Abstract
Osteoarthritis (OA) is a degenerative joint disease characterized by loss of articular cartilage, inflammation and pain, which sometimes necessitates total joint arthroplasty (TJA). Profiling biomarkers of cartilage degradation and inflammation is a promising area of research to understand the pathogenesis of OA. This study aims to report the post-operative fluctuations of 3 biomarkers of OA, osteopontin (OPN), matrix metalloproteinase-9 (MMP-9), and ADAMTS4 (a disintegrin and metalloproteinase with thrombospondin motifs 4), in patients undergoing TJA to further define the interaction among these biomarkers and delineate their role in OA pathogenesis. OPN is an extracellular matrix (ECM) glycoprotein with increased activity in OA and joint damage and is upregulated by either inflammation or cleavage by MMPs and thrombin. MMP-9 is known to cleave OPN and is upregulated by inflammatory markers, such as IL-1, IL-6 and CRP. ADAMTS4 is an enzyme that degrades aggrecan, a major component of cartilage. These biomarkers were measured in deidentified blood samples collected on the day of surgery, 1 day post-operatively, and day 5-7 post-operatively. MMP-9 and OPN levels were significantly elevated at all times, and ADAMTS4 was significantly decreased at baseline versus controls. OPN and ADAMTS4 inversely fluctuated post-operatively, indicating an interrelation between these 2 biomarkers. This study suggests that the upregulation of MMP-9 and therefore OPN then results in the downregulation of ADAMTS4. The relationship between OPN and thrombin also highlights the importance of monitoring for thrombotic complications. These biomarkers, along with thrombin-mediated cleavage products, may be helpful in the prognostic management of OA patients.
Keywords: osteoarthritis, cartilage degradation, osteopontin, matrix metalloproteinase-9, a disintegrin and metalloproteinase with thrombospondin motifs 4
Background
Osteoarthritis (OA) is a degenerative joint disease characterized by loss of articular cartilage, inflammation and pain.1 It is estimated that OA affects 70% of the population aged 55-70 years old. Currently, treatment is limited to symptom relief or total joint arthroplasty (TJA), most commonly total knee arthroplasty (TKA) or total hip arthroplasty (THA).2 Degenerative processes in this syndrome are mediated through the activation of matrix degrading enzymes, heparanases and proteases, such as thrombin and matrix-metalloproteinases.3 Inflammation and its downstream effects are also contributing factors in the development of OA.1 Thus, cartilage remodeling and simultaneous activation of the hypercoagulable state in these patients results in the observed complications.
End stage OA is marked by inflammation, which directly effects levels of certain cartilage degradation biomarkers, such as matrix metalloproteinase-9 (MMP-9) and ADAMTS4 (a disintegrin and metalloproteinase with thrombospondin motifs 4).4 Osteopontin (OPN) is also a major player in regulation of both cartilage breakdown and the inflammatory cascade.5
OPN is a cytokine, hormone and an extracellular matrix (ECM) glycoprotein with many known functions, however it has been shown in previous studies to have increased mRNA expression in cartilage in individuals with OA and joint damage.5 This is thought to be due to an immune response that is initiated by inflammation.6 It is secreted by epithelial cells, smooth muscle cells and endothelial cells and can be increased in OA patients by several different mechanisms including the release from ECM, cartilage or bone, from inflammation or from cleavage by matrix metalloproteinases (MMPs), thrombin or heparanases.7 Specifically, MMP-2, 3, 7 and 9 are known to cleave OPN.5,8,9 OPN is primarily regulated by cleavage at various sites along the protein, which results in the exposure of different binding sites.9 Previous studies have shown that thrombin cleaves OPN at specific sites that are known to be involved in the pathogenesis of OA.10 This form of OPN is involved the inflammatory cascade and in many similar pathophysiologic conditions.10 Thrombin-cleaved OPN is known to play a substantial role in the pathogenesis of OA and is elevated in advanced stage OA.10 OPN cleaved by thrombin can be measured in the synovial fluid of OA patients and is a helpful marker for disease severity and progression.10 Additionally, this fragment has been postulated to play a role in the development of thrombotic complications in atherosclerotic plaques, providing a possible connection to the coagulopathy complications in OA patients as well.3,7,11 In inflammatory processes, OPN is known to be regulated by Syndecan-4, a sulfated glycosaminoglycan on cell surfaces. Syndecan-4 blocks various thrombin-cleaved sites on OPN, so it is not active and does not contribute to inflammation.12
MMP’s are a family of enzymes that are thought to be the primary actors that cause digestion of the articular cartilage in OA.13 They have a zinc-containing catalytic site that is used to cleave components of the ECM.14 MMP’s are well characterized in many previous studies and have been shown to destroy collagen, which is a major component of cartilage.13,14 The primary MMP’s that have been implicated in the pathogenesis of OA include MMP-1, -2, -3, -8, -9, -13, -14, and -16.14 MMP-9 is known to be elevated in cartilage of moderate to severe OA patients and is known to contribute to the loss of articular cartilage, leading to increased severity of OA.13,14 It is upregulated by pro-inflammatory markers, such as IL-1, IL-6 and CRP. Not only does MMP-9 degrade collagen, but also aggrecan, which is also a major component of cartilage.13 Notably, MMP-9 is known to cleave OPN at approximately 30 different sites.8
ADAMTS4, also known as aggrecanase-1, is an enzyme that degrades aggrecan, a major component of matrix cartilage molecules.15,16 While this enzyme has been shown to be elevated in early stages of OA, in the later stages it is decreased, labeling ADAMTS4 as a possible diagnostic biomarker of OA.17,18 Also, genes associated with ADAMTS4 participate in cartilage degradation and the pathogenesis of OA.18 OPN has been shown to decrease ADAMTS4 mRNA expression, acting to protect against cartilage degradation in these cases.15
It was hypothesized that in patients undergoing TJA due to severe OA, circulating levels of OPN and MMP-9 may be elevated and ADAMTS4 may be decreased compared with healthy controls. Measurement of these biomarkers can help elucidate the pathogenesis of OA and the immediate post-surgical changes that occur after TJA. Additionally, the circulating levels of these biomarkers and thrombotic biomarkers may be of prognostic value in risk stratification of these patients pre-operatively.
Materials and Methods
Deidentified venous blood was collected at 3 different draw dates (day 0, day 1 post-operatively, and day 5-7 post-operatively) from patients undergoing TJA due to OA (n = 63). The patients ages ranged from 47-87 years old with mean age 68 ± 10.8 years. The samples were collected in vacutainer tubes containing sodium citrate (0.109 M) and sent to the Hemostasis & Thrombosis Laboratory of Loyola University Medical Center for processing. Plasma was separated from blood via centrifugation at 1500xg for 20 minutes. Samples were frozen at -80°C and were thawed in batches for analysis. The samples were analyzed using enzyme-linked immunosorbent assay (ELISA) kits for OPN (R&D Systems, Minneapolis, MN), MMP-9 (R&D Systems, Minneapolis, MN), and ADAMTS4 (LifeSpan BioSciences, Inc., Seattle, WA). Reagents, standards, and sample solutions were thawed, diluted, and prepared according to ELISA manufacturer’s specifications. A set of 50 plasma specimens from healthy individuals were purchased from George King BioMedical (Overland Park, KS) to use as control samples. The protocol was approved by the Loyola University Chicago IRB and patients were properly consented before blood samples were drawn.
Statistical Analysis
Data was analyzed using GraphPad Prism Version 8.0 (GraphPad Software, San Diego, CA), and Microsoft Excel. The results were stated as mean ± standard error of the mean. The Kruskal-Wallis 1-way ANOVA was used to determine statistical significance. P values < 0.05 were considered statistically significant.
Results
There were significant differences in levels of MMP-9 at all time points in the patients who underwent TJA due to late stage OA compared with healthy controls (Figure 1). MMP-9 at day 0: (957 ± 107 ng/mL vs. 77.4 ± 7.75 ng/mL; P < 0.0001), day 1 post-operatively: (1507 ± 84.8 ng/mL vs. 77.4 ± 7.75 ng/mL; P < 0.0001), day 5-7 post-operatively: (1264 ± 78.8 ng/mL vs. 77.4 ± 7.75 ng/mL; P < 0.0001). The overall mean MMP-9 level was significantly elevated compared to controls (1270 ± 56.7 ng/mL vs. 77.4 ± 7.75 ng/mL; P < 0.0001), as shown in Table 1. The minimum overall level of MMP-9 in OA patients was not detectable, the median level was 1252 ng/mL, while the maximum level was 2955 ng/mL. Meanwhile, the overall minimum level of MMP-9 in controls was 34.1 ng/mL, the median was 72.5 ng/mL and the maximum was 174 ng/mL.
Figure 1.

Box plot showing median levels of matrix metalloproteinase-9 (MMP-9) stratified by draw date (day 0, day 1, day 5-7) versus controls. Boxes show interquartile ranges and I bars demonstrate highest andlowest values. Day 0 versus control, P < 0.0001; day 1 versus control, P < 0.0001; day 5-7 versus control, P < 0.0001; day 0 versus day 1, P = 0.0011; day 0 versus day 5-7, P = 0.337; day 1 versus day 5-7, P = 0.716. (*P < 0.05).
Table 1.
Comparison of Biomarker Levels Between Controls and Osteoarthritis Patients.
| Biomaker | Control | OA | P | %Δ |
|---|---|---|---|---|
| MMP-9(ng/ml) | 77.4 ± 7.75 | 1270 ± 56.7 | <0.0001 | +1541 |
| OPN(ng\ml) | 66.3 ± 2.49 | 150 ± 7.20 | <0.0001 | +126 |
| ADAMTS4 (OD450 nm) | 0.099 ± 0.046 | 0.090 ± 0.016 | 0.347 | -9.09 |
There were also significant differences in OPN levels at all time points versus healthy controls (Figure 2). OPN at day 0: (155 ± 12.8 ng/mL vs. 66.3 ± 2.49 ng/mL; P < 0.0001), day 1 post-operatively: (152 ± 11.6 ng/mL vs. 66.3 ± 2.49 ng/mL, P < 0.0001), and at day 5-7 post-operatively: (140 ± 13.8 ng/mL vs. 66.3 ± 2.49 ng/mL, P = 0.0007). The overall mean of OPN was also significantly elevated compared to healthy controls (150 ± 7.20 ng/mL vs. 66.3 ± 2.49 ng/mL; P < 0.0001), as shown in Table 1. The overall minimum value of OPN in OA patients was not detectable, the median was 133 ng/mL, and the maximum was 392 ng/mL. The healthy controls had a minimum OPN level of 31 ng/mL, a median level of 63 ng/mL, and a maximum of 117 ng/mL.
Figure 2.
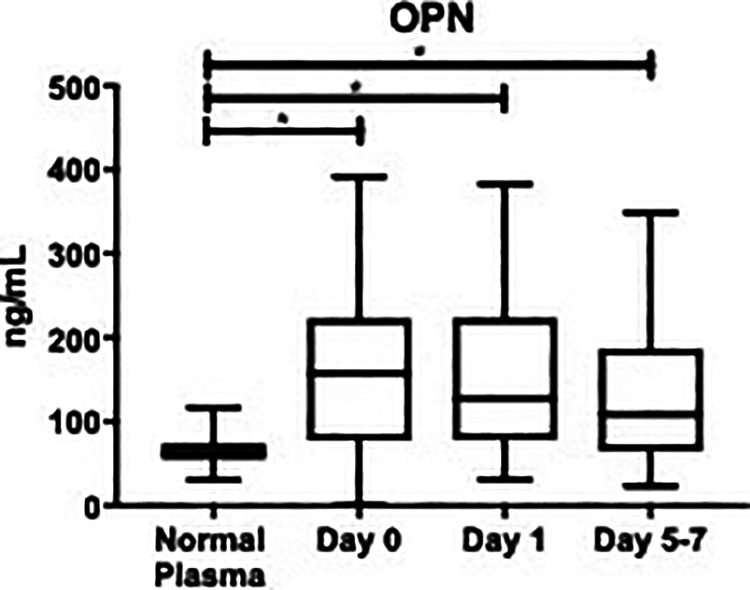
Box plot showing median levels of osteopontin (OPN) stratified by draw date (day 0, day 1, day 5-7) versus controls. Boxes show interquartile ranges and I bars demonstrate highest and lowest values. Day 0 versus control, P < 0.0001; day 1 versus control, P < 0.001; day 5-7 versus control, P = 0.007; day 0 versusday 1, P > 0.999; day 0 versus day 5-7, P > 0.999; day 1 versus day 5-7, P > 0.999. (*P < 0.05).
ADAMTS4 showed a significant decrease versus healthy controls at day 0 (-1.21 ± 0.028 log (OD450 nm) vs. -0.850 ± 0.169 log (OD450 nm); P = 0.0077). However, at day 1 post-operatively and day 5-7 post-operatively, the differences were not statistically significantly different than healthy controls. ADAMTS4 at day 1: (-1.15 ± 0.04 log (OD450 nm) vs. -0.850 ± 0.169 log (OD450 nm); P = 0.1038), and day 5-7 post-operatively (-1.05 ± 0.07 log (OD450 nm) vs. -0.850 ± 0.169 log (OD450 nm); P = 0.7393), as shown in Figure 3. In OA patients, the overall mean level of ADAMTS4 was decreased compared to controls (0.090 ± 0.016 OD 450 nm vs. 0.099 ± 0.046 OD 450 nm); P = 0.347). The percent change between control and OA patients was -9.09%, as shown in Table 1. The minimum overall ADAMTS4 level in OA patients was not detected, median was 0.055 OD 450 nm, and maximum was 1.59 OD 450 nm. Accordingly, in healthy controls the minimum was not detected, the median was 0.063 OD 450 nm, and the maximum was 0.502 OD 450 nm.
Figure 3.
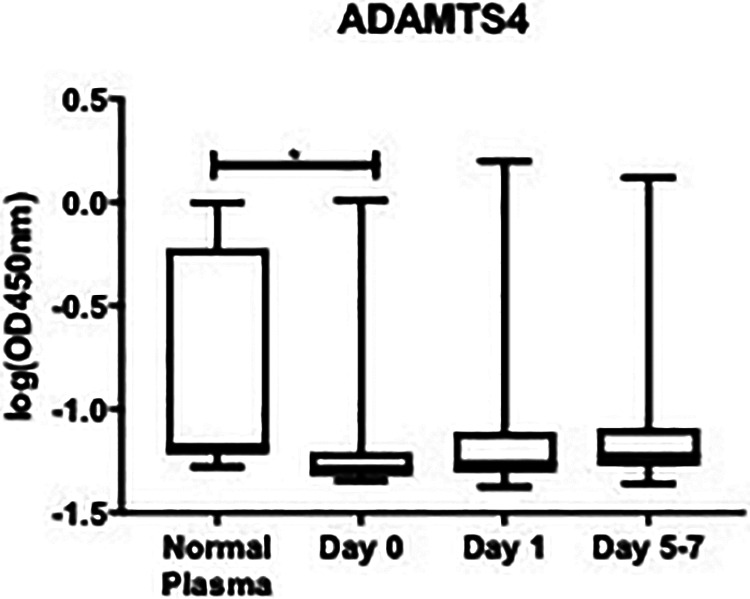
Box plot showing median levels of a disintegrin and metalloproteinase with thrombospondin motifs4 (ADAMTS4) stratified by draw date (day 0, day 1, day 5-7) versus controls. Boxes show interquartileranges and I bars demonstrate highest and lowest values. Day 0 versus control, P = 0.0077; day 1 versuscontrol, P = 0.1038; day 5-7 versus control, P = 0.7393; day 0 versus day 1, P = 0.7694; day 0 versus day 5-7, P > 0.999; day 1 versus day 5-7, P = 0.0900. (*P < 0.05).
Discussion
Previous studies have examined these inflammatory and cartilage degradation biomarkers and their relationship to the pathogenesis of OA.4,5,14 Our study shows results consistent with these findings meanwhile indicating a relationship between these biomarkers. The results of this study indicate that both inflammation and cartilage degradation are interrelated factors that play a role in the development and progression of OA and may even have an effect on post-operative recovery of patients that undergo TJA. The relationship between these markers may shed light on the chain of events in the pathogenesis of OA.
In this study, OPN was shown to be elevated in patients with OA versus the healthy controls. It is known that the mRNA expression of OPN is elevated in OA patients, as well as the fact that it is increased in response to inflammation and injury, leading to increased destruction of articular cartilage.5 As it is known that cleaved OPN upregulates NF-κB, which stimulates IL-1, IL-6, TNF-α and other inflammatory biomarkers, this shows that increased cleavage of OPN leads to upregulation of the inflammatory cascade.19,20 This proves there is an opportunity for intervention to break the progression of this positive feedback cycle once it is initiated in the pathogenesis of OA. The central role of thrombin-cleaved OPN in the upregulation of both the matrix degradation and hypercoagulable state is therefore important.
Our study also demonstrated that MMP-9 is elevated overall in patients with OA compared to controls, which is consistent with what has been shown in other studies. This is due to the fact that MMP-9 is upregulated by inflammatory cytokines and thrombin, which are elevated in OA.14,21 Thrombin stimulates both the inflammatory response and fibrin deposition in patients with OA, lending itself as an important intermediary in both hemostatic and cytokine activation pathways.21 As previously stated, OPN is also known to upregulate these inflammatory biomarkers, indicating that this pathway may contribute to adverse outcomes in a patient with OA.19 MMP-9, enterokinase and thrombin cleave OPN at various different sites.14 The biologic activity of the cleaved fragments of OPN is determined based on what site is exposed and therefore, which fragment is generated.22 MMP-9 has been shown to cleave OPN in cardiac fibroblasts, which has not been reported before in patients with OA.8 Importantly, MMP-9 produces a fragment that includes an arginyl-glycyl-aspartic acid (RGD)-containing sequence.8 Cellular interactions with OPN are mediated via integrins, and via the RGD-binding sequence, OPN interacts with the α5β1 integrin specifically.22 The α5 integrin is known to play a causative role in the pathogenesis of OA, indicating that this RGD-sequence, and therefore MMP-9, contribute to OA pathogenesis through its interaction with OPN.23,24 This study has proven that both MMP-9 and OPN are elevated in late-stage OA patients, and their relationship in the context of OA is a novel observation. Additional studies are necessary to further elucidate this causal relationship, but these new interactions are helpful next steps in our continued understanding of joint degradation.
ADAMTS4 levels were decreased overall compared to healthy controls. These results are consistent with a previous study that determined that ADAMTS4 expression is decreased in late stage OA.20 The patients in our study underwent THA due to severe OA, so these levels were expected to be low. In comparison to the control group, there was a wide scatter in the absorbance representing the ADAMTS4 antigen levels in plasma samples. Methodological differences in the commercially available ELISA methods for this analyte utilized different type of capturing antibodies. The assay kit used in our study may have relatively limited sensitivity, which resulted in the observed lower absorbance. For this reason, logarithmic transformation of the absorbance provided a clearer trend toward the decreased levels in OA patients. However, these results were upheld in other models of OA. Nevertheless, the decreased trend, as signaled by lower absorbance, validates the previously upheld observations.
In other studies, OPN has been shown to decrease the mRNA expression of ADAMTS4.15 Since OPN is elevated in our patients, it follows that ADAMTS4 expression would be lower for this reason. As previously noted in Figures 2 and 3, OPN and ADAMTS4 inversely fluctuate post-operatively. While OPN steadily decreases post-operatively from day 0, day 1, and day 5-7, ADAMTS4 steadily increases along these same time points. This further elucidates the interaction between these 2 biomarkers.
MMP-9 is known to be activated by thrombin, which amplifies the effects of MMP-9 on both the activation of OPN and loss of articular cartilage.25 Thrombin itself also cleaves OPN, leading to its upregulation and contribution to the pathogenesis of OA.10 The role of thrombin in both activation of MMP-9, OPN and the inflammatory cascade, as well as its central role in hemostasis makes it an important contributor to the progression of OA and a promising area of future study in relation to monitoring of disease progression, therapeutics and intervention strategies.10,25
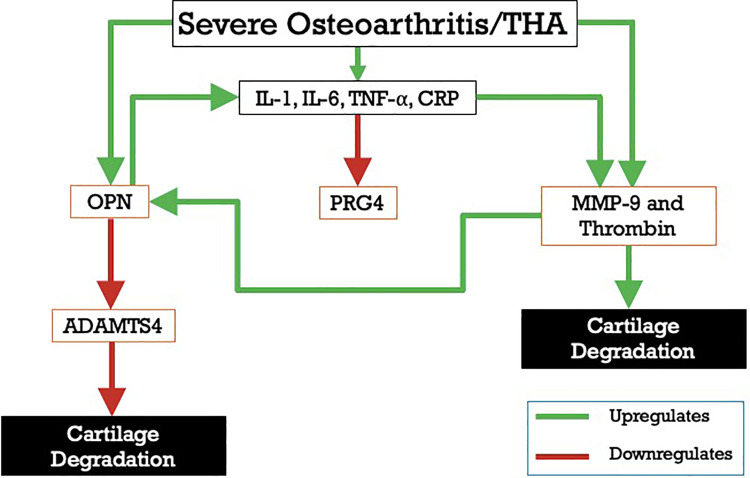
Figure 4 depicts the relationship between these biomarkers, inflammation and the cascade that contributes to late stage OA. An individual with severe OA is known to have upregulation of the inflammatory cascade, OPN, MMP-9 and thrombin.5,10,14,19,20 MMP-9 contributes to cartilage degradation by both inducing loss of articular cartilage itself while also cleaving OPN, exposing the RGD-binding site. OPN is also elevated in inflammatory states and is known to also induce inflammation via upregulation of NF-κB, which initiates a positive feedback cycle.8,13,19,20 Meanwhile, OPN is also known to inhibit the expression of ADAMTS4 in these patients, helping to prevent further degradation of cartilage by inhibiting continued digestion of aggrecan.15
Figure 4.
Interrelationship of OA biomarkers. MMP-9, matrix metalloproteinase-9; OPN, osteopontin; ADAMTS4, a disintegrin and metalloproteinase with thrombospondin motifs 4; PRG4, proteoglycan 4; IL-1,interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; CRP, C-reactive protein; THA, totalhip arthroplasty.
Conclusion
This study suggests that there is dysregulation between OPN, MMP-9 and ADAMTS4 in patients with OA. MMP-9 is elevated and cleaves OPN, which is then damaging to cartilage by upregulating the inflammatory cascade. OPN also downregulates the expression of ADAMTS4 in late stage OA. Measurement of these levels as OA progresses may be helpful in monitoring levels of inflammation and cartilage degradation, giving clinicians a point at which they can intervene with therapies. These results reported herein clearly demonstrate the interrelationship between thrombin and matrix degrading enzymes, both of which cleave OPN, which in turn downregulates ADAMTS4, depicting the interrelationship between these mediators. Thus, these biomarkers are implicated in the overall pathogenesis of OA, especially at the later stages. MMP-9, OPN and ADAMTS4, along with thrombin-related biomarkers, such as thrombin-antithrombin complex and D-dimer, may be of use in determining individualized treatment plans and surgical interventions for these patients.
Acknowledgments
The authors gratefully acknowledge the assistance of the staff of the Hemostasis Research Laboratories, the Department of Orthopaedic Surgery and Rehabilitation, and the Department of Pathology for their assistance in the collection and processing of the patients’ plasma samples used in this study. We are also thankful to Dr. Alexander Ghanayem of the Department of Orthopaedic Surgery and Rehabilitation of Loyola for his guidance and support for this work. This study was partly funded through grant number NIAID T35 AI125220.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics and patient consent: Ethical approval to collect residual blood samples from the clinical laboratories was granted by the Institutional Review Board of the Loyola University Medical Center (IRB# 9192 0510980).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hannah Slovacek  https://orcid.org/0000-0003-0678-9615
https://orcid.org/0000-0003-0678-9615
Mateja Jezovnik  https://orcid.org/0000-0002-5317-0148
https://orcid.org/0000-0002-5317-0148
Debra Hoppensteadt  https://orcid.org/0000-0001-9342-4213
https://orcid.org/0000-0001-9342-4213
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthr Cartil. 2014;12(Suppl A):S31–33. [DOI] [PubMed] [Google Scholar]
- 2. Ruan MZC, Erez A, Guse K, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5(176):176ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scatena M, Lucy L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27(11):2302–2309. [DOI] [PubMed] [Google Scholar]
- 4. Nguyen LT, Sharma AR, Chakraborty C, Saibaba B, Ahn ME. Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci. 2017;18(3):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao SG, Li KH, Zeng KB, Tu M. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr Cartil. 2010;18(1):82–87. [DOI] [PubMed] [Google Scholar]
- 6. Shang H, Hao Y, Hu W, Hu X, Jin Q. OPN gene locus is associated with the risk of knee osteoarthritis: a case–control study. Biosci Rep. 2019;39(2):BSR20182023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brien ERO, Garvin MR, Stewart DK, et al. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994;14(10):1648–1656. [DOI] [PubMed] [Google Scholar]
- 8. Lindsey ML, Zouein FA, Tian Y, Iyer RP. Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can J Physiol Pharmacol. 2015;93(10):879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renu A, Crawford HC, Haro H, Matrisian LM, Havrda MC. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem. 2001;276(30):28261–28267. [DOI] [PubMed] [Google Scholar]
- 10. Gao SG, Cheng L, Zeng C, et al. Usefulness of specific OA biomarkers, thrombin-cleaved osteopontin, in the posterior cruciate ligament OA rabbit model. Osteoarthr Cartil. 2013;21(1):144–150. [DOI] [PubMed] [Google Scholar]
- 11. Kurata M, Zeng T, Kumon Y, et al. Plasma thrombin-cleaved osteopontin elevation after carotid artery stenting in symptomatic ischemic stroke patients. Hypertension Res. 2012;35(2):207–212. [DOI] [PubMed] [Google Scholar]
- 12. Shigeyuki K, Ikesue M, Kimura C, et al. Syndecan-4 protects against osteopontin-mediated acute hepatic injury by masking functional domains of osteopontin. J Exp Med. 2008;205(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meszaros E, Malemud CJ. Prospects for treating osteoarthritis: enzyme–protein interactions regulating matrix metalloproteinase activity. Ther Adv Chronic Dis. 2012;3(5):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith GN., Jr The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front Biosci. 2006;11(3081):95. [DOI] [PubMed] [Google Scholar]
- 15. Gao SG, Zeng C, Tagawa M, et al. Effect of osteopontin on the mRNA expression of ADAMTS4 and ADAMTS5 in chondrocytes from patients with knee osteoarthritis. Exp Ther Med. 2015;9(5):1979–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang M, Jin H, Shen J, Im HJ, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li W, Wang H, Zhang C. Increased serum ADAMTS-4 in knee osteoarthritis: a potential indicator for the diagnosis of osteoarthritis in early stages. Genet Mol Res 2014;13(4):9642–9649. [DOI] [PubMed] [Google Scholar]
- 18. Ayanoglu T, Atalar H, Esen E, Ataoglu MB. The role of ADAMTS genes in the end stage of hip osteoarthritis. Acta Orthop Traumatol Turc. 2019;53(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kathryn WX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Reviews. 2008;19(5-6):333–345. [DOI] [PubMed] [Google Scholar]
- 20. Sun PF, Kong WK, Liu L, et al. Osteopontin accelerates chondrocyte proliferation in osteoarthritis rats through the NF-κb signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(6):2836–2842. [DOI] [PubMed] [Google Scholar]
- 21. Chou PY, Su CM. The characteristics of thrombin in osteoarthritic pathogenesis and treatment. Biomed Res Int. 2014;2014:407518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christensen B, Sørensen ES. Osteopontin is highly susceptible to cleavage in bovine milk and the proteolytic fragments bind the αVβ3-integrin receptor. J Dairy Sci. 2014;97(1):136–146. [DOI] [PubMed] [Google Scholar]
- 23. Candela ME, Wang C, Gunawardena AT, et al. Alpha 5 integrin mediates osteoarthritic changes in mouse knee joints. PLoS One. 2016;11(6):e0156783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. AlmonteBecerril M, Costell M, Kouri JB. Changes in the integrins expression are related with the osteoarthritis severity in an experimental animal model in rats. J Orthopaedic Res. 2014;32(9):1161–1166. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Jianmin L, Shaoheng H. Induction of MMP-9 release from human dermal fibroblasts by thrombin: involvement of JAK/STAT3 signaling pathway in MMP-9 release. BMC Cell Biol. 2007;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]