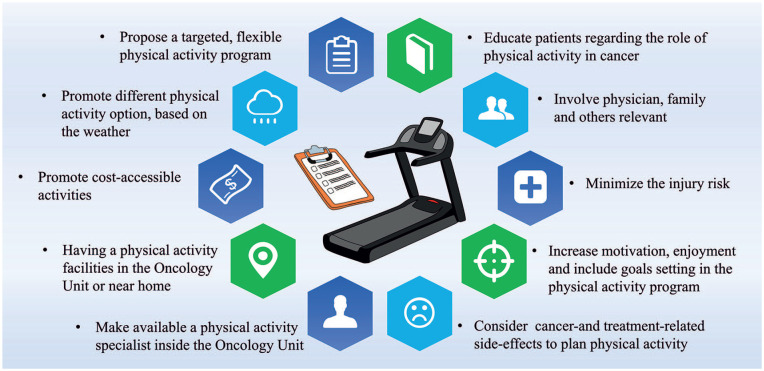
According to these findings, a series of recommendations to promote a successful physical activity program in this population were proposed (Figure 2).
Figure 2.
Strategies to implement a physical activity program in the oncological clinical setting.
Theme 1: Benefits
This theme reflected the belief of the patients regarding physical activity and was grouped into two main categories: physical and physiological benefits and socio-psychological benefits.
Physical and physiological benefits
All patients strongly expressed that physical activity is beneficial for overall well-being. The positive effects of being physically active were related to physical aspects, as Arianna (breast) told: “Physical activity has a beneficial impact on my body, also during the chemotherapy treatment: I have more strength, I can maintain my muscle mass and I am in a good mood.” Some subjects also recognized the impact of physical activity to counteract treatments related side-effects: “I suffered from constipation due to cancer therapies, and walking has been very beneficial for me against this disorder” (Tiziana, breast) or “I usually go running before my chemotherapy, and I do not experience any treatment-related side effects, while, if I do not, I feel exhausted, I have nausea and vomiting” (Matteo, pancreas). Finally, a patient underlined how to be active can help to fight cancer: “Physical activity keeps the estrogens at low levels. My cancer eats estrogens, so if I do physical activity, I starve my tumor” (Tiziana, breast).
Socio-psychological benefits
Participants identified several advantages related to psychological aspects: “When you perform physical activity, endorphins are released. This mechanism can help to fight the depression. . .and consequently to have a different attitude in approaching cancer” (Maria, pancreas). Physical activity was described as a “natural recharge,” “(Physical activity) makes you feel good, especially from the mental point of view. . .you feel charged and ready to face everything” (Martina, breast), and, as a “day organizer,” “(Physical activity) can really help you to plan your daytime, and even if something unexpected happens, you are able to manage it” (Salvatore, pancreas). Moreover, patients expressed that physical activity was a vehicle to increase their perception of control, in the cancer context, as Martina (breast) said, “when you suffer from cancer, you rely on the oncologists, surgeons, nurses, etc.; physical activity is something that you decide to do! You can take the reins of the situation and control it without being at the mercy of the events.” The subjects also recognized how to be physically active can help them to face the cancer disease also in the family context: “When I go walking with my husband, we talk about everything, we plan, and we organize our next steps; physical activity has truly reunited us, despite my cancer” (Maria, pancreas).
Theme 2: Barriers
Many factors were individuated as potential barriers, which can hinder physical activity. Obstacles were grouped into three categories: lack of motivation, disease condition, medical treatments and risk of injury, and weather, expenses, lack of time, and information.
Lack of motivation
Lack of motivation, emerging as self-described “laziness,” was the central concept reported about less than half of participants. “I am the perfect example of laziness. I do not like physical activity even if I recognize its importance,” said Luca (thymus), or “Physical activity is secondary for me. . .my head tells me that it is important, but I am lazy” (Elisa, breast). However, some of them recognized their predisposition and consequently tried to react positively, as Teresa (colorectal) expressed: “I am lazy, and I know it. Thus, I put myself in situations that force me to be physically active.”
Disease condition, medical treatments and risk of injury
Health conditions and cancer treatment-related side effects were identified as the major barriers to physical activity. Chemotherapy side-effects can impact on the ability to perform a regular physical activity, as Martina (breast) said “When you are undergoing cancer treatments, sometimes you are not able to get up from the sofa; neither with your willpower, you can!”, or Debora (pancreas) explained, “This chemotherapy malaise, expressed especially with fatigue, leads you to do less physical activity.” The presence of bone metastasis was identified a significant limitation to exercising, as Elisa (breast) expressed, “I have bone metastasis in the spine, and I know that I must avoid loading in that zone” or Michele (lung) elucidated, “I love cycling, but I cannot perform it because I have hip bone metastasis. I am on fracture risk, and I am afraid to get injured.” Patients indicated some medical procedures that interfere with their physical activity. The presence of ileostomy was emerged as an obstacle, seriously limiting the daily physical activity: “I have an ileostomy, which has generated a hernia; before I was able to walk for 10 kilometers, but now I can make only one or one and a half. Furthermore, I cannot do some activities, such as swimming, because I have a bag in my belly” (Maria, pancreas). Also, the central venous catheter (CVC), can impair the perception to be able to perform physical activity: “I have a psychological block regard the presence of CVC. I know that I could do physical activity without realizing to have the CVC, but this is a huge psychological obstacle for me” (Luca, thymus). Some concerns emerged about the risk of injury: “When I go walking, I experience back pain sometimes” Elisa (breast), or “You could get hurt, like injure your knee” Martina (breast). During the discussions, excess physical activity as a potentially harmful factor was also mentioned: “Exaggeration could potentially be dangerous” Luca (thymus), or “One time I overdid, and the day after I was sick, I felt confused,” expressed Matteo (pancreas).
Weather, expenses, lack of time and information
Rain, seasonality, or more, in general, extreme weather, can interfere with physical activity: “Weather is an obstacle, because if the downpour falls you prefer to stay at home” (Michele, lung), or “I am in crisis during the winter season, due to cold and little sunlight” (Sergio, head and neck). On the contrary, also heat can impede the physical activity predisposition: “I do not like performing physical activity during the summer, it is too hot; I feel exhausted” (Alessandro, melanoma). A common factor identified by the patients was the lack of information regarding physical activity in the oncological setting: “It is hard to find suitable information regarding physical activity” (Martina, breast) or “I have an exercise bike at home, but I do not know if it is beneficial for me to load my hips or my knees; I do not have information about this” (Ennio, pancreas). Lack of time was an obstacle prevalently emerged in patients that were still working: “Between family and work, it is really difficult to find some time to dedicate in exercising” (Sergio, head and neck). Finally, one subject expressed some concerns about the cost related to physical activity: “I would like to try archery, but is too expensive, 500 euros for three lessons. It seems that some sports are only for a small elite group of people” (Luca, thymus).
Theme 3: Cues to Action
Six categories were used to group the stimuli that trigger the decision-making process to perform physical activity: counseling, social support, enjoyment, goals, and pets, targeted physical activity programs, an available specialist, supporting information, and organizational aspects.
Counseling and social support
Interpersonal factors were important to motive patients to start physical activity. On the one side, the initial advice of oncologists or other relevant figures was a considerable incentive to be physically active, as Alessandro (colorectum) mentioned, “Talking with doctors can really help you to start exercising,” or Daniela (breast) said “When I started my chemotherapy, my physical and psychological status were bad. After the advice of medical staff, I began walking, and I bought a cyclo-ergometer.” On the other side, another aspect regarded strong support from family and friends. “My son calls me 100 times per day to remind me to stay active. . .my strength derives from my sons because they tell me that I am a wonder woman; they give me so much energy” said Debora (pancreas) or “I appreciate that my husband come walking with me because I usually do not take many initiatives alone” specified Marta (ovary). About the friends’ support, Alessandro (melanoma) declared: “My friends stimulate me to restart cycling with them, they are a positive support for me!.”
Enjoyment, goals, and pets
Patients referred to be positively encouraged if they set goals, or a targeted objective, as Gigliola (breast) said, “During my cancer therapies I gained weight, so I decided to begin physical activity with the aim to improve my body composition. Since January, I have lost 10 kilograms!.” Some participants reported enjoyment as an incentive to perform physical activity: “I love walking; it is a positive activity! Maybe I could have started it before!” (Marta, ovary) or “Be physically active gives you great satisfaction. I like it” (Matteo, pancreas). A patient reported that having a pet can help to maintain an active lifestyle: “Having a dog helps you stay active because you must get it out every day, even when the weather is bad” (Alessandro, melanoma).
Targeted physical activity programs
A targeted, flexible program was identified as a key facilitator by the patients. Almost half of the patients reported that physical activity should be programmed at the beginning of cancer treatments, as Marta (ovary) explained: “. . .when you start chemotherapy, you do not think about physical activity. It should be programmed at the beginning, after diagnosis, and included in the therapeutic plan.” Different preferences have emerged regarding the modality to perform physical activity: “I want (exercising) alone” (Michele, lung) or “Due to work, family and other reason I would prefer training at home, but with monthly counseling with a physical activity specialist” Sergio (head and neck) or “I like to do physical activity in-group, with other patients” Salvatore (pancreas). All the participants agreed that a physical activity program should be targeted on the patient’s disease and the comorbidities: “The activity should be diversified according to the different type of disease; I have had breast surgery with axillary dissection, and some exercises may be better for me but not for those patients that experienced another type of cancer” Martina (breast) or “I have a herniated disc so the activity should be adequate also to my comorbidity” Salvatore (pancreas).
An available specialist
Several subjects have expressed the necessity to have a qualified specialist inside the Oncology Unit. Although the initial advice by medical staff can encourage subjects to start physical activity, some patients desired more specific instructions: “When I received the diagnosis, I asked the oncologists: what do I do? Should I perform physical activity? They usually answered me: do what you feel like doing. . .but this kind of response is not satisfying!” Salvatore (pancreas). From their perspectives, an available specialist could strongly help them to be more self-confident in performing physical activity: “Now the only activity that I perform is walking, because I am afraid of getting injured. I must be cautious due to my bone metastases. In my opinion, having a qualified specialist inside the hospital would give me more confidence” Arianna (breast) or “When you are diagnosed with cancer, you do not know whom to ask information. It is helpful to have in the same place oncologist, dietitian, psychologist, surgeon, etc., so even for the physical activity could be the same” Martina (breast).
Supporting information
Promote physical activity, through credible and suitable information, including the benefits and practical consideration to exercising, can facilitate the engagement: “Promoting the information regarding the benefits of physical activity, specifying the advantages for the body systems and your disease” Ennio (pancreas) and “I need information about how to perform physical activity because I am 60 years old and I do not think to begin to do an uphill race” Sergio (head and neck). Participants reported the importance of having specific information: “Maybe it would be necessary to give patients detailed, and more targeted information, based on the type of disease” Maria (pancreas) or “ The information should be addressed on the key points that specify the benefits of an active lifestyle related to disease because this can help to consider physical activity as real medicine.”
Organizational aspects
Suitable structures can facilitate an active lifestyle. Some patients would perform physical activity outdoor, as Daniela (breast) supported, “I prefer to do physical activity outside if the weather is good.” Others would desire a gym inside the hospital: “In each oncological unit there should be a gym, it is necessary, because it is important such as the chemotherapy, and could simplify the approach to physical activity” Debora (pancreas). Moreover, another important characteristic was the distance from the fitness center: “Having a gym near home is extremely important because if I have to drive, I will not go” Gigliola (breast).
Discussion
The present study explored factors that influence physical activity engagement in cancer patients suggests a series of recommendations to promote a successful physical activity program in this population (Figure 2).
Benefits
We found that patients generally have a positive perception of physical activity, reporting a variety of related benefits, from physical factors to those physiological and socio-psychological. These results are consistent with previous studies.16-18,20,24,28 Smith et al16 reported that patients described physical activity as positive behavior for general health, to manage some treatment-related side effects and other chronic conditions (eg, cardiovascular disease). Older breast cancer survivors mentioned that an active lifestyle help to reduce the stress levels and combat depression.17 At the same time, Mazzoni et al28 found that patients exercising during oncological treatments reported a feeling of self-control.
Barriers
Several barriers inhibiting cancer patients’ physical activity participation were identified. Some of them are related to cancer and its treatments. These results are comparable with previous investigations.15,16,19,21,23,29 A recent, qualitative meta-synthesis has confirmed that the most relevant obstacles of adopting an active lifestyle were linked to cancer- and treatment-related side effects.21 Fatigue is one of the most reported impediments which hinder the engagement in physical activity in this study, but also in other works.16,20,29 Nevertheless, a recent meta-analysis, including 113 studies with a total of 11 525 patients, showed that exercise was more effective than pharmaceutical interventions to ameliorate the level of cancer-related fatigue.30 To date, the role of physical activity to improve fatigue in cancer is well consolidated, so much so that also the recent ESMO clinical practice guidelines for cancer-related fatigue include exercise as an efficacious tool to manage this symptom.31 Thus, informing patients regarding the beneficial effect of physical activity on fatigue is crucial. Although similar reports noted safety issues, including the risk of injury,20,21,29 as features hindering physical activity, this appears the first investigation finding bone metastasis as a factor seriously limiting the engagement in physical activity. Exercise oncology in advanced cancer is rapidly increasing during recent years. Promising results, such as the safety aspects relating to fracture risk and the preliminary efficacy of exercise in patients with bone metastasis,32 were shown also for resistance training, an activity traditionally considered detrimental for fracture risk.33 Patients with bone metastasis expressed their will to be active, but also the impediment of not knowing which exercises are safe and whom to rely on to practice physical activity. In this regard, reassuring patients about safety and adapting activities to their ability should be considered. In line with prior studies,15,29 some procedures, as ileostomy and CVC were recognized as potential obstacles to exercising. Henriksson and colleagues found a similar concern about the peripherally inserted central catheter in patients during adjuvant treatments.15 One possible explanation could be that this type of procedure undermines patients’ confidence on their ability, thus raising doubts on the possibility of performing physical activity.Another related concept is the availability ofl information on physical activity. Patients referred to the lack of specific material and suggest that having targeted information may facilitate engagement. The lack of accessible and evidence-based information about physical activity in cancer is a persistent problem in the current literature. A recent study reviewed e websites in the English language that provide information on maintaining or improving physical activity after a cancer diagnosis.34 The results showed that the importance of physical activity is frequently mentioned, while the available information suffers from a lack of comprehensive, specific advice as well as detailed mentions on practice.34 Thus, in this work authors suggested that written or online health information, to be efficacious, should be high quality and accessible, evidence-based, should motivate and guide the engagement, outlining the risk and advising the seeking of professional advice.34
Although not surprising, the presence of obstacles related to external situations (eg, bad weather, expenses, etc.), consistently mentioned in previous works,23,35 the lack of motivation, self-described by patients as “laziness,” was not so common. In other investigations23,35 patients reported laziness as a feature hindering their willingness to exercising, suggesting that planning a physical activity intervention aimed also to appropriately build patient’s motivation could be an effective way to overcome this state of mind.
Cues to Action
Regarding cues to action, social support by family and friends motivated patients to stay active. However, the literature showed mixed results. On the one side, social support was a vehicle to increase the physical activity motivation16; on the other, an investigation found indifferent the relationship with the family and others.24 Focusing on counseling, patients reported medical advice as an important motivator to support an active lifestyle. A previous work found that patients preferred to receive initial information from their oncologists,10 and a randomized trial indicated that a 30-seconds oncologist recommendation was able to significantly increase exercise levels in breast cancer survivors attending a primary adjuvant treatment consultation,36 thus supporting the importance of the endorsement by the oncologist. However, the advice of medical staff can be the first step to managing behavior; in our study, having the opportunity to consult a physical activity specialist can encourage patients to receive a program adapted to their necessity and conditions and can consequently stimulate them to improve their lifestyle. This agrees with prior investigations,16,21 and, interestingly, also with the oncology care providers’ perspectives. Indeed, from the medical staff point of view, having a physical activity specialist as part of the clinical team and educating patients about physical activity are considered the optimal strategies to engage patients in exercising.37
Moreover, enjoyment and setting goals are also effective strategies to support patients and to increase adherence to a physical activity program and contrast lack of motivation. According to the present research, and also in the study of Mikkelsen and colleagues, patients with advanced cancer reported the importance of enjoyment, setting goals, and tracking progress as a strategy to increase motivation.20 Interestingly, our work found that having a pet can act as a motivator to promote physical activity. To our knowledge, no prior research found a similar result. Nevertheless, the use of pet therapy, that is, offer several activities, using an animal in order to maintain both mental and physical human health, is a broader topic, which can provide numerous benefits. Contact with pets may reduce loneliness, offering security, and giving encouragement. Moreover, a recent systematic review showed that animal-assisted interventions in oncology settings have a positive association with physiological and psychological outcomes, including oxygen saturation, quality of life, perceived satisfaction, and depression, and other negative mood states.38
We found heterogeneity, regarding the place to perfom physical activity. For some patients, the program should be delivered in the hospital, while for others in a gym near home. This was reported in other studies on preferences too,14,19 and one possible explanation could be that some patients, especially with advanced disease, may feel more confident in a hospital setting. Consistent with previous research,35 patients declared that physical activity programs should be tailored according to their cancer type and the presence of comorbidities. Moreover, different preferences were found regarding the modality to perform the physical activity; some patients preferred to exercise individually, others with a program to follow at home, while some choose a group-based program. This finding supports a survey-research, which found that 40% of cancer patients preferred a group-based physical activity program, 25% an individual program with a personal trainer, and 27% chose an individual program to follow at home.10 Overall, these results suggest that offering different programs, according to patients’ preferences and disease status, may optimize uptake and adherence.
Limitations
Our study had some limitations, including the low response rate, which may have introduced a selection bias, even if the main reasons to decline the participation in the study did not support this hypothesis. In our investigation, about half of the patients met the current exercise guidelines, while literature usually reports low physical activity levels in this population.10,39 Nevertheless, differently from previous investigations applying the prior guidelines version, this study has followed the recent American College of Sports Medicine guidelines for cancer patients, released in October 2019.3 Therefore, although we cannot exclude that our sample was positively biased towards physical activity, we assume that the obtained results may at least partially mirror the current situation in general cancer population. Moreover, in our study, patients had different cancer types, and consequently, the results may not be generalizable. Nevertheless, our research aimed to investigate the features that affect the engagement in physical activity in a “real world” context of the Oncology Unit. For this reason, we believe that including in the sample participants with different socioeconomic status, demographic characteristics, and various cancer types was a strength of our investigation.
Finally, in our sample, more than half of the participants were affected by an advanced stage disease. In this light, our findings may be partially biased, being more applicable to those patients dealing with more burdensome symptoms and treatment issues than those at earlier stages. From the other side, this population is usually understudied regarding physical activity predispositions and practice supporting the relevance of this data in providing a specific intervention potentially able to preserve their quality of life and autonomy.
Conclusion
In conclusion, physical activity has been demonstrated to represent an essential complementary tool in cancer patients. In order to develop a lifestyle intervention, exploring factors influencing physical activity behavior is particularly important, especially in Italy, where the literature on this field is scarce. Overall, we found that patients had positive beliefs, reporting a variety of benefits. The barriers were mainly oriented to the disease conditions and treatment-related side effects. Nevertheless, several strategies were identified to support and motivate cancer patients to start or maintain a physical activity program.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alice Avancini  https://orcid.org/0000-0003-2439-4799
https://orcid.org/0000-0003-2439-4799
References
- 1. AIOM-AIRTum. I numeri del cancro in Italia. IX ed Intermedia Editore; 2019. [Google Scholar]
- 2. IARC. Cancer Tomorrow. World Health Organization; 2020. [Google Scholar]
- 3. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel AV, Friedenreich CM, Moore SC, et al. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51:2391-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleckner IR, Dunne RF, Asare M, et al. Exercise for toxicity management in cancer-a narrative review. Oncol Hematol Rev. 2018;14:28-37. [PMC free article] [PubMed] [Google Scholar]
- 7. Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36:2297-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017;11:339-349. [DOI] [PubMed] [Google Scholar]
- 9. Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91-104. [DOI] [PubMed] [Google Scholar]
- 10. Avancini A, Pala V, Trestini I, et al. Exercise levels and preferences in cancer patients: a cross-sectional study. Int J Environ Res Public Health. 2020;17:5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granger CL, Connolly B, Denehy L, et al. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer. 2017;25:983-999. [DOI] [PubMed] [Google Scholar]
- 12. Jones LW, Guill B, Keir ST, et al. Using the theory of planned behavior to understand the determinants of exercise intention in patients diagnosed with primary brain cancer. Psychooncology. 2007;16:232-240. [DOI] [PubMed] [Google Scholar]
- 13. De Luca V, Minganti C, Borrione P, et al. Effects of concurrent aerobic and strength training on breast cancer survivors: a pilot study. Public Health. 2016;136:126-132. [DOI] [PubMed] [Google Scholar]
- 14. Blaney J, Lowe-Strong A, Rankin J, Campbell A, Allen J, Gracey J. The cancer rehabilitation journey: barriers to and facilitators of exercise among patients with cancer-related fatigue. Phys Ther. 2010;90:1135-1147. [DOI] [PubMed] [Google Scholar]
- 15. Henriksson A, Arving C, Johansson B, Igelström H, Nordin K. Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient Educ Couns. 2016;99:1220-1226. [DOI] [PubMed] [Google Scholar]
- 16. Smith L, Croker H, Fisher A, Williams K, Wardle J, Beeken RJ. Cancer survivors’ attitudes towards and knowledge of physical activity, sources of information, and barriers and facilitators of engagement: a qualitative study. Eur J Cancer Care (Engl). 2017;26:e12641. [DOI] [PubMed] [Google Scholar]
- 17. Owusu C, Antognoli E, Nock N, et al. Perspective of older African-American and Non-Hispanic white breast cancer survivors from diverse socioeconomic backgrounds toward physical activity: a qualitative study. J Geriatr Oncol. 2018;9:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avancini A, Skroce K, Tregnago D, et al. “Running with cancer”: a qualitative study to evaluate barriers and motivations in running for female oncological patients. PLoS One. 2020;15:e0227846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox L, Wiseman T, Cahill D, et al. Barriers and facilitators to physical activity in men with prostate cancer: a qualitative and quantitative systematic review. Psychooncology. 2019;28:2270-2285. [DOI] [PubMed] [Google Scholar]
- 20. Mikkelsen MK, Nielsen DL, Vinther A, Lund CM, Jarden M. Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment - a qualitative interview study. Eur J Oncol Nurs. 2019;41:16-23. [DOI] [PubMed] [Google Scholar]
- 21. Lavallée JF, Abdin S, Faulkner J, Husted M. Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: a qualitative metasynthesis. Psychooncology. 2019;28:468-476. [DOI] [PubMed] [Google Scholar]
- 22. Wurz A, St-Aubin A, Brunet J. Breast cancer survivors’ barriers and motives for participating in a group-based physical activity program offered in the community. Support Care Cancer. 2015;23:2407-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun V, Raz DJ, Kim JY, et al. Barriers and facilitators of adherence to a perioperative physical activity intervention for older adults with cancer and their family caregivers. J Geriatr Oncol. 2020;11:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheill G, Guinan E, Neill LO, Hevey D, Hussey J. The views of patients with metastatic prostate cancer towards physical activity: a qualitative exploration. Support Care Cancer. 2018;26:1747-1754. [DOI] [PubMed] [Google Scholar]
- 25. Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q. 1984;11:1-47. [DOI] [PubMed] [Google Scholar]
- 26. O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251. [DOI] [PubMed] [Google Scholar]
- 27. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazzoni AS, Carlsson M, Berntsen S, Nordin K, Demmelmaier I. “Finding my own motivation” - a mixed methods study of exercise and behaviour change support during oncological treatment. Int J Behav Med. 2019;26:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clifford BK, Mizrahi D, Sandler CX, et al. Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support Care Cancer. 2018;26:685-700. [DOI] [PubMed] [Google Scholar]
- 30. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. Published online March 12, 2020. doi: 10.1016/j.annonc.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 32. Sheill G, Guinan EM, Peat N, Hussey J. Considerations for exercise prescription in patients with bone metastases: a comprehensive narrative review. PM R. 2018;10:843-864. [DOI] [PubMed] [Google Scholar]
- 33. Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:328-335. [DOI] [PubMed] [Google Scholar]
- 34. Goodwin BC, Crawford-Williams F, Castro O, Rowe A, De Cocker K. Online physical activity and sedentary behaviour information for cancer survivors. J Cancer Surviv. 2020;14:677-688. [DOI] [PubMed] [Google Scholar]
- 35. Hardcastle SJ, Maxwell-Smith C, Kamarova S, Lamb S, Millar L, Cohen PA. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support Care Cancer. 2018;26:1289-1295. [DOI] [PubMed] [Google Scholar]
- 36. Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28:105-113. [DOI] [PubMed] [Google Scholar]
- 37. Nadler M, Bainbridge D, Tomasone J, Cheifetz O, Juergens RA, Sussman J. Oncology care provider perspectives on exercise promotion in people with cancer: an examination of knowledge, practices, barriers, and facilitators. Support Care Cancer. 2017;25:2297-2304. [DOI] [PubMed] [Google Scholar]
- 38. Holder TRN, Gruen ME, Roberts DL, Somers T, Bozkurt A. A systematic literature review of animal-assisted interventions in Oncology (Part I): methods and results. Integr Cancer Ther. 2020;19:1534735420943278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2018;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]