Abstract
Approximately half of heart failure patients in the US have heart failure with preserved ejection fraction (HFpEF). HFpEF impairs physical performance and thus reduces quality of life. Increasing dietary protein intake can increase lean body mass and physical performance in healthy elderly individuals, but the effect of a high-quality protein supplement, with or without a structured exercise program, has not been investigated in HFpEF patients. Twenty-three obese elderly HFpEF patients with grade 1 or 2 diastolic dysfunction were randomized into three groups: control, protein supplementation alone, and protein plus exercise. Protein supplementation involved providing sufficient whey protein so that total intake was 1.2 g protein/kg/day. The exercise intervention was 2 days of hydrotherapy and 1 day of gym sessions per week under supervision of a fitness expert. Physical parameters and functional tests were performed at baseline and at 12 weeks. Protein supplementation alone failed to improve physical performance. However, when combined with light exercise, there was significant improvement in some (6-minute walk, 10 m walking speed, quadriceps strength), but not all, physical function measurements. The results of this pilot study suggest that further exploration of potential interactive effects between protein supplementation and light exercise in individuals with HFpEF is warranted.
Keywords: heart failure, dietary supplements, exercise therapy, aging, obesity
Introduction
Approximately half of the HF patients in the US have heart failure with preserved ejection fraction (HFpEF), a health condition that currently places a burden of over $15 billion on the US economy (Virani et al., 2020). One of the key clinical features of HFpEF is poor exercise tolerance, which leads to decreased functional capacity and poor quality of life (Gupte & Hamilton, 2016; Kitzman et al., 2014). Pharmacotherapies have successfully improved clinical and mortality outcomes in HF with reduced ejection fraction, but that is not the case for HFpEF. As a result, non-pharmacological therapies for HFpEF, including dietary and exercise interventions, are appealing. A number of clinical trials involving exercise have been performed in HFpEF patients (Pandey et al., 2015), but investigations of dietary interventions have been limited.
There is a tendency to develop protein malnutrition with increasing age or chronic medical conditions, including HF (Cheng et al., 2017; Picetti et al., 2017; Wolfe et al., 2008). Increasing dietary protein consumption in older healthy individuals to as much as twice the recommended dietary allowance of 0.8 g/kg/day has been widely recommended to maximize lean body mass and physical performance in healthy elderly individuals (Baum et al., 2016; Borsheim et al., 2008; Dillon, 2013; Wolfe et al., 2008). However, “anabolic resistance”, wherein the normal stimulatory effect of dietary protein on muscle protein synthesis is diminished, is a prominent feature of HF (Azhar & Wei, 2013; Baum et al., 2016; Dillon, 2013; Kim et al., 2019). Anabolic resistance may limit the benefit of increasing dietary protein consumption in HFpEF.
Exercise therapy has also been studied in the elderly and its beneficial effects have been established in overweight patients with HFpEF (Nolte et al., 2015; Upadhya & Kitzman, 2019). However, there is limited data exploring the effects of protein supplementation on the functional capacity of overweight or obese HFpEF patients with adjuvant aerobic exercises. One of our previous studies examined an at-home aerobic exercise program paired with protein supplementation in elderly HF patients, who reported reduced perception of fatigue and physical limitations at the end of the study (George et al., 2017). However, it has also been reported in the literature, by us and by others, that people tend to reduce their protein intake with age. This reduced protein intake compounds the weakness and muscle loss associated with aging, and exacerbates the functional impairment often accompanying HF (Baum et al., 2016; Dillon, 2013; Kim et al., 2019; Picetti et al., 2017; Wolfe et al., 2008). It was the goal of this study to determine if supplementation of the diet of older individuals with whey protein improves physical capacity. It was our further goal to determine if a mild structured exercise program could amplify the effect of whey protein supplementation.
Methods
Subjects
After the informed consent was signed, 23 subjects met the inclusion and exclusion criteria. Subjects were 55 to 85 years of age with a body mass index (BMI) between 28 and 45. All subjects also had recent echocardiographic evidence of preserved ejection fraction (EF > 50%) and grade 1 or grade 2 diastolic dysfunction. In addition, the subjects had clinical symptoms characteristic of HF such as poor exercise tolerance and dyspnea on moderate exertion. They were ambulatory, able to perform light exercise and were independent in all Activities of Daily Living (ADL) as well as Instrumental Activities of Daily Living (IADL). Exclusion criteria included allergy to whey protein, renal failure, skin conditions, inability to participate in hydrotherapy and any other unstable medical conditions (Figure 1, Table 1).
Figure 1.
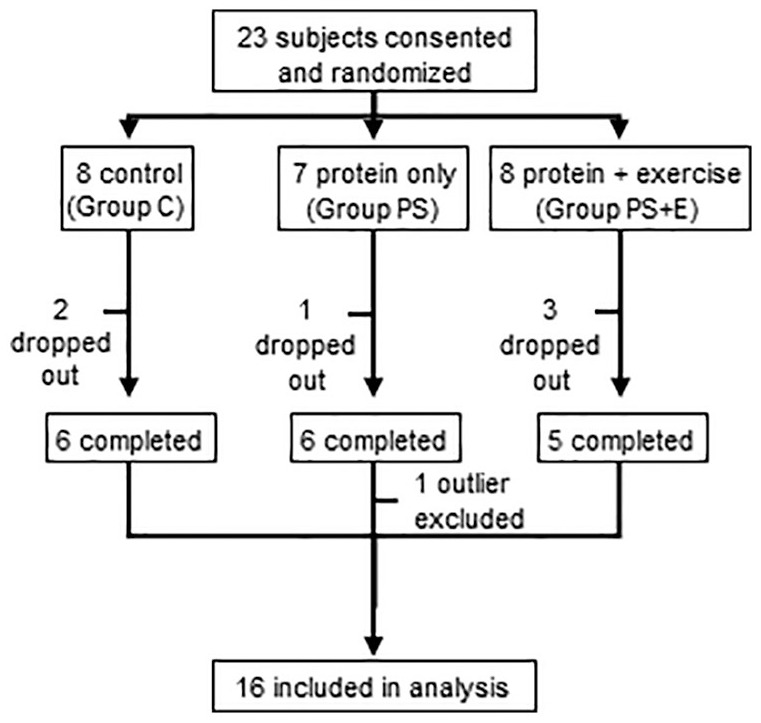
Study schema. There were three groups, control (C), protein supplement only (PS), and protein supplement plus exercise (PS+E).
Table 1.
Demographics and attendance for each treatment group.
| Group C | Group PS | Group PS + E | All | |
|---|---|---|---|---|
| Age (±SE) | 72 (±3.5) | 67 (±3.9) | 71 (±2.8) | 70.1 (±1.9) |
| Gender | ||||
| Female | 3 | 2 | 3 | 8 |
| Male | 3 | 3 | 2 | 8 |
| Race | ||||
| African American | 0 | 4 | 1 | 5 |
| White | 6 | 1 | 4 | 11 |
| Study visits completed (±SE) | 90.9% (±3.7) | 86.7% (±6.2) | 91.7% (±3.1) | 89.8% (±2.5) |
| Ejection fraction (±SE) | 56.7 (±2.5) | 59 (±1.0) | 58.2 (±1.1) | 57.9 (±1.0) |
| Diastolic dysfunction | + | + | + | |
Note. C = control; PS = protein only; PS + E = protein + exercise.
Study Design
This was an open labeled, randomized controlled trial conducted over 12 weeks with three arms of elderly obese patients with HFpEF. After screening and enrollment, subjects were randomized into 1 of the 3 groups: Control (C), Protein Supplementation (PS), or Protein Supplementation & Exercise (PS+E).
Control group (C)
Subjects in group C (N = 6) received no intervention and were monitored weekly without any feedback on diet or exercise.
Protein Supplementation group (PS)
In the PS group (N = 5), whey protein was supplemented to optimize each subject’s daily protein intake to maintain an ideal protein intake, which included their usual diet and up to a maximum of 50 g of whey protein supplement per day. The calculated protein in their diet plus the protein supplement was estimated to be approximately 1.2 g/kg bodyweight per day. They were provided with diet log books and were required to maintain daily logs of all their meals for the entire study period. These logs were photocopied on a weekly basis by the study physician and reviewed by the research dietician to keep track of the subjects’ daily caloric and protein intake. The subjects were counseled to increase or cut back on their protein intake if they exceeded or fell short of their daily protein requirements. Subjects in the PS group were not given any instructions on exercise.
Protein Supplementation + Exercise group (PS+E)
In addition to the protein supplement, subjects in PS+E (N = 5) received a 35-minute gym session once a week (Friday) and a 35-minute water-based exercise session two times a week (Monday and Wednesday) for the entire 12-week study period. Vital signs and weights were measured immediately before and after every gym and hydrotherapy session; once immediately prior to the session (after a 5-minute rest), and again at the end of the session (out of the pool after a 5-minute rest). The second vitals check was to assess the immediate effect of water immersion on central hemodynamics and document any changes in cardiovascular function. The gym exercise regimen (35-minute session each) was comprised of stretching and warm up (3 minutes), resistance training (25 minutes) and balance exercises (7 minutes). Hydrotherapy sessions (35 minutes each) included 5 minutes of warm up, 15 minutes of upper body exercises and 15 minutes of lower body exercises. There was a 20 to 30-minute cool-down period included after each gym or hydrotherapy session.
Functional Assessment Methods
Subjects in all three groups (C, PS, and PS+E) underwent a physical exam (including waist circumference and body composition using a handheld bioelectrical impedance device), electrocardiogram (ECG), measurement of resting blood pressure and heart rate, Saint Louis University Mental Status (SLUMS) cognitive function test and physical functional tests at baseline and end of the 12-week trial (Tariq et al., 2006). All other tests were done as previously described and included the stair climbing test, get up and go (GUG), six-minute walk test (6MWT), finger tapping test, 10 m walking speed (10MWS), quadriceps strength, and grip strength (Kortebein et al., 2008; Mizner et al., 2005; Sallinen et al., 2010; Schatz, 2011; Steffen et al., 2002).
Statistical Methods
The mean (±SE) of the baseline study measures, the end of study measures, and their difference (post-study minus pre-study) were calculated by treatment group. For calculations by treatment group, the difference for each measure was calculated as difference = post – pre. These differences were then compared between treatment groups to determine if the change from pre to post differed in individuals in group C, group PS, or group PS+E. The Mann–Whitney U Test was used to evaluate if treatment groups showed significant differences. Analyses were performed using SAS version 9.4. A significance level of 0.05 was applied to one-sided hypotheses.
Results
Demographics
A total of 23 subjects were consented and randomized (Figure 1). Seventeen subjects successfully completed the 12-week study period. Reasons for non-completion of study included inability to participate in exercises due to worsening angina (n = 1), severe arthritic pain (n = 1), personal reasons (n = 2) and transport problems (n = 2). One subject was excluded as an outlier for having study measures greater than 2 standard deviations outside of the group mean. Of those who completed the study and were included in the analysis, study visit attendance exceeded 89% (Table 1). Subjects were matched for comorbidities in addition to HF; the distribution of comorbidities was similar in all three groups (Table 2).
Table 2.
Number of subjects in each treatment group diagnosed with congestive heart failure and associated comorbidities.
| Group C | Group PS | Group PS + E | All | |
|---|---|---|---|---|
| Congestive heart failure | 6 | 5 | 5 | 16 |
| Hypertension | 6 | 5 | 5 | 16 |
| Hyperlipidemia | 5 | 5 | 4 | 14 |
| Coronary artery disease | 3 | 3 | 1 | 7 |
| Diabetes mellitus | 4 | 4 | 3 | 11 |
| Osteoarthritis | 6 | 4 | 1 | 11 |
| GERD | 3 | 4 | 3 | 10 |
Note. C = control; PS = protein only; PS + E = protein + exercise; GERD = Gastroesophageal reflux disease.
Hemodynamic Changes
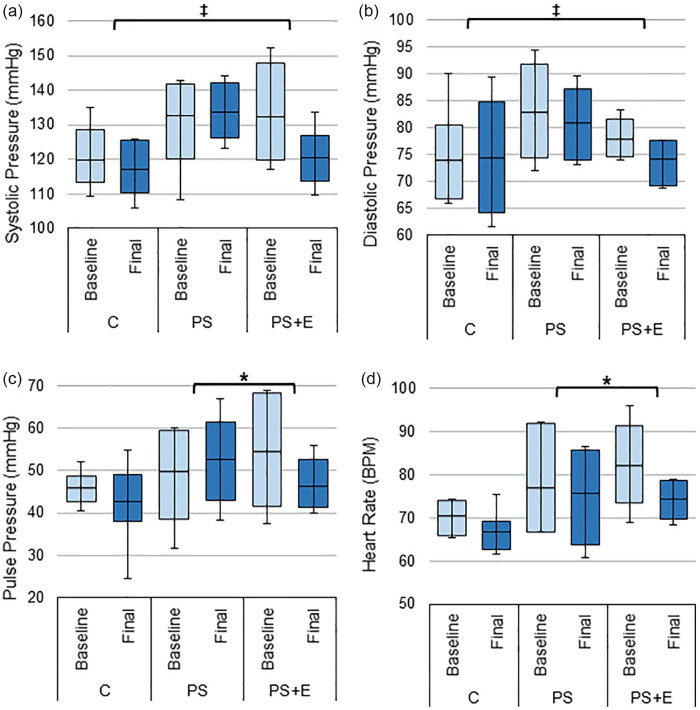
The PS+E group had the most improvement in hemodynamic measures. The change from baseline in systolic and diastolic blood pressure for PS+E was significantly different compared to group C. Group PS+E showed an improvement with decrease in both systolic and diastolic blood pressure (PS+E vs. C, systolic BP: 11.9 ± 4.7 vs. 2.8 ± 2.7, p < 0.05; diastolic BP: 3.6 ± 3.1 vs. –0.4 ± 2.6, p < 0.05, Figure 2a and b).
Figure 2.
Hemodynamic changes. The average: (a) systolic pressure, (b) diastolic pressure, (c) pulse pressure, and (d) heart rate from baseline and final study visits for each treatment group.
Source. Pulse pressure is defined as systolic pressure minus diastolic pressure. Data presented as box plots with mean, 1st and 3rd quartile ranges, and maximum/minimum values.
Note. C = control; PS = protein only; PS + E = protein + exercise.
*Significantly different from PS (p-value < .05). ‡Significantly different from C (p-value < .05).
PS+E also showed an improvement with a decrease in pulse pressure and heart rate from baseline while PS had an increase in pulse pressure and only a slight decrease in heart rate (PS+E vs. PS, pulse pressure: 8.3 ± 3.9 vs. –1.7 ± 3.9, p < 0.05; heart rate: 7.8 ± 3.1 vs. 1.2 ± 2.0, p < 0.05, Figure 2c and d).
Cognition and HF Quality of Life
Subjects in PS group showed a slight tendency toward an improvement or increase in the SLUMs scores, but it was not significant (0.5 ± 0.8, NS) (Table 3).
Table 3.
Functional assessments: average change from baseline (±SE) for each functional assessment in each treatment group. Bolded text indicates most improvement or least decline for each measure.
| Group C Mean (±SE) | Group PS Mean (±SE) | Group PS+E Mean (±SE) | |
|---|---|---|---|
| SLUMs | −0.33 (±1.4) | 0.60 (±0.9) | −0.20 (±1.1) |
| 6-minute walk test, m | −9.55 (±11.0) | −24.08 (±12.1) | 36.63 (±16.5) *‡ |
| Stair climbing test, sec | 0.75 (±3.1) | 0.08 (±1.8) | −3.97 (±5.8) |
| Get up & go test, sec | −0.71 (±0.3) | 0.13 (±0.4)‡ | −1.29 (±1.0) |
| 10 meter walk speed, m/s | −0.04 (±0.0) | −0.23 (±0.2) | 0.13 (±0.1) *‡ |
| Finger tapping test, taps per 10 seconds | |||
| Dominant hand | −1.75 (±4.1) | 0.74 (±1.8) | 5.06 (±3.6) |
| Non-dominant hand | −4.55 (±5.3) | 0.64 (±1.3) | 4.34 (±2.8) |
| Body composition | |||
| BMI | 0.74 (±0.6) | 1.02 (±0.2) | 0.58 (±0.7) |
| Body fat % | −1.00 (±0.6) | 1.72 (±1.1)‡ | −0.60 (±0.5) |
| Hand grip strength, kg | |||
| Dominant hand | 0.90 (±1.4) | 3.68 (±1.6)‡ | 4.06 (±2.7) |
| Non-dominant hand | −0.28 (±1.6) | 1.59 (±1.4) | 2.98 (±1.1) |
| Quadriceps strength, kg | 1.00 (±2.6) | 12.60 (±3.6)‡ | 21.52 (±7.6) ‡ |
Note. C = control; PS = protein only; PS + E = protein + exercise; SLUMs = Saint Louis University Mental status examination.
Significantly different from PS (p-value < .05). ‡Significantly different from C (p-value < .05).
Physical Function Tests
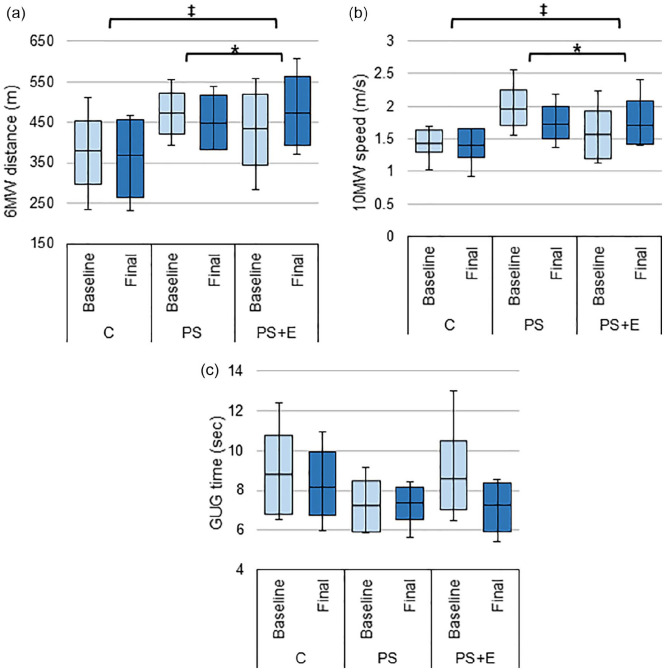
In all of the physical tests, PS+E group showed the most improvement from baseline. Improvement in 6MW distance for PS+E was significantly different from C (36.63 ± 16.5 vs. –9.55 ± 11.0, p < 0.05) and from PS (36.63 ± 16.5 vs. –24.08 ± 12.1, p < 0.01; Figure 3a). Additionally, 10MW speed was significantly improved for PS+E versus C (0.13 ± 0.1 vs. –0.04 ± 0.0, p < 0.05) and PS+E versus PS (0.13 ± 0.1 vs. –0.23 ± 0.2, p < 0.05; Figure 3b). Subjects in both the C and PS+E groups were able to complete the GUG test in a reduced period of time as compared to baseline, which indicated improved speed, however, the results were not significant (Figure 3c). Group PS did not improve and showed a prolonged GUG time (Figure 3c). There were no significant differences between groups for the stair climbing or for the finger-tapping test (Table 3).
Figure 3.
Changes in stamina and endurance. The average baseline and final: (a) 6 minutes walk distance, (b) 10 m walk speed, and (c) get-up-and-go time for each treatment group.
Source. A reduced Get-up-and-go time indicates improvement. Data presented as box plots with mean, 1st and 3rd quartile ranges, and maximum/minimum values.
Note. C = control; PS = protein only; PS + E = protein + exercise.
*Significantly different from PS (p-value < .05). ‡Significantly different from C (p-value < .05).
BMI and Body fat
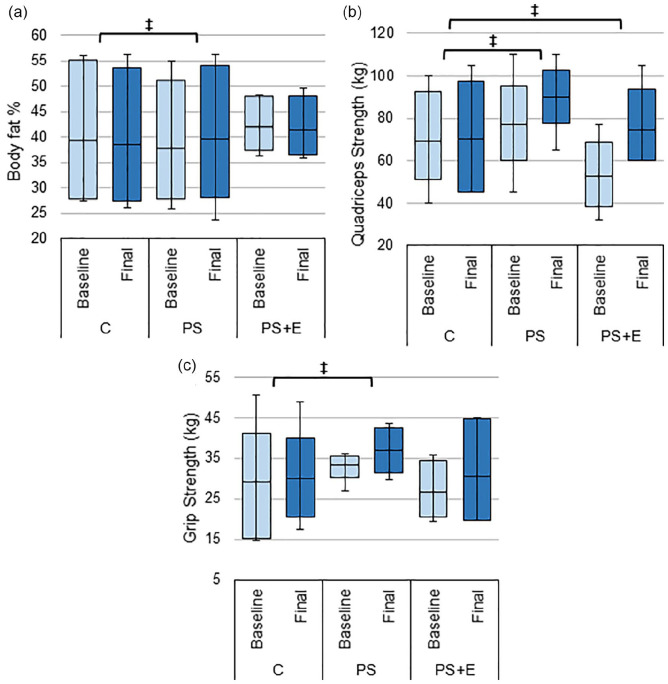
There was no significant difference in BMI within each group from baseline to final. However, the PS group showed a trend in increased weight and BMI versus the other groups (Table 3). PS group also had a significantly higher increase in fat versus C group (p < 0.05, Figure 4a, Table 3).
Figure 4.
Changes in composition and strength. The average baseline and final: (a) body fat %, (b) Quadriceps strength, and (c) handgrip strength test results for subjects’ dominant hand in each treatment group.
Source. Body fat % measured using handheld bioelectric impedance device. Data presented as box plots with mean, 1st and 3rd quartile ranges, and maximum/minimum values.
Note. C = control; PS = protein only; PS + E = protein + exercise.
‡Significantly different from C (p-value < .05).
Strength Tests
Quadriceps strength increased significantly in the PS+E group as compared to C group (21.52 ± 7.6 vs. 1.00 ± 2.6 p < 0.05). Quadriceps strength also increased in the PS + E group as compared to C group (12.60 ± 3.6 vs. 1.00 ± 2.6 p < 0.05; Figure 4b). There was no significant difference between PS and PS+E for this measure (Table 3).
Change in dominant hand grip strength for PS group was significantly different from C group (3.68 ± 1.6 vs. 0.90 ± 1.4, p < 0.05), but PS+E group was not significantly different from C group (Figure 4c).
Discussion
The beneficial effect of structured exercise programs on physical performance in overweight/obese patients with HFpEF has been reported (Nolte et al., 2015; Pandey et al., 2015; Upadhya & Kitzman, 2019). However, many individuals with HFpEF are incapable of performing the level of exercise intensity generally thought to be necessary to elicit beneficial training effects, and even those who benefit significantly from participation in an exercise program usually do not continue with exercise after withdrawing from a structured program (Henderson et al., 2018). For those reasons, in this pilot study we have tested the effect on a variety of physical performance parameters as well as cardiovascular function of a low-intensity exercise program that most individuals with HFpEF can perform and in which long-term participation is a reasonable expectation. We have found that individuals with HFpEF who are consuming approximately 1.2 g protein/kg/day may benefit both in terms of physical and cardiovascular function from an exercise program that most would consider likely to be insufficient to promote significant physiological adaptations.
Systolic, diastolic, and pulse pressures as well as heart rate improved to a greater extent in the PS + E group than in either of the other groups, which reflected possible underlying cardiac re-conditioning. Additionally, involving hydrotherapy along with land-based aerobic exercise may have helped to improve cardiovascular function by redistributing blood from the legs to the central circulatory system, thus improving diastolic filling, heart rate, stroke volume and ejection fraction (Neto et al., 2015).
We ensured that each participant was consuming an adequate amount of dietary protein to maximize the beneficial effect of exercise. We did this because the optimal intake of protein in older individuals has been proposed to be significantly greater than the recommended dietary allowance of 0.8 g protein/kg/day (Baum et al., 2016; Borsheim et al., 2008; Dillon, 2013; George et al., 2017; Wolfe et al., 2008), and many elderly fail to consume even 0.8 g protein/kg/day. In addition to gains in strength, increasing protein intake may improve body composition and quality of life (QOL). Butler showed that using a high protein hypo-energetic diet may help reduce weight and body fat, improve O2 saturation and improve QOL. (Butler, 2016). Additionally, Coker et al., found that improving protein intake may improve lipids and insulin levels even before loss of adipose tissue becomes apparent (Coker et al., 2015). In our current study, we observed weight gain in nearly all subjects who consumed protein, regardless of whether they were engaged in exercise.
Interactive effects between the level of dietary protein intake and the benefits of exercise is well-established, and a deficiency in dietary protein might have limited the adaptions of muscle protein to exercise. However, dietary guidelines for obese elderly with HFpEF have not been addressed. Heart failure of all types is accompanied by “anabolic resistance”, meaning that the usual anabolic response to dietary protein is blunted (Azhar & Wei, 2013; Baum et al., 2016; Dillon, 2013; Kim et al., 2019). It is therefore possible that total dietary protein must be greater than 1.2 g protein/kg/day to observe significant beneficial effects on physical performance. It is possible that we failed to see an effect of PS alone because we did not provide enough supplemental protein.
The major shortcoming of our study was the small number of subjects. In particular, the failure of protein supplementation alone to elicit beneficial effects is at odds with other publications (Borsheim et al., 2008; Coker et al., 2015; Kim et al., 2019), and changes may become statistically significant with more subjects. Conversely, PS+E elicited statistically significant beneficial effects on physical and cardiovascular function with a very small number of subjects. In order to achieve statistical significance with such a small number of subjects requires a robust response. Consequently, the results of this pilot study strongly suggest that a larger study would be likely to show beneficial effects of a low-intensity exercise program including hydrotherapy in individuals with HFpEF consuming an adequate amount of dietary protein.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the Claude D. Pepper Older American Independence Center grant (NIH 1P30AG28718) as well as the Nutritional Therapy in Elderly with Heart Failure grant (NIH 2R42AG050375).
ORCID iDs: Gohar Azhar  https://orcid.org/0000-0002-6810-5189
https://orcid.org/0000-0002-6810-5189
Amanda Pangle  https://orcid.org/0000-0001-5974-618X
https://orcid.org/0000-0001-5974-618X
References
- Azhar G., Wei J. Y. (2013). New approaches to treating cardiac cachexia in the older patient. Current Cardiovascular Risk Reports, 7(6), 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J. I., Kim I. Y., Wolfe R. R. (2016). Protein consumption and the elderly: What is the optimal level of intake? Nutrients, 8(6), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsheim E., Bui Q. U., Tissier S., Kobayashi H., Ferrando A. A, Wolfe R. R. (2008) Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clinical Nutrition, 27(2), 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T. (2016). Dietary management of heart failure: Room for improvement? British Journal of Nutrition, 115(7), 1202–1217. [DOI] [PubMed] [Google Scholar]
- Cheng Y. L., Sung S. H., Cheng H. M., Hsu P. F., Guo C. Y., Yu W. C., Chen C. H. (2017). Prognostic nutritional index and the risk of mortality in patients with acute heart failure. Journal of the American Heart Association, 6(6), e004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker R. H., Deutz N. E., Schutzler S., Beggs M., Miller S., Wolfe R. R., Wei J. (2015). Nutritional supplementation with essential amino acids and phytosterols may reduce risk for metabolic syndrome and cardiovascular disease in overweight individuals with mild hyperlipidemia. Journal of Endocrinology, Diabetes & Obesity, 3(2), 1029. [PMC free article] [PubMed] [Google Scholar]
- Dillon E. L. (2013). Nutritionally essential amino acids and metabolic signaling in aging. Amino Acids, 45(3), 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M., Azhar G., Pangle A., Peeler E., Dawson A., Coker R., Coleman K. S., Schrader A., Wei J. (2017). Feasibility of conducting a 6-month long home-based exercise program with protein supplementation in elderly community-dwelling individuals with heart failure. Journal of Physiotherapy & Physical Rehabilitation, 2(2), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte A. A., Hamilton D. J. (2016). Exercise intolerance in heart failure with preserved ejection fraction. Methodist DeBakey Cardiovascular Journal, 12(2), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. M, Miller M. E, Fielding R. A, Gill T. M, Glynn N. W, Guralnik J. M, King A., Newman A. B., Manini T. M., Marsh A. P., Pahor M, McDermott M. M., Rejeski J., Tudor-Locke C., Kritchevsky S. B., et al. (2018) Maintenance of physical function 1 year after exercise intervention in at-risk older adults: Follow-up from the LIFE study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 73(5), 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. Y., Park S., Smeets E. T., Schutzler S., Azhar G., Wei J. Y., Ferrando A. A., Wolfe R. R. (2019). Consumption of a specially-formulated mixture of essential amino acids promotes gain in whole-body protein to a greater extent than a complete meal replacement in older women with heart failure. Nutrients, 11(6), 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman D. W., Nicklas B., Kraus W. E., Lyles M. F., Eggebeen J., Morgan T. M., Haykowsky M. (2014). Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. American Journal of Physiology-Heart and Circulatory Physiology, 306(9), H1364–H1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortebein P., Symons T. B., Ferrando A., Paddon-Jones D., Ronsen O., Protas E., Conger S., Lombeida J., Wolfe R., Evans W. J. (2008). Functional impact of 10 days of bed rest in healthy older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(10), 1076–1081. [DOI] [PubMed] [Google Scholar]
- Mizner R. L., Petterson S. C., Snyder-Mackler L. (2005). Quadriceps strength and the time course of functional recovery after total knee arthroplasty. Journal of Orthopaedic & Sports Physical Therapy, 35(7), 424–436. [DOI] [PubMed] [Google Scholar]
- Neto M. G., Conceição C. S., de Jesus F. L. A., Carvalho V. O. (2015). Hydrotherapy on exercise capacity, muscle strength and quality of life in patients with heart failure: A meta-analysis. International Journal of Cardiology, 198, 216–219. [DOI] [PubMed] [Google Scholar]
- Nolte K., Herrmann-Lingen C., Wachter R., Gelbrich G., Düngen H. D., Duvinage A., Hoischen N., von Oehsen K., Schwarz S., Hasenfuss G., Halle M., Pieske B., Edelmann F. (2015). Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: The Ex-DHF-P trial. European Journal of Preventive Cardiology, 22(5), 582–593. [DOI] [PubMed] [Google Scholar]
- Pandey A., Parashar A., Kumbhani D., Agarwal S., Garg J., Kitzman D., Levine B., Drazner M., Berry J. (2015) Exercise training in patients with heart failure and preserved ejection fraction: Meta-analysis of randomized control trials. Circulation: Heart Failure, 8(1), 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti D., Foster S., Pangle A., Schrader A., Wei J., Azhar G. (2017). Knowledge and consumption of protein in older adults: Opportunity for improvement. International Journal of Development Research, 01, 12519. [Google Scholar]
- Sallinen J., Stenholm S., Rantanen T., Heliövaara M., Sainio P., Koskinen S. (2010). Hand-grip strength cut points to screen older persons at risk for mobility limitation. Journal of the American Geriatrics Society, 58(9), 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. (2011). Finger tapping test. Encyclopedia of Clinical Neuropsychology, 1, 1050–1051. [Google Scholar]
- Steffen T. M., Hacker T. A., Mollinger L. (2002). Age-and gender-related test performance in community-dwelling elderly people: Six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Physical Therapy, 82(2), 128–137. [DOI] [PubMed] [Google Scholar]
- Tariq S. H., Tumosa N., Chibnall J. T., Perry M. H., III, Morley J. E. (2006). Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder - a pilot study. The American Journal of Geriatric Psychiatry, 14(11), 900–910. [DOI] [PubMed] [Google Scholar]
- Upadhya B., Kitzman D. W. (2019). Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clinical Cardiology, 43(2), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani S. S., Alonso A., Benjamin E. J., Bittencourt M. S., Callaway C. W., Carson A. P., Chamberlain A. M., Chang A. R., Cheng S., Delling F. N., Djousse L, Elkind M. S. V., Ferguson J. F., Fornage M., Khan S. S, Kissela B. M., Knutson K. L., Kwan T. W., Lackland D. T., et al. (2020). Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation, 141(9), e139–e596. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R, Miller S. L, Miller K. B. (2008). Optimal protein intake in the elderly. Clinical Nutrition, 27(5), 675–684. [DOI] [PubMed] [Google Scholar]